Abstract
A total of 108 blood samples obtained from 28 male and 80 female patients diagnosed with ME were diluted in sterile, Ringer’s Solution and forced (by suction) through 0.2 µm filters. Of the 28 male samples, 4 yielded filterable bacteria and of the 80 female samples, 18 gave filterable bacteria; as a result, of the total of 124 samples, 22 yielded FB. Filterable (0.4 and 0.2, but not 0.1micron filterable) bacteria were also isolated from the nose throat and skin of paediatric patients and from the throat and skin of staff at an emergency paediatric hospital. The highest percentage of bacterial passage occurred through the largest (0.4 µm) pores. The results show that ultrasmall bacteria occur in ME patients and in paediatric patients and nurses. The potential pathogenic role of such filterable bacteria is briefly discussed.
Keywords: Ultrasmall bacteria, Filterable bacteria, Nanobacteria, Myalgic encephalomyelitis (ME), Infectious disease
1. Introduction
Ultrasmall bacteria (USBs) which are capable of passing through micro-pore filters (0.2 µm and less) have long been recognized. Such bacteria are also referred to as “filterable”, or “ultra-bacteria” (Al-Sulaiman, 2006, Alshammari, 2010, Fawaz Alshammari et al., 2011). Filterable bacteria were widely studied during the first half of the last century, although much of the early literature on filterable bacteria was criticised on the basis that the porcelain filters used during this period were “leaky” and allowed the passage of relatively large bacteria (Hadley et al., 1931, Heinberger-Nobel, 1951). Reports of the existence of bacteria being able to pass through modern membrane filters have however, confirmed that filterable bacteria are a reality. It is noteworthy that the term ‘filterable virus’ was originally applied to ultra-small, culture able bacteria and that most species of bacteria were said to produce filterable small forms, which were generally thought to be components of a complex bacterial life cycle (Mellon, 1920, Sherman and Safford, 1920, Sherman and Safford, 1931, Wainwright, 1997). Those who doubted the ability of bacteria to develop small forms suggested that such filterability could be explained by assuming that the application of suction occasionally forced normal-sized bacteria through filters. The fact that ultrasmall bacteria exist has however, been confirmed by more recent studies (Jones et al. 1999). Interest in filterable bacteria has also recently been renewed under the term ‘nanobacteria’. However, nanobacteria are considered to be somewhat unusual bacteria (e.g. Brevunimonas (formerly Pseudomonas diminuta), rather than small forms of common bacteria (Blosse et al., 1998, Wainwright, 1999). Despite such recent studies little is known about the distribution of ultrasmall bacteria in the general environments including hospitals, or the role they play as causal agents of human disease (Enby, 1984); of particular importance, is the suggestion that ultrasmall bacteria may play a role in the aetiology of cancer (Wainwright, 1997, Wainwright, 1999, Wainwright, 2000, Wainwright and Al Talih, 2003; Fawaz Alshammari et al., 2011).
The present study was initiated when the opportunity arose to study 1) samples of blood from patients suffering from Myalgic Encephalomyelitis (ME) and 2) children and nursing staff respectively of a paediatric hospital.
The aetiology of ME (or chronic fatigue syndrome) has yet to been determined, and despite often being regarded as a psychotic illness, recent studies point to an infectious aetiology, possibly a type of Lyme Disease, or a non-specific Borreliaosis. Myalgic encephalomyelitis is a syndrome of unknown and possibly multiple aetiologies, affecting the central nervous system, immune system, and many other systems and organs (Tarello, 2001a, Tarello, 2001b). There is no simple diagnostic test and most definitions involve the recognition of a number of features, most commonly severe mental and physical exhaustion or depletion which is “not relieved by rest” and is often worsened by trivial exertion. Myalgic encephalomyelitis occurs more often, but not exclusively, in women, possibly due to immunological factors, but has also been reported in persons of all ages, notably teenagers.
The majority of bacteria, including most pathogens, are around 1micron in size, but much smaller bacteria, exist which can pass through 0.4, 0.2 and possibly 0.1 µm micropore filters. These ultrasmall bacteria have not been widely studied but, because of their small size are likely to play a significant role in human pathogenicity, not least because they are small enough to enter the human cell or even the cell nucleus. As a prelude to determining what, if any role, USBs play in paediatric infection medicine it is first essential that we do a broad survey of the type and occurrence of these bacteria in relation to childhood disease; this is the second aim of the work detailed here.
The purpose of the research work described here was to attempt to isolate ultrasmall bacteria from the blood of ME patients and from the noses, throats and skin of children and from the throats and skin of staff at a paediatric hospital in Saudi Arabia.
2. Materials and methods
2.1. Isolation of ultrasmall bacteria from blood samples
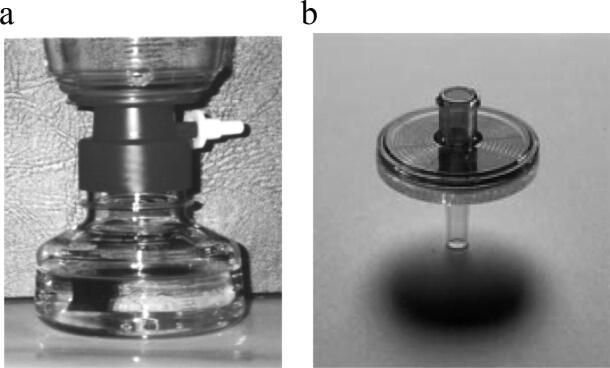
Filters, 0.2 µm, supplied by Nalgene (contained inside sterile, plastic bags) were used (Fig. 1). All manipulations were conducted inside these bags and near flame to avoid any contamination.
Fig. 1.
Membrane filter apparatus used to process a) ME blood samples, b) filter used for samples obtained from patients and staff of a paediatric hospital.
Firstly the screw lid was removed while the filter apparatus (0.2 µm pore size, Fig. 1a) was inside the sterile polythene bag. The medium (L.B) was then injected with a sterile. Hypodermic sterile syringe (20 ml) and a piece of autoclaved tape was used to cover the hole. The filter was kept in vertical position. A blood sample (1 ml in 5 ml of sterile Ringer’s Solution), was then injected into the filter funnel using a 2.5 ml sterile hypodermic syringe and the hole in the polythene bags covered with autoclaved tape. Suction was applied until the sample passed through the filter. The filter funnel was removed, and the bottle containing the medium and culture was closed by the screw cap. Finally, the plastic bag was removed and the bottle was incubated at 37 °C for periods ranging from 1 day to five weeks. After a week, turbidity due to bacterial growth appeared in the medium. This turbid growth was then inoculated on LB plates and any bacterial growth was then sub-cultured. No attempt was made to identify the bacterial isolates.
2.2. Isolation of ultrasmall bacteria for paediatric patients
Clinical samples were obtained from emergency paediatric patients (suffering from a variety of medical problems) from the nose, skin and throat and from the skin and throat of staff at the King Khalid University Hospital, Riyadh. The samples were grown in nutrient broth for 24 h and then passed by syringe pressure through micropore filters of pore size 0.4, 0.2 and 0.4 (Fig. 1b) micron into fresh, sterile Nutrient broth which was incubated until cloudy. An aliquot of the cloudy medium (0.1 ml) was then transferred to Nutrient Agar and any the bacteria of colonies which grew were identified using classical techniques.
3. Results and discussion
-
A).
Ultrasmall bacteria in ME blood samples
A total of 108 blood samples were diluted in sterile, Ringer’s Solution and forced (under suction) through 0.2 µm filters (Fig. 1). The samples were obtained from 28 male and 80 female patients diagnosed with ME (3 replicates). Of the 28 male samples, 4 yielded filterable bacteria and of the 80 female samples, 18 gave filterable bacteria; as a result, of the total of 124 samples, 22 yielded FB.
-
B).
Ultrasmall bacteria isolated from emergency paediatric patients and hospital staff
The results of Table 1 show that 0.4 and 0,2 µm-filterable bacteria were isolated from the nose throat and skin of paediatric patients and from the throat and skin of nursing staff at King Khalid University Hospital, Riyadh; they were mainly gram negative cocci, although an occasional gram negative rod was also found . In contrast no bacteria, from any source, passed through 0.1 µm filters (Table 1). Not surprisingly the highest percentage of bacterial passage occurred through the largest (0.4 µm) pores. Bacteria cultivated in the laboratory are generally of a size around 1micron. The results of Table 1 show that some bacterial element passed through 0.4 and 0.2 µm pores. We are unable to state if such passage was brought about by sub- 0.4 and 0. 2- intact bacterial cells, smaller, life cycle forms, or viable cell fragments. What is however clear, is that a bacterial form capable of growing in a liquid medium below the membrane was cable of passing through the membrane pores.
Table 1.
Number of filterable bacteria isolated (using 0.4, 0.2 and 0.1 µm filters) from paediatric patients and hospital staff (total number tested given in parentheses), ND, not determined.
| Pore size of filter | 0.4 µm |
0.2 µm |
0.1 µm |
|||
|---|---|---|---|---|---|---|
| Samples sources | Paediatric | Staff | Paediatric | Staff | Paediatric | Staff |
| Nose | 4P (5) T | ND | 2P (5) T | ND | 0P (5) T | ND |
| Throat | 10P (19) T | 4P (9) T | 5P (19) T | 1P (9) T | 0P (19) T | 0P (9) T |
| Skin | 4 P (5) T | 4P (5) T | 1P (5) T | 0P (5) T | 0P (5) T | 0P (5) T |
Positive blood samples; () T Total of blood samples; ND, not determined
We also tested a variety of environmental samples for the presence of filterable bacteria, including rainwater, snow, soils, river and lake water and household vacuum cleaner dust, and faeces (cow, sheep, rabbit, human). Except for the vacuum cleaner dust, none of the above environmental samples yielded bacteria (or other microorganisms) when passed through a 0.2 μm filter, with or without the addition of suction pressure. This was evidenced by the fact that no growth occurred in the liquid medium beneath the membrane. A bacterium did however, pass through the filter from the dust suspension and grow in the medium below, after overnight incubation which was independently identified by 16SrRNA (NCIMB, Aberdeen, as Staphylococcus haemolyticus). It should be noted that only one medium was used; other media may have supported the growth of filterable bacteria from these environmental samples. The same medium was used here as was used in the blood studies; other media may of course have isolated filterable bacteria. The fact that environmental samples failed to yield bacteria is both intriguing and highly noteworthy, particularly in relation to the above-mentioned studies showing the presence of filterable bacteria in some blood samples. The results clearly show that filterable bacteria are not widely distributed in environmental samples, such as soil water, and a variety of animal as well as human faces (Al-Sulaiman, 2006, Alshammari, 2010). Of the environmental samples, only vacuum cleaner dust yielded a filterable bacterium, a result which is not readily explainable (although it could be a membrane-damage artifact); its presence might on the other hand be relevant to human pathogenicity, notably in relation to asthma.
Filterability of bacteria through membranes is a statistical event and some expanded pores of a filter may allow the occasional, large, bacterium to pass through. As a result, filterability is ideally confirmed by the demonstration (e.g. by scanning electron microscopy) of the presence of filterable forms. (Wainwright, 1997). As these bacteria are sub-micron in size they represent examples of what have recently been termed nanobacteria. This term can however, lead to confusion, since it is generally used in reference to certain calcified forms, which are often considered bacteria (Kajander, 2006). There, there is no doubt however, that filterable bacteria exist, and can be found, notably in seawater, and may be of considerable medical importance (Lillis and Bissonnette, 2001, McLaughlin et al., 2002).
The fact that most of the environmental samples studied by us lack filterable bacteria provides an important indicator of the validity of the results relating to the blood samples. This is because it could be argued that the detection of filterable bacteria in such samples was due to the presence of holes, larger than the notional size of the filter, which allowed “normal-sized” bacteria to pass through. If this were the case however, one would expect to find filterable bacteria to be apparently widely distributed across a wide range of environmental samples, since the isolation of such bacteria would depend not on the presence of filterable forms, but on the presence of holes, randomly distributed amongst membranes. The fact that filterable bacteria were not widely isolated demonstrates that small, filterable forms are present in the blood samples of ME and paediatric patients, and are unlikely to result from artefacts caused by “leaky membranes”.
The results described above were not derived from a clinical trial and the majority of blood samples from ME patients did not yield ultrasmall bacteria. As a consequence, although blood from M.E. patients can contain filterable bacteria, this finding cannot be used to diagnose the condition. The fact remains that filterable bacteria do exist in human blood, although it is not clear from these results what, if any role they play in the aetiology of M.E. Our results also show that USBs can be isolated from the nose, throat and skin of paediatric patents and the skin and throats of hospital nursing staff. Again, USBS were not isolated from all samples, so it is clear that further work s required to determine the link, if any, between the occurrence of USBs and diseases, including possibly cancer.
Acknowledgements
This project was supported by Researchers Supporting Project number (RSP-2019/5) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alshammari F. University of Sheffield; 2010. Application of Molecular Identification and Other Modern Approaches to the Study of Ultrasmall Bacteria. PhD Thesis. [Google Scholar]
- Al-Sulaiman M.A. University of Sheffield; UK: 2006. Studies on Pleomorphic and Filterable Bacteria in Blood and Environmental Samples. PhD Thesis. [Google Scholar]
- Blosse P.T., Boulter E.M., Sundaram S. Diminutive bacteria-implications for validating sterile filtration processes. Amer. Biotech. Lab. 1998;16:38–40. [Google Scholar]
- Enby E.O.H. Microbe-like formations in the blood of patients with chronic of diseases. Swedish. J. Biol. Med. 1984;8:1–12. [Google Scholar]
- Alshammari Fawaz, Wainwright Milton, Alabri Khalid, Alharbi Sulamain A. Studies on ultrasmall bacteria in relation to the presence of bacteria in the stratosphere. International J. Astrobiol. 2011;10(2):99–103. [Google Scholar]
- Hadley P., Delves E., Klimer J. The filterable forms of bacteria: a filterable stage in the life history of the Shiga dysentery bacillus. J. Infect. Dis. 1931;48:1–159. [Google Scholar]
- Heinberger-Nobel E. Filterable forms of bacteria. Bacteriol. Rev. 1951;15:77–103. doi: 10.1128/br.15.2.77-103.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.R., Chamberlain A.H., Adams M.R. An investigation of the presence of ultra-microcells in natural mineral water. Lett. Appl. Microbiol. 1999;28:275–279. doi: 10.1046/j.1365-2672.1999.00526.x. [DOI] [PubMed] [Google Scholar]
- Kajander E.O. Nanobacteria-propagating calcifying nanoparticles. Lett. Appl. Microbiol. 2006;42:549–552. doi: 10.1111/j.1472-765X.2006.01945.x. [DOI] [PubMed] [Google Scholar]
- Lillis T.O., Bissonnette G.K. Detection and characterization of filterable heterotrophic bacteria from rural groundwater supplies. Lett. Appl. Microbiol. 2001;32:268–272. doi: 10.1046/j.1472-765x.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin R.W., Vali H., Lau P.C.K., Palfree R.G.E., De Ciccio A., Sirois M., Ahmad D., Villemur R., Desrosiers M., Chan E.C.S. Are there naturally occurring pleomorphic bacteria in the blood of healthy humans? J. Clin. Microbiol. 2002;40:4771–4775. doi: 10.1128/JCM.40.12.4771-4775.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon R. Life cycles of the bacteria and their possible relation to pathology. Amer. J. Med. Sci. 1920;159:874–882. [Google Scholar]
- Sherman J.M., Safford C.F. Primitive or filterable forms of bacteria. Science. 1920;74:602–603. doi: 10.1126/science.74.1928.602. [DOI] [PubMed] [Google Scholar]
- Sherman J.M., Safford C.F. The occurrence of filterable forms of bacteria in nature. Science. 1931;79:448–449. doi: 10.1126/science.73.1895.448-a. [DOI] [PubMed] [Google Scholar]
- Tarello W. Chronic fatigue syndrome (CFS) associated with Staphylococcus spp. bacteremia, responsive to potassium arsenite 0.5% in a veterinary surgeon and his co-working wife, handling with CFS animal cases. Comp. Immunol. Microbiol. Infect. Dis. 2001;24:233–246. doi: 10.1016/s0147-9571(01)00012-1. [DOI] [PubMed] [Google Scholar]
- Tarello W. Chronic fatigue syndrome (CFS) in 15 dogs and cats with specific biochemical and microbiological anomalies. Comp. Immunol. Microbiol. Infect. Dis. 2001;24:165–185. doi: 10.1016/s0147-9571(00)00025-4. [DOI] [PubMed] [Google Scholar]
- Wainwright M. Extreme pleomorphism and the bacterial life cycle-a forgotten controversy. Persp. Biol. Med. 1997;40:207–425. [Google Scholar]
- Wainwright M. Nanobacteria and associated 'elementary bodies' in human disease and cancer. Microbiol. 1999;145:2623–2624. doi: 10.1099/00221287-145-10-2623. [DOI] [PubMed] [Google Scholar]
- Wainwright M. Highly pleomorphic Staphylococci as a cause of cancer. Med. Hypoth. 2000;54:91–94. doi: 10.1054/mehy.1998.0827. [DOI] [PubMed] [Google Scholar]
- Wainwright M., Al Talih A. Is this the historical “cancer germ”? Med. Hypoth. 2003;60:290–292. doi: 10.1016/s0306-9877(02)00388-2. [DOI] [PubMed] [Google Scholar]