Abstract
Cactaceae plant family comprises over 130 genera and 2000 species of succulent flowering plants. The genera Mammillaria and Notocactus (Parodia), which have medicinal and nutritional applications as well as aesthetic appeal, are considered to be among the major genera of the family. Several species of both genera show morphological and chemical similarities and diversities according to environmental conditions and genotypes. Here, we assessed the genetic relationships of nine species belonging to two major genera Mammillaria and Notocactus under the family Cactaceae, using two modern gene-targeting marker techniques, the Start Codon Targeted (SCoT) Polymorphism and the Conserved DNA-Derived Polymorphism (CDDP). Besides, we screened the various phytochemicals and evaluated the antioxidant activities of the nine species of cacti. Five out of the 10 SCoT and eight CDDP primers used to screen genetic variations within the nine species yielded species-specific reproducible bands. The entire 156 loci were detected, of which 107 were polymorphic, 26 were monomorphic, and 23 were unique loci. The nine species were categorized into two groups based on the dendrogram and similarity matrix. Phytochemical profiling revealed that sterols, triterpenes, flavonoids, and tannins were found in all the tested species. Additionally, two Notocactus species (N. shlosserii and N. roseoluteus) and one Mammillaria species (M. spinosissima) revealed a considerable antioxidant activity. Our results demonstrated that gene-targeting marker techniques were highly powerful tools for the classification and characterization of the nine investigated species, despite displaying high similarities at both morphological and phytochemical levels.
Keywords: Cactaceae, Authentication, Genetic diversity, SCoT, CDDP, Phytochemical screening
1. Introduction
The family Cactaceae includes approximately 130 genera and 2000 species that were originally native to the New World. Cacti are the best representatives of plants that are good improved toward dry lands and a variety of weathers. Cacti remain famous for their ability to not only grow, but also thrive under stressful environments in numerous parts worldwide, including Australia, India, Mediterranean basin, Middle East, and South Africa. As they do not need much water, they exhibit unusual physiological and morphological characteristics (Osuna-Martínez et al., 2014, Abdelaziz et al., 2019). Different trends in the adaptation of the plants to conditions of water deficit have been identified. Although they are mainly cultivated for their edible fruits, dissimilar parts of these plants are used in the nutrition and cosmetic manufacturing in some countries, thus giving this family a strong economic relevance. They have been globally commercialized as ornamental plants (Hernández-Hernández et al., 2011, Boshara, 2014). Cacti are known to contain several chemical compounds with nutritionally and medicinally desirable properties (Solórzano et al., 2014). In Mexico, Cuba, Colombia, and the United States, the fruits of cacti are used as human food, and some species have a cultural value. In certain rural communities, cactus is predominantly utilized as folk medicine to treat illnesses such as the flu, infections, worms, and urethral problems. Cacti possess a therapeutic effect against cancer cells (Boshara, 2014). The family Cactaceae consists of two major genera, the Echinocactus that represents distinctive cacti of the deserts of Mexico and the USA, and Mammillaria, which is among the largest genera of North American cacti. They are little-rising, usually globose, and have tuberculate shoots. Together these genera were preciously enclosed in the core classification systems of the 19th century (Vázquez-Sánchezet al., 2013).
The genus Mammillaria, which constitutes about 168–171 species, is mostly endangered at the global level. Some species of this genus have a small range of distribution and correspondingly a small population size (Butterworth, 2003, Solórzano et al., 2014). These cacti are fairly small, usually elongated or globular, and profusely flowering. They have colored spines that vary significantly in figure, size, and color. The flowers are typically short and arranged abundantly in rings round the apex of the shoot like a garland. They are famous for their flat, juicy, club-shaped berries, typically of bright red color, which gives a colorful vision during fall. Therefore, Mammillaria represents a permanent source of admiration for collectors as well as nature lovers (Mattagajasingh et al., 2006). This appearance can be attributed to their abundant content of anthocyanin pigments. Some species of Mammillaria, such as M. spinosissima, M. theresae, and M. flavicentra, are rich in flavonoids such as quercetin, luteolin, and kaempferol and their glycosides (Cecília de. Fátima et al., 2006, Formagio et al., 2014). Most species of Mammillaria are reported to be nontoxic and are used to treat ear-aches, dysentery, and as insecticide, poison (either as poison or for treatment against poisoning), purgative, pulicide, and snake repellent. Notocactus or Parodia (the type genus of the family) is a genus of flowering plants in the family Cactaceae native to the uplands of Argentina, Bolivia, Peru, Colombia, Uruguay, and Brazil. Notocactus was included in Parodia by the International Organization for Succulent Plant Study at the end of the 1980s, a decision which is still controversial even today (Machado et al., 2008). The traditional taxonomy of plants is based on shared morphological, biochemical, cytological, and ecological characteristics/traits. In recent years, several novel molecular marker approaches have been developed and applied in numerous plant genetic studies. The development of these methods is mainly as a result of fast growing in genomic study and has initiated a new tendency away from arbitrary markers toward the development of gene-targeting markers (Osuna-Martínez et al., 2014). As a result of rapidly increasing availability of genomic databases, the progress of efficient markers positioned within or near certain genes has become relatively easier (Hernández-Hernández et al., 2011, Atia et al., 2020). These gene-targeting markers have many other applications, such as in studies on genetic variability and diversity, phylogenetics and systematics, molecular ecology, conservation biology, and developmental biology. At the end of the 2000s, Collard and Mackill successfully developed two of the most effective novel gene-targeting marker systems in plants. These marker systems were named the Start Codon Targeted (SCoT) Polymorphism and Conserved DNA-Derived Polymorphism (CDDP). The principle of the SCoT marker system depended on the small, conserved region flanking the ATG start codon in plant genes. SCoT markers were found to be highly reproducible, which could be due to the use of a single 18-mer primer with an annealing temperature of 50 °C (Butterworth, 2003). SCoT is a dominant marker similar to the random amplified polymorphic DNA (RAPD) and inter-simple sequence repeats (ISSR). It was successfully used in numerous genetic analyses, mapping of quantitative trait loci (QTL), and in studies on bulk segregation analysis. SCoT markers yielded tremendous success in fingerprinting and diversity analyses in peanut, potato, grapes, and several medicinal plants (Solórzano et al., 2014). On the other hand, the CDDP approach for producing DNA markers in plants was settled grounded on data mining for small conserved amino acid sequences that were found in proteins. It used a single (15 to 19-mer) primer with a fixed annealing temperature of 50 °C for PCR amplification (Cecília et al., 2006) Indeed, gene-targeted fingerprinting techniques such as SCoT and CDDP were developed to combine the well-established practice of arbitrary marker techniques with new procedural novelties, through the combination of gene or promoter in their primers (Mokhtar and Atia, 2019). These features provided the advantages of good reproducibility and improved resolution to gene-targeted markers, by the simultaneous occurrence of dominant and co-dominant bands. Therefore, this study aims to perform a molecular characterization and fingerprinting of nine species of cacti belonging to two genera; Notocactus and Mammillaria, by using two novel gene-targeting markers (SCoT and CDDP) in order assess their genetic relatedness. Additionally, a preliminary screening of their phytochemical constitutions and evaluation of their antioxidant activities was carried out to obtain baseline information on their pharmacological activities.
2. Materials and methods
2.1. Plant material
All plants were obtained from Private Cactus Farm in Shibin El Qanater (Qalyubia, north of Cairo in the Nile Delta region), Egypt. The plants were collected in the flowering stage in the middle of August 2018. Voucher specimens of altogether cactus plants were placed in the Pharmacognosy Department, Faculty of Pharmacy, Cairo University, Cairo, Egypt. Facts about the names of collected samples, their respective codes and voucher numbers are presented in Table 1 and Fig. 1.
Table 1.
The cactus species, code numbers, and voucher specimen code.
| Name of cactus species | Code Number | Voucher Specimen Code |
|---|---|---|
| M. hahniana Werderm | 1 | 14816 II |
| M. spinosisima Lem. | 2 | 14816 IV |
| M. supertexta Hort. | 3 | 14816 V |
| M. polythele Mart. | 4 | 14816 VI |
| M. springlei J.M.Coult | 5 | 14816 VII |
| N. shlosserii Vliet | 6 | 14816 III |
| N. magnificus F.Ritter | 7 | 14816 IX |
| N. roseoluteus Vliet | 8 | 14816 VIIⅠ |
| N. Leninghausii A.Berger | 9 | 14816 I |
Fig. 1.
The cactus species; 1- Notocactus Leninghausii A. Berger; 2- N. shlosserii Vliet; 3- N. roseoluteus Vliet; 4- N. magnificus F. Ritter; 5- Mammillaria spinosisima Lem;6- M. supertexta Hort.;7- M. polythele Mart.;8- M. springlei J.M. Coult; 9- M. hahniana Werderm.
2.2. Molecular analysis
2.2.1. Extraction of plant DNA
Genomic DNA was isolated from plants (100 mg) of all the nine-plant species via a DNeasy Plant Mini Kit (QIAGEN, Santa Clarita, CA), rendering to the manufacturer’s protocol. The extracted DNA concentration was measured using a Qubit® 3.0 Fluorometer (Thermo Fisher Scientific Inc.). The DNA concentrations were adjusted to 10 ng/µL in all samples for subsequent molecular analyses.
2.2.2. Analysis of SCoT polymorphism
PCR-based amplification of SCoT polymorphism was done as outlined by the technique described by Collard and Mackill (2009a). A set of 10 SCoT primers was screened against the nine species of cacti. PCR was carried out on reaction mixtures of 25 μL comprising 1X PCR buffer, 1.5 mM MgCl2, 0.2 μM of each dNTPs, 1 μM of primer, 1U Go-Taq Flexi polymerase (Promega), and 25 ng of genomic DNA. The PCR amplification was set at 94 °C for 5 min for preliminary denaturation, trailed by 35 cycles (94 °C for 1 min, 50 °C for 1 min, and 72 °C for 90 s), and a final elongation at 72 °C for 7 min. The amplification fragments were resolved using electrophoresis in 1.5% agarose gel comprising ethidium bromide (0.5 µg/mL) in 1× TBE buffer. A 100 bp DNA ladder was used. The PCR products were visualized in UV light and photographed.
2.3. Analysis of CDDP
PCR-based amplification of CDDP was done as described by Collard and Mackill (2009b). A set of eight CDDP primers were screened for the nine cacti species. The amplification reactions were done on 25 μL containing 1X PCR buffer, 1.5 mM MgCl2, 0.2 μM of each dNTP, 1 μM of primer, 1U Go-Taq Flexi polymerase (Promega), and 25 ng of genomic DNA.
A PCR cycle was done: a preliminary denaturation at 94 °C for 3 min, and then 35 cycles of 94 °C for 1 min, 50 °C for 1 min, and 72 °C for 2 min; the final elongation was carried out for 5 min. All the PCR magnification fragments were electrophoresed on 1.5% agarose gels in 1X TBE buffer stained with ethidium bromide. A 100 bp DNA ladder was used as a DNA marker. The PCR products were visualized on UV light and photographed.
2.4. Data analysis
For the analysis of molecular data, the amplified bands were scored visually. To reduce errors, only clear and distinguishable bands were scored. The fragments were counted as present (1) or absent (0) to generate a binary data set. Percentage of polymorphism was calculated by dividing the sum of amplified polymorphic bands by the entire quantity of amplified fragments by the same primer or primer mixture. To estimate the genetic similarity, Jaccard’s coefficient (Jaccard, 1908) was used. A dendrogram was produced by cluster analysis using the unweighted pair group technique of the arithmetic averages (UPGMA) for all the marker systems. Principal component analysis (PCA) was conducted using a D center module (Jaccard, 1908).
2.5. Phytochemical screening
Phytochemicals of all the samples under investigation were screened to identify the main secondary metabolites, such as alkaloids, tannins, saponins, anthraquinones, flavonoids, sterols and/or triterpenes, and cardiac glycosides (Cecília et al., 2006).
2.6. Extraction procedure
The plants were cut into small pieces and extracted with 70% ethanol for three days. The solvent was evaporated using a rotating evaporator (Buchi®R-300, USA) at 45 °C, and kept at 20 °C till used for phytochemical analysis.
2.7. Determination of phenolic content
The entire phenolic contented of cactus extracts was estimated using Folin–Ciocalteu's reagent Machado et al., 2008. Briefly, 1 mg/mL of each cactus extract was liquified in methanol and used as the stock solution. An aliquot of 100 μL of each extract was mixed with 0.5 mL of Folin–Ciocalteu's reagent, after which 1.0 mL of distilled water and supplemented with1.5 mL of 2% aqueous sodium bicarbonate. The reaction combination was left for 30 min with frequent shaky. Finally, the absorbance of the mixture was calculated at 765 nm. All the experiments were repeated three times and the readings were stated as milligrams of gallic acid correspondent (GAE) per gram of dry plant extract.
2.8. Estimation of total flavonoid and flavanol contents
The entire flavonoid contents were estimated using 10% aluminum trichloride hexahydrate in methanol (Machado et al., 2008). Briefly, each extract (500 µL) was diluted with 2.80 mL of distilled water and allowed to react with 0.1 mL of AlCl3 and 0.1 mL of 1 M sodium acetate. The reaction mix was kept for 40 min, after which its absorbance was measured at 415 nm. On the other hand, the flavanol content was measured spectrophotometrically using 2% AlCl3. Of each extract, 2 mL was mixed with 2 mL of AlCl3 and 3 mL sodium acetate (50 g/L) at 20 °C. The resulting mixture was kept for 2.5 h and the absorbance was measured at 440 nm using quercetin as standard. Both flavonoids and flavanols were stated as milligrams of quercetin equals per gram of dry weight (mg QE/g extract).
2.9. Antioxidant activity
2.9.1. Estimation of DPPH free radical scavenging activity
Free radical scavenging capacities of the tested extracts were determined using steady DPPH (Harborne, 1984, Hwang et al., 2014). The final concentration of DPPH was 200 µM and the total reaction was 3.0 mL. After 60 min of incubation in dark conditions, the absorbance was calculated at 517 nm compared to pure methanol as the blank. The percentage of inhibition of free DPPH radicals was measured via the equation: Inhibition (%) = 100 × [(Control – Sample)/Control]. The standard curve was prepared using Trolox as positive control. Results were stated as milligrams of Trolox equivalents per gram of sample (mg TE/g sample).
2.10. Determination of ABTS free radical scavenging activity
The standard resolution of ABTS reagent was prepared by adding equal amounts of a 7 mM aqueous resolution of ABTS with 2.45 mM potassium persulfate and incubating the mixture for 16 h at (25 °C) in the shady. The final resolution was then set by water down using 1 mL of ABTS resolution with 60 mL of ethanol: water (50:50, v/v) to get an absorbance of 1.0 ± 0.02 units at 734 nm. The extracts (50 µL) permitted to react with 4.95 mL of the ABTS resolution for 1 h in shady. The absorbance was then calculated at 734 nm (Harborne et al., 1984). Percentage of inhibition of the ABTS free radical was measured using the equation: Inhibition (%) = 100 × [(Control – Sample)/Control]. The standard curve was arranged using Trolox as positive control. The results were stated as milligram of Trolox equivalent per gram of sample (mg TE/g sample).
3. Results
3.1. Analysis of CDDP and SCoT
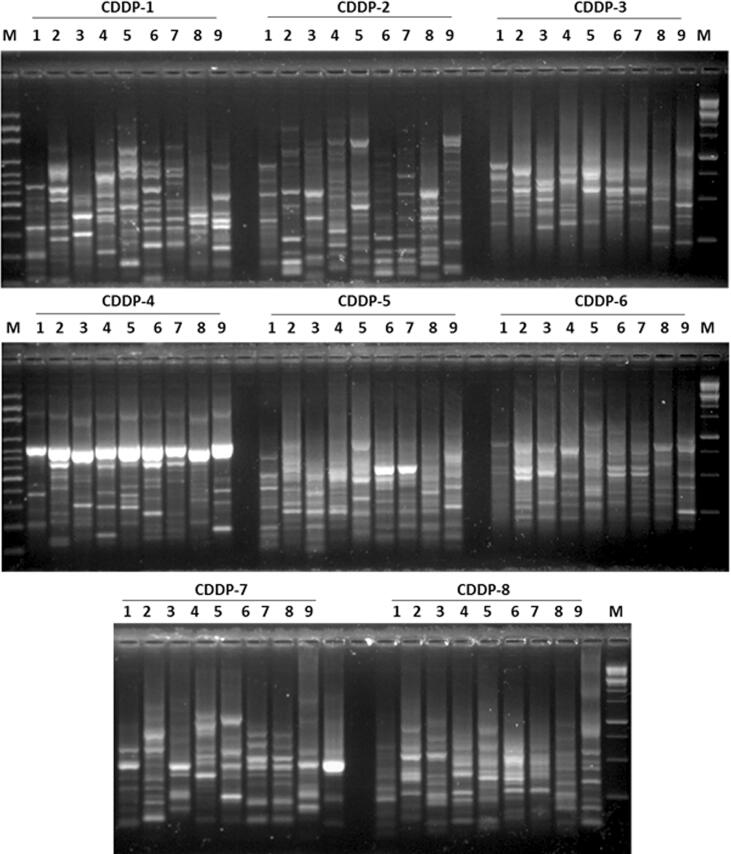
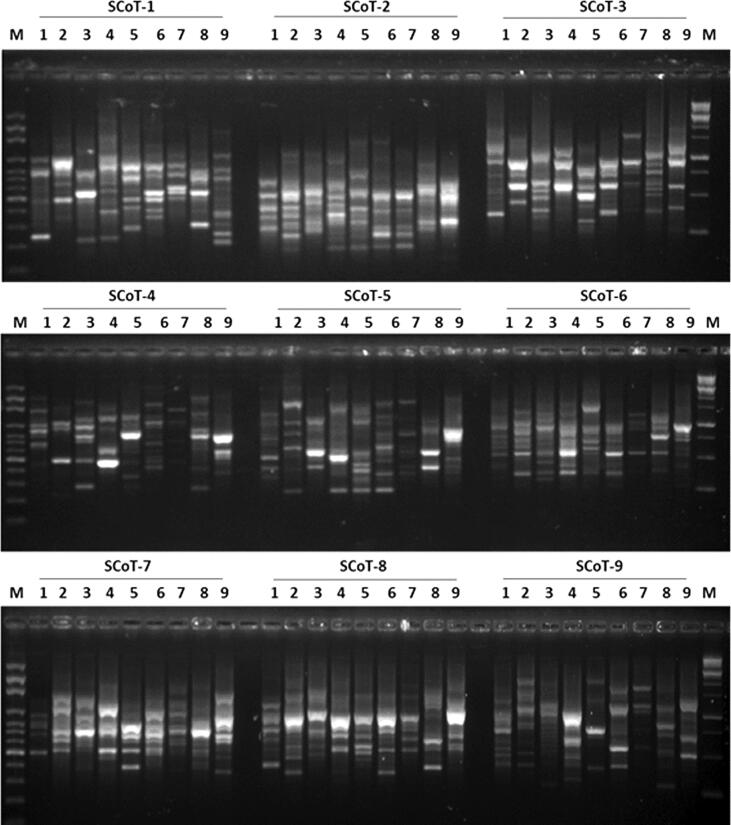
In order to investigate the genetic diversity and evaluate the degree of polymorphism between the nine species of cacti (four of Notocactus and five of Mammillaria), 10 SCoT and eight CDDP primers were used (Table 2). For CDDP analysis, CDDP sequences from the nine species of cacti were amplified using eight CDDP primers (Fig. 2). A total of 190 amplification products were obtained in the form of scorable bands, all of which were polymorphic (100%) (Table 2). The number of amplified DNA fragments per primer ranged from 19 (primer CDDP–8) to 27 (primer CDDP–2), and the percentage of polymorphism for all the used primers was 100%. For SCoT analysis, SCoT sequences from species of both the genera were amplified using 10 SCoT primers (Fig. 3). A total of 179 amplification products were obtained in the form of scorable bands, out of which 175 (98.0%) were found to be polymorphic, with an average 17.9 bands/PC (Table 2). The number of amplified DNA fragments per primer ranged from 13 (primers SCoT–2 and SCoT–9) to 26 (primer SCoT–1), and the percentage of polymorphism ranged from 86% (primers SCoT–7) to 100% (primers SCoT–3, 4, 5, 6, 8, 9, and 10).
Table 2.
Primer code, primer sequences, number of total bands, polymorphic bands, and percentage of polymorphism in the CDDP and SCoT primers.
| Code | Primer Sequence | Number of bands |
% of polymorphism | |
|---|---|---|---|---|
| Total | Polymorphic | |||
| CDDP | ||||
| CDDP-1 | TGGCGSAAGTACGGCCAG | 25 | 25 | 100 |
| CDDP-2 | GTGGTTGTGCTTGCC | 27 | 27 | 100 |
| CDDP-3 | GCCCTCGTASGTSGT | 20 | 20 | 100 |
| CDDP-4 | GGCAAGGGCTGCCGC | 23 | 23 | 100 |
| CDDP-5 | GGCAAGGGCTGCCGG | 24 | 24 | 100 |
| CDDP-6 | CACTACCGCGGSCTSCG | 23 | 23 | 100 |
| CDDP-7 | GCSGAGATCCGSGACCC | 22 | 22 | 100 |
| CDDP-8 | TGGCTSGGCACSTTCGA | 19 | 19 | 100 |
| Total | 190 | 190 | 100 | |
| Average | 19 | 19 | ||
| SCoT | ||||
| SCoT-1 | CAACAATGGCTACCACCA | 26 | 25 | 96 |
| SCoT-2 | CAACAATGGCTACCACCC | 13 | 12 | 92 |
| SCoT-3 | CAACAATGGCTACCACCG | 16 | 16 | 100 |
| SCoT-4 | CAACAATGGCTACCACCT | 19 | 19 | 100 |
| SCoT-5 | CAACAATGGCTACCACGA | 23 | 23 | 100 |
| SCoT-6 | CAACAATGGCTACCACGC | 21 | 21 | 100 |
| SCoT-7 | CAACAATGGCTACCACGG | 14 | 12 | 86 |
| SCoT-8 | CAACAATGGCTACCACGT | 18 | 18 | 100 |
| SCoT-9 | CAACAATGGCTACCAGCA | 13 | 13 | 100 |
| SCoT-10 | CAACAATGGCTACCAGCC | 16 | 16 | 100 |
| Total | 179 | 175 | 98 | |
| Average | 17.9 | 17.5 | ||
Fig. 2.
Comparison of the eight CDDP-PCR profiles of the four Notocactus species and five Mammillaria species. Lane on both, the right and left side, is the DNA size marker (100 bp plus). Samples order (1 to 9; left to right).
Fig. 3.
Comparison of the nine SCoT-PCR profiles of the four Notocactus and five Mammillaria species. Lane on both the right and left side is the DNA size marker (100 bp plus). Samples order (1 to 9; left to right).
3.2. Analysis of molecular phylogeny
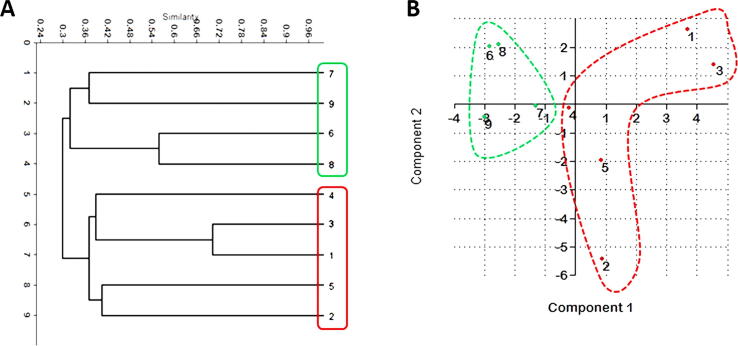
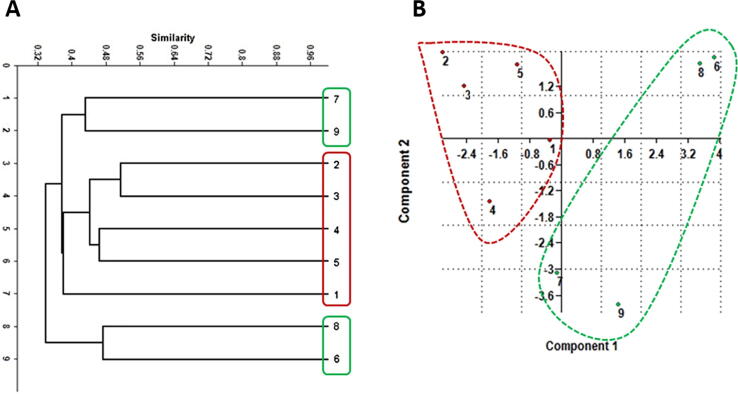
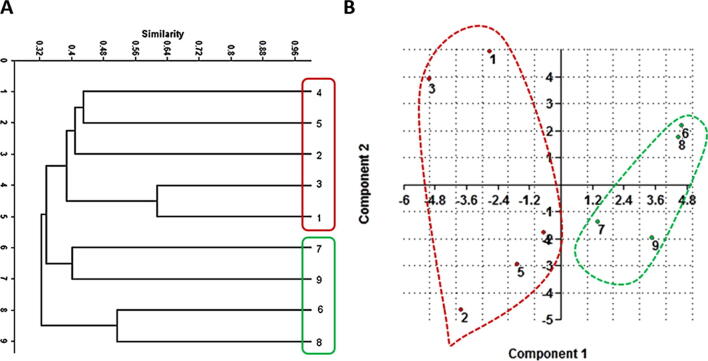
Dendrograms based on UPGMA analysis of SCoT, CDDP, and combined data were constructed for the nine species of cacti (Fig. 4, Fig. 5, Fig. 6). For CDDP, the dendrogram comprised two main clusters. The first cluster successfully grouped all Mammillaria species as two species (3 and 1) that were the most genetically similar and grouped them with species no. 4 in one sub-cluster, while the second sub-cluster comprised two species (2 and 5) (Fig. 4). On the other hand, the second cluster grouped four Notocactus species as two species (6 and 8) that were the most genetically similar and grouped the species 7 and 9 in the second sub-cluster. Furthermore, the PCA analysis of CDDP data revealed highly similar results that were comparable to the cluster analysis (dendrogram). The PCA results indicated that the grouping remained similar to that shown by the cluster analysis (Fig. 4 and Table 3). For SCoT, the dendrogram comprised two main clusters; the first cluster was further divided into two sub-clusters, the first of which grouped all the Mammillaria species as two species (2 and 3) that were the most genetically similar, followed by species 4 and 5, and finally the species no. 1, which was the most diverged among all the tested Mammillaria species (Fig. 5). The second sub-cluster grouped two Notocactus species as 7 and 9, while the rest of the Notocactus species were grouped as species 6 and 8. Furthermore, PCA analysis of the SCoT data exhibited consistent results, as they showed comparable groups to the ones obtained from the cluster analysis (dendrogram) (Fig. 5 and Table 4). For the combined data, the cluster analysis of the nine species of cacti using the above marker systems revealed two dendrograms exhibiting unique topology with some similarities. The data scored from SCoT and CDDP were combined and analyzed to generate deeper relationships based on the wider and more versatile genome coverage. The combined dendrogram comprised two main clusters with a high topology that matched with the SCoT dendrogram. The first cluster was further divided into two sub-clusters; one grouped all the Mammillaria species in addition to two Notocactus species (no. 7 and 9). On the other hand, the rest of the Notocactus species (no. 6 and 8). Furthermore, PCA analysis of the combined data exhibited consistent results that were comparable with the grouping obtained from cluster analysis (dendrogram) (Fig. 6 and Table 5).
Fig. 4.
(A) UPGMA cluster analysis based on Jaccard’s similarity coefficient of CDDP analysis of the four Notocactus species and five Mammillaria species. (B) Principal Component Analysis (PCA) of the CDDP-PCR data of the four Notocactus species and five Mammillaria species showing the two-dimensional (PC1 and PC2) plot.
Fig. 5.
(A) UPGMA cluster analysis based on Jaccard’s similarity coefficient of SCoT analysis of the four Notocactus species and five Mammillaria species. (B) Principal Component Analysis (PCA) of the CDDP-PCR data of the four Notocactus species and five Mammillaria species showing the two-dimensional (PC1 and PC2) plot.
Fig. 6.
(A) UPGMA cluster analysis based on Jaccard’s similarity coefficient of combined analysis (CDDP + SCoT) of the four Notocactus species and five Mammillaria species. (B) Principal Component Analysis (PCA) of the combined data (CDDP + SCoT) of the four Notocactus species and five Mammillaria species showing the two-dimensional (PC1 and PC2) plot.
Table 3.
Jaccard’s similarity matrix based on the CDDP analysis of the four Notocactus species and five Mammillaria species.
| Sample-1 | Sample- 2 | Sample-3 | Sample-4 | Sample-5 | Sample-6 | Sample-7 | Sample-8 | Sample-9 | |
|---|---|---|---|---|---|---|---|---|---|
| Sample-1 | 100% | ||||||||
| Sample- 2 | 30% | 100% | |||||||
| Sample-3 | 27% | 27% | 100% | ||||||
| Sample-4 | 39% | 38% | 39% | 100% | |||||
| Sample-5 | 34% | 40% | 30% | 39% | 100% | ||||
| Sample-6 | 70% | 41% | 31% | 39% | 38% | 100% | |||
| Sample-7 | 30% | 29% | 30% | 24% | 31% | 28% | 100% | ||
| Sample-8 | 35% | 31% | 56% | 35% | 22% | 27% | 25% | 100% | |
| Sample-9 | 25% | 34% | 38% | 38% | 28% | 28% | 37% | 34% | 100% |
Table 4.
Jaccard’s similarity matrix based on the SCoT analysis of the four Notocactus species and five Mammillaria species.
| Sample-1 | Sample- 2 | Sample-3 | Sample-4 | Sample-5 | Sample-6 | Sample-7 | Sample-8 | Sample-9 | |
|---|---|---|---|---|---|---|---|---|---|
| Sample-1 | 100% | ||||||||
| Sample- 2 | 35% | 100% | |||||||
| Sample-3 | 33% | 25% | 100% | ||||||
| Sample-4 | 35% | 43% | 30% | 100% | |||||
| Sample-5 | 31% | 42% | 34% | 46% | 100% | ||||
| Sample-6 | 51% | 51% | 35% | 46% | 44% | 100% | |||
| Sample-7 | 40% | 38% | 27% | 46% | 38% | 40% | 100% | ||
| Sample-8 | 33% | 37% | 47% | 31% | 39% | 37% | 41% | 100% | |
| Sample-9 | 32% | 29% | 33% | 40% | 33% | 39% | 43% | 36% | 100% |
Table 5.
Jaccard’s similarity matrix based on the combined analysis (CDDP + SCoT) of the four Notocactus species and five Mammillaria species.
| Sample-1 | Sample- 2 | Sample-3 | Sample-4 | Sample-5 | Sample-6 | Sample-7 | Sample-8 | Sample-9 | |
|---|---|---|---|---|---|---|---|---|---|
| Sample-1 | 100% | ||||||||
| Sample- 2 | 32% | 100% | |||||||
| Sample-3 | 30% | 26% | 100% | ||||||
| Sample-4 | 37% | 40% | 34% | 100% | |||||
| Sample-5 | 32% | 41% | 32% | 43% | 100% | ||||
| Sample-6 | 61% | 46% | 33% | 43% | 41% | 100% | |||
| Sample-7 | 35% | 33% | 28% | 35% | 34% | 34% | 100% | ||
| Sample-8 | 34% | 34% | 51% | 33% | 31% | 32% | 34% | 100% | |
| Sample-9 | 28% | 32% | 36% | 39% | 30% | 33% | 40% | 35% | 100% |
3.3. Preliminary phytochemical screening
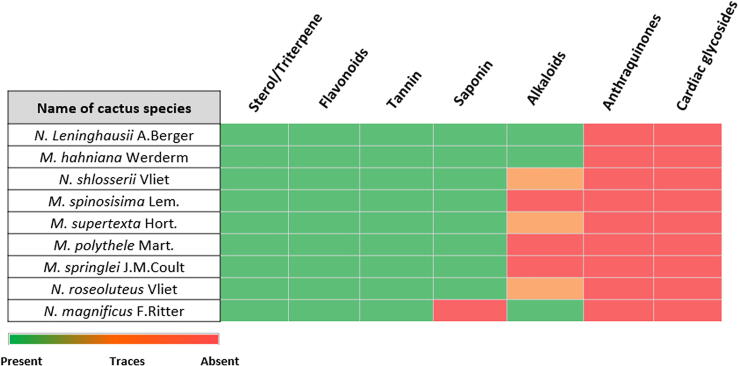
The results of the phytochemical screening are presented in Fig. 7. The presence of sterol, triterpene, tannin, and flavonoids was observed in all samples. Saponins were present in all the extracts, except in that of N. magnificus. On the other hand, alkaloids were abundant in N. leninghausii, M. hahniana Werderm, and N. magnificus F. Ritter, present in traces in N. shlosserii, M. supertexta Hort., and N. roseol, and absent in M. spinosis, M. polythele Mart., and M. spriglei. Anthraquinones and cardiac glycosides were not present in any of the specimens under investigation.
Fig. 7.
Heatmap representing the identified levels of phytochemical screening within the four Notocactus species and five Mammillaria species.
3.4. Determination of the total phenolic, total flavonoid, and total flavanol contents
The total phenolic contents of different species of cacti were determined spectrophotometrically using Folin–Ciocalteu's reagent and are presented in Table 6. The total phenolic content ranged from 16.58 ± 0.14 to 87.15 ± 0.01 µg/g of plant extract. N. leninghausii yielded the highest phenolic content, while M. supert and N. magnificus F. Ritter displayed comparable amounts of the compound (58.64 ± 0.73 µg/g and 58.93 ± 0.14 µg/g, respectively). M. spriglei yielded the lowest phenolic content among the cactus species. In contrast, N. leninghausii and N. magnificus F. Ritter showed the least flavonoid and flavanol content among the species of cacti (Table 6).
Table 6.
The concentration of total phenolic compounds, total flavonoids, and total flavanol along with the estimation of the antioxidant activity of the cactus species determined by the ABTS and DPPH methods.
| Name of species | Conc. of total phenolic compounds | Conc. of total flavonoid | Conc. of total flavanol | ABTS | DPPH |
|---|---|---|---|---|---|
| N. Leninghausii A.Berger | 87.15 ± 0.01 | 5.71 ± 0.01 | 5.63 ± 0.01 | 31.333 ± 0.079 | 7.158 ± 0.230 |
| M. hahniana Werderm | 36.43 ± 0.11 | 26.41 ± 0.01 | 19.80 ± 0.01 | 17.783 ± 0.145 | 4.849 ± 0.213 |
| N. shlosserii Vliet | 33.73 ± 0.01 | 11.83 ± 0.01 | 9.21 ± 0.01 | 29.443 ± 0.081 | 9.591 ± 0.248 |
| M. spinosisima Lem. | 41.05 ± 0.01 | 19 ± 0.01 | 12.40 ± 0.09 | 30.935 ± 0.082 | 10.600 ± 0.036 |
| M. supertexta Hort. | 58.64 ± 0.73 | 29.11 ± 0.01 | 6.88 ± 0.01 | 27.762 ± 0.208 | 7.232 ± 0.219 |
| M..polythele Mart. | 27.39 ± 0.01 | 11.04 ± 0.01 | 7.18 ± 0.04 | 28.622 ± 0.291 | 7.157 ± 0.154 |
| M. springlei J.M.Coult | 16.58 ± 0.14 | 8.55 ± 0.01 | 3.92 ± 0.01 | 21.411 ± 0.524 | 6.408 ± 0.177 |
| N. roseoluteus Vliet | 36.74 ± 0.14 | 6.79 ± 0.01 | 5.68 ± 0.05 | 40.324 ± 0.295 | 9.518 ± 0.288 |
| N. magnificus F.Ritter | 58.93 ± 0.14 | 5.19 ± 0.01 | 5.15 ± 0.01 | 31.663 ± 0.096 | 5.552 ± 0.112 |
3.5. Antioxidant activity
The generation of reactive oxygen species (ROS) in our body is widely known to play a significant role in the progression of several oxidative stress-related diseases (Mosmann, 1983, Elaasser and El Kassas, 2011). We determined the capacities of different cactus extracts to scavenge the free radicals, using ABTS and DPPH methods, against Trolox (water-soluble analog of vitamin E) as the positive control. The ABTS assay showed a higher percentage of inhibition, ranging from 40.324 ± 0.295 mg/g to 17.783 ± 0.145 mg/g sample, compared to the DPPH assay. The data presented in Table 6 indicated that N. roseol exhibited the highest percentage inhibition of ABTS solution, while N. magnificus F. Ritter and N. leninghausii exhibited nearly similar values (31.663 ± 0.096 and 31.333 ± 0.079 mg/g sample, respectively). M. polythele Mart. and M. supert showed moderate inhibition capacity, while M. hahni displayed the least inhibition capacity against ABTS solution (17.783 ± 0.145 mg/g sample). In contrast, all the cactus extracts exhibited a low percentage of inhibition against the DPPH solution. The antioxidant capacity of the species was in the order: M. spinosis > N. shlosserii > N. roseol > M. supert > N. leninghausii > M. polythele > M. spriglei > N. magnificus > M. hahni.
4. Discussion
To the best of our knowledge, this study is the first to investigate and analyze the genetic diversity within and between the genera Mammillaria and Notocactus using modern approaches involving functional markers such as SCoT and CDDP, in addition to the preliminary screening of their phytochemical constitution and antioxidant activities that represent an initial image of their pharmacological activities. Genome-conserved regions across different plant species have facilitated the development of molecular markers such as SCoT and CDDP. These markers utilize longer primers with higher annealing temperatures, which makes them more reliable, reproducible, and easier to design than other arbitrary markers such as RAPD or DAF. Moreover, they focus on gene regions, which makes them preferable to random markers in QTL mapping applications (Collard and Mackill, 2009, de Lucena et al., 2013). Collard and Mackill (2009b) exploited conserved DNA regions within the selection of well-known plant genes that were mainly involved in response to biotic and abiotic stresses or plant development to design CCDP primers. Finally, it was recommended that since SCoT and CDDP markers were generated from the functional region of the genome, the genetic analyses utilizing them would be highly useful for crop improvement programs, such as QTL mapping, assessment of genetic diversity, construction of linkage maps, and identification of different genotypes (Zibaee et al., 2011). We applied the SCoT and CDDP techniques for the first time to analyze the genetic diversity between nine species belonging to the genera Mammillaria and Notocactus (Parodia), which are considered to be major genera of the family Cactaceae. Both the markers were able to differentiate between the nine species, although the percentage of polymorphism for SCoT and CDDP was different (98% and 100%, respectively). This indicated that CDDP was more capable of discriminating between different genotypes, which is in agreement with the findings of a study on chickpea, that also reported that CDDP was more effective than SCoT and SSR markers in showing the diversity patterns across different genotypes (Hahm et al., 2010, Ezzat et al., 2016). Our results also indicate that CDDP has a higher rate of reproducibility than SCoT, although fewer primers were used for CDDP (eight primers) compared to SCoT (10 primers). Poczai et al., (2013) reported that the reproducibility of CDDP was higher than that of traditional arbitrarily amplified DNA markers and that the technique could easily generate functional markers (FM) related to a given plant phenotype. Furthermore, this study is the first to highlight the major secondary metabolites of these species of cacti. Several phyto-constituents, such as sterols, triterpenes, tannins, flavonoids, and saponins were detected in these species, except for N. magnificus. On the other hand, alkaloids were only observed in two species of Notocactus (N. leninghausii Berger and N. magnificus) and one of Mammillaria (M. hahniana Werderm). M. supertexta Hort. and N. Leninghausii Berger showed the highest phenolic content. However, they exhibited the lowest content of total flavonoids and flavanol. Recently, a similar finding was reported by Figueroa-Pérez et al., (2018), who observed a considerable amount of saponin in cladodes of medium age, which decreased with age. Our results agreed with those of with Dib et al. (2013), who reported a moderate number of flavonoid compounds in Opuntia ficus-indica. L. To the best of our knowledge, there are no reports regarding the quantitative determination of polyphenolic content in these cactus species. Several epidemiological studies have established an inverse relationship between the intake of dietary antioxidants and the incidence of several diseases such as cancer, Alzheimer’s, inflammation, and cardiovascular diseases (Li et al., 2009, Bhattacharyya et al., 2013, Atia et al., 2016). Therefore, the antioxidant activity of different cactus species was evaluated using two complementary methods (ABTS and DPPH). Both the reagents are known to be stable against free radicals and the resulting colors show characteristic absorptions at 734 and 519 nm, respectively (Figueroa-Pérez et al., 2018). The scavenging activity of DPPH depends mainly on proton donation to produce reduced DPPH, which can be evaluated by a decrease in its absorbance (Dib et al., 2013, Shalaby and Shanab, 2013). The inhibition potential in DPPH assay can be attributed to the level of flavonoids and tannins in several cactus species (Abdel-Hameed et al., 2009). Among the two methods, the ABTS method appeared to be more sensitive than DPPH, as it gave reproducible results at several pH levels (unlike DPPH, which was affected by acidic media). In addition, ABTS solution reacted rapidly to the sample, as compared to the slow reactivity of DPPH solution (Bendary et al., 2013, Ammar et al., 2015). Among the nine investigated species, N. roseoluteus exhibited the strongest antioxidant potential according to ABTS method. Other phytoconstituents such as alkaloids and saponins may participate in higher antioxidant activity, as measured using the ABTS method. Future studies in the identification and isolation of the active compounds from cacti are recommended. Further pharmacological studies are required to explore the medicinal uses of cacti in more detail.
Acknowledgment
We are grateful to Dr. Usama K. Abdel-Hameed, Associated professor of the taxonomy of flowering plants, Botany Department, Faculty of Science, Ain Shams University for assistance in the identification of the cactus species.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohamed A.M. Atia, Email: matia@ageri.sci.eg.
Gamal H. Osman, Email: geosman@uqu.edu.sa.
References
- Abdelaziz M., Abdelsattar M., Abdeldaym E., Atia M., Mahmoud A., Saad M., Hirt H. Piriformospora indica alters Na+/K+ homeostasis, antioxidant enzymes and LeNHX1 expression of greenhouse tomato grown under salt stress. Sci. Hortic. 2019;256:108532. [Google Scholar]
- Abdel-Hameed E.-S.S. Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem. 2009;114(4):1271–1277. [Google Scholar]
- Ammar I., Ennouri M., Attia H. Phenolic content and antioxidant activity of cactus (Opuntia ficus-indica L.) flowers are modified according to the extraction method. Ind. Crops Products. 2015;64:97–104. [Google Scholar]
- Atia M.A., Abdeldaym E.A., Abdelsattar M., Ibrahim D.S., Saleh I., Elwahab M.A., Osman G.H., Arif I.A., Abdelaziz M.E. Piriformospora indica promotes cucumber tolerance against Root-knot nematode by modulating photosynthesis and innate responsive genes. Saudi J. Biol. Sci. 2020;27(1):279–287. doi: 10.1016/j.sjbs.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atia M.A., Osman G.H., Elmenofy W.H. Genome-wide in silico analysis, characterization and identification of microsatellites in Spodoptera littoralis multiple nucleopolyhedrovirus (SpliMNPV) Sci. Rep. 2016;6:33741. doi: 10.1038/srep33741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendary E., Francis R., Ali H., Sarwat M., El Hady S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013;58(2):173–181. [Google Scholar]
- Bhattacharyya P., Kumaria S., Kumar S., Tandon P. Start Codon Targeted (SCoT) marker reveals genetic diversity of Dendrobium nobile Lindl., an endangered medicinal orchid species. Gene. 2013;529(1):21–26. doi: 10.1016/j.gene.2013.07.096. [DOI] [PubMed] [Google Scholar]
- Boshara O.A.A. University of Gezira; 2014. Phytochemical Screening for Leaves, Cortex and Pith of the Cactus Euphorbia trigona L. [Google Scholar]
- Butterworth, C.A. 2003. Phylogenetic studies of Tribe Cacteae (Cactaceae) with special emphasis on the genus Mammillaria.
- Cecília de Fátima, De Amorim C.B.R., De Albuquerque E.L.C., Maia M.B.S. Medicinal plants popularly used in the Xingó region–a semi-arid location in Northeastern Brazil. J. Ethnobiol. Ethnomed. 2006;2(1):15. doi: 10.1186/1746-4269-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard B.C., Mackill D.J. Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol. Biol. Report. 2009;27(1):86. [Google Scholar]
- Collard B., Mackill D. Conserved DNA-derived polymorphism (CDDP): a simple and novel method for generating DNA markers in plants. Plant Mol. Biol. Report. 2009;27(4):558. [Google Scholar]
- de Lucena C.M., de Lucena R.F.P., Costa G.M., Carvalho T.K.N., da Silva Costa G.G., da Nóbrega Alves R.R., Pereira D.D., da Silva Ribeiro J.E., Alves C.A.B., Quirino Z.G.M., Nunes E.N. Use and knowledge of Cactaceae in Northeastern Brazil. J. Ethnobiol. Ehnomed. 2013;9(1):62. doi: 10.1186/1746-4269-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib H., Beghdad M.C., Belarbi M., Seladji M., Ghalem M. Antioxidant activity of phenolic compound of the cladodes of Opuntia ficus-indica MILL. From northwest Algeria. Int. J. Med. Pharm. Sci. 2013;3:147–158. [Google Scholar]
- Elaasser M., El Kassas R. Detoxification of aflatoxin B1 by certain bacterial species isolated from Egyptian soil. World Mycotoxin J. 2011;4(2):169–176. [Google Scholar]
- Ezzat S.M., El Sayed A.M., Salama M.M. Use of random amplified polymorphic DNA (RAPD) technique to study the genetic diversity of eight aloe species. Planta Medica. 2016;82(15):1381–1386. doi: 10.1055/s-0042-108208. [DOI] [PubMed] [Google Scholar]
- Figueroa-Pérez M.G., Pérez-Ramírez I.F., Paredes-López O., Mondragón-Jacobo C., Reynoso-Camacho R. Phytochemical composition and in vitro analysis of nopal (O. ficus-indica) cladodes at different stages of maturity. Int. J. Food Prop. 2018;21:1–16. [Google Scholar]
- Formagio A.S.N., Volobuff C.R.F., Santiago M., Cardoso C.A.L., Vieira M.D.C., Valdevina Pereira Z. Evaluation of antioxidant activity, total flavonoids, tannins and phenolic compounds in Psychotria leaf extracts. Antioxidants. 2014;3(4):745–757. doi: 10.3390/antiox3040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne J. Springer; 1984. Methods of Plant Analysis, in Phytochemical Methods; pp. 1–36. [Google Scholar]
- Hahm S.W., Park J., Son Y.S. Opuntia humifusa partitioned extracts inhibit the growth of U87MG human glioblastoma cells. Plant Foods Human Nutrition. 2010;65(3):247–252. doi: 10.1007/s11130-010-0188-y. [DOI] [PubMed] [Google Scholar]
- Hernández-Hernández T., Hernández H.M., De-Nova J.A., Puente R., Eguiarte L.E., Magallón S. Phylogenetic relationships and evolution of growth form in (Cactaceae (Caryophyllales, Eudicotyledoneae) Am. J. Bot. 2011;98(1):44–61. doi: 10.3732/ajb.1000129. [DOI] [PubMed] [Google Scholar]
- Hwang E.-S., Do N., Thi H. Effects of extraction and processing methods on antioxidant compound contents and radical scavenging activities of laver (Porphyra tenera) Prevent. Nutr. Food Sci. 2014;19(1):40. doi: 10.3746/pnf.2014.19.1.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaccard P. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 1908;44:223–270. [Google Scholar]
- Li X., Wu X., Huang L. Correlation between antioxidant activities and phenolic contents of radix Angelicae sinensis (Danggui) Molecules. 2009;14(12):5349–5361. doi: 10.3390/molecules14125349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado M.C., Nyffeler R., Eggli U., Larocca e Silva J.F. A new species of Parodia (Cactaceae, Notocacteae) from Rio Grande do Sul, Brazil. Novon: J. Bot. Nomenclature. 2008;18(2):214–219. [Google Scholar]
- Mattagajasingh I., Mukherjee A.K., Das P. Genomic relations among 31 species of Mammillaria Haworth (Cactaceae) using random amplified polymorphic DNA. Zeitschrift für Naturforschung C. 2006;61(7–8):583–591. doi: 10.1515/znc-2006-7-819. [DOI] [PubMed] [Google Scholar]
- Mokhtar M., Atia M. SSRome: an integrated database and pipelines for exploring microsatellites in all organisms. Nucl. Acids Res. 2019;47:D245–D252. doi: 10.1093/nar/gky998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Osuna-Martínez U., Reyes-Esparza J., Rodríguez-Fragoso L. Cactus (Opuntia ficus-indica): a review on its antioxidants properties and potential pharmacological use in chronic diseases. Nat. Prod. Chem. Res. 2014 [Google Scholar]
- Poczai P., Varga I., Laos M. Advances in plant gene-targeted and functional markers: a review. Plant Meth. 2013;9:6. doi: 10.1186/1746-4811-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby, E.A., Shanab, S.M., 2013. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. 42(5), 556–564
- Solórzano S., Cuevas-Alducin P.D., García-Gómez V., Dávila P. Genetic diversity and conservation of Mammillaria huitzilopochtli and M. supertexta, two threatened species endemic of the semiarid region of central Mexico. Revista Mexicana de Biodiversidad. 2014;85(2):565–575. [Google Scholar]
- Vázquez-Sánchez M., Terrazas T., Arias S., Ochoterena H. Molecular phylogeny, origin and taxonomic implications of the tribe Cacteae (Cactaceae) Syst. Biodivers. 2013;11(1):103–116. [Google Scholar]
- Zibaee A., Zibaee I., Sendi J.J. A juvenile hormone analog, pyriproxyfen, affects some biochemical components in the hemolymph and fat bodies of Eurygasterintegriceps Puton (Hemiptera: Scutelleridae) Pest Biochem. Physiol. 2011;100(3):289–298. [Google Scholar]