Abstract
Supplementation of the growing substrate by nitrogenous additives has been known to improve the production of oyster mushroom (Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm. (1871)). However, the application of nano-additives has not been reported in such cultivation yet. The study investigated the effect of nano-urea added in two different doses (3 g and 5 g per kg substrate), once (at spawning or after first flush) or twice (at spawning and after first flush) to the growing substrate consisting of wheat straw and spent oyster substrate (1:1, w/w). Results showed that the application of nano-urea once has induced the highest number of mushroom flushes (four flushes) despite the dose applied. Contrarily to early findings, where high doses of nitrogen have caused inhibition of mushroom growth and production, nano-urea application has had better effects when applied twice. With 5 g/kg, it induced the shortest period between the first and the third flush (15 days). With 3 g/kg, it resulted in the highest biological and economic yields at the third flush (332.7 g/bag and 283.1 g/bag respectively), in total (973.4 g/bag and 854.0 g/bag respectively), the highest biological efficiency (109.6%), and pileus diameter/stipe length ratio (2.8). Experimental findings of the current study may be potentially applied at commercial scale.
Keywords: Oyster mushroom, Spent substrate, Nano-urea, Biological yield, Mushroom characteristics
1. Introduction
Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm. (1871), commonly known as oyster mushroom, is the second most cultivated edible mushroom worldwide after Agaricus bisporus (Sánchez, 2010). During the past two decades, the mushroom has been gaining importance as health promoter and environmental restorer resulting in an upsurge in their research and development activities (Patel et al., 2012). P. ostreatus can be easily cultivated on different agricultural and industrial waste products (Mikiashvili et al., 2006, Bellettini et al., 2019). Cereal straw (mainly wheat straw) is the common substrate used in commercial production (Rühl and Kües, 2007). Such substrate is naturally poor in nitrogen (0.5–0.8%) (Upadhyay et al., 2002).
Spent mushroom substrate (SMS) generated from mushroom cultivation forms an abundant, nutritive, easily available and cheap source of substrate in mushroom producing regions (Grimm and Wösten, 2018). It is composed of agro-residues and fungal mycelium left after harvesting of mushrooms (Jordan et al., 2008). There are references to the use of such wastes for the production of a variety of edible mushroom species like Agaricus, Auricularia, Lentinula, Pleurotus and Volvariella (Pardo-Giménez, 2008). SMS does not always support good yields when spawned over it (Sharma and Jandaik, 1985). On the other hand, supplementation of SMS is a good solution to employ it as a base material to grow P. ostreatus (Pardo-Giménez et al., 2012, Ashrafi et al., 2014). Supplementation consists of the application of nutritional amendments to the substrates employed for mushroom cultivation (Carrasco et al., 2018). Most importantly, the choice of supplement and its application time are essential for obtaining the expected results (Desrumaux et al., 1999). Various nitrogen-rich supplements were reported to enhance yield (Bonatti et al., 2004, Naraian et al., 2009) and hasten the production process (Royse, 2002). Nitrogen is an essential element for the mushroom growth and cellular metabolism, particularly protein and enzymes synthesis. It is transported into the living cell in an inorganic (nitrate, nitrite, ammonia) or an organic form (amino acids, urea and other nitrogen compounds) (Mikeš et al., 1994). Experiments proved the interest of supplements based on soybean or other nitrogen sources for the cultivation of P. ostreatus in substrates that are naturally poor in this compound (Zied et al., 2014). For instance, the application of urea as an organic nitrogen source has come out with different results according to the concentration of urea applied and type of substrate used (Kanhar et al., 2007, Déo and Faustin, 2015). Supplementation with urea was mainly reported at spawning.
In the last decade, nanotechnology has gained momentum in agriculture (Mukhopadhyay, 2014). The agricultural field benefits from nanotechnology by nano-fertilizers that play a greater role in crop production (Duhan et al., 2017). Nano particles have extensive surface area and capable of holding abundance of nutrients and release it slowly and steadily such that it facilitates uptake of nutrients matching the crop requirement without any associated ill-effects of customized fertilizer inputs (Selva and Balakrishnan, 2017). The application of nano-fertilizers was largely reported on plant crops like tomato (Sajyan et al., 2018, Sajyan et al., 2019), but not until present date on mushrooms. Therefore, the present study was carried out in order to determine how nano-urea applied in different timing and concentrations would affect growth and yield of P. ostreatus.
2. Materials and methods
The present study was carried out at the Agricultural and Veterinary Research Center of the Lebanese University, Faculty of Agricultural Engineering and Veterinary Medicine, at Ghazir station.
2.1. Substrate preparation
The substrate used was a 1:1, w/w mixture of wheat straw (WS) and spent oyster substrate (SOS). SOS was used previously in one growing cycle of P. ostreatus at a local mushroom farm “Gourmet”. It was sun-dried for a week and shopped prior to use. Properties of the substrate (Table 1) including carbon (%), nitrogen (%), organic matter (%) and moisture (%) contents, C/N ratio and pH were determined at the Lebanese Agricultural Research Institute (LARI)-Tal Amara station. The determination of crude fiber (%) (AOAC 962.09 standards), total carbohydrates (%) (Anthrone method) and total protein (%) (Kjeldhal method) was performed at the LFDCA (Lebanese Food Drugs and Chemicals Administration)-Lebanese University-Hadath. The substrate was pasteurized at 60–65 °C for 8 h and then cooled down for 15 h to the spawning temperature (25 °C) (Pardo-Giménez et al., 2012).
Table 1.
Physico-chemical properties of the substrate.
| Physico-chemical properties | WS | WS + SOS (1:1) |
|---|---|---|
| Moisture (%) | 11.0 | 15.6 |
| Organic matter (%) | 92.7 | 82.8 |
| Carbon (%) | 54.0 | 48.1 |
| Nitrogen (%) | 1.1 | 1.1 |
| C/N ratio | 50:1 | 43:1 |
| pH (1:5) | 5.8 | 5.2 |
| Total carbohydrates (%) | 38.54 | 30.54 |
| Total protein (%) | 5.5 | 7.5 |
| Crude fiber (%) | 38.44 | 30.44 |
2.2. Experimental treatments
This experiment investigated the effect of nano-urea consisting of 21% of total nitrogen nano-scale (Lithovit®-Urea50) added in two different doses (C1: 3 g/kg and C2: 5 g/kg), once (t1: at spawning or t2: after first flush) or twice (t3: at spawning and after first flush). Experimental treatments were: T1: C1t1, T2: C1t2, T3: C2t1, T4: C2t2, T5: C1t3 and T6: C2t3. Two controls were adopted; the first (T0′) was an experimental control consisting of non-treated substrate WS + SOS (1:1) and the second was a commercial control (T0) consisting of WS. The experimental design was incomplete factorial design with three factors (substrate type, nano-urea dose and timing of application), eight treatments and ten replicates (bags) per treatment.
2.3. Spawning and cropping
P. ostreatus spawn of M 2175 strain was prepared at the laboratory of Food Technology of the Faculty of Agricultural Engineering and Veterinary Medicine. It consisted on wheat grain spawn prepared in glass jars of 200 mL. Spawning was done at a rate of 5%, corresponding to 50 g per kg of substrate. Inoculated substrates were filled into perforated transparent polyethylene bags of 60 cm length and 40 cm width. Five holes of 20 mm diameter were evenly made in the sides of the bags. Inoculated bags were incubated in dark conditions at 23–25 °C until complete mycelial colonization. The cropping chamber was moistened to a relative humidity of 80–90%. At complete substrate colonization, fruiting was induced by ventilation (to keep CO2 levels between 900 and 2300 ppm), reduction of room temperature to around 15 °C and lighting.
2.4. Measurements
Spawn run initiation (SRI) was recorded as the time (in days after spawning: DAS) when first white patches of growing mycelium were observed. Squares of 5 × 5 cm were drawn on bags (prior to filling). The time when half of squares were covered by mycelium was recorded as 50% mycelial colonization (50% MC). The time to full colonization (100% MC) was recorded when all squares became white. The surface mycelial density (SMD) corresponded to the degree of mycelial colonization of the substrate. It was evaluated at the time of full colonization by assigning (1) to poor running growth, (2) to mycelium growing throughout the bag but not uniformly white and (3) to mycelium growing throughout the bag and uniformly white (Yang et al., 2013). Time to pin head formation (PN) (DAS) was recorded for each mushroom flush. Earliness was determined as the number of days between spawning and harvest of first flush. Number of bunches (NB), weight of bunches (WB), number of effective fruit bodies (NEFB) and weight of effective fruit bodies (WEFB) were determined per flush. Biological yield (BY) and economic yield (EY) were evaluated per flush and in total. EY corresponded to the total weight of effective fruit bodies after removal of the base of stalks (Girmay et al., 2016). Biological efficiency (BE) was calculated per treatment as follows: total fresh weight of EFB (g)/initial dry weight of substrate (g) × 100 (Oseni et al., 2012). Physical characteristics of mushrooms were evaluated per flush by measuring pileus diameter (PD), pileus length (PL), stipe diameter (SD) and stipe length (SL), on 10 representative samples, using a sliding caliper.
2.5. Statistical analysis
Data analysis was performed using SPSS 25®. One-way ANOVA and Duncan tests were applied. In addition, stepwise multiple regression analysis was used to evaluate the relation between biological yield per flush (as dependent variable) and weight of effective fruit bodies, number of effective fruit bodies, stipe length, stipe diameter, pileus length and pileus diameter (as predictors) with 95% level of confidence.
3. Results
3.1. Effect of nano-urea application once at spawning
Results of ANOVA showed that at the first flush, the effect of treatment was statistically significant (p < 0.05) on averages of NB, number of days to first harvest (earliness), NEFB, WB, WEFB, BY, EY, PD, SL, PL and PD/SL. On the other hand, averages of remaining indicators (SRI, 50% MC, 100% MC, SMD, PN and SD) were not significantly affected by treatment. In all treatments, SRI started at around 2 DAS, time to 50% MC ranged between 5 and 8 DAS, time to 100% MC ranged between 8 and 10 DAS, and SMD was around 3 (corresponding to mycelium growing throughout the bag and uniformly white).
Findings in Table 2 showed that harvest of first flush in T0′ was earlier by around 5 days compared to T0. Application of nano-urea at spawning has significantly delayed the time to harvest of first flush compared to experimental control (T0′) despite the concentration applied. The product application with the lowest dose (3 g/kg) resulted in the lowest NB and the highest WB per bag. NEFB was significantly reduced following nano-urea application at spawning compared to experimental control by 8.7 and 6.7 in T1 and T3 respectively compared to T0′). The application of nano-urea with 5 g/kg resulted in significantly higher WEFB compared to its application with 3 g/kg. Biological and economic yields obtained at the first flush were significantly higher in commercial control by 8.6 g and 40.4 g respectively compared to the experimental control. In addition, BY and EY were significantly decreased in treated substrates compared to both controls and despite the concentration applied.
Table 2.
Earliness and productive indicators at the first flush.
| Treatment | Earliness | NB/bag | WBg/bag | NEFB/bag | WEFB/bag | BYg/bag | EYg/bag |
|---|---|---|---|---|---|---|---|
| T0 | 40.0b | 5.0b | 131.9a | 14.7b | 31.0b | 438.2c | 415.3c |
| T0′ | 35.3a | 3.7a | 177.3b | 20.0c | 21.0a | 429.6c | 374.9b |
| T1 C1t1 | 39.7b | 1.7a | 241.2c | 11.3a | 23.8a | 299.4b | 277.6a |
| T3 C2t1 | 38.7b | 3.0ab | 117.2a | 13.3ab | 29.2b | 269.7a | 263.2a |
NB: Number of bunches, WB: weight of bunches, NEFB: number of effective fruit bodies, WEFB: weight of effective fruit bodies, BY: Biological yield and EY: economic yield.
(Means within the same column followed by the same letters are not significantly different at p < 0.05 according to Duncan’s multiple range test).
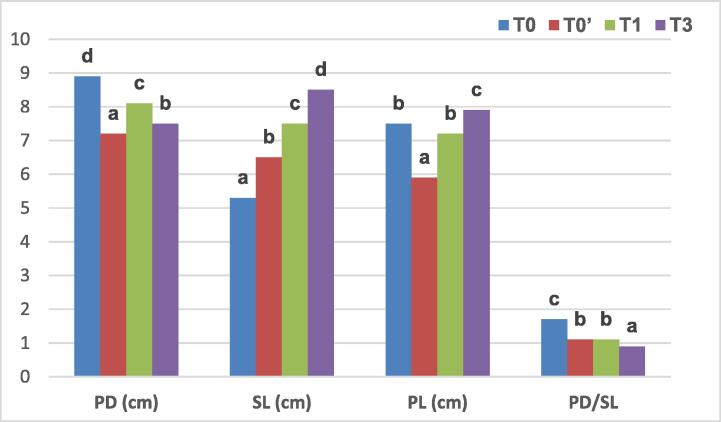
With respect to physical characteristics of mushrooms produced at the first flush (Fig. 1), PD was significantly lower in T0′, T1 and T3 compared to T0. On the other hand, SL was significantly increased in T0′, T1, and T3 compared to T0. PL was significantly increased in T3 by 0.7 cm comparing to T1, and in both treatments the increase was between 1.3 and 2 cm comparing to T0′. The ratio PD/SL was significantly lower in all treatments compared to commercial control compared to T0).
Fig. 1.
Physical characteristics of mushrooms at the first flush PD: Pileus diameter, SL: Stipe length, PL: Pileus length and PD/SL: Pileus diameter/Stipe length ratio (Means followed by the same letters, relative to each indicator, are not significantly different at p < 0.05 according to Duncan’s multiple range test).
3.2. Effect of nano-urea application on production of second and third flush
ANOVA test showed a significant effect (p < 0.05) of the treatment on all indicators assessed in the second and third flushes. Three mushroom flushes were obtained in all treatments except in T0 (2 flushes), T1 and T3 (4 flushes). The application of nano-urea once with 3 g/kg (T1 and T2) has hastened the pin head formation at second flush by 3–7 days, however it has delayed the pin head formation with 5 g/kg (T4 and T3) by 6–8 days compared to both controls. Double application of the product with 3 g/kg has most significantly delayed the time to pin head formation at second flush. Harvest of second flush was earlier when the product was applied once with 3 g/kg compared to both controls (by around 3 and 6 days in T1 and T2 compared to T0 and T0′). Harvest of third flush was delayed in all treated substrates compared to T0′ with the exception of T6. Overall, harvest of the three mushroom flushes was the latest in T5 (Table 3).
Table 3.
Number of flushes, time to pin head formation and harvest in second and third flushes.
| Treatment | NF | PN (DAS) flush 2 | PN (DAS) flush 3 | Harvest (DAS) flush 2 | Harvest (DAS) flush 3 |
|---|---|---|---|---|---|
| T0 | 2.0a | 50.0b | – | 54.7c | – |
| T0′ | 3.0b | 50.0b | 61.0b | 54.0c | 65.3b |
| T1 C1t1 | 3.3b | 47.0b | 65.0c | 51.0b | 70.3c |
| T2 C1t2 | 3.0b | 43.0a | 64.0c | 48.3a | 73.0c |
| T3 C2t1 | 3.3b | 58.7c | 65.3c | 64.3d | 71.0c |
| T4 C2t2 | 3.0b | 56.7c | 75.0d | 62.0d | 79.0d |
| T5 C1t3 | 3.0b | 67.0d | 77.0d | 71.7e | 80.0d |
| T6 C2t3 | 3.0b | 49.0b | 55.3a | 53.3bc | 59.7a |
NF: Number of flushes, PN: time to pin head formation (Means within the same column followed by the same letters are not significantly different at p < 0.05 according to Duncan’s multiple range test).
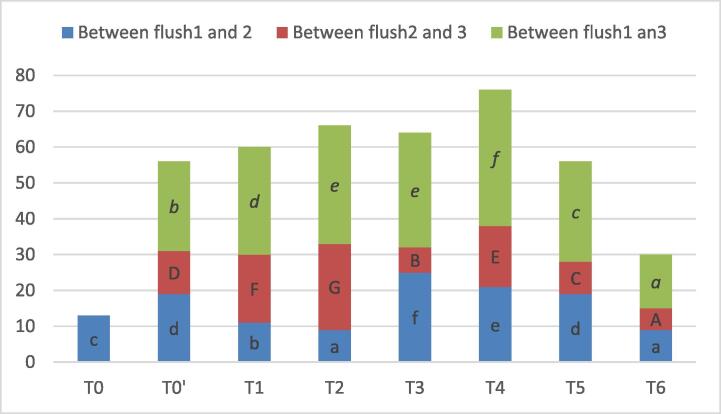
The shortest periods between harvests of consecutive flushes (Fig. 2) were obtained following double application of nano-urea with 5 g/kg (T6), where the second flush had been harvested 9 days after the first flush, and the third one had been harvested 6 days after the second. Such treatment (T6) has shortened the period between harvest of first and third flushes among all treatments by 10, 15, 18, 17, 23 and 13 in T0′, T1, T2, T3, T4, and T5 respectively).
Fig. 2.
Periods between consecutive flushes (Means followed by the same letters of lower case (period between flush 1 and 2), upper case (period between flush 2 and 3), or italic case (period between flush 1 and 3) relative to each treatment, are not significantly different at p < 0.05 according to Duncan’s multiple range test).
At the second flush, NB was significantly lower or comparable in treated substrates to that of commercial control, despite the timing and the dose of application. However, WB was significantly improved in T1, T2 and T3 (by 17.4%, 46.1%, and 38.6% respectively compared to T0). At the third flush, NB was significantly higher in T2 and T5 compared to the T0′ by 3 and 5 bunches respectively. At the same flush, WB was significantly higher in T3 and T6 compared to T0′ by 33.5 and 19.4 g respectively. Single application of nano-urea has significantly increased NEFB at the second flush despite the dose applied compared to T0′. On the other hand, double application of the product with 3 g/kg or 5 g/kg has significantly increased WEFB at the same flush, by around 7 g compared to T0 and T0′, despite the dose applied. Moreover, the lowest dose applied after first flush (T2) or twice (T5) resulted in significantly higher NEFB at the third flush, in comparison with remaining treatments including T0′. In general, WEFB was reduced with consecutive flushes in all treatments except in T0′ and T6 where it showed an opposite trend (Table 4).
Table 4.
Number and weight of bunches and effective fruit bodies in second and third flush.
| Treatment | NB flush 2 | NB flush 3 | NEFB flush 2 | NEFB flush 3 | WB (g) flush 2 | WB (g) flush 3 | WEFB (g) flush 2 | WEFB (g) flush 3 |
|---|---|---|---|---|---|---|---|---|
| T0 | 5.0c | – | 14.0bc | – | 67.8b | – | 19.0b | – |
| T0′ | 3.3abc | 3.0ab | 9.0a | 6.3b | 74.9bc | 51.2d | 19.6b | 33.4c |
| T1 C1t1 | 4.0bc | 3.3b | 12.7b | 7.0b | 82.1c | 23.9a | 21.1b | 11.3a |
| T2 C1t2 | 2.0a | 6.0c | 13.0bc | 13.0c | 125.8e | 25.1a | 19.1b | 10.2a |
| T3 C2t1 | 4.3bc | 1.7a | 16.0c | 7.0b | 110.5d | 84.7f | 18.8b | 11.5a |
| T4 C2t2 | 4.7bc | 2.0ab | 12.3b | 7.0b | 40.5a | 34.2b | 13.3a | 12.6a |
| T5 C1t3 | 4.0bc | 8.0d | 8.7a | 15.0c | 75.6bc | 41.4c | 25.9c | 18.7b |
| T6 C2t3 | 3.0ab | 2.0ab | 9.0a | 2.7a | 50.0a | 70.6e | 25.6c | 44.5d |
NB: Number of bunches, WB: weight of bunches, NEFB: number of effective fruit bodies, WEFB: weight of effective fruit bodies.
(Means within the same column followed by the same letters are not significantly different at p < 0.05 according to Duncan’s multiple range test).
The effect of nano-urea on PD was the most significant with double application of the product, mainly with 5 g/kg (T6), which resulted in the highest values of this indicator at the second flush and the third. At the second flush, there was a significant increase in PL following double application of the product with both doses in comparison with control; in between, T5 increased significantly this parameter by 0.4 cm comparing to T6. Single application after first flush T2 increased significantly PL by 1 cm in comparison with T0 and 0.4 cm in T0′. At the third flush, the application of nano-urea has significantly reduced both PD and PL compared to T0′, except in T6. At the second flush, SD was significantly increased in T1, T2 and T6 compared to T0. At the third flush, SD was significantly reduced in all treatments compared to T0′, except in T1 and T6. With respect to SL, it was increased in all treatments at the second flush compared to T0, except in T4 and T5. At the third flush, SL was decreased in T4 and T6 compared to T0′ (Table 5).
Table 5.
Mushroom physical characteristics in second and third flush.
| Treatment | PD (cm) flush 2 | PD (cm) flush 3 | PL (cm) flush 2 | PL (cm) flush 3 | SD (cm) flush 2 | SD (cm) flush 3 | SL (cm) flush 2 | SL (cm) flush 3 |
|---|---|---|---|---|---|---|---|---|
| T0 | 7.7ab | – | 6.5a | – | 1.0a | – | 3.6a | – |
| T0′ | 7.8ab | 12.2c | 7.1b | 8.5e | 1.4bc | 1.5c | 5.4c | 5.1b |
| T1 C1t1 | 7.5a | 6.6a | 6.4a | 6.5b | 2.0d | 1.6c | 5.2c | 3.7a |
| T2 C1t2 | 8.4b | 6.9a | 7.5c | 5.4a | 1.5c | 0.8a | 5.8d | 3.6a |
| T3 C2t1 | 8.1ab | 6.4a | 7.3bc | 6.4b | 1.2abc | 0.9ab | 6.1d | 3.6a |
| T4 C2t2 | 7.4a | 7.2ab | 6.4a | 7.8d | 1.0a | 1.0b | 3.9a | 5.0b |
| T5 C1t3 | 10.0c | 7.9b | 7.9d | 7.0c | 1.2ab | 0.8a | 3.7a | 3.8a |
| T6 C2t3 | 11.2d | 13.5d | 7.5c | 9.1f | 1.5c | 1.6c | 4.6b | 5.8c |
PD: pileus diameter, PL: pileus length, SD: stipe diameter and SL: stipe length.
(Means within the same column followed by the same letters are not significantly different at p < 0.05 according to Duncan’s multiple range test).
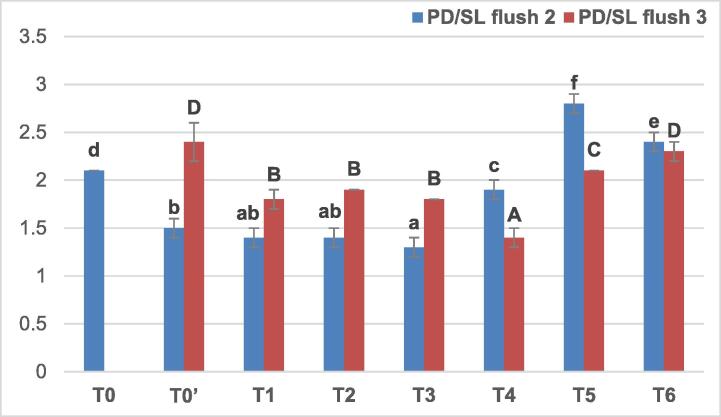
The ratio PD/SL (Fig. 3) was significantly increased at the second flush compared to both controls following double application of the product despite the dose of application. At the third flush, PD/SL obtained in all treatments was significantly lower or comparable to T0′, with the lowest value in T4.
Fig. 3.
The ratio PD/SL in the second and third flush (Means followed by the same letters of lowercase (corresponding to flush 2) or uppercase (corresponding to flush 3) are not significantly different at p < 0.05 according to Duncan’s multiple range test).
At the second flush, biological yield of T0 was significantly higher than that of T0′ by 48.3 g/bag. The application of nano-urea with 5 g/kg at spawning (T3) has improved BY of the second flush by around 10.3% and 25.6% respectively compared to T0 and T0′. Moreover, the application of the product with 3 g/kg at spawning (T1) or after first flush (T2), and with 5 g/kg at spawning (T3) has significantly increased average economic yield of second flush compared to T0′ (by around 14.8%, 20.2% and 31.1% respectively). A significant improvement in BY of third flush was obtained following the product application with 3 g/kg after the first flush and twice compared to experimental control (improvement by 43 g/bag and 195.9 g/bag respectively in T2 and T5 compared to T0′). At the same flush, EY obtained in T2, T5 and T6 was also increased by 45.0 g/bag, 167.9 g/bag and 18.3 g/bag compared to experimental control. In general, biological yield was reduced with consecutive flushes. However, double application of nano-urea with 3 g/kg has caused an improvement of 38.5% in biological yield of third flush compared to that of second flush. Values of total biological yield (TBY) were comparable in both controls. TBY obtained in T5 was significantly higher than both controls. Similarly, total economic yield (TEY) was superior in T5 compared to both controls by 186.2 g/bag and by 162.5 g/bag in T0 and T0′ respectively. TBY in T6 was comparable to that of T0′, but significantly higher than that of T0. TEY obtained in the same treatment was significantly higher than that of T0. The lowest TBY was in T4, where it was reduced by around 24.5% compared to T0 and 32.0% compared to T0′. Biological efficiency in T5 was significantly higher than all remaining treatments (Table 6).
Table 6.
Economic and biological yields and biological efficiency in second and third flushes.
| Treatment | BY (g/bag) flush 2 | EY (g/bag) flush 2 | BY (g/bag) flush 3 | EY (g/bag) flush 3 | Total BY (g/bag) | Total EY (g/bag) | BE (%) |
|---|---|---|---|---|---|---|---|
| T0 | 281.5d | 252.5d | – | – | 719.6bc | 667.8bc | 81.4bc |
| T0′ | 233.2c | 201.5c | 136.8d | 115.2c | 799.5bcd | 691.5bcd | 90.0 cd |
| T1 C1t1 | 273.7d | 236.6d | 82.2b | 72.4b | 695.9b | 625.6b | 78.4b |
| T2 C1t2 | 288.4d | 252.6d | 180.2e | 160.2e | 771.9 cd | 703.2 cd | 86.9bcd |
| T3 C2t1 | 313.7e | 292.4e | 118.7c | 78.6b | 736.8bc | 661.9bc | 83.0bc |
| T4 C2t2 | 197.4b | 173.8b | 57.9a | 51.9a | 543.3a | 479.7a | 61.2a |
| T5 C1t3 | 204.3b | 179.4b | 332.7f | 283.1f | 973.4e | 854.0e | 109.6e |
| T6 C2t3 | 152.2a | 132.8a | 141.2d | 133.5d | 828.8d | 743.9d | 93.3d |
BY: Biological yield and EY: Economic yield.
Means within the same column followed by the same letters are not significantly different at p < 0.05 according to Duncan’s multiple range test.
From the results of stepwise multiple regression (Table 7), the most explanatory models (highest coefficient of determination: R2) obtained at first flush (model 2; R2 = 0.98), second flush (model 4, R 2 = 0.79), and third flush (model 4, R2 = 0.88) showed that the biological yield was the most significantly affected by stipe length of mushrooms. Specifically, the increase in stipe length caused a decrease in biological yield (negative coefficient) at the first and third flushes. These results reflect the possibility to use mushroom physical characteristics, weight and number depending on the flush in order to predict biological yield and consequently the approximate economical income.
Table 7.
Models obtained with stepwise regression between biological yield and mushroom number, weight and its physical characteristics.
| predictive models | predictors | equation | Corrected R2 | |
|---|---|---|---|---|
| flush 1 | model 1 | SL | BY = −58.7 × SL + 768.1 | 0.84 |
| model 2 | SL, NEFB | BY = −48.8 × SL + 8.75 × NEFB + 569.4 | 0.98 | |
| flush 2 | model 1 | NEFB | BY = 13.4 × NEFB + 84.1 | 0.53 |
| model 2 | NEFB, SL | BY = 11.7 × NEFB + 18.3 × SL + 16.6 | 0.61 | |
| model 3 | NEFB, SL, PD | BY = 8.7 × NEFB + 18.6 × SL-12.7 × PD + 159.7 | 0.68 | |
| model 4 | NEFB, SL, PD, WEFB | BY = 10 × NEFB + 15.6 × SL −29.1 × PD + 7.3 × WEFB + 151 | 0.79 | |
| flush 3 | model 1 | NEFB | BY = 14.4 × NEFB + 30.5 | 0.48 |
| model 2 | NEFB, WEFB | BY = 21.5 × NEFB + 4.3 × WEFB-116.6 | 0.78 | |
| model 3 | NEFB, WEFB, SL | BY = 18.5 × NEFB + 6.7 × WEFB-50.2 × SL + 79.7 | 0.84 | |
| model 4 | NEFB, WEFB, SL, PD | BY = 19.2 × NEFB + 13.4 × WEFB-39.9 × SL-33.3 × PD + 181.6 | 0.88 | |
BY: biological yield, SL: stipe length, NEFB: number of effective fruit bodies, PD: pileus diameter, WEFB: weight of effective fruit bodies.
4. Discussion
The first flush of mushroom was harvested at 35 DAS when the substrate WS + SOS (1:1) was used. On similar substrate, Pardo-Giménez et al. (2012) have obtained earliness at around 32 DAS. Biological and economic yield of P. ostreatus obtained from wheat straw in the present study were higher than those reported earlier by Girmay et al. (2016) on same mushroom species. Cueva et al. (2017) proved a high dependency of biological efficiency on C/N ratio, with best results obtained in a C/N range of 37:1–53:1. In the current study, values of biological efficiency were comparable in tested substrates although WS had higher C/N ratio (50:1) than WS + SOS: 1:1 (43:1) (Table 1), which can be attributed to the initially higher protein content in the second substrate.
The ability of the fungus to use a certain substrate depends on its capacity to produce a lignocellulolytic enzyme complex (Buswell et al., 1995) which includes oxidative enzymes (laccase and manganese peroxidase), involved in lignin degradation (Galliano et al., 1991), and hydrolytic enzymes (xylanase and cellulase), involved in hemicellulose and cellulose degradation (Liguori et al., 2015). Nitrogen source is a major factor that affects the enzyme production by mushroom mycelium for biodegradation of a certain substrate (Singh et al., 2008). Commanday and Macy (1985) reported that ligninolytic activity in P. ostreatus is indeed suppressed by excess substrate nitrogen. Kanhar et al. (2007) had earlier reported an inhibition of spawn run resulting from the application of 5 g/kg of urea, which contradicts findings of the present study. Nano-urea did not as well affect the surface mycelial density, contrarily to findings of Hoa and Wang (2015) with urea. The yield decline with consecutive flushes could be due to either depletion of nutrients or accumulation of toxic substances unfavorable to fruiting. Most of nitrogen in the substrate is utilized for mycelial growth, and at the time of fruit formation it becomes inadequate and limits mushroom yield (Upadhyay et al., 2002). The application of nano-urea with a high dose could have facilitated nitrogen absorption by mycelium and hastened fruit formation at consecutive flushes. Furthermore, number of bunches obtained in treated substrates, once with both tested doses, was higher than that reported by Kanhar et al. (2007) following the application of urea with the same doses. Cap diameter resulting from nano-urea treatment was lower than that obtained by Déo and Faustin (2015) with 100 g of urea applied on different substrates. According to Synytsya et al. (2008) fruit bodies with larger pileus and shorter stipes are better than that with smaller pileus and longer stipes. They are more acceptable at the market. Mushroom obtained after nano-urea was applied twice, had the highest PD/SL ratio, which means that they were more marketable than those obtained after nano-urea was applied only once.
5. Conclusion
The re-use of spent oyster substrate may provide a nutritious, low-cost substrate with a potential to produce comparable yield to that obtained in conventionally used wheat straw. Added benefits may be acquired from such substrate when treated with nano-urea, mostly twice during the production cycle. The current study provided evidences on shortening in periods between consecutive flushes and improvement in biological yield, depending on the applied dose of nano-urea, and looking back to the need of confirmation in commercial production. Future studies may investigate the effect of nano-urea applied at lower doses on general performance of the mushroom.
Acknowledgements
Authors would like to acknowledge Tribodyn AG Company, Northelm, Germany, for offering Lithovit®-Urea50.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Layla Naim, Email: layla.naim@st.edu.lb.
Mohammed A. Alsanad, Email: mamalsanad@kfu.edu.sa.
Zeina El Sebaaly, Email: zeina.sebaaly@st.ul.edu.lb, zeinasebaaly1@hotmail.com.
Youssef N. Sassine, Email: ysassine@kfu.edu.sa.
References
- Ashrafi R., Mian M.H., Rahman M.M., Jahiruddin M. Recycling of spent mushroom substrate for the production of oyster mushroom. Res. Biotechnol. 2014;5(2):13–21. https://updatepublishing.com/journal/index.php/rib/article/view/2452 [Google Scholar]
- Bonatti M., Karnopp P., Soares H.M., Furlan S.A. Evaluation of Pleurotus ostreatus and Pleurotus sajor-caju nutritional characteristics when cultivated in different lignocellulosic wastes. Food Chem. 2004;88(3):425–428. [Google Scholar]
- Bellettini M.B., Fiorda F.A., Maieves H.A., Teixeira G.L., Àvila S., Hornung P.S., Júnior A.M., Ribani R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019;26:633–646. doi: 10.1016/j.sjbs.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buswell J., Cai Y.J., Chang S.T. Effect of nutrient nitrogen and manganese or manganese peroxidase and laccase production by Lentinula (Lentinus) edodes. FEMS Microbiol. Lett. 1995;128:81–88. [Google Scholar]
- Carrasco J., Zied D.C., Pardo J.E., Preston G.M., Giménez A.P. Supplementation in mushroom crops and its impact on yield and quality. AMB Expr. 2018;8(1):146–156. doi: 10.1186/s13568-018-0678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commanday F., Macy J.M. Effect of substrate nitrogen on lignin degradation by Pleurotus ostreatus. Arch. Microbiol. 1985;142:61–65. doi: 10.1007/BF00409238. [DOI] [Google Scholar]
- Cueva M.B.R., Hernández A., Niño-Ruiz Z. Influence of C/N ratio on productivity and the protein contents of Pleurotus ostreatus grown in different residue mixtures. Revista de la Facultad de Ciencias Agrarias. 2017;49(2):331–344. http://www.redalyc.org/articulo.oa?id=382853527023 [Google Scholar]
- Déo N., Faustin K. Effect of substrates and doses of urea on growth and yield of an oyster mushroom (Pleurotus ostreatus) in greenhouse. Int. J. Agric. Policy Res. 2015;3(8):314–322. doi: 10.15739/IJAPR.055. [DOI] [Google Scholar]
- Desrumaux B., Seydeyn P., Werbrouck A., Lannoy P. Supplémenter dans la culture du champignon de couche: experience comparative avec quelques produits de supplémentation du commerce. Bull FNSACC. 1999;81(1):789–802. [Google Scholar]
- Duhan J.S., Kumar R., Duhan S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017;15(15):11–23. doi: 10.1016/j.btre.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliano H., Gas G., Seris J.L., Boudet A.M. Lignin degradation by Rigidoporus lignosus involves synergistic action of two oxidizing enzymes: Mn peroxidase and laccase. Enzyme Microb. Tech. 1991;13:478–482. [Google Scholar]
- Girmay Z., Gorems W., Birhanu G., Zewdie S. Growth and yield performance of Pleurotus ostreatus (Jacq. Fr.) Kumm (oyster mushroom) on different substrates. AMB Expr. 6. 2016;87:1–7. doi: 10.1186/s13568-016-0265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D., Wösten H.A.B. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018;102:7795–7803. doi: 10.1007/s00253-018-9226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoa H.T., Wang C.L. The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus) Mycobiology. 2015;43(1):14–23. doi: 10.5941/MYCO.2015.43.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S.N., Mullen G.J., Murphy M.C. Composition variability of spent mushroom compost in Ireland. Bioresour. Technol. 2008;99:411–418. doi: 10.1016/j.biortech.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Kanhar Q.D., Jiskani M.M., Pathan M.A., Nizamani Z.A. Effect of urea on growth and yield of oyster mushroom, Pleurotus ostreatus (JACQ.EX.FR.) Kummer. Pak. J. Phytopathol. 2007;19(2):214–223. [Google Scholar]
- Liguori L., Ionata E., Marcolongo L., de Souza Vandenberghe L.P., La Cara F., Faraco F. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/951871. Article ID 951871, 14 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikeš V., Zofall M., Chytil M., Fulneček J., Scháně L. Ammonia-assimilating enzymes in the basidiomycete fungus Pleurotus ostreatus. Microbiology. 1994;140(1):977–982. doi: 10.1099/00221287-140-4-977. [DOI] [Google Scholar]
- Mikiashvili N., Wasser S., Nevo E., Elisashvili V. Effects of carbon and nitrogen sources on Pleurotus ostreatus ligninolytic enzyme activity. WorldJ. Microbiol. Biotechnol. 2006;22:999–1002. doi: 10.1007/s11274-006-9132-6. [DOI] [Google Scholar]
- Mukhopadhyay S.S. Nanotechnology in agriculture: Prospects and constraints. Nanotechnol. Sci. Appl. 2014;7:63. doi: 10.2147/NSA.S39409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naraian R., Sahu K., Kumar S., Garg S.K., Singh C.S., Kanaujia R.S. Influence of different nitrogen rich supplements during cultivation of Pleurotus florida on corn cob substrate. Environmentalist. 2009;29:1–7. doi: 10.1007/s10669-008-9174-4. [DOI] [Google Scholar]
- Oseni O., Dube S., Wahome P., Masarirambi M., Earnshaw D.M. Effect of wheat bran supplement on growth and yield of oyster mushroom (Pleurotus Ostreatus) on fermented pine sawdust substrate. Exp. Agric. Horticult. 2012;12(4):30–40. [Google Scholar]
- Patel Y., Naraian R., Singh V. Medicinal properties of Pleurotus species (oyster mushroom): a review. World J. Fungal Plant Biol. 2012;3:1–12. doi: 10.5829/idosi.wjfpb.2012.3.1.303. [DOI] [Google Scholar]
- Pardo-Giménez A., Picornell Buendía M.R., de Juan Valero J.A., Pardo-González J.E., Cunha Zied D. Cultivation of pleurotus ostreatus using supplemented spent oyster mushroom substrate. Acta Hortic. 2012;(933):267–272. doi: 10.17660/ActaHortic.2012.933.33. [DOI] [Google Scholar]
- Pardo-Giménez A. Reutilización del sustrato agotado en la producción de hongos comestibles cultivados. ITEA. 2008;104:360–368. [Google Scholar]
- Royse D.J. Influence of spawn rate and commercial delayed release nutrient levels of Pleurotus cornucopiae (oyster mushroom) yield, size and time to production. Appl. Microbiol. Biotechnol. 2002;58:527–531. doi: 10.1007/s00253-001-0915-2. [DOI] [PubMed] [Google Scholar]
- Sánchez C. Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl. Microbiol. Biotechnol. 2010;85(5):1321–1337. doi: 10.1007/s00253-009-2343-7. [DOI] [PubMed] [Google Scholar]
- Rühl M., Kües U. Mushroom production. In: Kües U., editor. Wood Production, Food Technology, and Biotechnological Impacts. Universitatsverlag Gottingen; Germany: 2007. pp. 555–586.http://webdoc.sub.gwdg.de/ [Google Scholar]
- Selva P.P., Balakrishnan N. A review of nano fertilizers and their use and functions in soil. Int. J. Curr. Microbiol. App. Sci. 2017;6:3117–3133. doi: 10.20546/ijcmas.2017.612.364. [DOI] [Google Scholar]
- Singh M.P., Pandey V.K., Pandey A.K., Srivastava A.K., Vishwakarma N.K., Singh V.K. Production of xylanase by white rot fungi on wheat straw. Asian J. Microbiol. Biotechnol. Environ. Exp. Sci. 2008;10(4):859–862. [Google Scholar]
- Sharma V.P., Jandaik C.L. Studies on recycling of Pleurotus waste. Mushroom J. Trop. 1985;6(2):13–15. [Google Scholar]
- Synytsya A., Mícková K., Jablonsky I., Sluková M., Copíková J. Mushrooms of genus Pleurotus as a source of dietary fibres and glucans for food supplements. Czech J. Food Sci. 2008;26(6):441–446. doi: 10.17221/1361-CJFS. [DOI] [Google Scholar]
- Sajyan T.K., Shaban N., Rizkallah J., Sassine Y.N. Effects of Monopotassiumphosphate, Nano-calcium fertilizer, Acetyl salicylic acid (Aspirin) and Glycinebetaine application on growth and production of tomato (Solanum lycopersicum) crop under salt stress. Agron. Res. 2018;16(3):872–883. [Google Scholar]
- Sajyan T.K., Naim L., Sebaaly Z., Rizkallah J., Shaban N., Sassine Y.N. Alleviating the adverse effects of salinity stress on tomato crop (Solanum lycopersicum) using nano-fertilizer as foliar application. Acta Hortic. 2019;1253 doi: 10.17660/ActaHortic.2019.1253.5. 33 40. [DOI] [Google Scholar]
- Upadhyay R.C., Verma R.N., Singh S.K., Yadav M.C. Effect of organic nitrogen supplementation Pleurotus sp. The 4th ICMBP. Solan, India. Mushroom Biol. Mushroom Prod. UAEM. 2002;12:325–332. [Google Scholar]
- Yang W.J., Guo F.L., Wan Z.J. Yield and size of oyster mushroom grown on rice/wheat straw basal substrate supplemented with cotton seed hull. Saudi Journal of Biological Sciences. 2013;2013(20):333–338. doi: 10.1016/j.sjbs.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zied D.C., Pardo-Giménez A., Pardo J.E., Dias E.S., Carvalho M.A., Minhoni M.T.A. Effect of cultivation practices on the β-glucan content of Agaricus subrufescens basidiocarps. J. Agric. Food Chem. 2014;62:41–49. doi: 10.1021/jf403584g. [DOI] [PubMed] [Google Scholar]