Abstract
Vibrio parahaemolyticus is a foodborne bacterial pathogen that may cause gastroenteritis in humans through the consumption of seafood contaminated with this microorganism. The emergence of antimicrobial and multidrug-resistant bacteria is another serious public health threat worldwide. In this study, the prevalence and antibiotic susceptibility test of V. parahaemolyticus in blood clams, shrimps, surf clams, and squids were determined. The overall prevalence of V. parahaemolyticus in seafood was 85.71% (120/140), consisting of 91.43% (32/35) in blood clam, 88.57% (31/35) in shrimps, 82.86% (29/35) in surf clams, and 80% (28/35) in squids. The majority of V. parahaemolyticus isolates from the seafood samples were found to be susceptible to most antibiotics except ampicillin, cefazolin, and penicillin. The MAR indices of V. parahaemolyticus isolates ranged from 0.04 to 0.71 and about 90.83% of isolates were found resistant to more than one antibiotic. The high prevalence of V. parahaemolyticus in seafood and multidrug-resistant isolates detected in this study could pose a potential risk to human health and hence appropriate control methods should be in place to minimize the potential contamination and prevent the emergence of antibiotic resistance.
Keywords: Antibiogram, MAR, Multidrug-resistant, Seafood, Vibrio
1. Introduction
Vibrio parahaemolyticus is a facultative, anaerobic, Gram-negative, curved rod-shaped bacterium commonly found in marine and estuary environments. It is a moderate halophilic bacterium capable of survival and multiplication at a concentration of 1 to 9% sodium chloride (NaCl) while the optimal growth happens at 3% NaCl (Whitaker et al., 2010, Kalburge et al., 2014). The ecological habitat of V. parahaemolyticus can be free to live as bacterioplankton, associated with the seafood surface, and a parasite in the gastrointestinal tract of fish. Higher organisms such as crustacean and molluscan shellfish are frequently found to be associated with V. parahaemolyticus (Kirs et al., 2011, Rodgers et al., 2014, Malcolm et al., 2015, Mala et al., 2016, Yu et al., 2016). Shellfish and other aquatic organisms are therefore often used as a vehicle for the transmission of this microorganism. Although V. parahaemolyticus is a well-known halophile, some reports have shown that V. parahaemolyticus can also be found in freshwater organisms (Nair et al., 2007, Nelapati and Krishnaiah, 2010, Noorlis et al., 2011, Otomo et al., 2013).
Ingestion of food contaminated with V. parahaemolyticus can lead to gastrointestinal illness, including symptoms such as watery diarrhoea, abdominal cramps, nausea, vomiting, fever, headache and/or bloody diarrhoea (CDC, 2013). Open wounds in contact with V. parahaemolyticus may also result in wound infection and life-threatening septicemia. Based on the number of vibriosis infections reported to the Cholera and Other Vibrio Illness Surveillance (COVIS) system and Centers for Disease Control and Prevention (CDC) from 1996 to 2014, V. parahaemolyticus was identified as the most common foodborne pathogen which caused 39–51% of Vibrio infection compared to other Vibrio species such as V. vulnificus, V. cholerae (non-O1 and non-O139), V. alginolyticus, V. fluvialis, V. mimicus, and V. hollisae (Newton et al., 2012, Centers for Disease Control and Prevention (CDC), 2019). The high infection rate caused by V. parahaemolyticus results in high medical costs worldwide. For example, the annual health cost of ingestion of seafood contaminated with V. parahaemolyticus in the United States was estimated to be $21 million (Ralston et al., 2011).
Another global health concern in recent years has been the rising cost of the medical costs of antibiotic-resistant infections. Timely surveillance of antibiotic-resistant bacteria and dissemination of surveillance data are therefore essential to address these public health issues (Johnson, 2015). Antibiotic resistance profiles of bacteria are usually determined through phenotypic assays such as agar dilution, broth dilution and Kirby-Bauer disk diffusion. Agar and broth dilution methods are used to determine the minimal inhibitory concentration (MIC) of antimicrobial agents by inoculating the defined number of bacterial cells at different concentrations of the antimicrobial substance (Wiegand et al., 2008). Kirby-Bauer disk diffusion method involves different kinds of the antibiotic disc placed on a bacterium agar plate and the antimicrobial profile of the bacteria is interpreted as sensitive, intermediate and resistance based on the inhibition zone. This latter method is routinely used in many clinical microbiology laboratories to test common and fast-growing pathogens due to its simplicity, well standardized, and easily interpreted (Jorgensen and Ferraro, 2009, Syal et al., 2017).
The majority of V. parahaemolyticus strains isolated from clinical and environmental samples reported high resistance to multiple antibiotics such as amoxicillin, ampicillin, carbenicillin, cefazolin, ceftazidime, cephalothin, colistin, gentamicin, and tobramycin (Zanetti et al., 2001, de Melo et al., 2011, Al-Othrubi et al., 2014, Sudha et al., 2014, Yano et al., 2014). Extensive use and misuse of prophylactic antibiotics in aquaculture for the prevention of bacterial infection and rapid spread of disease is most likely the main cause of the emergence and widespread of multiple drug resistance (MDR) in V. parahaemolyticus isolates. In addition, the overuse of antibiotics in aquaculture not only increases the selection of antibiotic-resistant bacteria and the dissemination of the antibiotic-resistant genes but also results in the presence of antibiotic residues in aquatic organisms such as fish (Miranda et al., 2018). The presence of V. parahaemolyticus in seafood samples and the occurrence of V. parahaemolyticus antibiotic resistance should be evaluated frequently. The aim of this study was to determine the prevalence and antibiotic-resistant patterns of V. parahaemolyticus isolated from different types of seafood in Malaysia.
2. Materials and methods
2.1. Sample collection
A total of 140 seafood samples consists of 35 samples for each blood clam (Anadara granosa), shrimp (Penaeus spp.), surf clam (Paphia undulata), and squid (Loligo spp.) were purchased from different wet markets in Selangor, Malaysia, for a period of 6 months from January 2018 to June 2018. All samples were transported to the laboratory and analysed immediately on the same sampling date.
2.2. Enrichment and isolation
Samples were examined according to the US FDA Bacteriological Analytical Manual (BAM) for Vibrio species with some modifications (Kaysner and DePaola, 2004). Twenty-five grams of each sample was weighed and transferred to a sterile stomacher bag containing 225 mL of alkaline peptone water (APW; Merck, Germany). Sample in the stomacher bag was mixed with Stomacher Lab-Blender 400 (Seward Medical, UK) for 2 min. Serial 10-fold dilution was carried out up to 10−5 by transferring 1 mL of the mixture to 9 mL of APW. Each dilution tube in triplicate was incubated at 37 °C overnight. After incubation, one loopful of the sample in each tube was streaked onto the CHROMagar™ Vibrio (CV) plate and the plates were incubated at 37 °C for overnight. Bacterial colonies in mauve colour were considered to be presumptive V. parahaemolyticus. The mauve colony was picked, purified by streaking back onto the CV plate and incubated at 37 °C overnight. A single colony was transferred to tryptic soy broth (TSB; Merck, Germany) supplemented by 2.5% NaCl (Merck, Germany) and incubated at 37 °C overnight. Colonies growth in the TSB were subjected to DNA extraction and V. parahaemolyticus species-specific confirmation through PCR assay. Pure V. parahaemolyticus colonies were stored on tryptic soy agar (TSA; Merck, Germany) slanted and kept at room temperature until further analysis.
2.3. DNA extraction and PCR confirmation
Bacterial DNA extraction was done by boiling and freeze-thawing extraction procedures. Briefly, 1.5 mL of overnight culture growth in the TSB supplemented by 2.5% NaCl was centrifuged at 13,400 × g for 3 min. The supernatant was discarded and the pellet was suspended in 200 µL TE buffer (10 mM Tris-HCl and 1 mM EDTA•Na2 at pH 8.0). The suspension was boiled at 100 °C for 15 min in a dry bath (Labnet, USA) and immediately kept in a −20 °C freezer for 15 min. The suspension was then again centrifuged at 13,400 × g for 1 min and the supernatant was used as a DNA template for the PCR assay.
Presumptive V. parahaemolyticus colony growth on the CV plate was confirmed by amplification of V. parahaemolyticus species-specific gene with the toxR primers (F: 5′-GTCTTCTGACGCAATCGTTG-3′ and R: 5′-ATACGAGTGGTTGCTGTCATG-3′) (Kim et al., 1999). The presence of thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH) genes was also detected by the use of the tdh primers (F: 5′-CCACTACCACTCTCATATGC-3′ and R: 5′-GGTACTAAATGGCTGACATC-3′) and trh primers (F: 5′-TTGGCTTCGATATTTTCAGTATCT-3′ and R: 5′-CATAACAAACATATGCCCATTTCCG-3′), respectively (Tada et al., 1992, Bej et al., 1999). The amplification of toxR, tdh and trh genes was performed in a single reaction according to the multiplex PCR procedure described by Malcolm et al. (2015).
A total of 25 µL of each reaction mixture consists of 7 µL of 1.4 × PCR buffer, 2.5 µL of 2.5 mM MgCl2, 0.5 µL of 0.2 mM dNTPs, 0.5 µL of 0.2 μM primers mix, 0.4 µL of GoTaq® Taq polymerase (Promega, USA), 2 μL of DNA template, and 12.1 µL of sterilized distilled water. The amplification was performed in the Kyratec SuperCycler Trinity (Australia) and the following conditions were applied: initial denaturation at 95 °C for 5 min for 1 cycle, 30 cycles consisting of denaturation at 95 °C for 30 s, annealing at 60 °C for 45 s, extension at 68 °C for 1 min, and final extension cycle at 72 °C for 3 min.
2.4. Antibiotic susceptibility test (AST)
A single V. parahaemolyticus isolate from each positive sample was selected for the antibiotic susceptibility test. A total of 24 types of antibiotics including amikacin (30 μg), amoxicillin-clavulanic acid (20/10 μg), ampicillin (10 μg), ampicillin-sulbactam (10 μg), cefazolin (30 μg), cefepime (30 μg), cefotaxime (30 μg), cefoxitin (30 μg), ceftazidime (30 μg), cefuroxime sodium (parenteral) (30 μg), cephalothin (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), doxycycline (30 μg), gentamicin (10 μg), imipenem (10 μg), levofloxacin (5 μg), meropenem (10 μg), ofloxacin (5 μg), penicillin G (10 unit), piperacillin (100 μg), piperacillin-tazobactam (100/10 μg), tetracycline (30 μg), and trimethoprim-sulfamethoxazole (1.25/23.75 μg) was used in this study. All the antimicrobial susceptibility discs were purchased from Oxoid (England). Selection of antibiotics was based on their frequent usage in clinical practices and according to the Clinical and Laboratory Standards Institute (CLSI) M45 guideline for Vibrio spp. (not V. cholerae) (CLSI, 2010). Disk susceptibility testing was according to the Kirby-Bauer disk diffusion method and CLSI M45 guidelines (Bauer et al., 1966, CLSI, 2010).
Briefly, a direct colony suspension was prepared by suspending the bacterial colony in 0.85% NaCl solution and adjusting equivalent to 0.5 McFarland standard. The inoculum was swabbed uniformly on the Mueller-Hinton agar plate (MHA; Merck, Germany) using a sterile cotton swab and allowed to dry for 5–10 min before placing the antibiotic disks on the MHA plate by using Oxoid™ disk dispenser. The plates were then incubated at 35 °C for 16–20 h. Escherichia coli ATCC® 25922 was included and used as a quality control organism in this study to monitor the accuracy of disk diffusion tests.
2.5. AST interpretive criteria
The diameter of any inhibition zone around the antibiotic disk was measured in the nearest millimetre. The zone diameter value was used to categorize each isolate as susceptible, intermediate, and resistant according to the CLSI recommendation breakpoint (CLSI, 2010). The multiple antibiotic resistance (MAR) index was also determined by using the formula, a/b, where “a” is the number of antibiotics to which the particular isolate was resistant, and “b” is the total number of antibiotics tested (Krumperman, 1983).
3. Results
3.1. Prevalence of V. parahaemolyticus
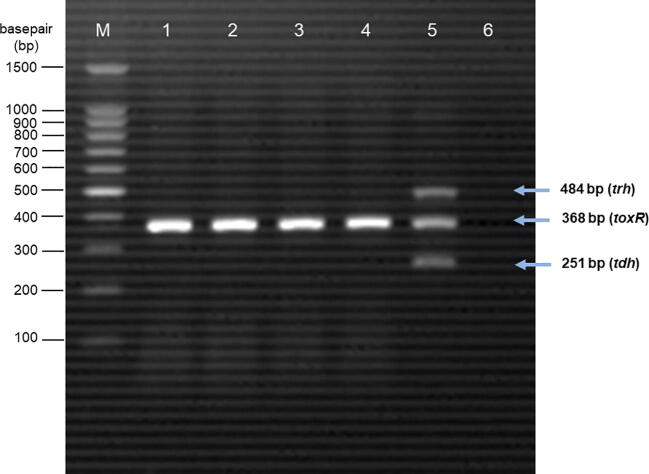
A total of 120 (85.71%) samples were found to be positive for V. parahaemolyticus. V. parahaemolyticus was isolated from 91.43% (32/35) of blood clams, 88.57% (31/35) of shrimps, 82.86% (29/35) of surf clams, and 80% (28/35) of squids. The prevalence of V. parahaemolyticus in four different types of seafood samples is summarised in Table 1. A 368 bp DNA fragment was developed from the PCR assay detection of V. parahaemolyticus species-specific toxR gene (Fig. 1). None of the V. parahaemolyticus isolates isolated from seafood samples were positive for pathogenic tdh and trh genes.
Table 1.
Prevalence of Vibrio parahaemolyticus in blood clam, shrimp, surf clam and squid.
| Sample | No. of sample | Number of positive samples | (%) of positive samples |
|---|---|---|---|
| Blood clam | 35 | 32 | 91.43 |
| Shrimp | 35 | 31 | 88.57 |
| Surf clam | 35 | 29 | 82.86 |
| Squid | 35 | 28 | 80.00 |
| Total | 140 | 120 | 85.71 |
Fig. 1.
Agarose gel electrophoresis of PCR products. M = 100 bp DNA marker; Lane 1: blood clam sample with toxR positive; Lane 2: shrimp sample with toxR positive; Lane 3: surf clam sample with toxR positive; Lane 4: squid sample with toxR positive; Lane 5: positive control; Lane 6: negative control.
3.2. Antibiotic susceptibility test (AST)
A total of 120 V. parahaemolyticus isolates collected from all positive samples were confirmed by a pre-tested PCR assay against 24 types of antibiotics. Antibiotic susceptibility profiles of 120 V. parahaemolyticus isolates are presented in Table 2. All the V. parahaemolyticus isolates are 100% penicillin G resistant. The majority of V. parahaemolyticus (84.17%) isolated from seafood samples were also found to be highly resistant to ampicillin and cefazolin. Antibiotic sensitivity profiles of V. parahaemolyticus have shown that chloramphenicol inhibits the growth of all isolates. Ampicillin-sulbactam, imipenem, meropenem, tetracycline, trimethoprim-sulfamethoxazole, and doxycycline were also found to be effective against more than 90% of V. parahaemolyticus isolates. The antibiotic resistance profile of each V. parahaemolyticus was found to differ with MAR indices ranging from 0.04 to 0.71 (Table 3). The BC4 isolate showed the highest MAR index of 0.71 that was resistant to 17 antibiotics. In this study, majority of V. parahaemolyticus isolates from seafood samples demonstrated resistance to at least 3 antibiotics.
Table 2.
Antibiotic susceptibility profiles of V. parahaemolyticus isolated from seafood samples tested by disk diffusion method.
| Antibiotics | Code | Interpretive criteria |
||
|---|---|---|---|---|
| Resistant (%) | Intermediate (%) | Sensitivity (%) | ||
| Amikacin (30ug) | Ak | 45 (37.50) | 40 (33.33) | 35 (29.17) |
| Amoxicillin-clavulanic acid (20/10ug) | Amc | 1 (0.83) | 28 (23.33) | 91 (75.83) |
| Ampicillin (10ug) | Amp | 101 (84.17) | 13 (10.83) | 6 (5.00) |
| Ampicillin-sulbactam (10ug) | Sam | 1 (0.83) | 7 (5.83) | 112 (93.33) |
| Cefazolin (30ug) | Cz | 101 (84.17) | 17 (14.17) | 2 (1.67) |
| Cefepime (30ug) | Fep | 4 (3.33) | 30 (25.00) | 86 (71.67) |
| Cefotaxime (30ug) | Ctx | 6 (5.00) | 72 (60.00) | 42 (35.00) |
| Cefoxitin (30ug) | Fox | 15 (12.50) | 57 (47.50) | 48 (40.00) |
| Ceftazidime (30ug) | Caz | 6 (5.00) | 29 (24.17) | 85 (70.83) |
| Cefuroxime sodium (parental) (30ug) | Cxm | 62 (51.67) | 45 (37.50) | 13 (10.83) |
| Cephalothin (30ug) | Kf | 65 (54.17) | 43 (35.83) | 12 (10.00) |
| Chloramphenicol (30ug) | C | – | – | 120 (100) |
| Ciprofloxacin (5ug) | Cip | 16 (13.33) | 80 (66.67) | 24 (20.00) |
| Doxycycline (30ug) | Do | – | 2 (1.67) | 118 (98.33) |
| Gentamicin (10ug) | Cn | 8 (6.67) | 35 (29.17) | 77 (64.17) |
| Imipenem (10ug) | Ipm | – | 2 (1.67) | 118 (98.33) |
| Levofloxacin (5ug) | Lev | 2 (1.67) | 30 (25.00) | 88 (73.33) |
| Meropenem (10ug) | Mem | – | 2 (1.67) | 118 (98.33) |
| Ofloxacin (5ug) | Ofx | 3 (2.50) | 33 (27.50) | 84 (70.00) |
| Penicillin G (10 unit) | P | 120 (100) | – | – |
| Piperacillin (100ug) | Prl | 43 (35.83) | 28 (23.33) | 49 (40.83) |
| Piperacillintazobactam (100/10ug) | Tzp | 19 (15.83) | 35 (29.17) | 66 (55.00) |
| Tetracyline (30ug) | Te | – | 7 (5.83) | 113 (94.17) |
| Trimethoprimsulfamethoxazole (1.25/23.75ug) | Sxt | – | 6 (5.00) | 114 (95.00) |
Table 3.
Antibiotics resistance profile and multiple antibiotic resistance (MAR) index of V. parahaemolyticus isolated from seafood samples.
| MAR Index | Antibiotics Resistance Profile | Isolatesa | Percentage of Isolate (%) |
|---|---|---|---|
| 0.71 | Amp, Amc, Sam, Prl, Tzp, Cz, Fep, Ctx, Fox, Cxm, Kf, Ak, Cn, Cip, Lev, Ofx, P | BC4 | 0.83 |
| 0.63 | Amp, Prl, Tzp, Cz, Fep, Ctx, Fox, Caz, Cxm, Kf, Ak, Cip, Lev, Ofc, P | SC2 | 0.83 |
| 0.58 | Amp, Prl, Tzp, Cz, Ctx, Fox, Caz, Cxm, Kf, Ak, Cn, Cip, Ofx, P | SQ3 | 0.83 |
| 0.54 | Amp, Prl, Tzp, Cz, Fep, Ctx, Fox, Caz, Cxm, Kf, Ak, Cip, P | BC10 | 0.83 |
| 0.50 | Amp, Prl, Tzp, Cz, Fep, Ctx, Fox, Cxm, Kf, Ak, Cip, P | SH3 | 0.83 |
| 0.46 | Amp, Prl, Tzp, Cz, Fox, Cxm, Kf, Ak, Cn, Cip, P | BC16, SH19 | 1.67 |
| 0.42 | Amp, Prl, Tzp, Cz, Cxm, Kf, Ak, Cn, Cip, P | SQ13, SH20 | 1.67 |
| 0.38 | Amp, Prl, Tzp, Cz, Fox, Caz, Cxm, Kf, P Amp, Prl, Tzp, Cz, Cxm, Kf, Ak, Cip, P Amp, Prl, Tzp, Cz, Cxm, Kf, Ak, Cn, P Amp, Prl, Cz, Ctx, Fox, Cxm, Kf, Ak, P |
SH2 BC1 SQ4 SC15 |
0.83 0.83 0.83 0.83 |
| 0.33 | Amp, Cz, Fox, Cxm, Kf, Ak, Cip, P Amp, Prl, Tzp, Cz, Cxm, Kf, Cip, P Amp, Prl, Tzp, Cz, Cxm, Kf, Ak, P Amp, Prl, Tzp, Cz, Fox, Cxm, Kf, P Amp, Prl, Tzp, Cz, Caz, Cxm, Kf, P Amp, Prl, Cz, Cxm, Kf, Ak, Cn, P |
SH1 SC3 SC11 SC12 BC13 SC19 |
0.83 0.83 0.83 0.83 0.83 0.83 |
| 0.29 | Amp, Prl, Cz, Fox, Cxm, Kf, P Amp, Prl, Cz, Cxm, Kf, Cip, P Amp, Prl, Tzp, Cz, Cxm, Kf, P Amp, Cz, Cxm, Kf, Ak, Cip, P Amp, Prl, Cz, Cxm, Kf, Ak, P |
BC3 BC29 SQ2, BC2, SQ7 SH9, SQ21, BC30 SH4, SH5, BC12, SQ4, SH18, SQ15, SQ23, BC23 |
0.83 0.83 2.50 2.50 6.67 |
| 0.25 | Amp, Cz, Cxm, Kf, Cip, P Amp, Prl, Cz, Cxm, Ak, P Amp, Cz, Caz, Cxm, Kf, P Amp, Prl, Cz, Fox, Ak, P Amp, Cz, Fox, Cxm, Kf, P Amp, Prl, Cz, Cxm, Kf, P Amp, Prl, Cz, Cxm, Ak, P Amp, Cz, Cxm, Kf, Ak, P |
SQ1 SQ5 SC13 SH13 SQ10, BC22 SC17, SQ22 SH16, SH17 SH11, SH14, SH15, SH25, SH28, SC24, SC26 |
0.83 0.83 0.83 0.83 1.67 1.67 1.67 5.83 |
| 0.21 | Amp, Prl, Cz, Ak, P Amp, Cz, Cxm, Ak, P Amp, Cz, Kf, Ak, P Amp, Cz, Cxm, Kf, P |
SQ11, SQ24 BC17, BC32 SH6, SQ6, SQ26 SC1, BC5, SQ8, SC10, SQ12, BC25, BC27, SC29 |
1.67 1.67 2.50 6.67 |
| 0.17 | Amp, Cz, Ak, P Amp, Cz, Kf, P Amp, Prl, Cz, P |
SQ17 SC6, SH30, SH31 SH8, SC14, BC15 |
0.83 2.50 2.50 |
| 0.13 | Cz, Kf, P Amp, Cz, P |
SC21, BC21 SC4, BC6, SC7, SC8, BC7, SQ9, SC9, BC11, BC14, BC18, SH21, BC19, SH24, SQ20, SH27, SQ25, SH29, BC26, SC23, SC25, SC28 |
1.67 17.50 |
| 0.08 | Kf, P Cxm, P Cz, P Amp, P |
SC18 SH7 SH10, SH22, SH23, SC27 BC9, SH12, SC16, SH26, BC24, SQ28 |
0.83 0.83 3.33 5.00 |
| 0.04 | P | SC5, BC8, SQ16, SQ18, SQ19, BC20, SC20, SC22, SQ27, BC28, BC31 | 9.17 |
BC – Blood clam; SH – Shrimp; SC – Surf clam; SQ – Squid.
4. Discussion
The prevalence of V. parahaemolyticus in different types of seafood samples ranged from 80 to 91.43% with an average of 85.71% in this study. Blood clam was detected at the highest prevalence rate (91.43%) followed by shrimp (88.57%), surf clam (82.86%), and squid (80.00%). The higher occurrence of V. parahaemolyticus in seafood was mainly due to the widely disseminated of V. parahaemolyticus in estuarine, marine and coastal environments (Su and Liu, 2007, Johnson et al., 2012, Givens et al., 2014, Wu et al., 2014). The warm and tropical climate of Malaysia is also likely to promote and favour the growth of V. parahaemolyticus. Schwab et al. (2014) reported that the warmer the weather, the higher the incidence density of gram-negative bacteria. Not only that, Sterk et al. (2015) reported that an average temperature increases by 3.7 °C could lead to the changes in the concentration of V. parahaemolyticus and increase the risk of illness by two to three times higher.
The results obtained from this study were found to be comparable to the findings of Tran et al. (2018). Tran et al. (2018) reported that 332 of 385 (86.2%) seafood samples, including molluscan shellfish and shrimp collected in Vietnam, had been contaminated with V. parahaemolyticus. On the other hand, Malcolm et al. (2015) reported a slightly higher prevalence of V. parahaemolyticus in seafood samples where all blood clams (84/84), 98.7% (75/76) surf clams, and 97.2% (70/72) shrimps were positive for V. parahaemolyticus. In contrast, Letchumanan et al. (2015) reported only 44% (200/450) shellfish samples, including mud crab, flower crab, carpet clam, hardshell clam, and mud creeper collected in Malaysia, were found to be positive for V. parahaemolyticus species-specific toxR gene. Similarly, Li et al. (2019) reported a low prevalence rate of V. parahaemolyticus in which 15.7% (365/2328) fish, 27.6% (164/594) crustaceans and 27.6% (84/304) molluscs collected in China were identified for V. parahaemolyticus.
Although a high prevalence of V. parahaemolyticus was detected in seafood samples, the majority of isolates were found to be non-pathogenic to humans due to lack of pathogenic tdh and trh genes. In this study, no seafood samples were detected with tdh and/or trh genes. Previous studies have also reported a low occurrence of V. parahaemolyticus isolates for tdh and trh genes, in accordance with the present results. Tran et al. (2018) reported that 25 out of 385 (6.5%) molluscan shellfish and shrimp samples were detected with pathogenic tdh and/or trh genes. Malcolm et al. (2015) revealed that 33.1% (77/232) and 6.9% (16/232) of seafood samples were detected with the presence of tdh and trh genes, respectively. Letchumanan et al. (2015) reported that only 6.5% (13/200) of the V. parahaemolyticus isolates collected from shellfish samples were trh-positive and none of the samples was tdh-positive. Consequently, majority of V. parahaemolyticus strains isolated from the seafood samples are found with the absence of tdh and trh genes. However, the pathogenicity of V. parahaemolyticus is complex and interactive (Sun et al., 2019). The significance of V. parahaemolyticus and its host-pathogen interactions for human infection is still questionable (Ghenem et al., 2017). V. parahaemolyticus strain without the virulence tdh and trh genes have been also isolated from clinical specimens and reported in several studies (Bhoopong et al., 2007, Jones et al., 2012, Li et al., 2014, Pazhana et al., 2014). Besides the predominant tdh and trh hemolysin genes, the other factors contributing to human pathogenesis of V. parahaemolyticus infection have been addressed in some previous research studies. For instance, Makino et al. (2003) suggested that the presence of Type III Secretion Systems (T3SS) in V. parahaemolyticus strain is one of the important virulence factors closely related to V. parahaemolyticus pathogenicity. Urease present in many other foodborne pathogens, including V. parahaemolyticus, is defined as an enterovirulence factor that hydrolyses urea and increases pH in the immediate environment within the host (Okuda et al., 1997, Hongping et al., 2011, Berutti et al., 2014).
V. parahaemolyticus isolated from different types of seafood samples in this study were found to be highly resistant to the penicillin class of antibiotics, including penicillin G (10 unit) and ampicillin (10 µg). This finding is consistent with previous studies that the majority of V. parahaemolyticus isolates from seafood, such as grouper, shellfish, small mackerel and shrimp, were found to be highly resistant to penicillin (92.54–100%) and ampicillin (82.09–88%) (Srinivasan and Ramasamy, 2009, Letchumanan et al., 2015, Tan et al., 2017, Amalina et al., 2019). A total of 84.17% of V. parahaemolyticus isolates from this study were also found to be resistant to cefazolin (30 µg), which is a cephalosporin antibiotic. From the results, it can be concluded that V. parahaemolyticus strains isolated from different types of seafood were found to be highly resistant to beta-lactam class antibiotics, including penicillin and cephalosporins. Therefore, ampicillin, penicillin and cefazolin should be phased-out for treating V. parahaemolyticus infections. Likewise, cefotaxime and ciprofloxacin are not a good choice in the treatment regimens for Vibrio infections because of its associated intermediate resistance by 60% and 66.67% of V. parahaemolyticus isolates, respectively.
Although V. parahaemolyticus isolates from this study displayed high levels of resistance to ampicillin, cefazolin, and penicillin, as well as intermediate levels of resistance to cefotaxime and ciprofloxacin, the antibiogram revealed that most of the V. parahaemolyticus isolates were susceptible to ampicillin-sulbactam (93.33%), chloramphenicol (100%), doxycycline (98.33%), imipenem (98.33%), meropenem (98.33%), tetracycline (94.17%), and trimethoprim-sulfamethoxazole (95%). These findings are comparable to the results of Letchumanan et al. (2015) in which V. parahaemolyticus isolates from shrimp samples were found to be highly susceptible to ampicillin-sulbactam (96%), chloramphenicol (95%), imipenem (98%), tetracycline (82%), and trimethoprim-sulfamethoxazole (93%). Lopatek et al. (2015) also reported that all isolates of V. parahaemolyticus isolated from raw shellfish were susceptible to chloramphenicol and tetracycline. Similarly, Xu et al. (2016) demonstrated that majority of V. parahaemolyticus isolates from retail aquatic products in North China were susceptible to chloramphenicol (95%), ciprofloxacin (92%), gentamicin (63%), tetracycline (83%), and trimethoprim-sulfamethoxazole (75%). These antibiotics could, therefore, be used effectively in the treatment of V. parahaemolyticus infections.
V. parahaemolyticus isolates tested in this study demonstrated MAR indices ranging from 0.04 to 0.71, with an average of 0.22. One isolate from blood clam exhibited the highest MAR index value of 0.71 which showed resistance to 17 antibiotics. Overall, 90.83% of V. parahaemolyticus isolates were multidrug resistance (MDR) which exhibited resistance to more than one antibiotic used in this study. A total 67 of 120 isolates (55.83%) had MAR indices of more than 0.20. The MAR index greater than 0.2 indicates that the bacterial strain tested originated from the high-risk sources where antibiotics are frequently used (Krumperman, 1983, Gufe et al., 2019). According to the study conducted by Ahmed et al. (2018) which reported all V. parahaemolyticus isolates showed MDR for at least 7 antibiotics, MAR indices ranging from 0.58 to 1, and an average MAR index value of 0.77. Yu et al. (2016) showed the MAR indices ranging from 0.11 to 0.22 and V. parahaemolyticus isolate with the highest MAR index exhibited resistance to 4 antibiotics. From these results, it is noted that MAR indices vary between the studies and are not suitable for comparison due to the number of antibiotics and types of antibiotics used in the tests.
5. Conclusion
High prevalence of V. parahaemolyticus was detected in various types of seafood samples collected in Selangor, Malaysia. Although pathogenic V. parahaemolyticus strains with hemolysin tdh and trh genes have not been detected in this study, the risk of V. parahaemolyticus infection cannot be disregarded as the pathogenesis of vibriosis caused by tdh- and trh-negative strains of V. parahaemolyticus is still open to question. Infections caused by multidrug-resistant V. parahaemolyticus strains also pose a significant risk to human health. Antimicrobial resistance surveillance programmes should be continuous and aggregated at the national level in order to detect the emerging resistance and access the burden of antimicrobial resistance.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by Fundamental Research Grant Scheme (FRGS) of the Ministry of Higher Education (MOHE), Malaysia (01-01-18-2015FR), Grant Putra IPS from Universiti Putra Malaysia (GP-IPS 9438703) and partly by the Japan Agency for Medical Research and Development, AMED (JP15km0908001).
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed H.A., El Bayomi R.M., Hussein M.A., Khedr M.H.E., Abo Remela E.M., El-Ashram A.M.M. Molecular characterization, antibiotic resistance pattern and biofilm formation of Vibrio parahaemolyticus and V. cholerae isolated from crustaceans and humans. Int. J. Food Microbio. 2018;274:31–37. doi: 10.1016/j.ijfoodmicro.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Al-Othrubi S.M., Kqueen C.Y., Mirhosseini H., Hadi Y.A., Radu S. Antibiotic resistance of Vibrio parahaemolyticus isolated from cockles and shrimp sea food marketed in Selangor, Malaysia. Clin. Microbial. 2014;3:148–154. doi: 10.4172/2327-5073.1000148. [DOI] [Google Scholar]
- Amalina N.Z., Santha S., Zulperi D., Amal M.N.A., Yusof M.T., Zamri-Saad M., Ina-Salwany M.Y. Prevalence, antimicrobial susceptibility and plasmid profiling of Vibrio spp. isolated from cultured groupers in Peninsular Malaysia. BMC Microbiol. 2019;19:251. doi: 10.1186/s12866-019-1624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A.W., Kirby W.M.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Path. 1966;45:493. [PubMed] [Google Scholar]
- Bej A.K., Patterson D.P., Brasher C.W., Vickery M.C.L., Jones D.D., Kaysner C.A. Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tlh, tdh and trh. J. Microbiol. Methods. 1999;36:215–225. doi: 10.1016/s0167-7012(99)00037-8. [DOI] [PubMed] [Google Scholar]
- Berutti T., Williams R., Shen S., Taylor M., Grimes D. Prevalence of urease in Vibrio parahaemolyticus from the Mississippi Sound. Lett. Appl. Microbiol. 2014;58:624–628. doi: 10.1111/lam.12237. [DOI] [PubMed] [Google Scholar]
- Bhoopong P., Palittapongarnpim P., Pomwised P., Kiatkittipong A., Kamruzzaman M., Nakaguchi Y., Nishibuchi M., Ishibashi M., Vuddhakul V. Variability in properties of Vibrio parahaemolyticus strains isolated from single patients. J. Clin. Microbiol. 2007;45:1544–1550. doi: 10.1128/JCM.02371-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Signs & symptoms: Vibrio parahaemolyticus illnesses associated with consumption of shellfish, United States, 2013. 2013. https://www.cdc.gov/vibrio/investigations/vibriop-09-13/signs-symptoms.html accessed 20 October 2019.
- Centers for Disease Control and Prevention (CDC) Cholera and Other Vibrio Illness Surveillance (COVIS) 2019. https://www.cdc.gov/vibrio/investigations/vibriop-09-13/signs-symptoms.html accessed 22 October 2019.
- CLSI, 2010. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; Approved Guideline – Second Edition. CLSI document M45-A2. Clinical and Laboratory Standards Institute, Pennsylvania.
- de Melo L.M., Almeida D., Hofer E., Dos Reis C.M., Theophilo G.N., Santos A.F., Vieira R.H. Antibiotic resistance of Vibrio parahaemolyticus isolated from pond-reared Litopenaeus vannamei marketed in natal, Brazil. Braz. J. Microbiol. 2011;42(4):1463–1469. doi: 10.1590/S1517-838220110004000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghenem L., Elhadi N., Alzahrani F., Nishibuchi M. Vibrio Parahaemolyticus: a review on distribution, pathogenesis, virulence determinants and epidemiology. Saudi. J. Med. Med. Sci. 2017;5(2):93–103. doi: 10.4103/sjmms.sjmms_30_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens C.E., Bowers J.C., Depaola A., Hollibaugh J.T., Jones J.L. Occurrence and distribution of Vibrio vulnificus and Vibrio parahaemolyticus - potential roles for fish, oyster, sediment and water. Lett. Appl. Microbiol. 2014;58(6):503–510. doi: 10.1111/lam.12226. [DOI] [PubMed] [Google Scholar]
- Gufe C., Hodobo T.C., Mbonjani B., Majonga O., Marumure J., Musari S., Jongi G., Makaya P.V., Machakwa J. Antimicrobial profiling of bacteria isolated from fish sold at informal market in Mufakose, Zimbabwe. Int. J. Food Microbiol. 2019 doi: 10.1155/2019/8759636. 8759636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongping W., Jilun Z., Ting J., Yixi B., Xiaoming Z. Insufficiency of the Kanagawa hemolytic test for detecting pathogenic Vibrio parahaemolyticus in Shanghai, China. Diagn. Microbiol. Infect. Dis. 2011;69(1):7–11. doi: 10.1016/j.diagmicrobio.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Johnson A.P. Surveillance of antibiotic resistance. Phil. Trans. R. Soc. B. 2015;370:20140080. doi: 10.1098/rstb.2014.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.N., Bowers J.C., Griffitt K.J., Molina V., Clostio R.W., Pei S., Laws E., Paranjpye R.N., Strom M.S., Chen A., Hasan N.A., Huq A., Noriea N.F.I.I.I., Grimes D.J., Colwell R.R. Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the coastal and estuarine waters of Louisiana, Maryland, Mississippi, and Washington (United States) Appl. Environ. Microbiol. 2012;78(20):7249–7257. doi: 10.1128/AEM.01296-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.L., Ludeke C.H., Bowers J.C., Garrett N., Fischer M., Parsons M.B., Bopp C.A., DePaola A. Biochemical, serological, and virulence characterization of clinical and oyster Vibrio parahaemolyticus isolates. J. Clin. Microbiol. 2012;50:2343–2352. doi: 10.1128/JCM.00196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J.H., Ferraro M.J. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin. Infect. Dis. 2009;49:1749–1755. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
- Kalburge S.S., Whitaker W.B., Boyd E.F. High-salt preadaptation of Vibrio parahaemolyticus enhances survival in response to lethal environmental stresses. J. Food Prot. 2014;77(2):246–253. doi: 10.4315/0362-028X.JFP-13-241. [DOI] [PubMed] [Google Scholar]
- Kaysner, C.A., DePaola, A.J., 2004. Bacteriological analytical manual: methods for specific pathogens. U.S. Food and Drug Administration. Chapter 9 Vibrio. https://www.fda.gov/food/laboratory-methods-food/bam-vibrio (accessed 12 March 2018).
- Kim Y.B.U., Okuda J.U.N., Matsumoto C., Takahashi N., Hashimoto S., Nishibuchi M. Identification of Vibrio parahaemolyticus strains at the species level by PCR targeted to the toxR gene. J. Clin. Microbiol. 1999;37:1173–1177. doi: 10.1128/jcm.37.4.1173-1177.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirs M., DePaola A., Fyfe R., Jones J.L., Krantz J., Van Laanen A., Cotton D., Castle M. A survey of oysters (Crassostrea gigas) in New Zealand for Vibrio parahaemolyticus and Vibrio vulnificus. Int. J. Food Microbiol. 2011;147(2):149–153. doi: 10.1016/j.ijfoodmicro.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Rodgers C., Parveen S., Chigbu P., Jacobs J., Rhodes M., Harter-Dennis J. Prevalence of Vibrio parahaemolyticus and Vibrio vulnificus in blue crabs (Callinectes sapidus), seawater and sediments of the Maryland Coastal Bays. J. Appl. Microbiol. 2014;117(4):1198–1209. doi: 10.1111/jam.12608. [DOI] [PubMed] [Google Scholar]
- Krumperman P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983;46(1):165–170. doi: 10.1128/aem.46.1.165-170.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchumanan V., Yin W.F., Lee L.H., Chan K.G. Occurrence and antibiotic resistance of Vibrio parahaemolyticus from shellfish in Selangor, Malaysia. Front. Microbiol. 2015;6:1417. doi: 10.3389/fmicb.2015.01417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xie X., Shi X., Lin Y., Qiu Y., Mou J., Chen Q., Lu Y., Zhou L., Jiang M., Sun H., Ma H., Cheng J., Hu Q. Vibrio parahaemolyticus, southern coastal region of China, 2007–2012. Emerg. Infect. Dis. 2014;20:685–688. doi: 10.3201/eid2004.130744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Pei X., Yan J., Liu D., Zhang H., Yu B., Li N., Yang D. Prevalence of foodborne pathogens isolated from retail freshwater fish and shellfish in China. Food Control. 2019;99:131–136. doi: 10.1016/j.foodcont.2018.12.024. [DOI] [Google Scholar]
- Lopatek M., Wieczorek K., Osek J. Prevalence and antimicrobial resistance of Vibrio parahaemolyticus isolated from raw shellfish in Poland. J. Food Prot. 2015;78(5):1029–1033. doi: 10.4315/0362-028x.jfp-14-437. [DOI] [PubMed] [Google Scholar]
- Mala W., Alam M., Angkititrakul S., Wongwajana S., Lulitanond V., Huttayananont S., Kaewkes W., Faksri K., Chomvarin C. Serogroup, virulence, and molecular traits of Vibrio parahaemolyticus isolated from clinical and cockle sources in northeastern Thailand. Infect. Genet. Evol. 2016;39:212–218. doi: 10.1016/j.meegid.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Malcolm T.T.H., Cheah Y.K., Radzi C.W.J.W.M., Kasim F.A., Kantilal H.K., John T.Y.H., Martinez-Urtaza J., Nakaguchi Y., Nishibuchi M., Son R. Detection and quantification of pathogenic Vibrio parahaemolyticus in shellfish by using multiplex PCR and loop-mediated isothermal amplification assay. Food Control. 2015;47:664–671. doi: 10.1016/j.foodcont.2014.08.010. [DOI] [Google Scholar]
- Makino K., Oshima K., Kurokawa K., Yokoyama K., Uda T., Tagomori K., Iijima Y., Najima M., Nakano M., Yamashita A., Kubota Y., Kimura S., Yasunaga T., Honda T., Shinagawa H., Hattori M., Iida T. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V cholerae. Lancet. 2003;361:743–749. doi: 10.1016/S0140-6736(03)12659-1. [DOI] [PubMed] [Google Scholar]
- Miranda C.D., Godoy F.A., Lee M.R. Current status of the use of antibiotics and the antimicrobial resistance in the Chilean salmon farms. Front. Microbiol. 2018;9:1284. doi: 10.3389/fmicb.2018.01284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair G.B., Ramamurthy T., Bhattacharya S.K., Dutta B., Takeda Y., Sack D.A. Global dissemination of Vibrio parahaemolyticus serotype O3:K6 and its serovariants. Clin. Microbiol. Rev. 2007;2(1):39–48. doi: 10.1128/CMR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelapati S., Krishnaiah N. Detection of total and pathogenic Vibrio parahaemolyticus by polymerase chain reaction using toxR, tdh and trh genes. Vet. World. 2010;3(6):268–271. [Google Scholar]
- Newton A., Kendall M., Vugia D.J., Henao O.L., Mahon B.E. Increasing rates of vibriosis in the United States, 1996–2010: review of surveillance data from 2 systems. Clin. Infect. Dis. 2012;54:S391–S395. doi: 10.1093/cid/cis243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorlis A., Ghazali F.M., Cheah Y.K., Tuan Zainazor T.C., Ponniah J., Tunung R., Tang J.Y.H., Nishibuchi M., Nakaguchi Y., Son R. Prevalence and quantification of vibrio species and Vibrio parahaemolyticus in freshwater fish at hypermarket level. Int. Food Res. J. 2011;18:689–695. [Google Scholar]
- Okuda J., Ishibashi M., Abbot S.L., Janda J.M., Nishibuchi M. Analysis of the thermostable direct hemolysin (tdh) gene and the tdh-related hemolysin (trh) genes in urease- positive strains of Vibrio parahaemolyticus isolated on the west coast of the United States. J. Clin. Microbiol. 1997;35:1965–1971. doi: 10.1128/jcm.35.8.1965-1971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otomo Y., Hossain F., Rabbi F., Yakuwa Y., Ahsan C.R. Pre-enrichment of estuarine and fresh water environmental samples with sodium chloride yields in better recovery of Vibrio parahaemolyticus. Adv. Microbiol. 2013;3:21–25. doi: 10.4236/aim.2013.31003. [DOI] [Google Scholar]
- Pazhana G.P., Bhowmik S.K., Ghosh S., Guin S., Dutta S., Rajendran K., Saha D.R., Nandy R.K., Bhattacharya M.K., Mukhopadhyay A.K., Ramamurthy T. Trends in the epidemiology of pandemic and non-pandemic strains of Vibrio parahaemolyticus isolated from diarrheal patients in Kolkata, India. PLoS Negl. Trop. Dis. 2014;8(5):e2815. doi: 10.1371/journal.pntd.0002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston E.P., Kite-Powell H., Beet A. An estimate of the cost of acute health effects from food- and water-borne marine pathogens and toxins in the USA. J. Water Health. 2011;9(4):680–694. doi: 10.2166/wh.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab F., Gastmeier P., Meyer E. The warmer the weather, the more Gram-negative bacteria - Impact of temperature on clinical isolates in intensive care units. PLoS One. 2014;9(3):e91105. doi: 10.1371/journal.pone.0091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan P., Ramasamy P. Occurrence, distribution and antibiotic resistance patterns of Vibrio species associated with viral diseased shrimp of South Indian aquaculture environment. Int. J. Agric. Sci. 2009;1:1–10. doi: 10.9735/0975-3710.1.2.1-10. [DOI] [Google Scholar]
- Sterk A., Schets F.M., de Roda Husman A.M., de Nijs T., Schijven J.F. Effect of climate change on the concentration and associated risks of Vibrio Spp. in Dutch recreational waters. Risk Anal. 2015;35(9):1717–1729. doi: 10.1111/risa.12365. [DOI] [PubMed] [Google Scholar]
- Su Y.C., Liu C. Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol. 2007;24(6):549–558. doi: 10.1016/j.fm.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Sudha S., Mridula C., Silvester R., Hatha A.A. Prevalence and antibiotic resistance of pathogenic vibrios in shellfishes from Cochin market. Indian J. Geo Mar. Sci. 2014;43:815–824. [Google Scholar]
- Sun Y., Guo D., Hua Z., Sun H., Zheng Z., Xia X., Shi C. Attenuation of multiple Vibrio parahaemolyticus virulence factors by Citral. Front. Microbiol. 2019;10:894. doi: 10.3389/fmicb.2019.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syal K., Mo M., Yu H., Iriya R., Jing W., Guodong S., Wang S., Grys T.E., Haydel S.E., Tao N. Current and emerging techniques for antibiotic susceptibility tests. Theranostics. 2017;7(7):1795–1805. doi: 10.7150/thno.19217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada J., Ohashi T., Nishimura N., Shirasaki Y., Ozaki H., Fukushima S., Takano J., Nishibuchi M., Takeda Y. Detection of the thermostable direct hemolysin gene (tdh) and the thermostable direct hemolysin-related hemolysin gene (trh) of Vibrio parahaemolyticus by polymerase chain reaction. Mol. Cell. Probes. 1992;6:477–487. doi: 10.1016/0890-8508(92)90044-X. [DOI] [PubMed] [Google Scholar]
- Tan C.W., Malcolm T., Kuan C.H., Thung T.Y., Chang W.S., Loo Y.Y., Premarathne J.M.K.J.K., Ramzi O.B., Norshafawatie M.F.S., Yusralimuna N., Rukayadi Y., Nakaguchi Y., Nishibuchi M., Radu S. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from short mackerels (Rastrelliger brachysoma) in Malaysia. Front. Microbiol. 2017;8:1087. doi: 10.3389/fmicb.2017.01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T., Yanagawa H., Nguyen K.T., Hara-Kudo Y., Taniguchi T., Hayashidani H. Prevalence of Vibrio parahaemolyticus in seafood and water environment in the Mekong Delta, Vietnam. J. Vet. Med. Sci. 2018;80(11):1737–1742. doi: 10.1292/jvms.18-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker W.B., Parent M.A., Naughton L.M., Richards G.P., Blumerman S.L., Boyd E.F. Modulation of responses of Vibrio parahaemolyticus O3:K6 to pH and temperature stresses by growth at different salt concentrations. Appl. Environ. Microbiol. 2010;76(14):4720–4729. doi: 10.1128/AEM.00474-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand I., Hilpert K., Hancock R. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- Wu Y., Wen J., Ma Y., Ma X., Chen Y. Epidemiology of foodborne disease outbreaks caused by Vibrio parahaemolyticus, China, 2003–2008. Food Control. 2014;46:197–202. doi: 10.1016/j.foodcont.2014.05.023. [DOI] [Google Scholar]
- Xu X., Cheng J., Wu Q., Zhang J., Xie T. Prevalence, characterization, and antibiotic susceptibility of Vibrio parahaemolyticus isolated from retail aquatic products in North China. BMC Microbiol. 2016;16(32):1–9. doi: 10.1186/s12866-016-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano Y., Hamano K., Satomi M., Tsutsui I., Aue-umneoy D. Diversity and characterization of oxytetracycline-resistant bacteria associated with non-native species, white-leg shrimp (Litopenaeus vannamei), and native species, black tiger shrimp (Penaeus monodon), intensively cultured in Thailand. J. Appl. Microbiol. 2014;110:713–722. doi: 10.1111/j.1365-2672.2010.04926.x. [DOI] [PubMed] [Google Scholar]
- Yu Q., Niu M., Yu M., Liu Y., Wang D., Shi X. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shellfish in Shanghai. Food Control. 2016;60:263–268. doi: 10.1016/j.foodcont.2015.08.005. [DOI] [Google Scholar]
- Zanetti S., Spanu T., Deriu A., Romano L., Sechi L.A., Fadda G. In vitro susceptibility of Vibrio spp. isolated from the environment. Int. J. Antimicrob. Agents. 2001;17:407–409. doi: 10.1016/s0924-8579(01)00307-7. [DOI] [PubMed] [Google Scholar]