Abstract
Nearly half of Veterans have obesity, fueling chronic diseases. The Department of Veterans Affairs (VA) offers an evidence-based behavioral weight management intervention called MOVE!, mostly delivered through in-person group sessions. Few eligible Veterans participate due to factors like distance and preferences, mirroring barriers in the general population. Practical alternatives to standard in-person programs are needed to improve access and engagement. A self-directed lifestyle intervention called D-ELITE—delivered through pre-recorded videos by DVD or online streaming—previously efficacious in a general primary care population, may provide such an alternative. This pragmatic clinical trial will evaluate whether D-ELITE improves weight and general health status among Veterans with obesity, relative to VA usual care. The yearlong intervention includes one orientation by phone, supplemental lifestyle coaching primarily via technology-based messages, 12 DVD or online streaming sessions over 3 months, and continued self-directed weight management for months 4–12. Participants use MyFitnessPal.com or paper booklets for self-monitoring weight, diet, and physical activity. Follow-up assessments at 12 and 24 months are administered by mail or phone. The study hypothesis is that compared with usual care, D-ELITE will lead to greater improvements in 12-month weight loss, per VA electronic health records, and general physical health status, assessed using the self-reported SF-12 physical composite score. We will also explore D-ELITE's effects on secondary biometric (e.g., HbA1c) and intermediate (e.g., diet) outcomes, reach, and budget impact. If effective, D-ELITE will offer a potentially scalable, low-cost alternative to VA's existing weight loss interventions by mitigating barriers presented by distance and technology.
Keywords: Obesity, Pragmatic randomized controlled trial, Behavioral weight loss, Technology, Veteran
Abbreviations: VA, Department of Veterans Affairs; SF-12, 12-item Short Form Survey; DPP, Diabetes Prevention Program; EHR, electronic health record; GLB, Group Lifestyle Balance; PCS, SF-12 physical composite score; MCS, SF-12 mental composite score; MCA, VA Managerial Cost Account System; HERC, Health Economic Resource System
1. Introduction
Nearly half of Americans are predicted to have obesity by 2030 [1], fueling chronic disease burden [2]. Because most Veterans receiving healthcare from the Department of Veterans Affairs (VA) have obesity (41%) or are classified as overweight (37%) (vs. 38% and 13% of the general population) [3], they should receive behavioral weight management interventions that promote healthy diet and exercise. Such programs can produce clinically meaningful weight loss, reducing chronic disease risk [4].
VA widely offers its evidence-based behavioral weight management program called MOVE! [5], which supports healthy diet and physical activity, primarily through in-person group visits [6,7]. Like behavioral weight management programs in the general population [4], nearly one-third of Veterans who attended at least eight MOVE! visits had clinically meaningful weight loss after 6 months [7]. However, only 2–12% of eligible patients participate in MOVE! [5], and prior research found that, comparable to the general population [8], factors like travel distance [9] and scheduling inflexibility [10] inherent with interactive group sessions interfere with MOVE! participation. To improve reach, healthcare systems like VA are expanding use of synchronous telemedicine, mobile applications, and web-based programming [5]. While 90% of the general population report using the internet [11], only 41% of Veterans using VA healthcare use the internet [12]. Access to weight management services may improve if delivered using pre-recorded videos available via web-based streaming and low-technology (e.g., DVD) modalities.
A self-directed low-technology program that was tested as part of a 3-arm randomized controlled trial (RCT) called E-LITE, holds promise as a MOVE!-alternative that could address barriers to reach and engagement. The RCT was conducted among non-Veteran adults who were classified as being at least overweight, with prediabetes or metabolic syndrome. The self-directed intervention was based on the Diabetes Prevention Program (DPP)’s [[13], [14], [15]] real-world translation called the Group Lifestyle Balance (GLB) program. Educational content was delivered by DVD and self-directed written material and supplemented with standardized remotely-delivered lifestyle coaching messages, and as-desired personalized coaching. The self-directed GLB-based DVD-delivered intervention was compared to a more intensive coach-led group intervention and usual primary care [16]. It led to reduced weight, cardiovascular disease (CVD), and metabolic risk factors, comparable to the more intensive coach-led intervention and superior to usual care, sustained over two years [16,17]. This self-directed intervention has not yet been tested among Veterans, whose outcomes may be different due to potential barriers to behavioral weight management, including higher rates of psychiatric conditions and medical complexity [[18], [19], [20]].
This pragmatic clinical trial tests the previously studied self-directed intervention—now in DVD and online-streaming formats—among Veterans with obesity, comparing 12-month weight and general health status, relative to usual care. Secondary endpoints include biometric outcomes (blood pressure and Hba1c) and self-reported behavioral and psychological intermediate outcomes (e.g., physical activity). We anticipate D-ELITE will have low costs and be accessible, so we assess budget impact and reach, informative for potential dissemination. If effective, D-ELITE could be offered as part of VA's MOVE! intervention suite, while further informing its utility for the general population.
2. Methods
D-ELITE is a pragmatic clinical trial in which 500 Veterans with obesity are randomized to usual care or the D-ELITE intervention, which consists of usual care enhanced with the self-directed E-LITE program for 12 months. This trial is registered in clinicaltrials.gov (NCT03260140) and approved by the VA Puget Sound Healthcare System Institutional Review Board (IRB).
2.1. Study design
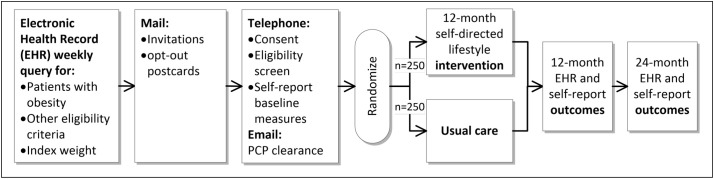
The study design is depicted in Fig. 1 with each element detailed in the following sections.
Fig. 1.
D-ELITE study design overview.
2.1.1. Aim 1
Determine the effectiveness of the D-ELITE intervention on 12-month weight, extracted from the VA electronic health record (EHR) database, and self-reported general health status on the SF-12, compared with usual VA care.
2.1.2. Aim 2
Examine the D-ELITE intervention compared with usual care on exploratory biometric outcomes (EHR-based weight and self-reported general health status at 24 months and EHR-based blood pressure and HbA1c at 12 and 24 months), which will inform whether D-ELITE improves downstream metabolic and cardiovascular effects, important to clinicians and decision-makers. We also will explore select self-reported intermediate psychological and behavioral outcomes (physical activity, sedentary behavior, diet quality, sleep quality, mental health status, and dietary self-efficacy at 12 and 24 months), which will provide insights about mechanisms of change if D-ELITE is effective (or areas in need of better targeting if the intervention is not effective). Lastly, understanding D-ELITE's reach and budget impact will inform decision-makers and potential dissemination.
2.2. Eligibility criteria
Eligibility criteria are aimed at ensuring participant safety and balancing internal validity with real world applicability (see Table 1 ). To be eligible and recruited for the study, Veterans must have a prior-week VA primary care visit-measured Body Mass Index (BMI) of 30.0–44.9 kg/m2, which becomes the study's index weight for those participants who ultimately enroll. To identify these Veterans, this pragmatic trial uses the VA's EHR database [21].
Table 1.
Eligibility criteria.
| Inclusion criteria To be included in the study, potential participants must meet the following criteria:
People meeting the following criteria are excluded from study participation:
|
We use several methods to ensure we obtain plausible, high-quality index weights and weight data from the medical record, guided by prior work [9]. We require at least one valid weight measure in the past year in addition to the index weight, and exclude any index weight indicating excessive weight change prior to the index date: > 40-pound loss in any 30-day period in the year prior or more than an average of two pounds lost per week over periods greater than 30 days. To exclude plausible but likely invalid index weights, we exclude weights if the standard deviation of the difference between index and any past-year weight is greater than 5% of the average weight, if the weight change is greater than ±100 pounds, or if the BMI change is greater than ±15. We also exclude individuals with weights <90 or > 500 pounds, height < 49 or > 84 in., and BMI <15 or > 65 (calculated using the most recent height measurement between 49 and 84 in.). We apply the same algorithms for obtaining weights for outcome measurement, described further in Section 2.9.1.
2.3. Recruitment and screening
Invitation letters and information statements are sent to a random sample of potentially eligible Veterans identified through VA's EHR, oversampling for women in order to enroll at least 30% women in the study. For individuals who return an opt-in card, research staff send baseline surveys with a cover letter explaining that research staff will follow-up by phone. Eligible participants confirm consent to participate over the phone under a waiver of documentation of written informed consent. Research staff then administer baseline surveys, or participants may return the surveys by mail. Prior to randomization, we send consented participants' primary care providers secure emails informing them of their patients' desire to participate and ask if their patient should not participate. If a provider does not reply after three contacts, we inform them that we will enroll their patient in three business days if they do not reply. If any primary care provider informs us after randomization that their patient should not participate, we cease any contraindicated intervention activities, but continue conducting follow-up assessments. Screening, enrollment, and randomization activities are carried out as soon as possible, but no longer than 12 weeks after the EHR-identified index weight date.
2.4. Randomization and blinding
We randomize participants to usual care or usual care enhanced with the D-ELITE self-directed intervention using a structured query language SQL-based computer procedure programmed to produce a 1:1 ratio using permuted-blocks within 12 strata categorized by: population density [23] (urban: ≥30% of population residing in an urbanized area as defined by the Census Bureau; rural: areas not defined as urban), BMI obesity category (Class I: 30.0–34.9.0; Class II: 35.0–39.9; Class III: 40.0–44.9), and age (<65, ≥65). All staff are blinded to treatment assignment at the time of randomization. Subsequently, assignments are identifiable to participants, interventionists, and those reviewing adverse events, while the other investigators, data monitoring committee members, outcome assessors, and data analysts remain blinded throughout the trial. We notify participants about their randomization assignment by mail, along with a weight scale and water bottle with a study logo (and D-ELITE program self-study materials for intervention participants).
2.5. Continuation of usual care
After study enrollment, the study does not interfere with ongoing patient care, and no participants are restricted from seeking any type of weight loss treatment. Participants' post-enrollment care is monitored through the EHR and self-report measures, which will be described in study reports.
2.6. Intervention
2.6.1. Evidence-based D-ELITE intervention format, structure and content
The D-ELITE intervention is based on the self-directed arm of the previously described E-LITE trial that was conducted in a non-Veteran population [16,24]. The E-LITE intervention was based on the DPP's real-world translation Group Lifestyle Balance, which is grounded in Social Cognitive Theory and uses self-regulation strategies (goal setting, self-monitoring, action planning, and problem-solving), delivering behavioral weight management support consistent with clinical practice guidelines [4]. Aside from minor modifications, D-ELITE is nearly identical to the self-directed intervention tested in the prior E-LITE trial.
In D-ELITE, the lifestyle coach initiates the intervention with a telephone orientation (replacing the in-person group orientation in E-LITE) to describe the self-directed program. The program curriculum is detailed in Table 2 . During the call the coach collaborates with the participant to choose a safe, realistic weight loss goal in order to achieve one-to-two pounds lost per week. They also set a corresponding personalized daily calorie intake goal that takes baseline weight and weight loss goals into account, using standard evidence-based caloric intake recommendations. Consistent with GLB guidelines, coaches recommend against weight loss exceeding 3 pounds per week or very low caloric diets (< 1200 kcal/day). The core curriculum (months 1–3) focuses on self-monitoring of weight, diet, and physical activity, and watching pre-recorded ~25-min GLB video session (via DVD or online streaming) each week for 12 weeks, in conjunction with completing corresponding self-study handouts. The main objective of this phase is to facilitate gradual weight loss through successive and progressive changes in 1) diet, including portion control, choosing low-energy and nutrient-dense meals and snacks (e.g., fruit, vegetables, whole grains, and low-fat, non-sweetened dairy products), reduced consumption of refined and/or added carbohydrates/sugars, healthy food preparation techniques, and careful selection of restaurant items; 2) physical activity, targeting at least 150 min of weekly moderate-intensity physical activity consistent with the Physical Activity Guidelines for Americans [25], which is safe and attainable for most adults, including those with chronic health conditions; and 3) behavioral skills training. Months 4–12 focus on continued self-directed/monitored gradual weight loss and maintenance, and include 10 additional self-study handouts on topics including: Strengthen Your Physical Activity Plan, Take Charge of Lifestyle, Mindful Eating and Movement, Managing Your Stress, Sitting Less for Your Health, More Volume Fewer Calories, Stay Active, Balancing Your Thoughts, Heart Health, and Look Back and Look Forward.
Table 2.
D-ELITE intervention core curriculum session topics.
|
Throughout the 12-month intervention period, participants use the MyFitnessPal.com website or GLB paper booklets, depending on preference, for self-monitoring of weight, diet, and physical activity. As in E-LITE, study coaches send standardized reminders (via MyFitnessPal or mail) every other week to complete designated sessions and continue self-monitoring but otherwise do not initiate contact with the participant. Because of the self-directed nature of this intervention, the choice to engage with a lifestyle coach after the phone orientation is strictly up to the participant and can occur as seldom or often as the participant chooses. Notably, E-LITE study participants in the self-directed arm had a median of 31 secure messages over the course of the intervention [16], suggesting that contact outside the 26 scheduled reminders/messages was limited.
2.6.2. Staffing and training
We carefully select staff and conduct standardized training and regular supervision regarding the informed consent process, protocol procedures, rapport building, interviewing techniques, trial-specific protocols, and problem-solving techniques as appropriate to study roles. Three lifestyle coaches located at VA Puget Sound provide the intervention orientation, coaching messages, and as-needed coaching that supplement the self-directed intervention. They are certified GLB health coaches, having participated in a two-day training led by the DPP training center at the University of Pittsburgh. Coaches are experienced research staff with bachelors' or higher degrees in allied health or biology-related fields with no prior experience delivering a weight-related intervention. Coaches meet regularly to review participant communications and ensure messaging is consistent with the GLB curriculum, seeking input from behavioral weight management expert investigators for issues not clear in GLB materials. They maintain a spreadsheet for documenting decisions, available for future reference.
2.7. Participant safety
In addition to our eligibility criteria, we screen potential participants via chart review, and seek primary care provider clearance prior to enrollment, to avoid enrolling Veterans for whom participation would likely be inappropriate. Participants diagnosed with any exclusionary condition following randomization may continue unless withdrawn by their doctor. In addition to events discovered during intervention encounters or other contacts, all participants are assessed for potential adverse events (AE) at 12 and 24 month follow-up, asking whether they have had any hospitalizations or ER visits, or new or worsening diagnosis for Cardiovascular and Circulatory, Gastrointestinal, Mental Health, Musculoskeletal, Neurologic, Lung, or other significant concerns. Documentation in the EHR is used to verify patient self-reports. We review these events for seriousness, study relatedness, and expectedness. The Data Monitoring Committee, consisting of a PhD and MD health services investigator, review any event considered serious, unexpected, and related. Adverse events are reported according to IRB requirements. This trial is anticipated to be minimal risk, given the excellent safety data in E-LITE [16]. We plan no formal interim analysis of efficacy or futility but provide interim safety reports semi-annually to the Data Monitoring Committee.
2.8. Retention
We regularly review best practices for retaining participants, including staff and participant roles and responsibilities, and conveying appreciation for participation and study affiliation. We train staff to conduct effective informed consent to ensure participants fully understand the demands and nature of the study before enrolling, including the concept of random assignment and what each treatment involves, and the importance of follow-up assessment even if they are not adhering to their assigned treatment.
To promote retention, each participant receives a study water bottle and body weight scale valued at $20 after randomization. At 4, 8, 16, and 20 months post-randomization, we send participants letters thanking them for their participation and reminding them about when to expect the next set of surveys. At the end of their 24-month involvement, we compensate participants $20 if they complete either the 12- or 24-month outcomes surveys, and $30 if they return both sets of surveys. The amount of compensation was determined by the IRB to be appropriate given that no in-person participant visits were required, and that surveys take only about 20 min to complete. VA policy requires payments be no less than $25 at a time due to administrative burden of processing, so compensation was combined for the two assessments.
2.9. Study measures and data collection schedule
We send 12- and 24-month surveys to participants via mail starting one month prior to the due date. If the participant does not return the questionnaires within two weeks, we send a reminder letter with another set of surveys and follow-up by telephone for up to three consecutive unanswered calls, to remind them to complete measures or offer for them to complete them by phone with study staff. Table 3 summarizes measures and the data collection schedule.
Table 3.
Measures and timing of data collection.
| Variable | Data source | Baseline | 3 months | 12 months | 24 months |
|---|---|---|---|---|---|
| Aim 1: Primary outcomes | |||||
| Weight | All primary care weights from index (baseline) through 15 mos. in EHR | x | x | ||
| SF-12 PCS | Self-report | x | x | ||
| Aim 2: Biometric outcomes | |||||
| Weight | All primary care weights from index through 27 mos. in EHR (or participant measured by mail or phone if no EHR) | x | x | ||
| Blood pressure | Primary care-based EHR | x | x | x | |
| HbA1c | Outpatient lab values in EHR | x | x | x | |
| SF-12 PCS | Self-report | x | x | ||
| Aim 2: Intermediate outcomes | |||||
| SF-12 MCS | Self-report | x | x | x | |
| Physical activity | Self-report | x | x | x | |
| Diet quality/self-efficacy | Self-report | x | x | x | |
| Sleep | Self-report | x | x | x | |
| Aim 2: Reach and budget impact | |||||
| Reach | x | ||||
| Budget impact | Manual accounting logs, VA databases | Ongoing through 12 mos | |||
| Descriptive data: characteristics, D-ELITE engagement, co-intervention | |||||
| Demographics | EHR; Self-report | x | |||
| Obesity-related comorbidities | EHR; Self-report | x | x | x | |
| Smoking status | Self-report | x | x | x | |
| Distance of residence from primary care clinic | EHR | x | |||
| Other weight loss interventions | EHR/self-report | Ongoing | |||
| FDA-approved Weight Loss Medications [22] | EHR | Ongoing | |||
| D-ELITE engagement | Self-report and program data | x | x | ||
|
Ongoing through 12 mos plus 3- and 24-month surveys | |||||
2.9.1. Primary outcomes
This study specifies two primary outcomes: 12-month weight and general physical health status (measured with the SF-12 PCS), in order to place equal emphasis on the targeted clinical measure of weight and a more patient-centered outcome.
2.9.1.1. 12-month weight
All primary care-based weights from index weight through 15 months of follow-up are pulled from the EHR, selecting the index weight as baseline. The weight within the 9–15-month post-randomization window closest to their 12-month post-randomization date is selected as the 12-month follow-up weight. This method for obtaining medical record-based weights has been used in prior work that showed medical record-based weights did not differ from research protocol-obtained weights [26]. Participants without a post-index primary care-associated weight 9 months following randomization are sent a letter asking them to visit their primary care clinic to be weighed, and to request the weight be entered into their medical record. VA Primary care clinics generally support Veterans presenting to have a weight taken as part of ongoing prevention and health promotion, even without a scheduled visit. Measurements are typically taken by nurses and other medical support staff.
2.9.1.2. General physical health status
At baseline and 12 months, participants complete the SF-12, a health-related quality of life (HRQoL) instrument that captures general health, functioning, and well-being [27]. The SF-12 was selected as a co-primary outcome in this weight loss trial because first, weight-related clinical practice guidelines [4] and systematic reviews [28] have called for more research to examine effects of weight loss on general health status and functioning. In addition, general HRQoL is potentially more personally meaningful to some patients than the clinical measure of weight alone, making this measure valuable to VA clinicians, given VA emphasizes providing “whole health,” patient-centered outcomes [29]. Because prior studies have found benefit of weight loss on the SF-12 physical composite score (PCS), but not the mental composite score (MCS) [30,31], PCS but not the MCS is included as a co-primary outcome in the present study.
2.9.2. Secondary outcomes
We carefully selected a subset of a priori biometric and intermediate outcomes to evaluate in exploratory secondary analyses. We selected biometric outcomes known to be associated with weight loss [4], which will help us evaluate whether D-ELITE results in important downstream metabolic and cardiovascular benefits. We also measure a subset of psychological and behavioral indices known to be intermediate outcomes in weight loss interventions [32,33], which will provide insights about mechanisms of change if D-ELITE is effective (or areas in need of better targeting if the intervention is not effective). Additionally, reach and budget impact analyses will inform decision-makers and potential dissemination.
2.9.2.1. Biometric outcomes
2.9.2.1.1. Weight
We assess all weights obtained from primary care clinics from randomization through 27 months of follow-up as a secondary outcome, using procedures outlined above for the primary outcome. We also will assess the proportion achieving 5% loss of baseline weight (i.e., clinically meaningful weight loss) [4] at 12 and 24 months. To be used only in secondary analyses and in the unexpected case of significant missing EHR data, we ask any participants who do not have a primary care-based weight by 12 months post-randomization to report their weight based on the study-provided scale over the telephone or by returning a mailed survey. We provide written and verbal instruction on measuring themselves in a fashion that approximates clinic-based weights.
2.9.2.1.2. Blood pressure
Diastolic and systolic values in the EHR from randomization through 24 months for primary care-based blood pressure measurements.
2.9.2.1.3. HbA1c
All HbA1c laboratory values associated with any VA outpatient visit in the EHR from randomization through 24 months.
2.9.2.1.4. General physical health status
We assess 24-month general health status using the physical composite score of the SF-12 as described above.
2.9.3. Psychological and behavioral intermediate outcomes
2.9.3.1. Mental health status
We assess 12- and 24-month mental health status using the mental composite score of the SF-12 [27].
2.9.3.2. Physical activity
7-item short form of the International Physical Activity Questionnaire, which evaluates weekly walking, vigorous and moderate-intensity activity [34].
2.9.3.3. Diet quality and self-efficacy
Starting the Conversation is an 8-item self-report measure of diet quality that assesses intake of various types of food (e.g., fruit and vegetable, sugary beverages) [35]. We also include three questions about diet self-efficacy [36,37].
2.9.3.4. Sleep-related disturbance and impairment
We include 4-item sleep disturbance and 8-item sleep-related impairment scales from the NIH PROMIS measures [38].
2.9.4. Outcomes relevant to future implementation
2.9.4.1. Reach
We selected the most relevant measure for the present trial from the well-established RE-AIM framework [39]: “reach,” defined as the “number, proportion, and representativeness of individuals willing to participate in a given…intervention.” First, we estimate the proportion of those eligible who agreed to participate, with total recruitment letters sent, and total eligibility screenings conducted as denominators. Second, we capture “representativeness” by comparing participants' demographic characteristics to the VA patient population and to those who were eligible for the study. Also consistent with RE-AIM, we assess reasons for non-participation among those screened in order to understand how to better promote reach to those who did not participate in the future.
2.9.4.2. Budget impact analysis
Aim 2 also involves assessing the cost implications of D-ELITE, compared with those of usual care. We will perform a budget impact analysis from the VA perspective using a 12-month intervention period. First, we will compare costs of care potentially associated with weight loss therapies between the intervention and control groups: VA nutrition and behavioral weight management healthcare visits and weight loss medication from the VA Managerial Cost Account (MCA) System and Health Economic Resource System (HERC) [40,41]. Second, we will estimate potential future D-ELITE implementation costs, combining estimated potential patients based on trial reach findings with costs of identifying and recruiting patients; training lifestyle coaches; and activities performed by the lifestyle coach using detailed project logs, incorporating data on staff salaries [42].
2.9.5. Descriptive measures
Several measures will characterize the sample, and degree of participation in D-ELITE and other co-interventions.
2.9.5.1. Sociodemographic, health characteristics, and utilization measures
Using a combination of EHR and self-report measures, socio-demographic characteristics and distance to primary care clinics are measured at baseline. Obesity-related conditions, tobacco use, and weight loss treatment participation (e.g., MOVE!, dietician services, weight loss medication prescriptions) are captured from the EHR. We also assess self-reported non-VA weight management services and medications with the 12- and 24-month surveys. We expect low participation rates in other weight loss programs, dietitian services, and weight loss medication prescriptions as was found in E-LITE [16], and given few Veterans participate in MOVE! [9], receive dietitian care [43], and/or are prescribed weight loss medications [44].
2.9.5.2. D-ELITE engagement
We assess engagement in D-ELITE among intervention participants by assessing number of sessions viewed, modality used (DVD vs. online), and use of session handouts with a mailed survey at 3 and 12 months. During the telephone orientation, the coach guides the participant through the process of adding the coach as a MyFitnessPal friend and granting the coach access to view their food and exercise diaries, which they consented to during the enrollment visit. This allows us to assess self-monitoring data stored in MyFitnessPal or recorded on paper trackers that participants are asked to return monthly in pre-addressed, postage-paid envelopes we provide. We record the number of times lifestyle coaches are contacted, number of minutes spent on the contact and the indication for making contact. Engagement metrics will allow us to explore the relationship between engagement and weight loss outcomes in post-hoc analyses.
2.9.6. Statistical analysis
2.9.6.1. Analytic plan
We will use intention to treat (ITT) for all primary and secondary analyses. We will evaluate the dropout and missing data patterns for informative missingness [45]. If we find no evidence of informative missingness we will be able to rely on our use of well-specified mixed models to test effects of the intervention (described below) as these are known to lead to valid conclusions even when data are missing at random (MAR) [46,47]. If we detect informative missing data patterns, we will apply sensitivity analyses using pattern mixture models [45]. Complete case analysis will only be performed as a sensitivity analysis, to provide useful inference if the data are mostly complete and the bias introduced by dropping the small proportion of incomplete cases is negligible.
To test this study's effect on weight, we will compare mean weights between intervention and control groups predicted at 12 months using this repeated-measures mixed-effects linear model [[48], [49], [50]].
T corresponds to a continuous time variable with values between 6 and 15 months, Y is weight at follow-up time t on a participant randomized to arm X (intervention or UC). Y0 is the weight of a patient at baseline. By including a random intercept in the model (Z), the analysis will be clustered by patient. The analysis will also include as precision variables the covariates used in the stratified randomization (Q), detailed in Section 2.4 [51]. The random error, ε, accounts for the non-independence of repeated measures using an unstructured covariance within participants. Between-group differences in SF-12 will be examined using tests of mean difference in groups after adjusting for baseline (Y0) measures using a linear model. The model for SF-12 will not include a time effect or a random effect for patient as there is only one measurement after baseline. As with the 12-month weight analysis, randomization covariates will be included in the SF-12 model.
We will adjust the co-primary outcome analyses for multiple testing using a Bonferroni adjustment [52], which will allow for individualized assessment of our two primary outcomes at significance level 0.025 while maintaining a familywise error rate equal to 0.05. If exactly one hypothesis test leads to a null hypothesis rejection, we will infer that the intervention had a significant effect on that primary outcome but not the other. Two null hypothesis rejections and two failures to reject are also possibilities, in which case we will infer that the intervention had a significant effect on both or neither of our primary outcomes, respectively.
Exploratory analyses of Aim 2's biometric, psychological, and behavioral outcomes will be examined using tests of mean difference between groups after adjusting for baseline measures and randomization covariates in linear or logistic models, drawing on models used for primary analyses. We also will examine effects among women, and have estimated—with the assumptions of 80% power, ICC of 0.9, and 150 women participants—that we will have power to detect a similarly-sized effect among women as in the prior E-LITE trial [16]. Given these outcomes are exploratory and are not focused on hypothesis testing, adjustment for multiple comparisons will not be performed [53]. Rather, these analyses will report effect sizes and 95% confidence intervals, along with p-values, to aid with interpretation of clinical significance.
2.9.6.2. Sample size and power calculation
This study is powered on weight and SF-12 PCS outcomes, using ANCOVA for 12-month follow-up weight adjusted for baseline weight, and applying a t-test for the 12-month SF-12 [54]. See Table 4 for a summary. A two-sided type I error rate of α = 0.025 was chosen based on a multiple comparisons Bonferroni adjustment. In order to ensure adequate power for this pragmatic trial, we are enrolling 500 Veterans.
Table 4.
Sample size requirements for 12-month weight, SF-12 PCS.
| 12-Month outcome (Testing Method) | Significance level | Power | Scenario type | Mean Diff. (SD) Cohen's d | Sample Size (w/ 20% Attrition) |
|
|---|---|---|---|---|---|---|
| Per-Group | Total | |||||
| Weight (ANOVA, with adjustment for baseline weight) |
0.025 | 0.90 | Mean Diff. = 7.7 SD = 50.0 Corr. b/w Baseline, 12-Mo. Measures = 0.97 |
7.7 (50.0) d = 0.154 |
78 | 156 |
| Conservative (25% Cohen's d reduction, Corr. = 0.95) |
6.7 (57.7) d = 0.116 |
225 | 450 | |||
| SF-12 PCS (t-test) |
0.025 | 0.90 | MCID = 5.00 SD = 8.88 |
5.00 (8.88) d = 0.563 |
100 | 200 |
| Conservative (25% Cohen's d reduction) |
4.33 (10.25) d = 0.422 |
176 | 352 | |||
We based expected outcomes in D-ELITE on clinically meaningful outcomes. Regarding weight loss, we based it on the weight lost in E-LITE's self-directed arm among male participants (since we anticipate the majority of our Veteran participants will be male): 11 ± 2 lbs. [16]. This is an amount of weight loss likely to be clinically meaningful [4]. We expect weight loss in the control condition to be similar to that achieved in the standard MOVE! program: 3.3 pounds [55]. Thus, we expect to be able to detect a 7.7-pound absolute difference in 12-month mean weight between treatment arms, which requires 78 patients per treatment arm (156 patients in total), assuming a 12-month weight standard deviation of 50 lbs. [56], correlation of 0.97 between baseline and 12-month weight, 90% power, two-sided Type I error rate α = 0.025, and 20% attrition at 12 months [16]. This scenario has a Cohen's d effect size of 0.154 [57]. A more conservative set of assumptions with a 6.7-pound difference (25% reduction in Cohen's d effect size) and a weaker correlation of 0.95 between baseline and 12-month weight measures requires 225 patients per treatment arm (450 patients in total) with all other assumptions unchanged.
For SF-12 PCS at 12 months, we assume a Minimal Clinically Important Difference (MCID) of a 5.00-point absolute mean difference for the SF-12 PCS [58] between treatment arms, with a standard deviation of 8.88 points based on unpublished E-LITE data, which corresponds to a Cohen's d effect size of 0.563 [57]. Also assuming 90% power, two-sided Type I error rate α = 0.025, and 20% attrition at 12 months, this would require 100 patients per treatment arm (200 patients in total). A more conservative scenario that assumes a 4.33-point absolute mean difference (25%-reduced effect size) requires 176 patients per treatment arm (352 patients in total) with all other assumptions unchanged.
2.10. Pragmatic trial
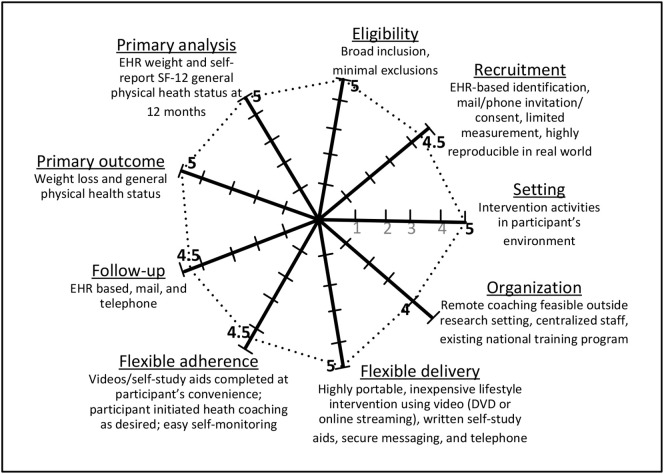
This study was designed as a pragmatic trial to test whether a previously proven intervention, as administered under usual standard care circumstances, enhances outcomes for Veterans. To ensure the study is pragmatic in nature, we applied the Pragmatic Explanatory Continuum Indicator Summaries (PRECIS-2) Wheel [59]. As displayed in Fig. 2 , this trial is highly pragmatic, with a score of 42.5 out of a possible 45. Specifically, this study is enrolling participants representative of VA patients; addresses a significant public health issue of clinical importance; uses outcomes meaningful and important to patients, clinicians, and health systems; includes practical recruitment and outcome assessment methods; and tests an intervention comparable to how it would be implemented in the real-world.
Fig. 2.
PRECIS-2 Wheel: pragmatic study design of the D-ELITE Trial.
D-ELITE is highly pragmatic as shown by the dotted circle (score = 42.5 out of 45 possible). Studies are rated for 9 domains on a 1–5 scale with 1 = extremely explanatory and 5 = extremely pragmatic.
3. Discussion
Current standard models of care such as those used in VA that rely on provider time—often delivered in-person—simply cannot reach a sufficient number of people who need the care, due to a variety of contextual and patient-level barriers [[8], [9], [10]]. Convenient care models are urgently needed to ensure people who are classified as overweight or with obesity can access evidence-based behavioral weight management services, to ensure population-level chronic disease risk reduction [4]. The present study tests whether a likely low-cost/resource, scalable, pragmatic, previously proven intervention can provide such a needed alternative.
The VA is one setting in great need of enhanced behavioral weight management access [60], given the high proportion of Veterans classified as overweight or with obesity [3], coupled with low engagement in the current standard of care [5]. Only one large study has examined technology-delivered behavioral weight management in VA, in a non-randomized trial of a different web-based version of DPP [61]. Those researchers found that an online version of DPP holds promise for increasing access and promoting weight loss among Veterans. The present study builds on that work to test another program based on the DPP, but in a pragmatic RCT. If D-ELITE is effective, this low technology intervention could likely be readily integrated into VA care with relatively minimal resource requirements.
Given D-ELITE is designed to rely minimally on the VA healthcare system, if effective it could also help reach the millions of Veterans classified as overweight or with obesity who do not receive VA care [62]. In addition, findings from the present trial will add to those from the prior E-LITE trial, with implications for how weight management services can be delivered to the general population. Because D-ELITE aligns with the Centers for Medicare and Medicaid Services reimbursement policy, which promotes brief, lower-intensity, behavioral counseling within a limited timeframe [63], D-ELITE would likely be viewed favorably for reimbursement.
The need for such highly pragmatic, accessible care is made even more pressing as we now face the SARS-CoV-2 pandemic. Demand for telemedicine services is soaring to reduce spread of SARS-CoV-2, while many healthcare systems are ill-prepared to equitably deliver traditional telemedicine at the scale required [64]. Despite increased need for weight management services to reduce risk of obesity-related chronic diseases associated with severe COVID-19, weight management services could fall by the wayside without programs like D-ELITE that can fill needed care gaps relatively easily [65]. This is especially important so that gaps in access to traditional telemedicine services don't worsen health disparities [64].
4. Limitations
Several potential study limitations warrant mention. Participants could be enrolled up to 12 weeks after their index weight was identified. Rather than include potential subsequent weights prior to randomization as baseline weight, we selected this approach to ensure consistency across participants regarding how baseline weight was ascertained. We will assess the degree to which dates of weight measurement, weight values, and analytic findings change if using most-recent pre-randomization weight instead of index weight in sensitivity analyses. Still, we do not expect this to have an impact, given we would expect such changes to be allocated in an equivalent fashion across treatment conditions due to randomization. Another potential limitation is reliance on the EHR for the primary outcome. Not having research staff obtain weight assessments could lead to missing data and/or bias. However, this method has been used in prior work that showed medical record-based weights did not differ from research protocol-obtained weights [26]. In addition to weight, this study selected a co-primary outcome of physical health status measured with the SF-12, which is not routinely collected in primary care. This study's findings may lend support to the value of administering the SF-12 in that setting, to align more with VA's emphasis on whole health and functioning [29].
5. Conclusions
Given how prevalent obesity is in VA [3], better addressing overweight and obesity through D-ELITE could reduce VA care costs, and substantially improve Veteran health. The proposed study not only contributes to the evidence base needed to inform and guide VA policy change, but will also provide valuable information to non-VA healthcare providers, particularly critical in the context of a rapidly growing need for effective and pragmatic, remotely-delivered, low-technology interventions.
Funding
The project described is supported by VA Health Services Research and Development (HSR&D) HX002113-01A2 (10/2017–6/2021) DVD Lifestyle Intervention (D-ELITE). Dr. Hoerster is supported by VA HSR&D Career Development Award 12-263 (4/2015-5/2020). The funders had no role in the collection, analysis and interpretation of data; in the writing of the report; nor in the decision to submit the article for publication.
Declaration of Competing Interest
Dr. Ma serves as a paid scientific consultant for Health Mentor, Inc. (San Jose, CA).
Dr. Au in the past 3 years has received personal remuneration from:
1. Novartis - member of DMC.
2. ABIM - Pulmonary Board Exam Writing Committee.
3. American Thoracic Society – Annals of the ATS, Deputy Editor.
This has not had any influence over the conceptualization or plan, nor will it influence the execution, of this study. Otherwise, the authors have no conflicts of interest to disclose.
Acknowledgments
We appreciate the contributions of Dr.Chuan-Fen Liu, for her work on conceptualizing the budget impact analysis, and Eric Gunnink for his help with conceptualizing the statistical analysis plan.
Contributor Information
Katherine D. Hoerster, Email: Katherine.Hoerster@va.gov.
Margaret P. Collins, Email: Margaret.Collins@va.gov.
David H. Au, Email: David.Au@va.gov.
Amber Lane, Email: Amber.Lane2@va.gov.
Eric Epler, Email: Eric.Epler@va.gov.
Jennifer McDowell, Email: Jennifer.McDowell@va.gov.
Anna E. Barón, Email: Anna.Baron@cuanschutz.edu.
Peter Rise, Email: Peter.Rise@va.gov.
Robert Plumley, Email: Robert.Plumley@va.gov.
Tanya Nguyen, Email: Tanya.Nguyen@va.gov.
Mary Schooler, Email: Mary.Schooler@va.gov.
Linnaea Schuttner, Email: Linnaea.Schuttner@va.gov.
Jun Ma, Email: maj2015@uic.edu.
References
- 1.Ward Z.J., Bleich S.N., Cradock A.L., Barrett J.L., Giles C.M., Flax C., Long M.W., Gortmaker S.L. 381(25) 2019. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity; pp. 2440–2450. [DOI] [PubMed] [Google Scholar]
- 2.Nyberg S.T., Singh-Manoux A., Pentti J., Madsen I.E.H., Sabia S., Alfredsson L., Bjorner J.B., Borritz M., Burr H., Goldberg M., Heikkilä K., Jokela M., Knutsson A., Lallukka T., Lindbohm J.V., Nielsen M.L., Nordin M., Oksanen T., Pejtersen J.H., Rahkonen O., Rugulies R., Shipley M.J., Sipilä P.N., Stenholm S., Suominen S., Vahtera J., Virtanen M., Westerlund H., Zins M., Hamer M., Batty G.D., Kivimäki M. Association of healthy lifestyle with years lived without major chronic diseases. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breland J.Y., Phibbs C.S., Hoggatt K.J., Washington D.L., Lee J., Haskell S., Uchendu U.S., Saechao F.S., Zephyrin L.C., Frayne S.M. The obesity epidemic in the veterans health administration: prevalence among key populations of women and men veterans. J. Gen. Intern. Med. 2017;32(Suppl. 1):11–17. doi: 10.1007/s11606-016-3962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen M.D., Ryan D.H., Apovian C.M., Ard J.D., Comuzzie A.G., Donato K.A., Hu F.B., Hubbard V.S., Jakicic J.M., Kushner R.F., Loria C.M., Millen B.E., Nonas C.A., Pi-Sunyer F.X., Stevens J., Stevens V.J., Wadden T.A., Wolfe B.M., Yanovski S.Z., Jordan H.S., Kendall K.A., Lux L.J., Mentor-Marcel R., Morgan L.C., Trisolini M.G., Wnek J., Anderson J.L., Halperin J.L., Albert N.M., Bozkurt B., Brindis R.G., Curtis L.H., DeMets D., Hochman J.S., Kovacs R.J., Ohman E.M., Pressler S.J., Sellke F.W., Shen W.K., Smith S.C., Jr., Tomaselli G.F. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the obesity society. Circulation. 2014;129(25 Suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maciejewski M.L., Shepherd-Banigan M., Raffa S.D., Weidenbacher H.J. Systematic review of behavioral weight management program MOVE! For veterans. Am. J. Prev. Med. 2018;54(5):704–714. doi: 10.1016/j.amepre.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Kinsinger L.S., Jones K.R., Kahwati L., Harvey R., Burdick M., Zele V., Yevich S.J. Design and dissemination of the MOVE! Weight-management program for veterans. Prev. Chronic Dis. 2009;6(3):A98. [PMC free article] [PubMed] [Google Scholar]
- 7.Kahwati L.C., Lance T.X., Jones K.R., Kinsinger L.S. RE-AIM evaluation of the veterans health Administration’s MOVE! Weight Management Program. Transl. Behav. Med. 2011;1(4):551–560. doi: 10.1007/s13142-011-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batsis J.A., Pletcher S.N., Stahl J.E. Telemedicine and primary care obesity management in rural areas - innovative approach for older adults? BMC Geriatr. 2017;17(1) doi: 10.1186/s12877-016-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Littman A.J., Boyko E.J., McDonell M.B., Fihn S.D. Evaluation of a weight management program for veterans. Prev. Chronic Dis. 2012;9 doi: 10.5888/pcd9.110267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McVay M.A., Yancy W.S., Bennett G.G., Jung S.-H., Voils C.I. Perceived barriers and facilitators of initiation of behavioral weight loss interventions among adults with obesity: a qualitative study. BMC Public Health. 2018;18(1):854. doi: 10.1186/s12889-018-5795-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pew Research Center . 2019. Internet/Broadband Fact Sheet. https://www.pewresearch.org/internet/fact-sheet/internet-broadband/. 2020. [Google Scholar]
- 12.Houston T.K., Volkman J.E., Feng H., Nazi K.M., Shimada S.L., Fox S. Veteran internet use and engagement with health information online. Mil. Med. 2013;178(4):394–400. doi: 10.7205/MILMED-D-12-00377. [DOI] [PubMed] [Google Scholar]
- 13.Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., Nathan D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knowler W.C., Fowler S.E., Hamman R.F., Christophi C.A., Hoffman H.J., Brenneman A.T., Brown-Friday J.O., Goldberg R., Venditti E., Nathan D.M. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamman R.F., Wing R.R., Edelstein S.L., Lachin J.M., Bray G.A., Delahanty L., Hoskin M., Kriska A.M., Mayer-Davis E.J., Pi-Sunyer X., Regensteiner J., Venditti B., Wylie-Rosett J. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J., Yank V., Xiao L., Lavori P.W., Wilson S.R., Rosas L.G., Stafford R.S. Translating the diabetes prevention program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern. Med. 2013;173(2):113–121. doi: 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao L., Yank V., Wilson S.R., Lavori P.W., Ma J. Two-year weight-loss maintenance in primary care-based diabetes prevention program lifestyle interventions. Nutr. Diabetes. 2013;3 doi: 10.1038/nutd.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoerster K.D., Lehavot K., Simpson T., McFall M., Reiber G., Nelson K.M. Health and health behavior differences: U.S. Military, veteran, and civilian men. Am. J. Prev. Med. 2012;43(5):483–489. doi: 10.1016/j.amepre.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 19.Lehavot K., Hoerster K.D., Nelson K.M., Jakupcak M., Simpson T.L. Health indicators for military, veteran, and civilian women. Am. J. Prev. Med. 2012;42(5):473–480. doi: 10.1016/j.amepre.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Agha Z., Lofgren R.P., VanRuiswyk J.V., Layde P.M. Are patients at veterans affairs medical centers sicker?: a comparative analysis of health status and medical resource use. Arch. Intern. Med. 2000;160(21):3252–3257. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Veterans Affairs . 2020. Corporate Data Warehouse (CDW), VA Informatics and Computing Infrastructure. https://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm. Accessed Feburary 6. [Google Scholar]
- 22.National Institute of Diabetes and Digestive and Kidney Diseases Prescription Medications to Treat Overweight and Obesity. January, 2020. https://www.niddk.nih.gov/health-information/weight-management/prescription-medications-treat-overweight-obesity (Accessed February 6 2020)
- 23.U.S. Department of Veterans Affairs Office of Rural Health April 8, 2019. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp (Accessed February 6 2020)
- 24.Diabetes Prevention Support Center DPP Group Lifestyle Balance Curriculum. http://www.diabetesprevention.pitt.edu/index.php/for-the-public/for-health-providers/group-lifestyle-balance-curriculum/ (Accessed February 6 2020)
- 25.Centers for Disease Control and Prevention How Much Physical Activity do Adults need? 2020. https://www.cdc.gov/physicalactivity/basics/adults/index.htm (Accessed Feburary 6 2020)
- 26.Xiao L., Lv N., Rosas L.G., Au D., Ma J. Validation of clinic weights from electronic health records against standardized weight measurements in weight loss trials. Obesity (Silver Spring) 2017;25(2):363–369. doi: 10.1002/oby.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ware J., Jr., Kosinski M., Keller S.D. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med. Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Warkentin L.M., Das D., Majumdar S.R., Johnson J.A., Padwal R.S. The effect of weight loss on health-related quality of life: systematic review and meta-analysis of randomized trials. 2014;15(3):169–182. doi: 10.1111/obr.12113. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Department of Veterans Affairs . 2020. Whole Health. https://www.va.gov/wholehealth/. Accessed May 8. [Google Scholar]
- 30.Sarwer D.B., Moore R.H., Diewald L.K., Chittams J., Berkowitz R.I., Vetter M., Volger S., Wadden T.A. The impact of a primary care-based weight loss intervention on the quality of life. Int. J. Obes. 2013;37(Suppl. 1):S25–S30. doi: 10.1038/ijo.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson D.A., Rejeski J., Lang W., Van Dorsten B., Fabricatore A.N., Toledo K., L.A.R. Group Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch. Intern. Med. 2009;169(2):163–171. doi: 10.1001/archinternmed.2008.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varkevisser R.D.M., van Stralen M.M., Kroeze W., Ket J.C.F., Steenhuis I.H.M. Determinants of weight loss maintenance: a systematic review. 2019;20(2):171–211. doi: 10.1111/obr.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St-Onge M.-P. Sleep–obesity relation: underlying mechanisms and consequences for treatment. 2017;18(S1):34–39. doi: 10.1111/obr.12499. [DOI] [PubMed] [Google Scholar]
- 34.Craig C.L., Marshall A.L., Sjostrom M., Bauman A.E., Booth M.L., Ainsworth B.E., Pratt M., Ekelund U., Yngve A., Sallis J.F., Oja P. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.Mss.0000078924.61453.Fb. [DOI] [PubMed] [Google Scholar]
- 35.Paxton A.E., Strycker L.A., Toobert D.J., Ammerman A.S., Glasgow R.E. Starting the conversation performance of a brief dietary assessment and intervention tool for health professionals. Am. J. Prev. Med. 2011;40(1):67–71. doi: 10.1016/j.amepre.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Norman G.J., Carlson J.A., Sallis J.F., Wagner N., Calfas K.J., Patrick K. Reliability and validity of brief psychosocial measures related to dietary behaviors. Int. J. Behav. Nutr. Phys. Act. 2010;7:56. doi: 10.1186/1479-5868-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sallis P.R., Grossman R.M., Patterson T.L., Nader P.R. The development of self-efficacy scales for health-related diet and exercise behaviors. Heath Educ Res. 1988;3:283–292. [Google Scholar]
- 38.Yu L., Buysse D.J., Germain A., Moul D.E., Stover A., Dodds N.E., Johnston K.L., Pilkonis P.A. Development of short forms from the PROMIS sleep disturbance and sleep-related impairment item banks. Behav. Sleep Med. 2011;10(1):6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glasgow R.E., Harden S.M., Gaglio B., Rabin B., Smith M.L., Porter G.C., Ory M.G., Estabrooks P.A. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. 2019;7(64) doi: 10.3389/fpubh.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phibbs C.S., Barnett P.G., Fan A. Health Economics Resource Center; Menlo Park, CA: VA Palo Alto: 2015. Research Guide to the Managerial Cost Accounting National Cost Extract. Guidebook. [Google Scholar]
- 41.Smith M.W., Chow A. Health Economics Resource Center; Menlo Park, CA: VA Palo Alto: 2010. Fee Basis Data: A Guide for Researchers. [Google Scholar]
- 42.Liu C.F., Rubenstein L.V., Kirchner J.E., Fortney J.C., Perkins M.W., Ober S.K., Pyne J.M., Chaney E.F. Organizational cost of quality improvement for depression care. Health Serv. Res. 2009;44(1):225–244. doi: 10.1111/j.1475-6773.2008.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Annis A.M., Harris M., Kim H.M., Rosland A.M., Krein S.L. Trends in primary care encounters across professional roles in PCMH. Am. J. Manag. Care. 2018;24(7):e222–e229. [PubMed] [Google Scholar]
- 44.Thomas D.D., Waring M.E., Ameli O., Reisman J.I., Vimalananda V.G. 27(7) 2019. Patient Characteristics Associated with Receipt of Prescription Weight-Management Medications Among Veterans Participating in MOVE! pp. 1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fairclough D. Chapman and Hall/CRC; Boca Raton, F: 2010. Design and Analysis of Quality of Life Studies in Clinical Trials. [Google Scholar]
- 46.Fitzmaurice G.M., Laird N.M., Ware J.H. 2nd ed. Wiley; New York: 2011. Applied Longitudinal Analysis. [Google Scholar]
- 47.Allison P.D. Handling missing data by maximum likelihood. SAS Global Forum. 2012:1–12. [Google Scholar]
- 48.Littell R.C., Milliken G.A. SAS Institute Inc; Cary, NC: 1996. SAS System for Mixed Models. [Google Scholar]
- 49.Brown H., Prescott R. John Wiley & Sons, Inc.; Chichester, England: 1999. Applied Mixed Models in Medicine. [Google Scholar]
- 50.Verbeke G., Molenberghs G. Springer-Verlag; New York: 2000. Linear Mixed Models for Longitudinal Data. [Google Scholar]
- 51.Shao J., Yu X., Zhong B. A theory for testing hypotheses under covariate-adaptive randomization. Biometrika. 2010;97(2):347–360. [Google Scholar]
- 52.Westfall P.H., Young S.S. John Wiley & Sons; New York, NY: 1993. Resampling-Based Multiple Testing: Examples and Methods for P-Value Adjustment. [Google Scholar]
- 53.Althouse A.D. Adjust for multiple comparisons? It’s not that simple. Ann. Thorac. Surg. 2016;101(5):1644–1645. doi: 10.1016/j.athoracsur.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 54.Clifton L., Birks J., Clifton D.A. Comparing different ways of calculating sample size for two independent means: a worked example. Contemp. Clin. Trials Commun. 2018;13:100309. doi: 10.1016/j.conctc.2018.100309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Littman A.J., Damschroder L.J., Verchinina L., Lai Z., Kim H.M., Hoerster K.D., Klingaman E.A., Goldberg R.W., Owen R.R., Goodrich D.E. National evaluation of obesity screening and treatment among veterans with and without mental health disorders. Gen. Hosp. Psychiatry. 2015;37(1):7–13. doi: 10.1016/j.genhosppsych.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Hoerster K.D., Lai Z., Goodrich D.E., Damschroder L.J., Littman A.J., Klingaman E.A., Nelson K.M., Kilbourne A.M. Weight loss after participation in a national VA weight management program among veterans with or without PTSD. Psychiatr. Serv. 2014;65(11):1385–1388. doi: 10.1176/appi.ps.201300404. [DOI] [PubMed] [Google Scholar]
- 57.Cohen J. 2nd ed. Lawrence Erbaum Associates; Hillsdale, New Jersey: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 58.Jayadevappa R., Cook R., Chhatre S. Minimal important difference to infer changes in health-related quality of life-a systematic review. J. Clin. Epidemiol. 2017;89:188–198. doi: 10.1016/j.jclinepi.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 59.Loudon K., Treweek S., Sullivan F., Donnan P., Thorpe K.E., Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 60.Department of Veterans Affairs and Department of Defense VA/DoD Clinical Practice Guideline for Screening and Management of Overwieght and Obesity. Version 2.0. 2014. http://www.healthquality.va.gov/guidelines/CD/obesity/CPGManagementOfOverweightAndObesityFINAL041315.pdf (Accessed February 6 2020)
- 61.Moin T., Damschroder L.J., AuYoung M., Maciejewski M.L., Havens K., Ertl K., Vasti E., Weinreb J.E., Steinle N.I., Billington C.J., Hughes M., Makki F., Youles B., Holleman R.G., Kim H.M., Kinsinger L.S., Richardson C.R. Results from a trial of an online diabetes prevention program intervention. Am. J. Prev. Med. 2018;55(5):583–591. doi: 10.1016/j.amepre.2018.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.U.S. Department of Veterans Affairs . 2020. About VHA. https://www.va.gov/health/aboutvha.asp. Accessed May 8. [Google Scholar]
- 63.Centers for Medicare and Medicaid Services Decision Memo for Intensive Behavioral Therapy for Obesity (CAG-00423N) 2011. http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?&NcaName=Intensive%20Behavioral%20Therapy%20for%20Obesity&bc=ACAAAAAAIAAA&NCAId=253& (Accessed December 6 2011)
- 64.Nouri S., Khoong E.C., Lyles C.R., Karliner L. Addressing Equity in Telemedicine for Chronic Disease Management during the Covid-19 Pandemic. Innov. Care Deliv. 2020;1(3) doi: 10.1056/CAT.20.0123. [DOI] [Google Scholar]
- 65.Hoerster K.D., Gray K., Raffa S.D. Weight matters: why we must not abandon weight management services amid COVID-19 [letter to the editor] JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.2765. (In Press) [DOI] [PubMed] [Google Scholar]