As the Covid-19 pandemic rages on, black Americans seem to be at a greater risk of adverse outcome. While comorbidities and socioeconomic circumstances certainly have an impact on these perceived discrepancies, we hypothesize that additional contributing factors underlie the divergence in morbidity and mortality between ethnic groups. Given the significance of immunopathology in Covid-19 mortality, we pondered whether specific immune disparities might directly contribute to sensitizing patients of certain ethnicities. Indeed, we found evidence of inherent differences in the immune system, which may increase the predisposition of black Americans to a severe cytokine storm. We hope that these observations may contribute to the prognosis of this population and prompt the utilization of anti-cytokine biologics to treat patients in the immune phase of Covid-19.
Using RNAseq gene analysis data from the Genotype-Tissue Expression (GTEx) project [1], we compared the expression of cytokines and other central immune modulators between healthy black and white Americans. We found that inflammasome-derived interleukin 1 beta (IL1β) and interleukin 18 receptor 1 (IL18R1) were up-regulated in black individuals (fold change (FC) = 2.25 and 2.21, respectively; P < .01), other cytokines such as interleukin-12 receptor beta 1 (IL12Rβ1) expression was also elevated (FC = 1.62)1 . Furthermore, we detected elevated expression of innate immunity receptors responsible for ssRNA recognition (TLR7) and non-methylated CpG sequence binding of bacterial or viral DNA (TLR9). (FC = 1.81; FC = 1.22; respectively, P < .01).
These variation in gene expression prompted us to investigate biochemical pathway discrepancies that would support our hypothesis and would help illuminate personalized treatment options. We identified several immune-related pathways that show significant deviation between different ethnic groups, including interleukin-6 production regulation pathways (Gene Set Enrichment Analysis (GSEA); P < .01) as well as type I interferon (IFN, GSEA; P < .01) response. Both IL6 and IFN are cardinal factors in human immune response to invading pathogens. In fact, IL6 inhibition has already been suggested as a possible treatment course for improving immune mediated hyperactivity in patients with severe Covid-19.
Interestingly, these results may also explain known epidemiological disparities in other immune-mediated conditions. For example, black Americans are more commonly implicated with sepsis-related mortality [2], as well as developing systemic lupus erythematosus. These conditions are associated with elevated IL1/IL18 and TLR7/TLR9 respectively. Likewise, the detected elevation in the IL12 axis is directly related to the increased prevalence of severe cutaneous psoriasis also seen in black Americans.
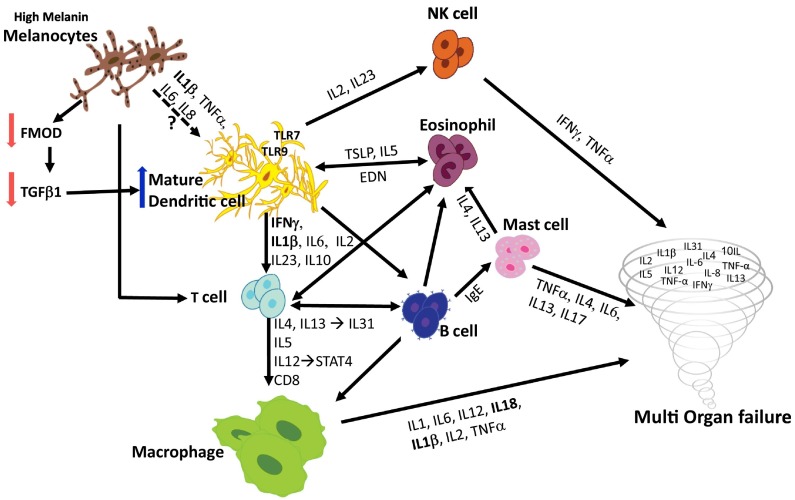
Nevertheless, such a significant elevation of immune mediators was intriguing as it indicates a strong correlation to Dendritic cells (DC) activation and function. DC are antigen-presenting cells that act as prominent mediators in the activation of numerous immune cell types and various innate and acquired immune responses (Fig. 1 ). For instance, IL1 and IL18 co-initiate the priming of DC activation as the primary step in an overwhelming immune response. IL12, which binds IL12Rβ1, secreted by activated DCs and, along with IL23, are pivotal for T-helper 17 cell (Th17) and natural killer cell activation, thus contributing to immune over-activation. Similarly, DCs also secrete IL6 and INF, which play a significant and well documented role in immune response against pathogens. Finally, TLR7 and TLR9, which are pertinent DCs receptors, present another route for DC activation by binding to pathogenic nucleic acids.
Fig. 1.
Postulated immune response mechanism in the COVID-19 pandemic.
Notably, DC activation is also implicated in prompting eosinophil and mast cell migration, proliferation and activation. Mast cell and eosinophil activation further contribute to end-organ damage, with the latter secreting eosinophil-derived neurotoxin (EDN) which acts as a positive feedback DC activator. Elevation of type 2 cytokine (Th2) levels was reported in severe SARS cases [3]. The hallmark of a Th2 response is manifested by IL4 and IL13, directly linked to inflammation and resultant skin rashes. Atopic dermatitis and prurigo nodularis are classic Th2-mediated diseases, both of which are more severe in black Americans. Interestingly, a rash was recently suggested as a possible symptom of COVID-19 (Fig. 1).
We thus postulated that DCs are likely to be a central player in the enhanced immune response in black Americans. While the mechanisms leading to racial disparities in cytokine expression levels seen in Covid-19 remain to be established, our experimentations in mice models suggest that in some cases, melanogenesis can directly impact the immune system through DC activation. Melanocytes are antigen-presenting cells and plays a dynamic role in the immune system. We previously described that fibromodulin (FMOD), a pigment-dependent angiogenic factor secreted by melanocytes, leads to proliferation of endothelial cells, followed by an increase in TGFβ1 levels [4]. Recent results have led us to hypothesize that melanocytes use FMOD/ TGFβ1 signaling to substantially modulate the immune system and more specifically, DC maturation and activity. By inoculating mice with CpG (oligodeoxynucleotides containing nonmethylated CpG) or lipopolysaccharide (major membrane components express on Gram-negative bacteria), we were able to provoke inflammation and promote DCs migration to the spleen. Strikingly, induced DCs in the spleen were more numerous and mature (as revealed by their increased expression of MHCII and CD86) in pigmented Black-C57 mice than in their under-melaninated congenic Yellow-C57 mice counterparts. Knockdown of FMOD in lightly pigmented mice recapitulated the phenotype, supporting causality. Finally, as T-cell activation is directly associated with an elevated level of mature DCs, the dark-pigmented mice may be predisposed to a more severe cytokine storm.
In conclusion, our findings suggest that black individuals may have the tendency to develop a harsher pro-inflammatory cytokine response. Accordingly, we posit that when confronted with SARS-cov-2, black Americans would be more prone to develop a rapid and more aggressive cytokine storm. This may necessitate earlier administration of biologics to block the ensuing overwhelming immune response in an attempt to improve their prognosis.
Acknowledgments
This study was supported in part by a grant from the NIH R01EY024046.
Footnotes
RNAseq levels: (i) Interleukin 1 beta (IL1β): fold change (FC) = 2.25, adjusted P = 2.03 × 10−3 in postmortem stomach from 28 black vs 166 white individuals. (ii) Interleukin 18 Receptor 1 (IL18R1): FC = 2.21, adjusted P = 8.25 × 10−4 in EBV-transformed lymphocytes from 25 black vs 103 white individuals. (iii) Interleukin-12 receptor beta 1 (IL12Rβ1) FC = 1.62, adjusted P = 8.69 × 104 in postmortem tibial artery from 50 black individuals as compared to 286 white individuals. (iv) Toll-like receptor 7 (TLR7) FC = 1.81, adjusted P = 5.49 × 10−3 in postmortem tibial artery. (v) Toll-like receptor 9 (TLR9): FC = 1.22, adjusted P = 6.83 × 10−3 in whole blood from 112 black vs 703 white individuals.
Contributor Information
Yuval Tal, Email: yuvalt@hadassah.org.il.
Avner Adini, Email: avner.adini@childrens.harvard.edu.
Alal Eran, Email: alal_eran@hms.harvard.edu.
Irit Adini, Email: iadini@mgh.harvard.edu.
References
- 1.Consortium GT Human genomics. The genotype-tissue expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348:648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matter M.L., Shvetsov Y.B., Dugay C. High mortality due to sepsis in native Hawaiians and African Americans: the multiethnic cohort. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li C.K., Wu H., Yan H. T cell responses to whole SARS coronavirus in humans. J. Immunol. 2008;181:5490–5500. doi: 10.4049/jimmunol.181.8.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adini I., Ghosh K., Adini A. Melanocyte-secreted fibromodulin promotes an angiogenic microenvironment. J. Clin. Invest. 2014;124:425–436. doi: 10.1172/JCI69404. [DOI] [PMC free article] [PubMed] [Google Scholar]