Abstract
Background
Conflicting recommendations exist related to whether masks have a protective effect on the spread of respiratory viruses.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement was consulted to report this systematic review. Relevant articles were retrieved from PubMed, Web of Science, ScienceDirect, Cochrane Library, and Chinese National Knowledge Infrastructure (CNKI), VIP (Chinese) database.
Results
A total of 21 studies met our inclusion criteria. Meta-analyses suggest that mask use provided a significant protective effect (OR = 0.35 and 95% CI = 0.24–0.51). Use of masks by healthcare workers (HCWs) and non-healthcare workers (Non-HCWs) can reduce the risk of respiratory virus infection by 80% (OR = 0.20, 95% CI = 0.11–0.37) and 47% (OR = 0.53, 95% CI = 0.36–0.79). The protective effect of wearing masks in Asia (OR = 0.31) appeared to be higher than that of Western countries (OR = 0.45). Masks had a protective effect against influenza viruses (OR = 0.55), SARS (OR = 0.26), and SARS-CoV-2 (OR = 0.04). In the subgroups based on different study designs, protective effects of wearing mask were significant in cluster randomized trials and observational studies.
Conclusions
This study adds additional evidence of the enhanced protective value of masks, we stress that the use masks serve as an adjunctive method regarding the COVID-19 outbreak.
Keywords: Facemask, Respiratory virus, Influenza, SARS-CoV, SARS-CoV-2, Prevention
Highlights
-
•
At present, the epidemic of COVID-19 has caused global concern.
-
•
Masks protect HCWs and other populations against respiratory viral infection.
-
•
The effectiveness of masks has been proven in different settings, populations, and areas.
1. Introduction
Facemasks are recommended for diseases transmitted through droplets and respirators for respiratory aerosols, yet recommendations and terminology vary between guidelines. The concepts of droplet and airborne transmission that are entrenched in clinical practice recently are more complex than previously thought. The concern is now increasing in the face of the Coronavirus Disease 2019 (COVID-19) pandemic [1]. The spread of respiratory viral infections (RVIs) occurs primarily through contact and droplet routes. And new evidence suggests severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can remain viable and infectious in aerosols for hours [2]. Therefore, the use of masks as appropriate personal protective equipment (PPE) is often considered when preventing the spread of respiratory infections. Experimental data shows that the micropores of mask block dust particles or pathogens that are larger than the size of micropores [3]. For example, the micropores of N95 masks materials are only 8 μm in diameter, which can effectively prevent the penetration of virions [4,5].
Although the aforementioned studies support the potential beneficial effect of masks, the substantial impact of masks on the spread of laboratory-diagnosed respiratory viruses remains controversial [6]. Smith et al. indicated that there were insufficient data to determine definitively whether N95 masks are superior to surgical masks in protecting healthcare workers (HCWs) against transmissible acute respiratory infections in clinical settings [7]. Another meta-analysis suggested that facemask provides a non-significant protective effect (OR = 0.53, 95% CI 0.16–1.71, I 2 = 48%) against the 2009 influenza pandemic [8]. Xiao et al. concluded that masks did not support a substantial effect on the transmission of influenza from 7 studies [6]. On the contrary, Jefferson et al. suggested that wearing masks significantly decreased the spread of SARS (OR = 0.32; 95% CI 0.25–0.40; I2 = 58.4%) [9]. Up to date, existing evidence on the effectiveness of the use of masks to prevent respiratory viral transmission contradicts each other.
Therefore, we performed a systematic review and meta-analysis to evaluate the effectiveness of the use of masks to prevent laboratory-confirmed respiratory virus transmission.
2. Methods
2.1. Identification and selection of studies
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement was consulted to report this systematic review. A comprehensive searching strategy was carefully designed to select eligible studies, published before March 2020, from multiple electronic databases, including PubMed, Web of Science, Cochrane Library, and Chinese National Knowledge Infrastructure (CNKI), VIP (Chinese) database. Relevant Chinese technical terms for the Chinese databases were used to search for published articles (see Appendix 1, for search details). Furthermore, references of all relevant articles and reviews were retrieved to search for additional eligible studies with full-texts. After removing duplicates, all abstracts and titles were filtered independently by two reviewers (M.L.; L.G.) and the full texts were downloaded and meticulously appreciated. The two reviewers compared and discussed the results and consulted with the third reviewer (C.Y.S.), if necessary, to reach a consensus.
2.2. Inclusion and exclusion criteria
The studies meeting the following criteria were included: (1) concerning the relationship between the face mask and preventing RVIs; (2) diagnosis of respiratory virus must have laboratory evidence, or the local clinical diagnostic criteria are applied during an acute large-scale infectious disease when laboratory evidence might be not available; (3) complete data available of both cases and controls to calculate an odds ratio (OR) with 95% confidence interval (CI); (4) appropriate study design; (5) no language restrictions applied. The exclusive criteria were as follows: (1) conferences/meetings abstracts, case reports, editorials, and review articles; (2) duplicate publication or overlapping studies; (3) studies with unavailable full texts.
2.3. Study quality assessment
The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the case-control study and cohort study: study ratings of seven to nine stars corresponded to high-quality, five to six stars to moderate quality, and four stars or less to low quality [10]. The Jadad scale was used to evaluate the quality of randomized controlled study: study ratings of three to five corresponded to high-quality, and two or less to low quality [11]. Three reviewers (M.L.; L.G.; C.Y.S.) completed assessments independently and the disagreements were resolved by a panel discussion with other reviewers.
2.4. Statistical analysis
The association of mask use with subsequent RVIs was assessed with odds ratios (ORs) with a 95% confidence interval (CI). P values less than 0.05 were considered statistically significant. Considering the potential for inter-study heterogeneity, subgroup analysis were carried out based on stratification by occupations (HCWs or Non-HCWs), countries, virus types, and study designs. Sensitivity analysis was performed by omitting individual studies to assess the stability of the meta-analysis. The heterogeneity was assessed using the I 2 statistic. The heterogeneity was considered insignificance when P > 0.10 and I 2 < 50%. If the study lacked heterogeneity, the pooled OR estimate was calculated using the fixed-effects model, otherwise the random-effects model was used [12]. Begg's and Egger's test were performed to quantitatively analyze the potential publication bias by Stata (version 14.0; Stata Corp, College Station, TX) software. The P values of Begg's and Egger's test more than 0.05 implied no obvious publication bias in this meta-analysis [13,14]. The meta-analysis was performed using Revman 5.3.5 (http://tech.cochrane.org/revman) [15].
3. Results
3.1. Characteristics of eligible studies
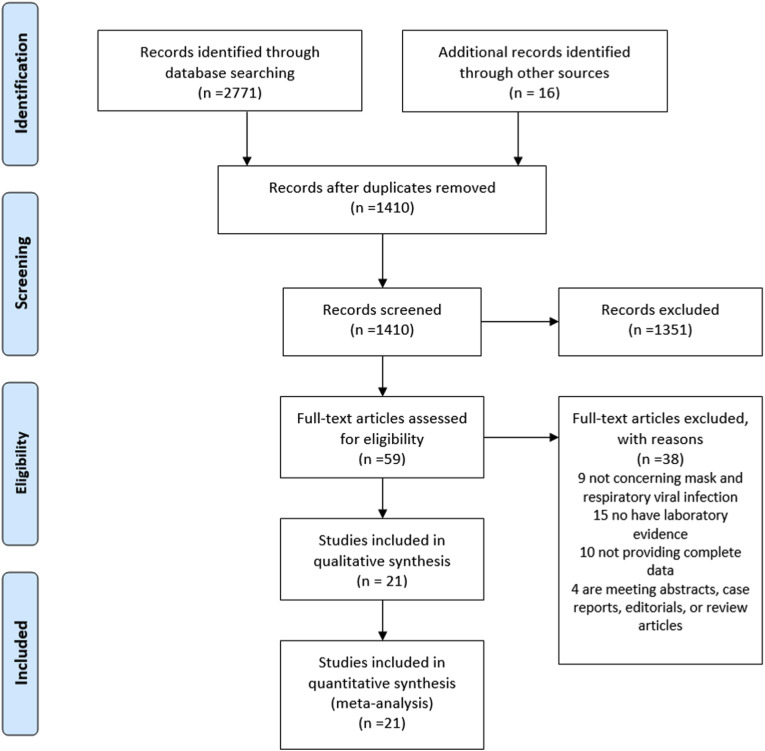
Following the literature search and screening (Fig. 1 ), a total of 21 studies which included 13 case-control studies, 6 cluster randomized trials, and 2 cohort studies met our inclusion criteria [4,[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]] (Table 1 ). Among them, 12 studies investigated HCWs, 8 studies investigated non-healthcare professional populations, and the remaining one study investigated HCWs and relatives of patients. Eleven studies were conducted in China (including 4 studies from Hong Kong, China), 6 in Western countries, and 4 in other Asian countries. And 4 studies investigated patients with respiratory virus, 7 studies investigated Severe acute respiratory syndrome coronavirus (SARS-CoV), 12 studies investigated influenza virus including 5 investigating the H1N1 virus, and 1 study investigated SARS-CoV-2.
Fig. 1.
Flow diagram of the study search and selection process.
Table 1.
Characteristics of eligible studies.
| Study | Year | Country | Virus | Mask type | Type of Study | Population | Main findings & comments | |
|---|---|---|---|---|---|---|---|---|
| 1 | Yin et al. | 2004 | China | SARSa | Paper mask, cotton mask | Case-control study | Healthcare workers | Wearing a mask is effective for medical personnel in preventing SARS hospital infections. |
| 2 | Wu et al. | 2004 | China | SARSa | Mask | Case-control study | Population | The mask use lowered the risk for disease supports the community's use of this strategy |
| 3 | Ma et al. | 2004 | China | SARSa | Mask | Case-control study | Healthcare workers | Wearing masks is of great significance to prevent respiratory infections. There are many types of masks used clinically. |
| 4 | Loeb et al. | 2004 | Canada | SARS | Medical Mask, N95 | Case-control study | Healthcare workers | Consistently wearing a mask (either surgical or particulate respirator type N95) while caring for a SARS patient was protective for the nurses. |
| 5 | Teleman et al. | 2004 | Singapore | SARSa | N95 | Case-control study | Healthcare workers | Both hand washing and wearing of N95 masks remained strongly protective but gowns and gloves did not affect. |
| 6 | Nishiura et al. | 2005 | Vietnam | SARS | Surgical mask | Case-control study | Employees and relative | Masks and gowns appeared to prevent SARS transmission. |
| 7 | Wilder-Smith et al. | 2005 | Singapore | SARS | N95 | Case-control study | Healthcare workers | Asymptomatic SARS was associated with lower SARS antibody titers and higher use of masks when compared to pneumonic SARS. |
| 8 | MacIntyre et al. | 2011 | China | Respiratory virus | Medical Mask, N95 Fit tested, N95 non-fit tested | Cluster randomized trial | Healthcare workers | There was no significant difference in outcomes between the N95 arms with and without fit testing. |
| 9 | Barasheed et al. | 2014 | Australia | Respiratory virus | Mask | Cluster randomized trial | Pilgrims | The laboratory results did not show any difference between the ‘mask’ group and ‘control’ group. |
| 10 | Sung et al. | 2016 | USA | Respiratory virus | Mask | Cohort study | HSCT patients | The requirement that all individuals in direct contact with HSCT patients wear surgical masks will reduce RVI. |
| 11 | Zhang et al. | 2017 | China | Respiratory virus | Masks | Case-control study | Healthcare workers | Choosing the right disposable respirator also plays an important role in controlling hospital viral infections. |
| 12 | Cowling et al. | 2008 | China (Hong Kong) | Influenza virus | Mask | Cluster randomized trial | Household | The laboratory-based or clinical secondary attack ratios did not significantly differ across the mask group and control group. Adherence to interventions was variable. |
| 13 | Cowling et al. | 2009 | China (Hong Kong) | Influenza virus | Mask | Cluster randomized trial | Household | Hand hygiene and facemasks seemed to prevent household transmission of influenza virus when implemented within 36 h of index patient symptom onset. |
| 14 | Suess et al. | 2012 | Germany | Influenza virus | Mask | Cluster randomized trial | Household | The secondary infection in the mask groups was significantly lower compared to the control group. |
| 15 | Aiello et al. | 2012 | USA | Influenza virus | Mask | Cluster randomized trial | Student | Face masks and hand hygiene combined may reduce the rate of ILI and confirmed influenza in community settings. |
| 16 | Cheng et al. | 2010 | China (Hong Kong) | H1N1 | Surgical mask | Case-control study | Healthcare workers | Not wearing a surgical mask during contact with the index case were found to be significant risk factors for nosocomial acquisition of S-OIV. |
| 17 | Jaeger et al. | 2011 | USA | H1N1 | Mask or N95 | Cohort study | Healthcare workers | The use of a mask or N95 respirator was associated with remaining seronegative. |
| 18 | Chokephaibulkit et al. | 2012 | Thailand | H1N1 | Mask | Case-control study | Healthcare workers | During the H1N1 outbreak in 2009, the wearing of masks by medical personnel was not related to the infection. There was a weak association in the nurse subgroup. |
| 19 | Zhang et al. | 2012 | China | H1N1 | Mask | Case-control study | Healthcare workers | The results suggest that the protective effect of wearing a mask is not significant. |
| 20 | Zhang et al. | 2013 | China (Hong Kong) | H1N1 | Mask | Case-control study | Population | Wearing masks is a protective factor against H1N1 infection when taking a plane. |
| 21 | Wang et al. | 2020 | China | SARS-CoV-2 | N95 | Case-control study | Healthcare workers | The 2019-nCoV infection rate for medical staff was significantly increased in the no-mask group compared with the N95 respirator group (adjusted odds ratio (OR): 464.82, [95% CI: 97.73-infinite]). |
Patients met local clinical diagnostic criteria during an acute large-scale infectious disease.
3.2. Quality of studies
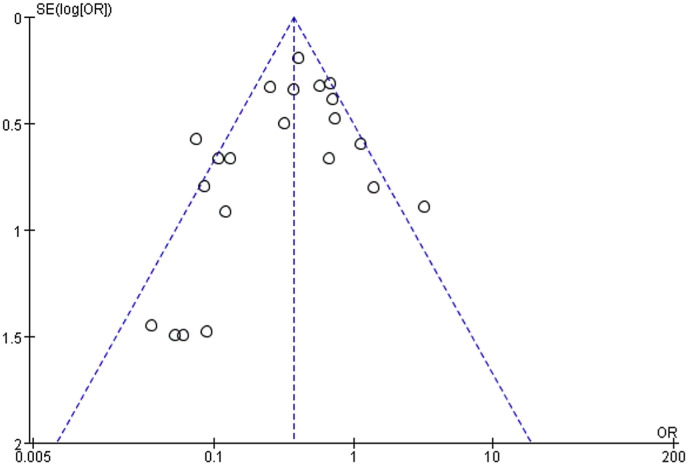
Inter-rater agreement of the quality of included studies was strong. Table 2, Table 3 summarize the quality evaluations of the included studies. Funnel plots assessing the risk of publication bias are included in Fig. 2 . Neither Begg's test (z = 0.45, p = 0.651) nor Egger's test (t = −0.65, p = 0.524) manifested any distinct evidence of the publication bias. The sensitivity analyses did not substantially alter the pooled ORs by excluding one-by-one study, indicating that the meta-analysis was generally robust.
Table 2.
The quality of the case-control studies and cohort studies.
| Study | Year | Selection | Comparability | Outcome | Starsa | |
|---|---|---|---|---|---|---|
| 1 | Yin et al. | 2004 | 3 | 2 | 2 | 7 |
| 2 | Wu et al. | 2004 | 4 | 2 | 2 | 8 |
| 3 | Ma et al. | 2004 | 3 | 2 | 2 | 8 |
| 4 | Loeb et al. | 2004 | 3 | 2 | 2 | 7 |
| 5 | Teleman et al. | 2004 | 3 | 2 | 3 | 8 |
| 6 | Wilder-Smith et al. | 2005 | 3 | 2 | 3 | 8 |
| 7 | Nishiura et al. | 2005 | 4 | 2 | 1 | 7 |
| 8 | Cheng et al. | 2010 | 3 | 2 | 3 | 8 |
| 9 | Jaeger et al. | 2011 | 3 | 2 | 2 | 7 |
| 10 | Chokephaibulkit et al. | 2012 | 3 | 2 | 2 | 7 |
| 11 | Zhang et al. | 2012 | 3 | 2 | 3 | 8 |
| 12 | Zhang et al. | 2013 | 4 | 2 | 1 | 7 |
| 13 | Sung et al. | 2016 | 3 | 2 | 2 | 7 |
| 14 | Zhang et al. | 2017 | 3 | 2 | 1 | 6 |
| 15 | Wang et al. | 2020 | 3 | 1 | 1 | 5 |
Scoring by Newcastle-Ottawa Scale.
Table 3.
The quality of randomized controlled studies.
| Study | Year | Randomization | Double-blind | Description of inclusion/exclusion criteria | Scoresa | |
|---|---|---|---|---|---|---|
| 1 | Cowling et al. | 2008 | 2 | 0 | 1 | 3 |
| 2 | Cowling et al. | 2009 | 2 | 0 | 1 | 3 |
| 3 | MacIntyre | 2011 | 2 | 0 | 1 | 3 |
| 4 | Suess et al. | 2012 | 2 | 1 | 1 | 4 |
| 5 | Ailello | 2012 | 2 | 1 | 1 | 4 |
| 6 | Barasheed et al. | 2014 | 2 | 0 | 1 | 3 |
Scoring by Jadad scale.
Fig. 2.
Funnel plot of mask-wearing and risk of laboratory-confirmed respiratory viral infection.
3.3. General protective effects
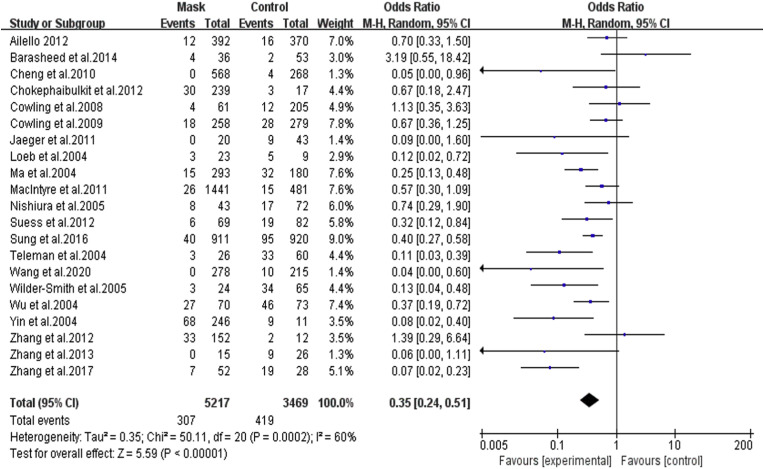
The 21 studies, involving 8,686 participants, showed that masks were generally effective in preventing the spread of respiratory viruses. After wearing a mask, the risk of contracting RVIs was significantly reduced, with the pooled OR was 0.35 and 95% CI = 0.24–0.51 (I 2 = 60%, M − H Random-effect model) ( Fig. 3 ).
Fig. 3.
Forest plot of the overall effect of mask-wearing on laboratory-confirmed respiratory viral infection.
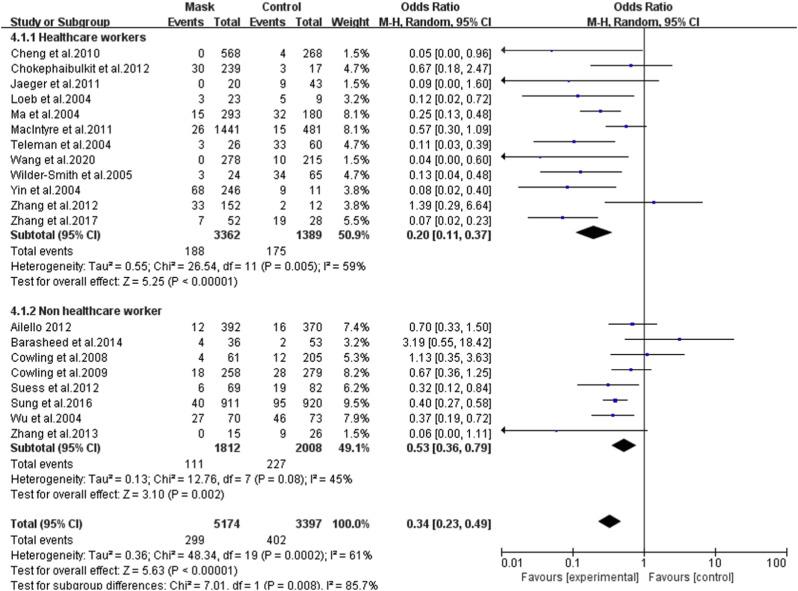
3.4. HCWs vs. non-HCWs
In the subgroup of HCWs, a more obvious protective effect was identified with the pooled OR of 0.20 (95% CI = 0.11–0.37, I 2 = 59%) ( Fig. 4 ). In one study investigating COVID-19, the OR was 0.04 (95%CI = 0.00–0.60) [35]. In the subgroup of non-HCW, also a protective effect was found with the pooled OR of 0.53 (95% CI = 0.36–0.79, I 2 = 45%). A more detailed analysis found significant effects in both the household subgroup (OR = 0.60, 95% CI = 0.37–0.97,I 2 = 31%), and the non-household subgroup (OR = 0.44, 95% CI = 0.33–0.59,I 2 = 54%) (Table 4 ). One study included both health care workers and family members of patients, with the OR of 0.74 (95% CI: 0.29–1.90) [22].
Fig. 4.
Forest plot of the effect of mask-wearing on laboratory-confirmed respiratory viral infection among HCW and non-HCW.
Table 4.
Meta-analysis results of effect of masks on laboratory-confirmed respiratory viral infection among different subgroups.
| Subgroup | Study numbers | OR | 95%CI | Heterogeneity | |
|---|---|---|---|---|---|
| Overall | – | 21 | 0.35 | 0.24–0.51 | 60% |
| HCW vs Non-HCWs | HCWs | 12 | 0.20 | 0.11–0.37 | 59% |
| Non-HCWs | 8 | 0.53 | 0.36–0.79 | 45% | |
| Non-HCWs | Household | 3 | 0.60 | 0.37–0.97 | 31% |
| Non-household | 5 | 0.44 | 0.33–0.59 | 54% | |
| Countries | Asian | 15 | 0.31 | 0.19–0.50 | 65% |
| Western | 6 | 0.45 | 0.24–0.83 | 51% | |
| HCWs based on countries | Asian | 10 | 0.21 | 0.11–0.41 | 64% |
| Western | 2 | 0.11 | 0.02–0.51 | 0% | |
| Non- HCWs based on countries | Asian | 4 | 0.51 | 0.34–0.78 | 64% |
| Western | 4 | 0.46 | 0.34–0.63 | 57% | |
| Virus types | Influenza virus | 12 | 0.55 | 0.39–0.76 | 27% |
| SARS-CoV | 7 | 0.26 | 0.18–0.37 | 47% | |
| SARS-CoV-2 | 1 | 0.04 | 0.00–0.60 | 0% | |
| H1N1 | 5 | 0.30 | 0.08–1.16 | 51% | |
| Study designs | Cluster RCTs | 6 | 0.65 | 0.47–0.91 | 20% |
| Observational studies(cohort and case control studies) | 15 | 0.24 | 0.15–0.38 | 52% |
HCW: Healthcare workers; Non-HCWs: Non-healthcare workers; RCT: Randomized control trial.
3.5. Asian countries vs. Western countries
By geographic locations, beneficial protective effects of wearing masks were found both in Asia (OR = 0.31, 95% CI = 0.19–0.50, I 2 = 65%), and in Western countries (OR = 0.45, 95% CI = 0.24–0.83, I 2 = 51%) ( Table 4 ). For HCWs, wearing mask can significantly reduce the risk of RVIs in both Asian (OR = 0.21, 95% CI = 0.11–0.41, I 2 = 64%) and Western countries (OR = 0.11, 95% CI = 0.02–0.51, I 2 = 0%). For non-HCWs, similar protective effects were also observed in Asian (OR = 0.51, 95% CI = 0.34–0.78, I 2 = 45%) and Western countries (OR = 0.46, 95% CI = 0.34–0.63, I 2 = 57%).
3.6. Subgroup analyses based on different virus types
Masks had a protective effect against influenza viruses (OR = 0.55, 95% CI = 0.39–0.76, I 2 = 27%), SARS (OR = 0.26, 95% CI = 0.18–0.37, I 2 = 47%), and SARS-CoV-2 (OR = 0.04, 95% CI = 0.00–0.6, I 2 = 0%) ( Table 4 ). However, no significant protective effects against H1N1 was shown (OR = 0.30, 95% CI = 0.08–1.16, I 2 = 51%).
3.7. Subgroup analyses based on different study designs
In the subgroups based on different study designs, protective effects of wearing mask were significant in cluster randomized trials (OR = 0.65, 95% CI = 0.47–0.91, I 2 = 20%) and observational studies (OR = 0.24, 95% CI = 0.15–0.38, I 2 = 54%) ( Table 4 ).
4. Discussion
This meta-analysis of the 21 studies provided the latest state-of-art evidence on the efficacy of masks in preventing the transmission of RVIs. Our data show that the protective effects of masks against RVIs were not only significant for both HCWs and non-HCWs, but also consistent between Asian and Western populations.
4.1. Mechanism of physical protection of masks
The physical barrier provided by a mask can effectively prevent the respiratory tract from contacting the outside virus, thereby reducing the risk of respiratory virus infections [36]. A recent study showed that SARS-CoV-2 can travel up to 4 m (≈13 feet) from patients and be widely distributed on daily objects (e.g. floors, computer mice, trash cans) [37]. Surgical masks are able to reduce influenza virus RNA in respiratory droplets and coronavirus RNA in aerosols [38]. The SARS-CoV-2 aerosol, mainly appearing in submicron region (d p between 0.25 and 1.0 μm) and supermicron region (d p > 2.5 μm) [39], can be effectively filtered out from the inhaled air by either surgical masks or N95 masks [3,40]. Comparison of the incidence of COVID-19 in Hongkong, China with Spain, Italy, Germany, France, U.S., U.K., Singapore, and South Korea showed that community-wide mask wearing may assist in controlling COVID-19 with reduced emission of infected saliva and respiratory droplets from mildly symptomatic patients [41].
4.2. Protective effects for both HCWs and non-HCWs
During the current COVID-19 pandemic, HCWs are facing the dangers inherent in close contact with index-patients [42]. In Italy, more than 2,600 HCWs have been infected by March 19, 2020, accounting for 8.3% of the country's total cases [43]. According to our analysis, wearing masks significantly reduced the risk of infection among HCWs by 80%. It is noteworthy that, none of the 278 HCWs wearing N95 masks in quarantined areas were infected by SARS-CoV-2 yet, 10 of the 215 HCWs who did not wear masks in the open areas were infected [35]. Therefore, universal masking of HCWs at clinical settings is likely to provide great benefits for HCWs. especially during current COVID-19 pandemic. Moreover, protective benefits were also reported in hematopoietic stem cell transplant (HSCT) patients [33]. Besides, Sokol's study also found a reduced risk of hospital-acquired RVIs by putting surgical masks on all workers and visitors in every patient room on the bone marrow transplant unit [44]. Accumulative data showed that people with older age, immunosuppressed state and systematic commodities are at higher risk for severe COVID-19 infection [[45], [46], [47]], and therefore, should be protected with proper measures (e.g. prophylactic masking) during the current pandemic. In addition, those who have close contact with those populations at high risks of contracting RVIs should consider wearing masks as well.
More importantly, our data showed that masks worn by non-HCWs can also effectively prevent the spread of respiratory viruses and reduce the risk of virus infection by 56% in non-household settings, indicating the potential benefits of wearing masks for the general public. Moreover, significant protective effects were found in the study conducted in the general population [17], indicating the potential benefits of wearing masks for the general public. Interestingly, a recent COVID-19 dynamics modeling study suggested that broad adoption of even relatively ineffective non-medical grade “social” masks may meaningfully reduce the community transmission and decrease peak hospitalizations and deaths during the current COVID-19 pandemic [48]. Although laboratory-confirmed virus results show no difference between the mask group and the control group in a study investigating the wearing of masks by pilgrims, wearing masks reduced the risk of influenza-like illness when people gather [4]. This difference between laboratory-confirmed cases and clinically diagnosed influenza-like illness cases were likely due to an under-diagnosis of real cases caused by too few nasal swabs collected for laboratory confirmation. Zhang et al. conducted a case-control study and found that none of the passengers always wearing masks on an international flight were infected with H1N1 [32], and a recent case report [49] described a man who was wearing a mask at international flight and then tested positive for COVID-19, while 25 other people closest to him on the plane and flight attendants were all tested negative, further demonstrating the benefits of wearing masks during public transportations [50].
Protective effects were also found among household settings showing a 40% reduced risk of RVIs. However, masking with prudent implementation and high compliance is a prerequisite to ensure the successful protection, which is practically challenging especially for non-HCWs. Two household studies included in our analysis reported low facemask adherence among household contacts [23,24], which might explain the poor protective effects from these studies. In contrast, Suess et al. reported a good compliance, which showed a significant protective effect [29]. These findings implicated that proper use of masks has an impact on the effectiveness of preventing RVIs. Given that most people in household settings were unlikely to strictly follow hand hygiene and mask use recommendations [23], re-evaluating the home quarantine strategy might be essential during the current COVID-19 pandemic [51,52].
4.3. Protective effects against influenza, SARS, and COVID-19
The risk of influenza, SARS, and COVID-19 infection were reduced by 45%, 74%, and 96% by wearing masks, respectively, which were consistent with previous meta-analyses during the SARS outbreaks [9,53]. The previous systematic review from Xiao et al., though reporting non-significant protection of masks against the influenza virus [6], did not strictly follow the PRISMA statement and represented merely non-aggregated data. For example, there was one study [54] included by Xiao et al. did not report a significantly protection by wearing masks. However, it should be noted that the result of this study was not convincing because the H1N1 pandemic broke out during the study period, and the national hygiene campaign implemented at this time influenced all participants to wear masks [54].
The insignificantly reduced risk of H1N1 infection following masking could be partially explained by the relatively small sample size and multiple confounding factors (e.g. prior vaccination, hand hygiene, age, gender, and culture). Jeager et al., 2009 indicated that overall PPE use among HCWs was low; as more than 25% of HCWs in this study reported they never used PPE in any patient encounter, and only 17% reported wearing masks with every H1N1 patient encounter, which could significantly lower the sample size of data collected [27]. Also, the same study [27] indicated that the majority of HCWs had received regular seasonal influenza vaccination, which could play a role of confounding factor contributing to protective effects toward control group. Additionally, during acute outbreak of H1N1, specific preventive measures were lagged behind H1N1 exposures. This could suggest that HCWs might already have been infected before wearing masks, further decreasing the powers of data collected.
4.4. Consistent protective effects between Asian and western countries
Due to current controversial guidelines between different countries and areas, regarding the general public wearing masks. We also analyzed its effects based on different geographic locations, showing that wearing masks does provide protective effects in both Asian countries and western countries by 69% and 55%, respectively. Among HCWs, it reduced the risk in both Asian and western countries. Especially, for non-healthcare populations, reduced risk of 54% was found in western countries, and a reduced risk of 49% was found in Asia. This would suggest that the proper use of masks might play a significant role in public health efforts to suppress the spread of RVIs, regardless of the geographic locations, especially during an outbreak.
4.5. Limitations and future perspective
The present meta-analysis still has several limitations. First, well-designed high-quality prospective studies and studies of masking in the general public are still insufficient. Second, Droplet-borne and airborne viruses are likely to cause large-scale transmissions among the passengers within closed transportation vehicles [55]. However, relevant studies are relatively rare [32]. Third, this article included some studies of SARS patients diagnosed according to clinical diagnostic criteria for SARS due to a low detection rate of RT-PCR [56]. The lack of sufficient virologic evidence may affect our conclusions. However, this effect might not be significant, as 92% of patients with clinical SARS for whom paired sera were available had a >4-fold rise in antibody titer to SARS-CoV [57]. Fourth, control subjects without masking are generally lacking in studies conducted in healthcare settings mainly due to the ethical issue. Future studies might choose HCWs from departments without needs of masking as controls [26]. Fifth, our study didn't have sufficient data for subgroup analysis of different mask types since our inclusion criteria mainly focused on masks versus no masks, which might inherently omit studies that focused on effectiveness of different mask types. Though there were published studies that had shown different specifications of masks and different wearing methods may affect the protective effect of masks [17,32]. And when the included studies divided the time/frequency of wearing masks, we only included the group of masks with the longest wearing/highest wearing frequency. This might also ignore effects of the short/infrequent mask-wearing. In addition, the studies we included were mainly conducted in Asia, especially China, and more evidence from other countries is needed to support our views. Last but not least, information about other confounding biases, such as vaccination, hand hygiene, age, gender, and culture, may affect the protective effect of masks.
5. Conclusion
The present systematic review and meta-analysis showed the general efficacy of masks in preventing the transmission of RVIs. Such protective effects of masking are evidentiary for both healthcare and non-healthcare workers and consistent between Asian and Western populations. More evidences are still needed to better clarify the effectiveness of masking in various circumstances.
Funding
This work was not supported by any funding.
CRediT authorship contribution statement
Mingming Liang: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing - original draft, Writing - review & editing. Liang Gao: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Ce Cheng: Investigation, Writing - original draft, Writing - review & editing. Qin Zhou: Writing - review & editing. John Patrick Uy: Writing - review & editing. Kurt Heiner: Writing - review & editing. Chenyu Sun: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing - original draft, Writing - review & editing.
Declaration of competing interest
We declare no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tmaid.2020.101751.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization . 2020. WHO Director-General's opening remarks at the media briefing on COVID-19-11 March 2020.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [cited 2020 March 10] [Google Scholar]
- 2.Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies A., Thompson K., Giri K., Kafatos G., Walker J., Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med Public Health Prep. 2013;7:413–418. doi: 10.1017/dmp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barasheed O., Almasri N., Badahdah A., Heron L., Taylor J., McPhee K. Pilot randomised controlled trial to test effectiveness of facemasks in preventing influenza-like illness transmission amo ng Australian Hajj pilgrims in 2011. Infect Disord - Drug Targets. 2014;14:110–116. doi: 10.13140/RG.2.1.1889.9048. [DOI] [PubMed] [Google Scholar]
- 5.Lee S.A., Grinshpun S.A., Reponen T. Respiratory performance offered by N95 respirators and surgical masks: human subject evaluation with NaCl aerosol representing bacterial and viral particle size range. Ann Occup Hyg. 2008;52:177–185. doi: 10.1093/annhyg/men005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao J., Shiu E.Y., Gao H., Wong J.Y., Fong M.W., Ryu S. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings-personal protective and environmental measures. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2605.190994. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith J.D., MacDougall C.C., Johnstone J., Copes R.A., Schwartz B., Garber G.E. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta-analysis. CMAJ (Can Med Assoc J) 2016;188:567–574. doi: 10.1503/cmaj.150835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saunders-Hastings P., Crispo J., Sikora L., Krewski D. Effectiveness of personal protective measures in reducing pandemic influenza transmission: a systematic review and meta-analysis. Epidemics. 2017;20:1–20. doi: 10.1016/j.epidem.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Jefferson T., Foxlee R., Del Mar C., Dooley L., Ferroni E., Hewak B. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ. 2008;336:77–80. doi: 10.1136/bmj.39393.510347.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells G., Shea B., O'Connell D., Peterson J., Welch V., Losos M. 2009. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [cited 2020 February5] [Google Scholar]
- 11.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J.M., Gavaghan D.J. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Contr Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R., Laird N. Meta-analysis in clinical trials. Contr Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 14.Egger M., Davey S.G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Cochrane Collaboration . 2014. Review manager (RevMan) Version 5.3. [Google Scholar]
- 16.Teleman M.D., Boudville I.C., Heng B.H., Zhu D., Leo Y.S. Factors associated with transmission of severe acute respiratory syndrome among health-care workers in Singapore. Epidemiol Infect. 2004;132:797–803. doi: 10.1017/S0950268804002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J., Xu F., Zhou W., Feikin D.R., Lin C., He X. Risk factors for SARS among persons without known contact with SARS patients, Beijing, China. Emerg Infect Dis. 2004;10:210–216. doi: 10.3201/eid1002.030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loeb M., McGeer A., Henry B., Ofner M., Rose D., Hlywka T. SARS among critical care nurses, Toronto. Emerg Infect Dis. 2004;10:251–255. doi: 10.3201/eid1002.030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma H., Wang H., Fang L., Jiang J., Wei M., Liu W. A case-control study on the risk factors of severe acute respiratory syndromes among health care workers. Zhonghua Liuxingbingxue Zazhi. 2004:13–16. [PubMed] [Google Scholar]
- 20.Yin W., Gao L., Lin W., Du L., Zhang X., Zou Q. Effectiveness of personal protective measures in prevention of nosocomial transmission of severe acute respiratory syndrome. Zhonghua Liuxingbingxue Zazhi. 2004:26–30. [PubMed] [Google Scholar]
- 21.Wilder-Smith A., Teleman M.D., Heng B.H., Earnest A., Ling A.E., Leo Y.S. Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerg Infect Dis. 2005;11:1142–1145. doi: 10.3201/eid1107.041165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishiura H., Kuratsuji T., Quy T., Phi N.C., Van Ban V., LE Dang H.A. Rapid awareness and transmission of severe acute respiratory syndrome in Hanoi French Hospital, Vietnam. Am J Trop Med Hyg. 2005;73:17–25. doi: 10.4269/ajtmh.2005.73.17. [DOI] [PubMed] [Google Scholar]
- 23.Cowling B.J., Fung R.O., Cheng C.K., Fang V.J., Chan K.H., Seto W.H. Preliminary findings of a randomized trial of non-pharmaceutical interventions to prevent influenza transmission in households. PloS One. 2008;3 doi: 10.1371/journal.pone.0002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowling B.J., Chan K., Fang V.J., Cheng C.K., Fung R.O., Wai W. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med. 2009;151:437–446. doi: 10.7326/0003-4819-151-7-200910060-00142. [DOI] [PubMed] [Google Scholar]
- 25.Cheng V.C., Tai J.W., Wong L., Chan J.F., Li I.W., To K. Prevention of nosocomial transmission of swine-origin pandemic influenza virus A/H1N1 by infection control bundle. J Hosp Infect. 2010;74:271–277. doi: 10.1016/j.jhin.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacIntyre C.R., Wang Q., Cauchemez S., Seale H., Dwyer D.E., Yang P. A cluster randomized clinical trial comparing fit‐tested and non‐fit‐tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influenza Other Respir Viruses. 2011;5:170–179. doi: 10.1111/j.1750-2659.2011.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaeger J.L., Patel M., Dharan N., Hancock K., Meites E., Mattson C. Transmission of 2009 pandemic influenza A (H1N1) virus among healthcare personnel—southern California, 2009. Infect Contr Hosp Epidemiol. 2011;32:1149–1157. doi: 10.1086/662709. [DOI] [PubMed] [Google Scholar]
- 28.Aiello A.E., Perez V., Coulborn R.M., Davis B.M., Uddin M., Monto A.S. Facemasks, hand hygiene, and influenza among young adults: a randomized intervention trial. PloS One. 2012;7 doi: 10.1371/journal.pone.0029744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suess T., Remschmidt C., Schink S.B., Schweiger B., Nitsche A., Schroeder K. The role of facemasks and hand hygiene in the prevention of influenza transmission in households: results from a cluster randomised trial; Berlin, Germany, 2009-2011. BMC Infect Dis. 2012;12:12–26. doi: 10.1186/1471-2334-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Seale H., Yang P., MacIntyre C.R., Blackwell B., Tang S. Factors associated with the transmission of pandemic (H1N1) 2009 among hospital healthcare workers in Beijing, China. Influenza Other Respir Viruses. 2013;7:466–471. doi: 10.1111/irv.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chokephaibulkit K., Assanasen S., Apisarnthanarak A., Rongrungruang Y., Kachintorn K., Tuntiwattanapibul Y. Seroprevalence of 2009 H1N1 virus infection and self‐reported infection control practices among healthcare professionals following the first outbreak in bangkok, Thailand. Influenza Other Respir Viruses. 2013;7:359–363. doi: 10.1111/irv.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L., Peng Z., Ou J., Zeng G., Fontaine R.E., Liu M. Protection by face masks against influenza A (H1N1) pdm09 virus on trans-Pacific passenger aircraft, 2009. Emerg Infect Dis. 2013;19:1403–1410. doi: 10.3201/eid1909.121765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sung A.D., Sung J.A., Thomas S., Hyslop T., Gasparetto C., Long G. Universal mask usage for reduction of respiratory viral infections after stem cell transplant: a prospective trial. Clin Infect Dis. 2016;63:999–1006. doi: 10.1093/cid/ciw451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L. Study on correlation between nosocomial respiratory virus infection and use of disposable respirator in medical staff. Nurs Pract Res. 2017;14:118–119. doi: 10.3969/j.issn.1672-9676.2017.22.046. [DOI] [Google Scholar]
- 35.Wang X., Pan Z., Cheng Z. Association between 2019-nCoV transmission and N95 respirator use. J Hosp Infect. 2020:30097–30099. doi: 10.1016/j.jhin.2020.02.021. S0195-6701(20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson D.F., Druce J.D., Birch C., Grayson M.L. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin Infect Dis. 2009;49:275–277. doi: 10.1086/600041. [DOI] [PubMed] [Google Scholar]
- 37.Guo Z.D., Wang Z.Y., Zhang S.F., Li X., Li L., Li C. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020 doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung N.H.L., Chu D.K.W., Shiu E.Y.C., Chan K.-H., McDevitt J.J., Hau B.J.-P. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y., Ning Z., Chen Y., Guo M., Liu Y.L., Gail N.K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020 doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 40.Garcia Godoy L.R., Jones A.E., Anderson T.N., Fisher C.L., Seeley K.M.L., Beeson E.A. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng V.C., Wong S.C., Chuang V.W., Chuang V.W., So S.Y., Chen J.H. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz J., King C., Yen M. Protecting health care workers during the COVID-19 coronavirus outbreak–lessons from taiwan's SARS response. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stickings T., Dyer C. 2020. Five more Italian doctors die battling coronavirus: thirteen medics have now lost their lives, with 2,629 health workers infected-8.3% of country's total.https://www.dailymail.co.uk/news/article-8129499/More-2-600-medical-workers-infected-coronavirus-Italy.html [cited 2020 March 21] [Google Scholar]
- 44.Sokol K.A., De la Vega-Diaz I., Edmondson-Martin K., Kim S., Tindle S., Wallach F. Masks for prevention of respiratory viruses on the BMT unit: results of a quality initiative. Transpl Infect Dis. 2016;18:965–967. doi: 10.1111/tid.12608. [DOI] [PubMed] [Google Scholar]
- 45.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murthy S., Gomersall C.D., Fowler R.A. Care for critically ill patients with COVID-19. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- 47.Center for Disease Control and Prevention . 2020. Coronavirus disease 2019 (COVID-19)https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/high-risk-complications.htm [cited 2020 March 17] [Google Scholar]
- 48.Eikenberry S.E., Mancuso M., Iboi E., Phan T., Eikenberry K., Kuang y. To mask or not to mask: modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infect Disease Model. 2020;5:293–308. doi: 10.1016/j.idm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz K.L., Murti M., Finkelstein M., Leis J.A., Fitzgerald-Husek A., Bourns L. vol. 192. 2020. p. E410. (Lack of COVID-19 transmission on an international flight). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Health Commission of the Prc . 2020. General prevention guidelines of 2019-nCoV infected pneumonia.http://www.nhc.gov.cn/xcs/kpzs/202001/3a13637e1a9249a2b6047f34b772b5e6.shtml [cited 2020 March 8] [Google Scholar]
- 51.Chan J.F., Yuan S., Kok K., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang R., Xia J., Chen Y., Shan C., Wu C. A family cluster of SARS-CoV-2 infection involving 11 patients in Nanjing, China. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30147-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Offeddu V., Yung C.F., Low M.S.F., Tam C.C. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta-analysis. Clin Infect Dis. 2017;65:1934–1942. doi: 10.1093/cid/cix681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmerman J.M., Suntarattiwong P., Levy J., Jarman R.G., Kaewchana S., Gibbons R.V. Findings from a household randomized controlled trial of hand washing and face masks to reduce influenza transmission in Bangkok, Thailand. Influenza Other Respir. Viruses. 2011;5:256–267. doi: 10.1111/j.1750-2659.2011.00205.x. Findings from a household randomized controlled trial of hand washing and face masks to reduce influenza transmission in Bangkok, Thailand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Health Commission of the Prc Notice on issuing a new coronary virus pneumonia diagnosis and treatment plan (Trial Version 7) 2020. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml [cited 2020 March 17]
- 56.Hui D., Cheong S., Wong P.C., Wang C. SARS: clinical features and diagnosis. Respirology. 2003;8:S20–S24. doi: 10.1046/j.1440-1843.2003.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan K.H., Poon L.L., Cheng V., Guan Y., Hung I., Kong J. Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis. 2004;10:294–299. doi: 10.3201/eid1002.030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.