Abstract
Objective
The objective was to investigate the expression of macrophage migration inhibitory factor (MIF) in non-small cell lung cancer (NSCLC), as well as the effects of macrophage MIF on tumor cells.
Methods
The human NSCLC cell strains H358 and H524 were selected as research objects. The Real-Time Polymerase Chain Reaction (RT-PCR) and Western Blot were utilized to detect the expression levels of MIF in human NSCLC cell strains. The lentiviral plasmid was utilized for MIF-mRNA interference. The expression levels of MIF before and after transfection were compared. The cell strains were cultured and proliferated for cell count and comparison.
Results
H358 showed MIF high expression while H524 showed MIF low expression. Once the H358 cells were constructed as silent MIF expression, compared with the original H358 cells, the difference was statistically significant. Once the H524 cells were constructed as high MIF expression, compared with original H524 cells, the difference was statistically significant. Being cultured for respective 3, 5, and 7 days, the transfected H358 cells showed a significant decrease in proliferative activity compared with original H358 cells, while the transfected H524 cells showed a significant increase in proliferative activity compared with original H524 cells.
Conclusion
MIF has high expression in H358 cells while low expression in H524 cells. The expression of MIF could enhance the proliferative activity of NSCLC tumor cells.
Keywords: Macrophage migration inhibitory factor, Non-small cell lung cancer, H358, H524, Transfection
1. Introduction
Lung cancer is one of the most malignant tumors with the fastest growth in incidence and mortality rates, which poses the greatest threat to the health and lives of human beings (Yamaguchi et al., 2020). According to histopathology, lung cancer is mainly divided into two categories, i.e., small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) (Jamalhanjani et al., 2017). Specifically, NSCLC accounts for about 80% of all lung cancers. Due to the slow growth rate of cancer cells and the late spread of metastasis, once NSCLC is clinically diagnosed, it is usually in its advanced stage; therefore, the 5-year survival rate of NSCLC patients is extremely low (Antonia et al., 2017). Therefore, the identification of the metastasis and progression of NSCLC has become a research hot spot for medical scholars. In tumor diseases, the internal and external environment in which tumor cells are located has an important influence on the occurrence, growth, and metastasis of tumors (Wang et al., 2017). Heterogeneous tumor cells and non-tumor cells coexist in tumors, and the living environment provided by non-tumor cells for protooncogenes is called tumor microenvironment (Ibáñez-Vea et al., 2017). In the tumor microenvironment, tumor cells can change and maintain their own survival and development conditions through autocrine and paracrine, thereby assisting the growth and development of tumor (Zhang et al., 2017). Studies have found that the macrophage migration inhibitory factor (MIF) can assist tumor microenvironment and participate in tumor development (Heidari et al., 2017). MIF is a protein molecule with multiple potencies that is constitutively expressed in a variety of immune and non-immune cells (Qian et al., 2017). Several studies have observed high expression levels of MIF in a variety of cancers (Luedike et al., 2018). Pantouris et al. (2018) reported that MIF plays an important role in the angiogenesis of tumor diseases (Pantouris et al., 2018). Xu et al. (2018) significantly inhibited the development of lung adenocarcinoma by knocking down the MIF expression (Xu et al., 2018). These studies suggest that MIF plays an important role in tumor cells, but how MIF participates in the development of NSCLC has not been fully described. To further explore the role of MIF in the pathogenesis of NSCLC, based on previous studies, this study speculates that the expression of MIF may affect the cell proliferation activity in the pathogenesis of NSCLC.
In summary, the intrinsic mechanism of NSCLC is still unclear. In order to further study the effects of MIF in the pathogenesis of NSCLC, in this study, the human NSCLC cell strains H358 and H524 were selected as research objects to explore the expression of MIF in NSCLC, providing a reference for the clinical treatment of NSCLC.
2. Materials and methods
2.1. Experimental cells
Human NSCLC cell strains H358 and H524 (ATCC (Artwork Tracing Certification Chain, USA)), which were stored in liquid nitrogen jars.
2.2. Extraction of total RNA by the Trizol method
The human NSCLC cell strains were taken out, and the medium was discarded. The cells were washed thrice with pre-cooled phosphate buffer saline (PBS) (Tianjin Guangcheng Chemical Reagent, China), with 5 min for each time. Each hole was added with 1 mL of Trizol (Jiaozuo LFFBio, China). The cells were stood on the ice for 5 min and repeatedly blown to make them evenly mixed. After the cells were fully lysed, they were transferred to a pre-cooled centrifuge tube (Nanjing Sbjbio, China). Then, the cells were added with 100 μL of chloroform (Sinopharm Chemical Reagent, China), mixed evenly with a shaker (Shanghai Xi-Yuan Bio, China), and stood for 5 min. The cells were centrifuged (12,000g/min, 4 °C) for 15 min in a high-speed centrifuge (Beckman Coulter, USA). Then, the uppermost solution was carefully pipetted and transferred to a new centrifuge tube. The same amount of isopropanol as the uppermost solution (Tianjin Guangcheng Chemical Reagent, China) was added. The centrifuge tube was evenly mixed with a shaker, stood for 15 min, and centrifuged (12,000g/min, 4 °C) for 10 min. The supernatant was carefully pipetted and discarded. The diethylpyrocarbonate (DEPC) water (Shanghai Yuanye Biotechnology, China) was utilized to formulate with the 75% ethanol (Tianjin Guangcheng Chemical Reagent, China), which should be immediately used after formulation. Then, the formulated 75% ethanol was added to the extracted white sedimentation. The sedimentation was centrifuged (7500g/min, 4 °C) for 5 min. The supernatant was carefully pipetted and discarded. The centrifuge tube was placed upside down on the filter paper and dried in the air. After 10 min, the DEPC water (20 μL, Shanghai Huyu Biological Technology, China) was added.
2.3. Detection of MIF-mRNA expression in human NSCLC cell strains by real-time polymerase chain reaction (RT-PCR)
Reverse transcription reaction: After quality verification, the extracted total RNA was subjected to reverse transcription reaction to generate cDNA, which was operated according to the specification system of the reverse transcription kit (Takara Corporation, Japan). The reaction system was as follows: 2 μL of 5 × PrimeScript® Buffer (for Real Time), 0.5 μL of PrimeScript® RT Enzyme Mix I, 0.5 μL of Oligo dT Primer (50 uM)*1, 0.5 μL of Random 6 mers (100 uM)*1, and 500 ng of Total RNA. The total volume was supplemented to 10 μL by RNase Free dH2O (Shanghai KM Biotechnology, China). The reverse transcription was reacted at 37 °C for 15 min, and the reverse transcriptase inactivation was reacted at 85 °C for 5 s.
Amplification reaction: The primer sequences of the target gene MIF-mRNA and the reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed, as shown in Table 1.
Table 1.
Primer sequence.
| Gene | Primer sequence |
|---|---|
| Target gene MIF-mRNA | The upstream primer: 5′-CACCGTCTCTTGTAGCAATCG-3′ The downstream primer: 5′-TAGCCTGGGCACCAGATAGC-3′ The upstream primer: 5′-cgtaaactttgccgcctatga-3′ The downstream primer: 5′-ttcttcattgcctgagtagcatttat-3′ |
| Reference gene GAPDH | The upstream primer: 5′-ATCAGCAATGCCTCCTGCAC-3′ The downstream primer: 5′-GGCATGGACTGTGGTCATG-3′ |
The reverse transcription reaction was operated according to the specification system of the SYBR®Premix Extaq™ II kit (Takara Corporation, Japan). The reaction system was as follows: 10 μL of SYBR (sygreen), 0.4 μL of PCR Forward Primer (10 μM), 0.4 μL of PCR Reverse Primer (10 μM), 1.6 μL of cDNA template, and the total volume was supplemented to 20 μL by DEPC water. Pre-denaturation was carried out at 95 °C for 10 min. Denaturation was carried out at 95 °C for 15 s. The reaction was repeated for 40 cycles. Annealing extension was carried out at 60 °C for 1 min. The calculation was performed by using ΔCt, and the final results were expressed by the 2−ΔCt method.
2.4. Detection of MIF protein expressions in human NSCLC cell strains by Western Blot
Preparation of protein samples: cells in the logarithmic growth phase of 2–6 generations were selected and inoculated in a 60 mm culture dish (Beijing Baiao Laibo Technology, China) at a density of 104/mm2. Samples could be taken until the cells were overgrown with approximately 65% of the culture bottom area. The culture solution was discarded, the culture dish was placed upside down on the absorbent paper to absorb the excess culture solution. The sample was washed thrice with 1 mL of pre-cooled 0.01 M PBS, with 1 min for each time. Then, the washing solution was discarded. The culture dish was placed upside down on the absorbent paper to absorb the residual washing solution. The sample was added with 80 μL of cell lysate (Shanghai Xinyu Biotechnology, China) and shaken for 20 min at 4 °C on a shaker. After the cells were completely lysed, they were quickly scraped on one side of the culture dish by using a cell scraper, and the cell debris and cell lysate were transferred to a pre-cooled centrifuge tube and centrifuged (4 °C, 12,000 rpm) for 15 min. The supernatant was transferred to a new pre-cooled centrifuge tube and stored at −20 °C.
Determination of protein concentration by the bicinchoninic acid (BCA) method: Different concentrations of bovine serum albumin (BSA) protein standard solutions were prepared in a 96-hole plate according to Table 2. The AB mixture solution was prepared from reagent A (1% BCA disodium salt, 2% anhydrous sodium carbonate, 0.16% sodium tartrate, 0.4% sodium hydroxide, 0.95% sodium bicarbonate, adjusted to a pH value of 11.25) and reagent B (4% copper sulfate) in a volume ratio of 50:1.
Table 2.
The detection of the standard curve.
| 1 μg/μL BSA protein standard solution (μL) | Lysis buffer solution (μL) | AB mixture solution (μL) | BCA working fluid (μL) | BSA protein content (μg/μL) |
|---|---|---|---|---|
| 0 | 5 | 25 | 200 | 180 |
| 1 | 4 | 25 | 200 | 180 |
| 2 | 3 | 25 | 200 | 180 |
| 3 | 2 | 25 | 200 | 180 |
| 4 | 1 | 25 | 200 | 180 |
| 5 | 0 | 25 | 200 | 180 |
The formulated BSA protein standard solutions with different concentrations were incubated in a 37 °C incubator. 15 min later, an automatic enzyme mark instrument (Molecular Devices, USA) was applied to measure the absorbance value at the wavelength of 595 nm. The standard curve was drawn from the absorbance of the working fluid, and the protein concentration of the samples was calculated from the standard curve.
Detection of protein expression: In accordance with the molecular weight of the protein samples to be assayed, the 10% separation gel and 5% stacking gel were prepared. Samples were loaded. Both sides of sample holes were loaded with protein marker. The power supply was connected. The constant voltage for stacking gel electrophoresis was 1200 V. After 30 min, the voltage was changed into a constant voltage of 90 V, and the electrophoresis was finished after 120 min. The polyvinylidene fluoride (PVDF) membrane was used in the Bio-Rad standard wet-type membrane transfer device. The membrane transfer was performed at a constant current of 300 mA. The time for membrane transfer was determined according to the size of protein. Generally, protein over 100 kDa required at least 120 min for membrane transfer, and protein less than 100 kDa required 90 min for membrane transfer, and protein of 50 kDa required 60 min for membrane transfer. After the membrane transfer was completed, the PVDF membrane was taken out, added with 5% skimmed milk, shaken slowly on a shaking bed for 90 min, and washed thrice with Tris-Buffered Saline and Tween 20 (TBST, Shanghai Yuduo Biotechnology, China), with 5 min for each time. The first antibody dilution solution (diluted 1:1000, Shanghai Hengfei Biotechnology, China) was added. The membrane was shaken slowly on a shaker overnight at 4 °C. Then, the membrane was washed thrice with TBST, with 10 min for each time. Next, the membrane was added with 5% skimmed milk and the second antibody dilution (diluted 1:5000, Shanghai Guduo Biotechnology, China), shaken slowly for 45 min at room temperature, and washed thrice with TBST, with 5 min for each time. In the dark box, the electrochemiluminescence (ECL) luminescent liquid (Nanjing Enogene Biotech, China) was dripped on the target protein region of the PVDF membrane, and the PVDF membrane was kept wet by dripping the ECL luminescent liquid continuously. The gel imaging system (Axygen, USA) was applied to scan and take photos of the membrane; the gray values of the protein bands were calculated.
2.5. Construction of H524 cell strains with high MIF expression by recombinant reverse transcription viral vector
Construction of lentiviral vector TG005-MIF vector: Humanized MIF gene primers were designed based on gene information, with Mlu I as the upstream primer and SpeI as the downstream primer. The empty vector pWPXL-MOD (TG005) and the recovered product of PCR were double-digested with Mlu I and SpeI. Then, they were ligated with the target gene fragment to transform the Escherichia coli. The plasmid was screened, amplified, and extracted. The lentiviral vector packaging was performed by using the Genechem gene lentiviral vector system.
Viral biological titer assay: Cell density of 1 × 105/well was used for passage in 24 well plate. After 24 h, the cell density was approximately 75%, and the cell culture medium was changed to serum-free medium. A mixed solution of DNA and Lipofectamine 2000 was added, and the cell was cultured in an incubator (37 °C, 5% CO2) for 8 h. The medium was discarded. The cells were washed with 20 mL of PBS, and the washing solution was discarded. 25 mL of cell culture medium containing 10% serum was added, and the cells were cultured in an incubator (37 °C, 5% CO2) for 48 h. The cell supernatant was collected and centrifuged (4000g, 4 °C) for 10 min. Then, the cell debris was discarded. Filtration was carried out by using a 0.45 μm filter (Shanghai Yiyan Biotechnology, China), and the collected filtrate was centrifuged (4000g) for 2 min to obtain the virus concentrate. The virus concentrate was kept in a virus tube and stored in a −80 °C freezer.
Viral biological titer assay: The virus was passaged in a 24-hole plate at a cell density of 1 × 105 per hole. After 24 h, 10 Eppendorf (EP) tubes were prepared and added with 90 μL of the medium. The 1st tube was added with 10 μL of the virus primary liquid to be determined. After being mixed evenly, 10 μL of the mixture in the 1st tube was pipetted and added into the 2nd tube. The operation was repeated until the last tube. The diluted virus solution was added to the cells, cultured in an incubator (37 °C, 5% CO2) for 48 h, and gently added with 500 μL of fresh medium. After 4 days, RNA was extracted for RT-PCR detection.
Lentiviral transfection: The cell strains were plated in 24-hole plates at a cell density of 1 × 105/hole. After 12 h, the medium was discarded. Then, 2 mL of fresh medium containing 5 μg/mL polybrene and an appropriate amount of pre-cooled lentiviral suspension was added. The plates were incubated in an incubator (37 °C, 5% CO2) for 24 h. The medium was discarded. Then, a medium containing 10% fetal bovine serum was added, and the cells were cultured in an incubator (37 °C, 5% CO2) for 72 h. A semi-lethal dose of screening drug was added. Then, after cell proliferation, protein expression levels were detected by Western Blot.
2.6. Construction of H358 cell strains with low MIF expression by the plasmid transfected cells
Extraction of the plasmid: The competent bacteria were taken out from the −80 °C refrigerator. After being melted in an icebox, the plasmid DNA having a volume of not more than 10 μL was quickly added, gently mixed uniformly, and ice-bathed for 30 min. Then, 100 mL of the lysate broth culture solution, a suitable antibiotic, and 1 mL of the bacterial solution containing the plasmid were added to the Erlenmeyer flask, and the solution was shaken overnight at 37 °C. When the bacteria grew near saturation, it was taken out and packed into two centrifuge tubes. One of the tubes was centrifuged (6000 rpm/min) for 10 min, and the medium in it was discarded. The other tube was first re-suspended by adding 20 mL of triple distilled water and then centrifuged (6000 rpm/min) for 10 min; the water was discarded. The cells were resuspended by adding a self-made solvent, centrifuged (4 °C, 10,000 rpm/min) for 10 min. Then, the supernatant was pipetted and added with 15 mL of isopropanol. After being mixed, the supernatant was allowed to stand for 15 min. Then, it was centrifuged (4 °C, 10,000 rpm/min) for 10 min. The supernatant was discarded. 600 μL of Tris-Ethylenediaminetetraacetic (TE) acid buffer and 10 μL of RNaseA were added and incubated at 37 °C for 1 h. The solution was transferred to a new EP tube and centrifuged (12,000 rpm) for 5 min. Then, the supernatant was discarded. The tube was washed with 300 μL of 70% ethanol. Next, the washing solution was discarded, and the tube was blown to dry. 400 μL of TE buffer was added. The aqueous phase was extracted. 40 μL of 3 M sodium acetate and 400 μL of isopropanol were added and allowed to stand for 20 min. The tube was centrifuged (12,000 rpm) for 5 min and washed with 70% ethanol. Next, the washing solution was discarded, and the tube was blown to dry. Then, 300 μL of TE acid buffer was added and assayed on a NanoDrop 2000 ultra-micro spectrophotometer (Thermo Fisher Scientific, USA).
Plasmid stable transfection: H358 cells in the middle of logarithmic growth were taken and added to DMEM medium for the preparation of a cell suspension of 1.5 × 105/mL. The cells were added to a 96-hole plate at 10 μL/well and allowed to stand overnight. The old medium was replaced with fresh neomycin solution at concentrations of 0, 200, 400, 600, 800, 1000 μg/mL, and replaced every 3 days thereafter.
Plasmid transfection: Cells were seeded in a hole of a 6-hole plate at a cell density of 105. Then, 1.5 mL of DMEM complete medium was added. After 24 h, the medium was replaced with Opti-MEM serum-free medium. Afterward, 2 μg of DNA was diluted with 250 μL of serum-free medium, and 5 μL of Lip-2000 was diluted in 250 μL of serum-free medium. They were incubated for 5 min at room temperature, mixed, and incubated for 20 min at room temperature. The cells were added with 500 μL of the mixed solution, mixed, and placed in an incubator for 48 h.
Neomycin-stabilized cell strain: The drug was added to each hole according to the optimal screening drug concentration of the cells, and the drug-containing medium was replaced every 3 days. After the cells that had not been transfected with the plasmid were completely dead, the medium was replaced with a medium containing no neomycin. Once the cell growth reached a 60% confluence rate, a medium containing neomycin was added. In addition, once the cell growth reached a 90% confluence rate, the cells were transferred to a culture flask for cultivation. The screening concentration of the medium was changed every 4 days, and after 3 weeks of transfection, the samples were collected. Western Blot was used to detect the silenced and high expressions of MIF protein.
2.7. Cell proliferation before and after RNA interference
Cell culture: Human NSCLC cell strains H358 and H524 were cultured in the DMEM high-glucose medium at 37 °C under 5% CO2 and stably passaged for 3 times.
Cell passage: Once the cells reached 80% of confluence, the medium was pipetted and transferred to a centrifuge tube, washed with a sterile PBS balance solution, and the washing solution was also transferred to a centrifuge tube. The appropriate amount of membrane protease (Shanghai Zhenyu Biotechnology, China) was added to digest the cells for 2 min. Then, the medium was added to terminate the digestion after 2 min. The cells were mixed evenly and centrifuged (1000 rpm) for 5 min. The supernatant was discarded. The cells were added with the appropriate amount of complete medium and mixed by pipetting.
Cell count: After mixing 100 mL of cell suspension with 100 mL of Trypan Blue (Shanghai Hengfei Biotechnology, China), 10 mL of the solution was added to the counting plate and allowed to stand for 1 min. Cells were plated on 96-hole plates for the detection and count of cells. Respective on the 1st, 3rd, 5th, and 7th day after transfection, 10 μL of CCK-8 solution was added to each hole, and the mixture was incubated at 37 °C for 2 h. A 450 nm spectrometry (Benson (Tianjin) Health Technology, China) was used to measure the absorbance value. The absorbance was directly proportional to the cell concentration. A was used to express the absorbance, and its calculation equation is A = abc, where a is the absorbance coefficient, unit: L/(g·cm), b is the distance of light passing through the sample (usually the thickness of the cuvette), unit: cm, and c is the solution concentration, unit: g/L. The cell concentration was calculated and the cell number was calculated.
2.8. Statistical method
The SPSS 26.0 statistical software package was used for data processing and analysis. The quantitative data in normal distribution were expressed with mean ± standard deviation. Intra-group comparisons were tested by independent sample t or the rank-sum. Inter-group comparisons were tested by Chi-square test.
3. Results
3.1. Expression of MIF in human NSCLC cell strains
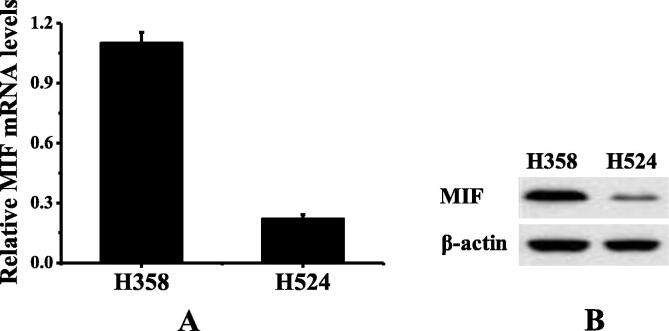
The detection results of MIF expression in human NSCLC cell strains were shown in Fig. 1. Fig. 1A showed the RT-PCR results, and Fig. 1B showed the Western Blot results. The H358 cell strains showed MIF high expression while the H524 cell strains showed the MIF low expression.
Fig. 1.
The detection results of MIF expression in human NSCLC cell strains (A showed the RT-PCR results; B showed the Western Blot results).
3.2. Expression of MIF in human NSCLC cell strains after RNA interference
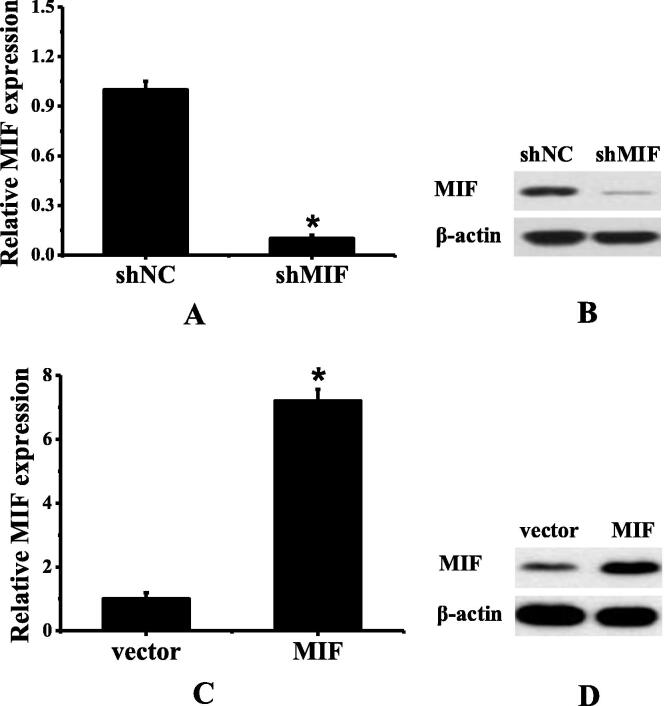
The results of Western Blot were shown in Fig. 2. Once the H358 cells were constructed as silent MIF expression (shMIF), compared with the original H358 cells (shNC), the difference was statistically significant (*P < 0.01). Once the H524 cells were constructed as high MIF expression (MIF), compared with original H524 cells, the difference was statistically significant (*P < 0.01).
Fig. 2.
Expression of MIF in human NSCLC cell strains before and after transfection (A showed the expression levels of MIF in H358 cell strains before and after transfection; B showed the Western Blot results of H358 cell strains before and after transfection; C showed the expression levels of MIF in H524 cell strains before and after transfection; D showed the Western Blot results of H524 cell strains before and after transfection).
3.3. Proliferation of human NSCLC cell strains
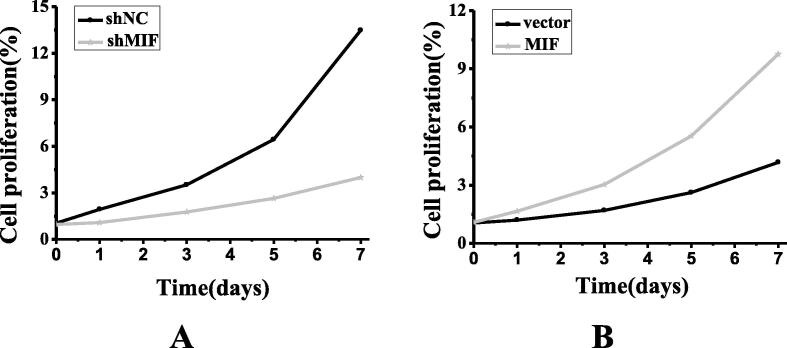
The results were shown in Fig. 3. Being cultured for 1 day, compared with the non-transfected H358 and H524 cell strains, the proliferation of transfected human NSCLC cell strains was not statistically significant (P > 0.05). Being cultured for respective 3, 5, and 7 days, the transfected H358 cells showed a significant decrease in proliferative activity compared with the non-transfected cells (shNC), and the difference was statistically significant (*P < 0.01). In addition, the transfected H524 cells (MIF) showed a significant increase in proliferative activity compared with the non-transfected cells (vector), and the difference was statistically significant (*P < 0.01).
Fig. 3.
The proliferation of human NSCLC cell strains (A showed the proliferation comparison of H358 cell strains before and after transfection; B showed the proliferation comparison of H524 cell strains before and after transfection).
4. Discussion
The recurrence and metastasis of NSCLC is an important factor affecting the survival of patients. The internal structure of the tumor is very complex, and the tumor microenvironment in which the tumor cells are located plays an important role in the development of the disease (Kim et al., 2017, Yamaguchi et al., 2020, Lalitha et al., 2020). Studies have found that MIF has important links with tumor microenvironment and tumor development (Zhang et al., 2020, Qiao et al., 2018, Wang et al., 2017). In order to investigate the expression of macrophage MIF in NSCLC, the human NSCLC cell strains H358 and H524 were selected as research objects. The RT-PCR and Western Blot were utilized to detect the expression levels of MIF in human NSCLC cell strains. The results showed high MIF expression in H358 cell strains and low MIF expression in H524 cell strains. The results are consistent with the previous findings that MIF in the lung tissues of patients with lung adenocarcinoma is highly expressed, which has reached the experimental expectations. The RNA transfection technology was utilized to compare the MIF expression levels and cell proliferation before and after the transfection. Once the H358 cells were constructed as silent MIF expression, compared with the original H358 cells, the difference was statistically significant. Once the H524 cells were constructed as high MIF expression, compared with original H524 cells, the difference was statistically significant. Being cultured for respective 3, 5, and 7 days, the transfected H358 cells showed a significant decrease in proliferative activity compared with original H358 cells, while the transfected H524 cells showed a significant increase in proliferative activity compared with original H524 cells. Therefore, MIF could enhance the proliferative activity of NSCLC tumor cells. Wang et al. (2017) reported that MIF was highly expressed in lung tissues of patients with lung adenocarcinoma. This is consistent with the results of this study, suggesting that MIF plays an important role in the pathogenesis of lung cancer (Wang et al., 2017).
In summary, MIF has high expression in H358 cells while low expression in H524 cells. The expression of MIF could enhance the proliferative activity of NSCLC tumor cells. The expression of macrophage MIF in NSCLC was explored and analyzed, which provided a reference for the research on NSCLC and the development of the disease. However, the research on MIF in this study is still in the initial stage. In the subsequent research, the related active mechanism of MIF will be explored in further, which will make the obtained results more valuable.
5. Conclusion
NSCLC cell lines H358 and H524 are used as research objects to detect the expression of MIF in human NSCLC cell lines. MIF mRNA is interfered by lentiviral plasmid, and the expression level of MIF and cell proliferation before and after transfection ire compared. It is found that MIF is highly expressed in H358 cells and low expressed in H524 cells, which can enhance the proliferation of NSCLC cells. This paper expounds the expression of MIF in NSCLC cells, which has important reference value and application significance, but there are also some deficiencies in the research process. For example, the small amount of sample collection leads to a certain degree of deviation of the results. Therefore, in the later research process, the data capacity will be further increased, making the results more valuable.
Footnotes
Peer review under responsibility of King Saud University.
References
- Antonia S.J., Villegas A., Daniel D. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 2017;377(20):1919. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- Heidari Z., Mahmoudzadeh-Sagheb H., Hashemi M. Association of macrophage migration inhibitory factor gene polymorphisms with chronic periodontitis in a South Eastern Iranian population. Dental Res. J. 2017;14(6):395–402. doi: 10.4103/1735-3327.218563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez-Vea M., Zuazo M., Gato M. Myeloid-derived suppressor cells in the tumor microenvironment: current knowledge and future perspectives. Archivum Immunologiae Et Therapiae Experimentalis. 2017;66(2):1–11. doi: 10.1007/s00005-017-0492-4. [DOI] [PubMed] [Google Scholar]
- Jamalhanjani M., Wilson G.A., Mcgranahan N. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med. 2017;376(22):2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Lee J., Bae S.J. NADPH oxidase 4 is required for the generation of macrophage migration inhibitory factor and host defense against Toxoplasma gondii infection. Sci. Rep. 2017;7(1):6361. doi: 10.1038/s41598-017-06610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalitha L.J., Sales T.J., Clarance P.P. In-vitro phytopharmacological and anticancer activity of Loranthus Longiflorus Desv. Var. Falcatuskurz against the human lung cancer cells. J. King Saud Univ. Sci. 2020;32(1):1246–1253. [Google Scholar]
- Luedike P., Alatzides G., Papathanasiou M. Predictive potential of macrophage migration inhibitory factor (MIF) in patients with heart failure with preserved ejection fraction (HFpEF) Eur. J. Med. Res. 2018;23(1):22. doi: 10.1186/s40001-018-0321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantouris Georgios, Bucala Richard, Lolis Elias J. Structural plasticity in the C-terminal region of macrophage migration inhibitory factor-2 is associated with an induced fit mechanism for a selective inhibitor. Biochemistry. 2018;57(26):3599–3605. doi: 10.1021/acs.biochem.8b00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L., Wang X.Y., Thapa S. Macrophage migration inhibitory factor promoter polymorphisms (−794 CATT5–8): relationship with soluble MIF levels in coronary atherosclerotic disease subjects. BMC Cardiovasc. Disord. 2017;17(1):144. doi: 10.1186/s12872-017-0570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C., Li S., Lu H. Laminar flow attenuates macrophage migration inhibitory factor expression in endothelial cells. Sci. Rep. 2018;8(1):2360. doi: 10.1038/s41598-018-20885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li Y., Chen Y. Urinary macrophage migration inhibitory factor as a noninvasive biomarker in pediatric Henoch-Schönlein purpura nephritis. J. Clin. Rheumatol. 2017;23(5):258. doi: 10.1097/RHU.0000000000000570. [DOI] [PubMed] [Google Scholar]
- Wang M., Zhao J., Zhang L. Role of tumor microenvironment in tumorigenesis. J. Cancer. 2017;8(5):761–773. doi: 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Pan Y., Xu Q. Porphyromonas gingivalis ATCC 33277 promotes intercellular adhesion molecule-1 expression in endothelial cells and monocyte-endothelial cell adhesion through macrophage migration inhibitory factor. BMC Microbiol. 2018;18(1):16. doi: 10.1186/s12866-018-1156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi F., Minakata T., Miura S., Suzuki T., Sagara H. Heterogeneity of latent tuberculosis infection in a patient with lung cancer. J. Infect. Public Health. 2020;13(1):151–153. doi: 10.1016/j.jiph.2019.07.009. [DOI] [PubMed] [Google Scholar]
- Yamaguchi F., Minakata T., Miura S. Heterogeneity of latent tuberculosis infection in a patient with lung cancer. J. Infect. Public Health. 2020;13(1):151–153. doi: 10.1016/j.jiph.2019.07.009. [DOI] [PubMed] [Google Scholar]
- Zhang C., Guo H., Shen M. Comparison of clinical effectiveness of nurse led care among Chinese patients with cancer: a prospective study evaluating effective patient care compared to consultant oncologist. J. Infect. Public Health. 2020;13(2):159–163. doi: 10.1016/j.jiph.2019.07.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kurupati R., Liu L. Enhancing CD8+ T cell fatty acid catabolism within a metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy. Cancer Cell. 2017;32(3):377–391. doi: 10.1016/j.ccell.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]