Abstract
The world is witnessing tumultuous times as major economic powers including the US, UK, Russia, India, and most of Europe continue to be in a state of lockdown. The worst-hit sectors due to this lockdown are sales, production (manufacturing), transport (aerospace and automotive) and tourism. Lockdowns became necessary as a preventive measure to avoid the spread of the contagious and infectious “Coronavirus Disease 2019” (COVID-19). This newly identified disease is caused by a new strain of the virus being referred to as Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS CoV-2; formerly called 2019-nCoV). We review the current medical and manufacturing response to COVID-19, including advances in instrumentation, sensing, use of lasers, fumigation chambers and development of novel tools such as lab-on-the-chip using combinatorial additive and subtractive manufacturing techniques and use of molecular modelling and molecular docking in drug and vaccine discovery. We also offer perspectives on future considerations on climate change, outsourced versus indigenous manufacturing, automation, and antimicrobial resistance. Overall, this paper attempts to identify key areas where manufacturing can be employed to address societal challenges such as COVID-19.
Keywords: SARS CoV-2, Manufacturing, Sensing, Climate change, Nanotechnology, PPE
Graphical abstract
Highlights
-
•
We review the coronavirus pandemic and discuss salient aspects of the disease, its impact, propagation and treatment options.
-
•
Nanotechnology and ultra-precision engineering hold the promise in developing resilient measures to tackle the pandemic.
-
•
Digital manufacturing and automation can help productivity and modelling and simulation can aid drug and vaccine discovery.
-
•
We explore design for manufacturing approaches required for developing new face masks and PPE's.
-
•
We discuss the short- and long-term impact of COVID-19 on supply chains, climate change and lifestyles.
1. Introduction
Coronaviruses (CoVs) belong to the family Coronaviridae which includes four genera: α, β, γ and δ as well as several subgenera and species [1]. SARS CoV-2 is a β-coronavirus with a single-stranded RNA genome of ~30 kb [2]. Recent topical research has revealed several new CoVs (three α-coronaviruses, three new β-coronaviruses, and one previously described α-coronavirus) from bats captured from Myanmar and future emergence of new diseases caused by these CoVs due to change of land use has been speculated [3]. Furthermore, newer mutations of the virus that originally spread from Wuhan were confirmed as deadlier in some countries compared to others, which has led to added confusion and concern [4].
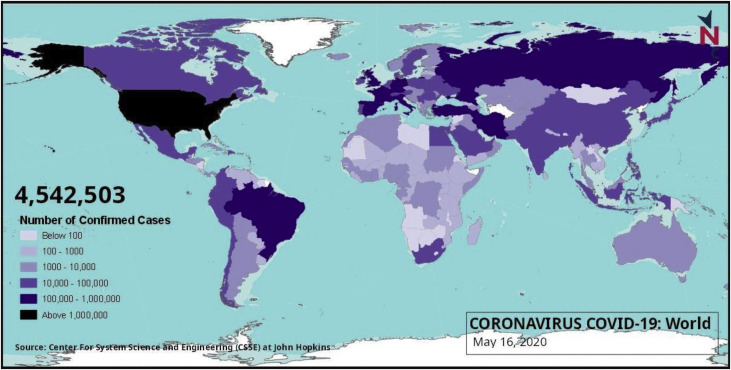
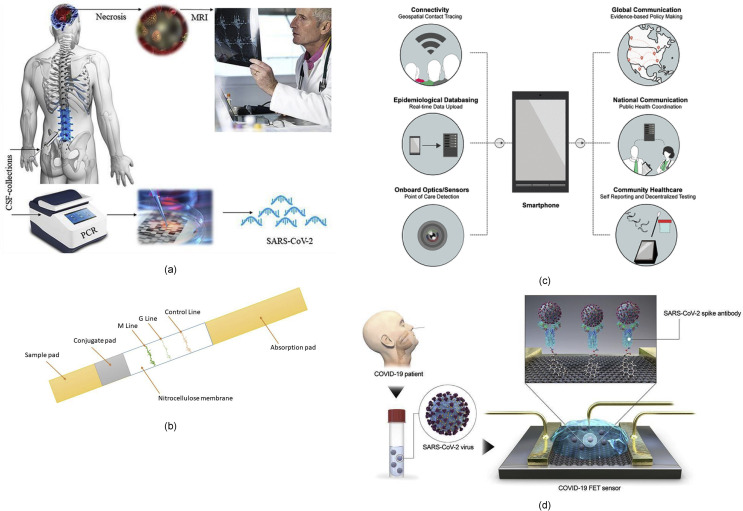
SARS CoV-2 was first identified from the outbreak of respiratory illness cases in Wuhan City in the Hubei Province of China. Initial reports of the virus were made to the World Health Organisation (WHO) on December 31, 2019. This was followed by the WHO declaring COVID-19 as a global health emergency on January 30, 2020 due to rapid spreading, and a later pandemic declaration on March 11, 2020. The disease has quickly engulfed most of the world and has caused severe infection to populations across numerous countries as shown in Fig. 1 .
Fig. 1.
Reported number of COVID-19 infected persons shown on the world map as on May 16, 2020 (Source: newsclick.in).
2. COVID-19: disease, fatality, propagation and treatment
2.1. The COVID-19 disease
COVID-19 is shorthand for “Coronavirus Disease 2019" which is caused by the SARS CoV-2 virus, also called coronavirus in typical usage. Viruses cause disease by binding to receptors on cells in the human body and then replicating at a rapid rate which triggers a variety of pathogenic processes. In the case of COVID-19, the structures on the surface of the virus bind to receptors in the airway or the lungs of human beings. The lungs may become inflamed, making breathing more difficult. For some people, the infection becomes severe and leads to critical care requirements.
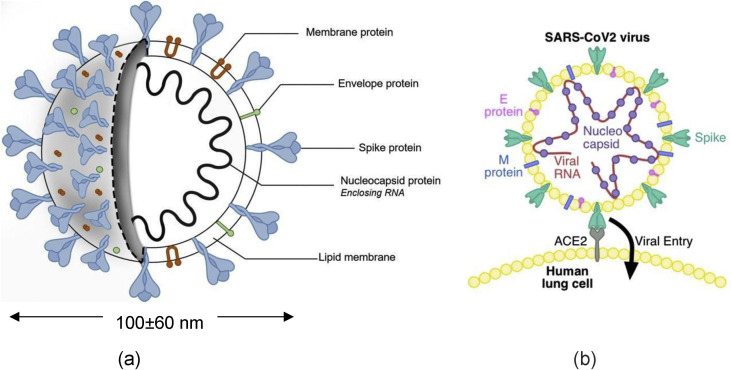
The anatomy of SARS CoV-2, including its internal biological structure of spike protein legs, envelope protein, and membrane protein, surrounding the genomic RNA is shown in Fig. 2 (a). The virus shown in Fig. 2(a) highlights a large diameter of about 0.1 μm with variations in sizes reported by different researchers. The spike-shaped protein legs that make up a portion of the outer capsule of the virus create the crown-like or corona-like appearance. Thus, the name coronavirus.
Fig. 2.
(a) Anatomy of SARS CoV-2 (courtesy biorender.com) and (b) likely mechanism of infection [5] showing Envelope (E) Protein, Membrane (M) Protein, Spike (S) Protein, Nucleocapsid (N) Protein (blue dots) and genomic RNA shown by red chain (reprinted with permission).
Loeffelholz et al. [1] described the biological structure of the virus by stating.
“Coronaviruses are enveloped viruses containing a single strand of positive-sense RNA. Virions are mostly spherical, with pronounced spiked glycoprotein (S) embedded in the envelope. Additional structural proteins include envelope (E), matrix (M), and nucleocapsid (N). Intra- and inter-species transmission of CoVs and genetic recombination events contribute to the emergence of new CoV strains”.
The mechanism of infection due to the surface interactions between the spike protein and the lung cells is depicted in Fig. 2(b). This understanding was gathered from the genetic analysis which revealed that the mutations located in the spike surface glycoprotein might induce conformal changes and play an essential role in binding to receptors on the host cell (lungs) and determine host tropisms by leading to possible changing antigenicity [7].
Viruses are fundamentally different to bacteria so the classically developed work on nano-structuring and biomimicking surfaces, such as the cicada wing, primarily targeting bacterial killing, should not be confused as a readily available knowledge to kill SARS CoV-2. Physically, a bacterium is larger than a virus (the biggest size of a virus is smaller than the smallest known bacteria). Bacteria are living cells which are capable of prolific reproduction independently, whereas viruses are non-living particles, requiring a host cell for replication. Also, bacteria possess a cell-wall engulfing a chromosome, whereas viruses consist of genetic material, either DNA or RNA, covered by a protein coating. Bacterial infections can therefore be treated with high success using antibiotic drugs. Although some antiviral medicines are now available, the susceptibility of viruses to react to the treatment rate of medicines is significantly reduced as they are non-living. Hence, the only long-term option to eradicate viruses is the development of vaccines that stimulate the natural production of antibodies.
Studies suggest that the source of SARS CoV-2 could either be RaTG13 from horseshoe bats (Rhinolophus affinis), pangolins (Manis javanica), or a mix of both. Their transmission has occurred by zoonotic mechanisms [6,7]. However, the coronavirus isolated from pangolins is 99% similar in a specific region of the Spike protein, which corresponds to the 74 amino acids involved in the Angiotensin- Converting Enzyme 2 (ACE 2) receptor binding domain, which allows the virus to enter human cells to infect them as shown in Fig. 2(b). The virus RaTG13 isolated from Rhinolophus Affinis bats is highly divergent in this specific region (only 77% similarity). This observation indicates that the coronavirus isolated from pangolins can enter human cells, whereas coronavirus isolated from Rhinolophus affinis bats is unable to enter human cells.
2.2. Fatality of COVID-19
The SARS CoV-2 has a high transmissible efficiency and COVID-19 has high morbidity and mortality. A popular but possibly flawed measure for assessing fatality of disease is the use of deaths/case counts. This measure would yield a fatality rate of 6.13% for COVID-19 as of May 17, 2020 [8]. The problem with this measure is that case counts reflect the number of tests that were done rather than infections, and the deaths lag the cases because fatality (if observed) may happen several days after the case is identified. A lag in reporting case numbers and incorrect tests may also occur. An alternative measure is the Case Fatality Rate (CFR), which is the ratio of deaths/(deaths + recovered cases). This measure would yield a fatality rate of 12.3% [9].
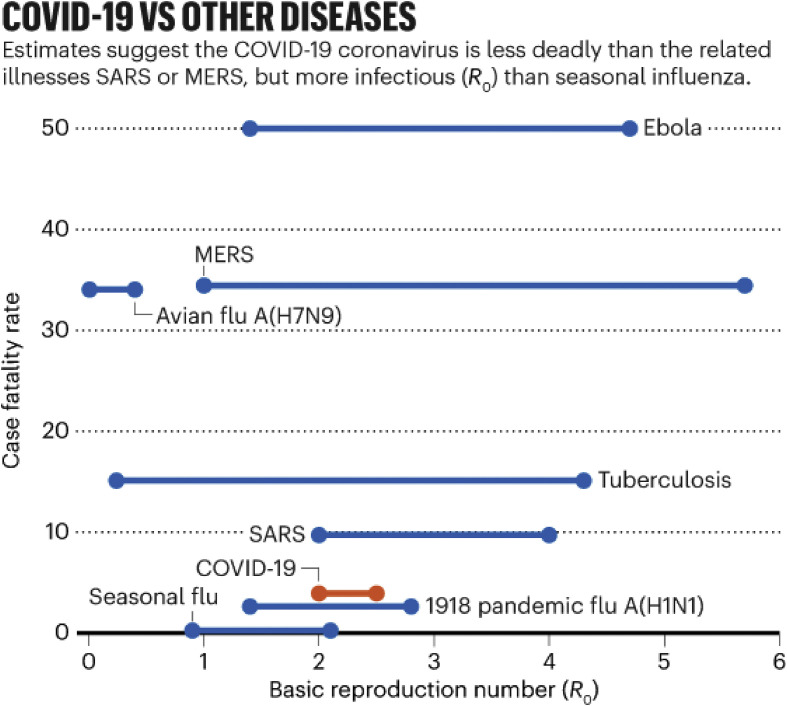
The consensus is that the COVID-19 disease has high fatality and can exceed the fatality ratio of the century-old “Spanish flu”, which had a 10% CFR [11]. However, the data analysis of Callaway et al. [10] shown in Fig. 3 suggests that COVID-19's CFR is lower than that of MERS and Ebola and that its infection rate (R0-the expected number of cases directly generated by one case in a population where all individuals are susceptible to infection) suggests that the infection can spread more easily than other diseases, including seasonal influenza.
Fig. 3.
Data showing SARS CoV-2 is less deadly than other pathogens such as SARS CoV-1, Ebola etc. Reprinted with permission from Ref. [10].
A further comparison of three different episodes of epidemics and pandemics caused by the family of coronavirus, namely, SARS CoV-1, MERS and SARS CoV-2, is depicted in Table 1 .
Table 1.
| Disease type | Year | Fatality ratio | Total deaths reported | Total affected countries |
|---|---|---|---|---|
| Severe acute respiratory syndrome (SARS CoV-1) | 2003 | 14–15% | 813 | 32 |
| Middle East respiratory syndrome (MERS) | 2012 | 34.3% | 858 | 27 |
| Severe acute respiratory syndrome 2 (SARS CoV-2) | 2019 | 12.3% ↑ | 362,081↑ | 213 ↑ |
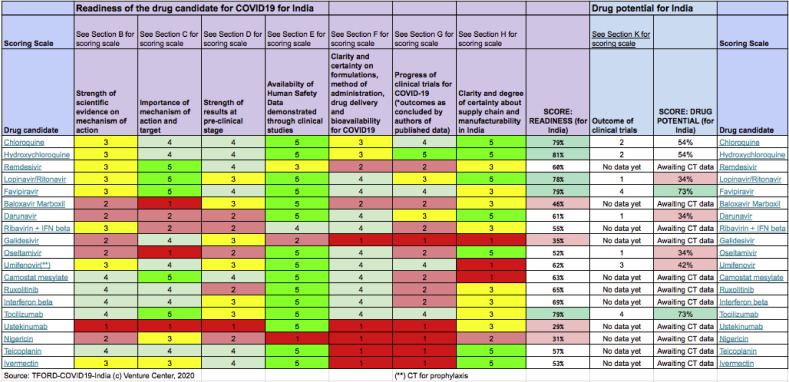
As an agile response to the pandemic, a few drugs are gaining popularity. Recently, the Indian Government task force for the repurposing of drugs (TFORD) has proposed that Favipiravir and Tocilizumab are the most promising drugs to treat COVID-19. A rigorous survey of about 70 drugs showed that the originally thought game changing HCQ scored only 54% at the readiness level in comparison to Favipiravir (73%) and Tocilizumab (73%) drugs. A ready list of this comparison is shown in Table 2 [12]. Also, trials are underway to test the efficacy of natural compounds such as the Ashwagandha (Withania somnifera) and Propolis to cure COVID-19 (Footnote: https://doi.org/10.1080/07391102.2020.1772108).
Table 2.
Readiness level of drugs against COVID 19 compared by TFORD [12].
The exact mechanism of these drugs towards the virus is yet to be completely understood. However, the mechanism where a drug affects the replication of viruses in-vivo is a broadly accepted concept. In general, viruses replicate via protein processing using endosomes within Golgi bodies. A drug such as HCQ increases the pH of the Golgi bodies making them more basic, thus disrupting the integrity of the internal Nucleocapsid protein (see Fig. 2(a)). This denatures the protein of the Coronavirus rendering it dysfunctional. Therefore, the rationale for using HCQ is built on the premise that the change in pH brought about by the drug inhibits the endosomal transport necessary to spread the infection, hence the patient recovers.
Another short-term treatment being implemented by some hospitals across the world is to infuse patients with the antibody-rich blood plasma of people who have recovered from COVID-19 [13]. This approach has been used during disease outbreaks for over a century. However, the approach carries significant risk in terms of the already immunocompromised patients’ immune response after administration, and results may vary between patients.
2.3. Propagation of COVID-19
As an early effort of investigation of the problem from scratch, researchers at Toho University in Japan used high-sensitivity cameras and laser beam guidance experiments to deduce that saliva spray during a sneeze (potentially containing thousands of viruses) can be classified into large vs small droplets or droplets vs aerosols. The droplets, due to their heavier weight, fall off, whereas aerosols remain airborne for a few hours due to their relatively small size. Prima facie evidence suggests that the coronavirus has escalated to a pandemic due to the high contagion occurring via this ‘airborne’ spread model. Recently, the possibility of asymptomatic or oligosymptomatic infection has also been highlighted [14].
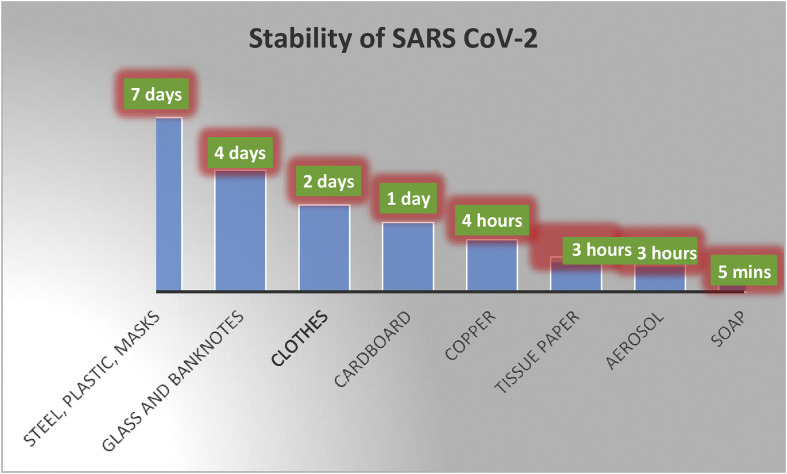
The aerosols may circulate near an infected patient in an airborne condition depending on the local conditions (airflow rate, humidity, dryness) for up to a few hours, even after the infected person has left the location. Hence, the chances of contracting coronavirus are relatively high by merely occupying the same vicinity where an infected person has been or passed through. Researchers have experimentally evaluated the stability of SARS CoV-2 recently, and these comparisons are shown in Fig. 4 [15,16].
Fig. 4.
Chart comparing the stability of SARS CoV-2 on various media. Reprinted with permission from Ref. [15,16].
It is noteworthy that SARS CoV-2 was stable in the aerosol (airborne) form for up to 3 h. As opposed to this, the virus appears to be stable on surgical masks or stainless-steel surfaces for up to 7 days and for up to 4 days on smooth surfaces such as glass or currency notes. The same research also shows that the application of hand soap does not immediately deactivate the virus but rather takes up to 5 min. Therefore, a 5-min waiting period after a handwash is required before bringing hands in contact with face, mouth or nose.
SARS CoV-2 is not only present in the airway secretions of infected patients when they sneeze, but also when they simply breathe out or speak. Studies have shown that SARS CoV-2 is also present in other body fluids of infected people, such as faeces, blood [17], oral fluids, anal secretions [18], tears [19], and urine [20]. The virus primarily attacks the respiratory system, however, new evidence shows the virus is not filtered by the kidneys, as traces of virus can be seen in sewage systems [8].
Therefore, as a mitigation strategy, testing of these fluids, which can be available and abundant in the wider population can be done for identifying the most effective areas.
Overall, when people are in close contact with one another, then transmission is more likely. Most sources for infection worldwide have happened in an enclosed space, including homes, offices, public transport and restaurants. A second spreading pathway for the virus is through touching a surface or object that has the virus on it and then touching one's own mouth, nose, or eyes. If one is in a well-ventilated space with fewer people, even for a longer period, the risk of infection is low. Sustained contact with an infected person, even for a short period, even without a cough or sneeze, can spread the infection.
2.4. Attack mechanism of SARS CoV-2 and immunity of the body
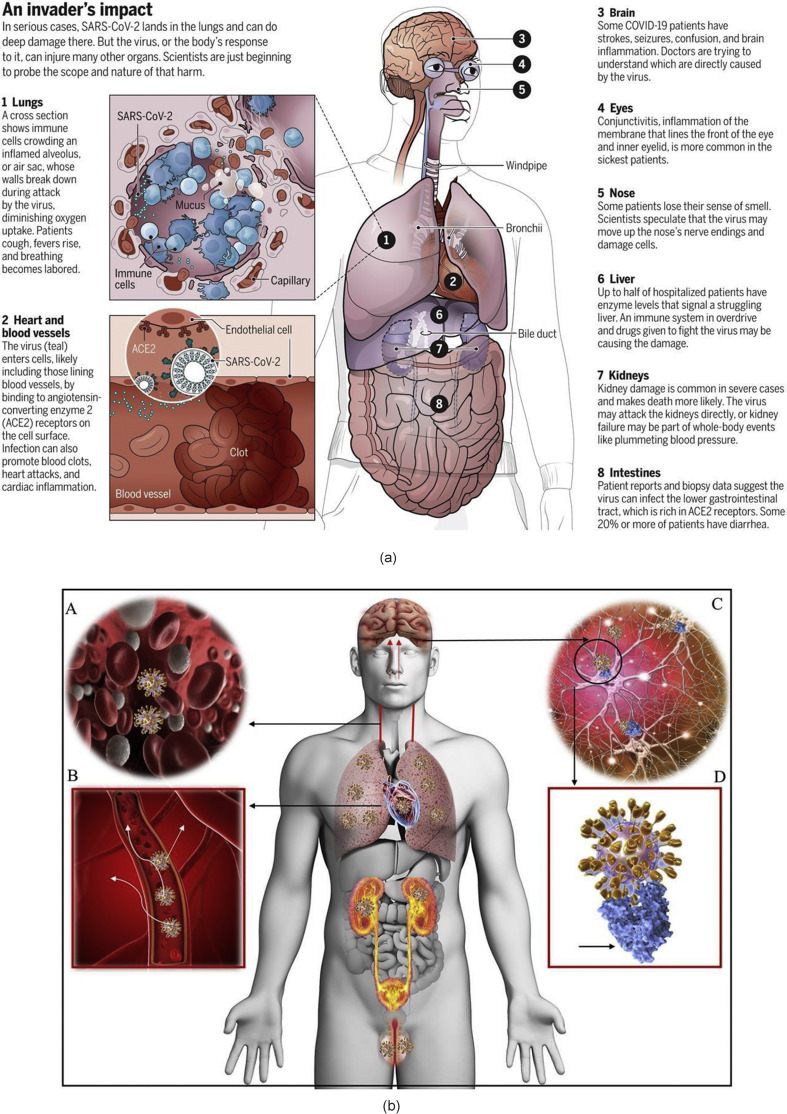
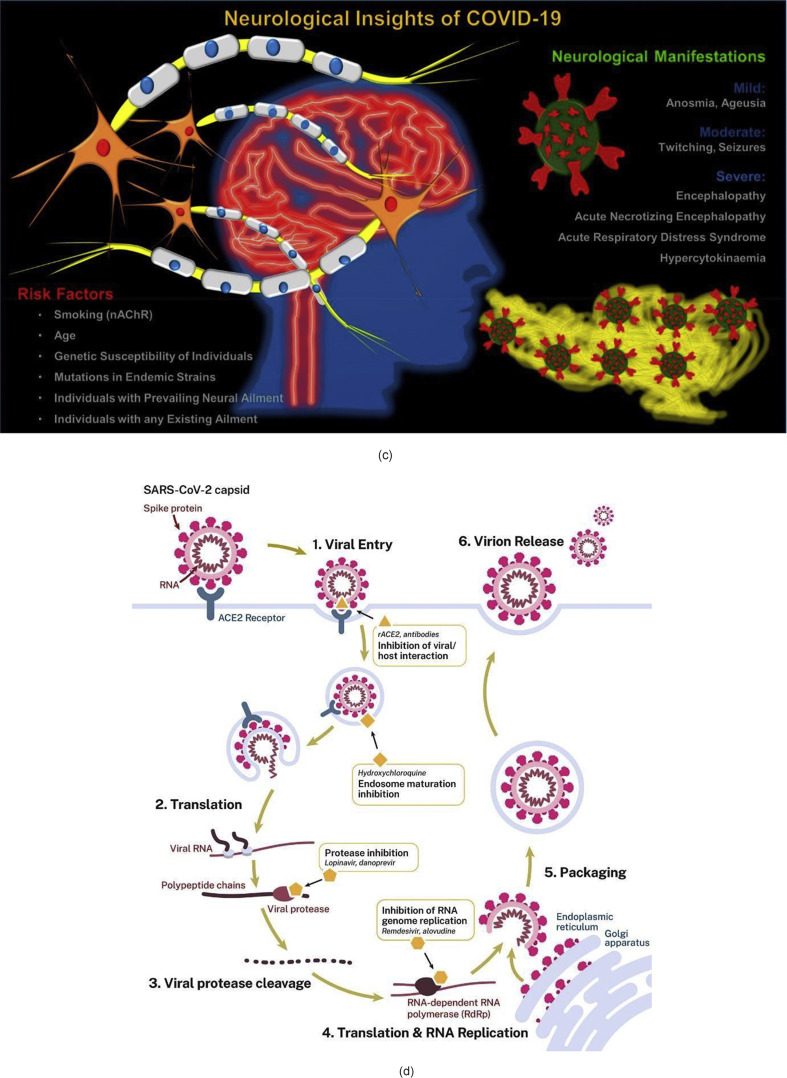
By the virtue of disturbing the cell programming, SARS CoV-2 possesses the capability to cause a surprise attack on almost any part of the body ranging from lungs, heart, blood vessels, brain, eyes, nose, liver, kidneys and intestine. A schematic representation of this adverse impact is shown in Fig. 5 (a) [21]. Recent literature suggests that apart from the airway, the human brain may also be targeted by the virus - see Fig. 5, Fig. 6 [22]. Patients have reported having mild (anosmia and ageusia) to severe (encephalopathy) neurological manifestations, and if true, it makes SARS CoV-2 more lethal.
Fig. 5.
(a) Various damage sites in the body reported to be caused by COVID-19 [21]. (b) Tissue distribution of ACE2 receptors in humans [23] (c) Possible neuro disorders due to SARS CoV-2 [22] and (d) Life cycle of SARS CoV-2 in host cells [24]. (figures reprinted with permission).
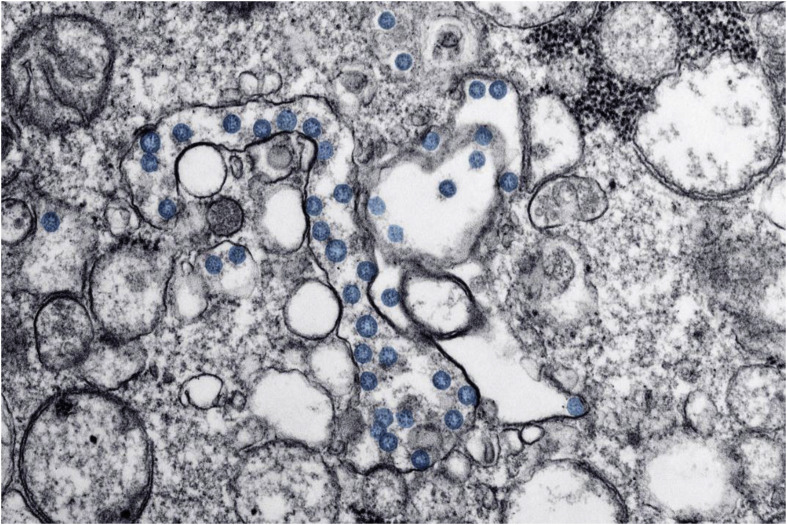
Fig. 6.
TEM image showing the presence of spherical coronavirus particles. Reprinted with permission from Ref. [26].
Our nasal lining tissue contains a rich number of cell receptors called angiotensin-converting enzyme 2 (ACE2), which are favourable sites for the SARS CoV-2 to attach its spiked protein to, thus paving way for the entrance of the virus inside the body. Once attached to the cell, SARS CoV-2 can change cell programming, replicate itself, and attack new cells at a very rapid pace. Within a week of entrance into the body, the virus exhibits effects. During this time, the person may show early symptoms such as fever, dry cough, sore throat, loss of smell and taste, or head and body aches. At this point, the failure of the immunity in defeating the virus means giving the virus a way forward to enter into the respiratory system, where the lungs are attacked. The lungs also have a cell lining rich in ACE2 [22].
As the immune system continues to fight the SARS CoV-2, the supply of healthy oxygen is disrupted. As the disease progresses inside the body, the white blood cells release inflammatory molecules or chemokines, which attack and kill the virus-infected immune cells, leaving the dead cells and pus behind. This affects healthy oxygen transfer in the lungs and is the stage where the patient starts to complain of pneumonia, coughing, fever, and rapid, shallow respiration. Acute respiratory distress syndrome may develop. At this stage, the lungs start to be filled with opaque black spots signifying closure of the air pores, and such patients require ventilator support. The survivors of this severity of infection can end up having long-term complications. A few hospitals have successfully applied artificial intelligence to monitor the recovery rate by monitoring the Coronascore – a general term used to quantify the extent of the blocked pores (in cm3) [25].
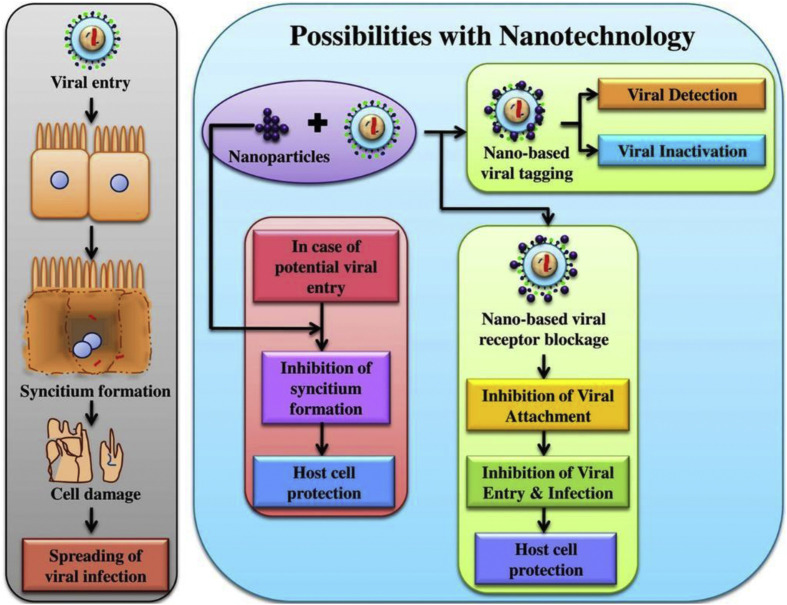
3. Proposed solutions from a manufacturing perspective
Long-term research measures, advanced manufacturing and metrology have pivotal roles to play in containing a pandemic. Specifically, the development of engineering innovations is timely for the control of the COVID-19 pandemic. For diagnostic testing to be useful for informing doctors and governments about the true incidence at any one time of coronavirus in the population, it needs to be inexpensive, high in sensitivity and specificity (sensitivity refers to the ability of a test to correctly identify those with the disease or true positive rate, whereas specificity refers to the ability of a test to correctly identify those without the disease or true negative rate) and easy to use for non-experts. As doctors are treating infected patients in this emergent time in an unprepared state, emergent manufacturing measures can help design better testing equipment and thus help in both short and long term to tackle this problem.
3.1. Testing and detection of coronavirus: sensors and instrumentation
3.1.1. Bulk testing using chest CT or RT-PCR tests
The most significant challenge now lies in probing early-stage symptoms of COVID-
19. For a relatively late stage detection in severe cases, chest Computer Tomography (CT) using X-ray probes (providing >90% sensitivity) has proved to be a more reliable test assay in comparison to the reverse-transcription polymerase-chain-reaction (RT-PCR) test and other sensor-based detection methods currently being pursued [27]. Since the nervous system and respiratory dysregulation are both likely to co-exist, COVID-19 testing should be done by combining brain MRI and RT- PCR tests as shown in Fig. 7 (a) [28]. However, caution is required in the analysis of results, such that COVID-19 should not be confused with drug-resistant tuberculosis (TB) as both would show symptoms of damages in the lungs. In an attempt to boost the testing in a populous country such as India, the Indian Council of Medical Research (ICMR) has approved the use of diagnostic machines used for testing drug-resistant tuberculosis under the guidance that the throat/nasal swabs are collected in the viral transport medium [29]. Interestingly, the PCR test cannot identify asymptomatic infections or those people who were exposed to or infected with COVID-19 in the past and did not suffer from the disease or have recovered from COVID-19 and may still be spreading the virus (carriers). Therefore, different tests may be required to collate vital information required by government bodies to carry out a risk-benefit analysis to relax lockdown measures to protect their economies.
Fig. 7.
(a) Need for more rigorous tests [28], (b) COVID-19 Detection Test cassette demonstrating the assay procedure (c) importance of IoT sensing [32] (d) Schematic diagram of COVID-19 field-effect transistor (FET) sensor operation procedure. Graphene as a sensing material is selected, and SARS-CoV-2 spike antibody is conjugated onto the graphene sheet via 1-pyrenebutyric acid N-hydroxysuccinimide ester [31] (figures reprinted with permission).
3.1.2. Lab-on-a-chip for individual tests
Immunodiagnostic kits such as a lab-on-a-chip are being developed for asymptomatic detection of COVID-19 [30]. A lab-on-chip (Fig. 7(b) works on the principle of detecting the presence of patient-generated antibodies against the virus that causes a specific disease, in this case, COVID-19. The test uses a lateral flow immunoassay to assess the presence of an analyte from a patient sample or specimen and detects two types of antibody isotypes: IgM and IgG. IgM antibodies are the first antibodies to appear in response to a novel antigen and imply a more recently initiated infection while IgG antibodies are generated later in the course of infection and possess a higher affinity for the target antigen, meaning they are more specifically able to bind the substance which caused the immune response. A test is declared positive if either one or both antibodies are detected during the test, similar to widely used pregnancy tests.
The test consists of an anti-human IgG coating in the G test line region and an IgM coating the M test line region (see Fig. 7(b). During testing, the sample (blood, urine etc) reacts with SARS-CoV-2 antigen-coated gold nanoparticles (AuNP) in the conjugation pad of the test cassette. Any antibody in the patient sample that recognises the SARS-CoV-2 antigen binds to the Antigen-AuNP complex. The mixture then migrates laterally across the membrane by capillary action/lateral flow. As these human antibody/antigen/AuNP complexes move across the test lines, they are captured at the anti-human IgM ‘M' Line, the anti-human IgG ‘G' Line, or both, depending on the antibody contents of the specimen. The sample first reaches the anti-human IgM antibodies which coat the M line. If the specimen contains IgM antibodies to SARS-CoV-2, a coloured line will appear in the M test line region. Next, the sample reaches the anti-human IgG antibodies which coat the G line. If a specimen contains IgG antibodies to SARS-CoV-2, the conjugate-specimen complex reacts with anti-human IgG. A coloured line appears in the G test line region as a result. The rabbit IgG-AuNP complexes are captured by the control line (which contains anti-rabbit-IgG). This visible line indicates that there has been successful lateral flow across the detection strip. The last check ensures that the sample had enough volume to move across the entirety of the test cassette. Only human antibody/SARS-CoV-2 Antigen/AuNP complexes will produce a visible red or pink line at the M or G line. Other antibodies produce no colour.
The accuracy of these lab-on-chip tests is still being debated as it depends on two key parameters, sensitivity and specificity. To date, IgG related sensitivity and specificity has been found to be higher than that of IgM.
A schematic diagram in Fig. 7(c) and (d) shows that unlike currently available diagnostic methods, field-effect transistor (FET)-based biosensing devices may have several advantages, including the ability to make highly sensitive and instantaneous measurements using small amounts of analytes [31]. The FET sensor shown in Fig. 7(d) makes use of a graphene sheet since graphene-based FET biosensors can detect surrounding changes on their surface and provide an optimal sensing environment for ultrasensitive and low-noise detection. From this standpoint, graphene-based FET technology is attractive for applications related to the sensitive immunological diagnosis.
3.2. Coronavirus sensitive fluorescent light emitting sensor embedded face masks
A group of researchers at MIT and Harvard are developing coronavirus-identifying sensor embedded face masks. This ongoing work is an extension to their previous work where they developed a low-cost method to detect a Zika Virus [33]. In this technique, the sensor is made by using genetic material such as the RNA or the DNA which binds to the viruses. The researchers used a lyophilizer to freeze-dry the genetic material onto the fabric of the mask. The material deposited onto the fabric remains stable for many months (at room temperature) and the detection process starts merely by the presence of moisture (such as saliva). The detection signal is small (in terms of voltage) and can be detected by an additional fluorimeter device that can quantify this signal and emit in form of a fluorescent light. Thus, one can expect to see light glowing masks to detect coronavirus in the future.
3.3. Fumigation chambers
Fumigation chambers allow quick disinfection of a person while visiting areas such as hospitals, universities or airports. The aim is to use tubes releasing hydrogen peroxide in a chamber designed to be five-feet wide and seven-feet tall. The fumigation chamber usually seals automatically after the person's entry to avoid any leakage outside of the chamber and has designated sensors facilitating the chamber entry. The disinfection lasts for 5 s. It is to be noted here that hydrogen peroxide has also been recommended by the Food and Drug Administration (FDA) to be used as a sterilisation material to decontaminate N95 or N95-equivalent respirators for reuse by health care workers in hospital settings [34].
3.4. Ultraviolet exposure-based inactivation of coronavirus
A populist view is that sunlight and high temperature during peak summers kills the coronavirus, which would help containment of its spread. It is reported that SARS CoV-2 does not survive beyond 5 min after being exposed to 70 °C [16]. However, it has yet to be ascertained as to how long does sunlight take to deactivate SARS CoV-2 and at what intensity. A more specific question is whether ultra-violet (UV) light can kill coronavirus? Sunlight usually contains three types of UV: UVA (320–400 nm), UVB (280–320 nm), and UVC (200–280 nm). UVA and UVB can both cause sunburn, however, UVC is shorter and a more energetic wavelength of light. While UVC is effective at destroying genetic material, whether in humans or viral particles, it is filtered by the Ozone layer and does not reach the earth's surface. Preliminary findings from the National Biodefense Analysis and Countermeasures Centre of the USA (which houses a BSL-4 level lab) indicate that “sunlight seems to be very detrimental to the virus … within minutes, the majority of the virus is inactivated on surfaces and in the air in direct sunlight.” [35].
Research on the use of UV as a treatment is still evolving and the behaviour of SARS CoV-2 under UV light is unknown. Results were relatively favourable for SARS CoV-1 treatment with UVC [36,37]. SARS CoV-1 was efficiently inactivated after 40 min of UVC exposure (at about a wavelength of 254 nm), whereas addition of psoralen (a light-sensitive drug) to UVA enhances inactivation of the SARS CoV-1. Popular press reports suggest that UV light booths are capable of deactivating coronavirus without any human contact and are proposed to be deployed (Fig. 8 ). One must be cautious, since UVC can be dangerous to the skin, causing burns within seconds and harmful to the eyes if observed directly. Therefore, risk assessment and associated precautions would need to be deployed that may render this strategy difficult in the short-term.
Fig. 8.
A passenger bus being disinfected by UVC in Shanghai, China. Reprinted with permission from Ref. [38].
3.5. Surface manufacturing (coatings)
3.5.1. Antimicrobial coatings
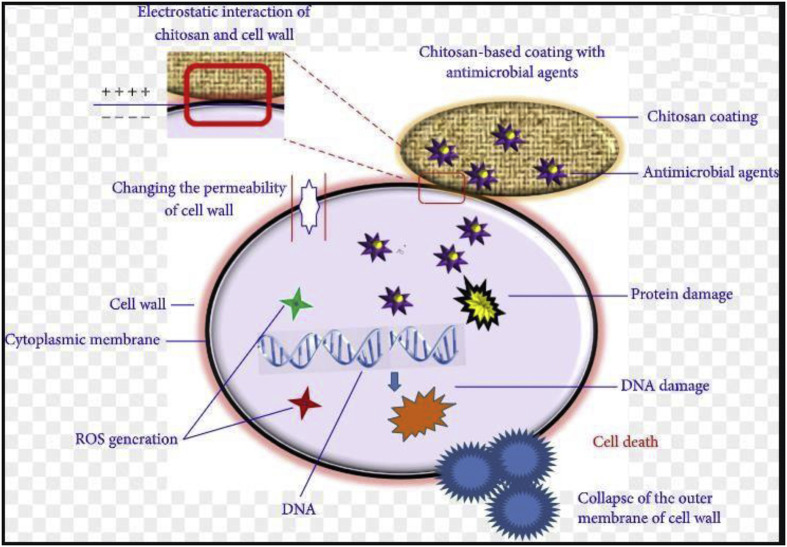
Chouirfa et al. [39] summarised nanomaterials based coatings for antibacterial applications and Xing et al. [40] have summarised the potential of natural polymer chitosan as an antimicrobial agent (Fig. 9 ). Their research on antimicrobial properties of a nanocomposite coating formed by polysaccharide 1-deoxylactit-1-yl chitosan (Chitlac) and silver nanoparticles (nAg) on methacrylate thermosets showed satisfactory results.
Fig. 9.
Antimicrobial mechanism of chitosan-based coating with antimicrobial agents. Reprinted with permission from Ref. [40].
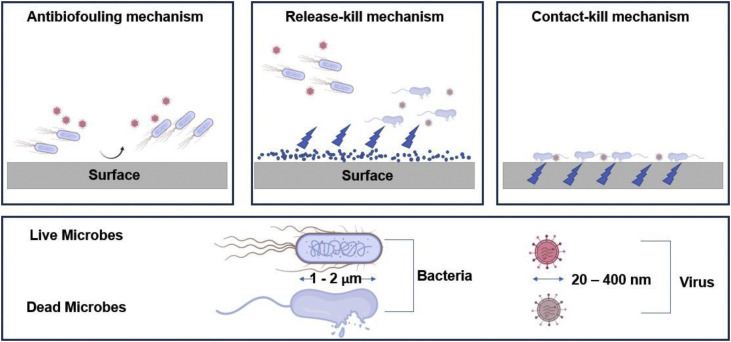
Antimicrobial surfaces are based on three main mechanisms (see Fig. 10 ): the anti-biofouling mechanism which repels microbes and prevents them from adhering to the surface, the release-killing mechanism where microbes are killed in the near-surface environment with a release of antimicrobial agents and the contact-killing mechanism where microbes adhere and are killed on the surface [41,42].
Fig. 10.
Mechanisms behind antimicrobial surfaces (courtesy biorender.com).
Researchers have also experimented with developing long-lasting antimicrobial surfaces to stop spreading pathogenic microbes through commonly touched surfaces or at-risk surfaces and have focused on the use of antimicrobial/antiviral materials such as copper. Experimental trials for copper oxide impregnated respiratory protective facemasks have yielded 100% efficiency in eradicating the infectivity of human and avian influenza A virus in simulated breathing conditions [43]. Warnes et al. [44] have shown that the surfaces of dry copper alloys are lethal to viruses such as MNV-1 at room temperature, with higher copper content being more time-efficient. Generally, the antimicrobial activity of copper is attributed to oxidative behaviour of copper and the solubility properties of copper oxides [45].
More recently, surface texturing of copper via cold spray methods has shown enhanced promise as an antiviral agent [46]. Combining the aforementioned antimicrobial contact-killing properties of copper, the anti-adhesion properties of polymeric micelles and the release-kill abilities of chlorine dioxide (ClO2), Li et al. [47] developed a multifunctional coating viricidal for influenza virus H1N1. This multifunctional coating is composed of ClO2 encapsulated in polymeric micelles (with slow on-demand release) on which copper nanoparticles were covalently tethered.
Other studies have investigated hydrophobic polycationic coatings as antimicrobial coatings [[48], [49], [50]]. Hsu et al. [51] investigated the mechanism by which the N,N- Dodecyl,methyl-Polyethelenimine (PEI) coated surfaces killed the influenza A virus. It was concluded that upon contact with the coated surface, the virus adheres irreversibly and through hydrophobic and electrostatic interactions, the virus’ disintegration is then initiated resulting in RNA leakage into the solution.
The incorporation of PEI into protective mask textiles has also been investigated with nearly a 1000-fold improvement in the capture of the T4D bacteriophage virus of Escherichia coli B [52]. Finally, the direct mechanical action of sharp nanostructures such as darts, blades and spikes as a kind “mechano-cide” has been shown to have some success for bacteria, but remains largely unexplored for viruses [53]. From a contact mechanics perspective, the combined effect of sharp (10's of nm) surface features combined with ubiquitous surface forces suggests the generation of potentially large disruptive stresses on relatively soft viral materials. A few manufacturing research laboratories including the Indian Institute of Technology Delhi (IIT) in India and Cranfield University, UK have taken the lessons from the past and developed manufacturing innovations to fight this pandemic [54,55].
3.5.2. Metallic nanoparticles and nanotechnology focussed solutions
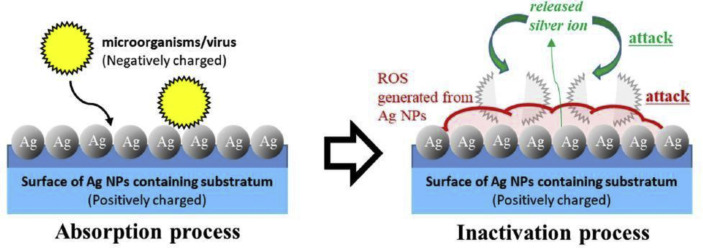
Somewhat surprisingly, while researchers have investigated bactericidal properties of nanoparticles of silver, yet the current understanding of the interaction of these nanoparticles with viruses is limited. However, some positive results are reported and, based on these reports, this direction of manufacturing research holds a good promise to tackle SARS CoV-2. Previous studies showed that the size of nanoparticle is critical to manifest a viricidal effect. For example, a nanoparticle size of less than 25 nm was found to be effective against Tacaribe virus [56], while a particle size of between 7 and 20 nm worked well against Herpes Simplex Virus (HSV) type 1/2 and human parainfluenza virus type-3 [57]. Nakamura et al. [58] reported that materials with immobilised silver nanoparticles possess enhanced microbicidal activities against the virus. These researchers developed a material using silver nanoparticle absorbed on a chitin sheet having a nanoscale fibre-like surface structure shown in Fig. 11 and obtained favourable results against H1N1influenza A virus.
Fig. 11.
The mechanism of viricidal activity of silver (Ag) Nanoparticles (NP) chitin nanofiber sheets showing strong antimicrobial activity via reactive oxygen species (ROS) and silver ions on the substrate. Reprinted with permission from Ref. [58].
Moreover, the unique characteristics of metallic nanoparticles such as high surface to volume ratio, surface-enhanced Raman scattering and localised surface plasmon resonance can be utilised for virus detection and therapy via destruction through laser-induced localised hypothermia as well. Some of the candidate nanoparticles are metallic and high entropy alloy nanoparticles (AgAuPtPdCu high entropy nanoparticles, FeO NPs, Ag NPs, TiO2 NPs, Au NPs, Ag–Au core-shell NPs) [59].
For this purpose, UV–Visible spectroscopy can be deployed to study the surface plasmon resonance, refractive index, and fluorescence change when nanoparticles interact with virus particles.
Nanoparticle-based investigation can help detect and inactivate viruses (Fig. 12 ). Specific to coronavirus, graphene oxide sheets with silver nanoparticles (GO-Ag NPs) were reported to inhibit the growth of feline coronavirus (FCoV) by up to 25%, in comparison to pure GO that inhibited it only up to 16% [60]. Additionally, chiral biosensor with self-assembled chiral gold nanohybrids (CAu NPs) on account of multiple plasmonic scattering showed better detection performance on coronavirus [61].
Fig. 12.
Nanoparticle based therapy for SARS CoV-2. Reprinted with permission from Ref. [59].
As with all fundamental and translation nanomaterial research, progress is not immediate. Caution is required with nanomaterial technologies, as the long-term impact on human health and the environment of free nanoparticles has not been ascertained, and this may ultimately pose greater risk, particularly in the liver and respiratory tract of human beings and in ecosystems. Lack of governmental regulation strategies for nanomaterials can also hinder the speed of approval and ultimately the availability in the marketplace. Nevertheless, a call has gone out to nanomedicine researchers to utilise their existing knowledge base and translate their technologies towards COVID-19, should the outbreak last more than 12 months [62].
3.6. Digital manufacturing of filters, antiviral masks using a combination of precision subtractive and additive processes
3.6.1. Rapid manufacturing of mask filters
Filtration efficiency is a strong measure of penetration prevention of aerosols through mask filters and is usually required to be above 98% for surgical face masks [63].
Ultrasonic welding is usually deployed to produce filters with sufficient filtration efficiency. Both polymer and textile-based masks are popular and can benefit from the adoption of sequential micromachining techniques [64]. A surgical mask (procedure mask) is worn by health professionals during surgery to avoid exposure to aerosols. Such masks are not designed to protect the wearer from inhaling airborne bacteria or virus particles and are less effective than respirators, such as N95 or NIOSH masks which provide better protection due to their material, shape and tight seal.
The WHO Laboratory Biosafety Manual necessitates Biosafety Level 2 (BSL-2) requirements for non-propagative diagnostic laboratories and BSL-3 for laboratories handling high concentrations of live SARS-CoV-2. According to the manual and the WHO biosafety guidance for SARS-CoV-2, the exhaust air from such laboratories should be discharged through high-efficiency particulate air (HEPA) filters. It is worth mentioning that particle collection efficiency of such mechanical filtering methods decreases to about 50% at particle sizes of 0.5 μm due to diffusion and diffusion-interception regimes of particles with sizes in the range from 0.05 up to 1 μm [21].
Therefore, HEPA filters are not ideal in screening the viruses like SARS CoV-2 which has a typical diameter from 60 nm to 160 nm [22].
Mass production of appropriate filters to screen the virus entry is a micromanufacturing challenge. This could potentially involve technologies such as direct laser micromachining or punch-based microstamping methods. For this purpose, a plasma spraying, or laser micromachining method may be used to obtain a well-suited punch which can be used to stamp (pierce) polymer sheets (e.g. PET), to achieve appropriate filter sizes. Another candidate process of subtractive manufacturing is metal anisotropic reactive ion etching with oxidation (MARIO) [65].
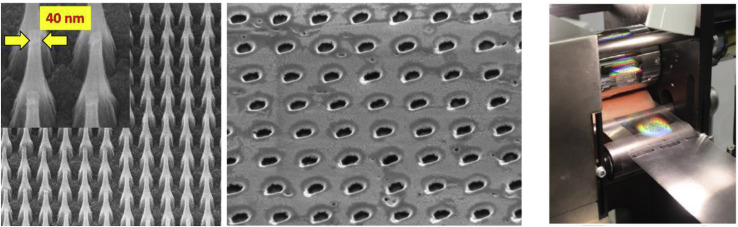
An illustration of how the proposed micromachining strategies can be deployed for scalable fabrication of filters to screen virus scale particles is shown in Fig. 13 . While self-assembly techniques such as block copolymers have shown promise filtering small viral particles such as human rhinovirus [66], direct printing methods allow greater engineering control over pore size and spacing, critical to tuning membrane efficiency, fluid resistance and mechanical strength.
Fig. 13.
Illustration of micro punch array with 40 nm diameter and inverse-pattern via stamping (left, center) of viral scale mask filter made possible by a ion implant die fabrication method [67,68] for scalable fabrication via e.g. roll to roll processing (right). Figures reprinted with permission.
3.6.2. New design and scalable manufacturing of antiviral coated masks
Protection of surrounding or nearby people is important and an infected patient can help to control the spread by wearing a mask, leading to the concept of social distancing suggested by various governments. The safe distance guideline is an important concept especially in populous countries where an assembly of people on the streets can cause the spread to grow exponentially. Keeping this in mind, the WHO urged people to maintain a safe social physical distance of about 3 ft (~1 m), while the Centre of Disease Control and Prevention recommends this to be 6 ft (~2 m). These limits are now challenged by recent research that suggests this safe distance should be 23–27 ft (7–8 m) [69]. The same study also suggested that the currently available commercial masks need to be redesigned. It was suggested that the masks available at present are not suitable for containment of sprayed aerosols (during a sneeze) travelling at the velocity of 108 kmph [70], as this increases the possibility of escape of viruses through contaminated droplets from edges of the mask making nearby people more vulnerable to contracting the coronavirus. Also, Chin et al. [16] tested the strength of the virus and suggested that the virus is highly stable at 4 °C as well as in the pH range of 3–10. The virus was found to be detectable on a mask surface even after 7 days. These findings suggest a product design strategy to develop more appropriate masks for handling exhalations travelling at high speeds by considering human ergonomics, material aspects, antimicrobial nature and structural aspect. While the design and strategies for developing these new types of masks are underway, a possible technique for deploying the extant fabrication methods would be to merge the two approaches, namely, additive and subtractive manufacturing methods described above. This development would mean that even in the current landscape, a new mask-making strategy would provide more protection than the currently available masks.
ASTM F2100-07 is the relevant International standard that details specifications of materials to be used in medical face masks. However, the current standards do not capture the techniques required to make the mask reusable especially as the current materials are yet to be tested against SARS CoV-2. As of now, CURAD antiviral isolation masks making use of a cocktail coating (citric acid, zinc and copper) are available as an option to disinfecting the outer surface of the mask. These are useful for medical professionals as they can come in contact with the virus infected aerosols more frequently.
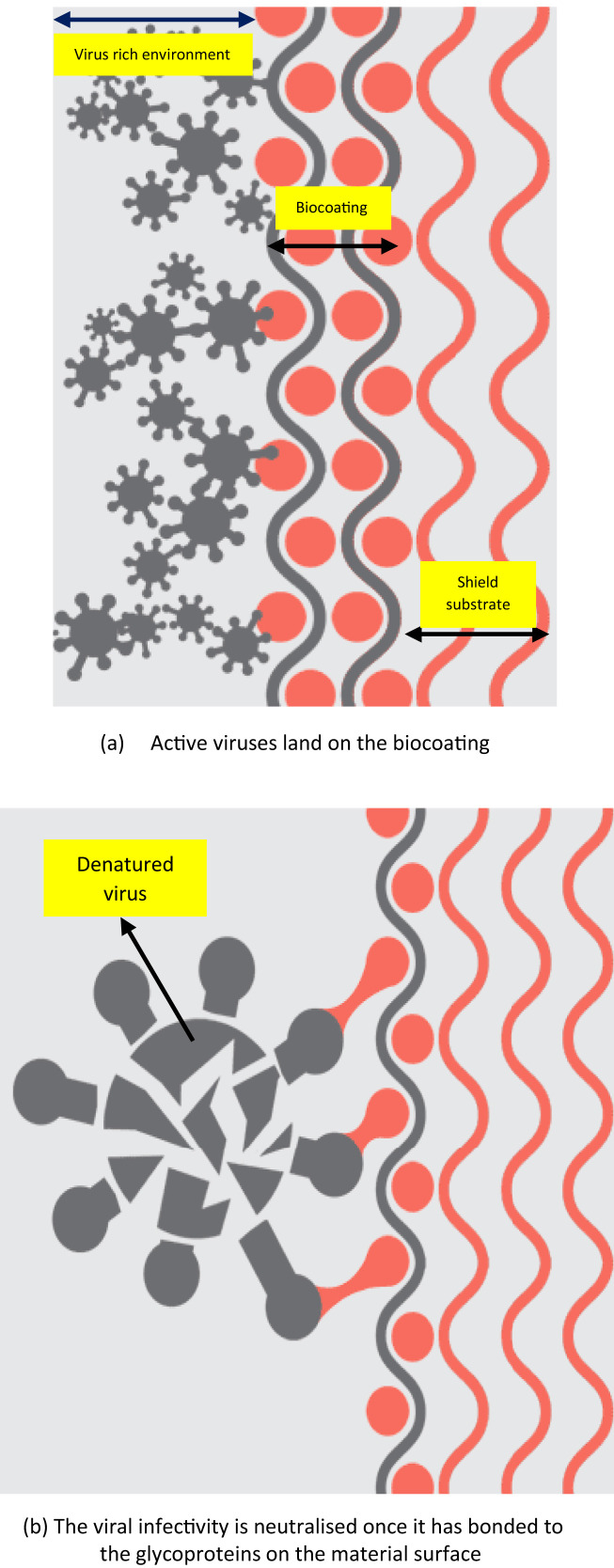
Another concept is that of a ‘Germ trap’ surface [71], which is akin to fooling the spike proteins of a virus. Virustatic Ltd., [72] ventured with the The University of Manchester, UK to identify specific glycoproteins that have carbohydrates attached to their surface, similar to those seen on the surface of the cells of the upper respiratory tract. Due to unavailability of any physical cells to invade on the material surface of the fabric, the virus would in theory decompose and become deactivated on the textile surface (see Fig. 14 ). The efficiency of germ trap coating in achieving its goal is yet to be tested and the experimental trials to verify this theory are underway.
Fig. 14.
Mechanism of germ trap fooling the virus into attaching to the protein surface [72].
Based upon the existing literature, an immediate measure to functionalise the Personal Protective Equipment's (PPEs) could be to introduce the use of antimicrobial coatings comprising of proven materials (e.g. zinc and sodium [73,74] based compounds) on the surface of PPEs, especially the shields and visors. Such coatings can be deposited by additive methods such as spraying (3D), dip coating or via Roll to Roll (R2R) manfacturing methods.
In terms of processing techniques, one limitation of thermal spraying is that it requires heat resistant feedstock materials, limiting the use of most of the chemical species traditionally used to functionalise surfaces, such as polyethylene glycol (PEG) [75]. In view of this limitation, a possible research avenue is to develop functional coatings with viricidal properties such that the coating ingredients do not degrade at high temperatures, thus providing a controlled release over time. With this goal in mind, a suitable processing and material matrix availability is crucial to obtain a coating to possess desirable advantages in the medical field.
On the other hand, the use of thermal sprayed hydroxyapatite (HA) coatings on orthopaedic implants has been widely adopted in the medical field since the late 1980s [76]. As reported in the early 2000s [77], the use of thermal sprayed HA coatings on metal implants presents several advantages and a positive potential for its use in the medical field [78]. Taking into consideration the status of HA as the standard in thermal sprayed coatings and the decades of research on its behaviour and response to living tissue [79], the choice of HA as a base material for the development of anti-microbial functional coatings has been most popular to date.
At a personal level, deploying smart PPE incorporating viral detection capability into masks, gloves or wearing “viral dosimeter” badges might help monitor the strength of direct transmission vectors as well as environmental exposure rates and accumulation. Conversely, masks could collect exhaled viral particles for early detection of infection [70]. Detection could be implemented as microfluidic channels functionalized with bioelectronic miniaturized detection schemes [80,81] with specific virus sensitivity [82,83]. Local area smart sensing filter textiles incorporated into mobile or in-place ventilation systems designed to both filter and detect could also help provide early alert alarms and protection in buildings and other areas of restricted air replacement.
The importance of wearing a mask by the infected person and by a healthy person coming in the vicinity of the infected person is ranked on the priority order shown by the cartoon model in Fig. 15 .
Fig. 15.
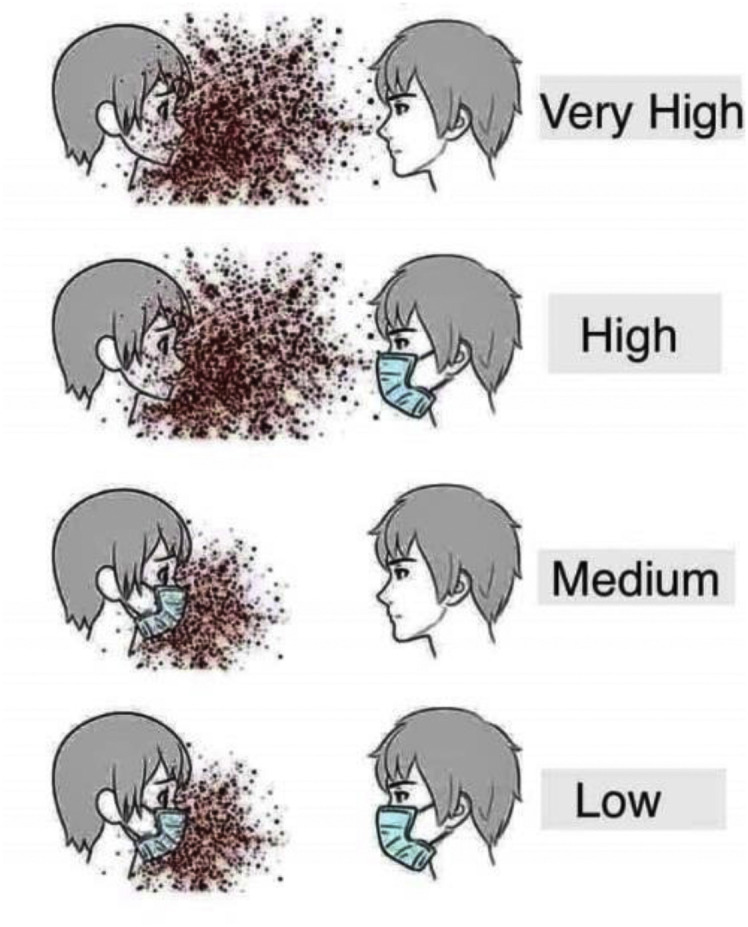
Cartoon model showing the importance of wearing a face shield/mask in avoiding the probable recurrent spread of coronavirus
3.7. Disposal of biomedical waste from COVID-19 patients
Safe disposal of materials used in the treatment of infected patients such as gloves, masks, test samples, clothes or the human waste, especially in ICU patients who may be closer to death is a challenging task. In addition to the possibility of an infection, this waste could lead to other secondary issues including emergence of a new category of virus/bacteria. Hence, safe disposal of biomedical waste is very important. Additionally, gaining support from members of the public to adequately dispose of their used personal masks etc. is essential. Recently, an absorbent gel with embedded disinfecting material has been developed at Sree Chitra Tirunal Institute for Medical Sciences and Technology, India for liquid respiratory and other body fluid solidification and disinfection for the safe management of infected respiratory secretions. This material helps to solidify the liquid respiratory secretions from ICU patients or those with copious secretions treated in the wards. The material so collected becomes fit for disposal through the usual incineration system for biomedical wastes. Newer guidelines are required to refine the existing risk assessments procedures [84].
4. Modelling and simulations
4.1. Artificial intelligence methods utilising existing genome databases: biological modelling with assistance from phylogenetic network
Big data analytics and extant research utilizes accelerated development of techniques for data mining, machine learning and use of Artificial Intelligence (AI) that shows promise in the treatment of SARS CoV-2. As a direct benefit to this approach, a digitalised shadow of the data to study plausible scenarios of certainty of an event becomes easier. As for SARS CoV-2, genome data now exist offering scope for soft-computing application researchers as well as those involved in AI based research to offer new insights into the area. As an example, the Global Initiative on Sharing All Influenza Data (GISAID) database (https://www.gisaid.org), made available in March 2020, contains a compilation of over 24,000 SARS-CoV-2 complete and partial genomes from all over the globe since December 2019 [85].
Recently, researchers from Cambridge used phylogenetic network on 160 complete genomes of SARS CoV-2 [85] to predict probable mutation and migration paths of the coronavirus.
4.2. Atomistic modelling and molecular docking
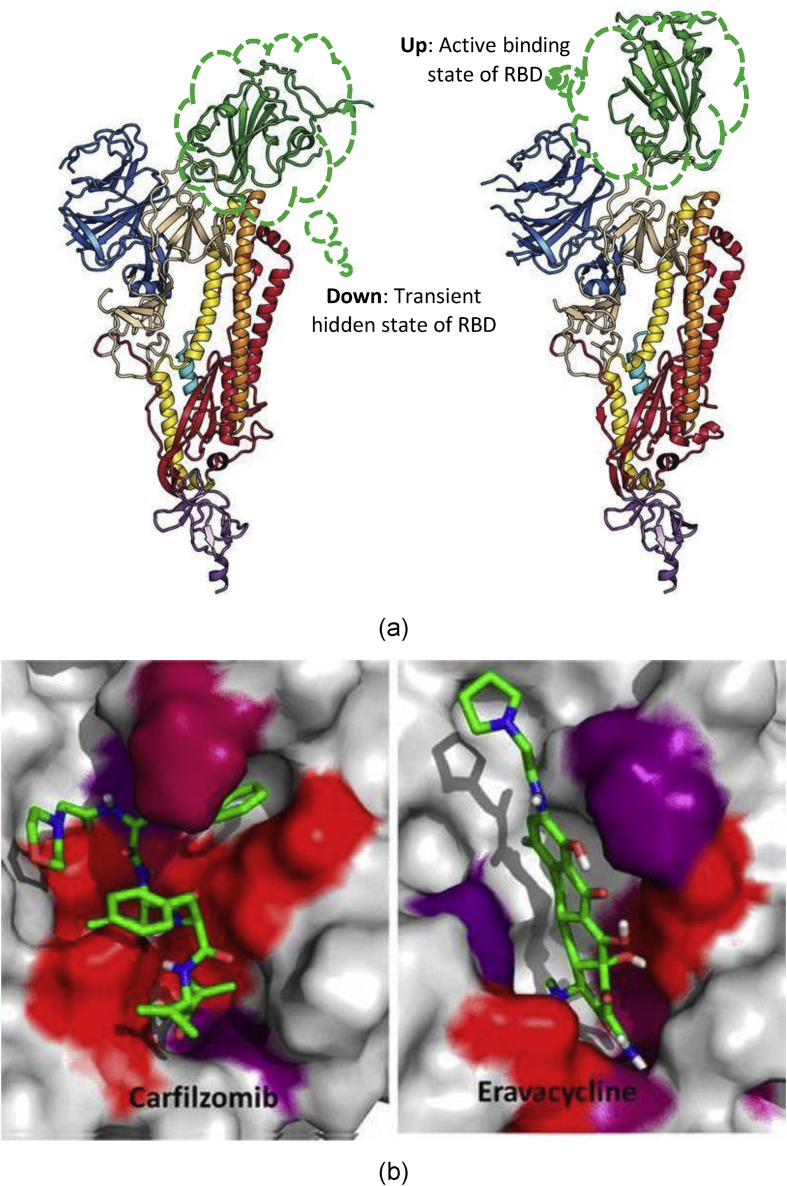
Atomistic modelling and molecular docking [86] can hold the key to a scientific breakthrough to address questions surrounding SARS CoV-2. The development of vaccines, antibodies and diagnostics is dependent in a large measure on our understanding of the interfacial science between the spike (S) glycoprotein of SARS CoV-2 and ACE2 receptor in human lung cell (as shown earlier in Fig. 2). Wrapp et al. [87] obtained a cryo-electron microscopy structure of the SARS CoV-2 S trimer in the metastable prefusion conformation, showing it undergoes a transient (hide and active states) structural rearrangement. Their results relying on surface plasmonic resonance elucidated a stronger affinity of SARS CoV-2 with ACE2 compared to SARS CoV-1, and shed light on two states, namely, a “down state” or receptor-inaccessible and an “up state” or receptor-accessible state shown in Fig. 16 (a).
Fig. 16.
(a) Single protomer of SARS CoV-2 with the RBD in the (a) “down” (receptor-inaccessible) conformation) and (b) “up” (receptor-accessible) configuration [87] (b) an example of molecular docking [88] (figures reprinted with permission).
In yet another interesting study [89], computer-aided drug screening was used to run a test assay over 10,000 compounds as inhibitors to a key CoV enzyme, Mpro.
Ebselen was found to exhibit antiviral activity and illustrate a way forward use of this potentially promising modelling strategy to discover targeted drug and vaccine development. A molecular docking example shown in Fig. 16(b) is yet another effective example of accelerating the drug discovery [88].
4.3. Aerodynamic analysis and role of computational fluid dynamics (CFD)
Recently, Liu et al. [90] carried out an aerodynamic analysis by measurement of the RNA of SARS CoV-2. They used pre-sterilized gelatin filters (pore size of 3 μm) to collect the aerosols from different areas of two Wuhan Hospitals. This study classified the sampling location in three areas:
-
(1)
Patient areas: These are the areas where COVID-19 patients are physically present such as intensive care units, coronary care units, ward rooms, a toilet and staff workstations
-
(2)
Medical staff areas: These are the areas that are exclusively accessed by the medical staffs who are in direct contact with the COVID-19 patients
-
(3)
Public areas: These are the venues open to the general public such as reception and waiting lounge areas.
Surprisingly, the researchers detected maximum stains of SARS CoV-2 in patients’ toilet areas, while the levels were low in the isolation wards and ventilated patient rooms. Also, the medical staff areas showed peaks of high concentrations with aerosol size ranging from 0.01 μm to 2.5 μm and they were neutralised after rigorous sanitisation procedures.
The study suggests that there is a likelihood of resuspension of virus-laden aerosols from the surface of medical staff's protective equipment including surgical masks during their removal while having lunch, visiting toilets or breaks etc. The source of these virus suspensions was speculated to be from the direct deposition of patient's respiratory droplets or airborne SARS-CoV-2 onto the protective apparel. The researchers also found that the SARS-CoV-2 in aerosol forms had relatively longer residence time, implying the infection remains for a relatively longer time causing further transmissions. This study serves as a practice guide on the requirements and specification after the lockdown procedures are eased to avoid recurrent infection.
The exit-strategy after the lockdown must consider carefully the ventilation of offices/classrooms, maximum use of open space (preferable sunlight), regular sanitisation of clothes, hairs and hands, and proper use and disinfection of toilet areas.
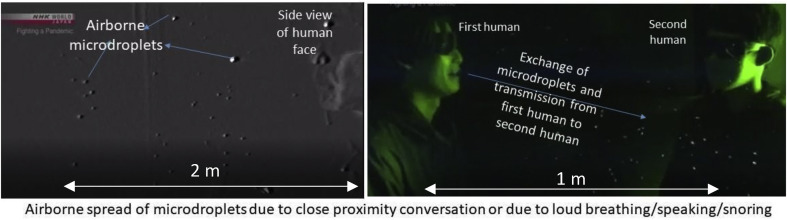
The data obtained by Toho University (Fig. 17 ) suggests that laser technology can be employed efficiently to monitor the streamlines and microdroplets movements from a sneeze. This monitoring could be a highly effective lab-scale activity to not just guide the futuristic CFD models but also to efficiently design new masks effective for preventing diseases caused by high velocity spray caused by a sneeze.
Fig. 17.
Airborne spread tracked by the laser experiments at Toho University, Japan.
5. Challenges and alternative approaches for COVID-19
While engineering and manufacturing will undoubtedly play their part over the coming years, the interventions proposed herein can help avoid the recurrent spread and emergence of new infections.
The patients who have survived while battling against COVID-19 showed that they have developed antibodies to the virus. Antibodies are virus-specific proteins, which have a memory of the exposure to the virus and will recognize the virus on a second exposure. Antibodies help prevent future infections by detecting the virus and binding to their surfaces signalling the body's immune system to destroy such viruses or virus-infected cells. While reasons are not clear, common wisdom is that the human body does not have an adequate immune response to HIV. For COVID- 19, an adequate immune response does exist. Therefore, a vaccine is more likely to be created successfully for SARS CoV-2. Additionally, the observation that a large majority of people recover, either without any symptoms or with minimal symptoms such as fever and body ache, also bodes well for the development of a vaccine.
Three major scenarios are currently being pursued or envisaged in tackling the disease.
-
(i)
Research and trials on vaccines: Clinical trials are being conducted across the globe into novel vaccines and fast-tracking approval processes are being deployed. However, scientists fear that the virus itself, like the flu, can mutate and form different strains. Therefore, immunity after immunisation or contracting and surviving COVID-19 may only last as short as two years.
-
(ii)
Therapeutic trials using available drugs: Attention is being focused to drug repurposing and advanced formulations, including antimalarial, HIV, and other antiviral and immune-modulating drugs and biomolecules.
-
(iii)
Nanotechnology: Study of molecules that show promise but exhibit poor physio-chemical properties or toxicity is currently being formulated across the globe into advanced nanomedicine platforms, translating the knowledge gained in drug targeting and efficacy enhancement from conditions such as cancer and cardiovascular disease. Such technologies aim to provide better treatment prognosis for those patients who fall ill with coronavirus related diseases. Attention to immune-modulating molecules is growing, as patients who contracted severe forms of COVID-19 were reported to experience notorious cytokine storms which ultimately lead to their death. Ability to suppress such immunological responses could be one key to controlling the progression of the virus from mild to severe infection.
Lessons learned during this pandemic will also impact the outlook for other viruses. These include governmental pandemic funding allocation, stockpiling of medical protective equipment and emergency aid strategies within and between countries across the globe. As the coronavirus problem increased across the globe in 2020, many questions have been raised regarding what more governments could have done, why they did not act more quickly, and what were the fundamental failures within their policy. Over time, addressing these issues will pave the way for a more streamlined and effective future response. In addition to this, contact tracing technology is being heavily invested in across the globe, with the intention that citizens would be able to download mobile applications, which could inform them (and the authorities) if they had been exposed to another person who tested positive to COVID-19. Whilst early trials seem positive, the longer-term question remains over privacy and ownership of personal data collected by such applications. Access to death records inside countries and across continents will allow identification of high-risk population categories which will aid risk prevention. The positive news is that this pandemic has cemented the importance of scientific integrity, the inclusion of scientific advisors into government, and the role of science in society. While this may offer little solace at such an upsetting time, yet, with the power of science, this disease will be managed unlike many other viral threats over the years. Currently, vaccine trials are ongoing at various phases of progress in different countries (see Table 3 ) and good news may be expected soon.
Table 3.
| Country | Vaccine | Phase |
|---|---|---|
| China | Ad5-nCoV | II |
| China | Unnamed (Inactivated SARS-CoV-2 virus) | I-II |
| China | Covid-19/aAPC | I |
| China | LV-SMENP-DC | I |
| China | Unnamed (inactivated COVID-19 virus) | II |
| USA | mRNA-1273 | I |
| USA/S. Korea | INO-4800 | I-II |
| USA/Germany | BNT162 | I-II |
| UK | ChAdOx1 nCoV-19 | I-II |
| Canada | bacTRL-Spike | I |
In the wake of this pandemic, many countries have developed new methods of digital communications that were not realised to be of much importance earlier. For example, in the UK, enhanced digital communication developed through structured formats, including the Discourse Digital Health Network, which is a ‘Discussion and collaboration for the UK and international digital health communities’ [95].
The world has seen a surge in the use of digital tools being growingly used for teaching, research, consultation, banking and day to day activities. Digital health innovation has been a prime example. These approaches culminated in the development of telemedicine consultation. The current situation has also driven many changes in work-life routines such as less travel and digital technology-based remote work.
6. Authors’ perspective
6.1. Impact on climate change and weather transitions
The coronavirus pandemic has brought significant disruption and caused severe emotional, sentimental and financial losses. There is even a chance that life on the planet will never be the same again and many stringent safety measures will continue to be followed in the time to come while using public transport, air travel, or while visiting touristic places. The continued extension of lockdowns (e.g., Lockdown 1.0 to Lockdown 4.0 in India) has resulted in making people restless when staying indoors. Countries who were not very resilient in adapting to the situation even saw disrupted supply chains, disturbed migrant workers, lack of food and other supplies and unavailability of essential items - a situation comparable to a natural disaster.
However, every disaster brings forth a new cycle of life and the episode of COVID-19 did bring some good things. Countries facing severe pollution in air, water, and land saw a life not seen in the last many decades. Social websites and digital tools were used heavily to share the beauty of nature. Rivers got cleaner, the air became more breathable, and the sky got clearer [96]. As a result of this transition, even climate change and transition in weather were observed in many places and it will be befitting to say that we witnessed how “nature self-heals and mends itself”.
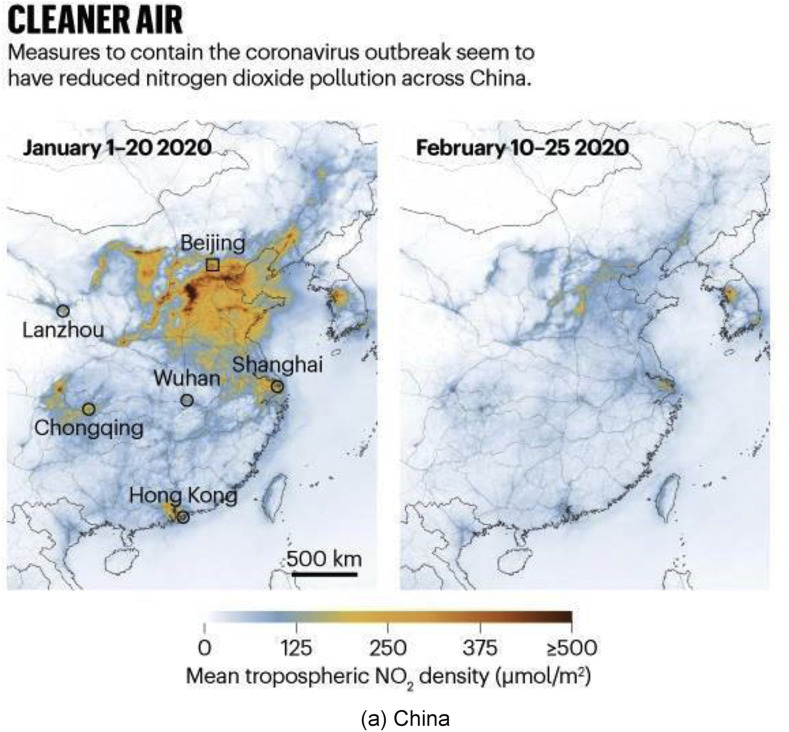
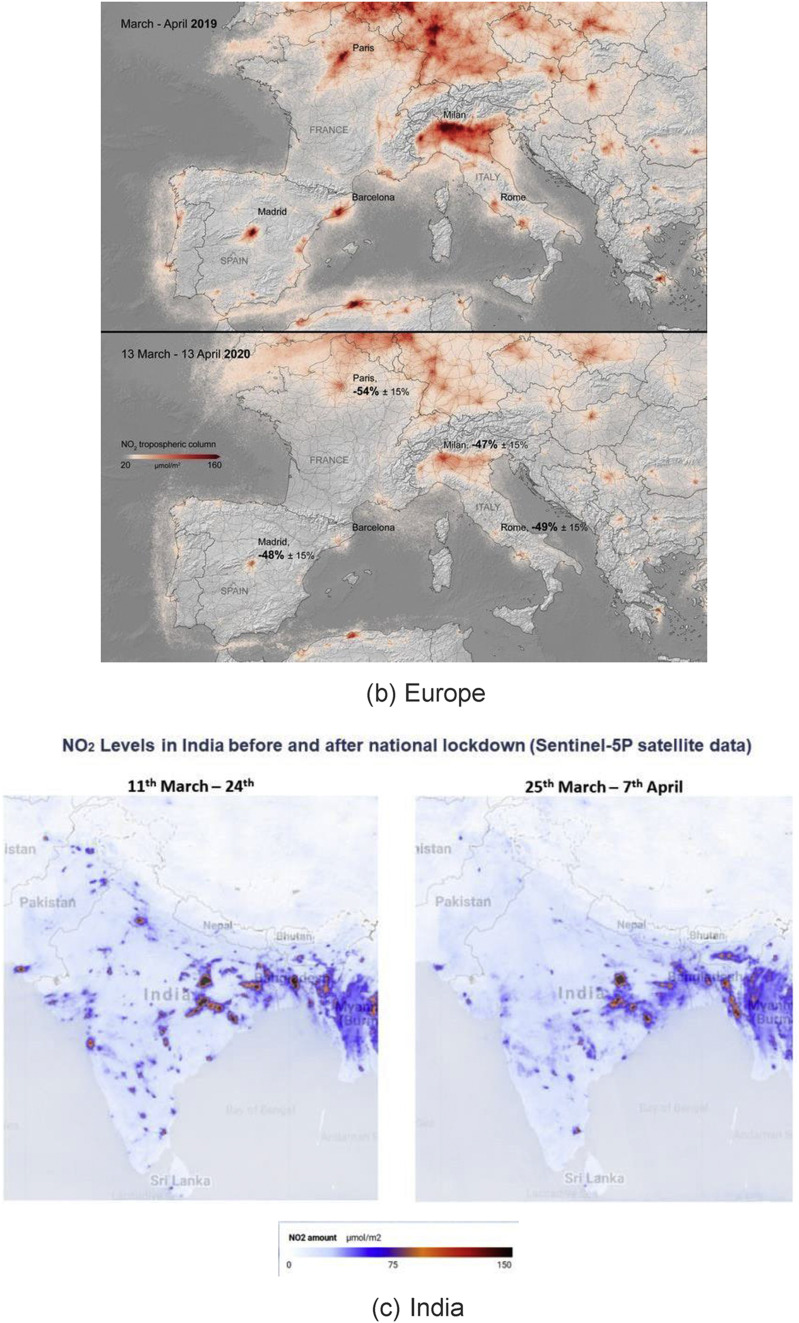
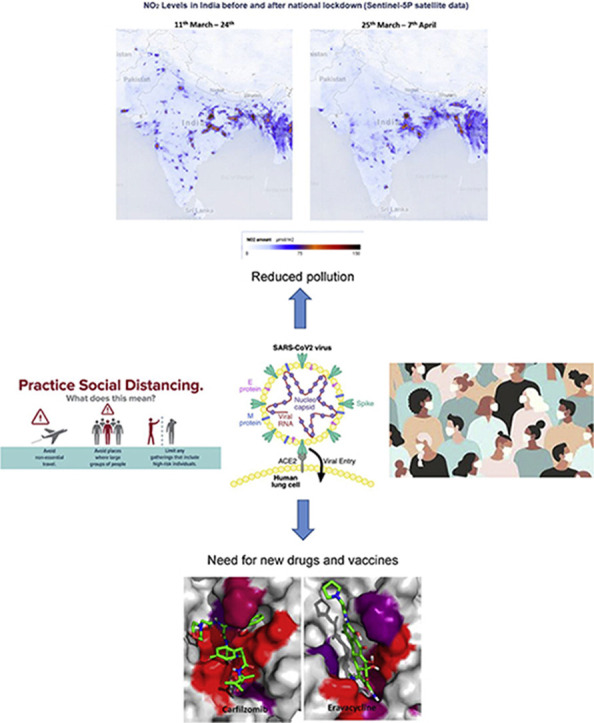
A prime example of climate change as a result of lockdown can be seen in China, Europe and India (see Fig. 18 ). India has faced two major challenges for many years in the wake of rapid urbanisation: alarming levels of air pollution and a rapid decrease in the availability of clean water, especially from rivers like the Ganga. The government of India had earlier set up a mega project called “Namami Gange” [97] for freeing the rivers from industrial wastes. The project is of such importance that when addressing the Indian community at Madison Square Garden in New York in 2014, the Prime Minister of India Mr Narendra Modi had said, “If we can clean the Ganga, it will be a huge help for 40% population of the country.” COVID-19 has rejuvenated the Ganga and many other rivers around the world, making them cleaner to a point that their water was reported to be potable [98].
Fig. 18.
Satellite data from NASA and the European Space Agency showing a dramatic decrease in the nitrogen dioxide (NO2) during pandemic peak. (NO2 is produced when fossil fuels are burned). Reprinted with permission from Ref. [10,99,100].
The learning from the episode of COVID-19 has been that resources offered by mother nature are meant to be used and not to be “exploited”. If this simple rule is forgotten, then the world may continue to witness a regular self-healing cycle. On this occasion it was COVID-19 but the next time, it could be readjustments in the ecosystem caused by a surge in the carbon emissions.
Researchers are reporting a decrease in solar activity leading to an increased flux of energetic particles in our galaxy [101]. Before it is too late to realize, and the entire human race wipes-off the face of the earth due to the climate change, we must take this pandemic as a wake-up call towards being considerate to the environment.
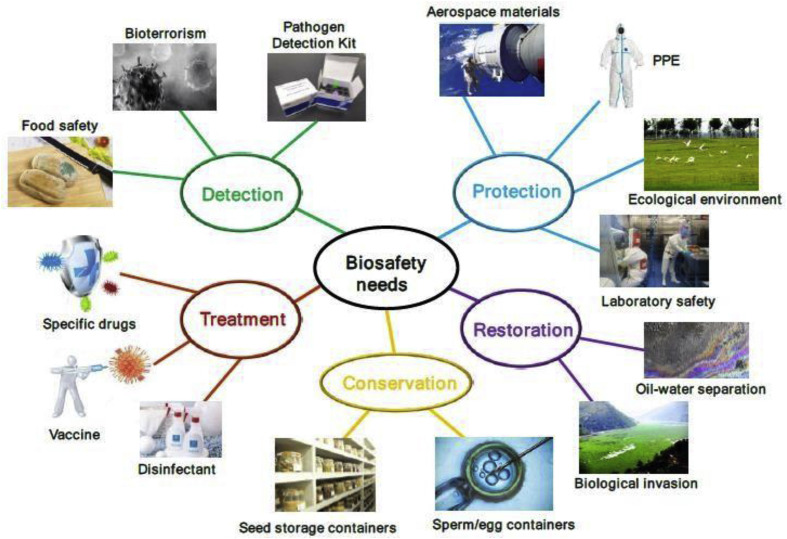
What has apparently come clearer though is that problems like biohazards primarily emerge due to our lifestyle (e.g. consumption patterns including eating habits) or mishandling of biowastes (see Fig. 19 ). There is an absolute necessity to pay utmost attention to biosafety and implementation of effective ISO standards worldwide. Health of people is also closely connected to the health of animals and our shared environment. This is because people, animals, plants, and our environment are interdependent [102] and this direction of research considers the concept of “One health”.
Fig. 19.
Biosafety needs and future guided bioresearch. Reprinted with permission from Ref. [103].
6.2. Move towards indigenous manufacturing and automation
6.2.1. Outsourcing vs. indigenous manufacturing
In the wake of the COVID-19 pandemic, the world witnessed an all-time-high demand of ventilators, PPE's, masks, bed liners, other essential health supplies and medicines. The pandemic struck everywhere, including the largest manufacturing economies – and pretty much all at once. As a result, most nations experienced manufacturing shocks. The two shocks that have impacted manufacturing worldwide are:
-
•
Supply shock: The containment measures (such as lockdowns and social distancing) kept the workers away and resulted in reduced productivity and output.
-
•
Demand shock: Customer consumption patterns started changing (e.g. movement away from “non-essentials”) and this affected the demand for a large variety of manufactured goods and associated services.
As the pandemic spread, a few countries were resilient enough to cope with the pace of transformed supply chain requirements while many others who had earlier (in pre- COVID-19 era) aligned themselves to outsourced or cloud-based manufacturing, primarily driven by low-cost, became hugely dependent for their essential supplies on other countries (primarily China). Stringent lockdowns enforced by different countries at different times had severe consequences on the supply chains where outsourcing-based dependencies existed. Indeed, China manufactures much of its pharmaceuticals for export in the Hubei province, which is where COVID-19 originated and this outbreak introduced a bottleneck in the global supply of several antibiotics. Even functioning factories that tried to increase their manufacturing productivity by increasing their inventory or logistics capability or increasing the number of operators, could not do so. Their efforts were negated by the strong social distancing measures.
Overall, unprecedented requirements arising out of COVID-19 could not be met by outsourced manufacturing systems. Under such scenarios, indigenous manufacturing programs have come into focus. Many examples of a sustained push to indigenous manufacturing were seen: The US ramped up indigenous production of masks, ventilators and test-kits and India increased indigenous PPE suits production capacity from practically nothing to more than 100,000 suits per day. Earlier, there were only a handful of firms such as Becton Dickinson, Philips, Hamilton Medical, Fisher & Paykel Healthcare, Draeger, Medtronic and GE systems who manufactured specialised ventilators. In the wake of the pandemic, new capabilities on this front have emerged, and many innovations were deployed at manufacturing firms. For example, in the US, automobile firms such as GM and Ford as well as other firms emerged with new designs and ventilator manufacturing capabilities.
The manufacturing related lesson learned from the COVID-19 episode is that pandemics can affect manufacturing in several geographical locations simultaneously and therefore can affect an individual nation's surge capacity to deliver essential supplies such as diagnostics, drugs and vaccines to its population. Going forward, this lesson will critically affect thoughts on manufacturing strategy and low-cost based outsourcing, and we may see a sustained push towards development of resilient indigenous manufacturing capabilities and a decreasing reliance on low-cost based outsourced manufacturing.
6.2.2. Promoting automation
A second trend that we perceive is an increasing focus on digitised automation and use of automated production systems including the use of robotics in day to day life as well as deployment of embedded robotics in manufacturing operations. An example of a futuristic automation capability was displayed at the Indian Institute of Technology Guwahati who developed remote controlled food delivery robot for COVID-19 isolation wards (see Fig. 20 ). The robot was designed for 6 h run when fully charged.
Fig. 20.
A prototype demonstrator of COPARBOT (Courtesy: Dr. S. Kanagaraj at IIT Guwahati, India).
6.2.3. Antimicrobial resistance (AMR) and increased chances of zoonotic diseases
Antimicrobial resistance (AMR) refers to the resistance of microbes to antimicrobial drugs or “microbial immunity”. The WHO as well as several national health organizations have identified antibiotic resistance as one of the greatest threats to global public health, economic growth, agriculture, economic security and national security. Around the globe, people are being admitted to hospitals with infections that do not respond to antibiotic treatment. The problem of AMR is rooted in the fact that when microbes are repeatedly exposed to antibiotics, they mutate to produce strains that are resistant to the antibiotic and are therefore able to resist the effects of medication that could successfully treat the microbe.
In 2014, Lord Jim O'Neill and his team published a review commissioned by the United Kingdom government entitled, “Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations” (the AMR Review) which is in line to the question that de Kraker et al. [104] asked “Will 10 million people die a year due to AMR by 2050?”
The problem of AMR is connected to that of COVID-19 with respect to the emergence of zoonotic diseases. The trend of overuse and/or misuse of antimicrobials, excess use of certain antibiotics in animals, and pharmaceutical industry pollution can lead to continuous emergence of zoonotic diseases.
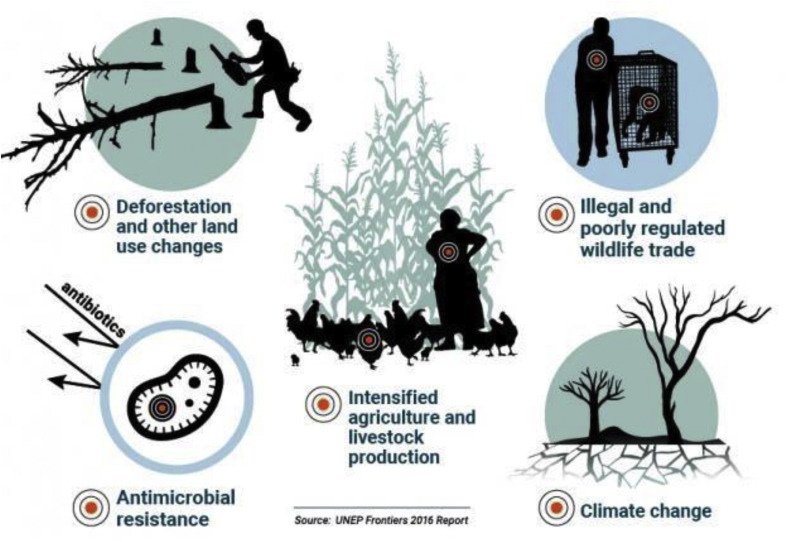
Excessive use of antimicrobials stresses the naturally occurring microbiome and allows for resistant bacteria to become dominant. Indeed, research has suggested that AMR might spread to humans through food products of animal origin, the environment, and by direct contact in the case of agricultural workers. Addressing the issue of AMR as well as others shown in Fig. 21 is thus a global priority as its impact on claiming lives is no less than that of COVID-19 [105].
Fig. 21.
A schematic showing various factors of concern leading to increasing zoonosis emergence [106].
7. Conclusions
The rapid emergence of the COVID-19 pandemic provided minimal time for well-directed resource mobilization, and almost every country was tested for its resilience in its medical and manufacturing abilities during the first half of 2020. The number of tragic health cases pushed organizations such as the FDA to allow Emergency Use Authorisation of various drugs without a systematic testing protocol, and among the 70 drugs tried, Favipiravir and Tocilizumab were rated most effective while further testing with natural compounds from Ashwagandha (Withania Somnifera) and Propolis is underway. Amidst the chaotic situation, the growing use of digital tools for professional communications and artificial intelligence to monitor the recovery of COVID-19 patients showed some positive outcomes. A major question remains open to date, “how can the symptoms of COVID-19 be detected in their early stages?” While more research is needed on the instrumentation and manufacturing sides, there are clear opportunities identified from the available scientific knowledgebase. In relatively advanced stages of the symptoms, rapid measurements by combining chest Computer Tomography (CT) and reverse-transcription polymerase-chain-reaction (RT-PCR) tests seem to provide a high confidence level in the screening. More work is needed on the sensing side for detection of the virus in the primitive stages. Definitive promise has been exhibited using laser technology to monitor the aerosol spread and to detect the RNA of the virus and gain a deeper understanding of the aerodynamic properties of emergent viruses.
Further development of fumigation chambers and ultra-violet chambers seem to hold promise as precautionary measures that can be implemented in public places like malls, airports, theatres and train stations etc.
In this paper, we have also highlighted the possibilities of developing novel tools such as lab-on-the-chip, in addition to developing advanced Personal Protective Equipment (PPE) using combinatorial additive and subtractive manufacturing techniques such as Thermal spray, dip coating and Roll-to-Roll (R2R) manufacturing. These technologies allow the capability to filter the coronavirus, which has a diameter in the range of 100 ± 60 nm. It is envisioned that in future, wearing masks and social distancing would be mandated to avoid the recurrent spread of the virus. In the long term, micro-manufacturers, medical professionals, metrology engineers, material scientists and virologists will need to work in a more interdisciplinary way to develop modelling informed fabrication strategies. Accelerated use of molecular modelling approaches - like molecular dynamics and molecular docking – also seems to hold promise in drug and vaccine discovery to end this pandemic from its root.
As society progresses to meet challenges such as COVID-19, many overarching issues will become important. Strong measures will need to be implemented to ensure careful handling of biowastes, indigenous capability-based manufacturing focus will become stronger and issues such as antimicrobial resistance will demand increased attention. Our hope is that the post-pandemic society will better heed the warning signs from nature and work on making manufacturing systems resilient to address emerging threats.
Data statement
All data in the manuscript will be available through Cranfield University open repository (https://doi.org/10.17862/cranfield.rd.12376889).
Declaration of competing interest
We hereby declare that the authors and co-authors of this work have no competing interests.
Acknowledgements
Authors are very much thankful to the Research support provided by the UKRI via Grants No.: (EP/K503241/1, EP/L016567/1, EP/S013652/1, EP/T001100/1, EP/S036180/1 and EP/T024607/1) as well as the GCRF/EPSRC supported SUNRISE program (EP/P032591/1). Additionally, we acknowledge the support received from H2020 (Cost Actions (CA18125, CA18224, CA17136 and CA16235) and EURAMET EMPIR A185 (2018)), Royal Academy of Engineering Grant No. IAPP18-19∖295 (Indo-UK partnership), Royal Academy of Engineering Grant No. TSP1332 (South Africa- UK partnership) and Newton Fellowship award from the Royal Society (NIF∖R1∖191571). Also, numerical calculations performed on the Isambard Bristol, UK and ARCHER HPC were made available by the EPSRC Resource Allocation Panel (RAP).
References
- 1.Loeffelholz M.J., Tang Y.-W. Laboratory diagnosis of emerging human coronavirus infections—the state of the art. Emerg. Microb. Infect. 2020:1–26. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim D., Lee J.-Y., Yang J.-S., Kim J.W., Kim V.N., Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181 doi: 10.1016/j.cell.2020.04.011. 914-921.e910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valitutto M.T. Detection of novel coronaviruses in bats in Myanmar. PloS One. 2020;(4):15. doi: 10.1371/journal.pone.0230802. p. e0230802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao Hangping, Lu Xiangyun, Chen Qiong, Xu Kaijin, Chen Yu, Cheng Linfang, Liu Fumin, Wu Zhigang, Wu Haibo, Jin Changzhong, Zheng Min, Wu Nanping, Jiang Chao, Li Lanjuan. Patient-derived mutations impact pathogenicity of SARS-CoV-2. medRxiv. 2020 doi: 10.1101/2020.04.14.20060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.https://www.healthline.com/health-news/heres-exactly-where-were-at-with-vaccines-and-treatments-for-covid-19#Antivirals. Accessed on 29/5/2020.
- 6.Andersen K.G. The proximal origin of SARS-CoV-2. Nat. Med. 2020:1–3. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.https://www.weforum.org/agenda/2020/03/coronavirus-origins-genome-analysis-covid19-data-science-bats-pangolins/. accessed on 29/5/2020.
- 8.https://www.worldometers.info/Coronavirus/. assessed on 29 May 2020.
- 9.Ghani A. Methods for estimating the case fatality ratio for a novel, emerging infectious disease. American journal of epidemiology. 2005;162(5):479–486. doi: 10.1093/aje/kwi230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callaway E. Coronavirus by the numbers. Nature. 2020;579:482. doi: 10.1038/d41586-020-00758-2. [DOI] [PubMed] [Google Scholar]
- 11.http://www.emro.who.int/health-topics/mers-cov/news.html. assessed on 29 May 2020.
- 12.https://www.news18.com/news/india/coronavirus-treatment-update-covid-19-cure-medicines-favipiravir-tocilizumab-hydroxchloroquine-2595057.html. accessed on 29/5/2020.
- 13.Maxmen A. How blood from coronavirus survivors might save lives. Nature. 2020 doi: 10.1038/d41586-020-00895-8. https://www.nature.com/articles/d41586-020-00895-8 [DOI] [PubMed] [Google Scholar]
- 14.Wölfel R. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020:1–10. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 15.van Doremalen N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chin A.W.H. Stability of SARS-CoV-2 in different environmental conditions. The Lancet. MAY 01, 2020;1(1):E10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms. assessed on 29/5/2020.
- 18.Zhang W. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microb. Infect. 2020;9(1):386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia J., Tong J., Liu M., Shen Y., Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020;92:589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng L., Liu J., Xu W. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swab specimens. J. Med. Virol. 2020:1–5. doi: 10.1002/jmv.25936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wadman M., Couzin-Frankel J., Kaiser J., Matacic C. A rampage through the body. Am. Assoc. Adv. Sci. 2020:356–360. doi: 10.1126/science.368.6489.356. [DOI] [PubMed] [Google Scholar]
- 22.Das Gaurav, Mukherjee Nabanita, Ghosh Surajit. Neurological insights of COVID-19 pandemic. ACS Chem. Neurosci. 2020;11(9):1206–1209. doi: 10.1021/acschemneuro.0c00201. [DOI] [PubMed] [Google Scholar]
- 23.Baig A.M. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 24.Eastman Richard T., Roth Jacob S., Brimacombe Kyle R., Simeonov Anton, Shen Min, Patnaik Samarjit, Hall Matthew D. Remdesivir: a Review of its Discovery and development Leading to emergency use Authorization for Treatment of COVID-19. ACS Cent. Sci. 2020;6(5):672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.https://spectrum.ieee.org/the-human-os/biomedical/imaging/hospitals-deploy-ai-tools-detect-covid19-chest-scans. accessed on 29/5/2020.
- 26.https://phil.cdc.gov/Details.aspx?pid=23354. accessed on 13/05/2020.
- 27.Ai T. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baig A.M. Updates on what ACS reported: emerging evidences of COVID-19 with nervous system involvement. ACS Chem. Neurosci. 2020;11(9):1204–1205. doi: 10.1021/acschemneuro.0c00181. [DOI] [PubMed] [Google Scholar]
- 29.https://www.thehindu.com/news/national/icmr-approves-use-of-diagnostic-machine-used-for-drug-resistant-tb-for-covid-19/article31308299. accessed on 29/5/2020.
- 30.https://dst.gov.in/dst-funded-startup-develops-kits-testing-asymptomatic-covid-19-infections-gears-vaccine-production. accessed on 29/05/2020.
- 31.Seo G. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14(4):5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 32.Udugama B. Diagnosing COVID-19: the Disease and Tools for detection. ACS Nano. 2020;14(4):3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 33.Pardee K. Rapid, low-cost detection of Zika virus using programmable biomolecular components. Cell. 2016;165(5):1255–1266. doi: 10.1016/j.cell.2016.04.059. [DOI] [PubMed] [Google Scholar]
- 34.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda- Issues-Second-Emergency-Use-Authorization-Decontaminate-N95. assessed on 12/4/2020.
- 35.https://www.nbcnews.com/news/us-news/Inside-Secret-Dhs-Lab-Testing-How-Long- Coronavirus-Can-Survive-N1201386). accessed on 14 May 2020.
- 36.Darnell M.E. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol Methods. 2004;121(1):85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darnell M.E., Taylor D.R. Evaluation of inactivation methods for severe acute respiratory syndrome coronavirus in noncellular blood products. Transfusion. 2006;46(10):1770–1777. doi: 10.1111/j.1537-2995.2006.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.https://www.bbc.com/future/article/20200327-can-you-kill-coronavirus-with-uv-light. accessed on 29/5/2020
- 39.Chouirfa H. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019;83:37–54. doi: 10.1016/j.actbio.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 40.Xing Y. Chitosan-based coating with antimicrobial agents: preparation, property, mechanism, and application effectiveness on fruits and vegetables. Int. J. Polym. Sci. 2016;2016 [Google Scholar]
- 41.Mukherjee M., De S. Antibacterial polymeric membranes: a short review. Environ. Sci.: Water Res. Technol. 2018;4(8):1078–1104. [Google Scholar]
- 42.Xie D., Howard L., Almousa R. Surface modification of polyurethane with a hydrophilic, antibacterial polymer for improved antifouling and antibacterial function. J. Biomater. Appl. 2018;33(3):340–351. doi: 10.1177/0885328218792687. [DOI] [PubMed] [Google Scholar]
- 43.Borkow G. A novel anti-influenza copper oxide containing respiratory face mask. PloS One. 2010;(6):5. doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warnes S.L., Keevil C.W. Inactivation of norovirus on dry copper alloy surfaces. PloS One. 2013;(9):8. doi: 10.1371/journal.pone.0075017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vincent M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018;124(5):1032–1046. doi: 10.1111/jam.13681. [DOI] [PubMed] [Google Scholar]
- 46.Sundberg K. Effectiveness of nanomaterial copper cold spray surfaces on inactivation of influenza A virus. J. Biotechnol. Biomater. 2015;5(205):2. [Google Scholar]
- 47.Li Y. A smart multi-functional coating based on anti-pathogen micelles tethered with copper nanoparticles via a biosynthesis method using l-vitamin C. RSC Adv. 2018;8(33):18272–18283. doi: 10.1039/c8ra01985a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haldar J. Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. Proc. Natl. Acad. Sci. Unit. States Am. 2006;103(47):17667–17671. doi: 10.1073/pnas.0608803103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haldar J. Hydrophobic polycationic coatings inactivate wild-type and zanamivir-and/or oseltamivir-resistant human and avian influenza viruses. Biotechnol. Lett. 2008;30(3):475–479. doi: 10.1007/s10529-007-9565-5. [DOI] [PubMed] [Google Scholar]
- 50.Haldar J., Weight A.K., Klibanov A.M. Preparation, application and testing of permanent antibacterial and antiviral coatings. Nat. Protoc. 2007;2(10):2412. doi: 10.1038/nprot.2007.353. [DOI] [PubMed] [Google Scholar]
- 51.Hsu B.B. Mechanism of inactivation of influenza viruses by immobilized hydrophobic polycations. Proc. Natl. Acad. Sci. Unit. States Am. 2011;108(1):61–66. doi: 10.1073/pnas.1017012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiliket G. A new material for airborne virus filtration. Chem. Eng. J. 2011;173(2):341–351. [Google Scholar]
- 53.Lin N. Nanodarts, nanoblades, and nanospikes: mechano-bactericidal nanostructures and where to find them. Adv. Colloid Interface Sci. 2018;252:55–68. doi: 10.1016/j.cis.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 54.https://www.cranfield.ac.uk/press/news-2020/cranfield-university-helps-create-new-low-cost-simple-ventilators-for-covid19-patients. accessed on 29/5/2020
- 55.https://timesofindia.indiatimes.com/Home/Education/News/How-Iits-Are-Experimenting-To- battle-against-covid-19/articleshow/75007602.Cms
- 56.Speshock J.L. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010;8(1):19. doi: 10.1186/1477-3155-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaikwad S. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013;8:4303. doi: 10.2147/IJN.S50070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakamura S. Synthesis and application of silver nanoparticles (Ag NPs) for the prevention of infection in healthcare workers. Int. J. Mol. Sci. 2019;20(15):3620. doi: 10.3390/ijms20153620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kerry R.G. Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomed. Nanotechnol. Biol. Med. 2019;18:196–220. doi: 10.1016/j.nano.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y.-N. Antiviral Activity of graphene–silver Nanocomposites against non- Enveloped and enveloped viruses. Int. J. Environ. Res. Publ. Health. 2016;13(4):430. doi: 10.3390/ijerph13040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmed S.R., Nagy É., Neethirajan S. Self-assembled star-shaped chiroplasmonic gold nanoparticles for an ultrasensitive chiro-immunosensor for viruses. RSC Adv. 2017;7(65):40849–40857. [Google Scholar]
- 62.Chan Warren C.W. Nano Research for COVID-19. ACS Nano. 2020;14(4):3719–3720. doi: 10.1021/acsnano.0c02540. [DOI] [PubMed] [Google Scholar]
- 63.Chellamani K., Veerasubramanian D., Balaji R.V. Surgical face masks: manufacturing methods and classification. J. Acad. Ind. Res. 2013;2:320–324. [Google Scholar]
- 64.Chavoshi S.Z., Goel S., Morantz P. Current trends and future of sequential micro- machining processes on a single machine tool. Mater. Des. 2017;127:37–53. [Google Scholar]
- 65.Aimi M.F. High-aspect-ratio bulk micromachining of titanium. Nat. Mater. 2004;3(2):103–105. doi: 10.1038/nmat1058. [DOI] [PubMed] [Google Scholar]
- 66.Yang S.Y. Virus filtration membranes prepared from nanoporous block copolymers with good dimensional stability under high pressures and excellent solvent resistance. Adv. Funct. Mater. 2008;18(9):1371–1377. [Google Scholar]
- 67.Cross G.L. The production of nanostructures by mechanical forming. J. Phys. Appl. Phys. 2006;(20):39. R363. [Google Scholar]
- 68.McKenzie W., Pethica J., Cross G. A direct-write, resistless hard mask for rapid nanoscale patterning of diamond. Diam. Relat. Mater. 2011;20(5–6):707–710. [Google Scholar]
- 69.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323(18):1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 70.Leung N.H. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020:1–5. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tortorici M.A. Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struct. Mol. Biol. 2019;26(6):481–489. doi: 10.1038/s41594-019-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.https://www.virustaticshield.com/. accessed on 17/5/2020.
- 73.Te Velthuis A.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quan F.-S. Universal and reusable virus deactivation system for respiratory protection. Sci. Rep. 2017;7(1):1–10. doi: 10.1038/srep39956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han S., Kim C., Kwon D. Thermal/oxidative degradation and stabilization of polyethylene glycol. Polymer. 1997;38(2):317–323. [Google Scholar]
- 76.Cheang P., Khor K. Addressing processing problems associated with plasma spraying of hydroxyapatite coatings. Biomaterials. 1996;17(5):537–544. doi: 10.1016/0142-9612(96)82729-3. [DOI] [PubMed] [Google Scholar]
- 77.Sun L. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. 2001;58(5):570–592. doi: 10.1002/jbm.1056. an Official Journal of the Society for Biomaterials, the Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. [DOI] [PubMed] [Google Scholar]
- 78.Vardelle A. The 2016 thermal spray roadmap. J. Therm. Spray Technol. 2016;25(8):1376–1440. [Google Scholar]
- 79.Geesink R.G., de Groot K., KLEIN C.P. Chemical implant fixation using hydroxyl-apatite coatings: the development of a human total hip prosthesis for chemical fixation to bone using hydroxyl-apatite coatings on titanium substrates. Clin. Orthop. Relat. Res. 1987;225:147–170. [PubMed] [Google Scholar]
- 80.Torsi L. Organic field-effect transistor sensors: a tutorial review. Chem. Soc. Rev. 2013;42(22):8612–8628. doi: 10.1039/c3cs60127g. [DOI] [PubMed] [Google Scholar]
- 81.Angione M.D. Interfacial electronic effects in functional biolayers integrated into organic field-effect transistors. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(17):6429–6434. doi: 10.1073/pnas.1200549109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hai W. Human influenza virus detection using sialyllactose-functionalized organic electrochemical transistors. Sensor. Actuator. B Chem. 2018;260:635–641. [Google Scholar]
- 83.Liao J. Functional sensing interfaces of PEDOT: PSS organic electrochemical transistors for chemical and biological sensors: a mini review. Sensors. 2019;19(2):218. doi: 10.3390/s19020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schröder Imke. COVID-19: a risk assessment perspective. ACS Chem. Health Saf. 2020;27(3):160–169. doi: 10.1021/acs.chas.0c00035. [DOI] [PubMed] [Google Scholar]
- 85.Forster P. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc. Natl. Acad. Sci. Unit. States Am. 2020:202004999. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sang P. Anti-HIV drug repurposing against SARS-CoV-2. RSC Adv. 2020;10(27):15775–15783. doi: 10.1039/d0ra01899f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wrapp D. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Junmei. Fast Identification of possible drug Treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study. J. Chem. Inf. Model. Article ASAP. 2020 doi: 10.1021/acs.jcim.0c00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jin Z., Du X., Xu Y. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature. 2020:2020. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 90.Liu Y. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020:1–6. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 91.https://www.trialsitenews.com/cansino-biologics-ad5-ncov-the-first-covid-19-vaccine-to- Phase-Ii-Clinical-Trials/. accessed on 13/5/2020.
- 92.https://www.clinicaltrialsarena.com/comment/covid-19-vaccine-development/. accessed on 13/5/2020.
- 93.https://www.nih.gov/news-events/news-releases/Nih-Clinical-Trial-Investigational-Vaccine- Covid-19-Begins. accessed on 13/5/2020.
- 94.https://clinicaltrials.gov/. accessed on 13/5/2020.
- 95.Robbins T. SAGE Publications Sage UK; London, England: 2020. COVID-19: A New Digital Dawn? [Google Scholar]
- 96.Jin Song. COVID-19, climate change, and renewable energy research: we are all in this together, and the time to act is now. ACS Energy Lett. 2020;5(5):1709–1711. doi: 10.1021/acsenergylett.0c00910. [DOI] [PubMed] [Google Scholar]
- 97.https://www.pmindia.gov.in/en/government_tr_rec/namami-gange/. accessed on 29/5/2020
- 98.Hallema D.W., Robinne F.-N., McNulty S.G. Pandemic spotlight on urban water quality. Ecol. Process. 2020;9(1):1–3. doi: 10.1186/s13717-020-00231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.https://www.esa.int/Applications/Observing_the_Earth/Copernicus/Sentinel- 5P/Coronavirus_lockdown_leading_to_drop_in_pollution_across_Europe. accessed on 18/5/2020.
- 100.http://www.esa.int/Applications/Observing_the_Earth/Copernicus/Sentinel- 5P/Air_pollution_drops_in_India_following_lockdown. accessed on 18/5/2020.
- 101.Zeitlin C. Update on galactic cosmic ray integral flux Measurements in lunar orbit with CRaTER. Space Weather. 2019;17(7):1011–1017. [Google Scholar]
- 102.https://www.cdc.gov/onehealth/basics/index.html. accessed on 15.5.2020.
- 103.Yu Y. Biosafety materials: an emerging new research Direction of materials Science from COVID-19 outbreak. Mater. Chem. Front. 2020 [Google Scholar]
- 104.de Kraker M.E., Stewardson A.J., Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;(11):13. doi: 10.1371/journal.pmed.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.http://www.euro.who.int/en/health-topics/disease-prevention/antimicrobial-resistance/education,-awareness-and-behaviour-change/infographics/infographic-amr-and-covid-19. accessed on 29/5/2020
- 106.https://www.unenvironment.org/news-and-stories/story/six-nature-facts-related-coronaviruses. accessed on 29/5/2020