Abstract
Objective
The effects of the periodontal intervention on rats with type-II diabetes mellitus (T2DM) and chronic periodontitis (CP) were explored through observing the changes in carotid artery pathology and interleukin-6 (IL-6) levels.
Methods
The rats were randomly divided into 5 groups, i.e. group A (the normal control group), group B (the T2DM control group), group C (the CP control group), group D (the T2DM + CP group), and group E (the periodontal intervention T2DM + CP group). Blood samples of rats were collected from angular veins respectively at the following 5 time nodes: 1 week before the intervention (T1), 1 week after the intervention (T2), 3 weeks after the intervention (T3), 5 weeks after the intervention (T4), and 7 weeks after the intervention (T5); IL-6 concentrations before and after the intervention were determined by the enzyme-linked immunosorbent assay (ELISA), and the pathology of carotid arteries were observed by the Hematoxylin-Eosin (HE) stain.
Results
The pathological results of carotid arteries showed that the blood vessels of rats in group A were normal in morphology; most of the carotid artery vessel walls of rats in groups B, C, and D were significantly thickened and the fibers were disorderly arranged; the increased thickness of vessel walls of rats in group E was reduced, a small number of foam cells and inflammatory cells were observed, and the irregular arrangement of fibers was improved. In terms of the IL-6 concentrations, during the period of T1-T5, in groups B, C, and D, the IL-6 concentrations in rats were increased (P < 0.05); after the periodontal intervention, in group E, the IL-6 concentrations in rats were first increased then decreased (P < 0.05).
Conclusion
In terms of the long-term effects, periodontal intervention may reduce the inflammations of patients with diabetes mellitus and periodontitis and improve the lesions of carotid arteries.
Keywords: Chronic periodontitis, Type-II diabetes mellitus, Carotid artery pathology, Interleukin-6, Enzyme linked immunosorbent assay
1. Introduction
Periodontitis is a chronic inflammatory disease characterized by periodontal tissue destruction, which leads to the loss of connective tissue attachments, the absorption of alveolar bone, the formation of pathological periodontal pockets that affects the periodontal tissues, and ultimately the loss of teeth (Hong et al., 2016). More than half of the adults all over the world have had chronic periodontitis, and the incidence rate of periodontitis among people over 60-year-old is as high as 80% (Rotimi et al., 2010). Periodontitis is not limited to periodontal tissues; it is also closely related to systemic health problems such as diabetes mellitus, cardiovascular diseases, and obesity (Meurman et al., 2004, Davé and Dyke, 2008). Type II diabetes mellitus (T2DM) is the most common form of diabetes mellitus, accounting for 95% of all diabetes cases (Oh et al., 2016). Most of the diabetes patients died of various complications of diabetes mellitus, in which cardiovascular and cerebrovascular complications account for the majority (Andrea et al., 2018). Atherosclerosis (AS) is the primary pathogenesis of the cardiovascular and cerebrovascular diseases (González-Quijada et al., 2018, Yazouli et al., 2018), while periodontal damages and diabetes mellitus are two factors that cause atherosclerosis. Therefore, periodontitis and diabetes mellitus share many same risk factors, and they are also high-risk factors of each other. Borgnakke and Wang (Borgnakke et al., 2013, Wang et al., 2013) found that chronic periodontitis (CP), T2DM, and AS are related due to the same host background of chronic inflammations; the inflammatory response and related vascular tissue endothelial responses are important to the evolution of the diseases.
Interleukin-6 (IL-6) is closely related to CP, T2DM, and AS; it is a predictor of macrovascular lesions in patients with T2DM, which is not only related to insulin resistance but also positively correlated with the degree of resistance; it may participate in the progression of diabetes mellitus (Pihlstrom et al., 2005, Chavarry et al., 2009). Inflammation has inevitable effects on health; therefore, the treatments of periodontitis should not only focus on solving the oral problems of patients but also formulate reasonable therapeutic plans to reduce the inflammation as much as possible (AlSohail et al., 2013). Studies on the effects of the oral intervention on diabetes mellitus and periodontitis are frequently seen; however, most of the studies focused on the control and improvement of periodontal inflammation and glucose metabolism indicators, while few studies focused on the effects on blood vessels. Based on the rat models of type-II diabetes mellitus and chronic periodontitis, changes in serum inflammatory factor IL-6 levels and carotid vessel wall pathology before and after periodontal treatment intervention were compared to explore and discuss the effects of periodontal intervention on rats with type-II diabetes mellitus and chronic periodontitis, hoping to provide experimental foundation for the diagnosis and treatments of patients with such diseases.
2. Materials and methods
2.1. Animals
A total of 60 6-week-old male SD rats, weighed 200 ± 10 g. After adaptive feed, the rats were randomly divided into 5 groups, i.e. group A (the normal control group, 10 rats), group B (the T2DM control group, 10 rats), group C (the CP control group, 10 rats), group D (the T2DM + CP group, 10 rats), and group E (the periodontal intervention T2DM + CP group, 20 rats). The animal treatment and experimental procedures conform to the national standards for experimental animals, and the experiment was reported to the ethics committee of the hospital.
2.2. Model construction and grouping
The normal control group: rats in the normal control group were given standard feed without any interventions.
The type-II diabetes mellitus (T2DM) control group: Rats in the T2DM control group were first given adaptive feed; then, the proportion of high-fat and high-glucose feed was gradually increased, and rats were fed for 8 consecutive weeks; the fasted rats were injected with streptozocin (STZ) solution intraperitoneally at a dosage of 25 mg/kg, after 72 h, the fasting plasma glucose (FPG) of each rat was assayed; if the assayed FPG exceeded 7.8 mmol/L, the model was successfully constructed, otherwise the rat was given STZ solutions continuously until the model was successfully constructed.
The chronic periodontitis (CP) control group: Rats in the CP control group were first given adaptive feed; then, each fasted rat was injected with 5% chloral hydrate, whose unilateral experimental teeth were treated with orthodontic wires (Changsha Tiantian Dental Equipment, China) to form gaps between teeth; after 2 weeks, the orthodontic wires were removed, covered with about 15 rings of threads, placed on the dental cervix of rat through the gaps between experimental teeth, and tied together in the shape of “8″. The porphyromonas gingivalis (P.g) suspension with formulated concentration was applied to the orthodontic wires and the gum gaps every 3 days; the experimental teeth of rats were observed every week until the manifestations and symptoms of periodontitis were seen.
The T2DM + CP group: Rats in the T2DM + CP group were modeled by the same methods as the T2DM control group and the CP control group, the diet was consistent with the T2DM control group.
Group E (the periodontal intervention T2DM + CP group): Periodontal mechanical treatments were given to rats in group E (the periodontal intervention T2DM + CP group); the specific procedures included the removal of orthodontic wires and threads, and the scaling and root planing of the experimental teeth.
2.3. Periodontal examinations
The periodontal probe (Shanghai New Medical Instrument Factory, China) was used to routinely examine the probing depth (PD), bleeding index (BI), tooth mobility (TM), and gingival recession of each experimental tooth of rats in each group; in addition, in the reference of Mazza scoring criteria, the BI value (0 indicated healthy gums without bleeding; 1 indicated mild inflammation of the gums, without bleeding; 2 indicated dotted bleeding; 3 indicated bleeding, and the blood extended along the edge of the gums; 4 indicated bleeding overflew the gingival sulcus; 5 indicated spontaneous bleeding) of the most severe point of each experimental tooth of each rat was recorded (Lockhart et al., 2012), and was checked once a week.
2.4. Serum IL-6 assay
Blood samples of rats were collected from angular veins respectively at the following 5 time nodes: 1 week before the intervention (T1), 1 week after the intervention (T2), 3 weeks after the intervention (T3), 5 weeks after the intervention (T4), and 7 weeks after the intervention (T5); the volume of blood samples collected from each rat each time was 1.5 mL. The samples were centrifuged at a low temperature of 4 °C to separate the serum, and the supernatant was sealed and stored at −20 °C. The serum IL-6 concentrations of rats in the 5 groups were determined by the enzyme-linked immunosorbent assay (ELISA). The specific steps were as described in the ELISA kit.
The specific steps were as follows: First, the sample diluent was diluted 10 times with distilled water. A total of 8 centrifuge tubes with a volume of 1.5 mL were prepared. The first tube was added with 900 μL of the sample diluent and 100 μL of the 80 ng/mL standard solution, and the mixture was well-mixed. The remaining tubes were added with 500 μL of the sample diluent in sequence. A total of 500 μL of the solution in the first tube was pipetted and added into the second tube by using a micropipette, and the mixture was well-mixed. The above operation was repeated to sequentially diluted\ the solutions in the remaining 5 tubes; finally, 500 μL of the mixed solution pipetted from the seventh tube was discarded. The eighth tube was the blank control group. The washing solution was diluted 20 times with double-distilled water. In a degressive concentration gradient of 8000 pg·mL−1, 4000 pg·mL−1, 2000 pg·mL−1, 1000 pg·mL−1, 500 pg·mL−1, 250 pg·mL−1, 125 pg·mL−1, and 0 pg·mL−1, the standard was added into the first 7 holes on the first row of the ELISA plate, with 0.1 mL in each hole; the remaining 1 hole on the first row was only added with 0.1 mL of sample diluent as the zero hole. Each remaining hole on the plate was directly added with 100 μL of the serum sample to be tested, and the reaction plate was thoroughly mixed. After the plate was sealed with a sealing membrane, it was reacted in a light-proof environment and a constant temperature of 37 °C for 40 min. After the plate was washed, each hole of the reaction plate was successively added with 50 μL of deionized water and 50 μL of the first antibody working solution; the mixture was mixed thoroughly, and the plate was sealed with sealing membrane; then, it was reacted in an environment of 37 °C for about 20 min. After the plate was washed, each hole was added with 100 µL of ELISA working solution; after being thoroughly mixed, the plate was sealed with sealing membrane and reacted for 10 min. After the plate was washed, each hole was added with 100 µL of substrate working solution and reacted in a light-proof environment at 37 °C for 15 min. The terminating solution was sequentially added to each hole in an amount of 100 μL per hole and mixed thoroughly. The absorbance OD value was measured at a wavelength of 450 nm within 0.5 h by using an enzyme mark instrument, and the data information such as the corresponding IL-6 content and the standard curves were calculated by using the relevant ELISA analysis software.
2.5. Hematoxylin-Eosin (HE) stain
Rats in each group were anesthetized and executed 7 weeks after the intervention. The carotid artery branches of the rats were harvested, soaked, rinsed, and fixed in 10% neutral formalin for 24 h to prepare the vascular paraffin tissue blocks, which were then sectioned at a thickness of about 2.0 μm. The paraffin sections were baked on a constant temperature baker at about 60 °C; then, the sections were stained with Hematoxylin-Eosin (HE). Next, the sections were dewaxed and hydrated in xylene I, xylene II, absolute alcohol I, absolute alcohol II, 90% ethanol I, 80% ethanol II, and distilled water successively. The hematoxylin staining lasted for 3–5 min; then, the sections were differentiated 1 s with hydrochloric acid alcohol, and the staining was blued by ammonia water for 1 s. The eosin staining lasted for 1–2 min; the sections were dehydrated successively in 80% ethanol, 90% ethanol, absolute alcohol, xylene, xylene II, and xylene III. After being slightly dried, the sections were sealed with gum, and the lesions of carotid artery vessel walls were observed under a microscope (OLYMPUS, Japan).
2.6. Statistical processing
Statistics software SAS V8 was used to process and analyze all data collected. All the quantitative data were described by mean number ± standard deviation ( ± s), and P < 0.05 was taken as the test criterion.
3. Results
3.1. Results of periodontal examinations
Teeth of rats in groups A and B were normal; after the models were successfully constructed, symptoms of periodontitis were observed on rats in groups C, D, and E; a large amount of soft scale and food residue were seen in the wire ligated position, with a small amount of dental calculus; after the orthodontic wires were removed, tissues around the gums were obviously inflamed, which were reddish; in addition, the edges of the gums were slightly thickened, the texture was softer, the elasticity disappeared, and the gums would bleed obviously with slight touches; the BI values reached 3–4, and the gingival recession of some of the rat gums reached 1/3 of the tooth roots, accompanied by II ~ III° of TM, and the probing depth (PD) was 0.4–0.5 cm. Before the rats were executed, the periodontal conditions of rats in each group were different. Compared with the inflammation observed at model construction, the periodontitis of rats in group C became more severe, the TM was aggravated, the BI basically reached 3 ~ 4, and the TM of experimental teeth was II ~ III°, and the PD was between 1.0 and 1.1 cm. Gums of rats in group D became dark red, the gingival recession became more severe, the BI basically reached 3 ~ 4, the TM was II ~ III°, and the teeth of a small number of rats were lost, and the PD was between 1.0 and 1.2 cm. In group E, the periodontitis was improved, the color of teeth was lighter, the swelling was relieved, the BI was reduced to 2–3, and the TM of the experimental teeth was recovered to I ~ II°, and the PD was between 0.6 and 1.1 cm. It can be seen that there was no significant difference in PD, BI, and TM between group A and group B (P < 0.05), and there was no significant difference in PD, BI and TM between groups C, D and E (P < 0.05), but PD, BI and TM of group A and group B had statistical difference with groups C and D, groups E and C, and groups D and E (P > 0.05). The details were shown in Table 1.
Table 1.
Periodontal changes of rats in each group during T1-T5.
| Groups | Items | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|
| Group A | PD | 0.45 ± 0.27 | 0.44 ± 0.25 | 0.47 ± 0.22 | 0.44 ± 0.28 | 0.46 ± 0.21 |
| BI | 0.26 ± 0.05 | 0.26 ± 0.04 | 0.28 ± 0.05 | 0.27 ± 0.04 | 0.28 ± 0.04 | |
| TM | 0° | 0° | 0° | 0° | 0° | |
| Group B | PD | 0.47 ± 0.25 | 0.45 ± 0.28 | 0.47 ± 0.26 | 0.46 ± 0.23 | 0.44 ± 0.24 |
| BI | 0.27 ± 0.04 | 0.31 ± 0.04 | 0.32 ± 0.03 | 0.32 ± 0.04 | 0.33 ± 0.05 | |
| TM | 0° | 0° | 0° | 0° | 0° | |
| Group C | PD | 1.01 ± 0.21 | 1.04 ± 0.19 | 1.03 ± 0.25 | 1.05 ± 0.37 | 1.10 ± 0.32 |
| BI | 3.39 ± 0.31 | 3.41 ± 0.22 | 3.45 ± 0.30 | 3.52 ± 0.34 | 3.55 ± 0.27 | |
| TM | II ~ III° | II ~ III° | II ~ III° | II ~ III° | II ~ III° | |
| Group D | PD | 1.07 ± 0.22 | 1.09 ± 0.28 | 1.10 ± 0.21 | 1.10 ± 0.33 | 1.13 ± 0.29 |
| BI | 3.45 ± 0.41 | 3.51 ± 0.36 | 3.55 ± 0.32 | 3.64 ± 0.21 | 3.67 ± 0.23 | |
| TM | II ~ III° | II ~ III° | II ~ III° | II ~ III° | II ~ III° | |
| Group E | PD | 1.08 ± 0.26 | 1.00 ± 0.35 | 0.91 ± 0.18 | 0.73 ± 0.45 | 0.66 ± 0.31 |
| BI | 3.44 ± 0.38 | 3.21 ± 0.33 | 3.01 ± 0.37 | 2.74 ± 0.43 | 2.41 ± 0.35 | |
| TM | II ~ III° | II ~ III° | II ~ III° | I ~ II° | I ~ II° |
3.2. Results of carotid artery pathological HE stains
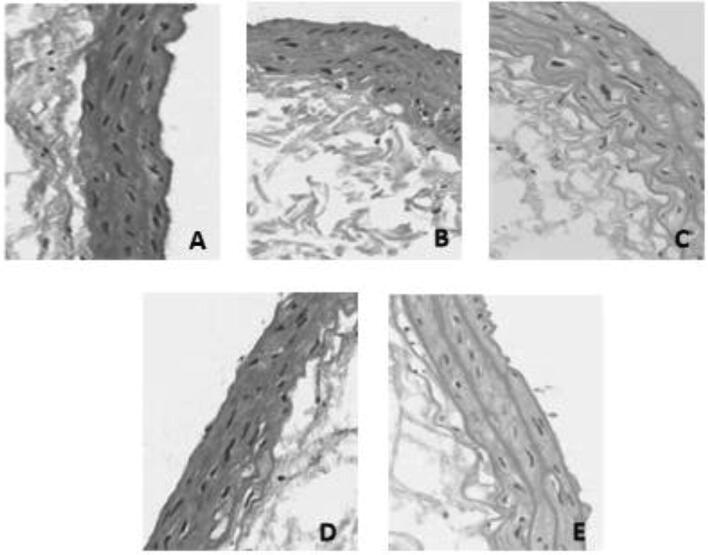
The morphology of vessel walls of rats in group A was normal; in groups B, C, and D, the changes in morphology and thickness of vessel walls were different. In group B, the thickness of rat vessel walls was increased in different degrees; the elastic fibers were irregularly arranged, and a lot of inflammatory cells were seen in the vessel walls, accompanied with vacuolar degeneration. In group C, the intima-media membranes of the vessel walls were thickened in different degrees; the inflammatory cells were widely distributed in the intima-media membranes of the vessel walls, the fibers were irregularly arranged with a few fractures, the smooth muscle cell (SMC) in the vessel walls was denatured, and foam cells appeared. In group D, the thickening degree of vessel walls was the most obvious, most of the SMC was atrophied and disappeared, a large number of foam cells and inflammatory cells was seen, which were in extensive distribution; the elastic fibers were irregularly arranged with fractures. In group E, the thickening of rat vessel walls was not as obvious as that in group D; most of the vessel conditions were improved, the vessel walls were slightly thickened, a small number of foam cells and inflammatory cells were distributed in the intima-media membranes of the vessel walls, and the disordered elastic fibers were improved. The details were shown in Fig. 1.
Fig. 1.
Changes in pathological results of carotid arteries of rats in each group after the 7-week periodontal intervention (HE, ×200).
4. IL-6 concentrations in rats before and after the periodontal intervention
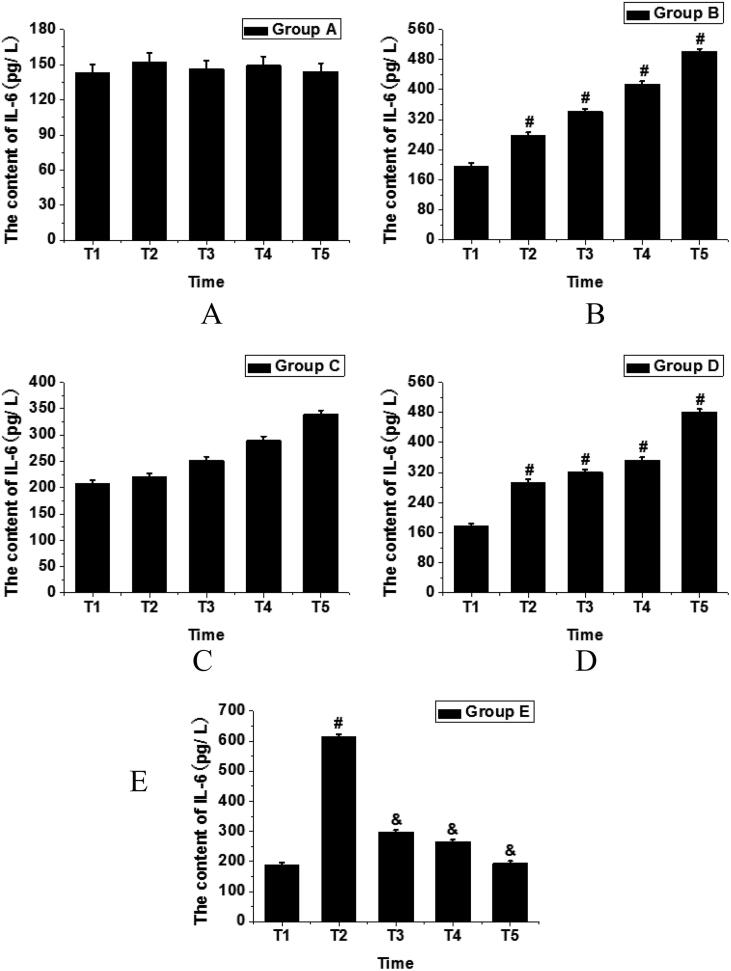
In group A, the IL-6 concentrations in rats fluctuated slightly during the T1-T5 time periods, with no obvious changes and thereby no statistical significance (P > 0.05). In group B, the IL-6 concentrations in rats were increased during the T1-T5 time periods, and the differences were statistically significant (P < 0.05). In group C, during the T1-T5 time periods, the IL-6 concentrations in rats were significantly higher than that in group A. In group D, during the T1-T5 time periods, the IL-6 concentrations also showed the same continuous increasing trend as group C (P < 0.005); the highest concentration appeared at T5, which was about 3.34 times of that in group A and about 2.49 times of that in group E; in addition, in group D, after reaching the T2 time node, the IL-6 concentrations were significantly higher than that in group C, and the differences were statistically significant (P < 0.05). In group E, respectively at the times nodes after intervention (time nodes T2, T3, T4, and T5), the concentrations of IL-6 were significantly higher than that in group A (P < 0.05); in addition, after the first periodontal intervention, the concentrations of IL-6 in group E increased sharply from T1 time node to a higher level of T2 time node, began to decrease at T3 time node, and dropped to the lowest at T5 time node, the differences were statistically significant (P < 0.05); besides, at the T5 time node, the concentrations dropped to that of T1 time node, and the differences were not statistically significant (P > 0.05); in group E, the IL-6 concentrations in rats at T4 and T5 time nodes were lower than those in group D at the same time nodes, with statistical differences (P < 0.05). The details were shown in Table 2 and Fig. 2.
Table 2.
Changes in IL-6 concentrations in rats during T1-T5 time periods (pg L-1).
| Groups | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|
| Group A | 143.10 ± 39.56 | 152.31 ± 35.91 | 146.22 ± 27.87 | 149.52 ± 33.69 | 144.04 ± 31.73 |
| Group B | 197.36 ± 32.95 | 278.55 ± 23.84 | 341.78 ± 17.52 | 414.81 ± 21.08 | 501.41 ± 29.97 |
| Group C | 207.50 ± 41.74 | 219.88 ± 29.21 | 251.33 ± 30.12 | 289.87 ± 32.51 | 339.27 ± 31.46 |
| Group D | 177.54 ± 51.22 | 294.71 ± 20.53 | 320.76 ± 28.97 | 353.66 ± 17.71 | 481.72 ± 20.03 |
| Group E | 189.24 ± 41.53 | 615.29 ± 52.77 | 297.35 ± 37.42 | 265.44 ± 29.05 | 193.78 ± 25.85 |
Fig. 2.
Analysis of IL-6 concentration change in T1-T5 time interval of each group (A. Group A; B. Group B; C. Group C; D. Group D; E. Group E. The data of each group were analyzed at different time intervals, in which # P < 0.05 represents the statistical difference of IL-6 concentration in other time intervals in the group compared with T1 time interval, and & P < 0.05 represents the statistical difference of IL-6 concentration in other time intervals in the group compared with T2 time interval).
5. Discussion
IL-6 can reflect the occurrence and development of AS lesions (Matsumoto et al., 2010). The results of the experiment showed that the rat IL-6 concentrations in group B increased at all the time nodes (P < 0.05), indicating that the inflammatory responses of T2DM would increase with time, which increased the risks of vascular diseases. Periodontal pathogens and the subsequent periodontitis can increase the IL-6 concentrations (Watarai et al., 2006). The IL-6 concentrations in group C during the T1-T5 periods were all higher than those in group A (P < 0.05); in addition, most of the intima-media membranes in rat vessel walls were increased, with a large number of inflammatory cells seen in the membranes, and the elastic fibers were disorderly arranged, indicating that if chronic periodontitis was not treated, the systemic inflammatory responses would be aggravated, and IL-6 may be related to vascular diseases. During T1-T5, the IL-6 concentrations in groups D and E were higher than those in group A at the same time node, and the degrees were different (P < 0.05); the inflammations in group D were aggravated as the time was prolonged, and the IL-6 concentrations continued to increase and reached the highest at T5. At the same time, the thickness of carotid artery vessel walls of the rats in group D was increased significantly, the foam cells were formed, the inflammatory cells appeared in large numbers, and the elastic fibers were arranged irregularly with partial fractures. All of these indicated that in terms of rats with T2DM and CP if the chronic periodontitis persisted for a long time, the risks of vascular lesions in rats would be increased, thereby the degree of the lesions was increased.
Studies on the correlations of periodontal treatments and inflammation factors were various. Higashi et al. (2008) proved that periodontal treatments could improve the conditions of periodontal health to a certain extent; however, the periodontal treatments would not affect the levels of vascular markers. The experimental results have shown that the levels of IL-6 in group E was increased significantly at T2 time node; however, the levels of IL-6 began to decrease at T3 time node and the decreasing trend was kept during the following time nodes, which approached the baseline level (the T1 level) at T5 time node; in addition, the pathological results showed that 7 weeks after intervention, the vascular lesions of rats were improved, indicating that the periodontal intervention in rats with T2DM and CP may lead to the temporary increases of serum IL-6 levels and the risks of vascular diseases in rats, which should be vigilant; however, as the treatment proceeded, the periodontal intervention could greatly improve the systemic inflammatory responses in rats, thereby further reducing the risks of vascular diseases. Therefore, clinically, while performing the periodontal intervention to patients with both T2DM and CP, appropriate medical treatments should also be given, which may be effective in controlling local infections and systemic inflammations, thereby achieving better treatment results.
In summary, in terms of the long-term effects, periodontal intervention may reduce the inflammations of patients with diabetes mellitus and periodontitis and improve the lesions of carotid arteries.
6. Conclusion
In this study, the level of serum validation factor IL-6 and the pathological changes of carotid artery wall were studied in rats with T2DM. 60 healthy rats were divided into five groups, and different treatment methods were adopted for each group and the model was established. Then, the pathological changes of carotid artery and the level of IL-6 were detected at 5 time points. The results showed that the vascular morphology of group A was normal, while in groups C, D and E, most carotid artery walls were thickened and fibers arranged in disorder. The thickness of vascular wall increased in group E, there was a small amount of foam cells and inflammatory cells, and the irregular arrangement of fibers was improved. IL-6 content in groups B, C and D showed an upward trend during T1-T5 (P < 0.05); the content of IL-6 in group E increased first and then decreased after intervention (P < 0.05). Therefore, this experiment can prove that periodontal intervention may reduce the inflammation of patients with T2DM and improve the carotid artery disease. However, there are still some deficiencies in this paper, like the small number of experimental rats, resulting in the contingency of experimental data. In the future research, the number of research samples will be increased to get more accurate results.
Declaration of Competing Interest
The authors declared that they have no conflicts of interest to this work.
Acknowledgement
This work was supported by the Medical and health research project of Zhejiang Province, China (2017RC011 and 2016KYB233), National Natural Science Foundation of Zhejiang province, China (Y2100578 and Y2090486), and the Development project of science and Technology of Hangzhou, China (20110833B09).
Footnotes
Peer review under responsibility of King Saud University.
References
- AlSohail A., Ciancio S., Alsuwyed A. The prevalence of oral Candida infections in periodontitis patients with type 2 diabetes mellitus. J. Infect. Public Health. 2013;6(4):296–301. doi: 10.1016/j.jiph.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Andrea L., Emőke K.T., Ágnes C. Screening risk factors for type 2 diabetes in overweight and obese adolescents in school settings of Hungary: a population-based study. J. King Saud Univ. – Sci. 2018;30(2):176–179. [Google Scholar]
- Borgnakke Wenche S., Ylöstalo Pekka V., Taylor George W., Genco Robert J. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J. Clin. Periodontol. 2013;40:S135–S152. doi: 10.1111/jcpe.12080. [DOI] [PubMed] [Google Scholar]
- Chavarry N.G., Vettore M.V., Sansone C., Sheiham A. The relationship between diabetes mellitus and destructive periodontal disease: a meta-analysis. Oral Health Preventive Dentistry. 2009;7(2):107–127. [PubMed] [Google Scholar]
- Davé S., Dyke T.V. The link between periodontal disease and cardiovascular disease is probably inflammation. Oral Dis. 2008;14(2):95–101. doi: 10.1111/j.1601-0825.2007.01438.x. [DOI] [PubMed] [Google Scholar]
- González-Quijada S., Pérez-González L., de Lagos M.D.Á.M. Non-diagnostic anti-C. burnetii phase I IgG titres: Should they be discarded in elderly patients? J. Infect. Public Health. 2018;11(6):851–855. doi: 10.1016/j.jiph.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Goto C., Jitsuiki D. Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension. 2008;51(2):446–453. doi: 10.1161/HYPERTENSIONAHA.107.101535. [DOI] [PubMed] [Google Scholar]
- Hong J.W., Hyun N.J., Dong-Jun K. The prevalence and associated factors of periodontitis according to fasting plasma glucose in the Korean adults: The 2012–2013 Korea National Health and Nutrition Examination Survey. Medicine. 2016;95(14) doi: 10.1097/MD.0000000000003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart P.B., Bolger A.F., Papapanou P.N. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012;125(20):2520–2544. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Sera Y., Ueki Y. Comparison of serum concentrations of soluble adhesion molecules in diabetic microangiopathy and macroangiopathy. Diabetic Med. 2010;19(10):822–826. doi: 10.1046/j.1464-5491.2002.00799.x. [DOI] [PubMed] [Google Scholar]
- Meurman J.H., Sanz M., Janket S.J. Oral health, atherosclerosis, and cardiovascular disease. Critical Rev Oral Biol. Med.: Off. Publ. Am. Assoc. Oral Biol. 2004;15(6):403–413. doi: 10.1177/154411130401500606. [DOI] [PubMed] [Google Scholar]
- Oh K.J., Lee D.S., Kim W.K. Metabolic adaptation in obesity and Type II diabetes: myokines, adipokines and hepatokines. Int. J. Mol. Sci. 2016;18(1):8. doi: 10.3390/ijms18010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Rotimi V.O., Salako N.O., Divia M. Prevalence of periodontal bacteria in saliva of Kuwaiti children at different age groups. J. Infect. Public Health. 2010;3(2):76–82. doi: 10.1016/j.jiph.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Wang X., Bao W., Liu J. Inflammatory markers and Risk of Type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36(1):166–175. doi: 10.2337/dc12-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai A., Nakashima E., Hamada Y. Aldose reductase gene is associated with diabetic macroangiopathy in Japanese Type-2 diabetic patients. Diab. Med.: J. British Diabetic Assoc. 2006;23(8):894–899. doi: 10.1111/j.1464-5491.2006.01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazouli L.E., Hejaji H., Elmdaghri N., Alami A.A., Dakka N., Radouani F. Investigation of Chlamydia pneumoniae infection in Moroccan patients suffering from cardiovascular diseases. J. Infect. Public Health. 2018;11(2):246–249. doi: 10.1016/j.jiph.2017.07.029. [DOI] [PubMed] [Google Scholar]