Abstract
Saprolegnosis of fresh water fishes caused by Saprolegnia diclina often results in serious economic losses to fish hatcheries. Despite the proven efficiency of malachite green as a potential fungicide in prevention and control of fish saprolegnosis, there is a strong debate about its safety aspects in use since it was documented to be responsible for many carcinogenic and teratogenic attributes. Bioactivity of four ethanolic plant extracts were assessed to attain a natural alternative to the traditional fungicide currently used in saprolegnosis control. Ethanolic extracts of Punica granatum and Thymus vulgaris exhibited a potential efficacy in suppressing mycelial growth of S. diclina at concentration of 0.5 mg/ml while extracts of Nigella sativa and Zingiber officinales were not effective respectively. The extract of pomegranate showed the highest antifungal potency with minimum inhibitory concentration (MIC) of 200 ppm while thyme extract was less effective and recorded MIC of 400 ppm against S. diclina. The acute fish toxicity of the plant extracts indicated the low toxicity of P. granatum and T. vulgaris extracts as no fish mortalities were detected at aquaria containing 200, 400 and 800 ppm of plant extracts respectively. Considering the low toxicity of these plant extracts, it may be concluded that 200 and 400 ppm of pomegranate and thyme extracts which suppressed the mycelial growth of the S. diclina could be safely used for saprolegniasis control.
Both of pomegranate and thyme extracts which proved to possess a potential antifungal activity can be considered as a natural alternative fungicides to control saprolegniasis avoiding carcinogenic malachite green application.
Keywords: Saprolegnosis, Plant extracts, Alternative fungicides, Punica granatum, Thymus vulgaris
1. Introduction
Saprolegniasis of fresh water fishes induced by Saprolegnia parasitica and S. diclina was regarded as the most serious fungal disease threaten fish industry causing a high fish mortality and huge economic losses to fish hatcheries (Thoen et al., 2011, Van Den Berg et al., 2013, Songe et al., 2016). Saprolegniasis was controlled with application of commonly available fungicides as malachite green, formaldehyde, hydrogen peroxide and copper oxysulfate (Barnes et al., 2001, Mitchell et al., 2009, Straus et al., 2009, Earle and Hintz, 2014). Despite the proven efficiency of these chemical fungicides in the prevention and controlling of mycotic fish diseases, their excessive application has resulted in the accumulation of residual toxicity in fish flesh (Sudova et al., 2007). Moreover, the overuse of these fungicides altered water biological balance by decimating beneficial hydrophytes and contaminated the environment (Battaglin and Fairchild, 2002). Also, these fungicides were proven to be responsible for many carcinogenic and teratogenic attributes (Corcoran et al., 2010). In an attempt to modify these conditions, efforts will be exerted to expose the natural sources of antimicrobial agents. These bioactive agent must be effective, inoffensive for fish, safe for human health and don't pose any environmental problems (Madhuri et al., 2012). The search has been extended to plant extracts which possess fungicidal properties, easily degradable by natural microbes and doesn't contributed with any environmental or health risk. Fungicidal activities of plant extracts have been investigated on controlling saprolegniasis of fish and their eggs by several investigators. For example; (Mori et al., 2002, Ghasemi Pirbalouti et al., 2009, Shin et al., 2017) tested suppression of aquatic fungi as Saprolegnia species by some plant extracts; (Rai et al., 2002) screened antifungal effect of five essential oils against the pathogenic Saprolegnia ferax strain isolated from diseased fish; (Tampieri et al., 2003) evaluated fungicidal activities of some selected essential oils on mycelial growth of S. parasitica; (Chukanhom et al., 2005) studied antifungal activities of Alpinia galange against water molds; Thymoquinone extracted from Nigella sativa was potentially effective against Saprolegnia spp. isolated from diseased fish (Hussein et al., 2002). Rohani et al. (2006) evaluated the antifungal activities of some essential oils of Geranium herbarum against Saprolegnia species isolated from diseased rainbow trout; (Ilondu et al., 2009) treated saprolegniasis of Clarias gariepinus (fresh water fish) with aqueous extract of Vernonia amygdalina; (Madhuri et al., 2012) reported antimycotic activity of some medicinal plants against saprolegniasis of fresh water fishes and (Agbebi et al., 2012) investigated the antifungal potential of Euphoriba kamerunica (spurge) against growth of Saprolegnia species isolated form diseased eggs of catfish. They concluded that, 25 ml and 50 ml concentration of spurge could be used to control Saprolgnia infection in fish hatchery. Furhermore, study by Salehi et al. (2015) demonstrated that Euclayptus globules, Thymus daenensis and another forty essential oils were effective in suppressing fungal growth of Saprolegnia parasitica with MIC of 2.5 and 5 ml respectively. Thymus vulgaris and Zingiber officinales extracts exhibited a highly antifungal efficacy and showed fungicidal potency against Rhizoctonia solani, Fusarium oxysporium and Pythium aphanidermatum with MIC of 4 mg/ml and MFC of 8 mg/ml (Mostafa et al., 2012). On the other hand, leaves extract of Thymus vulgaris suppress completely mycelial growth and degenerate hyphae of Pythium ultimum at plant extract concentration of 400 ppm (Ramanathan et al., 2004). However, Xue-Gang et al. (2013) recorded fungitoxicity of ginger (Z. officinale) extract against a range of fungi among which certain species of Oomycetes were present. The plant extracts of Nigella sativa, Z. officinale, P. granatum and T. vulgaris exhibited a potential antifungal efficiency against a number of phytopathogenic fungi such Aspergillus flavus, Fusarium oxysporium, Rhizoctonia solani and Pythium ultimum (Kumar et al., 2008, Pane et al., 2011). Research concerning the efficiency of the above mentioned plant extracts against aquatic fungi (Saprolegnia spp.) is scanty. Therefore, the main goal of the present study was to assess antifungal potency of some plant extracts as Nigella sativa, Punica granatum, Thymus vulgaris and Zingber officinale against saprolegniasis caused by S. diclina in vitro.
2. Materials and methods
2.1. Isolation of saprolegnoid strain
The fish pathogenic isolate Saprolegnia diclina was isolated from diseased tilapia fish (Oreochromis niloticus), purified and identified by the methods described by Beakes, 1994, Johnson et al., 2002. The culture of pathogenic S. diclina was maintained on Sabouraud’s dextrose agar (SDA) slant supplemented with (0.5 gm/L) of penicillin G and streptomycin. The identified strain, S. diclina was kept at 10 °C on SDA and renewed at regular intervals.
2.2. Plant extraction preparations
Phyto-materials of four plant species correlated to four botanical families (Table 1) were purchased from local markets of Riyadh, Saudi Arabia. These materials were identified and their identifications were confirmed by herbarium of botany dept. college of science, King Saud university. Phyto-materials were collected, washed with tap water, disinfected by sodium hypochlorite solution (0.5%). Plant materials were rinsed with distilled water to remove chlorine residues and finally dried in shade. The dried material of each plant species was ground into a fine powder using blender to pass 100 mm sieve. Fifty grams of the powdered material was immersed in 200 ml of ethanol with stirring for 48 hrs then filtered through double layers of muslin to discard plant debris. The filtrates were centrifuged at 8000 rpm for 10 min and finally filtered again through Whatman filter paper No.(41) to attain a clear filtrate. The filtrates were dried at 35 °C under reduced pressure. The extract yields were stored in small bottles and refrigerated at 4 °C till used.
Table 1.
Ethanobotanical data of employed plant species and their traditional medicine uses.
| Ethnobotanical data | Plant species | |||
|---|---|---|---|---|
| Scientific name | Nigella sativa | Punica granatum | Thymus vulgaris | Zingiber officinale |
| Family | Ranunculaceae | Lythraceae | Lamiaceae | Zingiberaceae |
| Common name | Black caraway | Pomegranate | Thyme | Ginger |
| Local name | Black seeds | Romman | Za'ater | Zanjabil |
| Plant organ used | Seeds | Peels | Leaves | Rhizome |
| pH of the extract | 6.8 | 4.7 | 5.3 | 7.1 |
| Main chemical composition | Linoleic acid, thymoquinone, nigellone, nigilline, melanthin, damascenine, and anethole. | Catechins, gallocatechins, prodelphinidins, punicalagins tannins, and anthocyanins | Thymol, linalol carvacrol, cymene, pinene, menthone, and borneol | Zingerone, shogaols, gingerols, cineol, zingiberene, citral bisabolene, farnesene, and β-phelladrene |
| Traditional use | It used as food spice and as a carminative in indigestion and bowel complaints. | It used against diarrhea, dysentery, intestinal parasites and as contraceptive | It used as Antiseptic, antispasmodic, tonic and carminative | It used frequently for dyspepsia, gastroparesis, slow motility symptoms, constipation, and colic and as carminative |
2.3. Preparation of zoospores suspension
Zoospore suspension of S. diclina was prepared by subculture the pathogenic isolate on potato dextrose agar supplemented with 0.2% yeast extract at 18 ± 2 °C for three days. Agar disc from the edge of actively growing S. diclina were cut and immersed in sterilized aquarium water flasks supplement with (0.5gm/L) chloromephenicol and (0.1%) tween 20 to eliminate bacterial contamination and facilitate zoospores emigration from fungal mycelium. Flasks were incubated at 18 ± 2 °C for 18 hrs and the agar discs were discarded. Zoospores of S. diclina was harvested, diluted and their absorbance were measured at wavelength (λ) 580 µm to 30% using spectrophotometer. The viable count of zoospores at this absorbance was approximately 105 zoospores/ml.
2.4. Fungicidal analysis of the plant extracts
2.4.1. Antifungal screening test
Agar disc diffusion method was achieved to detect antifungal potency of the plant extracts using potato dextrose agar medium (PDA) supplement with (0.2%) yeast extract and (0.5gm/L) of chloromephenicol to eliminate bacterial contamination. About 10 ml of PDA medium was dispensed into sterile petridishes (as basal medium) then overloaded with 15 ml of seeded medium previously inoculated with fungal zoospore suspension (2 ml of 105 zoospores/100 ml of medium) to attain (2.0 × 103) zoospores /ml of medium. The plant extracts were prepared by dissolving their intended amounts in 2 ml of methanol, sterilized through disposable Millipore syringe (0.22 µm) and loaded over sterilized filter paper discs of (7 mm) in diameter to attain final concentration of 0.5 mg/disc. Filter paper discs loaded with 1 µg of malachite green was used as a positive control and reference fungicide. The plates were refrigerated at 4 °C for two hours then incubated at 18 ± 2 °C for three days. The presence of inhibition zones were investigated, estimated by Vernier caliper and regarded as indication for antifungal potency.
2.4.2. Determination of minimum inhibitory concentration (MIC)
The effective extracts including P. granatum and T. vulgaris were achieved to detect their potency in the restriction of pathogenic S. diclina strain and to estimate their minimal inhibitory concentration (MIC). Different concentrations of each plant extract (0.2, 0.4, 0.6 and 0.8 mg/ml) were preformed by dissolving their intended amount in 2 ml of methanol, sterilized through Millipore filter (0.22 µm) and pipetted over filter paper discs of 7 mm in diameter. Ten ml of PDA basal medium followed by fifteen ml of seeded medium (previously injected with zoospores suspension of S. diclina) were poured into sterile petridishes. Requisite amounts of different concentrations of each plant extract were loaded on sterile filter paper discs and placed over the PDA plates at 3 cm from each other. The plates were refrigerated at 5 °C for 2 h to permit plant extract diffusion then incubated at 18 ± 2 °C for 3: 5 days. The presence of inhibition zones were recorded and tabulated against plant extracts concentrations.
2.5. Acute fish toxicity of the effective plant extracts
Acute toxicity test of the effective plant extracts on tilapia fish (Oreochromis niloticus) was achieved with fish fingerlings of average body weight (10 ± 0.5 gm). Stock solutions of P. granatum and T. vulgaris ethanolic extracts were prepared with the concentrations of (0.0, 200, 400, 800, 1200, and 1600 ppm). A total of 60 healthy tilapia fingerlings were held in six glass aquaria containing 60 L of water and supplemented with an air supply and dechlorinated tap water in a rate of 10 fish/aquarium. Through the experiment period, water pH was adjusted to 7.0 ± 0.5 and the temperature was maintained at 23 ± 2 °C. All fish were acclimatized to lab. conditions and were fed twice daily at a rate of 3% of its body weight (6.0 gm/treatment). Six experimental fish groups were exposed to different concentrations of P. granatum and T. vulgaris extracts for 96hrs without feeding. Dead fish were picked up immediately from the glass aquarium to avoid deterioration of water. The mortality percentage of fish was calculated after 24, 48, 72 and 96 hrs. of exposure for each plant extracts respectively.
2.6. Statistical analysis
The experiments were preformed in triplicates for each treatment. The obtained results were presented as mean ± SE (standard error) and analyzed statistically for significance by one-way ANOVA test at P ≤ 0.05.
3. Results
3.1. Identification of the saprolegnoid strain
Isolation of pathogenic S. diclina was performed from colonized dorsal muscle of diseased tilapia fish (Oreochromis niloticus) and the production of reproductive structures was achieved by growing the isolate on sesame seeds in sterilized aquarium water (Fig. 1). The purified strain was identified on the basis of macroscopic and microscopic characteristics with reproductive structures according to methods described by Beakes, 1994, Johnson et al., 2002.
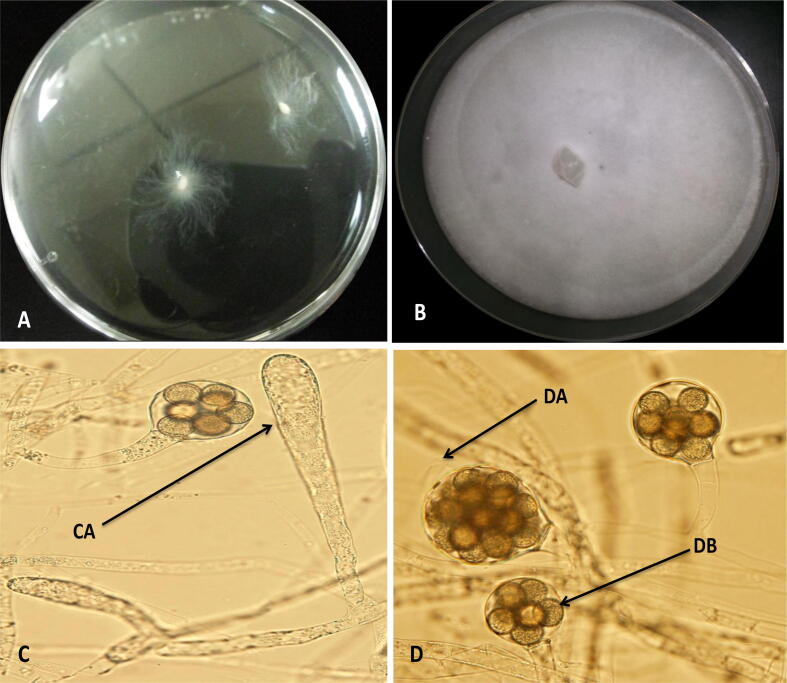
Fig. 1.
A) Isolation of Saprolegnia diclina using sesame seeds in sterilized aquarium water; B) Cultural characteristics of S. diclinia grown on PDA medium; C) Morphological characteristics of S. diclina showing clavate zoosporangium (CA); D) Sexual Structures of S. diclina showing diclinous antheridium (DA) and spherical oogonium with centric oospores (DB).
The purified strain showed a typical morphology of Saprolegnia diclina on sesame seeds and PDA plates (Fig. 1A & B). The isolate cultured in sesame seeds produced sexual structures with a similar morphological characteristic of S. diclina like, clavate zoosporangium (Fig. 1C), diclinous antheridium (Fig. 1DA) and spherical unpitted oogonium with centric oospores (Fig. 1DB).
3.2. Antifungal screening test
Scientific and classical name of the used plants and their traditional medicine uses are demonstrated in Table 1. Four plants correlated to four botanical families were investigated to detect their antifungal potency against etiological agent of fish saprolegniasis (Saprolgenia diclina) using disc diffusion method. Screening of antifungal activities of these plant extracts was represented in Table 2 and demonstrated in Fig. 2. Only, P. granatum and T. vulgaris extracts were potentially effective in preventing mycelial growth of S. diclina at concentration of 0.5 mg/ml while other plant extracts of Nigella sativa and Zingiber officinales were not effective. P. granatum was the most effective extract inhibiting mycelial growth of S. diclina and exhibiting inhibition zone of (17.81 mm) while T. vulgaris extract was less effective in suppressing mycelial growth of the pathogenic S. diclina and recorded inhibition zone of (15.75 mm).
Table 2.
Antifungal screening test of ethanolic plant extracts (0.5 mg/ml) against pathogenic Saprolegnia diclina isolate.
| Plant species | Inhibition zone diameter (mm) | Plant extract (mg) equivalent to (1 µg) of malachite green potency |
|---|---|---|
| Nigella sativa | 0.00* ± 0.0 | 0.00 |
| Punica granatum | 17.81* ± 0.6 | 0.97 |
| Thymus vulgaris | 15.75* ± 0.3 | 1.10 |
| Zingiber officinale | 0.00* ± 0.0 | 0.00 |
| Malachite green | 34.53 ± 1.1 | – |
Asterisks (*) referred to the significant values (P ≤ 0.05).
Data are mean of triplicates ± standard error.
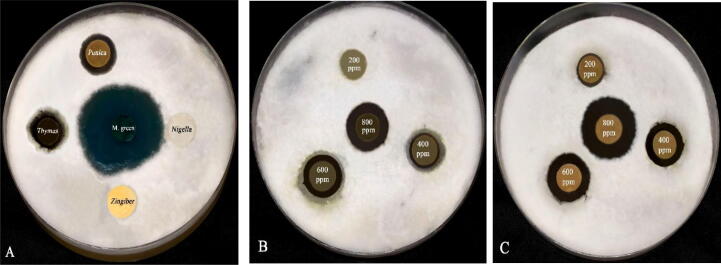
Fig. 2.
A) Screening of antifungal activities of some plant extracts against S. diclina; B) MIC of Thymus vulgaris extract against S. diclinia using disk diffusion method; C) MIC of punica granatum extract against S. diclinia using disk diffusion method.
On the other hand, malachite green was strongly effective against S. diclina suppressing its mycelial growth with inhibition zone of (34.53 mm). However, malachite green still the most effective fungitoxicant suppressing mycelial growth of S. diclina than extracts of P. granatum and T. vulgaris as inhibition zone was (34.53 mm) at concentration of 1 µg/ml while 0.97 and 1.10 mg of the previous plant extracts were required respectively to attain the same effect of malachite green. The MIC of the effective plant extracts (P. granatum and T. vulgaris) was employed by disc diffusion method to assess their fungicidal properties. The concentration influence of the functional plant extracts on growth parameter of S. diclina was represented in Table 3 and demonstrated in Fig. 2(a–b) as their inhibitory effect was increased in proportion to their concentrations. The results in Table 3 revealed that, P. garantum extract was strongly effective against S. diclina recording MIC of 200 ppm with inhibition zone of (11.34 mm) while T. vulgaris extract was less effective and no suppressive potency was detected at the same concentration. MIC of T. vulgaris extract against S. diclina was 400 ppm with inhibition zone of (15.05 mm). Thus we can conclude from the previous results that, the extract of P. granatum and T. vulgaris inhibited mycelial growth of the pathogenic S. diclina which was highly susceptible to P. granatum extract than that of T. vulgaris.
Table 3.
Mycelial growth inhibition (mm) of Saprolegnia diclina after treatment with the effective plant extracts at different concentrations.
| Plant extract conc. (ppm) | Plant species |
|
|---|---|---|
|
Punica granatum |
Thymus vulgaris |
|
| Inhibition zone diameter (mm) | Inhibition zone diameter (mm) | |
| 200 | 11.34 ± 0.4 | 0.00 ± 0.0 |
| 400 | 16.25 ± 1.1 | 15.05 ± 0.9 |
| 600 | 19.63 ± 0.6 | 17.82 ± 0.3 |
| 800 | 24.65 ± 0.3 | 22.41 ± 0.5 |
Data are mean of triplicates ± standard error.
3.3. Acute fish toxicity assay
The acute fish toxicity of the plant extracts was achieved through exposing of fish to different concentrations of P. granatum and T. vulgaris for 96 hrs. Results in Table 4 indicated the low toxicity of plant extracts as no fish mortalities were observed at aquaria containing 200, 400 and 800 ppm of plant extracts respectively. Only, 10 and 30% of fish accumulative mortalities were detected at 1200 ppm and 1600 ppm of pomegranate extract exposure for 96 hrs. On the other hand, 10 and 20% of fish mortalities were observed at 1200 and 1600 ppm of Thyme exposure. However, no mortalities were further detected in aquaria and the fish survivors from plant extracts toxicity test didn’t show any cytotoxic effect.
Table 4.
Accumulative mortalities of Tilapia sp. (O. niloticus) during acute exposure to plant extracts for 96 h.
| Plant extracts | Conc. (ppm) | No. of dead fish |
Percentage of mortality |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 24 h | 48 h | 72 h | 96 h | ||
| P. granatum | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 400 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 800 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1200 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 10 | |
| 160 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 30 | |
| T. vulgaris | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 200 | 0 |
0 | 0 | 0 | 0 |
0 | 0 | 0 | |
| 400 | 0 |
0 | 0 | 0 | 0 |
0 | 0 | 0 | |
| 800 | 0 |
0 | 0 | 0 | 0 |
0 | 0 | 0 | |
| 1200 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 10 | |
| 1600 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 20 | |
4. Discussion
Saprolegniasis of fresh water fish can be controlled by good water quality, air circulation of pond water, good fish nutrition, avoidance of fish crowding to minimize injury and finally application of chemical fungicides as malachite green, formalin and hydrogen peroxide (Sharma et al., 2012). However, excessive application of fungicides in fish disease treatment leads to accumulation of toxic residues in fish flesh, increase risk of environmental pollution and considered to be responsible for many carcinogenic and teratogenic attributes. So, the adverse impression of these fungicides on human health and environment are burning issues and it becomes necessary to develop effective and eco-friendly fungicides. Four ethanolic plant extracts were screened in vitro at 0.5 mg/ml to evaluate their antifungal activity in controlling saprolegiasis. Our results showed that, two plant extracts provided a significant inhibition of mycelial growth of the pathogenic S. diclina isolate and its sensitivity to a given plant extract varied greatly. P. granatum extract showed a potential suppressive effect on growth parameter of S. diclina at concentration of 0.5 mg/ml followed by extract of T. vulgaris while the other plant extracts have no suppressive potency at the same concentration. These results are coincident with that of ALsafah and AL-Faragi (2017) who ascertained that the dietary supplementation of T. vulgaris to fish increase survival rate of Cyprinus carpio challenged with Saprolegnia sp. Moreover, the results were compatible with that of Mostafa et al. (2012) who reported that both of P. granatum and T. vulgaris extracts were potentially active against Rhizoctonia solani, Fusarium oxysporum and a species of Oomycetes (Pythium aphanidermatum). A variation of antifungal efficacy of the concerned plant extracts may be attributed to considerable variation in their phytochemical constituents and variation in fungal diversity (Al-Rahmah et al., 2013). Thymol and Carvacrol were determined as the most effective antimicrobial component in T. vulgaris extract (Omidbeygi et al., 2007) while punicalagin, castagalagin and granatin were determined as the effective antimicrobial compounds in P. granatum extract (Dahham et al., 2010, Foss et al., 2014). Some researchers suggested that the effective plant extract contained antimicrobial components which were able to cross fungal cell membranes, blocking their vital enzymes and denaturizing proteins of their cell membrane that destroying selective permeability of fungal cell membrane and suppressing different biochemical process of fungal cell causing cell death (Pane et al., 2011, Madhuri et al., 2012). According to fish toxicity assay of the effective plant extracts, both of P. granatum and T. vulgaris extracts showed low toxicity to fish as no mortalities were detected at 800 ppm. Considering the low toxicity of these plant extracts, it may be concluded that 200 and 400 ppm of pomegranate and thyme extracts which suppressed the mycelial growth of the S. diclina could be safely used for saprolegniasis control through immersion of the diseased fish in aquaria containing the previous concentrations of the potentially active plant extracts for 30 min. Also, these plants extracts may be mixed with the fish feed decreasing their ability to be infested with S. diclina
Hence, the present study indicated that application of pomegranate and thyme extracts can be used as potential and applicable fungicide in fish disease treatment especially saprolegnasis caused by S. diclina. Also, the extracts of P. granatum and T. vulgaris can be considered as a safe alternative to teratogenic malachite green.
5. Conclusion
These effective plant extracts create a new opportunity in development process of safe and eco-friendly fungicides and they can be considered as alternative fungicides to control saprolegniasis avoiding carcinogenic malachite green application. However, further investigations on the effective plant extracts as prophylactic treatment during outbreak of saprolegniasis in vivo are required before practical use of these extracts in fish aquaculture.
Declaration of Competing Interest
None.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No. (RGP-1440-094).
Footnotes
Peer review under responsibility of King Saud University.
References
- Agbebi O., Oyeleke G., Agbon A. Use of Euphorbia kamerunica (Spurge) extract in the control of Saprolegnia species growth in incubated eggs of Clarias gariepinus. Global J. Sci. Front. Res. 2012;12:27–30. [Google Scholar]
- Al-Rahmah A., Mostafa A., Abdel-Megeed A., Yakout S., Hussein S. Fungicidal activities of certain methanolic plant extracts against tomato phytopathogenic fungi. African J. Microbiol. Res. 2013;7(6):517–524. [Google Scholar]
- ALsafah, A., AL‐Faragi, J., 2017. Influence of thyme (Thymus vulgaris) as feed additives on growth performance and antifungal activity on Saprolegnia spp. Cyprinus carpio, 1598–1602.
- Barnes M.E., Sayler W.A., Cordes R.J. Use of formalin treatments during incubation of eyed eggs of brown trout. North Am. J. Aquaculture. 2001;63(4):333–337. [Google Scholar]
- Battaglin W., Fairchild J. Potential toxicity of pesticides measured in midwestern streams to aquatic organisms. Water Sci. Technol. 2002;45(9):95–103. [PubMed] [Google Scholar]
- Beakes G. Features which characterize Saprolegnia isolates from salmonid fish lesions-a review. Salmon Saprolegniasis. 1994:33–66. [Google Scholar]
- Chukanhom K., Borisuthpeth P., Hatai K. Antifungal activities of aroma components from Alpinia galanga against water molds. Biocontrol Sci. 2005;10(3):105–109. [Google Scholar]
- Corcoran J., Winter M.J., Tyler C.R. Pharmaceuticals in the aquatic environment: a critical review of the evidence for health effects in fish. Crit. Rev. Toxicol. 2010;40(4):287–304. doi: 10.3109/10408440903373590. [DOI] [PubMed] [Google Scholar]
- Dahham S.S., Ali M.N., Tabassum H., Khan M. Studies on antibacterial and antifungal activity of pomegranate (Punica granatum L.) Am. Eurasian J. Agric. Environ. Sci. 2010;9(3):273–281. [Google Scholar]
- Earle G., Hintz W. New approaches for controlling Saprolegnia parasitica, the causal agent of a devastating fish disease. Trop. Life Sci. Res. 2014;25(2):101. [PMC free article] [PubMed] [Google Scholar]
- Foss S.R., Nakamura C.V., Ueda-Nakamura T., Cortez D.A., Endo E.H., Dias Filho B.P. Antifungal activity of pomegranate peel extract and isolated compound punicalagin against dermatophytes. Ann. Clin. Microbiol. Antimicrob. 2014;13(1):32. doi: 10.1186/s12941-014-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi Pirbalouti A., Taheri M., Raiesi M., Bahrami H., Abdizadeh R. In vitro antifungal activity of plant extracts on Saprolegnia parasitica from cutaneous lesions of rainbow trout (Oncorhynchus mykiss) eggs. J. Food Agric. Environ. 2009;7(2) [Google Scholar]
- Hussein M.M., El-Feki M.A., Hatai K., Yamamoto A. Inhibitory effects of thymoquinone from Nigella sativa on pathogenic Saprolegnia in fish. Biocontrol. Sci. 2002;7(1):31–35. [Google Scholar]
- Ilondu E.M., Arimoro F.O., Sodje A.P. The use of aqueous extracts of Vernonia amygdalina in the control of saprolegniasis in Clarias gariepinus, a freshwater fish. Afr. J. Biotechnol. 2009;8(24) [Google Scholar]
- Johnson T.W., Seymour R.L., Padgett D.E. University of North Carolina at Wilmington, Department of Biological Sciences; 2002. Biology and Systematics of the Saprolegniaceae. [Google Scholar]
- Kumar A., Shukla R., Singh P., Prasad C.S., Dubey N.K. Assessment of Thymus vulgaris L. essential oil as a safe botanical preservative against post harvest fungal infestation of food commodities. Innovative Food Sci. Emerg. Technol. 2008;9(4):575–580. [Google Scholar]
- Madhuri, S., Mandloi, A., Govind, P., Sahni, Y., 2012. Antimicrobial activity of some medicinal plants against fish pathogens.
- Mitchell A.J., Radomski A.A., Straus D.L., Carter R. The effect of hydrogen peroxide on the hatch rate and Saprolegnia spp. infestation of channel catfish eggs. North Am. J. Aquaculture. 2009;71(3):276–280. [Google Scholar]
- Mori T., Hirose H., Hanjavanit C., Hatai K. Antifungal activities of plant extracts against some aquatic fungi. Biocontrol Sci. 2002;7(3):187–191. [Google Scholar]
- Mostafa A.A., Al-Rahmah A., Abdel-Megeed A., Sholkamy E.N., Al-Arfaj A.A., El-Shikh M.S. Fungitoxic properties of some plant extracts against tomato phytopathogenic fungi. J. Pure Appl. Microbiol. 2012;6(4):1889–1898. [Google Scholar]
- Omidbeygi M., Barzegar M., Hamidi Z., Naghdibadi H. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control. 2007;18(12):1518–1523. [Google Scholar]
- Pane C., Spaccini R., Piccolo A., Scala F., Bonanomi G. Compost amendments enhance peat suppressiveness to Pythium ultimum, Rhizoctonia solani and Sclerotinia minor. Biol. Control. 2011;56(2):115–124. [Google Scholar]
- Rai M., Kaushal S., Acharya D. In vitro effect of five Asteraceous essential oils against Saprolegnia ferax, a pathogenic fungus isolated from fish. The Antiseptic. 2002;99(4):136–137. [Google Scholar]
- Ramanathan A., Marimuthu T., Raguchander T. Effect of plant extracts on growth in Pythium aphanideramtum. J. Mycol. Plant Pathol. 2004;34:315–317. [Google Scholar]
- Rohani, M., Ebrahimzadeh, M.H., Khosravi, A., Mokhayer, B., Mirzargar, S., Mehrabi, Y.E., 2006. Evaluation of Geranium herbarum escence application in control of fungal contamination in rainbow trout eggs.
- Salehi M., Soltani M., Islami H.R. In vitro antifungal activity of some essential oils against some filamentous fungi of rainbow trout (Oncorhynchus mykiss) eggs. Aquaculture, Aquarium, Conservat. Legislat. 2015;8(3):367–380. [Google Scholar]
- Sharma, M., Shrivastav, A., Sahni, Y., Pandey, G., 2012. Overviews of the treatment and control of common fish diseases.
- Shin S., Kulatunga D., Dananjaya S., Nikapitiya C., Lee J., De Zoysa M. Saprolegnia parasitica isolated from Rainbow Trout in Korea: Characterization, anti-Saprolegnia activity and host pathogen interaction in Zebrafish Disease Model. Mycobiology. 2017;45(4):297–311. doi: 10.5941/MYCO.2017.45.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songe M., Willems A., Wiik-Nielsen J., Thoen E., Evensen Ø., Van West P., Skaar I. Saprolegnia diclina IIIA and S. parasitica employ different infection strategies when colonizing eggs of Atlantic salmon, Salmo salar L. J. Fish Dis. 2016;39(3):343–352. doi: 10.1111/jfd.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D.L., Mitchell A.J., Carter R.R., Steeby J.A. Optimizing copper sulfate treatments for fungus control on channel catfish eggs. J. Aquatic Animal Health. 2009;21(2):91–97. doi: 10.1577/H07-057.1. [DOI] [PubMed] [Google Scholar]
- Sudova E., Machova J., Svobodova Z., Vesely T. Negative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: a review. Veterinarni Medicina-Praha- 2007;52(12):527. [Google Scholar]
- Tampieri M., Galuppi R., Carelle M., Macchioni F., Cioni P., Morelli I. Effect of selected essential oils and pure compounds on Saprolegnia parasitica. Pharm. Biol. 2003;41(8):584–591. [Google Scholar]
- Thoen E., Evensen Ø., Skaar I. Pathogenicity of Saprolegnia spp. to Atlantic salmon, Salmo salar L., eggs. J. Fish Dis. 2011;34(8):601–608. doi: 10.1111/j.1365-2761.2011.01273.x. [DOI] [PubMed] [Google Scholar]
- Van Den Berg A.H., McLaggan D., Diéguez-Uribeondo J., Van West P. The impact of the water moulds Saprolegnia diclina and Saprolegnia parasitica on natural ecosystems and the aquaculture industry. Fungal Biol. Rev. 2013;27(2):33–42. [Google Scholar]
- Xue-Gang H., Lei L., Cheng C., Kun H., Xian-Le Y., Gao-Xue W. In vitro screening of Chinese medicinal plants for antifungal activity against Saprolegnia sp. and Achlya klebsiana. North Am. J. Aquaculture. 2013;75(4):468–473. [Google Scholar]