Abstract
Objective
The objective was to investigate the anti-inflammatory effects of salidroside through the PI3K/Akt signaling pathway and its protective effects on acute hypoxia-induced myocardial injury in rats.
Methods
A total of 24 healthy Sprague-Dawley male rats were selected as the experimental subjects. All rats were divided into 4 groups by using the random number table method, with 6 rats in each group. The groups included the normal control group, the salidroside group, the hypobaric hypoxia group, and the hypobaric hypoxia + salidroside group. Rats in the salidroside group were fed in the original animal laboratory and were intragastrically administered with salidroside every morning at a dosage of 35 mg/kg. Rats in the normal control group were intragastrically administered with an equal dosage of saline. Rats in the hypobaric hypoxia + salidroside group were intragastrically administered with salidroside every morning at a dosage of 35 mg/kg, who were fed in the hypoxic experiment module for animals. The altitude was increased to 4000 m, and the rats were kept in the module for 24 h. Rats in the hypobaric hypoxia group were intragastrically administered with an equal dosage of saline in the same environment, and the altitude was increased to 4000 m after administration. Parameters of blood gas analysis, histopathological changes in cardiac tissues, cardiac indexes, and inflammatory factors IL-6 and TNF-α levels of rats in groups were compared.
Results
1. The cardiac indexes of rats in groups were compared. The differences between the hypobaric hypoxia group and the hypobaric hypoxia + salidroside group were statistically significant (P < 0.05). 2. The results of blood gas analysis of rats in groups were compared. The differences between the hypobaric hypoxia group and the hypobaric hypoxia + salidroside group were significantly different (P < 0.05). 3. In the hypobaric hypoxia group, the myocardial cells of rats were arranged disorderly and shaped differently, with cases such as edema, degeneration, necrosis, nucleus pyknosis, and massive infiltration of inflammatory cells. In the hypobaric hypoxia + salidroside group, the above-mentioned pathological changes in myocardial cells were relieved. 4. Compared with the hypobaric hypoxia group, in the hypobaric hypoxia + salidroside group, the concentrations of IL-6 and TNF-α in rats decreased apparently, and the differences were statistically significant (P < 0.05).
Conclusion
Salidroside had the repairing and protective effects on the hypobaric hypoxia-induced myocardial injuries in rats. The application of salidroside could reduce the inflammatory responses of rats with hypobaric hypoxia-induced myocardial injuries through PI3K/Akt signaling pathway, thereby protecting the myocardial cells.
Keywords: Salidroside, PI3K/Akt pathway, Acute hypoxia, Hypobaric hypoxia, Myocardial injury
1. Introduction
In recent years, with the changes in the living environment and living habits of human beings, the incidence rate of cardiovascular diseases has been increasing, as well as the mortality rate (Yazouli et al., 2018). The causes of cardiovascular disease are very complicated, in which the hypoxic environment at high altitude is an important cause of increased cardiac load. In China, the regions that the altitude is above 3000 m occupy a large amount of the total area. Due to the sparsely populated land and the need to invest a large amount of manpower in development and construction, many people go working in these areas every year. When people living in the plain area first arrive at high-altitude hypoxic areas, the body tends to be in an acute hypoxic state. In various systems and organs, the myocardial response to hypoxia is most sensitive (Banta et al., 2020). The long-term hypoxic environment can cause pulmonary hypertension, leading to pathological changes such as myocardial apoptosis, myocardial fibrosis, ventricular remodeling, and even pulmonary hypertension, pulmonary edema, heart failure, and other serious diseases (Zheng et al., 2020). As the heart adapts to the hypoxic environment, it will cause a series of disorders in the body, in which the excessive release of inflammatory factors plays an important role. Studies have shown that drugs that block the release of inflammatory factors can protect against myocardial damage caused by high altitude and low oxygen environment.
At present, the research on traditional Chinese medicine continues to be deepened. Especially in the treatment of cardiovascular diseases, Chinese medicine can be used as a safe and multi-target treatment in clinical practices (Xue et al., 2018). In terms of the protection of myocardial injuries, salidroside, and total ginsenoside ginseng root are effective extracts of traditional Chinese medicines, which have been proven to have apparent inhibitory and protective effects on damaged myocardial tissue through the formation of anti-oxygen free radicals and the inhibition of myocardial cell apoptosis (Yan et al., 2019). In terms of cardiovascular disease, salidroside can enhance and protect the cardiac functions by inhibiting the degeneration, necrosis, and apoptosis of myocardial cells. Myocardial cell injury is closely related to hypoxia and inflammation in the body. The phosphatidylinositol-3-kinase/protein kinase (PI3K/Akt) signaling pathway can regulate cardiac hypertrophy, myocardial fibrosis, and myocardial remodeling, which plays an essential role in the regulation and metabolism of cardiac functions (Li et al., 2016).
As a traditional Chinese medicine grown in high-altitude and the cold environment with strong ultraviolet irradiation, salidroside has been proven to be able to relieve the myocardial damages caused by various factors through PI3K/Akt signaling pathway, as well as inhibiting the ischemic reperfusion injury of the cerebral arteries (Guo et al., 2017). At present, the protective mechanism of salidroside on hypoxia-induced myocardial injury through the PI3K/Akt signaling pathway remains unclear. Therefore, in this study, the anti-inflammatory effect of salidroside through the PI3K/Akt signaling pathway will be explored further by constructing rat models.
2. The protective effects of salidroside on myocardial cells
Salidroside can be used as an anoxic adaptation to prevent and protect hypoxic-ischemic diseases caused by various stimulating factors (Qi et al., 2018). By studying the rat model of sepsis, it was found that salidroside could reduce the loss of bone mass by activating the HIF-1α pathway; in addition, salidroside could also inhibit the apoptosis of endothelial cells induced by cobalt dioxide. Akt belongs to a threonine protein kinase, which plays an important role in the angiogenesis of cardiovascular diseases and the metabolism of myocardial cells. Akt protein kinase is activated by PI3K and has a role in its upstream transcriptional level (Lu et al., 2017). During the pretreatment stage of salidroside, PI3K/Akt signaling pathway plays an important protective role in cardiac tissues in sepsis-induced myocardial injury, while salidroside can inhibit the release of inflammatory factors (Cai et al., 2017). Moderate activation of the PI3K/Akt signaling pathway has a positive effect on myocardial cell function; it can enhance the energy metabolism of myocardial tissues, promote neovascularization, and inhibit cell apoptosis. The apoptosis of myocardial cells can lead to cell damages and the development of heart disease. However, the continuous activation can cause deterioration of cardiac function.
The activation subunit of HIF-1 is HIF-1α. Therefore, HIF-1α is a downstream target of the PI3K/Akt signaling pathway and a regulator of the hypoxic environment, which can enhance the expression of vascular endothelial growth factor (VEGF) to complete the regulation of angiogenesis (Feng et al., 2018). Some scholars have found that HIF-1α can regulate the formation of atherosclerotic plaque and therefore plays an important role in the occurrence and progression of atherosclerosis, which also indicates that the PI3K/Akt signaling pathway has a certain correlation with the regulation of degradation and ubiquitination of HIF-1α protein (Sheng et al., 2017). The role of salidroside in protecting the heart is closely related to the inhibition of the release of inflammatory factors, which is inseparable from the activation of the PI3K/Akt/HIF-1α signaling pathway. In turn, a series of apoptotic signaling pathways are blocked, thereby the cell survival and proliferation are promoted.
In addition, salidroside has a role in relieving coronary arteries, which can increase the coronary blood flow and oxygen supply, enhance the myocardial contractility, and improve the microcirculation. Studies have shown that salidroside has a significant protective effect on cardiac damages caused by ischemia/reperfusion, which can reduce the apoptosis of myocardial cells caused by various factors (Li et al., 2018). Therefore, salidroside can be used as a drug target for the treatment of cardiovascular diseases. However, its underlying molecular mechanism is not yet clear.
3. Materials and methods
3.1. Experimental animals
A total of 24 SD healthy male rats were selected as experimental subjects, weighing 185–205 g, with similar ages. The adaptive feeding lasted for 1 week. The room temperature was controlled at 20–24 °C, and the relative humidity was controlled at 50–65%, with 12 h of the day-night cycle. All rats were free to move, eat, and drink during the experiment, and the feed was normal rat feed. This study was approved by the ethics committee of our hospital, and all the tests were conducted in accordance with the rules and regulations formulated by the ethics committee.
3.2. The construction and grouping of acute hypoxia rat models
All rats were divided into 4 groups by using the random number table method, with 6 rats in each group. The groups included the normal control group, the salidroside group, the hypobaric hypoxia group, and the hypobaric hypoxia + salidroside group. After 1 week of adaptive feeding, rats in the salidroside group were fed in the original animal laboratory (the altitude was about 800 m) and were intragastrically administered with salidroside every morning at a dosage of 35 mg/kg. Rats in the normal control group were fed in the same environment and intragastrically administered with an equal dosage of saline every morning for 3 consecutive days. Rats in the hypobaric hypoxia + salidroside group were intragastrically administered with salidroside every morning at a dosage of 35 mg/kg, who were fed in the hypoxic experiment module for animals. After administration, the altitude was increased to 4000 m at a velocity of 10 m/s, and the rats were kept in the module for 24 h. The next morning, at the same time, the altitude was descended to 800 m at a velocity of 10 m/s. Rats in the hypobaric hypoxia group were intragastrically administered with an equal dosage of saline in the same environment every morning, and the post-administration treatments were the same as those for rats in the hypobaric hypoxia + salidroside group. The salidroside used in the experiment was dissolved in saline and was used immediately after formulation. The blood volume pumped out from the heart was divided by the body surface area, the rats with different body sizes were directly compared, the cardiac output was calculated by the unit body surface area (m2), and the rat heart index was obtained.
3.3. The detection indicators and sample collection
(1) Comparison of blood gas analysis of rats groups: After the last administration, all the rats were taken out from the respective experimental environment and weighed. Then, the rats were treated with 10% chloral hydrate at a dosage of 0.3 mL/kg for anesthesia through intraperitoneal injections. After the anesthesia was successful, the abdominal fur of each rat was removed, the exposed skin was disinfected, and the abdominal aorta of the rats was fully exposed after laparotomy. Blood samples were collected and the collected blood samples were analyzed by a blood gas analyzer.
(2) Pathological observation and cardiac indexes of rat cardiac tissues: Rats in all four groups were put to death by decapitation after blood sample collection. The thoracic cavity of each rat was exposed. Then, the cardiac tissue was obtained, rinsed with saline, and weighed after dried with wipes. Afterward, the tissues were placed in preservation tubes and stored in a freezer at −80 °C. After being stationed in a 4% paraformaldehyde solution for 1 week, the cardiac tissue samples of rats were routinely subjected to alcohol gradient dehydration, xylene decoloring, and paraffin embedding, Then, the samples were sectioned, stained with Hematoxylin-Eosin (HE) staining, and sealed with neutral gum. The pathological changes in cardiac tissues of rats were observed by optical microscopy. Cardiac index (CI) = heart weight (HW)/body weight (BW).
(3) Comparison of serum inflammatory factor levels in rats: A total of 100 μg rat cardiac tissue was placed in an EP tube, lysed on ice, added with PBS, homogenized, and centrifuged. The supernatant was taken for the detection of IL-6 and TNF-α contents. The enzyme-linked immunosorbent assay was used to determine the OD value at 450 nm of each hole and the OD value of the standard by using a microplate reader, and a standard curve was drawn.
3.4. Statistical method
In this study, the data were statistically analyzed by SPSS 20.0 statistical software. The experimental data conforming to the normal distribution were expressed by (mean number ± standard deviation). The comparison between multiple groups was performed by one-way ANOVA. The variance was tested by the LSD method. Differences in variance were analyzed by Dunnett's T3 method. The difference was statistically significant at P < 0.05.
4. Results
4.1. The comparisons of general conditions and cardiac indexes between rats in groups
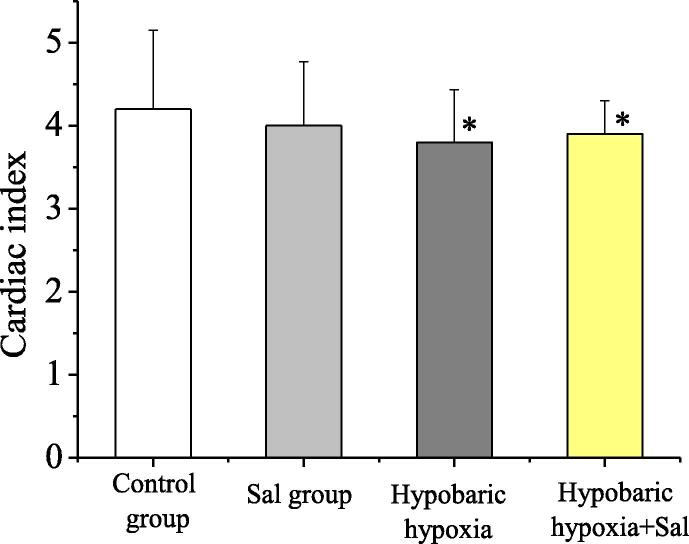
In this study, the survival and death of rats in groups were recorded. Except for the 2 death cases in the hypobaric hypoxia group, no death occurred in the other groups. The cardiac indexes of the normal control group (Control), the salidroside group (Sal), the hypobaric hypoxia group (4000 m), and the hypobaric hypoxia (4000 m) + salidroside group were respectively (4.2 ± 0.95), (4.0 ± 0.77), (3.8 ± 0.63), and (3.9 ± 0.40). The comparison of cardiac indexes between rats in groups was as shown in Fig. 1. Compared with the normal control group, in the hypobaric hypoxia group and the hypobaric hypoxia + salidroside group, the differences had statistical significance (P < 0.05). Compared with the hypobaric hypoxia group, in the hypobaric hypoxia + salidroside group, the difference had statistical significance (P < 0.05).
Fig. 1.
The comparison of cardiac indexes of rats in groups.
4.2. The comparison of blood gas analysis results of rats in groups
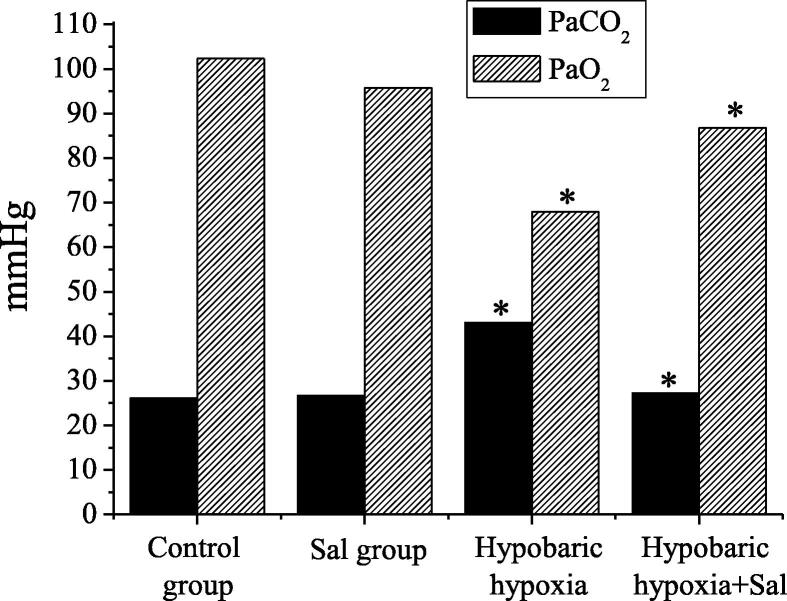
In this study, the blood gas parameters of rats in groups were tested, including lactic acid, PH value, oxygen partial pressure, carbon dioxide partial pressure, and blood oxygen saturation. The specific results were shown in Table 1. The oxygen partial pressure and carbon dioxide partial pressure results were compared, as shown in Fig. 2. Compared with the normal control group, in the hypobaric hypoxia group, the differences between parameters of blood gas analysis had statistical significance (P < 0.05). Compared with the hypobaric hypoxia group, in the hypobaric hypoxia + salidroside group, the differences between parameters of blood gas analysis had statistical significance (P < 0.05).
Table 1.
The detection results of blood gas analysis of rats in groups.
| Blood gas analysis | Control group | Sal group | Hypobaric hypoxia | Hypobaric hypoxia + Sal |
|---|---|---|---|---|
| PH | 7.29 ± 0.63 | 7.24 ± 0.23 | 7.27 ± 0.03* | 7.28 ± 0.03* |
| Lactic Acid (mmol/L) | 3.96 ± 0.70 | 3.87 ± 1.04 | 4.17 ± 0.60* | 4.12 ± 0.29* |
| PaCO2 (mmHg) | 26.16 ± 8.42 | 26.70 ± 2.69 | 43.02 ± 6.28* | 27.20 ± 2.71* |
| PaO2 (mmHg) | 102.35 ± 10.55 | 95.82 ± 10.78 | 67.96 ± 5.80* | 86.81 ± 6.25* |
| SaO2 (%) | 93.80 ± 13.02 | 94.82 ± 2.40 | 73.54 ± 1.78* | 86.57 ± 1.58* |
Note: among them * represents the difference with statistical significance.
Fig. 2.
The comparison of PaCO2 and PaO2 results of rats in groups.
Fig. 2 shows the comparison results of blood gas analysis indexes of rats between hypobaric hypoxia group and normal control group, and the results of blood gas analysis indexes between hypobaric hypoxia + salidroside group and hypobaric hypoxia group.
4.3. The histopathological changes in cardiac tissues of rats in groups
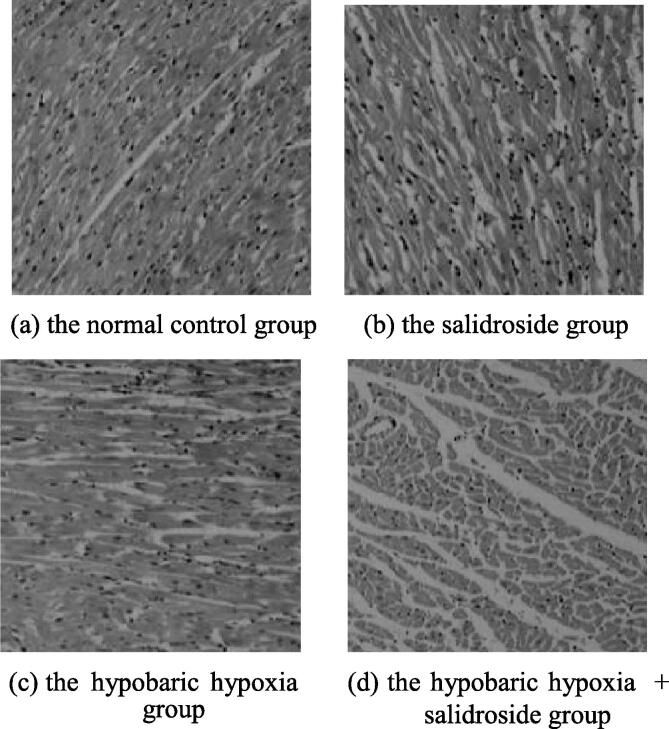
In this study, the structure of cardiac tissues of rats in groups was observed by optical microscopy, as shown in Fig. 3. In the normal control group and the salidroside group, the morphology and structure of myocardial cells were normal, with neat arrangements and clear nuclei. In the hypobaric hypoxia group, the myocardial cells of rats were arranged disorderly and shaped differently, with cases such as edema, degeneration, necrosis, nucleus pyknosis, and massive infiltration of inflammatory cells (mostly neutrophilic granulocytes). In the hypobaric hypoxia + salidroside group, the above-mentioned pathological changes in myocardial cells were relieved. It indicated that salidroside had repairing and protective effects on hypobaric hypoxia-induced myocardial injuries in rats.
Fig. 3.
The histopathological changes in cardiac tissues of rats in groups.
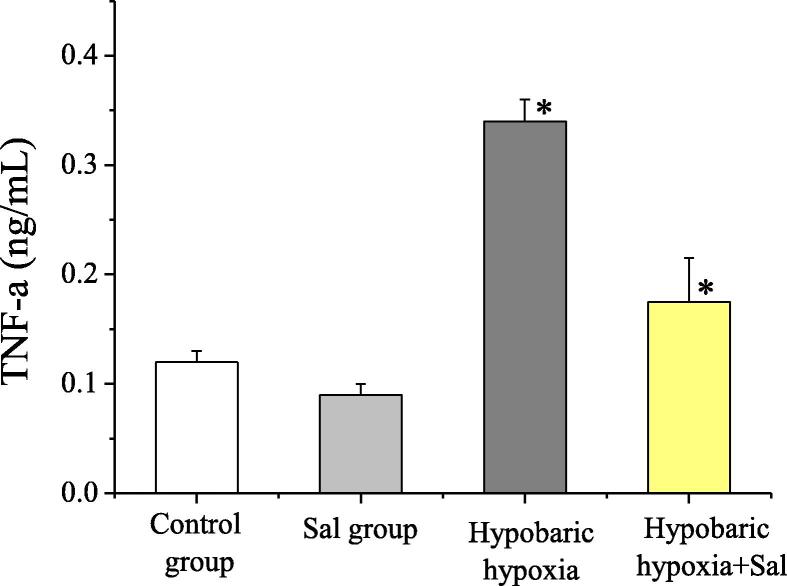
4.4. The comparison of inflammatory factor IL-6 and TNF-α levels of rats in groups
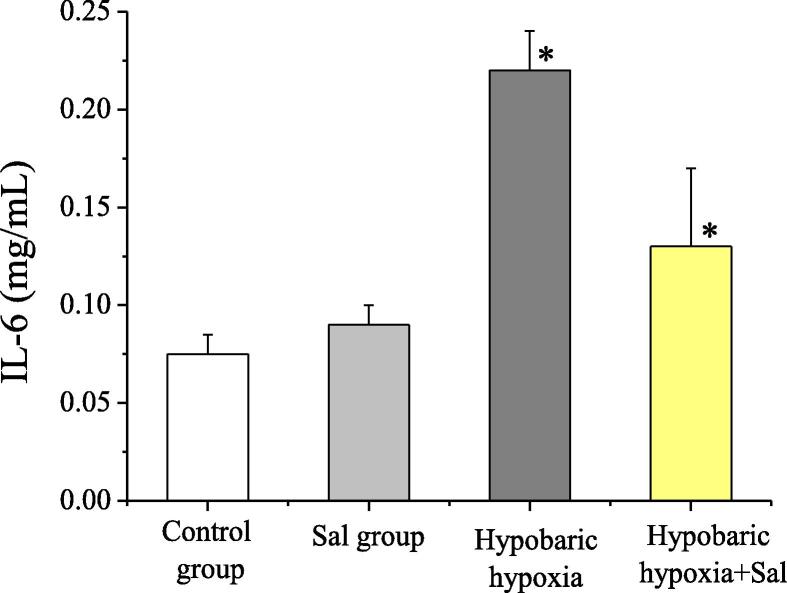
In this study, the levels of IL-6 and TNF-α were detected. Compared with the normal control group and the salidroside group, in the hypobaric hypoxia group, the IL-6 and TNF-α levels increased apparently, and the difference was statistically significant (P < 0.05). In the hypobaric hypoxia + salidroside group, compared with the hypobaric hypoxia group, the IL-6 and TNF-α levels decreased apparently, and the difference between the two groups was statistically significant (P < 0.05). The levels of inflammatory factor IL-6 and TNF-α of rats in groups were shown in Fig. 4 and Fig. 5. Therefore, the application of salidroside could reduce the inflammatory responses of rats with hypobaric hypoxia-induced myocardial injuries, thereby protecting the myocardial cells.
Fig. 4.
The comparison of IL-6 concentrations of rats in groups.
Fig. 5.
The comparison of TNF-α concentrations of rats in groups.
5. Discussion
In high-altitude areas, due to the low-pressure and oxygen-deficient environment, the insufficient oxygen supply to the oxygen body can lead to oxygen utilization barriers in tissues and organs, which will result in internal environmental disturbances and even lead to a series of pathological changes, especially myocardial damages. When the organism is hypoxic, the accumulation of reactive oxygen species in the myocardial cells causes oxidative damages, which is also a key factor leading to myocardial damages (Shaik et al., 2020). In addition, the hypobaric hypoxic environment will increase the vascular resistance of the lungs, leading to pulmonary hypertension, which will further aggravate the cardiac load and cause different degrees of myocardial damages.
From the perspective of traditional Chinese medicine, Rhodiola Rosea has the functions of replenishing vital energy, enhancing hemostasis, promoting blood circulation, and removing blood stasis. It is mainly used for the treatments of Qi deficiency, Qi weakness, lung-heat cough, bruises, burns, neurosis, and altitude sickness. Research of modern medicine has shown that Rhodiola Rosea contains 35 trace elements, 18 amino acids, vitamins A, D, E, and anti-aging active superoxides. Its nutritional composition is complete, and its compatibility is reasonable, which is rare in the plants found so far (Li et al., 2018). In pharmacology, Rhodiola Rosea has been proven to have a central excitatory effect and an “adapted to the original” effect, which can be used to prevent various hypoxic-ischemic diseases. Salidroside is one of the various active ingredients in Rhodiola Rosea. It can inhibit the proliferation of tumor cells by interfering with cell metabolism and changing the properties of the cell coat, which can also increase the conversion rate of T lymphocytes and the activity of phagocytes.
Akt is a threonine protein kinase, which plays an essential role in the angiogenesis of cardiovascular disease and the metabolism of myocardial cells. Moreover, PI3K/Akt signaling pathway has a significant protective effect on the heart in the myocardial injury induced by sepsis. Based on the pharmacological effects of salidroside, in this study, the anti-inflammatory effects of salidroside through the PI3K/Akt signaling pathway were explored by constructing the acute hypoxia models in rats. The results showed that the differences in blood gas analysis results between the hypobaric hypoxia group and the normal control group were statistically significant (P < 0.05). In addition, the differences in blood gas analysis results between the hypobaric hypoxia group and the hypobaric hypoxia + salidroside group were significantly different (P < 0.05). In the hypobaric hypoxia group, the myocardial cells of rats were arranged disorderly and shaped differently, with cases such as edema, degeneration, necrosis, nucleus pyknosis, and massive infiltration of inflammatory cells (mostly neutrophilic granulocytes). In the hypobaric hypoxia + salidroside group, the above-mentioned pathological changes in myocardial cells were relieved, and the concentrations of IL-6 and TNF-α in rats decreased significantly (P < 0.05). Therefore, salidroside had the repairing and protective effects on the hypobaric hypoxia-induced myocardial injuries in rats. The application of salidroside could reduce the inflammatory responses of rats with hypobaric hypoxia-induced myocardial injuries through PI3K/Akt signaling pathway, thereby protecting the myocardial cells. Through the research of this paper, it is concluded that salidroside has certain protective effect on cardiac myocytes. Although the paper has obtained certain research results, there are still some defects. For example, the number of research samples included is not enough, so the research results of this paper have certain limitations. In the next step, the basic research of salidroside and consolidate the research results will be continued.
Footnotes
Peer review under responsibility of King Saud University.
References
- Banta J.E., Ani C., Bvute K.M. Pulmonary vs. extra-pulmonary tuberculosis hospitalizations in the US [1998–2014] J. Infect. Public Health. 2020;13(1):131–139. doi: 10.1016/j.jiph.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Cai L., Li Y., Zhang Q. Salidroside protects rat liver against ischemia/reperfusion injury by regulating the GSK-3β/Nrf2-dependent antioxidant response and mitochondrial permeability transition. Eur. J. Pharmacol. 2017;806:32–42. doi: 10.1016/j.ejphar.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Feng J., Niu P., Chen K. Salidroside mediates apoptosis and autophagy inhibition in concanavalin A-induced liver injury. Exp. Therap. Med. 2018;15(6):4599–4614. doi: 10.3892/etm.2018.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.Q., Qi L., Yang J. Salidroside accelerates fracture healing through cell-autonomous and non-autonomous effects on osteoblasts. Cell Tissue Res. 2017;367(2):197–211. doi: 10.1007/s00441-016-2535-2. [DOI] [PubMed] [Google Scholar]
- Li J., Chen Q., He X. Dexmedetomidine attenuates lung apoptosis induced by renal ischemia–reperfusion injury through α 2 AR/PI3K/Akt pathway. J. Transl. Med. 2018;16(1):78. doi: 10.1186/s12967-018-1455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A., Liu Y., Zhai L. Activating peroxisome proliferator-activated receptors (PPARs): a new sight for chrysophanol to treat paraquat-induced lung injury. Inflammation. 2016;39(2):928–937. doi: 10.1007/s10753-016-0326-2. [DOI] [PubMed] [Google Scholar]
- Li G., Xing X., Yun L. Notoginsenoside R1 prevents H9c2 cardiomyocytes apoptosis against hypoxia/reoxygenation via the ERs/PI3K/Akt pathway. RSC Adv. 2018;8(25):13871–13878. doi: 10.1039/c8ra02554a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Li Y., Zhang T. Salidroside reduces high-glucose-induced podocyte apoptosis and oxidative stress via upregulating heme oxygenase-1 (HO-1) expression. Med. Sci. Monit. 2017;23:4067–4076. doi: 10.12659/MSM.902806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z., Tang T., Sheng L. Salidroside inhibits the proliferation and migration of gastric cancer cells via suppression of Src-associated signaling pathway activation and heat shock protein 70 expression. Mol. Med. Rep. 2018;18(1):147–156. doi: 10.3892/mmr.2018.8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaik A.H., Mohammed A.K., Sammeturi M. Maslinic acid ameliorate electrolytes, membrane bound ATPases, antioxidants and histopathology in isoprenaline attenuated myocardial toxicity in rats. J. King Saud Univ.-Sci. 2020;32(1):1055–1059. [Google Scholar]
- Sheng L., Mao X., Yu Q. Effect of the PI3K/AKT signaling pathway on hypoxia-induced proliferation and differentiation of bone marrow-derived mesenchymal stem cells. Exp. Therap. Med. 2017;13(1):55–62. doi: 10.3892/etm.2016.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Xing-He, Feng Zhen-Hua, Li Zhen-Xing. Salidroside inhibits steroid-induced avascular necrosis of the femoral head via the PI3K/Akt signaling pathway: In vitroandin vivostudies. Mol. Med. Rep. 2018;17(3):3751–3757. doi: 10.3892/mmr.2017.8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Li K., Buhe A. Salidroside inhibits the proliferation and migration of gastric carcinoma cells and tumor growth via the activation of ERS-dependent autophagy and apoptosis. RSC Adv. 2019;9(44):25655–25666. doi: 10.1039/c9ra00044e. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yazouli L.E., Hejaji H., Elmdaghri N., Alami A.A., Dakka N., Radouani F. Investigation of Chlamydia pneumoniae infection in Moroccan patients suffering from cardiovascular diseases. J. Infect. Public Health. 2018;11(2):246–249. doi: 10.1016/j.jiph.2017.07.029. [DOI] [PubMed] [Google Scholar]
- Zheng W., Yang J., Wang Y. Exosomal miRNA-93 and miRNA-205 expression in endometrial cancer. J. King Saud Univ.-Sci. 2020;32(1):1111–1115. [Google Scholar]