Abstract
Intratracheal instillation of apoptotic cells enhances resolution of experimental lung inflammation by incompletely understood mechanisms. We report that this intervention induces functional regulatory T lymphocytes (Tregs) in mouse lung experimentally inflamed by intratracheal administration of lipopolysaccharide. Selective depletion demonstrated that Tregs were necessary for maximal apoptotic cell–directed enhancement of resolution, and adoptive transfer of additional Tregs was sufficient to promote resolution without administering apoptotic cells. After intratracheal instillation, labeled apoptotic cells were observed in most CD11c+CD103+ myeloid dendritic cells migrating to mediastinal draining lymph nodes and bearing migratory and immunoregulatory markers, including increased CCR7 and β8 integrin (ITGB8) expression. In mice deleted for αv integrin in the myeloid line to reduce phagocytosis of dying cells by CD103+ dendritic cells, exogenous apoptotic cells failed to induce transforming growth factor-β1 expression or Treg accumulation and failed to enhance resolution of lipopolysaccharide-induced lung inflammation. We conclude that in murine lung, myeloid phagocytes encountering apoptotic cells can deploy αv integrin–mediated mechanisms to induce Tregs and enhance resolution of acute inflammation.
Although acute inflammation often resolves, persistent inflammation is a common and frequently intractable clinical problem.1, 2, 3 Nevertheless, a growing body of data indicates that inflammation may normally be kept in check by safe immunosuppressive clearance of cells dying by apoptosis.4, 5, 6, 7, 8, 9, 10 Both in vitro and in vivo approaches have demonstrated that myeloid phagocytes [both macrophages and immature dendritic cells (DCs)] employ various molecular complexes assembled by phagocyte cell surface αv integrin to bind cells dying by apoptosis11, 12, 13, 14, 15, 16 and trigger anti-inflammatory responses, such as elaboration of active transforming growth factor-β1 (TGF-β1) and induction of CD4+CD25+FoxP3+ regulatory T lymphocytes (Tregs) by CD103+ DCs.11,17, 18, 19, 20 A substantial body of data implicates αvβ3 and αvβ5 integrins in phagocytosis of apoptotic cells by macrophages and DCs, respectively, whereas myeloid αvβ8 integrin is required for efficient production of active TGF-β1 from the surface-bound latent form, a key immunosuppressive consequence of phagocytic clearance of cells dying by apoptosis.11, 12, 13, 14, 15, 16, 17, 18, 19, 20 However, whether such αv-dependent mechanisms can be deployed in the acutely injured lung to promote resolution of inflammation has been unknown.
Inhalation of bacterial endotoxins, such as lipopolysaccharide (LPS), can cause lung inflammation and is implicated in a range of lung diseases affecting humans, such as various occupational dust disorders,21 and animals (eg, equine heaves).22 Therefore, many have sought to dissect mechanisms of lung inflammation and its resolution by study of self-limited experimental lung inflammation induced by intratracheal administration of LPS. In this clinically relevant model, there is circumstantial evidence that clearance of cells dying by apoptosis is important in directing resolution of acute lung inflammation. Huynh et al10 demonstrated that intratracheal administration of apoptotic cells enhanced resolution of LPS-induced lung inflammation in a TGF-β1–dependent manner. Although TGF-β1 is now widely recognized as a key stimulus inducing Tregs,23 the role of such lymphocytes was not dissected by these investigators. Nevertheless, although D'Alessio et al24 did not examine effects of exogenous apoptotic cells, they did elegantly employ loss-of-function and gain-of-function approaches to demonstrate that Tregs are crucial for resolution of LPS-induced lung inflammation and are associated with increased TGF-β1 production and enhanced clearance of leukocytes by apoptosis and subsequent phagocytosis. Nevertheless, it was not known whether induction of Tregs is required for exogenous apoptotic cells to direct enhanced resolution of LPS-driven acute lung inflammation.
In this study, it was demonstrated that intratracheal administration of exogenous apoptotic cells not only enhances resolution of LPS-induced lung inflammation, but also induces functional Tregs in the lung, capable of suppressing T-cell proliferation in mixed cell culture. By selective inducible deletion of Tregs,25 it was confirmed that Tregs are necessary for maximal enhancement by administered apoptotic cells of resolution of lung inflammation. Adoptive transfer was also deployed to demonstrate that Tregs are sufficient for enhanced resolution, being able to substitute for exogenous apoptotic cells in promoting resolution of LPS-induced lung inflammation in wild-type mice. There is strong evidence in the gut that Tregs are induced in draining lymph nodes by immunoregulatory CD103+ myeloid dendritic cells that have migrated from the gut wall, having taken up cells dying by apoptosis at that site.26, 27, 28, 29, 30, 31, 32 The fate of labeled exogenous apoptotic cells that were administered intratracheally was therefore tracked. Most CD11c+CD103+ lung DCs migrating to draining mediastinal lymph nodes had ingested exogenous apoptotic cells and had acquired a migratory immunoregulatory phenotype expressing CCR7 and β8 integrin (ITGB8). The myeloid αv integrin is crucial for the induction of Tregs by immunoregulatory CD103+ DCs that have ingested apoptotic cells.11,18,20,32 Therefore, this study used mice selectively deficient for αv in the myeloid line, which is thought in myeloid DCs to inhibit αvβ5-mediated phagocytosis of apoptotic cells11,14,15 and αvβ8-directed activation of TGF-β1.11,33 It was observed that intratracheal administration of apoptotic cells neither induced TGF-β1 expression nor Treg accumulation and failed to enhance resolution of LPS-induced lung inflammation in mice lacking αv in the myeloid line.
We conclude that exogenous apoptotic cells promote resolution of experimental lung inflammation by migratory immunosuppressive CD103+ dendritic cells and myeloid αv integrin–dependent induction of regulatory T lymphocytes. We discuss the relevance of these findings to regulation of inflammatory responses in the lung in health and disease.
Materials and Methods
Animals
C57BL/6 mice (aged 6 to 8 weeks) were purchased from Charles River Laboratories (Tranent, Scotland, UK). FoxP3–green fluorescent protein reporter mice and FoxP3.LuciDTR-4 mice25 were obtained from the Anderton Group [Medical Research Council (MRC) Center for Inflammation Research, University of Edinburgh, Edinburgh, UK]. We have previously demonstrated11 targeting of αv deletion in αv-tie2 mice backcrossed to C57BL/6 backgrounds for 10 generations. In these experiments, control mice were wild-type genotype from the same litters at 6 to 8 weeks old. All mice were housed in conventional specific and opportunistic pathogen-free facilities. All experiments were performed after Research Ethics Committee and veterinary review at the University of Edinburgh, and were conducted in accordance with the UK Home Office Scientific Procedures Act (1986) under license P4871232F.
Generation of Apoptotic Cells
The mouse thymus was removed and disaggregated by passing through a 40-μm cell strainer in RPMI 1640 medium supplemented with 2 mmol/L l-glutamine, 100 U/mL penicillin, and 100 g/mL streptomycin, to yield a single-cell suspension. Sometimes, thymocytes were stained with CM-orange (Invitrogen, Carlsbad, CA) in serum-free RPMI 1640 medium in a 37°C incubator for 20 minutes before aging them. Thymocytes were aged overnight at 4 × 106/mL in RPMI 1640 supplemented medium with 1% fetal bovine serum and 1 μmol/L of dexamethasone, which yielded a largely annexin V–positive (>85% ± 5%) and low propidium iodide (PI)-positive (<15% ± 5%) cell population, as previously described.11,18,20
Airway Challenge and Treatment
Mice were lightly anesthetized with isoflurane and intratracheally instilled with 15 μg LPS (Sigma-Aldrich, St. Louis, MO; Escherichia coli serotype O26:B6). After 6 hours, 2 × 106 apoptotic cells in 50 μL phosphate-buffered saline (PBS) or PBS only as a control were intratracheally instilled. Control mice were challenged with 50 μL PBS. Mice were sacrificed at 24 hours and 6 days by cervical dislocation. For studies of possible transport of apoptotic cells by DCs, mice were intratracheally instilled with 50 μL of 8 mmol/L carboxyfluorescein succinimydl ester (CFSE) (Invitrogen) in PBS to label DCs; after 6 hours, 20 × 106 apoptotic cells stained with CM-orange in 50 μL PBS with 10 μg LPS were instilled intratracheally. Labeled apoptotic cells were administered at a larger dose to ensure they could be tracked, given that apoptotic cells are normally ingested and destroyed rapidly by phagocytes.5 Mice were sacrificed at 24 hours; lung draining mediastinal lymph nodes (dMLNs) were frozen into OCT compound and stored in −80°C freezer for section and microscopy analysis.
In Vivo Depletion of FoxP3+ T Cells
FoxP3.LuciDTR-4 mice (aged 6 to 8 weeks) were used.25 FoxP3+ T cells were depleted 2 days before intratracheal instillation by i.p. injection of diphtheria toxin17 (Sigma, St. Louis, MO) at 24 ng/g mouse body weight every day. Diphtheria toxin was injected intraperitoneally every 2 days (day 2 and day 4) at 24 ng/g mouse after intratracheal treatments (day 0). Control mice received the same volume of PBS by i.p. injection. Confirmation of FoxP3+ T-cell depletion was determined by staining peripheral blood 2 days after i.p. injection of diphtheria toxin (data not shown) and 6 days after intratracheal treatments. Mice were sacrificed by cervical dislocation after 6 days. Bronchoalveolar lavage (BAL), lung, and lung dMLN tissues were collected.
Isolation of Cells from BAL and Tissues
Mice were sacrificed by cervical dislocation. The trachea was exposed and cannulated with a 21-gauge needle encased in a silicon tube. Lungs were lavaged three times with 1 mL of cold PBS. Samples were centrifuged at 300 × g for 5 minutes at 4°C, and supernatant from the first lavage was stored in −80°C for cytokine detection. Cell counts were performed by using a Z2 Coulter counter (Beckman Coulter, Fullerton, CA), according to manufacturer's instructions. Single-cell suspensions were used for further analysis.
After performing BAL, lungs were perfused via the right ventricle of the heart with 5 mL PBS to deplete the intravascular pool of cells from the lung vasculature. Lung and lung dMLNs were removed, cut into pieces, and digested in RPMI 1640 medium containing DNase I (0.2 mg/mL) and Liberase TL (0.33 mg/mL) or collagenase D (2 mg/mL) (all from Roche, Welwyn Garden City, UK) for 30 minutes at 37°C and then incubated in PBS/0.5% bovine serum albumin/10 mmol/L EDTA for another 5 minutes. Single-cell suspensions were prepared from the predigested tissues by passing through 40-μm cells strainers (BD Falcon, Devon, UK) and washed cell strainer with PBS/0.5% bovine serum albumin/2 mmol/L EDTA. Red blood cells were lysed by using ammonium chloride buffer (Sigma). Cell count was performed with a Z2 Coulter counter.
Flow Cytometry and Antibodies
The single-cell suspensions from BAL, lung, and dMLNs were prepared as described above. Cells were pre-incubated with 5 μg/mL blocking antibody against CD16/CD32 (eBioscience, San Diego, CA) to reduce non-specific binding. The cells were stained in PBS containing 0.2% bovine serum albumin and 0.02% NaN3 with antibodies as follows: anti–CD11c-allophycocyanin (APC) or phosphatidylethanolamine (PE)–Cy7 (HL3) and anti–CD103-PE or PerCP-Cy5.5 (M290; BD Bioscience, Wokingham Berkshire, UK); anti–CD45-AF700 (30-F11), anti–CD3-APC-Cy7 (17A2), anti–CD64-PE-Cy7 (X54-5/7.1), anti–I-A/I-E-BV421 (M5/114.15.2), and Zombie Aqua Fixable Viability marker (all from BioLegend, San Diego, CA); and anti–CD11b-PerCP-Cy5.5 or APC-Cy7 (M1/70), anti–Ly6G (GR-1)–fluorescein isothiocyanate (RB6-8C5), CD4-PerCP-Cy5.5 (RM4-5), and mouse regulatory T-cell staining kit (FoxP3 FJK-16s-PE, CD4–fluorescein isothiocyanate, CD25-APC; all from eBioscience). Fluorescence-activated cell sorting (FACS) data acquisition was performed on a BD LSR Fortessa (BD Bioscience) running FACS Diva software version 8.0.1. FlowJo software version 9.9.4 (Tree Star, Ashland, OR) was used for data analysis. Cell sorting was performed on a FACSAria (BD Bioscience) flow cytometer.
Histologic Scores and Immunohistochemical Analysis of Lung Tissue
After BAL, lungs were perfused with PBS; and for histologic examination, lungs were inflated with and fixed in 10% neutral-buffered formalin. Organs were embedded in paraffin, and transversal sections (4 μm thick) were cut and stained with hematoxylin and eosin (Sigma-Aldrich). Inflammation was scored34 for each mouse at ×200 magnification by averaging the score of 10 consecutive fields where the lung was correctly inflated and the field contained a complete transaction of at least one bronchiole, blood vessels, and alveolar airway. Inflammation was scored on an increasing severity score of 1 to 5 in the perivascular and peribronchiolar alveolar tissue compartment (1, no cells; 2, <20 cells; 3, 20 to 50 cells; 4, 50 to 100 cells; and 5, >100 cells). Histologic scores were performed blinded (A.Z.) to experimental details. Lung hematoxylin and eosin staining images were obtained using a Zeiss Axis A1 (Carl Zeiss Ltd., Cambridge, UK) stereology microscope.
Cell Proliferation Assay
Single-cell suspensions from BAL and lung were harvested as described above. Lung cells were spun over Lympholyte-M (VHBio, Gateshead, UK) was used to isolate mononuclear leukocytes. Lung CD4+ T cells were sorted by magnetic positive selection separation (CD4+ T Cell Isolation Kit; Miltenyi Biotec, Bergisch Gladbach, Germany). The total BAL cell and CD4+ cells were washed and resuspended in complete medium (RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L l-glutamine, and 50 μmol/L 2-mercaptoethanol; Invitrogen) and plated into 96-well round bottom plates at 100 × 103 per well in triplicate. Cells were stimulated with anti-CD3e (5 μg/mL; BD Biosciences) for 72 hours. 3H-thymidine (0.5 μCi) was added to each well at the last 16 hours. Cell proliferation was analyzed by counting incorporation in a β scintillation counter (Wallac, Buckinghamshire, UK).
Quantification of mRNA Expression
Cells from BAL, lung, and dMLNs were harvested as described above. CD11c+ cells were sorted by using CD11c MACs microbeads (Miltenyi Biotech). Sorted cells were spun down and put into 0.5 mL TRIzol (Invitrogen) for RNA extraction. cDNA was synthesized using High Capacity Reverse Transcription kit (Applied Biosystems, Foster City, CA). For real-time PCR, all primer and probe mixes and TaqMan fast master mixes were purchased from Applied Biosystems. For genes of interest, mRNA expression was quantified by using ABI 7500 fast real-time PCR system, according to the manufacturer's instructions. The mRNA relative expression level of target gene was normalized to housekeeping gene 18s.
Isolation and Adoptive Transfer of CD4+ CD25+ T Cells and CD4+CD25− T Cells
Spleens from naïve C57BL6 mice were prepared for single-cell suspensions. CD4+CD25+ cells (Tregs) and CD4+CD25− cells (T cells) were isolated by using MACS bead mouse CD4+CD25+ Treg isolation kit (Miltenyi Biotec), according to the manufacturer's instructions. The purity of CD4+CD25+ T-cell and CD4+CD25− T-cell fractions was >93%, as assessed by flow cytometry. Single-cell suspensions (5 × 105 in 100 μL of PBS) were adoptively transferred at 6 hours after intratracheal instillation via tail vein injection.
Cytokine Production Measurements
BAL IL-10, active TGF-β1, tumor necrosis factor-α, and IL-6 levels were measured by using enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN).
In Vivo Assay of Phagocytosis of Apoptotic Thymocytes
To assess the ability of lung resident DCs to clear apoptotic cells in vivo, 10 × 106 fluorescently labeled (Cell Tracker Green CMFDA; Invitrogen) apoptotic thymocytes in 50 μL PBS were instilled intratracheally. Mice were sacrificed after 1 hour, and BAL lavage was collected. Cells were stained with anti-mouse CD11c-APC, major histocompatibility complex (MHC) II-BV421, and CD103-PE on ice for 20 minutes. Cells were analyzed by FACS, and DCs were gated on CD11c+MHCII+h cells. DC phagocytosis was assessed by fluorescence percentage.
Fluorescence Microscopy
Cryosections (5 μm thick) of dMLNs were cut and fixed in 4% paraformaldehyde and stained with DAPI (eBioscience). Images were obtained using an Axioskop microscope (Carl Zeiss Ltd.).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism software 6.0 (GraphPad Software, San Diego, CA). Data were analyzed by using an unpaired t-test when comparing two experimental groups. And one-way analysis of variance with Bonferroni or Tukey post-hoc test was used when comparing more than two experimental groups. Statistics were calculated using two-way analysis of variance Tukey post-hoc test when comparing more than two groups with two factors.
Results
Intratracheal Apoptotic Cells Enhance Resolution of LPS-Induced Lung Inflammation and Induce Regulatory T Cells
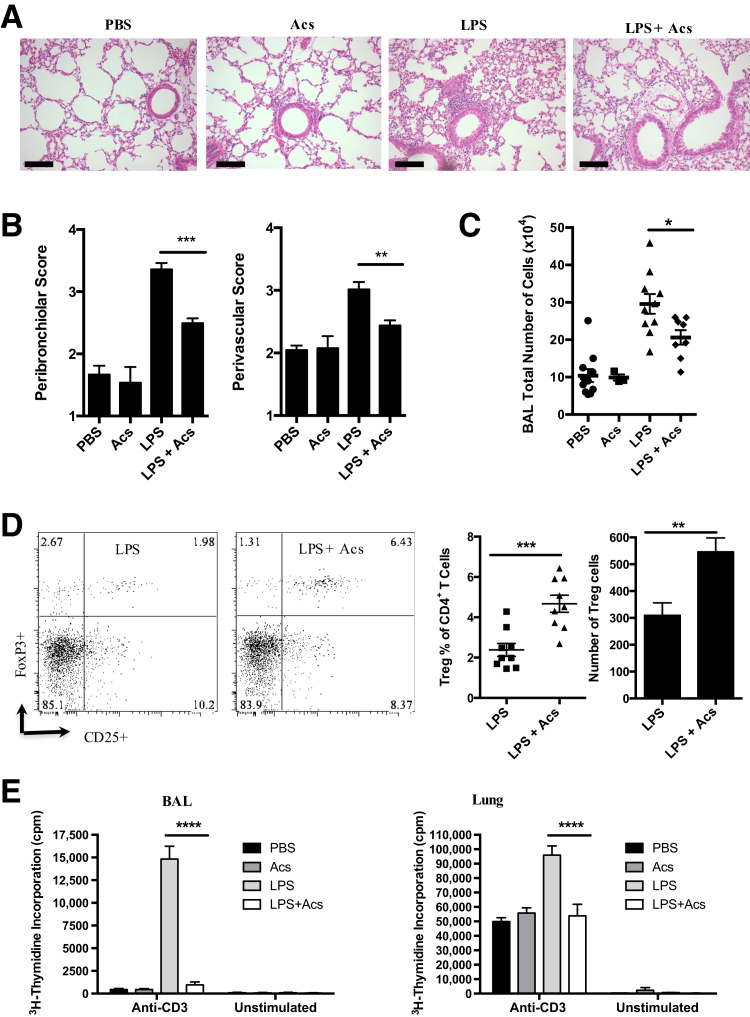
Exogenously administered apoptotic cells have been shown previously to modulate experimentally induced inflammation in several systems.9, 10, 11 Fifteen micrograms of LPS was administered via tracheal instillation in ether-anesthetized C57BL6 mice, after assessing lung inflammation at day 6, when granulocyte and mononuclear cell numbers are known to be declining in this model of self-limited lung inflammation2,10,24 (Figure 1). At 6 hours after intratracheal installation of LPS, when granulocyte numbers in BAL are known to be increasing at the fastest rate observed in this model,2,10,24 intratracheal administration of 2 × 106 mouse apoptotic thymocytes enhanced resolution of experimental lung inflammation, as assessed by histology (Figure 1, A and B) and total cell count in BAL (Figure 1C). Live thymocytes, instilled intratracheally at 2 × 106 at 6 hours as a control for apoptotic thymocytes, had no effect on LPS-induced lung inflammation at 6 days (eg, in BAL, total cell count in 3 mL was 49.9 ± 3.9 × 104 with LPS alone and 48.5 ± 3.8 × 104 with LPS plus live thymocytes versus 26.7 ± 2.8 × 104 with LPS plus apoptotic thymocytes; data are given as means ± SD; n = 6). When the presence of CD4+ T cells with the FoxP3+CD25+ regulatory phenotype was assessed by immunofluorescence flow cytometry of BAL, intratracheal administration of apoptotic thymocytes at 6 hours elicited an approximate doubling of Tregs (Figure 1D) versus LPS alone. In vitro evidence that this cell population included functional Tregs was obtained in mixed cell culture over 3 days of both total BAL cells or CD4+ cells purified from mouse lung obtained 6 days after intratracheal LPS; incorporation of ³H-thymidine was significantly reduced in both assays where animals had received intratracheal apoptotic cells at 6 hours (Figure 1E).
Figure 1.
Intratracheal administration of apoptotic cells promotes resolution of lipopolysaccharide (LPS)–induced lung inflammation and induces functional regulatory T cells (Tregs). As described in Materials and Methods, female C57BL/6 mice received control phosphate-buffered saline (PBS) or the stimulus LPS by intratracheal instillation at 0 days; 2 × 106 apoptotic thymocytes (Acs) intratracheally at 6 hours; and at 6 days, bronchoalveolar lavage (BAL) and lung tissue were harvested. A: Lung hematoxylin and eosin staining images. B: Lung injury score. C: Total BAL cell numbers. D: BAL regulatory T-cell percentage and number counts; representative fluorescence-activated cell sorting plots gated on CD3+CD4+ T cells. E: Total BAL cells (left panel) and lung CD4+ cells (right panel) were harvested from the four groups of experimental animals and were stimulated in vitro with anti-CD3 for 3 days. Cell proliferation was analyzed by counting incorporation of 3H-thymidine. One experiment representative of three is shown. Data are expressed as means ± SEM (B–E). n = 6 individual mice (B); n = 3 experiments (B–D); n = 3 to 4 mice per group per experiment (B–D). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001 (one-way analysis of variance with Dunnett post-hoc test). Scale bars = 100 μm (A).
Tregs Are Required and Sufficient for Enhancement of Resolution of Experimental Lung Inflammation
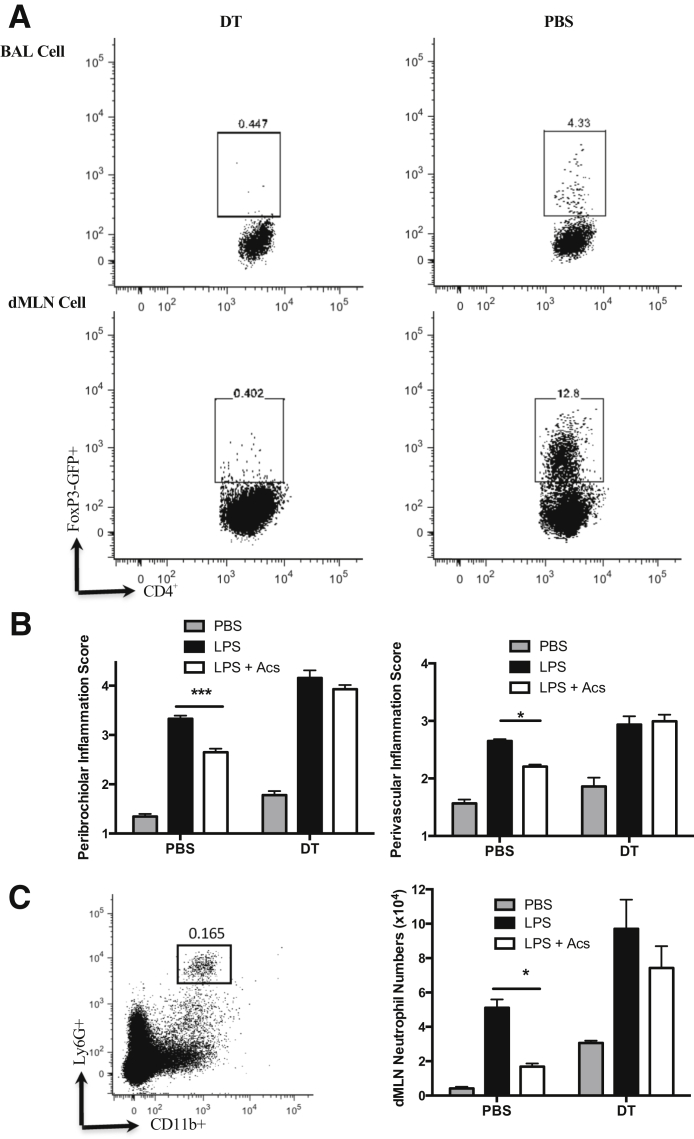
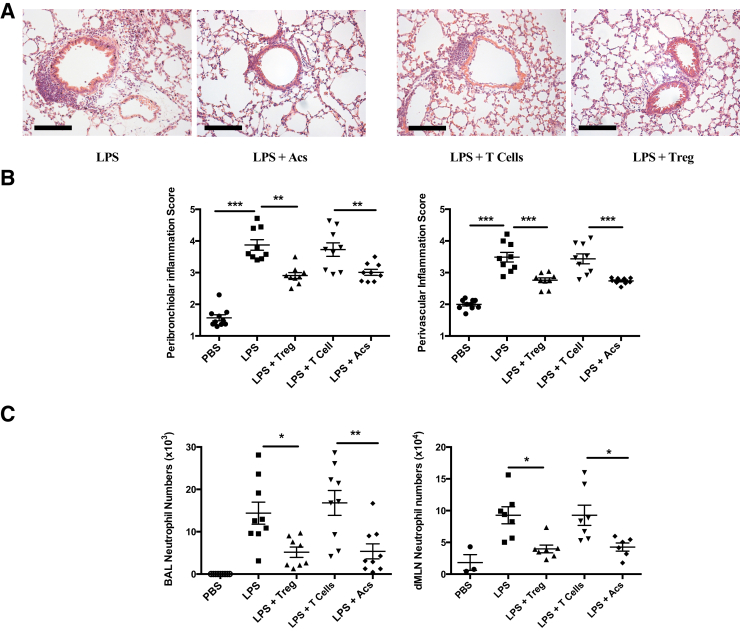
Mice25 transgenic for the diphtheria toxin receptor under the FoxP3 promoter were used to delete selectively FoxP3+ Tregs in mice receiving intratracheal LPS at day 0. Where diphtheria toxin was administered, as described above (Materials and Methods), FoxP3+CD4+ T cells were efficiently deleted in both BAL and cells isolated from mediastinal lymph nodes (Figure 2A). Deletion of Tregs abrogated the capacity of intratracheal apoptotic thymocytes to enhance resolution of lung inflammation assessed by histology (Figure 2B) or neutrophil counts in dissociated mediastinal lymph nodes (Figure 2C). Furthermore, this loss-of-function evidence was complemented with gain-of-function conditions achieved by adoptive i.v. transfer of CD4+CD25+ Tregs to wild-type mice, as described above (Materials and Methods). Administration of Tregs, but not CD4+CD25− control T cells, recapitulated the capacity of intratracheal apoptotic cells to enhance resolution of LPS-induced lung inflammation, as assessed by histology (Figure 3, A and B) and neutrophil counts in both BAL and dissociated mediastinal lymph nodes (Figure 3C).
Figure 2.
Regulatory T cells (Tregs) are required for apoptotic cell (Acs) direction of resolution of lung inflammation. FoxP3.LuciDTR-4 mice were treated by i.p. injection of diphtheria toxin (DT),17 as described in Materials and Methods, to secure depletion of FoxP3+ Tregs. Mice were sacrificed 6 days after intratracheal instillation of phosphate-buffered saline (PBS), lipopolysaccharide (LPS), or LPS with Acs; and tissues were analyzed by flow cytometry and histology. A: FoxP3-GFP+ expression flow cytometry plots after gating on CD3+CD4+ T cells in bronchoalveolar lavage (BAL) and lung draining mediastinal lymph nodes (dMLNs) after intratracheal instillation of LPS with Acs (one representative of two experiments). B: Lung injury score from two individual experiments. C: Single-cell suspensions from lung dMLNs were stained with anti-mouse Ly6G–fluorescein isothiocyanate and CD11b-PerCP-Cy5.5. Neutrophils were gated on Ly6G and CD11b double-positive cells; one indicative flow cytometry plot is presented. Chart shows neutrophil numbers in dMLNs. Data are expressed as means ± SEM (B and C). n = 6 individual mice from 2 experiments (B and C). ∗P < 0.05, ∗∗∗P < 0.001 (two-way analysis of variance with Tukey post-hoc test).
Figure 3.
Adoptive transfer of regulatory T cells (Tregs) mimics promotion of resolution of lipopolysaccharide (LPS)–induced lung inflammation by administered apoptotic cells (Acs). Single-cell suspensions of CD4+CD25+ T cells (Tregs) and CD4+CD25− T cells (T cells) prepared from naïve C57BL6 mouse spleen were adoptively transferred via tail vein injection at 6 hours after intratracheal treatments. Mice were sacrificed and tissues were collected at day 6. A: Lung hematoxylin and eosin staining images. B: Lung injury scores. C: Neutrophil numbers in bronchoalveolar lavage (BAL) and draining mediastinal lymph nodes (dMLNs) were measured by cell counter and flow cytometry, as described in Materials and Methods and legend to Figure 2. In all cases, data were combined from three individual experiments with at least three to nine individual mice. Data are expressed as means ± SEM (B and C). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (one-way analysis of variance with Dunnett post-hoc test). Scale bars = 100 μm (A). Acs, apoptotic cell; PBS, phosphate-buffered saline.
Phagocytosis of Intratracheal Apoptotic Cells Drives the Migration of Immunoregulatory CD103+ Myeloid Dendritic Cells to Mediastinal Lymph Nodes
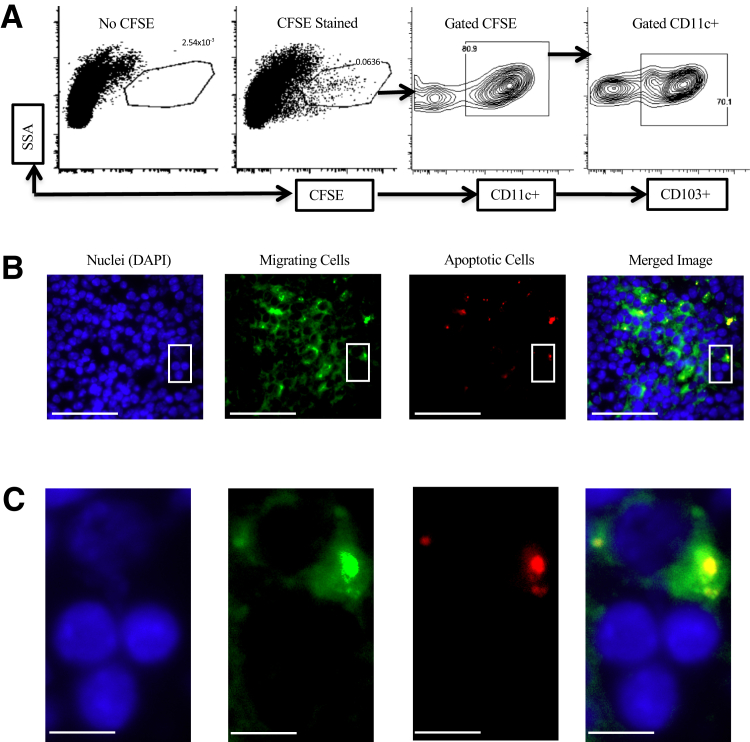
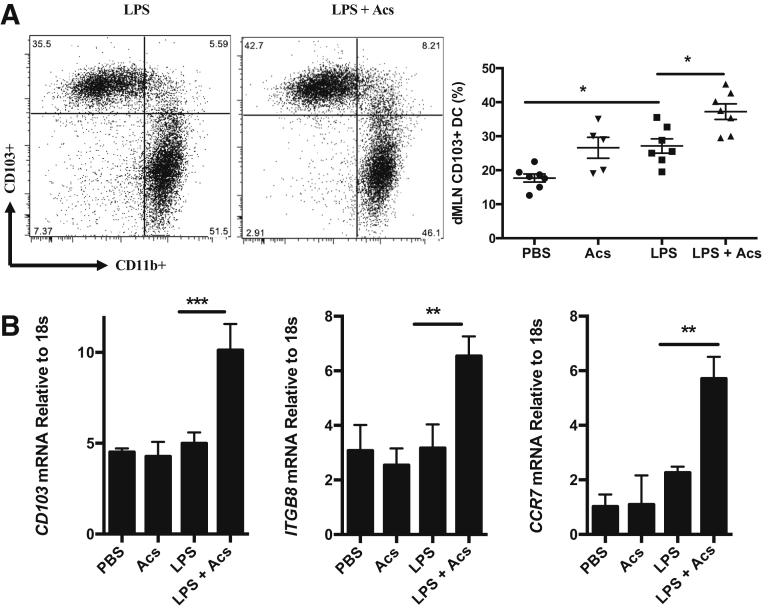
A considerable body of evidence in various experimental systems indicates that Tregs can be induced by immunoregulatory CD103+ (or species equivalent) myeloid DCs migrating to draining lymph nodes after phagocytosis of cells dying by apoptosis in the periphery.27, 28, 29, 30, 31, 32,35 Myeloid DCs were labeled in mouse lung by intratracheal administration of CFSE dye, followed 6 hours later by intratracheal LPS (10 μg) mixed with CM-orange–stained apoptotic thymocytes. Mice were then sacrificed at 24 hours (when DC migration to draining lymph nodes is maximal35), and flow cytometry of dissociated mediastinal lymph nodes demonstrated that CFSE-stained, CD11c+CD103+ DCs had migrated to lymph nodes from lung (Figure 4A). Fluorescence microscopy of frozen sections of mediastinal lymph nodes demonstrated that most DCs (means ± SEM, 67.2% ± 8.5%; n = 3) were associated with labeled apoptotic cells (Figure 4B), with higher-power microscopy strongly suggesting that CD103+ DCs had ingested exogenously administered apoptotic cells (Figure 4C). It was assessed whether such migrating CD103+ DCs had the expected migratory immunoregulatory phenotype11,18,20 by assessing cells dissociated from mediastinal lymph nodes 24 hours after intratracheal administration. Where apoptotic cells were administered, these significantly increased both the proportion of CD103+ DCs in the CD11c+MHCII+ population in mediastinal lymph nodes (Figure 5A) and mRNA expression for CD103, CCR7, and ITGB8 (Figure 5B), markers of the migratory immunoregulatory CD103+ DC population.11,18,20
Figure 4.
Lung dendritic cells (DCs) transport apoptotic cells to the draining mediastinal lymph nodes. C57BL6 mice received 50 μL carboxyfluorescein succinimydl ester (CFSE) (green) in phosphate-buffered saline by intratracheal administration to label lung DCs; 6 hours later, CM-orange–stained apoptotic cells were administered intratracheally with lipopolysaccharide. Mice were sacrificed after 24 hours, and draining mediastinal lymph nodes (dMLNs) were collected for single-cell suspensions or histology. A: Single-cell suspensions from lung dMLNs were stained with CD11c and CD103. Flow cytometry plots show one representative of three experiments. B: Fluorescence microscopy of sections of a typical lung draining mediastinal lymph node. dMLNs were stained with DAPI; migrated DCs are green, and apoptotic cells are red. Images were one representative of three. C: High-power images of the boxed areas from the relevant images in B. Scale bars: 35 μm (B); 5 μm (C). SSA, side scatter axis.
Figure 5.
Apoptotic cells enhance migration of CD103+ dendritic cells (DCs) with an immunoregulatory phenotype to draining mediastinal lymph nodes (dMLNs). Lung dMLN single-cell suspensions were prepared 24 hours after intratracheal administration of phosphate-buffered saline (PBS) as a control, apoptotic cells (Acs), lipopolysaccharide (LPS) plus PBS (LPS), or LPS plus apoptotic cells (LPS + Acs). A: Lung dMLN CD103+ DC percentage (CD103+CD11b−) measured by gating on CD11c+MHC-II+hi DCs. Flow cytometry plots display one representative of two experiments. Data were combined from two experiments. B: Lung dMLN DC mRNA expression relative to 18s measured by quantitative PCR. To obtain sufficient CD11c+ DCs, lung dMLNs were pooled from five animals per experimental group; CD11c+ DCs were isolated by using CD11c MACs beads and RNA prepared as described in Materials and Methods. Data are from three independent experiments. Data are expressed as means ± SEM (A and B). n = 5 to 7 mice per group (A). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (one-way analysis of variance with Dunnett post-hoc test).
Myeloid αv Integrin Is Essential for Induction by Intratracheal Apoptotic Cells of Tregs and Enhanced Resolution of Lung Inflammation
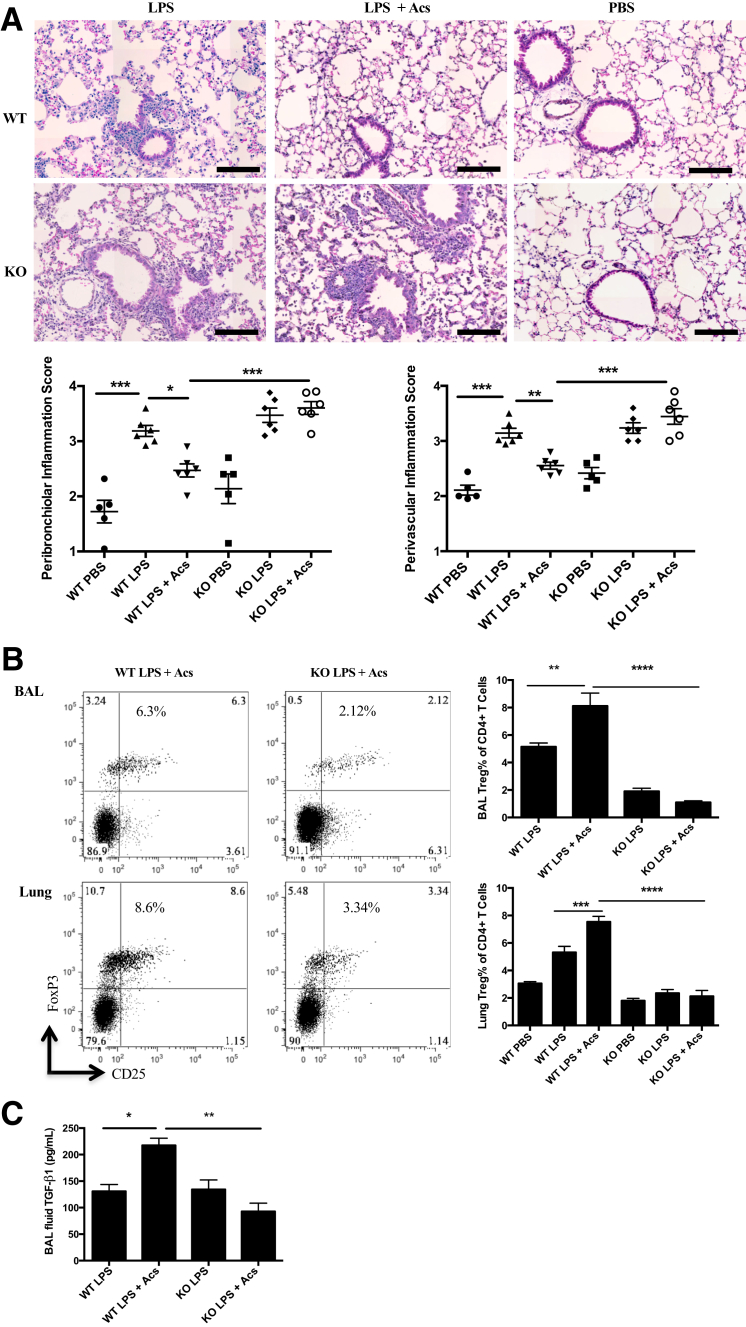
To determine whether myeloid αv integrin mediates enhancement of resolution of experimental lung inflammation by exogenous intratracheal apoptotic cells, LPS-induced inflammation was examined in mice deleted for αv in the myeloid line and controls. αv-tie2 mice, in which defective phagocytosis of apoptotic cells and associated uncontrolled inflammation have been shown to be specific to αv deficiency in the myeloid line, were used.11 In preliminary experiments in untreated mice, assessing BAL taken 1 hour after intratracheal administration of labeled apoptotic cells, it was confirmed that deletion of myeloid αv integrin resulted in reduced phagocytosis of apoptotic cells by CD11c+MHC II+hi DCs (means ± SEM, 18.07% ± 2.55% versus 32.43% ± 2.47% in wild-type mice; n = 6) and by the CD103+ subset of such DCs (means ± SEM, 14.89% ± 2.50% versus 29.82% ± 1.97% in wild-type mice; n = 6). In the αv-tie2 mice, apoptotic cells administered intratracheally failed to enhance resolution of inflammation, assessed by lung histology (Figure 6A), and failed to induce Tregs, as assessed by flow cytometry of BAL and dissociated lung tissue (Figure 6B). Furthermore, apoptotic cells did not induce expression of active TGF-β1 in mice lacking myeloid αv integrin (Figure 6C).
Figure 6.
Myeloid integrin αv is required for exogenous apoptotic cell direction of resolution of lung inflammation and local induction of transforming growth factor-β (TGF-β) and regulatory T cells (Tregs). Integrin αv-tie2 knockout (KO) and litter control wild-type (WT) mice received lipopolysaccharide (LPS) or phosphate-buffered saline (PBS) by intratracheal administration, followed at 6 hours with intratracheal administration of apoptotic thymocytes (Acs) or PBS. Tissues were collected at 24 hours to assess TGF-β expression or 6 days to assess cellular kinetics. A: Lung hematoxylin and eosin staining images and injury scores at 6 days. B: Bronchoalveolar lavage (BAL) and lung Treg percentage at 6 days. Representative flow cytometry plots show Treg percentage by gating on CD3+CD4+ T cells. C: BAL fluid TGF-β1 cytokine was measured by enzyme-linked immunosorbent assay 24 hours after intratracheal treatments. Chart shows one representative of three individual experiments. Data are expressed as means ± SEM of individual mice (A–C). n = 2 experiments (A); n = 3 experiments (B and C); n = 3 to 4 mice per group per experiment (A–C). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001 (one-way analysis of variance with Dunnett post-hoc test). Scale bars = 100 μm (A).
Discussion
Inhalation of inflammatory stimuli constitutes an ever-present threat to the structural and functional integrity of the lung. Yet, persistent inflammation of the lung is infrequent, suggesting that powerful mechanisms exist to suppress airway inflammation and promote its resolution. In this report, we demonstrate that one such mechanism is available to the experimentally inflamed lung. Intratracheal administration of exogenous apoptotic cells enhanced resolution of LPS-directed inflammation and induced functional Tregs, shown by depletion and supplementation to be necessary and sufficient for maximal acceleration of resolution. Labeled apoptotic cells were present within most CD11c+ CD103+ myeloid DCs migrating to draining lymph nodes, which exhibited migratory immunoregulatory markers, such as enhanced expression of CCR7 and β8 integrin (ITGB8). In mice deleted for αv integrin in the myeloid line, intratracheal administration of exogenous apoptotic cells failed to enhance resolution of LPS-induced lung inflammation, increase TGF-β1 expression, or induce Tregs. We conclude that a potent anti-inflammatory, proresolution mechanism is available to the lung, constituted by phagocytic clearance of apoptotic cells that leads to migration of immunosuppressive CD103+ myeloid DCs bearing apoptotic cells to draining lymph nodes and to myeloid αv integrin-dependent induction of anti-inflammatory Tregs.
This report in a clinically relevant model of lung inflammation builds on a growing body of data that indicate that CD103+ DC clearance of cells dying by apoptosis suppresses inflammation in the gut by mechanisms dependent on Tregs.11,26, 27, 28, 29, 30, 31, 32 Clearly, the microbiome of the gut constitutes an even greater threat of persistent inflammation than inhaled proinflammatory stimuli in the lung, so it is no surprise that this mechanism was first characterized in the gut. Working in the rat, MacPherson's group27 identified a potentially immunoregulatory population of DCs in intestinal lymph that had ingested apoptotic epithelial cells. This rat DC subset is likely to be equivalent to CD11c+ CD103+ DCs in the mouse, which have been shown by Powrie's group and others26, 27, 28, 29, 30 to migrate from the gut wall to draining lymph nodes and to be critical for TGF-β1–dependent induction of CD4+ CD25+ FoxP3+ Tregs. More recently, elegant studies from Blander's group31,32 deployed genetically directed in vivo labeling and inducible apoptosis in genetically targeted gut epithelial cells to demonstrate that CD11c+ DCs captured and ingested cells undergoing apoptosis. Single-cell genomic analysis demonstrated that CD11c+CD103+ DCs up-regulated expression of genes directing migration to lymph nodes (such as CCR7) and those involved in TGF-β1–mediated induction of Tregs (such as LRRC32).31,32 This important work supports our preceding analysis of mice deleted for αv integrin in the myeloid line.11 In the presence of gut microbiota, such mice developed spontaneous gut inflammation associated with diminished clearance of apoptotic cells, a marked reduction in Tregs in draining lymph nodes, and similarly impaired capacity of CD11c+ DCs isolated from such lymph nodes to induce FoxP3 Tregs ex vivo. We inferred that expression of αv by CD103+ DCs is necessary for αvβ5-mediated ingestion of apoptotic cells and subsequent αvβ8-mediated activation of TGF-β1 needed to induce regulatory T cells in the gut,11 a proposal supported by our subsequent work18,20 and by the work of Blander’s group.31,32 However, it was not known if such myeloid αv-dependent mechanisms operated in the lung, although Desch et al35 had demonstrated in a system geared to presentation of apoptotic cell-derived antigen to CD8 T cells that intranasally administered apoptotic cells were preferentially cleared by CD103+ DCs.
Before the current studies, there was circumstantial evidence that clearance of apoptotic cells by immunoregulatory DCs, leading to induction of Tregs, is a proresolution mechanism available to the inflamed lung. D'Alessio et al24 observed that in Rag-1−/− lymphocyte-deficient mice with delayed resolution of LPS-induced lung inflammation, rescue with CD4+ CD25+ FoxP3+ Tregs normalized resolution while increasing otherwise diminished levels of leukocyte apoptosis, clearance by phagocytes, and production of active TGF-β1, known to be necessary for induction of Tregs.23 Furthermore, Henson's group10 confirmed that TGF-β1 was induced in the lung by intratracheal administration of exogenous apoptotic cells, enhancing resolution of LPS-induced lung inflammation. However, neither study definitively linked phagocytic clearance of apoptotic cells with induction of Tregs, nor was the role of DC ingestion of apoptotic cells via αv integrin addressed. Nevertheless, despite providing new data on these issues, our study relied on exogenous administration of (labeled) apoptotic cells. Further work is needed, perhaps adapting to the lung the Blander group's32 genetically directed labeling and targeted cell death induction approaches developed for study of the gut, to demonstrate definitively that cells endogenous to the inflamed lung undergo apoptosis, are ingested by CD103+ DCs, and induce Tregs necessary for prompt resolution. Crossing such mice with animals lacking αv integrin in the myeloid line would be expected from the data reported herein to result in diminished capacity for DCs to i) ingest apoptotic cells, ii) elaborate active TGF-β1, iii) induce Tregs, and iv) promptly resolve experimental lung inflammation.
The current data point toward several future studies. It would be of interest to explore whether apoptotic cells of lineages different to the thymocytes used herein have αv/Treg-dependent proresolution effects in LPS-induced lung inflammation, although this seems likely given previous studies.1,10,36 Furthermore, although our data (Figures 1D, 2, and 3) strongly support the notion that numbers of Tregs at the inflamed site are deterministic in promoting resolution, increased suppressive potency of Tregs after apoptotic cell administration could be sought in the future. Although LPS as a proinflammatory stimulus is relevant to some types of clinical lung inflammation,21,22 further work with proinflammatory stimuli derived from Gram-positive organisms would clearly be of interest. Indeed, future work should determine whether resolution of persistent rather than self-limited lung inflammation can be promoted by administration of apoptotic cells, particulate ligands for myeloid αv integrin, or autologous Tregs. A limitation of the approach taken in the current work is the focus on cellular kinetics in acute lung inflammation, which are well defined in the intratracheal LPS model.2,10,24 Alternative models of lung inflammation will require study if effects of apoptotic cell administration on functional end points are to be determined. Last, further studies will also be needed to explore an intriguing idea that arises from the current data and our previous studies.11,18,20,37 We wonder whether mechanisms defined as promoting resolution of acute inflammation may also operate during health to provide tonic suppression of inflammation, as inferred by the transcriptional signatures described by Blander's group31 in myeloid phagocytes that have ingested an induced wave of apoptotic cells in vivo and by spontaneous inflammation in the (bacterially exposed) gut of mice lacking in the αv integrin in the myeloid line and with consequently diminished capacity to ingest apoptotic cells, elicit TGF-β1, and induce Tregs. Putative deficiencies in such mechanisms for tonic suppression of lung inflammation could be a hitherto unrecognized factor in the pathogenesis of diseases characterized by persistent lung inflammation.
To conclude, intratracheal administration of apoptotic cells to mice with self-limited lung inflammation induced by LPS demonstrates that powerful cellular mechanisms to suppress inflammation and promote its resolution are available to the lung. Exogenous apoptotic cells are ingested by CD103+ DCs that migrate from the airways to draining lymph nodes. Induction of Tregs follows, by mechanisms dependent on myeloid αv integrin, and is necessary and sufficient to enhance resolution of lung inflammation.
Acknowledgments
We thank Richard A. O'Connor for helping with FoxP3 reporter mice; Spike Clay and Gary Borthwick for assistance with animal experiments; the Queen's Medical Research Institute (QMRI) Flow Cytometry and Cell Sorting Facility for excellent technical assistance; Prof. Kev Dhaliwal, Prof. Adriano Rossi, Prof. Neil Henderson, and Prof. Jurgen Schwarze for supporting animal experiments; and Ruth Maclnnes and Eleanor Bonikowski for providing excellent support in preparation of the manuscript.
Footnotes
Supported by UK Medical Research Council grant G0802069 (J.S. and C.H.) and NIH grant U19 AI125378 (A.L.-H.).
Disclosures: None declared.
Author Contributions
C.H., S.A., A.L.-H., and J.S. conceived the study and edited the manuscript; A.Z. and A.L.-H. performed the experiments; A.Z., C.H., A.L.-H., and J.S. wrote the manuscript.
References
- 1.Savill J., Dransfield I., Gregory C., Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 2.Serhan C.N., Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 3.Fullerton J.N., Gilroy D.W. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15:551–567. doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- 4.Savill J.S., Wyllie A.H., Henson J.E., Walport M.J., Henson P.M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation: programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med. 1999;160:S5–S11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- 6.Tsuyuki S., Bertrand C., Erard F., Trifilieff A., Tsuyuki J., Wesp M., Anderson G.P., Coyle A.J. Activation of the Fas receptor on lung eosinophils leads to apoptosis and the resolution of eosinophilic inflammation of the airways. J Clin Invest. 1995;96:2924–2931. doi: 10.1172/JCI118364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milik A.M., Buechner-Maxwell V.A., Sonstein J., Kim S., Seitzman G.D., Beals T.F., Curtis J.L. Lung lymphocyte elimination by apoptosis in the murine response to intratracheal particulate antigen. J Clin Invest. 1997;99:1082–1091. doi: 10.1172/JCI119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dockrell D.H., Marriott H.M., Prince L.R., Ridger V.C., Ince P.G., Hellewell P.G., Whyte M.K. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J Immunol. 2003;171:5380–5388. doi: 10.4049/jimmunol.171.10.5380. [DOI] [PubMed] [Google Scholar]
- 9.Voll R.E., Herrmann M., Roth E.A., Stach C., Kalden J.R., Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 10.Huynh M.L., Fadok V.A., Henson P.M. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacy-Hulbert A., Smith A.M., Tissire H., Barry M., Crowley D., Bronson R.T., Roes J.T., Savill J.S., Hynes R.O. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savill J., Dransfield I., Hogg N., Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 13.Savill J., Hogg N., Ren Y., Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubartelli A., Poggi A., Zocchi M.R. The selective engulfment of apoptotic bodies by dendritic cells is mediated by the alpha(v)beta3 integrin and requires intracellular and extracellular calcium. Eur J Immunol. 1997;27:1893–1900. doi: 10.1002/eji.1830270812. [DOI] [PubMed] [Google Scholar]
- 15.Albert M.L., Pearce S.F., Francisco L.M., Sauter B., Roy P., Silverstein R.L., Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanayama R., Tanaka M., Miwa K., Shinohara A., Iwamatsu A., Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 17.Travis M.A., Reizis B., Melton A.C., Masteller E., Tang Q., Proctor J.M., Wang Y., Bernstein X., Huang X., Reichardt L.F., Bluestone J.A., Sheppard D. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paidassi H., Acharya M., Zhang A., Mukhopadhyay S., Kwon M., Chow C., Stuart L.M., Savill J., Lacy-Hulbert A. Preferential expression of integrin alphavbeta8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology. 2011;141:1813–1820. doi: 10.1053/j.gastro.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Worthington J.J., Fenton T.M., Czajkowska B.I., Klementowicz J.E., Travis M.A. Regulation of TGFbeta in the immune system: an emerging role for integrins and dendritic cells. Immunobiology. 2012;217:1259–1265. doi: 10.1016/j.imbio.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boucard-Jourdin M., Kugler D., Endale Ahanda M.L., This S., De Calisto J., Zhang A., Mora J.R., Stuart L.M., Savill J., Lacy-Hulbert A., Paidassi H. beta8 Integrin expression and activation of TGF-beta by intestinal dendritic cells are determined by both tissue microenvironment and cell lineage. J Immunol. 2016;197:1968–1978. doi: 10.4049/jimmunol.1600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castellan R.M., Olenchock S.A., Kinsley K.B., Hankinson J.L. Inhaled endotoxin and decreased spirometric values: an exposure-response relation for cotton dust. N Engl J Med. 1987;317:605–610. doi: 10.1056/NEJM198709033171005. [DOI] [PubMed] [Google Scholar]
- 22.Pirie R.S., Collie D.D., Dixon P.M., McGorum B.C. Inhaled endotoxin and organic dust particulates have synergistic proinflammatory effects in equine heaves (organic dust-induced asthma) Clin Exp Allergy. 2003;33:676–683. doi: 10.1046/j.1365-2222.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 23.Tran D.Q. TGF-beta: the sword, the wand, and the shield of FOXP3(+) regulatory T cells. J Mol Cell Biol. 2012;4:29–37. doi: 10.1093/jmcb/mjr033. [DOI] [PubMed] [Google Scholar]
- 24.D'Alessio F.R., Tsushima K., Aggarwal N.R., West E.E., Willett M.H., Britos M.F., Pipeling M.R., Brower R.G., Tuder R.M., McDyer J.F., King L.S. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suffner J., Hochweller K., Kuhnle M.C., Li X., Kroczek R.A., Garbi N., Hammerling G.J. Dendritic cells support homeostatic expansion of Foxp3+ regulatory T cells in Foxp3.LuciDTR mice. J Immunol. 2010;184:1810–1820. doi: 10.4049/jimmunol.0902420. [DOI] [PubMed] [Google Scholar]
- 26.Coombes J.L., Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang F.P., Platt N., Wykes M., Major J.R., Powell T.J., Jenkins C.D., MacPherson G.G. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Annacker O., Coombes J.L., Malmstrom V., Uhlig H.H., Bourne T., Johansson-Lindbom B., Agace W.W., Parker C.M., Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaensson E., Uronen-Hansson H., Pabst O., Eksteen B., Tian J., Coombes J.L., Berg P.L., Davidsson T., Powrie F., Johansson-Lindbom B., Agace W.W. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coombes J.L., Siddiqui K.R., Arancibia-Carcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blander J.M. Death in the intestinal epithelium-basic biology and implications for inflammatory bowel disease. FEBS J. 2016;283:2720–2730. doi: 10.1111/febs.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings R.J., Barbet G., Bongers G., Hartmann B.M., Gettler K., Muniz L., Furtado G.C., Cho J., Lira S.A., Blander J.M. Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature. 2016;539:565–569. doi: 10.1038/nature20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mu D., Cambier S., Fjellbirkeland L., Baron J.L., Munger J.S., Kawakatsu H., Sheppard D., Broaddus V.C., Nishimura S.L. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leech M.D., Benson R.A., De Vries A., Fitch P.M., Howie S.E. Resolution of Der p1-induced allergic airway inflammation is dependent on CD4+CD25+Foxp3+ regulatory cells. J Immunol. 2007;179:7050–7058. doi: 10.4049/jimmunol.179.10.7050. [DOI] [PubMed] [Google Scholar]
- 35.Desch A.N., Randolph G.J., Murphy K., Gautier E.L., Kedl R.M., Lahoud M.H., Caminschi I., Shortman K., Henson P.M., Jakubzick C.V. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J Exp Med. 2011;208:1789–1797. doi: 10.1084/jem.20110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel V.A., Lee D.J., Longacre-Antoni A., Feng L., Lieberthal W., Rauch J., Ucker D.S., Levine J.S. Apoptotic and necrotic cells as sentinels of local tissue stress and inflammation: response pathways initiated in nearby viable cells. Autoimmunity. 2009;42:317–321. doi: 10.1080/08916930902832124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stuart L.M., Lucas M., Simpson C., Lamb J., Savill J., Lacy-Hulbert A. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627–1635. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]