Abstract
The mitochondrial cytochrome c oxidase, the terminal enzyme of the respiratory chain, contains heme and copper centers for electron transfer. The conserved COX2 subunit contains the CuA site, a binuclear copper center. The copper chaperones SCO1, SCO2, and COA6, are required for CuA center formation. Loss of function of these chaperones and the concomitant cytochrome c oxidase deficiency cause severe human disorders. Here we analyzed the molecular function of COA6 and the consequences of COA6 deficiency for mitochondria. Our analyses show that loss of COA6 causes combined complex I and complex IV deficiency and impacts membrane potential-driven protein transport across the inner membrane. We demonstrate that COA6 acts as a thiol-reductase to reduce disulfide bridges of critical cysteine residues in SCO1 and SCO2. Cysteines within the CX3CXNH domain of SCO2 mediate its interaction with COA6 but are dispensable for SCO2–SCO1 interaction. Our analyses define COA6 as thiol-reductase, which is essential for CuA biogenesis.
Keywords: mitochondria, cytochrome c oxidase, CuA center, COA6, copper metallochaperones
Graphical abstract
Highlights
-
•
Loss of COA6 affects respiratory chain complexes IV and I.
-
•
Decreased membrane potential-driven protein import due to loss of COA6.
-
•
Cysteine residues in the CX3CXNH motif of SCO2 mediate COA6 interaction.
-
•
COA6 acts as thiol reductase for copper metallochaperones during CuA biogenesis.
Introduction
Mitochondria fulfill central functions in eukaryotic metabolism. In addition to participating in metabolite turnover and synthesis, mitochondria supply cells with the bulk of energy to drive cellular processes. At the heart of this process is the oxidative phosphorylation system (OXPHOS), which is located in the inner mitochondrial membrane. The protein complexes of the OXPHOS system derive from subunits of dual genetic origin. Thirteen subunits are encoded on human mitochondrial DNA (mtDNA), whereas the rest of the components are encoded in the nucleus, synthesized on cytosolic ribosomes, and transported into mitochondria [1,2]. Hence, the biogenesis of respiratory chain complexes and the F1Fo ATP synthase requires the assembly of proteins that reach the inner membrane through different supply routes [3,4]. In addition, during the assembly process, the redox-active cofactors need to be integrated. The cytochrome c oxidase (complex IV) contains two heme cofactors (a and a3) and two copper centers (CuA and CuB) required for the reduction of molecular oxygen to water.
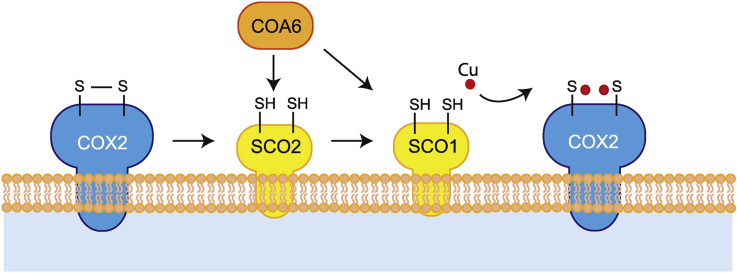
COA6 represents a conserved complex IV assembly factor required for the metalation of the COX2 subunit, which is critical for the formation of the CuA center in the intermembrane space domain [[5], [6], [7], [8], [9], [10]]. Loss of COA6 function is associated with hypertrophic cardiomyopathy and loss of either complex I and IV [11] or isolated complex IV deficiency [12]. COA6 was found in a complex with the metallochaperones SCO1 and SCO2, which are required for copper insertion into the CuA center [7,8] (Fig. 1A). SCO1 and SCO2 have distinct but cooperative functions in copper delivery to the CuA. While SCO2 is thought to deliver the copper molecule to COX2, SCO1 facilitates copper transfer to SCO2 from COX17 [13,14]. In addition, SCO2 is considered to regulate the thiol redox or metalation state of SCO1, thereby fulfilling a signaling role to regulate cellular copper efflux [15]. In contrast, recent in vitro studies suggested that copper transfer to the CuA site only requires SCO1, while SCO2 regulates the thiol redox state of COX2 but not of SCO1 [16]. Since cofactor insertion into the CuA center is thought to be coupled to COX2 insertion into the membrane, SCO1 and SCO2 have been found to interact with FAM36A [17]. In addition, SCO1, SCO2, and COA6 have been found to interact with COX16, a factor involved in delivering COX2 after metalation to the complex IV assembly intermediates (MITRAC complexes) [18]. The structural analysis of Coa6 revealed that the four Cys of the CX9C-CX10C motive form disulfide bridges. Based on the observation, that the Cys58-Cys90 disulfide bridge could be chemically reduced to free thiol groups, has been taken as an indication that these residues could be required for copper coordination [19]. However, the molecular function of COA6 in copper insertion into the CuA center and the question as to how the structural determinants of the protein, relate to its molecular function, remain unaddressed.
Fig. 1.
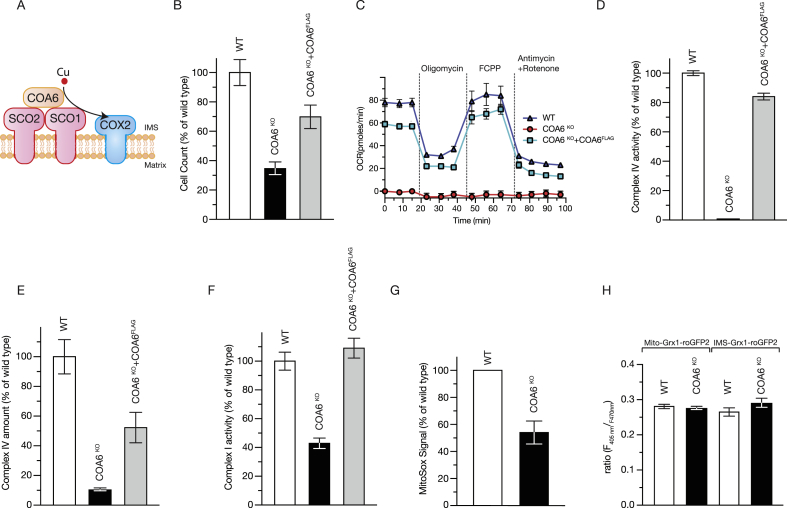
Lack of COA6 affects complex I and complex IV. A. Scheme of COA6 function in copper transfer to CuA center in COX2 (IMS, intermembrane space). B Cells were seeded in glucose-containing medium to equal density and counted after 72 h. Cell numbers were calculated and are presented as % of wild type (WT) (mean +/− SEM, n = 3). C. Representative real-time respirometry analysis of intact cells. Values are presented as mean +/− SEM, n = 6. D. Activity of complex IV (mOD/min) was measured photometrically; (mean +/− SEM, n = 3). E Complex IV amount was measured by ELISA (mean +/− SEM, n = 3). F. Complex I activity (mOD/min) was measured photometrically; (mean +/− SEM, n = 3). G. Reactive oxygen species in wild type (WT) and COA6 knock out cells (COA6KO) were assessed by MitoSOX staining and flow cytometry; (mean +/− SEM, n = 3). H. Glutathione redox potentials were measured in wild type (WT), and COA6 knock out cells upon transfection with either Mito-Grx1-roGFP2 or IMS-Grx1-roGFP2 and analyzed by live imaging. The ratio of fluorescence at 405/470 nm is presented as average +/− SEM, n > 44.
Here, we generated a COA6 knock out HEK293T cell line to analyze the COA6 function in CuA-site formation. Loss of COA6 function led to combined complex I and complex IV deficiency. A concomitant reduction of the inner membrane potential (ΔΨ) caused defects in membrane potential-driven protein transport across the inner mitochondrial membrane, whereas the mitochondrial intermembrane space import and assembly protein 40 (MIA40/CHCHD4)-dependent import into the intermembrane space was not compromised. Our analyses demonstrated that COA6 acts as a thiol-reductase to reduce disulfide bridges in the metallochaperones SCO1 and SCO2. The cysteines of the thioredoxin-like fold of SCO2 are required for the interaction with COA6 but dispensable for the dynamic interaction between SCO1 and SCO2. We conclude that the thiol reduction activity of COA6 is necessary for proper copper transfer to the CuA site.
Results
Lack of COA6 affects complex I and complex IV
COA6 participates in the copper insertion into COX2 through interactions with the copper chaperones SCO1 and SCO2 and the assembly factors COX18 and COX16 ([7,8,10,18]. However, the mechanism of copper transfer to COX2 and the role of COA6 in this process are ill-defined. For understanding COA6 function in this process, we generated a COA6 knock out HEK293T cell line (COA6KO) using the CRISPR/Cas9 system. Sequencing analyses revealed a deletion in Exon 2 that generates a premature Stop codon in the COA6 gene (Fig. Sup1A). The COA6KO displayed a strongly reduced growth rate compared to the wild type. Introduction of a FLAG-tagged COA6 version into the knock out partially restored cellular growth (Fig. 1B). Since patients carrying COA6 mutations displayed fatal hypertrophic cardiomyopathy and presented in one case with combined complex I and complex IV [11] and in another case with isolated complex IV deficiency [12], we assessed respiratory chain function in the mutant cells. Therefore, we measured oxygen consumption by real-time respirometry in intact cells. Respiration of COA6 knock out cells was drastically reduced compared to the wild type in agreement with a defect of oxidative phosphorylation (Fig. 1C). For defining how loss of COA6 affected the different complexes of the respiratory chain, we measured the activity and amounts of the cytochrome c oxidase. Complex IV activity and amount were drastically reduced in COA6 knock out cells as expected. Expression of a FLAG-tagged COA6 largely restored this defect (Fig. 1D and E), and in Western blot analyses was also found to rescue steady-state protein levels of complex IV components (Fig. Sup1B). We previously showed that silencing of COA6 by siRNA treatment also led to reduced activity and amounts of complex I [7]. In contrast, Stroud et al. reported that the loss of COA6 led to selective complex IV deficiency [8]. Therefore, we investigated if complex I was affected in COA6KO cells. To this end, we determined the enzymatic activity of complex I in a colorimetric assay. Interestingly, compared to wild type, the activity of complex I was strongly decreased in COA6KO cells (Fig. 1F). Under certain conditions, defects in oxidative phosphorylation can cause the production of reactive oxygen species (ROS) [20] and thus affect the mitochondrial glutathione redox potential. For addressing this, we measured superoxide production in wild type and COA6KO cells using the fluorescent dye MitoSOX. As depicted in (Fig. 1G), we found a decrease in superoxide levels in the absence of COA6. In addition, we analyzed the general redox buffering capacity of mitochondria by measuring glutathione redox potential using the Grx1-roGFP2 sensor targeted to the intermembrane space (IMS) or the mitochondrial matrix. These fluorescent sensors monitor the glutathione redox potential that is affected by ROS [21]. These analyses showed no differences in the redox potential between wild type and COA6KO cells (Fig. 1H). Accordingly, loss of COA6 leads to a combined complex IV and complex I deficiency in mitochondria. However, an increase in ROS production was not apparent in mutant cells, nor did we detect alterations in the mitochondrial glutathione redox potential.
Lack of COA6 impacts protein import
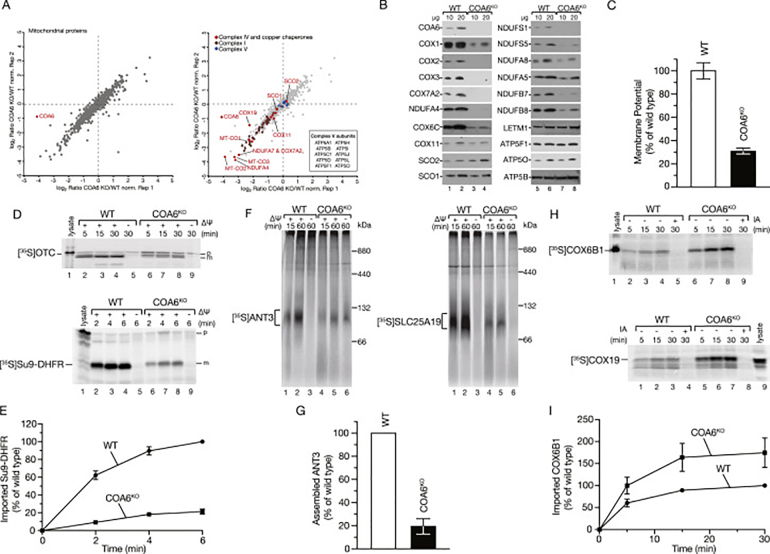
For addressing the extent to which the different components of complexes I and IV were altered in the absence of COA6 and whether other mitochondrial proteins were also affected, we assessed changes in the proteome of mitochondria isolated from wild type and COA6-deficient cells by quantitative mass spectrometry. Interestingly, the mitochondrial-encoded core components of cytochrome c oxidase COX1, COX2, and COX3 were strongly decreased in COA6KO mitochondria. In addition, other structural components of complex IV and of complex I were decreased in mitochondria in the absence of COA6. In contrast, the constituents of complex V were not significantly affected (Fig. 2A). These results were confirmed using SDS-PAGE and Western blotting (Fig. 2B). However, to our surprise, many other mitochondrial proteins not related to the OXPHOS system were decreased in COA6KO mitochondria. We reasoned that reduced activity of the respiratory chain would impact the transfer of protons to the intermembrane space, and thereby, affect the mitochondrial inner membrane potential, which drives the protein import into mitochondria. To this end, we measured the membrane potential by flow cytometry. COA6KO cells indeed displayed a strongly reduced membrane potential (Fig. 2C). Accordingly, it is conceivable that the loss of complexes I and IV, and the resulting decrease of the inner membrane potential reduced the import capacity of mitochondria leading to alterations in protein abundance. Therefore, we imported radiolabeled precursor proteins that follow different import routes into purified COA6KO mitochondria. The presequence-containing precursors OTC (ornithine-transcarbamylase) and Su9-DHFR (subunit 9 of the F1Fo ATP synthase) are transported by the TIM23 complex in a membrane potential-dependent manner into the mitochondrial matrix. Compared to wild type mitochondria, the import of both precursor proteins was reduced in the absence of COA6 (Fig. 2D and E). Metabolite carriers are imported and inserted into the inner mitochondrial membrane by the TIM22 complex in a membrane potential-dependent manner. We performed in vitro import analyses of the model carrier transport pathway substrates ANT3 (ADP/ATP carrier 3) and SLC25A19 (thiamine pyrophosphate carrier). After import, mitochondria were solubilized and imported proteins analyzed by Blue Native PAGE (BN-PAGE). In both cases, we observed a strongly decreased import in COA6KO mitochondria (Fig. 2F and G). In contrast to presequence-containing precursors and carriers, mitochondrial proteins of the intermembrane space that contain twin CXNC motifs, which are oxidized upon import forming disulfide bridges with the assistance of MIA40, are imported independently of membrane potential. Hence, we analyzed the import efficiency of the MIA40 substrates COX6B1 and COX19 in the absence of COA6. Interestingly, the import of precursors into the intermembrane space along the MIA40 pathway was not impaired in the absence of COA6 but rather accelerated (Fig. 2H and I). In summary, in the absence of COA6, the import routes that depend on the inner membrane potential are affected. However, the membrane potential independent import into the intermembrane space via MIA40 was not reduced but rather stimulated. Hence, a loss of membrane potential due to loss of complexes I and IV causes pleiotropic defects via compromised import of proteins that translocate across the inner membrane.
Fig. 2.
Lack of COA6 impacts protein import.A. Quantitative MS analysis of changes in the mitochondrial proteome in cells lacking COA6. B. Purified wild type (WT) and COA6KO mitochondria were analyzed by SDS-PAGE and Western blotting. C. Mitochondrial membrane potential was measured by flow cytometry using the dye JC-1. Fluorescence signals are depicted as mean +/− SEM, n = 4. D.In vitro import of radiolabeled precursor proteins into purified wild type (WT) and COA6KO mitochondria. Su9-DHFR and OTC were imported for indicated times in the presence or the absence of membrane potential (Δψ). Samples were Proteinase K (PK) treated after import, subjected to SDS-PAGE, and proteins visualized by digital autoradiography (p, precursor; m, mature). E. Quantification of Su9-DHFR import into mitochondria. Signals were quantified and are presented as a percent of the longest import timepoint in the wild type (WT) sample (mean, +/− SEM, n = 3). F.In vitro import of radiolabeled carrier precursors ANT3 (ADP/ATP carrier 3) and SLC25A19 (thiamine pyrophosphate carrier) into purified wild type (WT) and COA6KO mitochondria. Import was carried out for different times in the presence or absence of Δψ. After import, mitochondria were proteinase K (PK) treated, solubilized, and subjected to BN-PAGE separation and digital autoradiography. G. Quantification of ANT3 import into mitochondria after 60 min. Signals were quantified and are presented as a percent of wild type (WT) (mean, +/− SEM, n = 3). H.In vitro import of radiolabeled twin CXNC motifs containing precursors COX6B1 and COX19 into purified wild type (WT) and COA6KO mitochondria. Import was carried out for indicated times in the presence or absence of the cysteine modifying agent iodoacetamide (IA). Samples were subjected to proteinase K (PK) digestion and analyzed by SDS-PAGE and digital autoradiography. I. Quantification of COX6B1 import into mitochondria. Signals were quantified and are shown as percent of the longest import timepoint in the wild type (WT) sample (mean, +/− SEM, n = 3).
Cysteines in the CXXXC motif of SCO2 are required for binding to COA6
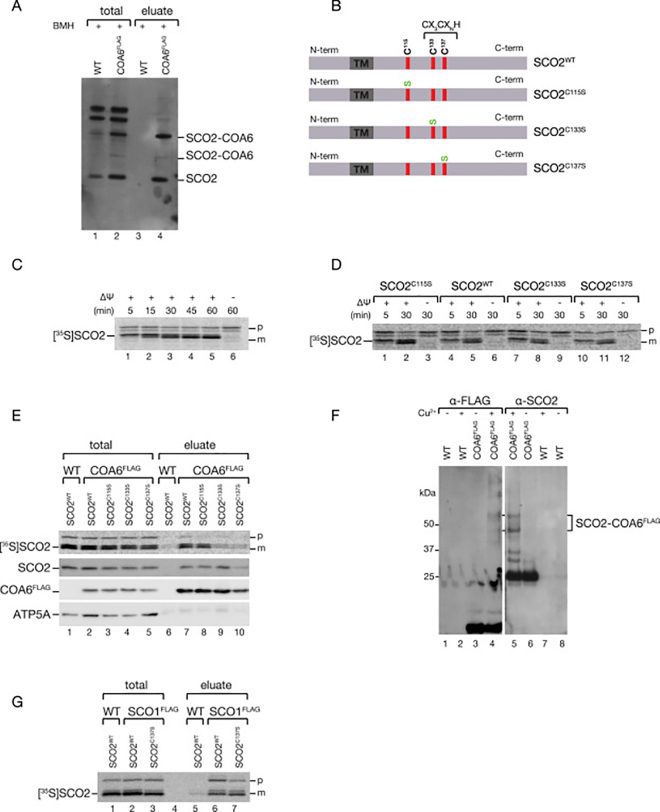
COA6 cooperates with SCO1 and SCO2 in the metalation of COX2 [7,8]. Mimicking pathologic mutations in either COA6 or SCO2 affected the co-immunoisolation of these proteins [7]. For addressing whether SCO2 and COA6 directly interacted with each other, we incubated COA6FLAG containing mitochondria with the sulfhydryl reactive crosslinker BMH. After FLAG immunoisolation, COA6-SCO2 crosslinks were recovered in the eluate, indicating that both proteins were covalently linked via crosslinking of cysteine residues (Fig. 3A).
Fig. 3.
Cysteines in the CXXXC motif of SCO2 are required for binding to COA6.A. Wild type (WT) and COA6FLAG mitochondria were subjected to chemical crosslinking using the homo-bifunctional crosslinker BMH. After crosslinking, mitochondria were solubilized and subjected to anti-FLAG immunisolation. Samples were analyzed by SDS-PAGE and Western blotting. B. Schematic representation of generated SCO2 mutants. Red, cysteines of the CX3CXNH motif; Green, exchanged residue; TM, transmembrane span. CIn vitro import of radiolabeled SCO2WT into purified wild type mitochondria in the presence or the absence of membrane potential (Δψ). Samples were subjected to proteinase K (PK) digestion and analyzed by SDS-PAGE and digital autoradiography. D.In vitro import of SCO2 cysteine mutants as in C. E. After in vitro import of indicated SCO2 variants into purified wild type (WT) and COA6FLAG mitochondria, samples were solubilized, subjected to anti FLAG immunoisolation, and eluates analyzed by SDS-PAGE, digital autoradiography and Western blotting. F. Wild type (WT) and COA6FLAG mitochondria were subjected to CuSO4 treatment. After treatment, mitochondria were solubilized and subjected to anti-FLAG immunisolation. Samples were analyzed by SDS-PAGE and Western blotting. G. SCO1FLAG immunoisolation after in vitro import of SCO2 variants into purified wild type (WT) and SCO1FLAG mitochondria. After the import of radiolabeled SCO2 variants, anti FLAG immunoisolation was performed and eluates analyzed by SDS-PAGE and digital autoradiography.
SCO1 and SCO2 contain a CX3CXnH motif that allows them either to bind Cu(I) with high affinity or to act as thiol-oxidoreductases [22,23]. Therefore, we analyzed the relevance of the cysteines of the CX3CXnH motif for the interaction with COA6, and consequently, for the dynamics of the copper transfer reactions. To this end, we generated cysteine mutants of SCO2. SCO2 contains three cysteines (C115, C133, and C137), with C133 and C137 being part of the CX3CXnH motif. We generated single mutants changing cysteine to serine residues (SCO2C115S, SCO2C133S, SCO2C137S) (Fig. 3B). In order to analyze the effect of the cysteine mutants, we generated radiolabeled versions of wild type, SCO2C115S, SCO2C133S, and SCO2C137S. SCO2 could be efficiently imported into purified wild type mitochondria in a membrane potential-dependent manner. Upon import, SCO2 was processed to the mature form (Fig. 3C). Similarly, the mutant versions of SCO2 were efficiently imported into wild type mitochondria (Fig. 3D). For analyzing how the cysteine mutants in SCO2 affected the interaction with COA6, we performed in vitro import of radiolabeled versions of SCO2 into isolated mitochondria from cells expressing C-terminal FLAG-tagged COA6. After import, COA6 was immunoisolated and bound SCO2 detected by autoradiography. Wild type SCO2 (SCO2WT) efficiently coprecipitated with COA6FLAG after in vitro import. SCO2C115S (not being in close proximity to the copper coordination center) interacted with COA6FLAG in similar amounts as wild type SCO2. Interestingly, the SCO2C133S and SCO2C137S mutants, which are affected in the CX3CXnH motif, displayed drastically decreased interactions with COA6FLAG by about 80% (Fig. 3E). Thus, the cysteines of the CX3CXnH motif are important for SCO2/COA6 interaction. Therefore, we addressed if COA6 and SCO2 directly interacted through disulfide bridge formation. For this, we treated samples with Cu2+ as redox catalyst to oxidize free sulfhydryls and to form disulfide bonds between cysteine residues in proximity to each other. After immunoisolation of COA6FLAG, we observed specific adducts detected by both SCO2 and FLAG antisera (Fig. 3 F).
SCO1 and SCO2 cooperate in the delivery of copper to the cytochrome c oxidase subunit COX2 [14]. For analyzing whether the cysteines in SCO2 determined not only the interaction with COA6 but also the dynamics of SCO1 and SCO2, we performed in vitro import of radiolabeled versions of SCO2 into mitochondria isolated from cells expressing FLAG-tagged SCO1. Interestingly, the interaction between SCO1 and the fully imported, mature SCO2C137S was similar to that of wild type SCO2 (Fig. 3G). Accordingly, the CX3CXnH motive of SCO2 was not relevant for the SCO1/SCO2 interaction. Thus, it is tempting to speculate that SCO1/SCO2 interaction does not occur through disulfide bridge formation. However, based on these observations, it is likely that COA6/SCO2 complex forms disulfide bridges during the copper transfer cycle.
COA6 acts as a thiol-reductase for copper metallochaperones SCO1 and SCO2
SCO2 has been shown to reduce cysteine residues in the copper coordination site of COX2 in vitro, whereas it was unable to oxidize cysteines in SCO1, implying that other factors are involved in these redox reactions in mitochondria [16]. Our analyses showed that the cysteines present in the CX3CXnH motive in SCO2 are determinants for the interaction with COA6. Therefore, we considered the possibility that COA6 might be involved in the regulation of the redox state of the copper metallochaperones during CuA biogenesis. For addressing this, we analyzed the redox state of cysteines in SCO2 in COA6KO mitochondria using a maleimide derivate coupled to a DNA probe. Upon reaction of the probe with a free cysteine, the target protein shows an apparent size shift of approximately 14 kDa in SDS-PAGE analysis. In wild type mitochondria, SCO2 displayed a molecular weight shift corresponding to three free thiol groups. In contrast, in COA6KO mitochondria, SCO2 accumulated in a state corresponding to a single reduced cysteine (Fig. 4A). A quantitative assessment of the ratio between the reduced and oxidized form of SCO2 revealed a drastic shift toward the oxidized state in mitochondria lacking COA6. For addressing whether the oxidized form of SCO2 in COA6KO mitochondria represented a form with a disulfide bridge, we preincubated COA6KO mitochondria with DTT prior to the maleimide-mediated modification. Upon chemical modification of DTT in COA6KO mitochondria, SCO2 displayed a wild type like migration pattern (Fig. 4A and B). For analyzing whether COA6 contributed to the oxidative state of SCO1 in organello, we assessed the availability of free cysteines by maleimide modification analyses in mitochondria lacking COA6. While a shifted form of SCO1 that corresponded to two free thiol groups was apparent in wild type mitochondria, COA6KO mitochondria displayed an accumulation of nonmodified SCO1, implying that the two cysteines were oxidized. The quantification of the ratio of reduced and oxidized SCO1 again showed a drastic decrease in the reduced state in the absence of COA6 (Fig. 4C). Upon treatment of COA6KO mitochondria with DTT, SCO1 could be fully reduced, and thus, be modified by the maleimide (Fig. 4C and D). For supporting the specificity of the observed redox phenotype upon lack of COA6, we expressed a FLAG-tagged version of COA6 in COA6KO cells. The redox phenotype of SCO1 and SCO2 in COA6KO cells could be rescued by expression of COA6 (Fig. 4E and F), confirming that COA6 is required for the reduction of the disulfide bridges in both proteins. In summary, our findings showed that COA6 is required to reduce a disulfide bridge in both SCO1 and SCO2 and that this effect is not indirectly caused by increased ROS production or an altered redox state of the intermembrane space.
Fig. 4.
COA6 acts as a thiol-reductase for copper metallochaperones SCO1 and SCO2.A. Wild type (WT) and COA6KO mitochondria were incubated in the presence or absence of DTT and subjected to cysteine modification. Samples were subjected to SDS-PAGE and Western blotting. B. Quantification of the ratio between most reduced and most oxidized form of SCO2 in wild type (WT) and COA6KO mitochondria presented as percent of WT; (mean +/− SEM, n = 3). C and D. Cysteine redox modification assay for SCO1 was carried out as described in A and B. E and F. Cysteine redox modification assay for SCO2 and SCO1 in cells expressing COA6FLAG. Wild type (WT), COA6KO and COA6KO+COA6FLAG mitochondria were subjected to cysteine modification and analyzed by SDS-PAGE and Western blotting.
Discussion
The catalytic core of the cytochrome c oxidase is conserved from bacteria to humans. The formation of the binuclear copper center CuA is, therefore, a common process, and the particular high reactivity of copper ions necessitates that the copper delivery is assisted by copper metallochaperones. However, the mechanisms of CuA biogenesis differ between organisms with regard to the participating copper-binding proteins. In bacteria, the activity of a periplasmic thioredoxin-like reductase TlpA maintains the active site cysteine pairs of CoxB and ScoI in the reduced state, and is, thus, required for Cu2+ binding. The copper chaperone ScoI reacts with apo-CoxB to establish a stable Cu2+ containing complex, which is released to form a CoxB-Cu2+ with the aid of the chaperone PcuC. A second round of PcuC action delivers Cu+ to CoxB, forming the CuA center [24]. In human mitochondria, other players among these COA6 have been identified as members of the copper insertion machinery for COX2 [7,8]. Interestingly, mammalian mitochondria lack a TlpA homolog. Therefore, the required thiol-reductase activity has to be a function of either SCO1, SCO2, or COA6. Our findings suggest that COA6 shows a thiol-reductase activity and is involved in the reduction of cysteines in the CX3CXnH motif of SCO1 and SCO2. Whereas SCO1 has been shown to transfer copper to COX2 in vitro in a two-step reaction, SCO2 presented a thiol-reductase activity that was able to reduce cysteinyl sulfurs of COX2 in a copper bound state [16]. Based on these observations, we propose that COA6 recycles SCO2 cysteines into a redox-active form, enabling further rounds of COX2 cysteinyl sulfur reduction. Remarkably, SCO2 reduced thiol groups of Apo-COX2 at a 2:1 stoichiometry in vitro [16]. Since COA6 cooperates with SCO2 during COX2 metalation, it is plausible that in vivo COA6 and SCO2, each provides one reducing equivalent per one COX2 molecule. Indeed, the redox potential of yeast COA6 (−349±1 mV) [19] indicated that COA6 could reduce the disulfide bonds in COX2 (−288±3 mV) [16], SCO2 (less than −300mV) [25], or SCO1 (−277±3 mV) [26]. However, the ability of COA6 to reduce thiol groups of COX2 in vivo could not be addressed in our model system, since cells lacking COA6 lack detectable levels of COX2. In addition, our analysis suggests that COA6 is involved in SCO1 reduction. In vitro, SCO1 can receive copper atoms from COX17 independently of the redox state of its cysteines. Whereas SCO2 necessarily needs to be in a reduced state to receive copper, SCO1 can be in either a reduced state or an oxidized state, being reduced by COX17 in a simultaneous step with copper transfer [27]. However, it is also conceivable that prior to copper transfer by COX17, SCO1 may be reduced by COA6. During the revision of this manuscript, Soma et al. solved the structure of human COA6 and showed in in vitro analyses that COA6 was able to reduce cysteine residues in purified SCO1, SCO2, and COX2. These results support our analyses of COA6 acting as a thiol-reductase in vivo. However, using COA6 patient-derived cells, they could only observe the effects on cysteine oxidation of SCO1 but not SCO2. Differences in the cellular models used for investigation may account for this difference with our analyses and need to be further studied in the future [28].
In conclusion, our results provide insights into the mechanistics of CuA biogenesis and define the function of COA6 as a thiol-reductase for copper metallochaperones. In addition, we demonstrate that the loss of COA6 not only affects complex IV assembly but also the formation of respiratory chain complex I. Remarkably, only one of the patients described with COA6 mutations showed a decreased complex I activity [11,12]. The COA6 knock out mutant used here displays reduced complex I and IV activity. However, at this point, the question as to how a loss of COA6 affects complex I remain open as no link between copper chaperone activity and complex I biogenesis has been observed.
Our analyses indicate thatloss of OXPHOS activity and the concomitant reduction of the membrane potential indirectly influences the mitochondrial proteome through protein import defects. Interestingly, the Δψ-independent import of substrates along the MIA40 pathway was not decreased but rather increased in the absence of COA6. The upregulation of mitochondrial import pathways has been frequently shown when one import pathway is defective [[29], [30], [31]]. However, the molecular reason for this phenomenon remains unclear. In addition, other additional mechanisms apart from reduced membrane potential may contribute to the altered mitochondrial proteome in COA6KO cells. For instance, alteration of copper homeostasis drastically alters the Fe/S cluster formation that involves the mitochondrial ISCA1/2 and GLRX5 proteins [32]. Interestingly, the protein levels of ISCA2, and to a lesser extent, those of GLRX5 are decreased in the absence of COA6 (see Supplemental table S1). Thus, it is conceivable that impaired iron-sulfur cluster biogenesis effects may impact the steady-state levels of different mitochondrial proteins, and thereby, cause alterations in protein abundance in many mitochondrial functions in addition to the respiratory chain e.g. citric cycle, heme biosynthesis, lipoic acid synthesis. Accordingly, COA6 dysfunction, e.g., in COA6 patients, leads to pleiotropic defects in mitochondrial function.
Material and Methods
Cell culture, generation of cell lines and proliferation assay
HEK293T Flp-In™ T-REX™ or HEK293 were cultured in DMEM media, supplemented with 10% (v/v) FBS, 2 mM L-glutamine, 1 mM sodium pyruvate and 50 μg/mL uridine at 37 °C under a 5% CO2 humidified atmosphere. The COA6KO HEK293T cell line was generated using CRISPR-Cas9 genome editing, as previously described [33]. Briefly, specific sgRNA was designed to target all isoforms of COA6. Oligonucleotides were then annealed and ligated into the pX458 vector, which contains GFP. Cells were transfected and sorted into single wells of a 96-well plate. Clones were screened for the absence of COA6 by immunoblotting. One clone was used for further analysis and sequencing after genomic DNA isolation confirmed the disruption of the COA6 gene. Rescue cell line expressing C-terminal tag generated using HEK293T Flp-In™ T-REX™ as previously described [18]. Cell lines stably expressing C-terminal FLAG-tagged versions of COA6 and SCO2 were generated using HEK293T Flp-In™ T-REX™ during previous studies [7]. C-terminal FLAG-tagged SCO1 was generated amplifying SCO1 (NM_004589.4) from cDNA and incorporating the FLAG sequence in the reverse primer. The amplicon was cloned into pcDNA5/FRT/TO vector, and HEK293T Flp-In™ T-REX™cells were transfected and selected as previously described [34]. For SILAC analysis, cells were grown for five passages on DMEM medium lacking arginine and lysine, supplemented with 10% (v/v) dialyzed fetal bovine serum, 600 mg/l proline, 42 mg/l arginine hydrochloride (13C6,15N4-arginine in “heavy” media), and 146 mg/l lysine hydrochloride (13C6, 15N2-lysine in “heavy” media) (Cambridge Isotope Laboratories, Tewksbury, MA, USA).
Cell proliferation experiments were performed by seeding 250000 cells in 6-well plates. After 72 h, cells were recovered in PBS and counted using an automated cell counter Countess™ (Invitrogen).
Real-time respirometry
Oxygen consumption rate (OCR) was measured with an XF96 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA, USA). HEK293T cells were seeded on the day of the measurement at a density of 40.000 cells/well. Baseline respiration was measured in DMEM supplemented with 1 mM pyruvate and 25 mM galactose after calibration at 37 °C in an incubator without CO2. Periodic measurements of oxygen consumption were performed, and OCR was calculated from the slope of change in oxygen concentration over time. Metabolic states were measured after subsequent addition of 3 μM oligomycin, 1 μM carbonyl cyanide 4 (trifluoromethoxy)phenylhydrazone (FCCP), 1 μM antimycin A, and 2 μM rotenone.
Enzymatic activities
A quantitative method (ELISA) for cytochrome c oxidase (CIV) specific activity and quantity of complex determinations (Abcam) was applied using the manufacturer's instructions, as previously described [35]. The lack of a negative slope due to Cytochrome c oxidation was considered as a complete lack of cytochrome c oxidase activity and set to 0. In the same way, Complex I activity measurements were performed according to the manufacturer's instructions (Abcam), as previously published [7].
Membrane potential and ROS measurements
For the estimation of membrane-potential of the mitochondria, cells were stained with 200 μM JC-1. The values of the two fluorescence readings gave a ratiometric comparison of mitochondrial membrane potential. Further, cells were also stained with 3 μM MitoSox Red for measuring mitochondrial ROS. In each case, 5 × 105 cells were stained with the respective dyes for 15 min at 37°C, washed twice with 1 × PBS, and then measured by flow cytometry.
Flow cytometric analyses were carried out using the BD-Canto flow cytometer (Becton Dickinson); 10,000 gated events were captured, and FACS-Diva software was used to compute the numerical data.
Redox potential measurements
For measuring Glutathione redox potential, WT or COA6KO cells (100,000–300,000) were seeded on 25 mm round glass coverslips 24–48 h before transfection. Genetically encoded protein sensors were transfected using Fugene® HD (Promega GmbH, Mannheim, Germany) along with 1 μg of plasmid DNA, according to the manufacturer's instructions. Imaging was performed 24 h after transfection. Plasmids pLPCX-mito-Grx1-roGFP2 and pLPCX-IMS-Grx1-roGFP2 were kindly provided by Dr. Tobias P. Dick, Heidelberg, Germany.
Imaging was performed 24 h after transfection using an inverted Olympus IX83 microscope, equipped with an MT20 Mercury–Xenon light source, CellSense Dimension software (Olympus), and a 40 × air objective (UPlanSApo 40× 0.95, Olympus). Measurements were performed at room temperature in Ringer's buffer (pH 7.4) containing 145 mM NaCl, 4 KCl, 10 mM Glucose, 10 mM HEPES, 2 mM MgCl2 and 0.25 mM CaCl2. The exposure time for both fluorescent channels was kept constant during the whole experiment. Excitation filters 405/20 and 470/40 were combined with a dual-band CFP/YFP emission filter (F58-017). Data are presented as background-corrected fluorescence ratios of F405 nm/F470 nm.
Mitochondrial isolation
Mitochondria used for Western blotting purposes were isolated by differential centrifugation as previously described [36]. For import experiments, mitochondria were isolated as previously described [37]. Briefly, cells were harvested and resuspended in ice-cold isotonic buffer (10 mM MOPS [pH 7.2], 75 mM mannitol, 225 mM sucrose, and 1 mM EGTA) supplemented with 2 mg/ml BSA and 2 mM PMSF, and subjected to centrifugation at 1000×g for 5 min at 4°C. The cell pellet was then resuspended in cold hypotonic buffer (10 mM MOPS [pH 7.2], 100 mM sucrose, and 1 mM EGTA) and incubated on ice for 5–7 min. The cell suspension was homogenized in a Dounce glass homogenizer (Sartorius). Cold hypertonic buffer (1.25 M sucrose and 10 mM MOPS [pH 7.2]) was added to the cell homogenate (1.1 ml/g of cells). The homogenate was subjected to centrifugation at 1000 × g for 10 min at 4°C to pellet the cellular debris. The supernatant that contained mitochondria was then carefully aspirated and centrifuged again. The supernatant was then subjected to high-speed centrifugation at 10,000 × g for 10 min at 4°C to pellet mitochondria. The pellet was resuspended in isotonic buffer without BSA and quantified using the Bradford assay.
Quantitative mass spectrometry and data analysis
Mitochondrial fractions isolated from differentially SILAC-labeled cells were mixed in a 1:1 ratio. Proteins were precipitated using acetone and resuspended in 8 M urea/10 mM ammonium bicarbonate (AmBic). Cysteine residues were reduced and alkylated by consecutive incubation with 5 mM tris(2-carboxyethyl)-phosphine/10 mM AmBic (30 min at 37°C) and 55 mM iodoacetamide/10 mM AmBic (45 min at room temperature in the dark). Urea concentration was adjusted to a final concentration of 2 M using 50 mM AmBic, and trypsin was added (1/30 protease-to-protein ratio) for proteolytic digestion overnight at 37°C. The experiment was performed in two individual replicates.
Peptide mixtures were analyzed on an Orbitrap Elite mass spectrometer coupled to an UltiMate 3000 RSLCnano HPLC system (Thermo Scientific), which was equipped with nanoEase™ M/Z Symmetry C18 pre-columns (length, 20 mm; inner diameter, 180 μm) for washing and preconcentration of the peptides and a nanoEase™ M/Z HSS C18 T3 analytical column (length, 250 mm; inner diameter, 75 μm; particle size, 1.8 μm; packing density, 100 Å) (Waters) for peptide separation. The solvent system for peptide elution consisted of 4% (v/v) dimethyl sulfoxide/0.1% (v/v) formic acid (solvent A) and 30% (v/v) acetonitrile/48% (v/v) methanol/4% (v/v) dimethyl sulfoxide/0.1% (v/v) formic acid (solvent B). Peptides were eluted by applying a gradient of 3–60% solvent B in 305 min, 60–95% B in 25 min, and 5 min at 95% B at a flow rate of 300 nl/min.
The Orbitrap Elite was operated in data-dependent mode. Survey scans ranging from m/z 370–1700 were acquired in the orbitrap at a resolution of 120,000 (at m/z 400) with automatic gain control (AGC) of 1 × 106 ions and maximum injection time (IT) of 200 ms. The 25 most intense multiply charged precursor peptides were selected for low energy collision-induced dissociation in the linear ion trap applying normalized collision energy of 35%, an activation q of 0.25, an activation time of 10 ms, an AGC of 5 × 103, and a maximum IT of 150 ms. Dynamic exclusion of previously fragmented precursor peptides was set to 45 s.
Mass spectrometric raw data were processed using MaxQuant/Andromeda (version 1.5.5.1 [38,39]; and searched against the UniProt human proteome set, including isoforms (downloaded December 2017) using default settings, except that the number of unique peptides and ratio counts required for protein identification and quantification, respectively, was set to one. Arg10 and Lys8 were selected as heavy labels. Carbamidomethylation of cysteine residues was set as fixed modification and methionine oxidation and acetylation of protein N-termini were considered variable modifications. The option “Requantify” was enabled to allow for the calculation of SILAC ratios even if only the isotope-labeled or unlabeled variant of a peptide is present in a sample by assigning a peptide intensity for the missing counterpart from the background signals in MS spectra at the expected m/z value. Information about proteins identified and COA6KO/WT SILAC ratios determined by MaxQuant are provided in Supplemental Table S1. Annotations for mitochondrial proteins are derived from the “Integrated Mitochondrial Protein Index” (IMPI), which was downloaded from the MitoMiner database (http://mitominer.mrc-mbu.cam.ac.uk; IMPI version Q2 2018; [40]. Only entries for known mitochondrial proteins were considered. Information about components of complex I, IV, and V (F1Fo ATP synthase) are derived from the Human Genome Organisation (HUGO) Gene Nomenclature Committee (HGNC; https://www.genenames.org/ [41];
Radioactive precursor synthesis and in organello import
Radiolabeled precursor proteins were synthesized using rabbit reticulocyte lysate (Promega) in the presence of [35S] methionine. The import of radiolabeled precursors into isolated mitochondria was performed at 30°C in import buffer (250 mM sucrose, 80 mM potassium acetate, 5 mM magnesium acetate, 5 mM methionine, 10 mM sodium succinate, 5 mM adenosine triphosphate, and 20 mM HEPES/KOH [pH 7.4]). For TIM22 substrates, import buffer was supplemented with 2 mM ATP, 1 mM DTT, 5 mM creatine phosphate, and 0.1 mg/mL creatine kinase. 2% Lysate was used for TIM23 and MIA40 proteins, whereas 10% was used for TIM22 imported carrier proteins. Samples were incubated with radiolabeled precursors for different times. Import of TIM23 and TIM22 substrates was stopped by the dissipation of membrane potential on ice using 8 mM antimycin A, 1 mM valinomycin, and 10 mM oligomycin. MIA40 import was stopped by the addition of 50 mM iodoacetamide and incubation on ice. Non-imported proteins were removed by proteinase K (20 μg/mL) treatment for 10 min on ice. PMSF (2 mM) was added to inactivate proteinase K for 10 min on ice. Mitochondria were collected, washed with SEM buffer (250 mM sucrose, 1 mM EDTA, 20 mM Mops [pH 7.2]), and used for SDS-PAGE analyses or BN-PAGE analyses. Results were visualized using digital autoradiography. Quantifications were performed using ImageQuantTL (GE Healthcare) using rolling ball background subtraction.
BN-PAGE Analyses
Mitochondria were solubilized in buffer containing 1% digitonin (20 mM Tris/HCl [pH 7.4], 0.1 mM EDTA, 50 mM NaCl, 10% (w/v) glycerol and 1 mM PMSF) to a final concentration of 1 mg/mL for 30 min at 4 °C. Lysates were cleared by centrifugation at 14,000 g for 15 min at 4 °C and 10× BN loading dye was added (5% Coomassie brilliant blue G-250,500 mM 6-aminohexanoic acid, and 100 mM Bis-Tris [pH 7.0]). Samples were loaded onto 6%–16% polyacrylamide gradient gels and separated as described [42].
Cysteine modification assay and crosslinking
Modification of free thiol groups was performed using the Sulfobiotics Protein Redox State Monitoring Kit Plus (Dojinjo) according to the manufacturer's indications with slight modifications. In brief, 200 μg mitochondria were solubilized at 10 μg/μl using provided detergent for 30 min at 4°C. After clearing samples by centrifugation at 14,000 g for 15 min at 4 °C, they were subjected to Cys modification for 30 min at 37°C and subsequently separated by SDS-PAGE. Acrylamide gel was irradiated with UV-light for 15 min, and Western blotting was performed afterward. In organello crosslinking was performed using the sulfhydryl crosslinker BMH at 1 mM concentration or 1 mM CuSO4 in crosslinker buffer (Sorbitol 0.6 M, 20 mM HEPES [pH 7.2]) for 45 min on ice. Samples were quenched by adding 50 mM Cysteine (for BMH) or 10 mM EDTA and N-ethylmaleimide (NEM) (final concentration). Mitochondria were re-isolated by centrifugation at 10,000×g 10 min at 4°C and subsequently washed with SEM buffer once in the case of BMH crosslinking. Afterward, FLAG immunoisolation and Western blotting under non-reducing conditions were performed.
FLAG Immunoisolation
Isolated mitochondria were solubilized in buffer (50 mM Tris-HCl, [pH 7.4], 150 mM NaCl, 1 mM EDTA, 10% (w/v) glycerol, and 2 mM phenylmethylsulfonyl fluoride [PMSF]) containing 1% (w/v) digitonin (Merck) or 0.2% triton-×100 (in the case of CuSO4 crosslinking) and incubated at 4°C. Samples were cleared by centrifugation, and supernatants applied to equilibrated anti-FLAG-agarose (Sigma). After washing, bound proteins were eluted with 1.5× Sample Buffer (94 mM Tris-HCl [pH 6.8], 3% SDS, 25% glycerol, 0.015% Bromophenol Blue) or FLAG peptide and subjected to SDS-PAGE and Western blotting.
Miscellaneous
Standard procedures were used for SDS-PAGE and Western blotting of proteins adsorbed to polyvinylidene fluoride membranes (Millipore). Primary antibodies were raised in rabbit or purchased (COX11, Proteintech. NDUFS1, Proteintech. NDUFS5, Abcam. NDUFA8, Abcam. NDUFB7, Abcam. NDUFA5, Proteintech, COX7A2, Proteintech. COX3, Proteintech. ATP5O, Proteintech. ATP5F1, Proteintech.). Antigen-antibody complexes were detected by HRP-coupled secondary antibodies and enhanced chemiluminescence detection on X-ray films.
Acknowledgements
We are indebted to M. Balleininger for technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft, the SFB1002 Project A06 (PR), the SFB1190 Project 17 (IB), Project ID 403222702/SFB 1381 (BW), the Excellence Strategy (CIBSS – EXC-2189 – Project-ID 390939984) (BW) as well as the European Research Council ERC335080 (PR) and ERC Consolidator Grant No. 648235 (BW), the Max Planck Society (PR), the Copernicus Award of the Foundation for Polish Science and Deutsche Forschungsgemeinschaft (PR & AC), the Humboldt Foundation (DPG). “Regenerative Mechanisms for Health” project MAB/2017/2, carried out within the International Research Agendas programme of the Foundation for Polish Science co-financed by the European Union under the European Regional Development Fund (AC), National Science Centre, Poland 2015/19/B/NZ3/03272 (MW).
Edited by M Yaniv
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmb.2020.01.036.
Contributor Information
David Pacheu-Grau, Email: David.Pacheu-Grau@med.uni-goettingen.de.
Peter Rehling, Email: peter.rehling@medizin.uni-goettingen.de.
Authors' contributions
David Pacheu-Grau. Conceptualization, methodology, investigation, visualization, writing-original draft and supervision. Michał Wasilewski. Methodology, investigation, visualization, and writing-review/editing. Silke Oeljeklaus. Investigation, visualization and writing-review/editing. Christine Silvia Gibhardt. Investigation and writing-review/editing. Abhishek Aich. Investigation. Sven Dennerlein. Investigation and visualization. Margarita Chudenkova. Investigation. Markus Deckers. Investigation and visualization. Ivan Bogeski. Resources, writing-review/editing and funding acquisition. Bettina Warscheid. Resources, writing-review/editing and funding acquisition. Agnieszka Chacinska. Conceptualization, resources, writing-review/editing and funding acquisition. Peter Rehling. Conceptualization, resources writing-original draft, project administration, funding acquisition and supervision.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Montoya J., López-Pérez M.J., Ruiz-Pesini E. Mitochondrial DNA transcription and diseases: past, present and future. Biochim. Biophys. Acta Bioenerg. 2006;1757:1179–1189. doi: 10.1016/j.bbabio.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Dudek J., Rehling P., van der Laan M. Mitochondrial protein import: common principles and physiological networks. Biochim. Biophys. Acta. 2013;1833:274–285. doi: 10.1016/j.bbamcr.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 3.Mick D.U., Fox T.D., Rehling P. Inventory control: cytochrome c oxidase assembly regulates mitochondrial translation. Nat. Rev. Mol. Cell Biol. 2011;12:14–20. doi: 10.1038/nrm3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soto I.C., Fontanesi F., Liu J., Barrientos A. Biochimica et Biophysica Acta. BBA-Bioenergetics. 2011:1–15. doi: 10.1016/j.bbabio.2011.09.005. [DOI] [Google Scholar]
- 5.Vögtle F.-N., Burkhart J.M., Rao S., Gerbeth C., Hinrichs J., Martinou J.-C. Intermembrane space proteome of yeast mitochondria. Mol. Cell. Proteomics. 2012;11:1840–1852. doi: 10.1074/mcp.M112.021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh A., Trivedi P.P., Timbalia S.A., Griffin A.T., Rahn J.J., Chan S.S.L. Copper supplementation restores cytochrome c oxidase assembly defect in a mitochondrial disease model of COA6 deficiency. Hum. Mol. Genet. 2014;23:3596–3606. doi: 10.1093/hmg/ddu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pacheu-Grau D., Bareth B., Dudek J., Juris L., Vögtle F.-N., Wissel M. Cooperation between COA6 and SCO2 in COX2 maturation during cytochrome c oxidase assembly links two mitochondrial cardiomyopathies. Cell Metabol. 2015;21:823–833. doi: 10.1016/j.cmet.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Stroud D.A., Maher M.J., Lindau C., Vögtle F.-N., Frazier A.E., Surgenor E. COA6 is a mitochondrial complex IV assembly factor critical for biogenesis of mtDNA-encoded COX2. Hum. Mol. Genet. 2015;24:5404–5415. doi: 10.1093/hmg/ddv265. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh A., Pratt A.T., Soma S., Theriault S.G., Griffin A.T., Trivedi P.P. Mitochondrial disease genes COA6, COX6B and SCO2 have overlapping roles in COX2 biogenesis. Hum. Mol. Genet. 2016;25:660–671. doi: 10.1093/hmg/ddv503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourens M., Barrientos A. Human mitochondrial cytochrome c oxidase assembly factor COX18 acts transiently as a membrane insertase within the subunit 2 maturation module. J. Biol. Chem. 2017;292:7774–7783. doi: 10.1074/jbc.M117.778514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvo S.E., Compton A.G., Hershman S.G., Lim S.C., Lieber D.S., Tucker E.J. Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Sci. Transl. Med. 2012;4 doi: 10.1016/j.jmb.2011.10.012. 118ra10–118ra10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baertling F., van den Brand M.A.M., Hertecant J.L., Al-Shamsi A., P van den Heuvel L., Distelmaier F. Mutations in COA6 cause cytochrome c oxidase deficiency and neonatal hypertrophic cardiomyopathy. Hum. Mutat. 2015;36:34–38. doi: 10.1002/humu.22715. [DOI] [PubMed] [Google Scholar]
- 13.Leary S.C., Sasarman F., Nishimura T., Shoubridge E.A. Human SCO2 is required for the synthesis of CO II and as a thiol-disulphide oxidoreductase for SCO1. Hum. Mol. Genet. 2009;18:2230–2240. doi: 10.1093/hmg/ddp158. [DOI] [PubMed] [Google Scholar]
- 14.Leary S.C. Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum. Mol. Genet. 2004;13:1839–1848. doi: 10.1093/hmg/ddh197. [DOI] [PubMed] [Google Scholar]
- 15.Leary S.C., Cobine P.A., Kaufman B.A., Guercin G.-H., Mattman A., Palaty J. The human cytochrome c oxidase assembly factors SCO1 and SCO2 have regulatory roles in the maintenance of cellular copper homeostasis. Cell Metabol. 2007;5:9–20. doi: 10.1016/j.cmet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Morgada M.N., Abriata L.A., Cefaro C., Gajda K., Banci L., Vila A.J. Loop recognition and copper-mediated disulfide reduction underpin metal site assembly of Cu Ain human cytochrome oxidase. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112:11771–11776. doi: 10.1093/emboj/19.8.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourens M., Boulet A., Leary S.C., Barrientos A. Human COX20 cooperates with SCO1 and SCO2 to mature COX2 and promote the assembly of cytochrome c oxidase. Hum. Mol. Genet. 2014;23:2901–2913. doi: 10.1093/hmg/ddu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aich A., Wang C., Chowdhury A., Ronsör C., Pacheu-Grau D., Richter-Dennerlein R. COX16 promotes COX2 metallation and assembly during respiratory complex IV biogenesis. Elife. 2018;7 doi: 10.7554/eLife.32572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maghool S., Cooray N.D.G., Stroud D.A., Aragão D., Ryan M.T., Maher M.J. Structural and functional characterization of the mitochondrial complex IV assembly factor Coa6. Life Sci. Alliance. 2019:2. doi: 10.26508/lsa.201900458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vukotic M., Oeljeklaus S., Wiese S., Vögtle F.-N., Meisinger C., Meyer H.E. Rcf1 mediates cytochrome oxidase assembly and respirasome formation, revealing heterogeneity of the enzyme complex. Cell Metabol. 2012;15:336–347. doi: 10.1016/j.cmet.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Meyer A.J., Dick T.P. Fluorescent protein-based redox probes. Antioxidants Redox Signal. 2010;13:621–650. doi: 10.1089/ars.2009.2948. [DOI] [PubMed] [Google Scholar]
- 22.Balatri E., Banci L., Bertini I., Cantini F., Ciofi-Baffoni S. Solution structure of Sco1: a thioredoxin-like protein Involved in cytochrome c oxidase assembly. Structure. 2003;11:1431–1443. doi: 10.1016/j.str.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Horng Y.-C., Leary S.C., Cobine P.A., Young F.B.J., George G.N., Shoubridge E.A. Human Sco1 and Sco2 function as copper-binding proteins. J. Biol. Chem. 2005;280:34113–34122. doi: 10.1074/jbc.M506801200. [DOI] [PubMed] [Google Scholar]
- 24.Canonica F., Klose D., Ledermann R., Sauer M.M., Abicht H.K., Quade N. Structural basis and mechanism for metallochaperone-assisted assembly of the CuA center in cytochrome oxidase. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aaw8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banci L., Bertini I., Ciofi-Baffoni S., Gerothanassis I.P., Leontari I., Martinelli M. A structural characterization of human SCO2. Structure. 2007;15:1132–1140. doi: 10.1016/j.str.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Banci L., Bertini I., Ciofi-Baffoni S., Leontari I., Martinelli M., Palumaa P. Human Sco1 functional studies and pathological implications of the P174L mutant. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15–20. doi: 10.1073/pnas.0606189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banci L., Bertini I., Ciofi-Baffoni S., Hadjiloi T., Martinelli M., Palumaa P. Mitochondrial copper(I) transfer from Cox17 to Sco1 is coupled to electron transfer. Proc. Natl. Acad. Sci. Unit. States Am. 2008;105:6803–6808. doi: 10.1073/pnas.0800019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soma S., Morgada M.N., Naik M.T., Boulet A., Roesler A.A., Dziuba N. COA6 is structurally tuned to function as a thiol-disulfide oxidoreductase in copper delivery to mitochondrial cytochrome c oxidase. Cell Rep. 2019;29:4114–4126. doi: 10.1016/j.celrep.2019.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denkert N., Schendzielorz A.B., Barbot M., Versemann L., Richter F., Rehling P. Cation selectivity of the presequence translocase channel Tim23 is crucial for efficient protein import. Elife. 2017;6 doi: 10.7554/eLife.28324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geissler A., Chacinska A., Truscott K.N., Wiedemann N., Brandner K., Sickmann A. The mitochondrial presequence translocase: an essential role of Tim50 in directing preproteins to the import channel. Cell. 2002;111:507–518. doi: 10.1016/s0092-8674(02)01073-5. [DOI] [PubMed] [Google Scholar]
- 31.Schulz C., Lytovchenko O., Melin J., Chacinska A., Guiard B., Neumann P. Tim50's presequence receptor domain is essential for signal driven transport across the TIM23 complex. J. Cell Biol. 2011;195:643–656. doi: 10.1073/pnas.1014918108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brancaccio D., Gallo A., Piccioli M., Novellino E., Ciofi-Baffoni S., Banci L. [4Fe-4S] cluster Assembly in mitochondria and its impairment by copper. J. Am. Chem. Soc. 2017;139:719–730. doi: 10.1021/jacs.6b09567. [DOI] [PubMed] [Google Scholar]
- 33.Callegari S., Müller T., Schulz C., Lenz C., Jans D.C., Wissel M. A MICOS-TIM22 association promotes carrier import into human mitochondria. J. Mol. Biol. 2019;431:2835–2851. doi: 10.1016/j.jmb.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Dennerlein S., Rozanska A., Wydro M., Chrzanowska-Lightowlers Z.M.A., Lightowlers R.N. Human ERAL1 is a mitochondrial RNA chaperone involved in the assembly of the 28S small mitochondrial ribosomal subunit. Biochem. J. 2010;430:551–558. doi: 10.1042/BJ20100757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pacheu-Grau D., Gómez-Durán A., López-Gallardo E., Pinós T., Andreu A.L., López-Pérez M.J. “Progress” renders detrimental an ancient mitochondrial DNA genetic variant. Hum. Mol. Genet. 2011;20:4224–4231. doi: 10.1093/hmg/ddr350. [DOI] [PubMed] [Google Scholar]
- 36.Lazarou M., Thorburn D.R., Ryan M.T., McKenzie M. Assembly of mitochondrial complex I and defects in disease. Biochim. Biophys. Acta. 2009;1793:78–88. doi: 10.1016/j.bbamcr.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Mohanraj K., Wasilewski M., Benincá C., Cysewski D., Poznanski J., Sakowska P. Inhibition of proteasome rescues a pathogenic variant of respiratory chain assembly factor COA7. EMBO Mol. Med. 2019;11 doi: 10.15252/emmm.201809561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 39.Cox J., Neuhauser N., Michalski A., Scheltema R.A., Olsen J.V., Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011;10:1794–1805. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
- 40.Smith A.C., Robinson A.J. MitoMiner v4.0: an updated database of mitochondrial localization evidence, phenotypes and diseases. Nucleic Acids Res. 2019;47:D1225–D1228. doi: 10.1093/nar/gky1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braschi B., Denny P., Gray K., Jones T., Seal R., Tweedie S. Genenames.org: the HGNC and VGNC resources in 2019. Nucleic Acids Res. 2019;47:D786–D792. doi: 10.1093/nar/gky930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wittig I., Braun H.-P., Schägger H. Blue native PAGE. Nat. Protoc. 2006;1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.