Abstract
Post-transcriptional modifications to messenger RNAs (mRNAs) have the potential to alter the biological function of this important class of biomolecules. The study of mRNA modifications is a rapidly emerging field, and the full complement of chemical modifications in mRNAs is not yet established. We sought to identify and quantify the modifications present in yeast mRNAs using an ultra-high performance liquid chromatography tandem mass spectrometry method to detect 40 nucleoside variations in parallel. We observe six modified nucleosides with high confidence in highly purified mRNA samples (N7-methylguanosine, N6-methyladenosine, 2′-O-methylguanosine, 2′-O-methylcytidine, N4-acetylcytidine, and 5-formylcytidine) and identify the yeast protein responsible for N4-acetylcytidine incorporation in mRNAs (Rra1). In addition, we find that mRNA modification levels change in response to heat shock, glucose starvation, and/or oxidative stress. This work expands the repertoire of potential chemical modifications in mRNAs and highlights the value of integrating mass spectrometry tools in the mRNA modification discovery and characterization pipeline.
Graphical Abstract

Cells face the daunting challenge of synthesizing the correct number of proteins at the right time with high fidelity. One way this is accomplished is through chemically modifying proteins and nucleic acids. Modifications change the topologies and chemistries accessible to biomolecules, altering their biogenesis, localization, function, and stability. There have been over 100 RNA modifications identified in noncoding RNAs and until recently post-transcriptional RNA modifications were thought to be largely limited to noncoding RNA species.1,2 Over the past eight years, this dogma has changed and there is a growing appreciation that modifications are also present in protein coding RNAs (mRNAs). The discovery of modified nucleosides in mRNAs has garnered a flurry of broad interest, because chemical modifications have the potential to modulate every step in the life cycle of an mRNA following transcription.
So far, only ~15 chemical modifications have been reported in mRNAs, including N6-methyladenosine (m6A), 5-methylcytidine (m5C), N1-methyladenosine (m1A), and pseudouridine (Ψ).1,3 Given the diversity of chemical modifications in noncoding RNAs, it is possible that the full catalog of mRNA modifications has not been uncovered. The current process for discovering and characterizing mRNA modifications is laborious; researchers hypothesize a particular mRNA modification exists and develop antibody- and/or reverse transcription-based tools to map the modification to the transcriptome.4 While this methodology has yielded multiple ground-breaking findings, it is not a tractable way to fully explore the breadth of possible mRNA modification chemical space and has a limited ability to establish absolute nucleoside concentrations. Accordingly, contradictory reports have emerged regarding the prevalence and location of several reported modifications.4 Thus, high-throughput quantitative tools able to directly detect mRNA modifications are needed to complement existing transcriptome-wide approaches for characterizing the cellular mRNA modification landscape.
Mass spectrometry has been used extensively to discover and study protein post-translational modifications and noncoding RNA post-transcriptional modifications.5,6 We believe that high-throughput mass spectrometry approaches have the potential to be similarly powerful for broadly screening and characterizing mRNA chemical modifications,7,8 akin to mass spectrometry studies of tRNA modifications that characterized dozens of nucleosides in parallel.9 We sought to quantitatively describe 40 possible nucleoside variations present in yeast mRNAs with a well-characterized ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) assay that uses standards to measure nucleoside levels with high accuracy, sensitivity, and selectivity simultaneously.6,10,11 In total, we assayed for the presence of 4 unmodified ribo- and deoxyribo-nucleosides, 29 naturally occurring modified nucleosides, and 3 non-naturally occurring modified nucleosides (as negative controls). A distinctive feature of this multiplexed approach is that it allows us to compare the relative levels of multiple modifications in a single experiment to exclude false positive hits that could arise from contaminating noncoding RNA species.
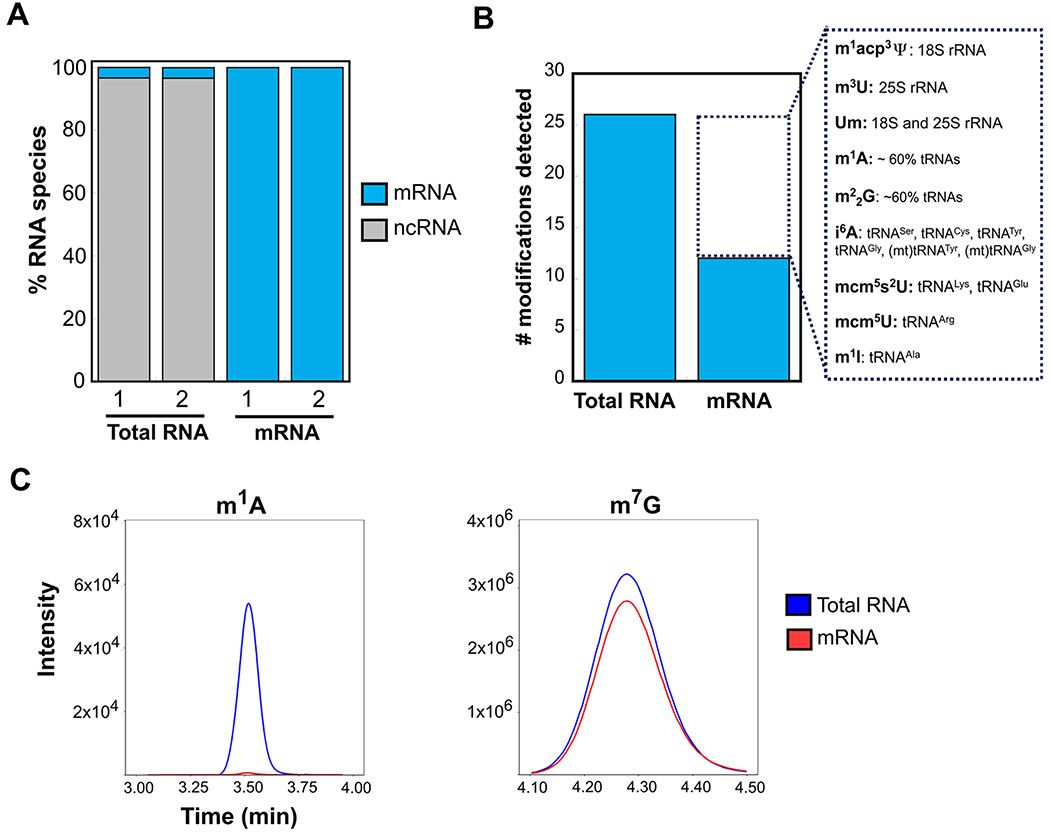
In our assays, all RNAs were enzymatically degraded into nucleosides for detection by UHPLC-MS/MS. Therefore, the limit of detection for identifying new mRNA modifications by this approach is largely determined by the purity of our mRNA. We tested four different purification schemes for yeast mRNAs and the most effective protocol is composed of two orthogonal steps (oligo-dT pull-down, followed by a RiboZero kit) (Figure S1 in the Supporting Information). We assessed the purity of our mRNA samples by Bioanalyzer, qRT-PCR, and RNA-seq (see Figure 1A, as well as Figures S1 and S2 in the Supporting Information). RNA-seq indicated that total RNA samples contained 3.4% ± 0.001% mRNA, while purified mRNA samples contained 99% ± 0.3% mRNA (Figure 1A). Although a previous study was unable to remove rRNA fragments from mRNA isolated from mouse ES cells using a similar protocol,12 our results demonstrate that mRNA can be purified from cells.
Figure 1.
mRNA sample purity. (A) Percentage of mRNA and noncoding RNA species (rRNA, snoRNA, snRNA, etc.) determined by RNA-seq for total RNA and mRNA samples in two biological replicates. (B) Number of modifications detected in total RNA and mRNA samples by UHPLC-MS/MS. Some of the modifications not detected are highlighted, and the RNA species where these modifications are found are indicated. (C) Overlaid extracted ion chromatograms of m1A and m7G in total RNA (blue line) and purified mRNA (red line) of no-stress condition. m7G, but not m1A, is present in our mRNA samples. The nucleases used in this study can liberate m7G found in the 5′ cap and internally in mRNAs.
We measured the concentrations of unmodified and modified RNA nucleosides in equal amounts of total RNA and purified mRNA by UHPLC-MS/MS (see Figure 1B, as well as Table S1 in the Supporting Information). All of the naturally occurring modifications in yeast RNAs for which we assayed were present in our total RNA; only modifications reported solely in bacterial RNA species (s2C and ho5U) were not detectable. In contrast, we did not observe signals for either bacterial RNA modifications or 14 naturally occurring yeast RNA modifications in any of our purified mRNA samples (i6A, m1A, m1I, cmnm5U, m3U, mnm5s2U, mnm5U, mo5U, s2U, m22G, m1acp3Ψ, Um, mcm5U, mcm5s2U). All of the modifications that were not detected in mRNAs have either been reported exclusively in noncoding RNAs or at very low levels in mRNAs. The inability to detect any signal in our mRNA samples for the majority of common and abundant tRNA modifications that we assayed (e.g., m1A and m22G) further indicates that our samples were highly purified, and essentially lacked detectable levels of tRNA modifications (see Figures 1B and 1C); this is an important observation because standard RNA-seq does not accurately report on tRNA levels.
All of the individual modifications that we detected were further scrutinized to discern the strongest positive “hits” from our assay. We reasoned that the bulk of the signal originating from modifications found primarily in mRNAs would be retained in our mRNA samples (retention of modification = 100% × ([modification in mRNA]/[unmodified nucleoside in mRNA])/([modification in total RNA]/[unmodified nucleoside in total RNA])) (see Figure 2A, as well as Figure S3 and Table S2 in the Supporting Information). As expected, most known mRNA modifications were well-retained, and 90% of the annotated noncoding RNA modifications were either not detectable or poorly retained (2.2%–6.8%), with an average retention of 4.8% ± 2.4% (see Figure S3 in the Supporting Information). Therefore, we empirically set a cutoff for denoting positive “hits” for new mRNA modifications at two standard deviations greater than the average retention value of most detectable noncoding modifications (10%). The m7G and m6A modifications known to exist predominantly in mRNAs, and previously reported 2′-O-methylcytidine (Cm) and 2′-O-methylguanosine (Gm) modifications were retained above this cutoff (see Figures 1C, 2, and 3A, as well as Figure S4 in the Supporting Information). Despite being present at detectable levels, reported mRNA modifications that are also highly abundant in noncoding RNA species, such as Ψ and m5C, were not retained at high levels. This makes sense because these modifications are estimated to occur in a low to modest percentage (<20%) of yeast mRNA sequences, but are overrepresented in the highly abundant tRNA and rRNA molecules that account for most of the RNA species in total RNA.12–14 Therefore, only a small fraction of Ψ and m5C should be expected to be retained, which is what we observe (Figure 2A). The analysis pipeline that we developed only permits us to draw firm conclusions regarding modifications that are highly abundant in mRNAs, relative to other RNA species.
Figure 2.
Modified nucleosides retained in mRNA samples. Modifications previously annotated in noncoding RNAs are shown in gray, those previously annotated mRNAs are in blue, and those that we denote as new mRNA modifications are displayed in red. (A) The percentage of different modifications retained in mRNA samples. (B) Phylogenetic distribution of the modified nucleosides whose levels we measured. All modifications retained in our mRNA samples are denoted with an asterisk (*). (C) Chemical structures of all of the modified nucleosides we observe in yeast mRNAs.
Figure 3.
Modified nucleosides present in mRNA samples. (A) Overlaid extracted ion chromatograms of f5C, m6A, and Cm in total RNA (blue line) and purified mRNA (red line). All three modifications were well-retained in purified mRNA. (B) Other modifications in noncoding RNAs where ac4C is found are not retained in mRNAs. We plotted the level of modified nucleoside in our mRNA sample, relative to the modification concentration present in total RNA. The plot shows the levels of ac4C (blue) and modifications on noncoding RNAs that contain ac4C retained in our mRNA samples grown under oxidative (H2O2) stress. *Am, Cm, and m1acp3Ψ are present in 18S rRNA. m1acp3Ψ is only present in 18S rRNA. (C) Overlaid extracted ion chromatogram of ac4C in wild-type mRNA (blue line) and rra1Δ mRNA (red line).
Initially, we were skeptical about finding m6A in mRNAs from yeast haploid cells, because the yeast RNA methyltransferase (MIS) complex responsible for m6A incorporation is reported to only be active during meiosis.15 However, countering this possibility, the catalytic component of the MIS complex (IME4) is expressed at low levels in cells not undergoing meiosis16 and a recent report indicates that IME4 catalyzes low levels of m6A incorporation into mRNAs in haploid yeast cells.17 These observations suggest that our finding is plausible. We also considered the possibility that our m6A signal resulted from the nonenzymatic Dimroth rearrangement of m1A to m6A during sample preparation.18 This seems unlikely because the rate constant for the conversion of m1A to m6A at neutral pH is slow at room temperature (RT) (kobs ≈ 5 × 10−5 min−1),18 and ~10 days would be needed to convert half of the m1A modifications to m6A; our samples were kept above −20 °C for <24 h. In addition, previous studies noted that the Dimroth rearrangements of m1A only go to 10%–50% completion at pH 7.19,20 Therefore, if our m6A signal originated from m1A, we would expect to observe a mixture of m1A and m6A products, and we do not (see Figure 1C). Hence, our results suggest that m6A can be incorporated at relatively low levels into haploid yeast mRNAs.
Two modifications annotated as noncoding RNAs were highly retained (>75%) in our mRNA samples under at least one experimental condition: 5-formylcytidine (f5C) (f5C/C = 0.04%) and N4-acetylcytidine (ac4C) (ac4C/C = 0.1%) (see Figure 2A, as well as Figure S3 and Tables S2 and S3 in the Supporting Information). f5C and ac4C are conserved in all kingdoms of life similar to most other reported mRNA modifications (Figure 2B). We verified the presence of f5C in our mRNA samples by using an antibody-based f5C detection kit (see Figure S5 in the Supporting Information). f5C is an in vivo oxidation product of m5C through 5-hydroxymethylcytidine (hm5C)21 observed in total- and polyA-enriched RNA.5,22,23 There has been speculation that f5C exists in eukaryotic mRNAs, and our results support this supposition.24 Ac4C is present in yeast tRNAs and rRNAs, and we considered that our mRNA samples could be enriched with contaminating noncoding RNAs containing ac4C. Our RNA-seq studies demonstrated that our samples were deplete of rRNA (Figure 1A), and we took advantage of the high level of modification in tRNAs and rRNAs to further assess our mRNA sample purity in the context of ac4C. If the ac4C signal that we measure originated from contaminating noncoding RNA fragments, then it is reasonable to expect that modifications located in close proximity to ac4C on the same molecule should also be highly retained in our mRNA samples. Our analyses demonstrate that other noncoding RNA modifications in ac4C-containing tRNAs and are either not detectable or poorly retained (<7%) in our mRNA samples (Figure 3B). In particular, m1acp3Ψ and Um modifications, which are present at stoichiometries similar to ac4C in 18S rRNA, were not detectable in our mRNA samples. We also investigated if the enzyme responsible for incorporating ac4C into 18S rRNA and tRNAs (Rra1) catalyzes its incorporation into mRNAs. The level of ac4C was measured in mRNAs purified from wild-type yeast and yeast lacking Rra1 (rra1Δ). No ac4C was detectable in mRNAs purified from the rra1Δ strain (see Figure 3C). Our results support a recent study that ac4C is enzymatically incorporated by the human homologue of Rra1, NAT10, into thousands of human mRNAs.25
Our systematic data analyses and exclusive assignment of highly retained, abundant modifications to mRNAs are particularly important in light of the current dialogue regarding prevalence and location of multiple reported mRNA modifications. While the groundbreaking studies that mapped m5C and m1A to the transcriptome indicated that these modifications are incorporated at thousands of sites, follow-up reports using different approaches reach opposing conclusions: some suggesting that these modifications are only in tens of mRNAs, while others support the initial finding that they are common.12,23,26–30 We do not detect m1A in our mRNA samples (Figure 1C) and our results suggest either that m1A is not in yeast mRNAs, or is incorporated at levels below our limit of detection. Since our studies were conducted in yeast, we do not exclude the possibility that m1A is in mammalian mRNAs, as previously reported.17,23,25 Our results are agnostic regarding the frequency of m5C in yeast mRNAs, because, while m5C is not highly retained in our mRNA samples, we observe the m5C oxidation product f5C. These controversies underscore the need for continued development and application of direct, quantitative methods—such as the UHPLC-MS/MS approach that we use here—to detect and characterize mRNA modifications.
Alterations in the modification status of noncoding RNA nucleosides, as a result of environmental stress, nutrition, and stage in the cell cycle progression, can impact their biological function.31,32 Similarly, the patterns of m6A and Ψ incorporation into mRNAs respond to a variety of conditions13,33,34 and the contributions of mRNA modifications to the cellular responses to environmental perturbations warrants additional characterization. We measured the levels of modifications in mRNAs collected from yeast grown under oxidative stress, heat shock, and glucose starvation and found that mRNA modifications exhibit statistically significant variations from basal conditions, in response to environmental challenges (see Figure 4, as well as Figure S6 and Table S4 in the Supporting Information). These changes in modification levels could result either from changes in modification stoichiometry or alterations in the expression levels of modified mRNAs. Our analyses revealed that m6A levels fluctuated under heat shock conditions (−24% ± 3%) and glucose starvation conditions (+34% ± 10%), consistent with previous reports.33 We also saw that the levels of Cm and Gm increased (+66% ± 12% and +31% ± 9%, respectively) under glucose starvation conditions. Interestingly, a signal for ac4C is present in total RNA in all of our samples but is only quantifiable at levels above background in mRNAs purified from yeast grown under oxidative stress. This observation leads us to propose that ac4C might have a role in the cellular response to oxidative stress in yeast.
Figure 4.
Post-transcriptional modifications in mRNA of S. cerevisiae exhibit differential responses to environmental stressors. The fold change for each modification upon stress induction was calculated as the ratio of nucleoside level in the mRNA sample of stress-treated condition ((H2O2 (+), heat shock (HS) (+), and glucose deprivation (Glu) (−)) and nucleoside level in the mRNA sample of no-stress condition (control). Error bars represent the standard error of mean. [Legend: (*) p < 0.05, (**) p < 0.01, and (***) p < 0.005, using Student’s t-test.
Our studies reveal two mRNA modifications in yeast—f5C and ac4C—and demonstrate the value of using quantitative UHPLC-MS/MS to augment transcriptome-wide mapping approaches for the discovery and characterization of mRNA modifications. This work also provides additional support for supposition that the mRNA post-transcriptional landscape is both complex and dynamic.
METHODS
Growth Conditions and Stress Experiments.
Wild-type and rra1Δ Saccharomyces cerevisiae cells were grown in YPD medium (nonstressed control, oxidative stress and heat shock conditions) or in defined synthetic complete medium (SC) with 2% glucose (glucose starvation). Before exposing cells to different stress conditions, cells grown in YPD medium (OD600 = 0.6) were collected and used as a control. Stress conditions were as follows: (1) oxidative stress (incubation of cells with 0.25 mM H2O2 (30 min, 30 °C)), (2) heat shock (cells grown at 37 °C for 45 min), and (3) glucose starvation (cells grown in SC (−) glucose media (30 °C, 60 min)). Details for each growth condition can be found in the Supporting Information.
Total RNA Extraction, mRNA Enrichment, and qRT-PCR.
Total RNA was extracted using hot acid phenol and treated with RNase-free DNase I. mRNA was isolated in two sequential steps: oligo-dT magnetic beads (Dynabeads) to isolate poly-adenylated RNAs, followed rRNA depletion (RiboZero Gold). The purity of the isolated mRNA was evaluated using Bioanalyzer RNA 6000 Pico Kit, qRT-PCR, analysis of modification levels, and RNA-seq (see Figure 1, as well as Figures S1 and S2 and Table S5 in the Supporting Information). The mRNA levels of CCT1, HSP30, and HXT2 genes were measured at different time points by qRT-PCR to verify that stress was induced under each condition (see Figure S7 in the Supporting Information). Luminaris HiGreen qRT-PCR Master Mix and gene-specific primers were used (see Table S6 in the Supporting Information), and ACT1 was the internal reference gene.
RNA-seq Data Analysis.
High-quality reads were saved in fastq files and deposited in the Gene Expression Omnibus (GEO) database at NCBI with Accession No. GSE126405 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE126405). Bowtie2 (v2.3.4.3)35 was used to align reads to Saccharomyces cerevisiae reference genome (R64-1-1), and mmquant tool (v0.1.0)36 was used to count the number of mapped reads for each transcript (see Table S5). The gene_biotype feature in S. cerevisiae GTF file (R64-1-1.95) was used to classify RNA species. Percentage of mapped reads to coding and noncoding RNA species was calculated by dividing the number of mapped reads to each RNA species by the total number of mapped reads (i.e., [number of mapped reads to mRNAs]/[number of mapped reads to the entire transcriptome]). Details are provided in the Supporting Information.
UHPLC-MS/MS Analysis.
RNA samples (100 ng/10 μL each) were analyzed as previously described.10 Briefly, digested RNA samples were separated by UHPLC interfaced to a triple quadrupole MS (Waters XEVO TQ-STEM) with sensitivity down to 23.01 femtograms (64.09 attomoles) in large excess of the sensitivity required to analyze and characterize RNA modifications. The instrument-selected reaction monitoring parameters were set to detect and quantify each of the 40 known nucleosides (see Table S7 in the Supporting Information). The resulting MS/MS signal relative to the internal standard was used to create calibration curves for quantification (see Figures S8 and S9 in the Supporting Information). Details of the technique, creation of standard curves of quantification (Table S8 and Figures S8 and S9), raw data, and data analyses are given in the Supporting Information. Each of our reported values reflects data collected from experiments performed with two biological replicates and three technical replicates of each biological sample.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge D. Eyler, M. Koutmos, R. Bailey, and A. Mapp for their thoughtful reading of the paper and comments.
Funding
This work was funded by the University of Michigan Start-Up Funds and National Institutes of Health Award No. R35 GM128836.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschembio.9b00369.
Supplemental tables (Tables S1–S8) (XLSX)
Additional experimental detail describing cell growth conditions, mRNA purification and analysis, RNA-seq data analysis, UHPLC-MS/MS analysis and data processing, f5C quantification, supplemental figures (Figures S1–S9), and associated references (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Gilbert WV, Bell TA, and Schaening C (2016) Messenger RNA modifications: Form, distribution, and function. Science 352, 1408–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Nachtergaele S, and He C (2017) The emerging biology of RNA post-transcriptional modifications. RNA Biol. 14, 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Roundtree IA, Evans ME, Pan T, and He C (2017) Dynamic RNA Modifications in Gene Expression Regulation. Cell 169, 1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Grozhik AV, and Jaffrey SR (2018) Distinguishing RNA modifications from noise in epitranscriptome maps. Nat. Chem. Biol 14, 215–225. [DOI] [PubMed] [Google Scholar]
- (5).Chen B, Yuan BF, and Feng YQ (2019) Analytical Methods for Deciphering RNA Modifications. Anal. Chem 91, 743–756. [DOI] [PubMed] [Google Scholar]
- (6).Gaston KW, and Limbach PA (2014) The identification and characterization of non-coding and coding RNAs and their modified nucleosides by mass spectrometry. RNA Biol. 11, 1568–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Xu L, Liu X, Sheng N, Oo KS, Liang J, Chionh YH, Xu J, Ye F, Gao YG, Dedon PC, and Fu XY (2017) Three distinct 3-methylcytidine (m(3)C) methyltransferases modify tRNA and mRNA in mice and humans. J. Biol. Chem 292, 14695–14703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Zhang LS, Liu C, Ma H, Dai Q, Sun HL, Luo G, Zhang Z, Zhang L, Hu L, Dong X, and He C (2019) Transcriptome wide Mapping of Internal N(7)-Methylguanosine Methylome in Mammalian mRNA. Mol. Cell, DOI: 10.1016/j.molcel.2019.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Su D, Chan CT, Gu C, Lim KS, Chionh YH, McBee ME, Russell BS, Babu IR, Begley TJ, and Dedon PC (2014) Quantitative analysis of ribonucleoside modifications in tRNA by HPLC-coupled mass spectrometry. Nat. Protoc 9, 828–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Basanta-Sanchez M, Temple S, Ansari SA, D’Amico A, and Agris PF (2016) Attomole quantification and global profile of RNA modifications: Epitranscriptome of human neural stem cells. Nucleic Acids Res. 44, No. e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Deng X, Chen K, Luo GZ, Weng X, Ji Q, Zhou T, and He C (2015) Widespread occurrence of N6-methyladenosine in bacterial mRNA. Nucleic Acids Res. 43, 6557–6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Legrand C, Tuorto F, Hartmann M, Liebers R, Jacob D, Helm M, and Lyko F (2017) Statistically robust methylation calling for whole-transcriptome bisulfite sequencing reveals distinct methylation patterns for mouse RNAs. Genome Res. 27, 1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Carlile TM, Rojas-Duran MF, Zinshteyn B, Shin H, Bartoli KM, and Gilbert WV (2014) Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature 515, 143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Machnicka MA, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, Helm M, Bujnicki JM, and Grosjean H (2012) MODOMICS: a database of RNA modification pathways-2013 update. Nucleic Acids Res. 41, D262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Schwartz S, Agarwala SD, Mumbach MR, Jovanovic M, Mertins P, Shishkin A, Tabach Y, Mikkelsen TS, Satija R, Ruvkun G, Carr SA, Lander ES, Fink GR, and Regev A (2013) High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell 155, 1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hongay CF, Grisafi PL, Galitski T, and Fink GR (2006) Antisense transcription controls cell fate in Saccharomyces cerevisiae. Cell 127, 735–745. [DOI] [PubMed] [Google Scholar]
- (17).Yadav PK, and Rajasekharan R (2017) The m(6)A methyltransferase Ime4 epitranscriptionally regulates triacylglycerol metabolism and vacuolar morphology in haploid yeast cells. J. Biol. Chem 292, 13727–13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Macon JB, and Wolfenden R (1968) 1-Methyladenosine. Dimroth rearrangement and reversible reduction. Biochemistry 7, 3453–3458. [DOI] [PubMed] [Google Scholar]
- (19).Russell SP, and Limbach PA (2013) Evaluating the reproducibility of quantifying modified nucleosides from ribonucleic acids by LC-UV-MS. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci 923–924, 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Dominissini D, Nachtergaele S, Moshitch-Moshkovitz S, Peer E, Kol N, Ben-Haim MS, Dai Q, Di Segni A, Salmon-Divon M, Clark WC, Zheng G, Pan T, Solomon O, Eyal E, Hershkovitz V, Han D, Dore LC, Amariglio N, Rechavi G, and He C (2016) The dynamic N(1)-methyladenosine methylome in eukaryotic messenger RNA. Nature 530, 441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Fu Y, Dominissini D, Rechavi G, and He C (2014) Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat. Rev. Genet 15, 293–306. [DOI] [PubMed] [Google Scholar]
- (22).Huber SM, van Delft P, Mendil L, Bachman M, Smollett K, Werner F, Miska EA, and Balasubramanian S (2015) Formation and abundance of 5-hydroxymethylcytosine in RNA. ChemBioChem 16, 752–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Guallar D, Bi X, Pardavila JA, Huang X, Saenz C, Shi X, Zhou H, Faiola F, Ding J, Haruehanroengra P, Yang F, Li D, Sanchez-Priego C, Saunders A, Pan F, Valdes VJ, Kelley K, Blanco MG, Chen L, Wang H, Sheng J, Xu M, Fidalgo M, Shen X, and Wang J (2018) RNA-dependent chromatin targeting of TET2 for endogenous retrovirus control in pluripotent stem cells. Nat. Genet 50, 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Wang R, Luo Z, He K, Delaney MO, Chen D, and Sheng J (2016) Base pairing and structural insights into the 5-formylcytosine in RNA duplex. Nucleic Acids Res. 44, 4968–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Arango D, Sturgill D, Alhusaini N, Dillman AA, Sweet TJ, Hanson G, Hosogane M, Sinclair WR, Nanan KK, Mandler MD, Fox SD, Zengeya TT, Andresson T, Meier JL, Coller J, and Oberdoerffer S (2018) Acetylation of Cytidine in mRNA Promotes Translation Efficiency. Cell 175, 1872–1886.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Safra M, Sas-Chen A, Nir R, Winkler R, Nachshon A, Bar-Yaacov D, Erlacher M, Rossmanith W, Stern-Ginossar N, and Schwartz S (2017) The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature 551, 251–255. [DOI] [PubMed] [Google Scholar]
- (27).Edelheit S, Schwartz S, Mumbach MR, Wurtzel O, and Sorek R (2013) Transcriptome-wide mapping of 5-methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs. PLoS Genet 9, e1003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Li X, Xiong X, Wang K, Wang L, Shu X, Ma S, and Yi C (2016) Transcriptome-wide mapping reveals reversible and dynamic N(1)-methyladenosine methylome. Nat. Chem. Biol 12, 311–316. [DOI] [PubMed] [Google Scholar]
- (29).Shen Q, Zhang Q, Shi Y, Shi Q, Jiang Y, Gu Y, Li Z, Li X, Zhao K, Wang C, Li N, and Cao X (2018) Tet2 promotes pathogen infection-induced myelopoiesis through mRNA oxidation. Nature 554, 123–127. [DOI] [PubMed] [Google Scholar]
- (30).Li X, Xiong X, Zhang M, Wang K, Chen Y, Zhou J, Mao Y , Lv J, Yi D, Chen XW, Wang C, Qian SB, and Yi C (2017) Base-Resolution Mapping Reveals Distinct m(1)A Methylome in Nuclear- and Mitochondrial-Encoded Transcripts. Mol. Cell 68, 993–1005.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Helm M, and Alfonzo JD (2014) Posttranscriptional RNA Modifications: playing metabolic games in a cell’s chemical Legoland. Chem. Biol 21, 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Chan CT, Pang YL, Deng W, Babu IR, Dyavaiah M, Begley TJ, and Dedon PC (2012) Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun 3, 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, and Qian SB (2015) Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Schwartz S, Bernstein DA, Mumbach MR, Jovanovic M, Herbst RH, Leon-Ricardo BX, Engreitz JM, Guttman M, Satija R, Lander ES, Fink G, and Regev A (2014) Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell 159, 148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Langmead B, Trapnell C, Pop M, and Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Zytnicki M (2017) mmquant: how to count multi-mapping reads? BMC Bioinformatics 18, 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.