Abstract
Substantia nigra (SN) hyperechogenicity measured by transcranial sonography (TCS) is a promising biomarker for Parkinson disease (PD). The aim of this study was to explore the diagnostic accuracy of SN hyperechogenicity (SN+) for differentiating PD from essential tremor (ET). A total of 119 patients with PD, 106 ET patients and 112 healthy controls that underwent TCS from November 2016 to February 2019 were included in this single-center retrospective case–control study. Two reviewers who were blinded to clinical information independently measured the SN+ by TCS imaging. The diagnostic sensitivity, specificity, and accuracy of TCS imaging were evaluated between the PD and healthy controls and between patients with PD and ET. Interrater agreement was assessed with the Cohen κ statistic. TCS imaging of the SN+ allowed to differentiate between patients with PD and ET with a sensitivity (91.6% and 90.8%) and specificity (91.5% and 89.6%) for readers 1 and 2, respectively. Interobserver agreement was excellent (к = 0.87). In addition, measurement of the SN+ allowed to differentiate between patients with PD and healthy subjects with a sensitivity (91.6% and 90.8%) and specificity (88.4% and 89.3%) for readers 1 and 2, respectively. Interobserver agreement was excellent (к = 0.91). Measurement of SN+ on TCS images could be a useful tool to distinguishing patients with PD from those with ET.
Keywords: essential tremor, Parkinson disease, substantia nigra hyperechogenicity, transcranial sonography
1. Introduction
Essential tremor (ET) is one of the most common movement disorder, affecting as high as 4% individuals,[1] and is characterized by rhythmic oscillation of agonist and antagonist muscle groups that typically affects the hands more than the head, trunk, legs, or voice.[2] Although ET does not influence life expectancy, it can affect the quality of life, socialization, mood, and functional activities.[3] In contrast, Parkinson disease (PD) is a progressively neurodegenerative disease characterized pathologically by the degeneration of pigmented dopaminergic neurons in the substantia nigra (SN), resulting in bradykinesia, rest tremor, rigidity, postural instability, and freezing of gait.[4]
The PD and ET are considered as distinct disorders that nevertheless have several overlapping nonmotor features, such as rapid eye movement sleep behavior disorder, constipation, hyposmia, and depression.[5] Clinically, differentiation of PD from ET may be challenging, especially in the early stages of the diseases. Although the clinical opinion of experts is still the gold standard, only 53% to 75% of their diagnoses agree with the definite pathologic diagnosis in some studies.[6] It is therefore crucial to identify markers that enable accurate early differential diagnosis of ET and PD,[7] especially in the premotor stage before most neurons are lost.
In 1995, Becker et al first reported the presence of a specific high-echogenicity area of the SN in patients with PD.[8] Since then, transcranial sonography (TCS) has been increasingly applied for the differential diagnosis of patients with PD and has drawn a lot of attention as a noninvasive, easily accessible, and inexpensive imaging method.[9] TCS parameters have been standardized in diverse ultrasound models, with good repeatability and reproducibility in distinct populations.
The SN hyperechogenicity (SN+), which is defined as an enlargement of the SN echogenic area, has been proposed as a diagnostic biomarker,[10] as it is stable and remains unchanged with PD progression.[11] In addition, SN+ is observable in patients with PD with very early or even premorbid stages[10] and is helpful for discriminating patients with PD from not only healthy controls (HCs)[12] but also those with major PD mimics such as ET and atypical parkinsonisms (APs).[13] It has been hypothesized that degeneration of nigrostriatal dopaminergic neurons increased SN iron content and SN+ may considered a early marker of increased vulnerability of nigrostriatal dopaminergic pathway.[9] Prior studies have suggested that up to 90% of PD patients with SN+, while only 12% of ET patients and 10% of HCs with SN+.[14]
Although some studies have evaluated the efficacy of TCS in the diagnosis of PD, most of them were conducted in European populations, and only few studies were conducted in Asian populations. In this study, we sought to assess the sensitivity and specificity of TCS imaging measurements of SN+ for differentiating PD from ET in Chinese population by using established consensus criteria as the standard of reference.
2. Materials and methods
All subjects provided written informed consent before testing. This single-center retrospective case–control study was approved by the institutional ethics review board of Tongji hospital, Tongji Medical College, Huazhong University of Science and Technology. The study did not receive any financial support from any industry.
2.1. Participants
This study was conducted in the movement disorder clinic at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) from November 2016 to February 2019. ET patients who fulfilled the clinical diagnostic criteria of the Movement Disorder Society Consensus Statement for ET were enrolled in this study.[15] In addition, age- and sex-matched patients with PD who fulfilled Movement Disorder Society (MDS) Clinical Diagnostic Criteria for Parkinson disease (MDS-PD criteria) were included in our study.[16] To evaluate motor symptoms and disease severity, assessments based on the unified Parkinson disease rating scale (UPDRS)[17] and Hoehn and Yahr rating scale[18] were performed in the entire study cohort. Magnetic resonance imaging was performed in each participant to exclude symptomatic parkinsonism. The diagnostic criteria for ET and PD were evaluated by 2 experienced neurologists (MZJ and GHL with 10 and 5 years of experience in clinical neurology, respectively).
For this study, we excluded participants with ET and PD who showed the following findings: dementia (as assessed according to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders criteria), corticobasal degeneration, tumors, infarction, hemorrhage, extensive white matter lesions, or history of trauma; clinical suspicion of secondary parkinsonism or APs, specifically vascular parkinsonism or drug-induced parkinsonism; multiple sclerosis, Huntington disease, and Wilson disease; cataract, oculomotor palsy and visual hallucinations; clinical suspicion of restless leg syndrome (according to questions prepared by International Restless Leg Syndrome Study Group in 1995), iron-deficiency anemia, peripheral neuropathy (according to nerve conduction), or low-serum ferrous levels (according to serum ferrous test).
In addition, age- and sex-matched HC participants with no history of neurologic or psychiatric diseases were selected among spouses, staff members, or healthy volunteers. All controls had a normal neurologic status and were screened for the absence of ET or PD by neurologists (MZJ and GHL). HCs were excluded if they had any neurologic diseases, psychiatric diseases, memory impairment or PD family history. All the participants were followed every 6 months to undergone clinical diagnosis and TCS after initial inclusion. We only choose the 1st TCS results to analysis, and the TCS results in the follow-up were not used in this study.
2.2. Transcranial sonography
The TCS examination was performed by 2 experienced sonographers (TAY and XRF, with 10 and 6 years of experience, respectively), who were blinded to the clinical data and other imaging results. To assess the intrarater reliability, a 2nd evaluation (subjects in a different order) was performed 2 hours after the 1st evaluation by the 2nd sonographer, who scored all the images independently. TCS was performed using Toshiba, Aplio-500 (Tochigi, Japan) Ultrasound System, with a 2.5- to 3.5-MHz phased-array transducer at a penetration depth of 14 to 16 cm and dynamic range of 45 to 55 dB. Individuals with sufficient temporal bone windows were included. The examination was performed bilaterally through the pre-auricular temporal acoustic window with the patient in the supine position. Time gain compensations and image brightness and were adapted as needed for each examination.
The examination was performed bilaterally through the transtemporal bone window. The butterfly-shaped midbrain area and surrounding basal cisterns were observed on the axial plane, and the echogenicity of the ipsilateral SN can be assessed. Once the SN was visible at its largest extension, the image was frozen and zoomed. The areas of SN echogenicity and midbrain were manually encircled on digitally stored images and planimetrically measured using the built-in area function of the ultrasound system. The larger of bilateral measurements of the SN+ of each participant, or the sole assessable measurement in cases involving a unilateral bone window, was used for analysis. In a diencephalic plane, the widths of the 3rd ventricles were measured between the inner boundaries of both hyperechoic lines of ependyma at the thalamus level. Mesencephalic echogenicity in the area of SN measured 3 times for each side, and the mean value was calculated. After stored in the computer, TCS images were removed the patient's name and were labeled with randomized numbers which generated from computer by a clinical worker (GZC who is blinded to the patients). The 2 sonographers (TAY and XRF) who were blinded to the randomized numbers read the TCS images and measured related parameters a month later. The 2 sonographers would discuss with the 3rd sonographer (DYD with 12 years of TCS experience) separately if they had any questions.
In this study, the primary outcome was to gain the accuracy of SN+ in PD diagnosis and the secondary outcome included the SN+ area in all the groups.
2.3. Statistical analysis
Continuous variables were summarized by using mean ± standard deviation and ranges. Categorical variables were summarized as numbers and percentages. Interreader agreement for visual evaluations was assessed by using the Cohen к intertest for nominal scales. к coefficient values of 0.81 to 1.00 indicated excellent agreement; 0.61 to 0.80, substantial agreement; 0.41 to 0.60, moderate agreement; 0.21 to 0.40, fair agreement; and 0.00 to 0.20, poor agreement.[19]
The Chi-squared test was used to evaluate differences in sex distribution among groups. The differences in clinical features among groups were evaluated with the Mann–Whitney U test. The Kruskal–Wallis test was used to evaluate intergroup differences in SN+ area. The independent t test for normally distributed continuous data, or the Mann–Whitney U test for nonnormally distributed continuous data when appropriate. Of all regions that were significantly altered in the main effect, Bonferroni correction was performed to compare multiple group comparisons. In our department, hyperechogenicity was defined as SN+ ≥ 0.20 cm2, while normoechogenicity was defined as SN+ < 0.20 cm2.[20] Sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) for the diagnosis of PD on the basis of SN+ measurements were calculated. Subgroup analysis were undergone according to sex (male or female) and age (≥60 or <60). PD incidence was much higher in patients larger than 60, so we want to see the accuracy of SN hyperechogenic in PD diagnosis in different age range. Unless otherwise stated, P < .05 was used as a statistically significant difference. The statistical analyses were performed using SPSS 22.0 (IBM Corp., Armonk, USA), and graphs were produced by using GraphPad Prism 6 (GraphPad Software, San Diego, USA).
3. Result
3.1. Study population
During enrollment, we screened 460 participants on the basis of the inclusion criteria. Figure 1 shows the patient flowchart for TCS examinations. Thirty-one participants were excluded under suspicion of other diseases. Thus, 155 patients with PD, 135 ET patients, and 139 HCs underwent following TCS examinations. Thirty-six patients (23.2%) in the PD group, 29 patients (21.5%) in the ET group, and 27 participants (19.4%) in the HC group had an insufficient temporal acoustic bone window (P = .31). Finally, 337 of 460 participants (73.3%) included in this study.
Figure 1.
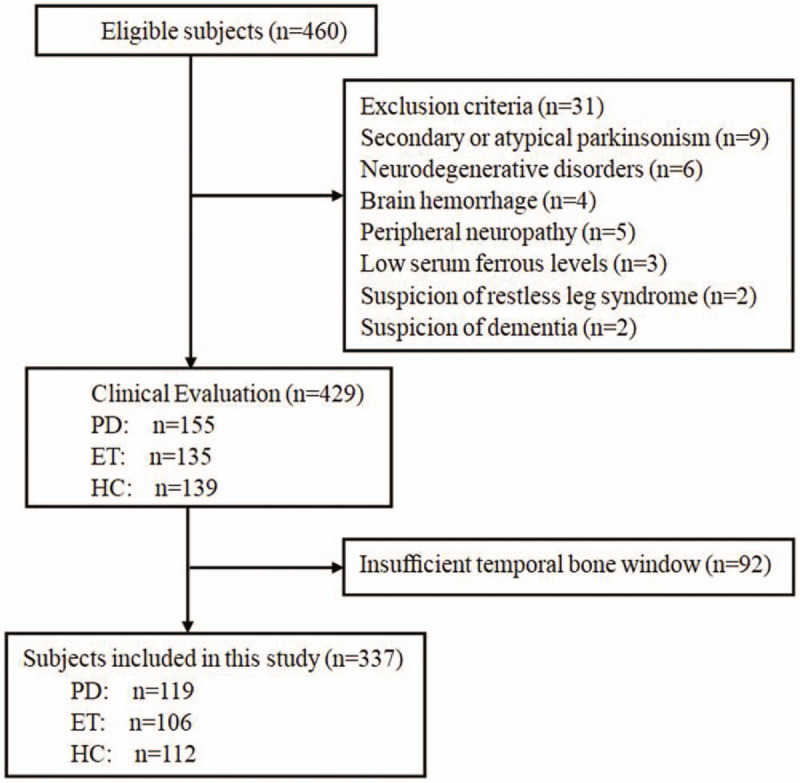
Diagram of the study design. ET = essential tremor, HC = health control, PD = Parkinson disease.
3.2. Clinical features
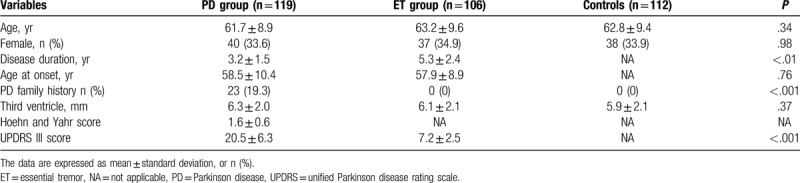
In this study, a total of 119 patients with PD (duration of PD ranges from 6 months to 5 years with an average of 3.2 years), 106 ET patients (duration of ET ranges from 2 to 8 years with an average of 5.7 years), and 112 HCs were screened for eligibility. Each participant was undergone clinical assessment every 6 months. Until now, there was no ET patients or health controls developed into PD in our follow-up. Baseline demographic and background characteristics are summarized in Table 1. The groups were well matched in terms of baseline characteristics, including age and sex. There were significant differences in disease duration (P < .01) and UPDRS III score (P < .001). In the PD group, Hoehn and Yahr staging system score was 1.6 ± 0.6 (range 1–3) and UPDRS III score was 20.5 ± 6.3.
Table 1.
Demographic and clinical features of the patients included.
3.3. Transcranial sonography
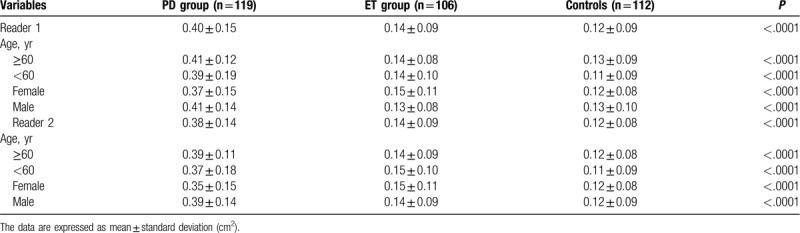
In assessments performed by reader 1, SN+ was significantly higher in patients with PD compared with those in both ET patients (0.40 ± 0.15 cm2 vs 0.14 ± 0.09 cm2; P < .0001) and in healthy volunteers (0.40 ± 0.15 cm2 vs 0.12 ± 0.09 cm2; P < .0001). No differences in SN+ were observed between ET patients and healthy volunteers (0.14 ± 0.09 cm2 vs 0.12 ± 0.09 cm2; P = .26) (Figs. 2 and 3). The widths of the 3rd ventricle in ET patients, patients with PD, and HCs were 6.1 ± 2.1 mm, 6.3 ± 2.0 mm, and 5.9 ± 2.1 mm, respectively, with no significant intergroup differences (P = .37)
Figure 2.
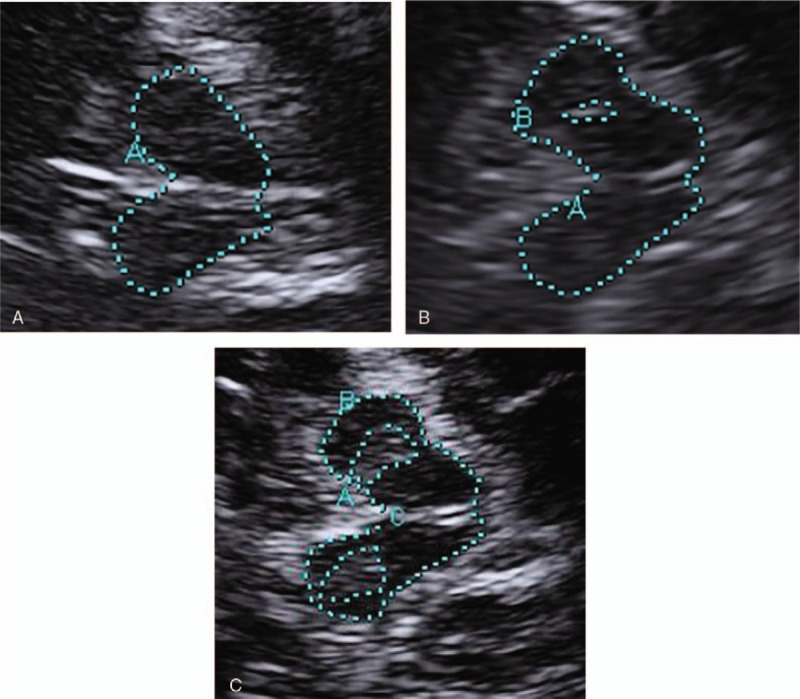
Transcranial sonographic images of the midbrain. (A) Image of normal SN echogenicity in healthy control. (B) Image of substantia nigra (SN) hyperechogenicity in Essential tremor patients. (C) Image of SN hyperechogenicity in Parkinson disease patient. In the graph, A represent midbrain area. The echogenic region of the substantia nigra is encircled with dotted line for better visualization (B and C).
Figure 3.
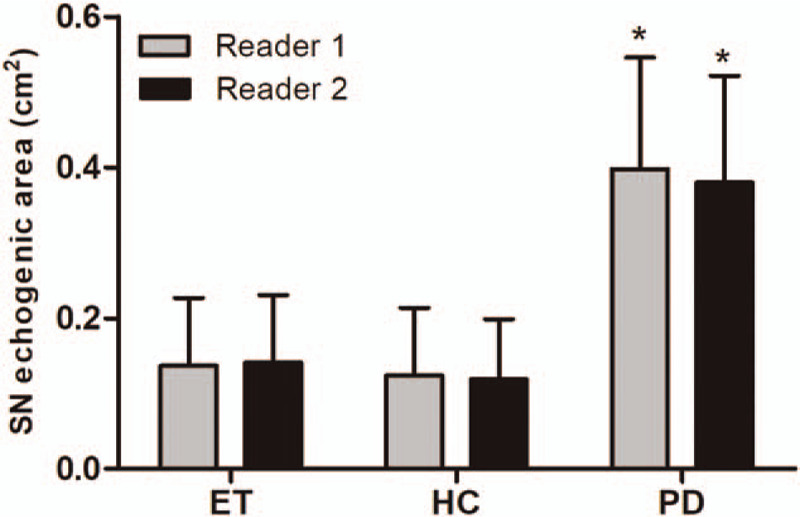
Transcranial sonographic substantia nigra (SN) hyperechogenicity in essential tremor (ET) patients, Parkinson disease (PD) patients, and healthy controls (HC) by reader 1 and reader 2. PD shows significant increased echogenicity than both ET and health control (∗P < .001). Data are expressed as mean ± standard deviation.
In assessments performed by reader 2, patients with PD showed significantly higher SN+ than both ET patients (0.38 ± 0.14 cm2 vs 0.14 ± 0.09 cm2; P < .0001) and HCs (0.38 ± 0.14 cm2 vs 0.12 ± 0.08 cm2; P < .0001). No differences in SN+ were observed between ET patients and HCs (0.14 ± 0.09 cm2 vs 0.12 ± 0.08 cm2; P = .07) (Fig. 3 and Table 2). The widths of the 3rd ventricle in ET patients, patients with PD, and HCs were 6.2 ± 1.9 mm, 6.3 ± 2.0 mm, and 5.9 ± 1.8 mm, respectively, with no significant intergroup differences (P = .21). Table 2 summarizes the SN+ area in different age groups and both sexes obtained by readers 1 and 2.
Table 2.
Transcranial sonographic substantia nigra (SN) echogenicity in healthy controls, Parkinson disease (PD), and essential tremor by subgroup analyses.
3.4. Quantitative analysis
Interreader agreement for SN+ detection between the 2 radiologists was excellent (к = 0.87). The McNemar test results showed no statistical difference between readers 1 and 2 in visual analysis of SN+ (P = .73).
For reader 1, the detection rate of SN+ by using TCS was 91.6% (109/119) in patients with PD, 8.5% (9/106) in ET patients, and 11.6% (13/112) in HCs. For differentiation of patients with PD from ET patients, the diagnostic sensitivity and specificity were 91.6% (95% confidence interval [CI], 86.5–96.7%) and 91.5% (95% CI, 86.1–96.9%), respectively. Positive and negative predictive values were 92.4% (95% CI, 87.5–97.2%) and 90.7% (95% CI, 85.1–96.3%), respectively. The accuracy was 91.6% (95% CI, 87.9–95.2%). For differentiation of patients with PD from HCs, the diagnostic sensitivity and specificity were 91.6% (95% CI, 86.5–96.7%) and 88.4% (95% CI, 82.4–94.4%), respectively. Positive and negative predictive values were 89.3% (95% CI, 83.8–94.9%) and 90.8% (95% CI, 85.3–96.3%), respectively. The accuracy was 90.0% (95% CI, 86.2–93.9%) (Table 3).
Table 3.
TCS for differentiation of patients with PD from patients with ET and health control participants.
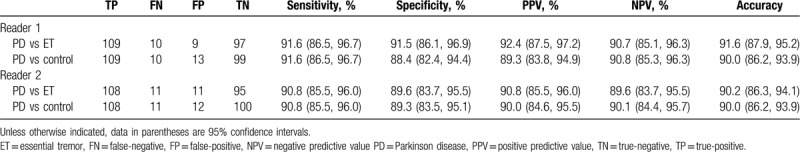
For reader 2, the detection rate of SN+ was 90.8% (108/119) in patients with PD, 10.4% (11/106) in ET patients, and 10.7% (12/112) in HCs. For differentiation of patients with PD from ET patients, the reviewer showed diagnostic sensitivity of 90.8% (95% CI, 85.5–96.0%), specificity of 89.6% (95% CI, 83.7–95.5%), PPV of 90.8% (95% CI, 85.5–96.0%), NPV of 89.6% (95% CI, 83.7–95.5%), and accuracy of 90.2% (95% CI, 86.3–94.1%). For differentiation of patients with PD from HCs, the reviewer showed diagnostic sensitivity of 90.8% (95% CI, 85.5–96.0%), specificity of 89.3% (95% CI, 83.5–95.1%), PPV of 90.0% (95% CI, 84.6–95.5%), NPV of 90.1% (95% CI, 84.4–95.7%), and accuracy of 90.0% (95% CI, 86.2–93.9%) (Table 3).
4. Discussion
This study demonstrated that TCS has good diagnostic accuracy for differentiating between patients with PD and ET, with a sensitivity of 91.6% and 90.8% and a specificity of 91.5% and 89.6% for readers 1 and 2, respectively. In addition, we reaffirmed that TCS imaging can differentiate between patients with PD and healthy individuals, with a sensitivity of 91.6% and 90.8% and a specificity of 88.4% and 89.3%for readers 1 and 2, respectively. These findings are consistent with those from our former meta-analysis, which included 3123 patients with PD from 39 studies and indicated that TCS can be an appropriate method for differentiation of patients with PD from both HCs and patients with other parkinsonism symptoms.[21]
The TCS through the preaurical bone window allows the depiction of characteristic abnormalities in the echogenicity of SN.[20] In this study, increased SN+ was detected in up to 91% (reader 1) and 90% (reader 2) of patients with PD and could be found unilaterally or asymmetrically bilaterally. In addition, 9 (8.5%, reader 1) and 11 (10.4%, reader 2) patients with ET had SN+ ≥0.20 cm2, which is consistent with the findings of the previous report.[9,22] Some studies pointed out that ET patients may have a higher risk of PD in the future,[23] so long-duration follow-up in ET patients with SN+ is needed. There was no significant difference in the SN+ area between ET patients and HCs, which is in agreement with the results of previous studies.[22] Readers 1 and 2 found SN+ in 11.6% and 10.7% of HCs, respectively, which is consistent with the result of a meta-analysis that reported that SN+ can be observed in 8–14% of the general population.[24] However, it is debatable whether the SN+ population has an increased risk of developing PD; hence, further follow-up is warranted for SN+ in HCs.[25]
The exact reason for the enlarged echogenic SN in patients with PD is still unknown. However, SN+ may reflect the alteration of dopaminergic cells and could be related to increased iron deposition within the SN.[26] In addition, increases in tissue calcium content, protein aggregation, and glial activation with increases in focal heavy metal deposits may also be implicated in SN+.[27] SN+ in PD cannot be used as a marker of progressive nigral degeneration, because it does not appear to be correlated with neither PD severity[28] nor dopaminergic deficit degree,[29] and the SN+ area remains stable throughout the disease course despite clinical disease progression.[30]
The ET is one of the most common movement disorders with an estimated prevalence of more than 5% in individuals older than 65 years.[31] The prevalence of PD in the same age range can be up to 1.8%.[32] Although ET and PD are thought to be mutually exclusive, clinical differentiation of ET from PD can be a real challenge and leads to misdiagnosis because of the presence of common disorders and overlapping features, especially in the aging population.[33] From the DATATOP (deprenyl and tocopherol antioxidative therapy of PD) study population (n = 800), 8% of patients were diagnosed with conditions other than PD on the basis of additional clinical, neuroimaging, or postmortem findings after 7 years’ follow-up.[34] In addition, in a postmortem study of 100 patients who had been diagnosed with idiopathic PD, only 76% of the patients had the typical histopathologic features of PD (i.e., Lewy bodies).[35] The 1st clinical symptoms of PD appear after at least 60–70% of dopaminergic neurons of the SN have degenerated and 80% of striatal dopamine content is reduced.[36] To date, no neuroprotective therapy has been shown to be adequately capable of modifying the clinical progression of the disease.[37] It is possible that a neuroprotective therapy will slow down the development of PD in the future. Thus, an independent diagnostic tool is needed to confirm the clinical diagnosis, especially in the early stage, when the motor symptoms have not yet become noticeable.[37]
Positron emission tomography and dopamine transporter single photon emission computed tomography are excellent techniques to improve clinical PD diagnosis, but these techniques are expensive, invasive, and not always available.[38] Computed tomography and magnetic resonance imaging have been proposed to differentiate idiopathic PD from atypical or secondary PD. However, the sensitivity and specificity are not very high.[39] TCS has several significant strengths, such as low cost, convenience, absence of radiation, noninvasiveness, and less dependence on patient compliance. However, there are some limitations for TCS: Its primary limitation is its dependence on the quality of the temporal acoustic bone window. The incidence of insufficient temporal windows in European populations is 4% to 15%,[40] while this may increase to 15% to 60% in the Asian population, especially in female patients with a small temporal window or advanced age.[41] A 2nd limitation of TCS is its dependence on the investigator's skill and experience. Another limitation is its dependence on the quality of the ultrasound system.
The current investigation also had some limitations. First, the patients included in this study had not been diagnosed with neuropathologic data, and it is possible that the clinical diagnoses may have been incorrect. However, this seems unlikely in our case for the following reasons: standardized criteria were used to evaluate all participants in this study by 2 experienced authors(GHL and MZJ) in movement disorders. The sensitivity and specificity of the clinical diagnostic criteria which applied for PD and ET diagnosing are high. Second, this was a pilot study that included a small number of patients who were not randomized at enrollment. Third, we included patients from November 2016 to February 2019 in this study, so the time since disease onset differs, which make that groups are not that comparable. Forth, we did not calculate the sample size in advance. Fifth, newly diagnosed patients were not included and the data for follow-up TCS were not analyzed in this study.
In conclusion, our findings indicate that TCS imaging is useful for distinguishing patients with PD from those with ET and control participants on an individual basis. A randomized controlled trial with a larger group of participants would be desirable in the future.
Author contributions
Conceptualization: Anyu Tao, Guangzhi Chen, Renfan Xu.
Data curation: Anyu Tao, Zhijuan Mao, Hongling Gao.
Formal analysis: Renfan Xu.
Funding acquisition: Renfan Xu.
Methodology: Youbin Deng, Guangzhi Chen.
Investigation: Anyu Tao, Renfan Xu.
Software: Guangzhi Chen
Supervision: Youbin Deng
Validation: Zhijuan Mao, Hongling Gao.
Writing – original draft: Anyu Tao, Renfan Xu.
Writing – review & editing: Anyu Tao, Renfan Xu.
Footnotes
Abbreviations: APs = atypical parkinsonisms, CIs = confidence intervals, ET = essential tremor, HCs = healthy controls, NPV = negative predictive value, PD = Parkinson disease, PPV = positive predictive value, SN = substantia nigra, SN+ = SN hyperechogenicity, TCS = transcranial sonography, UPDRS = unified Parkinson disease rating scale.
How to cite this article: Tao A, Chen G, Mao Z, Gao H, Deng Y, Xu R. Essential tremor vs idiopathic Parkinson disease: utility of transcranial sonography. Medicine. 2020;99:20(e20028).
This study was supported by the National Natural Science Foundation of China (No 81500293).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 2010;25:534–41. [DOI] [PubMed] [Google Scholar]
- [2].Elias WJ, Shah BB. Tremor. JAMA 2014;311:948–54. [DOI] [PubMed] [Google Scholar]
- [3].Louis ED, Machado DG. Tremor-related quality of life: a comparison of essential tremor vs. Parkinson's disease patients. Parkinsonism Relat Disord 2015;21:729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol 2009;8:464–74. [DOI] [PubMed] [Google Scholar]
- [5].Lacerte A, Chouinard S, Jodoin N, et al. Increased prevalence of non-motor symptoms in essential tremor. Tremor Other Hyperkinet Mov (N Y) 2014;4:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson's disease. Lancet Neurol 2006;5:75–86. [DOI] [PubMed] [Google Scholar]
- [7].Jellinger KA, Logroscino G, Rizzo G, et al. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 2016;87:237–8. [DOI] [PubMed] [Google Scholar]
- [8].Becker G, Seufert J, Bogdahn U, et al. Degeneration of substantia nigra in chronic Parkinson's disease visualized by transcranial color-coded real-time sonography. Neurology 1995;45:182–4. [DOI] [PubMed] [Google Scholar]
- [9].Berg D, Godau J, Walter U. Transcranial sonography in movement disorders. Lancet Neurol 2008;7:1044–55. [DOI] [PubMed] [Google Scholar]
- [10].Gaenslen A, Unmuth B, Godau J, et al. The specificity and sensitivity of transcranial ultrasound in the differential diagnosis of Parkinson's disease: a prospective blinded study. Lancet Neurol 2008;7:417–24. [DOI] [PubMed] [Google Scholar]
- [11].Berg D, Merz B, Reiners K, et al. Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson's disease. Mov Disord 2005;20:383–5. [DOI] [PubMed] [Google Scholar]
- [12].Busse K, Heilmann R, Kleinschmidt S, et al. Value of combined midbrain sonography, olfactory and motor function assessment in the differential diagnosis of early Parkinson's disease. J Neurol Neurosurg Psychiatry 2012;83:441–7. [DOI] [PubMed] [Google Scholar]
- [13].Lauckaite K, Rastenyte D, Surkiene D, et al. Specificity of transcranial sonography in Parkinson spectrum disorders in comparison to degenerative cognitive syndromes. BMC Neurol 2012;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stenc Bradvica I, Mihaljevic I, Butkovic-Soldo S, et al. Transcranial sonography and the pocket smell test in the differential diagnosis between Parkinson's disease and essential tremor. Neurol Sci 2015;36:1403–10. [DOI] [PubMed] [Google Scholar]
- [15].Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord 1998;13: Suppl 3: 2–3. [DOI] [PubMed] [Google Scholar]
- [16].Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30:1591–601. [DOI] [PubMed] [Google Scholar]
- [17].Goetz CG, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord 2007;22:41–7. [DOI] [PubMed] [Google Scholar]
- [18].Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–42. [DOI] [PubMed] [Google Scholar]
- [19].McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–82. [PMC free article] [PubMed] [Google Scholar]
- [20].Dong ZF, Wang CS, Zhang YC, et al. Transcranial sonographic alterations of substantia nigra and third ventricle in Parkinson's disease with or without dementia. Chin Med J (Engl) 2017;130:2291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tao A, Chen G, Deng Y, et al. Accuracy of transcranial sonography of the substantia nigra for detection of Parkinson's disease: a systematic review and meta-analysis. Ultrasound Med Biol 2019;45:628–41. [DOI] [PubMed] [Google Scholar]
- [22].Luo WF, Zhang YC, Sheng YJ, et al. Transcranial sonography on Parkinson's disease and essential tremor in a Chinese population. Neurol Sci 2012;33:1005–9. [DOI] [PubMed] [Google Scholar]
- [23].Louis ED, Frucht SJ. Prevalence of essential tremor in patients with Parkinson's disease vs. Parkinson-plus syndromes. Mov Disord 2007;22:1402–7. [DOI] [PubMed] [Google Scholar]
- [24].Vlaar AM, Bouwmans A, Mess WH, et al. Transcranial duplex in the differential diagnosis of parkinsonian syndromes: a systematic review. J Neurol 2009;256:530–8. [DOI] [PubMed] [Google Scholar]
- [25].Walter U, Niehaus L, Probst T, et al. Brain parenchyma sonography discriminates Parkinson's disease and atypical parkinsonian syndromes. Neurology 2003;60:74–7. [DOI] [PubMed] [Google Scholar]
- [26].Berg D, Hochstrasser H, Schweitzer KJ, et al. Disturbance of iron metabolism in Parkinson's disease -- ultrasonography as a biomarker. Neurotox Res 2006;9:1–3. [DOI] [PubMed] [Google Scholar]
- [27].Berg D, Roggendorf W, Schroder U, et al. Echogenicity of the substantia nigra: association with increased iron content and marker for susceptibility to nigrostriatal injury. Arch Neurol 2002;59:999–1005. [DOI] [PubMed] [Google Scholar]
- [28].Ressner P, Skoloudik D, Hlustik P, et al. Hyperechogenicity of the substantia nigra in Parkinson's disease. J Neuroimaging 2007;17:164–7. [DOI] [PubMed] [Google Scholar]
- [29].Berg D, Siefker C, Ruprecht-Dorfler P, et al. Relationship of substantia nigra echogenicity and motor function in elderly subjects. Neurology 2001;56:13–7. [DOI] [PubMed] [Google Scholar]
- [30].Behnke S, Runkel A, Kassar HA, et al. Long-term course of substantia nigra hyperechogenicity in Parkinson's disease. Mov Disord 2013;28:455–9. [DOI] [PubMed] [Google Scholar]
- [31].Deuschl G, Petersen I, Lorenz D, et al. Tremor in the elderly: essential and aging-related tremor. Mov Disord 2015;30:1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].de Rijk MC, Breteler MM, Graveland GA, et al. Prevalence of Parkinson's disease in the elderly: the Rotterdam study. Neurology 1995;45:2143–6. [DOI] [PubMed] [Google Scholar]
- [33].Algarni M, Fasano A. The overlap between essential tremor and Parkinson disease. Parkinsonism Relat Disord 2018;46: Suppl 1: S101–4. [DOI] [PubMed] [Google Scholar]
- [34].Jankovic J, Rajput AH, McDermott MP, et al. The evolution of diagnosis in early Parkinson disease. Parkinson Study Group. Arch Neurol 2000;57:369–72. [DOI] [PubMed] [Google Scholar]
- [35].Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 1991;114(Pt 5):2283–301. [DOI] [PubMed] [Google Scholar]
- [37].Tarakad A, Jankovic J. Diagnosis and management of Parkinson's disease. Semin Neurol 2017;37:118–26. [DOI] [PubMed] [Google Scholar]
- [38].Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun 2008;29:193–207. [DOI] [PubMed] [Google Scholar]
- [39].Schrag A, Good CD, Miszkiel K, et al. Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology 2000;54:697–702. [DOI] [PubMed] [Google Scholar]
- [40].Walter U, Behnke S, Eyding J, et al. Transcranial brain parenchyma sonography in movement disorders: state of the art. Ultrasound Med Biol 2007;33:15–25. [DOI] [PubMed] [Google Scholar]
- [41].Tsai CF, Wu RM, Huang YW, et al. Transcranial color-coded sonography helps differentiation between idiopathic Parkinson's disease and vascular parkinsonism. J Neurol 2007;254:501–7. [DOI] [PubMed] [Google Scholar]


