Abstract
To describe the epidemiological, clinical, laboratory, and radiological features and the management of adult patients who experienced a relapse between 2003 and 2015 of an acute hematogenous osteomyelitis acquired in childhood.
A retrospective multicentric cohort study was conducted in 5 centers in France.
Thirty-seven patients were included. The median age was 40 years (28–56), and 26 (70%) were male. The first site of infection was the distal femur (n = 23, 62%). The median time between the osteomyelitis in childhood and the relapse in adulthood was 26 years (13–45). Thirty-four (92%) patients reported inflammatory local clinical manifestations, 17 (46%) draining fistula, 10 (27%) fever. Most patients had intramedullary gadolinium deposition (with or without abscess) on magnetic resonance imaging. Most relapses were monomicrobial infections (82%). Staphylococcus aureus was the most commonly found microorganism (82%), expressing a small colony variant phenotype in 3 cases. Most patients (97%) had a surgical treatment, and the median duration of antibiotics for the relapse was 12 weeks. All patients had a favorable outcome, no patient died and no further relapse occurred. We count 2 femoral fractures on osteotomy site.
Osteomyelitis in childhood can relapse later in adulthood, especially in patients with lack of care during the initial episode. Osteotomy and prolonged antimicrobial therapy are required for clinical remission.
Keywords: adulthood, hematogenous osteomyelitis, relapse, Staphylococcus aureus
1. Introduction
Acute hematogenous osteomyelitis (AHO) is the most common type of bone infection in childhood. In high-income countries, AHO occurs in about 8 of 100,000 children per year.[1]
Clinical manifestations include fever, focal tenderness, limping or an inability to walk, sometimes visible redness and swelling around a long bone, more often in lower limbs. The patient's condition often deteriorates in the days preceding clinical presentation.[1,2,3] The treatment of AHO demands appropriate antimicrobial therapy in all cases and may require surgical incision and drainage. Extensive surgery is rarely needed, except if the patient is infected with a hypervirulent Staphylococcus aureus strain that produce a particular toxin, the Panton–Valentine leukocidin (PVL). The outcome of AHO in a child will usually be good, if the patient seeks treatment early and antibiotic therapy is started promptly.[4,5]
To our knowledge, few cases of relapse in adulthood are described in the literature, except in patients with sickle cell disease. S aureus reactivation osteomyelitis occurring many decades after the initial infection has been episodically reported.[6,7] One case of chronic relapsing Salmonella osteomyelitis was described in an immunocompetent patient with an infection in the same site 12 years later.[8] In chronic bone and joint infections, S aureus could express a particular phenotype called small colony variant (SCV), corresponding to pin-point colonies on agar in culture, that are associated with a capacity of intracellular persistence. The expression of this phenotype has been largely described in patients with chronic osteomyelitis.[9,10]
Accurate data about osteomyelitis that occurred in childhood and that relapsed during adulthood are not available in the literature. In this context, the present study aimed to describe the epidemiological, clinical, laboratory, and radiological features and the management of adult patients who experienced a relapse of a potential AHO acquired in childhood. We will focus also on the analysis of sub-populations: osteomyelitis caused by S aureus producing PVL and S aureus expressing a SCV phenotype.
2. Patients and methods
2.1. Study design and setting
This retrospective cohort study was conducted in 5 French centers including 4 regional reference centers for the management of complex Bone and Joint Infections (also called “Centre régional de Référence des Infections Ostéo–Articulaires complexes” [CRIOAc]). This study was approved by the “comité consultatif sur le traitement de l’information en matière de recherche dans le domaine de la santé” (No 15.1010).
2.2. Inclusion and exclusion criteria
Adult patients (≥18 years) who declared having developed an osteomyelitis in childhood and who was managed for a relapse on the same site between 2003 and 2015. Diagnosis of the osteomyelitis relapse was based on the multidisciplinary assessment in hospitals belonging to the CRIOAc network in France, taking into account clinical and physical examination, microbiology, histology, and radiographic examinations. For each selected cases, the multidisciplinary evaluation had to conclude that it was highly probable that the current osteomyelitis was a relapse of a childhood osteomyelitis.
2.3. Data collection
We selected patients from the hospital's electronic medical records. Index term “hematogenous osteomyelitis” and the time limit from January 1, 2003 to September 30, 2015 were set for the search criteria. The records initially retrieved were reviewed for eligibility assessment.
Data were retrospectively collected from medical records using a predefined protocol, which included demographic characteristics, clinical presentation, site of infection, radiological and microbiology data, management and outcome of such recurrence. Personal information was anonymized, as required by the “Commission Nationale de l’informatique et des Libertés” (DR-2016-207).
2.4. Statistical analysis
Categorical variables were described in numbers and percentages. Continuous variables were expressed as a median with interquartile 25–75 range (IQR). Fisher exact test and the Chi–squared test were used to compare categorical variables. Mann–Whitney test or Student t test was used to analyze continuous variables. P-values less than .05 were considered statistically significant.
3. Results
3.1. Patient demographics and characteristics
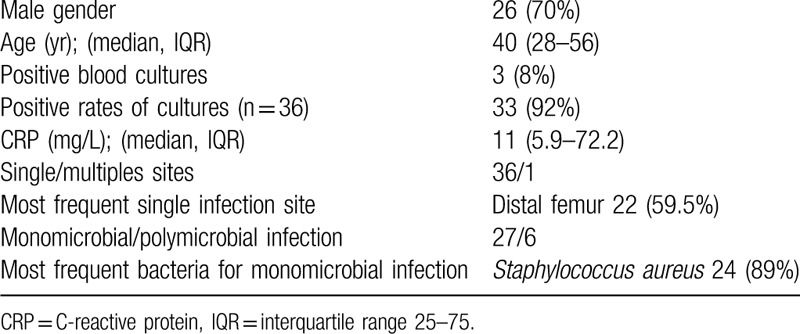
A total of 37 patients with potentially relapsing osteomyelitis were included during the study period. The median age was 40 years (IQR: 28–56 years), and 26 (70%) were male (Table 1). All patients were immunocompetent. Of the 37 cases, 13 were born in Europe (35%), 12 in tropical Africa, 7 in North Africa, and 3 in minor Asia. Two patients had sickle cell disease. The median time between the AHO in childhood and the potential relapse in adulthood was 26 years (IQR: 13–45). Eleven patients presented other potential relapses before the episode studied. They presented inflammatory local manifestations and/or fever for which they received antibiotic treatment.
Table 1.
Characteristics of hematogenous osteomyelitis relapsing in adulthood (n = 37).
3.2. Diagnosis and clinical presentations
Diagnosis and clinical presentations are detailed in the Figure 1. The most frequent site of infection was the femur (n = 23, 62.2%). One patient had bifocal disease (left femur and right distal tibia). At the diagnosis of relapse, the symptoms developed insidiously, but all patients remained free of symptoms during a median of 20 years (IQR: 9–35). Ten patients (27%) were febrile at diagnosis. Three patients were diagnosed with severe sepsis and 2 (5%) were hospitalized in intensive care. Thirty-four patients (92%) had inflammatory local clinical manifestations (tenderness, redness, and swelling), with draining fistula in 17 (46%) and soft tissue defect in 4 of them. Median C-reactive protein (CRP) was 11 mg/L (IQR: 5.9–72.2).
Figure 1.
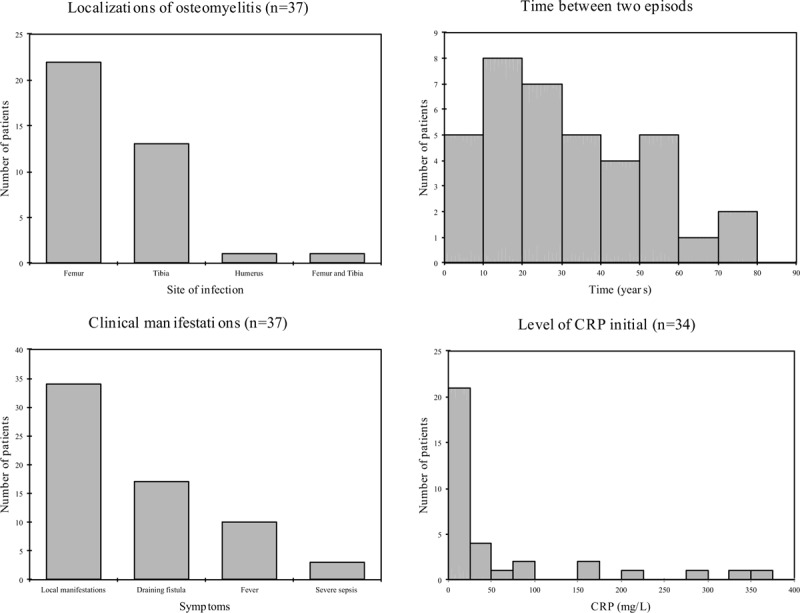
Localizations; time between the osteomyelitis in childhood and the relapse in adulthood; clinical manifestations and level of CRP. CRP = C-reactive protein.
3.3. Radiological data
On radiography (n = 33), the presence of thickening of the cortical bone (17 patients, 52%), periosteal reaction (12 patients, 36%) was the most often noted. No X-ray was normal. Magnetic resonance imaging (MRI) was performed in 30 patients. It showed signs of osteomyelitis in 27 cases (90%) and revealed an intramedullary contrast in 24 patients (80%) and/or intraosseous collection in 11 patients (37%). Typically, Figure 2 showed an intramedullary abscess of a 20-year-old patient having a relapse of right femoral osteomyelitis.
Figure 2.
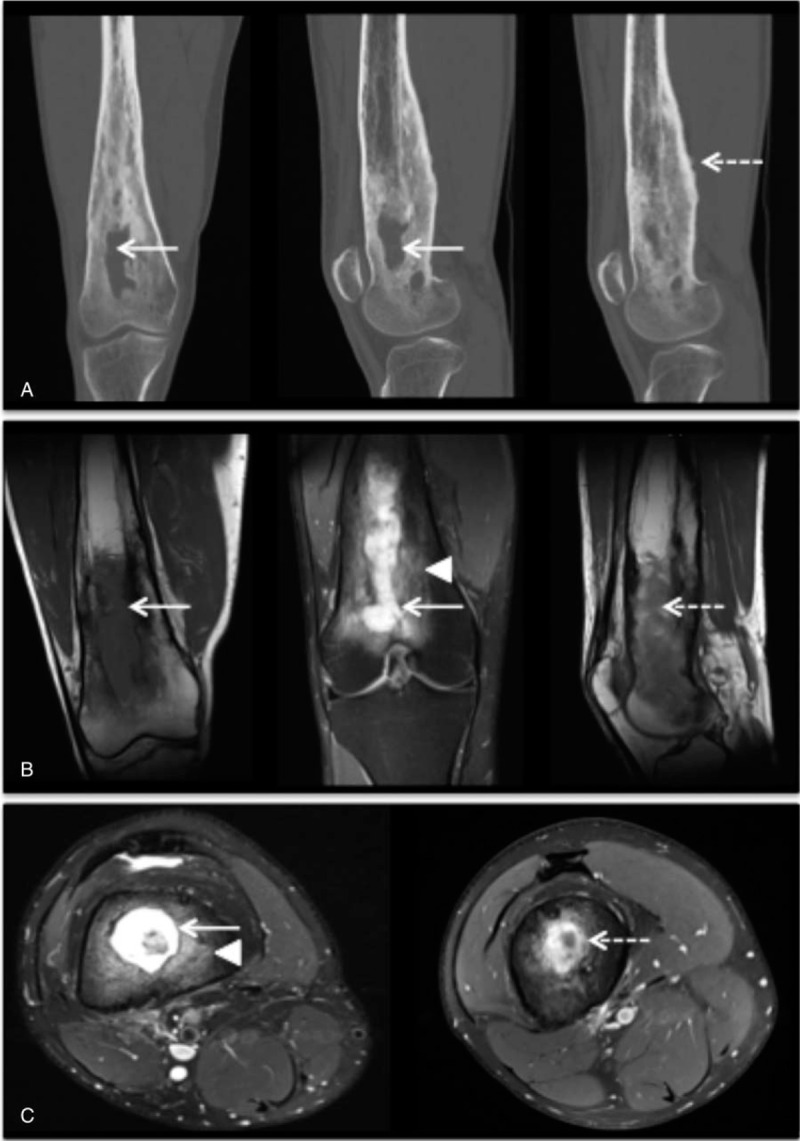
Osteomyelitis with intraosseous abcess. (A) Tomodensitometry of the right thigh, note a medullary defect (arrow), thickening of the cortex and periosteal reaction (dashed arrow). (B) Magnetic resonance imaging of the right thigh (from left to right), note a low-signal medullary image in T1-weighted sequence (arrow); hyperintensity medullary image (arrow) and bone marrow oedema (arrowhead) in T2-weighted sequence with fat suppression; and in T1-weighted post contrast image the heterogen enhancement associated with the intraosseous abscess (dashed arrow). (C) Magnetic resonance imaging of the right thigh (from left to right), T2-weighted fat suppressed image shows intraosseous abscess cavity (arrow) with rim of surrounding oedema (arrowhead); T1-weighted post contrast image with fat suppression shows peripherical enhancement associated with the intraosseous abscess (dashed arrow).
3.4. Surgical treatment
Thirty-six (97%) of patients had a surgical treatment. Among them, osteotomy was always performed, and the drainage of intraosseous abscess was done in 24 patients (68%). Four patients (11%) had repeated surgeries during the same hospital stay. In these, a first emergency surgery had been performed for evacuation of abscess or arthritis and then a second surgery with osteotomy was performed.
3.5. Microbiology
The positive rate of culture was in 33 patients (92%). Most of documented infections were monomicrobial infection (27 patients, 82%). The main bacteria involved were S aureus (n = 27, 82%), including 89% (n = 24) responsible for monomicrobial infection. Methicillin-resistant S aureus was found in 2 infections, 1 was associated with methicillin-sensitive S aureus (MSSA). Expression of SCV was found in 11% among S aureus isolates. PVL genes were detected in 2 cases among S aureus isolates. We gave further details on 2 of these patients, 1 infected with PVL S aureus and the other witch SCV. Other etiological agents include, 9% coagulase negative staphylococci isolates, 6% Streptococcus isolates, and 3% Salmonella. Enterobacteriacecae were isolated in 2 patients with polymicrobial infections. In 3 patients, no pathogenic microorganism had been isolated.
3.6. Treatment and outcome
All patients received intravenous antibiotics during their initial treatment for a period of 2 to 6 weeks (median of 6 weeks, IQR: 6–6). The median duration of antibiotics was 12 weeks. In monomicrobial MSSA osteomyelitis, intravenous antibiotics the most frequently prescribed were first-generation cephalosporins (13/24, [54%]) and anti-staphylococcal penicillin (6/24, [25%]) and oral antibiotics were rifampicin (19/24, [79%]), especially in association with a fluoroquinolone (11/24, [46%]) or clindamycin (7/24, [29%]). Five patients did not received rifampicin but clindamycin in association with a fluoroquinolone (2/5), or fusidic acid (2/5), or cycline (1/5). After a median follow-up of 24 months (IQR: 7.5–53.5; minimum 4, maximum 144), all patients had a favorable outcome with antibiotic treatment and surgery: no patient died and no further relapse occurred. We count 2 femoral fractures on osteotomy site. Two patients were lost to follow-up. To our knowledge, to date, there is no further relapse.
3.7. Childhood AHO
Concerning the management of childhood AHO, 17 patients remembered that they received antibiotics, 10 patients said they had not received antibiotics. Ten patients did not know if they had received medical treatment. And 24 (70%) had reached the operating room. Twelve have not received surgical treatment. Of note, 19% of our patients reported to have not been treated during the childhood, 35% of patients were managed with both antibiotic therapy and surgery.
3.8. Osteomyelitis caused by S aureus producing PVL
Case report no 1: A 69-year-old man, native of Portugal, presented with pain in both knees, limping, inability to walk and severe sepsis. He had a history of infection of the left femur 59 years ago; he was treated by surgery only. Laboratory studies showed leukocytosis (white cell count 18.4 × 109/L) and serum CRP level was clearly increased at 343 mg/L (normal level, <5). X-ray revealed the presence of periosteal reaction, sclerotic areas of the right distal femur (Fig. 3A). The MRI revealed necrotic right femoral osteomyelitis complicated by soft tissue abscess and arthritis; at left it revealed chronic diaphyseal osteomyelitis (Fig. 3B and C). Blood culture and articular punction identified MSSA. Transthoracic and transoesophageal echocardiography eliminated an infective endocarditis. He was hospitalized in intensive care and intravenous cloxacillin and gentamicin were administered. The first emergency surgery was performed for the right arthrotomy, intra-articular lavage, and curettage of the right femur. Then, 15 days later a new surgery left femorotomy with curettage, excision of fistula and drainage of abscess in the right thigh. Microbiology culture results showed MSSA PVL. He received an intravenous cloxacillin, clindamycin, and rifampicin for 6 weeks, followed by oral clindamycin and fusidic acid for 6 weeks. Three weeks after cessation of antibiotic therapy the patient had a recurrent chronic osteomyelitis of the right femur. Computed tomography showed right necrotic osteomyelitis with 2 soft tissues abscess. A new surgery was performed with excision and curettage of the right femur. All microbiological samples were sterile. Antibiotic therapy was conducted for another 12 weeks. Four years after the initial medical care, the patient has had no signs of recurrence.
Figure 3.
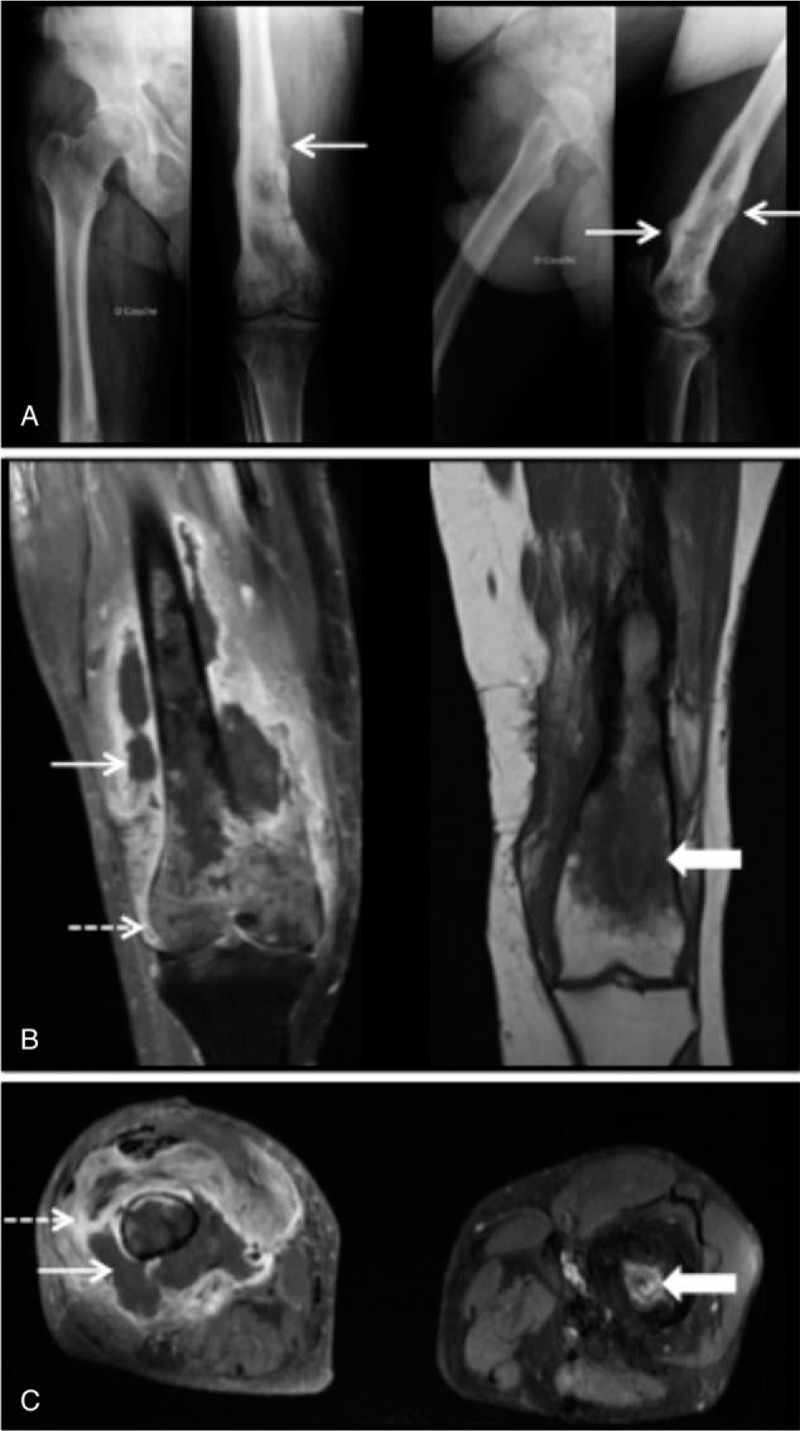
Right femoral osteomyelitis and left chronic femoral osteomyelitis. (A) Anteroposterior and lateral X-rays of the right femur, note periosteal reaction (arrow) and sclerotic areas. (B) (from left to right) Coronal magnetic resonance imaging in T1-weighted post contrast with fat suppression of the right femur, shows osteomyelitis complicated by a soft tissue abscess (arrow) and an arthritis (dashed arrow); Coronal magnetic resonance imaging, in T1-weighted post contrast of the left femur, shows chronic osteomyelitis and a marrow oedema (large arrow). (C) Axial magnetic resonance imaging of the right and the left femur, in T1-weighted post contrast with fat suppression, shows soft tissue fluid collection (arrow) and inflammation around the lesion (dashed arrow) to the right femur and intraosseous abscess to the left femur (large arrow).
In our study, fever was the major sign in patients who had presented osteomyelitis caused by MSSA PVL (Table 2). The median CRP level was higher: 319 mg/L (IQR: 308–331) versus 12 mg/L (IQR: 10–14) in other patients (Fig. 4).
Table 2.
Characteristics of osteomyelitis caused by MSSA PVL.
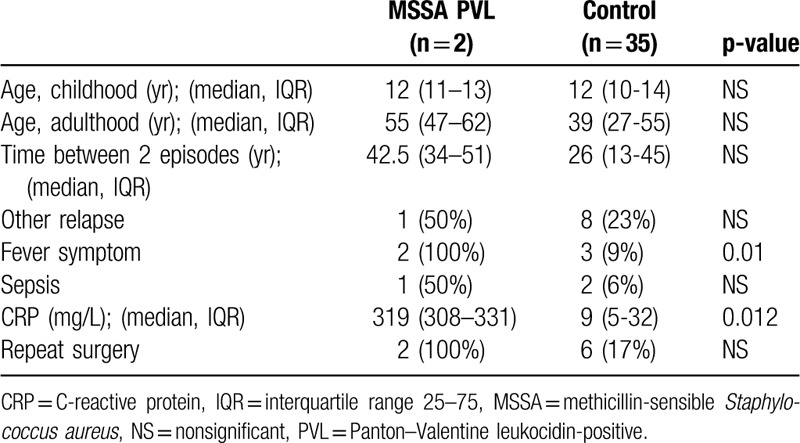
Figure 4.
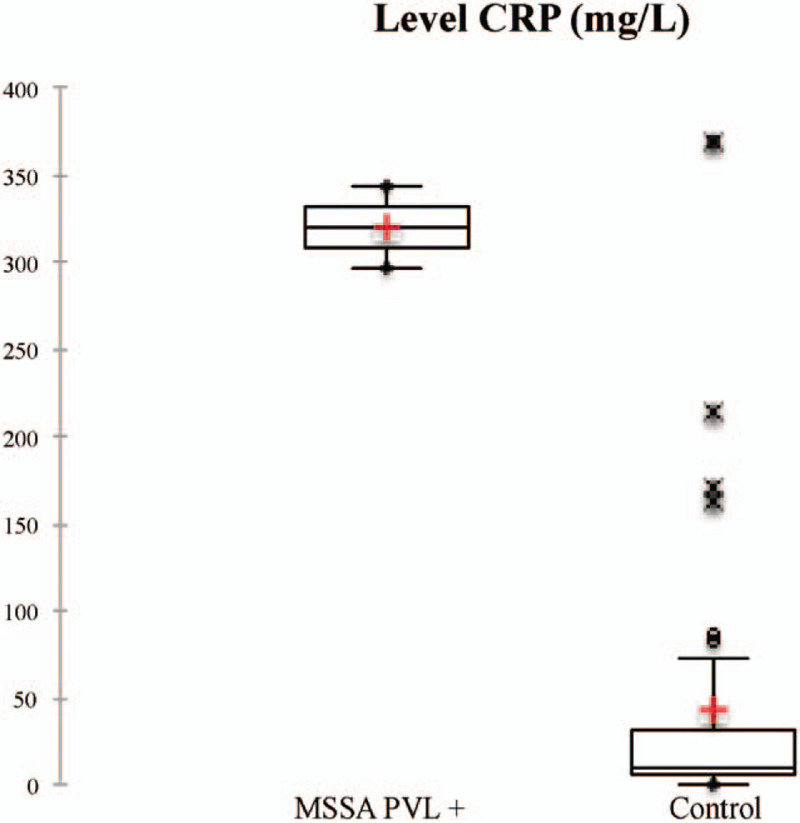
Comparison CRP level of osteomyelitis caused by MSSA PVL+ versus control. CRP = C-reactive protein, MSSA = methicillin-sensible Staphylococcus aureus, PVL + = Panton–Valentine leukocidin-positive.
3.9. Osteomyelitis caused by S aureus expression of SCV phenotype
Case report no 2: A 20-year-old man, native of Senegal, presented osteomyelitis of the left distal tibia, 11 years after a first episode of hematogenous osteomyelitis. During his childhood, he received antibiotics. X-rays and MRI showed intraosseous lesion suspicious for an intraosseous abscess (Fig. 5). A surgery with surgical debridement, cortical fenestrations, and curettage of the medullary canal was performed. Microbiology analysis revealed SCV of S aureus. Four samples were positive with different morphotypes. All isolates were resistant to penicillin G, 1 isolate was intermediate to cotrimoxazole and another intermediate to erythromycin. He received an intravenous cloxacillin therapy for 2 weeks, followed by oral rifampicin and ofloxacin for 10 weeks more. One year after the medical cure, the patient has had no signs of recurrence and only had a pain on palpation of the tibial crest.
Figure 5.
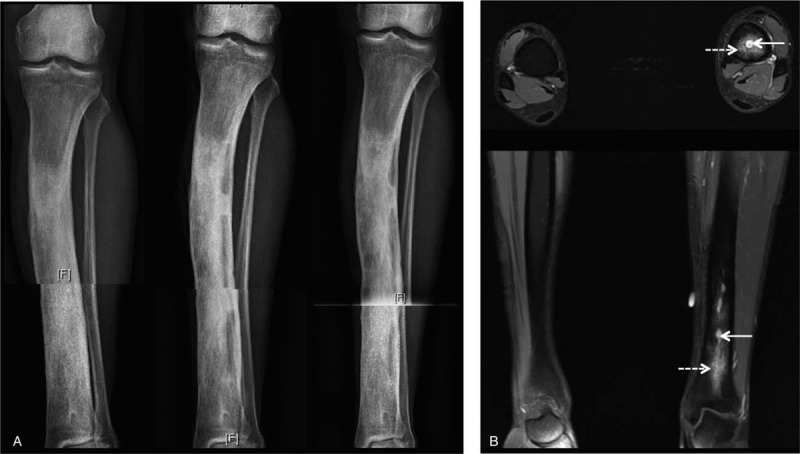
Left distal tibia osteomyelitis. (A) Radiographic features (from left to right): initial X-ray, after surgical debridement and after 3 mo. (B) Axial and coronal magnetic resonance imaging, T1-weighted post contrast with fat suppression, note an intraosseous abscess (arrow) and marrow oedema (dashed arrow).
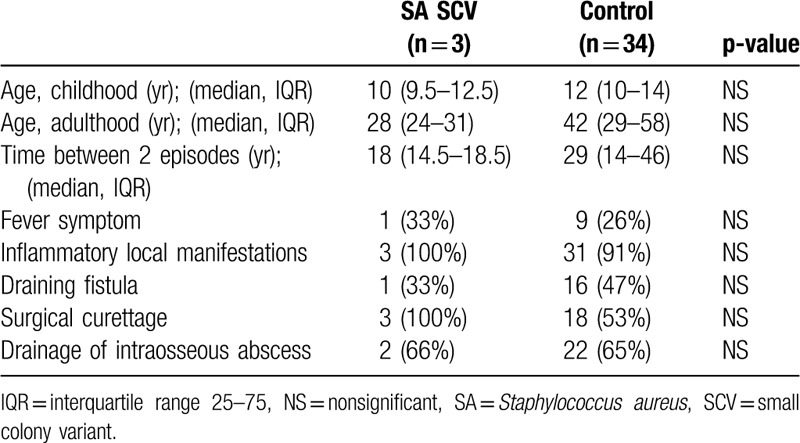
In our study, the characteristics of the 3 cases of osteomyelitis caused by S aureus SCV showed no difference with osteomyelitis due to other microorganism (Table 3). All patients with osteomyelitis caused by S aureus SCV had received rifampicin therapy. No patient relapsed.
Table 3.
Characteristics of osteomyelitis caused by small colony variants Staphylococcus aureus.
4. Discussion
The present study found that AHO in childhood might relapse after symptom-free periods lasting over 20 years. We report 37 cases in immunocompetent population, and this is the larger population in the literature. Inflammatory local signs with focal tenderness, redness, and swelling were the most common manifestations. Fever was infrequent and described by less than one-third of patients. Clinical outcome of most patients was favorable. All patients received antibiotics during 3 months. Ninety-five percent of patients had surgical treatment with osteotomy.
Our results underlined that AHO occurred predominantly in males and mainly affected the lower limbs with femur and tibia. Clinical presentation of relapse AHO was unspecific. We usually observed local symptoms or signs and most patients showed no fever. Three patients had neither fever, no inflammatory local signs, except a draining fistula. Three patients presented severe sepsis: 2 presented a soft tissue infection or arthritis and the last had a Staphylococcus epidermidis bacteremia.
Most of infections were monomicrobial. S aureus was the first pathogenic organism detected.[1,4,5,11] In our study, only 2 patients had sickle cell anemia, and one of them developed Salmonella osteomyelitis. Among the 6 cases of plurimicrobial infection, 1 had caused by MSSA and methicillin-resistant S aureus, 3 by coagulase negative staphylococci and 2 by enterobacteria. In the last 5 cases, there was a draining fistula and 1 patient had multiple recurrences and surgeries.
We found that S aureus was the first pathogen detected, usually methicillin sensitive. Two patients had infection due to S aureus PVL-producer. In bone and joint infections, it is considered one of the important virulence factors of S aureus responsible for destruction of white blood cells, necrosis and apoptosis and severe local damage, including bone distortion and abscesses, and is also associated with severe sepsis-related mortality.[12,13,14] In this series, patients infected with PVL S aureus had more fever and CRP levels were higher. The expression of SCV was found in 3 cases, this phenotype did not change the course but we had only very few cases. SCV of S aureus is a cause of persistant and relapsing infections.
In our study performed in a developed country, most of them were migrant, and the management of childhood AHO was not optimal. Only half of the patients remembered having received antibiotics and 27% did not remember having any medical treatment. A total of 19% of our patients reported to have not been treated during childhood while the clinical presentation suggested an AHO in childhood. Then, many studies describe that in low-income countries, the rate of AHO is probably higher, and frequently misdiagnosed or under-treated. Patients who did not remember receiving antibiotic treatment were either under-treated and the bone infection persisted in them in a dormant stage, but the most likely is that patients did not remember receiving treatment because they were young. Moreover, patients who usually present advanced diseases often have complications and could be under-treated. Early diagnosis, multidisciplinary care with appropriate antibiotic therapy, and timely surgical intervention all increase the chance of successful outcome for these patients.[15] The surgical excision must be thorough on the cortical bone as well as on the soft parts of the bone. Three to 5 tissues samples must be performed. Imagery before the operative planning can guide the surgical procedure in order to determine the infected area and the likely extend of debridement. The precise excision of the osteomyelitis requires a comfortable endomedullary access through corticotomy if the infected zone is not extensive, or on the contrary a bone flap, which then requires an associated osteosynthesis. In a recent series of 344 cases of osteomyelitis from Spain who included 52 cases (23%) of hematogenous osteomyelitis, authors highlight the importance of surgical management. At 12 months of follow-up, 4.7% of all cases of osteomyelitis relapsed, and patients that underwent surgery had significantly less relapses compared to those without surgery.[16] The main risk of the bone flap is postoperative fragility requiring discharge period of the limb and exposing the risk of fracture. In our study, during the follow-up, 2 patients had femoral fractures without trauma on osteotomy site when they began to walk again.
The time between both events was more than 20 years. This long and chronic course promotes bacterial biofilm formation and SCV formation; therefore, a longer antibiotic therapy is most appropriate for management of AHO relapse. Due to its strong activity against intracellular persistent bacteria rifampicin treatment may be indicated. Consequently, these elements justify a prolonged follow-up of patients after treatment to determine whether recovery was complete.
Apart from the retrospective design, the main limitation of this present study is the small number of patients, which is explained by the rarety of infection. Secondly, as the time between both episodes was longer and the initial management in childhood was mostly experienced abroad we had little information about the childhood AHO. As a consequence, the data collection on the childhood hematogenous osteomyelitis is based on oral data submitted to the patient memory and it is a potential bias. Then, the diagnosis of relapse of AHO is not certain, it is based on oral data patient and on the multidisciplinary assessment in 5 hospitals belonging to the CRIOAc network in France, taking into account clinical and physical examination, microbiology, histology, and radiographic examinations. For each cases, the multidisciplinary evaluation had to conclude that it was highly probable that the current osteomyelitis was a relapse of a childhood osteomyelitis. The duration of follow-up is quite short, considering the very long duration of these infections and the existence of very late relapses.[6,7] Because of the retrospective design of our study, no detailed data on the functional outcome of the patients were available.
5. Conclusions
In summary, osteomyelitis in childhood can relapse later in adulthood, especially in patients with lack of care during the initial episode. At relapse, S aureus was the first pathogenic organism detected, sometimes producing PVL or sometimes expressing the SCV phenotype. Surgical excision and prolonged antimicrobial therapy are required to cure the relapse. These patients are at risk to fracture when they resume walking.
Author contributions
Conceptualization: Axelle Clerc, Tristan Ferry.
Data curation: Axelle Clerc.
Formal analysis: Axelle Clerc.
Funding acquisition: Axelle Clerc.
Investigation: Axelle Clerc, Valerie Zeller, Frederic Laurent, Tristan Ferry.
Methodology: Axelle Clerc, Tristan Ferry.
Project administration: Axelle Clerc, Tristan Ferry.
Resources: Axelle Clerc, Simon Marmor, Eric Senneville, Bruno Marchou, Frederic Lucht, Nicole Desplaces, Sebastien Lustig, Tristan Ferry.
Software: Axelle Clerc.
Supervision: Christian Chidiac, Tristan Ferry.
Validation: Valerie Zeller, Simon Marmor, Frederic Laurent, Sebastien Lustig, Christian Chidiac, Tristan Ferry.
Visualization: Valerie Zeller, Simon Marmor, Eric Senneville, Bruno Marchou, Frederic Laurent, Frederic Lucht, Sebastien Lustig, Christian Chidiac, Tristan Ferry.
Writing – original draft: Axelle Clerc.
Writing – review and editing: Axelle Clerc, Valerie Zeller, Simon Marmor, Sebastien Lustig, Christian Chidiac, Tristan Ferry.
Footnotes
Abbreviations: AHO = acute hematogenous osteomyelitis, CRIOAc = Centre régional de Référence des Infections Ostéo–Articulaires complexes, CRP = C-reactive protein, MRI = magnetic resonance imaging, MSSA = methicillin-sensitive Staphylococcus aureus, PVL = Panton–Valentine leukocidin, SCV = small colony variant.
How to cite this article: Clerc A, Zeller V, Marmor S, Senneville E, Marchou B, Laurent F, Lucht F, Desplaces N, Lustig S, Chidiac C, Ferry T. Hematogenous osteomyelitis in childhood can relapse many years later into adulthood: a retrospective multicentric cohort study in France. Medicine. 2020;99:20(e19617).
The authors have no conflicts of interest to disclose.
References
- [1].Peltola H, Pääkkönen M. Acute osteomyelitis in children. N Engl J Med 2014;370:352–60. [DOI] [PubMed] [Google Scholar]
- [2].Dartnell J, Ramachandran M, Katchburian M. Haematogenous acute and subacute paediatric osteomyelitis: a systematic review of the literature. J Bone Joint Surg Br 2012;94:584–95. [DOI] [PubMed] [Google Scholar]
- [3].Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther 2010;8:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pääkkönen M, Peltola H. Antibiotic treatment for acute haematogenous osteomyelitis of childhood: moving towards shorter courses and oral administration. Int J Antimicrob Agents 2011;38:273–80. [DOI] [PubMed] [Google Scholar]
- [5].Ferroni A, Al Khoury H, Dana C, et al. Prospective survey of acute osteoarticular infections in a French paediatric orthopedic surgery unit. Clin Microbiol Infect 2013;19:822–8. [DOI] [PubMed] [Google Scholar]
- [6].Libraty DH, Patkar C, Torres B. Staphylococcus aureus reactivation osteomyelitis after 75 years. N Engl J Med 2012;366:481–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Al-Maiyah M, Hemmady MV, Shoaib A, et al. Recurrence of chronic osteomyelitis in a regenerated fibula after 65 years. Orthopedics 2007;30:403–4. [DOI] [PubMed] [Google Scholar]
- [8].Banky JP, Ostergaard L, Spelman D. Chronic relapsing Salmonella osteomyelitis in an immunocompetent patient: case report and literature review. J Infect 2002;44:44–7. [DOI] [PubMed] [Google Scholar]
- [9].Sendi P, Rohrbach M, Graber P, et al. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin Infect Dis 2006;43:961–7. [DOI] [PubMed] [Google Scholar]
- [10].Rit K. A case report of Small Colony variant of Staphylococcus aureus isolated from a patient with chronic oesteomyelitis in a tertiary care hospital of eastern India. Adv Biomed Res 2014;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Grammatico-Guillon L, Maakaroun Vermesse Z, Baron S, et al. Paediatric bone and joint infections are more common in boys and toddlers: a national epidemiology study. Acta Paediatr 2013;102:120–5. [DOI] [PubMed] [Google Scholar]
- [12].Dohin B, Gillet Y, Kohler R, et al. Pediatric bone and joint infections caused by Panton-Valentine leukocidin-positive Staphylococcus aureus. Pediatr Infect Dis J 2007;26:1042–8. [DOI] [PubMed] [Google Scholar]
- [13].Moumile K, Cadilhac C, Lina G, et al. Severe osteoarticular infection associated with Panton-Valentine leukocidin-producing Staphylococcus aureus. Diagn Microbiol Infect Dis 2006;56:95–7. [DOI] [PubMed] [Google Scholar]
- [14].Bocchini CE, Hulten KG, Mason EO, et al. Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics 2006;117:433–40. [DOI] [PubMed] [Google Scholar]
- [15].Pääkkönen M, Peltola H. Acute osteomyelitis in children. N Engl J Med 2014;370:1365–6. [DOI] [PubMed] [Google Scholar]
- [16].García Del Pozo E, Collazos J, Cartón JA, et al. Bacterial osteomyelitis: microbiological, clinical, therapeutic, and evolutive characteristics of 344 episodes. Rev Esp Quimioter 2018;31:217–25. [PMC free article] [PubMed] [Google Scholar]


