Abstract
Due to antimicrobial resistance and the public health hazard of antibiotic growth promoters, there is a grave need to find potential alternatives for sustainable poultry production. Piper betle (PB) and Persicaria odorata (PO) are herbs, which have been reported for antimicrobial, antioxidant, and anti-inflammatory properties. The present study aimed to estimate the influence of different dose supplementation of Piper betle leaf meal (PBLM) and Persicaria odorata leaf meal (POLM) on growth performance, ileal digestibility and gut morphology of broilers chickens. A total of 210 one day-old broiler chicks were randomly grouped into 7 treatments, and each treatment group has 3 replicates (n = 10) with a total number of 30 chicks. The treatments included T1 control (basal diet (BD) with no supplementation), T2 (BD + 2 g/kg PBLM); T3 (BD + 4 g/kg PBLM), T4 (BD + 8 g/kg PBLM), T5 (BD + 2 g/kg POLM), T6 (BD + 4 g/kg POLM), T7 (BD + 8 g/kg POLM). Growth performance, gut morphology and ileal digestibility were measured. Except for T4 (8 g/kg PBLM), graded dose inclusion of PBLM and POLM increased (P < 0.05) the body weight gain (BWG), positively modulated the gut architecture and enhanced nutrient digestibility in both stater and finisher growth phases of broiler chickens. Birds fed on PBLM 4 g/kg (T3), and POLM 8 g/kg (T7) had significantly higher (P < 0.05) BWG with superior (P < 0.05) feed efficiency in the overall growth period. Chickens fed on diets T3 and T7 had longer (P < 0.05) villi for duodenum as well as for jejunum. Furthermore, the birds fed on supplementations T3 and T7 showed improved (P < 0.05) digestibility of ether extract (EE), and dry matter (DM) compared to the control group. However, least (P < 0.05) crude protein (CP) digestibility was recorded for T4. In conclusion, dietary supplementations of PBLM 4 g/kg and POLM 8 g/kg were positively modulated the intestinal microarchitecture with enhanced nutrient digestibility, resulted in maximum body weight gain, thus improved the growth performance of broiler chickens.
Keywords: Broiler chickens, Growth performance, Gut morphology, Persicaria odorata, Piper betle
1. Introduction
For decades, the sub-therapeutic inclusion of antimicrobial growth promoters (AGPs) has been successfully and routinely practised in broiler chicken’s production. The AGPs increase the growth performance of birds by improving their intestinal health, enhancing nutrient absorption and utilisation. The inclusion of AGPs as a feed additive is the most common and economical way to improve poultry performance (Debnath et al., 2014). However, the use of AGPs has resulted in antimicrobial resistance and accumulation of residues in edible tissues, that may increase risks to human health (Cosby et al., 2015). This danger of antibiotic resistance has led to the ban against sub-therapeutic use of AGPs in food-producing animals (Toghyani et al., 2011). The threat of antibiotic-resistant pathogens leads to a growing dynamic in research and development of future alternative growth promoters for animal production (Al-Abd et al., 2015). Many alternatives to AGPs are introduced, while recently phytogenic feed additives (PFAs) gained consideration as a potential alternative in poultry production (Murugesan et al., 2015).
Natural substances like plants, herbs, plant extracts, essential oils, and spices, are referred to as PFAs or phytobiotics. Recently the phytobiotics got attention as potential feed additives in poultry production (Abudabos et al., 2016). The PFAs have numerous positive effects on poultry nutrition, which includes better feed intake, higher digestibility, and increased plasma total antioxidant capacity. They are potent antimicrobial, coccidiostats, anthelmintic, and immune-stimulating substances (Manafi, 2015). Supplementation of PFAs increases the apparent total tract nutrient digestibility (Murugesan et al., 2015) as well as improvement in the gut morphology of broilers (Ahsan et al., 2018), with a potential to favour the over-all health of broiler chickens (Paraskeuas et al., 2017). Various studies showed that PFAs might increase feed efficacy in broilers, as PFAs or phytobiotics, thinning the mucosal surface of gut resulted in increased diffusion of nutrients from the apical surface of epithelial cells, similar to AGPs (Yang et al., 2015). Many researchers are investigating phytogenic plants, including natural herbs, in terms of their nutraceutical, antimicrobial, and nutritive potential. Among such herbs of importance are Piper betle and Persicaria odorata.
The Piper betle belongs to the family Piperaceae, an essential herbal plant having significant medicinal value and variety of pharmacological activities. It is distributed throughout East Africa and the tropical regions of Asia and commonly known as “daun sirih”. The Piper betle leaves possess many valuable bioactivities and have been used in traditional medicinal systems (Patra et al., 2016). The Piper betle has been reported as potent antimicrobial (Syahidah et al., 2017), antioxidant (Jaiswal et al., 2014) and anthelmintic (Shah et al., 2016) herb. Previous phytochemical investigations reported the presence of high phenolic compounds in Piper betle; however, the reported significant bioactive compounds were hydroxychavicol and eugenol, and these bioactive compounds have been studied and reported as potent antimicrobial agents (Atiya et al., 2018, Syahidah et al., 2017).
The Persicaria odorata belongs to the family Polygonaceae, and it is also called as Polygonum minus Huds. The Persicaria odorata routinely used in Southeast Asian cuisine as a natural and traditional herb, and it is commonly named as “daun kesum” or “daun laksa” (Vikram et al., 2014). Previous studies shown that Persicaria odorata is a potent antioxidant (Cristapher et al., 2016), contains flavonoids and a high concentration of essential oils (Narasimhulu et al., 2014). Antiviral, antifungal, and antimicrobial activities of Persicaria odorata has also been reported (Vikram et al., 2014, Christapher et al., 2015). The said major bioactive compounds of Persicaria odorata include quercetin and myricetin. In poultry, quercetin has shown antiviral and antioxidant effects, additionally have a potential role in counteracting heavy metal toxicity (Saeed et al., 2017).
Very few studies are reported previously on the dietary inclusion of herbs in broiler chickens which contains tannin, as earlier it was assumed that tannin has anti-nutritional effects in monogastric animals. However, from the last few years, various studies revealed that the dietary supplementation of several tannin sources in low concentrations resulted in improved health status, thus enhanced the growth performance by positively modulating the gut and its eco-system in mono-gastric animals (Brus et al., 2013, Starčević et al., 2015). The Piper betle and Persicaria odorata have not been investigated in broiler chickens (to the best of our knowledge) for their effects on growth performance measures, gut health, and nutrient digestibility. Taking into account the medicinal potential of Piper betle and Persicaria odorata, this study has been anticipated to evaluate the influence of gradual level inclusion of PBLM and POLM on growth performance, gut morphology, and apparent ileal digestibility in broiler chickens.
2. Materials and methods
2.1. Ethical approval
Institutional Animal Care and Use Committee, Universiti Putra Malaysia approved the animal handling procedures for this experiment (UPM/IACUC/AUP-R033/2018).
2.2. Source and preparation method for diet
Fresh samples of Piper betle (PB) and Persicaria odorata (PO) were collected from herbarium garden, Universiti Putra Malaysia. The plant leaves were validated from Unit Biodiversity, Institute of Bio-Sciences, University of Putra Malaysia. Fresh sample (leaves) of both herbs were deposited with voucher no. SK3294/18 and SK3296/18 respectively for future reference. The collected leaves were subjected to dry at 50 °C for 72 h and pulverised to a fine powder. The prepared plant samples (fine powder) stored at 4 °C until further usage.
2.3. Management of birds
Two hundred and ten one day-old broiler chicks (Cobb 500) were procured from a local hatchery and individually wing-banded upon arrival. After being weighed, the chicks were randomly assigned into seven treatment groups; each treatment group consisted of 3 replicates of 10 chicks (n = 30). The chickens were raised into battery cages in an open-sided house, with free access to water and feed throughout the trial period. The cyclic temprature of the house were (maximum 34°C; minimum 24) and humidaty (maximum 91%; minimum 61%). Chicks were vaccinated with the following schedule:
-
i)
ND and IB (Newcastle disease and Infectious bronchitis) at the age of day 4 and day 21, administered via intra-ocular route.
-
ii)
IBD (Infectious bursal disease) at the age of day 7, administered via intra-ocular route.
2.4. Experimental design and supplemented treatments
The birds were raised on the starter diet during 0 to 21 days and finisher diet during 22–42 days. Treatments were: T1 (basal diet (BD) with no supplementation) control, T2 (BD + 2 g/kg PBLM); T3 (BD + 4 g/kg PBLM), T4 (BD + 8 g/kg PBLM), T5 (BD + 2 g/kg POLM), T6 (BD + 4 g/kg POLM), T7(BD + 8 g/kg POLM). The supplemented experimental diets meet or exceed the NRC (1994) recommendations, presented in (Table 1). Moreover, the basal diet was without any premixing of anticoccidial, antimicrobial, and antioxidant drugs or feed enzymes.
Table 1.
Ingredient (%age as feed) and nutrient composition of the basal diet.
| Ingredients (%) | Starter | Finisher |
|---|---|---|
| Corn | 54.40 | 58.95 |
| Soybean meal (SBM) (44%) | 33.90 | 28.00 |
| Fish Meal | 5.33 | 5.74 |
| Palm oil | 2.64 | 4.14 |
| Limestone | 1.06 | 0.86 |
| Salt | 0.38 | 0.28 |
| Dicalcium Phosphate | 1.09 | 0.84 |
| Mineral Mix* | 0.28 | 0.28 |
| Vitamin Mix** | 0.29 | 0.29 |
| Choline chloride | 0.10 | 0.10 |
| L-Lysine | 0.35 | 0.34 |
| DL-Methionine | 0.18 | 0.18 |
| Calculated analysis | ||
| (ME) MJ/kg | 13.10 | 13.40 |
| Crude Fat, % | 5.21 | 7.01 |
| Crude Protein, % | 22.00 | 20.00 |
| Calcium, % | 0.99 | 0.90 |
| Available P, % | 0.43 | 0.35 |
Premix supplied minerals per kg of dietary feed: Cu, 19.99 (mg); Zinc, 100.01 (mg); Mg, 16.0 (mg); iron, 120.0 (mg); I, 0.8 (mg); Co, 0.6 (mg).
Premix supplied vitamins per kg of dietary feed: Vitamin E, 0.02 (mg); Vitamin D3; 1200 (IU); Vitamin A, 6500 (IU); VitaminB1, 0.83(mg); riboflavin, 2.0(mg); Vitamin K (menadione) 1.33(mg); Vitamin B12, 0.03(mg); Vitamin B3 24 (mg); Vitamin B6, 1.37 (mg); Biotin, 0.03 (mg); Calcium D-Panthothenate, 3.69 (mg); Folic acid, 0.33 (mg).
2.5. Sample collection
Every week during the whole experiment, the live body weight (BW) and feed intake (FI) were recorded for each bird to measure growth performance. Furthermore, body weight gain (BWG) and feed conversion ratio (FCR) were calculated. On day 21 six chickens (n = 2 per replicate) and day 42 twelve chickens (n = 4 per replicate) were selected randomly, and slaughtered according to the procedure as described in “The Malaysian Standard (MS) 1500: 2009”. The intestinal samples were obtained to determine the gut morphology (villi height and crypt depth), while ileal digesta samples were collected to measure apparent ileal digestibility as described by (Alshelmani et al., 2016).
2.6. Gut morphology
The intestinal morphology was studied in line with the procedure described by (Alshelmani et al., 2016). About 5–6 cm long fragments of ileum, jejunum and duodenum were collected. The ileum segments were harvested (midway between the Meckel’s diverticulum to the anterior part of the ileocaecal junction). While the sections from the jejunum were collected (midway between the distal portion of the duodenal loop and Meckel's diverticulum), and duodenum segments were collected (pylorus to the distal part of the duodenal loop). A physiological solution was used for flushing intestinal fragments, and a 10% buffer formalin solution was used to fix the sample. The intestinal pieces were cut approximately up to 3.5 mm and then subjected to process, embedding with paraffin and stained using “hematoxylin and eosin” staining technique. The twelve villi and crypt unit were used for all observations. Furthermore, the morphometric parameter of each segment of intestine, including villus height (VH) and villus crypt depth (CD) were viewed under a light microscope installed with a digital camera (Leica DFC 295, Wetzlar, Germany). The measurement of villus height was performed from the villus tip to villus crypt junction, while the depth of invagination between two villi consider as crypt depth. For the measurement of villus height and crypt depth, the Image-Pro Plus software was used as described by Touchette, et al. (2002).
2.7. Nutrient digestibility
To determine the apparent ileal digestibility (AID), an indigestible marker “Titanium dioxide (TiO2)” was added in each treatment group at the dose rate of 3 g / kg of feed. The Marker (TiO2) was added in diets of broiler chickens on day18 to day 21 during the starter phase and on day 39 to day 42 of age during the finisher phase as described by (Alshelmani et al., 2017). After slaughtering the ileal digesta samples were collected (lower half of ileum) and kept at −80 °C until lab analysis. For digestibility estimation, digesta samples were freeze-dried, then dried in an oven at 80 °C to get constant weight, and finally homogenised. For determination of the dry matter (DM), ether extract (EE), crude protein (CP), and ash contents of digesta and feed; the method “proximate analysis of nutrients” was used as described by (AOAC, 1995). For determination of “TiO2” concentration of digesta and feed, the homogenised ileal digesta samples were ashed, then digested using sulphuric acid (7.4 M) and subjected to react with “H2O2”, and the absorbance was measured at 410 nm using a spectrophotometer (short et al., 1996).
2.8. Statistical analysis
The data were examined for their conformance to the normal distribution prior to analysis using the Kolmogorov-Smirnov test. The data were analysed as a 3 × 2 fractional arrangement with 3 levels of PBLM and POLM. The data were subjected to ANOVA using the general linear model, and the analysis was carried out with SAS (9.4) 2012 (SAS Institute Inc., Cary, NC, USA). The differences between the means of treatments were compared using the LSD, while the overall significance level was set at a value of P < 0.05.
3. Results and discussion
3.1. Growth performance
The influence of graded dose inclusion of PBLM and POLM on the growth performance measures of broiler chickens are presented in (Table 2). The main effects data on day 21 and day 42 showed that BWG, FI and FCR were not influenced with increased concentration of supplements dose. However, a significant interaction was observed for BWG (P < 0.05), indicating higher BWG with increased supplements dose. On day 21 increased (P < 0.05) body weight gain (BWG) was recorded in the chickens fed on supplemented diets T3 690.50 g and T7 688.10 g compared to the broiler chickens raised on other supplemented diet T4 and control diet. In the starter phase, dietary supplementation did not influence the FI in all experimental broilers, except those chickens, that fed on supplemented diet T4, thus resulted in significantly (P < 0.05) low FI. Also, superior feed efficiency was recorded in broilers fed on diets T3 and T7, with significantly reduced (P < 0.05) FCR 1.49 and 1.51, respectively, relative to other treatment groups and the control group.
Table 2.
Effects of different dose supplementation of PBLM and POLM on the growth parameter of broilers.
| 1–21 days |
21–42 days |
1–42 days |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatments | BWG (g) | Feed intake (g) | FCR | BWG (g) | Feed intake (g) | FCR | BWG (g) | Feed intake (g) | FCR |
| T1 | 638.39b | 1022.59a | 1.60a | 1639.93b | 3002.05a | 1.83a | 2277.32c | 4024.83a | 1.76 a |
| T2 | 657.82 ab | 1033.20 a | 1.57 a | 1692.46 ab | 3019.20 a | 1.78b | 2350.28b | 4052.40 a | 1.72b |
| T3 | 690.50 a | 1030.37 a | 1.49b | 1741.05 a | 3018.20 a | 1.73b | 2431.55 a | 4048.57 a | 1.67c |
| T4 | 633.04b | 981.14b | 1.55 a | 1655.21b | 2965.2b | 1.79b | 2288.85c | 3946.35b | 1.72b |
| T5 | 659.02 ab | 1035.20 a | 1.57 a | 1690.53 ab | 3026.0 a | 1.79b | 2349.49b | 4061.20 a | 1.73b |
| T6 | 670.70 ab | 1031.40 a | 1.54 a | 1694.33 ab | 3023.4 a | 1.78b | 2365.03b | 4054.80 a | 1.71b |
| T7 | 688.10 a | 1038.80a | 1.51b | 1733.10 a | 3013.8 a | 1.74b | 2421.20 a | 4052.60 a | 1.67c |
| SEM | 5.9 | 6.61 | 0.02 | 9.34 | 6.74 | 0.01 | 13.62 | 10.66 | 0.01 |
| Main effects! | |||||||||
| Supplement dose | |||||||||
| 2 g / kg | 658.42 | 1034.20 | 1.58 | 1691.50 | 3022.60 | 1.79 | 2349.91b | 4056.80 a | 1.73 |
| 4 g / kg | 680.60 | 1030.89 | 1.52 | 1717.69 | 3020.80 | 1.76 | 2398.29 a | 4051.69 a | 1.69 |
| 8 g / kg | 660.57 | 1009.97 | 1.53 | 1694.46 | 2989.50 | 1.77 | 2355.03ab | 3999.47b | 1.70 |
| Supplements | |||||||||
| P. betle | 660.45 | 1014.91 | 1.54 | 1696.44 | 3000.87 | 1.77 | 2356.90 | 4015.77 | 1.71 |
| P. odorata | 672.61 | 1035.13 | 1.54 | 1705.99 | 3021.07 | 1.77 | 2378.59 | 4056.20 | 1.71 |
| Source of variation | |||||||||
| Supplement dose | 0.251 | 0.335 | 0.396 | 0.282 | 0.107 | 0.493 | 0.067 | 0.048 | 0.136 |
| Supplements | 0.308 | 0.167 | 0.955 | 0.512 | 0.154 | 0.862 | 0.230 | 0.051 | 0.965 |
| Supplement × Supplement dose | 0.041 | 0.196 | 0.592 | 0.006 | 0.358 | 0.117 | 0.001 | 0.082 | 0.037 |
abcThe means with different superscripts within the same columns are significantly different (P < 0.05).
T1: (Basal diet (BD); control); T2:(BD + PBLM 2 g/kg); T3:(BD + PBLM 4 g/kg); T4:(BD + PBLM 8 g/kg); T5: (BD + POLM 2 g/kg); T6: (BD + POLM 4 g/kg); T7: (BD + POLM 8 g/kg)
Data were analysed as a 3 × 2 factorial arrangement, excluding control group.
The present findings were comparable with the outcomes of Aroche et al. (2018) who identified that feed supplementation of medicinal plants in the form of mix powder including; A. occidentale, P. guajava, and M. citrifolia enhanced the feed efficiency and resulted in superior FCR in starter phase. The reported results might be due to the potential of natural products containing useful bioactive compounds like eugenol and quercetin, which have the potential to improve the biological development of chickens. The secondary metabolites of herbs such as alkaloids, tannins, and flavonoids positively influence the birds' health, as they possess antimicrobial, anti-inflammatory, and antioxidant properties (Perez-Gregorio et al., 2014). Moreover, Aroche et al. (2018) reported that dietary inclusion of P. guajava and M. citrifolia leaf meal resulted in better utilisation of the nutrients with lower feed intake and high growth in the first growth phase of chickens. Further, he emphasised that P. guajava and M. citrifolia were reported antimicrobial and antioxidant (Ali et al., 2016); thus, their inclusion as a dietary supplement helps to improve the growth in chickens. The present study has shown that the BWG of broilers increased with an increasing level of POLM; a similar pattern was observed in PBLM supplemented treatments up to an inclusion level of 4 g/kg of feed. However, the further increase in PBLM (8 g/kg) resulted in significantly decreased BWG. This reduction in BWG at a higher inclusion level of PBLM might be due to high tannin concentrations. Excessive tannins may cause metabolic disturbances like precipitation of protein, that resulted in an anti-nutritional effect (Savón et al., 2006). It is assumed that the higher tannin concentrations resulted in lesser feed intake, and inhibiting the absorption of nutrients, ultimately leads to a decline in growth (Mansoori et al., 2015).
On day 42, the birds fed on diets T3 and T7 had maximum (P < 0.05) BWG relative to other supplemented birds of treatment T4 and the control group. Additionally, there was no influence (P > 0.05) of dietary inclusion of PBLM and POLM on FI; however, FI was recorded least (P < 0.05) for T4. The FCR was noted maximum (P < 0.05) in T1 1.83 in comparison with other dietary treatments, whereas no difference (P > 0.05) was recorded for FCR within supplemented groups. The increased BWG in broilers with PBLM and POLM supplementation might be due to the presence of polyphenols and flavonoids, primarily due to the secondary bioactive compound eugenol and quercetin, which were successfully quantified from PBLM and POLM, respectively (unpublished data). The secondary bioactive compound eugenol has been considered as an appetite stimulant (Kumar et al., 2014), and antioxidant (Kim et al., 2013), it can trigger the function of pancreatic and digestive enzymes (Pradhan et al., 2014). In a study, Paraskeuas et al. (2017) described the potential of eugenol, who reported that the supplementation of phytogenic feed additive including menthol, anethol and eugenol at 100 and 150 mg/kg in broiler chickens enhanced the growth performance, increased digestibility, improved serum total antioxidant capacity and reduced expression of pro-inflammatory cytokine (IL-18). On the other hand, bioactive compound quercetin is a flavone, that can improve the animal growth by up-regulating the growth hormone along with the hepatic growth hormone receptor, this stimulus increases the concentration of insulin-like growth factor-1 (Ouyang et al., 2016). Generally, the dietary supplementation of flavonoids in broiler chickens ration has potential to enhance growth performance of chickens (Starcevic, 2015). In another study, the role of a secondary bioactive compound like quercetin has been revealed by Kim et al. (2015), who narrates that the dietary inclusion of quercetin in broilers has positively improved the growth performance. The quercetin can limit the effects of oxidative stress (Ma et al., 2015), and pro-inflammatory cytokines such as TNF-α, cyclooxygenase-2 interleukin 6 and cyclooxygenase-2 (Zou et al., 2015). The highest BWG was recorded in broilers fed on diet T3 (PBLM 4 g/kg) with superior FCR of 1.73 in the finisher phase. Whereas, further increase in supplementation of PBLM at the dose of 8 mg/kg leads to decline (P < 0.05) in BWG, and least FI 2965.2 g. This decline in BWG and least FI might be due to high tannins concentration. These results endorsed the outcomes of Bee et al. (2017) who revealed that an increased level of chestnut tannins in the feed from 0.71% to 1.5%, resulted in reduced feed intake; thus, declined growth performance in chickens. Similarly, Oso et al. (2019) reported that the supplementation of phytogenic blend containing Piper betle, Cynodon dactylon, and Piper nigrum in broilers, resulted in better FCR and BWG in the treated group fed on 1% phytogenic blend compared to the broilers fed on 2% phytogenic blend. In monogastric animals especially in poultry, the positive influence of tannins depends on the balanced dose or inclusion level. Inclusion of higher tannin concentrations negatively affects the feed palatability. On the other hand, appropriate addition of tannins improves nutrient digestion through protein and enzyme complexation; also, tannins being a potent antimicrobial and antioxidant substances can positively modulate the gut ecosystem, thus enhance the growth performance (Huang et al., 2018). In terms of overall growth performance 1–42 day, the main effects data indicate that, as the dose of the supplement increased, BWG (P < 0.05) and FI (P < 0.05) increased; however, FCR was not affected with increased concentration of supplements. Furthermore, significant interaction was observed for BWG (0.000) and FCR (P < 0.05) highlighted that increased supplements‘ dose resulted in enhanced BWG with reduced FCR, indicating improved feed efficiency. The overall growth performance over 42 days shown that the supplemented diet T3 achieved the highest BWG 2431.55 g and superior feed efficiency with FCR 1.67 followed by T7 with BWG 2421.20 g that was maximum (P < 0.05) compared to other supplemented groups and recorded least (P < 0.05) in control diet T1 with BWG 2277.32 g. In general, phytobiotics treated groups showed significantly higher BWGs compared to the control group. Except for dietary group T4, supplementation did not influence the FI. The maximum (P < 0.05) FCR was noted in control group T1 1.76 relative to supplemented dietary treatments. While significantly least and better (P < 0.05) FCR was recorded for both T3 and T7. These results are parallel to Mpofu et al. (2016), who revealed that inclusion of phytobiotics like L. javanica at an inclusion level of 5 g/kg in broilers ration, has a positive impact on overall growth. In another study, Paraskeuas et al. (2017) highlighted similar results, who designated that inclusion of phytogenic feed additive including eugenol, menthol, and anethol at 100 to 150 mg/kg in feed, enhanced nutrient digestibility and thus enhanced growth measures in broiler chickens. Similarly, Salami et al. (2015) identified that the incorporation of medicinal herbs as a feed additive in the broiler’s diets improved the FCR in the last growing phase of birds.
3.2. Gut morphology
For the determination of nutrient response and gut health, the microscopic structure and status of the intestinal tract are considered good indicators (Viveros et al., 2011). The results of intestinal morphometrical parameters on day 21 and day 42 are presented in (Tables 3 and 4). On day 21, the main effects data highlighted that increased in supplements dose significantly (P < 0.05) increased the VH of duodenum and jejunum; additionally, increased the VH: CD of jejunum. However, no significant effect of increased supplements dose was observed for duodenum CD and VH: CD and jejunum CD. Also the VH, CD and VH: CD for ileum, were not influenced by increased supplements dose. Furthermore, Significant interaction was observed for duodenum and jejunum VH (P < 0.0001) and VH: CD (P < 0.05), that shown increased supplement dose increased the VH and VH: CD, in turn, resulted into improved gut health. The inclusion of different levels of PBLM and POLM in feed of birds resulted in an increased VH of all segments of the intestine. On day 21, supplemented diets T3 and T7 had increased (P < 0.05) VH in duodenum and jejunum as compared to other supplemented treatments and control group, followed by T2 and T6 that have longer (P < 0.05) villi of duodenum and jejunum compared to T5, T4, and control T1. Additionally, no differences (P > 0.05) were recorded in the villus height of ileum among supplemented and the control groups. The dietary inclusion has no influence (P > 0.05) on CD of the duodenum, while the chickens fed supplemented diet T3 shown least (P < 0.05) CD of jejunum as well as for ileum. The VH: CD of the duodenum as well as for ileum were recorded higher (P < 0.05) in all dietary treatments relative to the control group. While VH: CD of jejunum was observed higher (P < 0.05) in diets T3, T7, T6 and T2 followed by T5, T4, and the control T1.
Table 3.
Gut morphometric measures of broilers supplemented with different levels of PBLM and POLM on day 21.
| Villus height; (μm) |
Crypt depth; (μm) |
VH: CD |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatments | Duodenum | Jejunum | Ileum | Duodenum | Jejunum | Ileum | Duodenum | Jejunum | Ileum |
| T1 | 988.34c | 686.07c | 447.88 | 136.75 | 106.57 a | 94.34 a | 7.25b | 6.47b | 4.76b |
| T2 | 1044.07b | 720.61b | 459.85 | 139.06 | 106.89 a | 90.88 a | 7.52 a | 6.76 a | 5.09 a |
| T3 | 1097.63 a | 759.73 a | 464.79 | 128.25 | 99.92b | 85.29b | 8.6 a | 7.72 a | 5.47 a |
| T4 | 1001.91c | 680.25c | 448.66 | 136.76 | 112.36 a | 91.18 a | 7.34 ab | 6.14b | 4.95 ab |
| T5 | 1008.23c | 695.84c | 458.66 | 134.46 | 110.3 a | 89.67 a | 7.43 a | 6.31b | 5.14 a |
| T6 | 1018.04 bc | 726.99b | 454.63 | 136.14 | 104.77 a | 90.95 a | 7.45 a | 6.96 a | 5.02 a |
| T7 | 1084.45 a | 756.15 a | 464.83 | 128.18 | 105.28 a | 86.45b | 8.36 a | 7.25 a | 5.41 a |
| SEM | 9.06 | 4.55 | 3.36 | 2.03 | 1.56 | 2.06 | 0.15 | 0.13 | 0.16 |
| Main effects! | |||||||||
| Supplement concertation | |||||||||
| 2 g / kg | 1026.15b | 708.2245b | 459.26 | 137.11 | 108.55 | 90.33 | 7.61 | 6.66b | 5.29 |
| 4 g / kg | 1057.89 a | 743.36 a | 459.71 | 132.24 | 102.45 | 88.27 | 8.20 | 7.37 a | 5.51 |
| 8 g / kg | 1043.18 ab | 718.204b | 456.75 | 133.17 | 109.32 | 88.92 | 8.01 | 6.69 ab | 5.37 |
| Supplements | |||||||||
| P. betle | 1047.87 | 720.20 | 457.76 | 134.92 | 106.46 | 89.28 | 7.93 | 6.92 | 5.36 |
| P. odorata | 1036.94 | 726.33 | 459.38 | 133.43 | 107.08 | 89.06 | 7.95 | 6.90 | 5.42 |
| Source of variation | |||||||||
| Supplement dose | 0.015 | <0.0001 | 0.937 | 0.658 | 0.211 | 0.931 | 0.369 | 0.070 | 0.887 |
| Supplements | 0.209 | 0.323 | 0.824 | 0.746 | 0.856 | 0.960 | 0.973 | 0.950 | 0.865 |
| Supplement × Supplement dose | <0.0001 | <0.0001 | 0.326 | 0.361 | 0.325 | 0.647 | 0.040 | 0.019 | 0.677 |
abc The means with different superscripts within the same columns are significantly different (P < 0.05).
T1: (Basal diet (BD); control); T2:(BD + PBLM 2 g/kg); T3:(BD + PBLM 4 g/kg); T4:(BD + PBLM 8 g/kg); T5: (BD + POLM 2 g/kg); T6: (BD + POLM 4 g/kg); T7: (BD + POLM 8 g/kg).
Data were analysed as a 3 × 2 factorial arrangement, excluding control group.
Table 4.
Gut morphometric measures of broilers supplemented with different levels of PBLM and POLM on day 42.
| Villus height; (μm) |
Crypt depth; (μm) |
VH: CD |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatments | Duodenum | Jejunum | Ileum | Duodenum | Jejunum | Ileum | Duodenum | Jejunum | Ileum |
| T1 | 1372.94c | 967.31c | 645.12 | 185.11 a | 140.78 | 135.82 a | 7.44b | 6.88b | 4.76b |
| T2 | 1440.3b | 1035.79 a | 665.92 | 179.72 ab | 141.07 | 130.29 a | 8.05b | 7.37 ab | 5.12b |
| T3 | 1483.52 a | 1041.68 a | 671.49 | 168.81b | 137.05 | 121.33b | 8.81 a | 7.61 a | 5.54 a |
| T4 | 1393.84c | 970.04c | 649.11 | 184.43 a | 137.9 | 132.06 a | 7.57b | 7.04 ab | 4.92b |
| T5 | 1433.69b | 998.03b | 666.04 | 183.72 a | 139.87 | 131.57 a | 7.82b | 7.15 ab | 5.07b |
| T6 | 1439.21b | 1001.53b | 668.55 | 181.68 ab | 138.58 | 132.53 a | 7.94b | 7.24 ab | 5.06b |
| T7 | 1473.11 a | 1039.47 a | 670.5 | 168.94b | 139.57 | 122.9b | 8.73 a | 7.46 ab | 5.46 a |
| SEM | 12.29 | 4.28 | 2.67 | 1.74 | 1.31 | 1.33 | 0.1 | 0.08 | 0.06 |
| Main effects! | |||||||||
| Supplement concertation | |||||||||
| 2 g / kg | 1437b | 1017.11 ab | 665.96 | 181.47 | 140.47 | 131.33 | 7.98 | 7.30 | 5.11 |
| 4 g / kg | 1461.37 a | 1021.70 a | 670.06 | 175.24 | 137.46 | 127.03 | 8.41 | 7.48 | 5.32 |
| 8 g / kg | 1433.48b | 1004.81b | 659.81 | 176.68 | 138.74 | 127.08 | 8.19 | 7.30 | 5.24 |
| Supplements | |||||||||
| P. betle | 1439.22 | 1015.87 | 662.16 | 177.65 | 138.54 | 128.06 | 8.18 | 7.39 | 5.21 |
| P. odorata | 1448.67 | 1013.21 | 668.39 | 177.95 | 139.24 | 128.90 | 8.21 | 7.33 | 5.23 |
| Source of variation | |||||||||
| Supplement dose | 0.002 | 0.039 | 0.309 | 0.347 | 0.720 | 0.277 | 0.248 | 0.677 | 0.443 |
| Supplements | 0.157 | 0.626 | 0.257 | 0.936 | 0.818 | 0.738 | 0.862 | 0.751 | 0.913 |
| Supplement × Supplement dose | <0.0001 | <0.0001 | 0.150 | 0.008 | 0.907 | 0.006 | 0.001 | 0.183 | 0.012 |
abcThe means with different superscripts within the same columns are significantly different (P < 0.05).
T1: (Basal diet (BD); control); T2:(BD + PBLM 2 g/kg); T3:(BD + PBLM 4 g/kg); T4:(BD + PBLM 8 g/kg); T5: (BD + POLM 2 g/kg); T6: (BD + POLM 4 g/kg); T7: (BD + POLM 8 g/kg).
Data were analysed as a 3 × 2 factorial arrangement, excluding control group.
The main effects data of second-growth phase of broiler chickens showed that as supplements dose increased, VH of duodenum and jejunum significantly (P < 0.05) increased, however, CD and VH: CD of duodenum and jejunum were not affected by increased dose of supplements. Furthermore, increased supplementation dose did not influence the VH, CD and VH: CD of ileum. A significant interaction was observed for duodenum VH (P < 0.0001), CD (P < 0.008) and VH: CD (P < 0.001), as well as, for jejunum VH (P < 0.0001), also for ileum CD (P < 0.006), and VH: CD (P < 0.05), thus, indicating that increased supplement concentration positively modulate the gut. On day 42, except for dietary group T4 the increased (P < 0.05) VH were observed for duodenum and jejunum of all supplemented treatments in comparison with the control. Furthermore, significantly maximum (P < 0.05) VH were observed in T3 and T7, while recorded least (P < 0.05) VH in T4 among treatments. Additionally, the VH of ileum was not significantly influenced (P > 0.05) by dietary inclusion of PBLM and POLM in experimental chickens. The CD of the duodenum was recorded increased (P < 0.05) in T1, T4 and T5 as compared to other treatments T3 and T7; additionally the CD of ileum was recorded significantly lesser (P < 0.05) for T3 and T7. While the supplementation of PBLM and POLM has not affected the CD of jejunum. The dietary supplementation of PBLM and POLM resulted in higher (P < 0.05) VH: CD of duodenum and ileum in diets T3 and T7 relative to other treated and the control group. However, VH: CD of jejunum was recorded higher (P < 0.05) in T3 relative to the control group. The current findings endorsed the idea, that phytogenic feed additives (PFAs) or phytobiotics favourably modify the gut structure and digestive functions, thus enhanced the growth in broiler chickens. The morphological changes of gut under the influence of phytobiotics may provide the information on their beneficial role in the digestive tract. Generally, phytobiotics have potential to increased VH across the gut, decrease inflammatory events, and improve absorption efficiency (Murugesan et al., 2014). Endorsing this idea, Khan et al. (2017) in a study observed that the inclusion of Moringa oleifera at a rate of 1.2% in the diet of broiler chickens positively modulate the gut health through a developed intestinal microarchitecture. In another study, Karangiya et al. (2016) observed that supplementation of garlic in broilers at a dose rate of 1%, resulted in higher mean villi length and width. The significant factors of gut health are the height of villi and crypt depths. Longer villi resulted in bigger surface area, that increase absorption of nutrients and thus enhanced the growth performance and health of chickens (Kamboh et al., 2015). The improved villi length is related to a better expression of brush border enzymes and enhanced transport of nutrients (Viveros et al., 2011). In this study, the increased villus height, recorded in dietary groups T3 and T4, that could be an explanation for enhanced growth and better FCR obtained at supplementation level of PBLM 4 g/kg and POLM 8 g/kg.
Since intestinal crypts serve as a source of epithelial cells for villus, and their depth is directly related to epithelial cell turnover. Phytobiotics or PAFs supplementation resulted in shallow crypts of the gut, that indicates less cellular turnover and healthy intestine. It is worth mentioning that the process of cellular turnover is an energy-utilizing event; if this energy utilisation is saved by reducing cellular turnover might be used for enhancement in growth events. Thus, longer villi and shallow crypts are indicative of better productive performance (Ahsan et al., 2018).
In the present study, it was noted that the increasing concentration of PBLM 8 g/ kg (T4) resulted in reduced VH and increased CD, the current results corroborated the findings of Oso et al. (2019), who observed that inclusion of phytogenic blend that contained Piper betle in broiler chickens ration resulted in an increased duodenal VH at 1%, however decreased at a higher inclusion level of 2%; as well as this high concentration of phytogenic blend resulted in deepest crypt in jejunum; this suggested that the gut morphology improved at 1% inclusion beyond that the concentration get worsen.
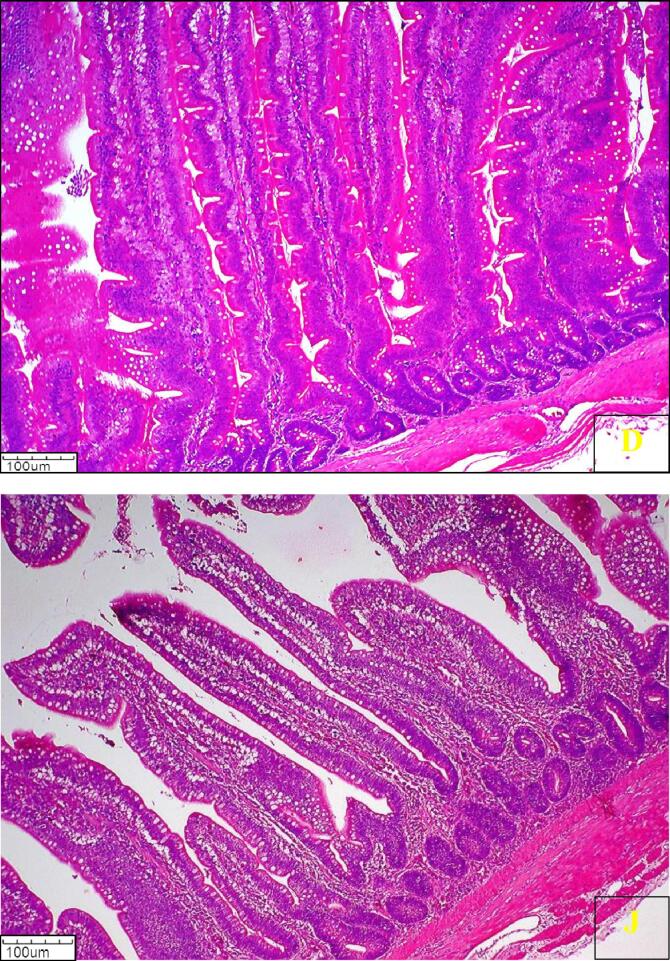
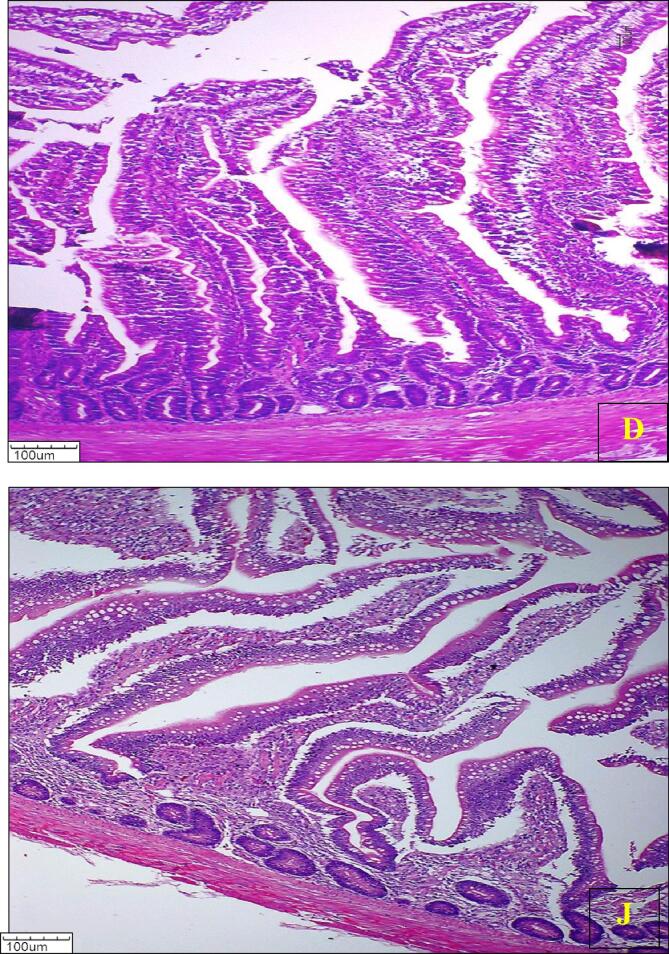
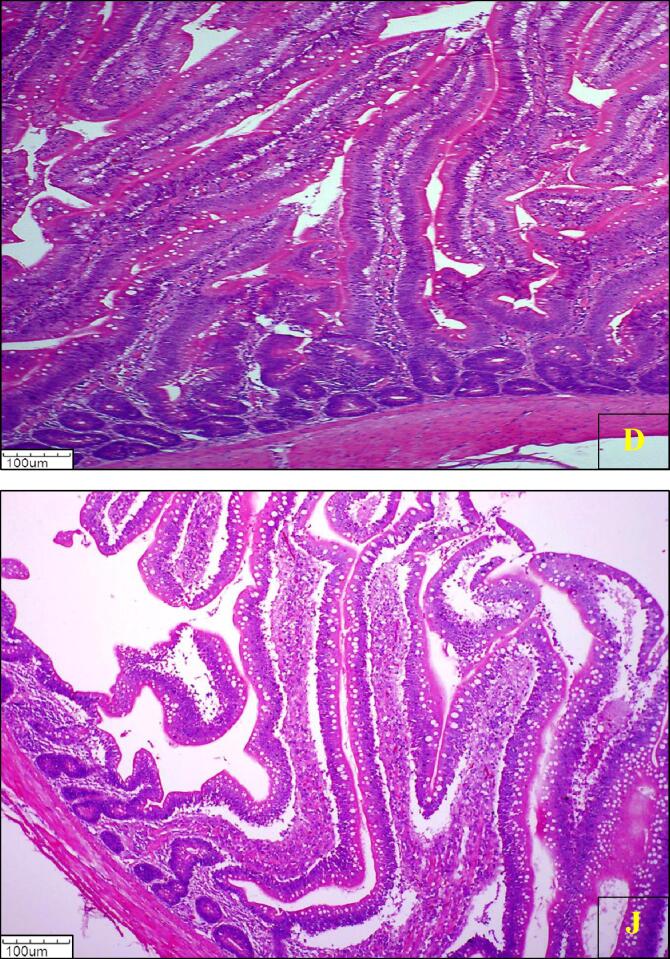
The histological photomicrograph of duodenum and jejunum villi of broiler chickens on day 42 from all groups is presented in (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7).
Fig. 1.
Photomicrograph of histology of Duodenum (D) and Jejunum (J) villi of broiler chickens from Control group T1 (H & E) 100X.
Fig. 2.
Photomicrograph of histology of Duodenum (D) and Jejunum (J) villi of broiler chickens from Control group T2 (H & E) 100X.
Fig. 3.
Photomicrograph of histology of Duodenum (D) and Jejunum (J) villi of broiler chickens from Control group T3 (H & E) 100X.
Fig. 4.
Photomicrograph of histology of Duodenum (D) and Jejunum (J) villi of broiler chickens from Control group T4 (H & E) 100X.
Fig. 5.
Photomicrograph of histology of Duodenum (D) and Jejunum (J) villi of broiler chickens from supplemented group T5 (H & E) 100X.
Fig. 6.
Photomicrograph of histology of Duodenum (D) and Jejunum (J) villi of broiler chickens from supplemented group T6 (H & E) 100X.
Fig. 7.
Photomicrograph of histology of Duodenum (D) and Jejunum (J) villi of broiler chickens from supplemented group T7 (H & E) 100X.
3.3. Nutrient digestibility
Supplementation of phytobiotics has been resulted in better feed intake and its utilisation, as they can enhance digestive enzymes concentrations; that promote nutrients digestion and increased absorption in the intestine (Khan et al., 2012, Abudabos et al., 2017). The results of apparent ileal nutrients digestibility (AID) are presented in (Tables 5 and 6). The main data effects of both growth phases of boiler chickens designated that DM, EE, CP and ash were not affected by the increased dose of supplementation, furthermore, no significant interaction was recorded. On day 21 and 42, the AID of dry matter (DM) and ether extract (EE) were observed higher (P < 0.05) in T3 and T7 among supplemented groups and relative to the control group. Furthermore, higher (P < 0.05) digestibility of crude protein (CP) was recorded in T7 followed by T3, T2, and T6 compared to dietary treatment T5 and the control group; however, significantly least (P < 0.05) CP value was noted in T4. This reduced digestion of crude protein CP in broilers fed on diet T4 might be due to the high concentration of tannins, that adversely influenced on nutrient digestion, especially protein digestion (Sav́on et al., 2006). The results of the current study endorsed the findings of Mansoori et al. (2015), who revealed that 0.2% supplementation of chestnut tannin in the broilers decreased animal growth performance, by minimising the nutrient digestion and absorption. Very much similar results narrated by Farahat et al. (2017) who revealed that the dietary supplementation of grape seed extract tannin in broiler chickens at the concentration of 2% positively promoted the digestion of the nutrients, while, further increment in level negatively influenced the protein and amino acid digestion. On day 21 higher (P < 0.05) value of ash was noted in supplemented treatments relative to the control; however, ash was unaffected by supplemented diets on day 42. Current study outcomes are in line with Murugesan et al. (2015), who reported that the growth enhancement of broiler birds fed PFAs, might be associated with the increased digestibility of nutrients. Similarly, Hassan et al. (2015) described that supplementation of R. officinalis, S. Officinalis, and D.vulgaris in broilers ration resulted in increased digestibility of DM, CP, ether extract, and organic material. Generally, flavonoids and polyphenols improve cellular and mucosal immunity by regulating the circulatory and endocrine markers; thus, dietary supplementation of phytobiotics containing secondary metabolite like flavonoids can improve the immunity and health of broiler chickens (Kamboh et al., 2018).
Table 5.
Apparent ileal nutrients digestibility of broilers supplemented with different levels of PBLM and POLM on day 21.
| Treatments | Dry matter (DM) | Ether extract (EE) | Crude protein (CP) | Ash |
|---|---|---|---|---|
| T1 | 69.34b | 64.97b | 68.20b | 30.97b |
| T2 | 72.08b | 68.28b | 70.94 a | 32.12 a |
| T3 | 76.15 a | 74.03 a | 74.02 a | 33.92 a |
| T4 | 70.70b | 65.85b | 65.63c | 32.51 a |
| T5 | 71.15b | 66.19b | 68.30b | 32.17 a |
| T6 | 71.17b | 68.07b | 70.83 a | 32.07 a |
| T7 | 75.30 a | 72.69 a | 74.17 a | 33.31 a |
| SEM | 1.33 | 1.33 | 1.37 | 0.46 |
| Main effects! | ||||
| Supplement concertation | ||||
| 2 g / kg | 71.61 | 67.24 | 69.62 | 32.15 |
| 4 g / kg | 73.66 | 71.05 | 72.48 | 32.99 |
| 8 g / kg | 73.00 | 69.27 | 69.90 | 32.91 |
| Supplements | ||||
| P. betle | 72.98 | 69.39 | 70.50 | 32.85 |
| P. odorata | 72.54 | 68.98 | 70.84 | 32.52 |
| Source of variation | ||||
| Supplement dose | 0.845 | 0.578 | 0.697 | 0.775 |
| Supplements | 0.882 | 0.892 | 0.911 | 0.758 |
| Supplement × Supplement dose | 0.418 | 0.209 | 0.165 | 0.582 |
abcThe means with different superscripts within the same columns are significantly different (P < 0.05).
T1: (Basal diet (BD); control); T2:(BD + PBLM 2 g/kg); T3:(BD + PBLM 4 g/kg); T4:(BD + PBLM 8 g/kg); T5: (BD + POLM 2 g/kg); T6: (BD + POLM 4 g/kg); T7: (BD + POLM 8 g/kg).
Data were analysed as a 3 × 2 factorial arrangement, excluding control group.
Table 6.
Apparent ileal nutrient s digestibility of broilers supplemented with different levels of PBLM and POLM on day 42.
| Treatments | Dry matter (DM) | Ether extract (EE) | Crude protein (CP) | Ash |
|---|---|---|---|---|
| T1 | 71.84b | 67.81b | 69.71 bc | 32.9 |
| T2 | 74.37b | 70.94b | 73.47 a | 34.71 |
| T3 | 77.23 a | 76.11 a | 75.01 a | 34.89 |
| T4 | 73.04b | 67.63b | 67.88c | 34.21 |
| T5 | 73.01b | 68.14b | 71.02b | 34.57 |
| T6 | 73.07b | 70.14b | 72.06b | 34.28 |
| T7 | 78.06 a | 74.07 a | 75.31 a | 34.74 |
| SEM | 1.18 | 1.3 | 1.35 | 0.44 |
| Main effects! | ||||
| Supplement concertation | ||||
| 2 g / kg | 73.69 | 69.54 | 72.25 | 34.64 |
| 4 g / kg | 75.15 | 73.13 | 73.69 | 34.73 |
| 8 g / kg | 75.55 | 70.85 | 71.60 | 34.47 |
| Supplements | ||||
| P. betle | 74.88 | 71.56 | 72.22 | 34.70 |
| P. odorata | 74.71 | 70.78 | 72.80 | 34.53 |
| Source of variation | ||||
| Supplement dose | 0.83 | 0.60 | 0.85 | 0.98 |
| Supplements | 0.95 | 0.79 | 0.85 | 0.86 |
| Supplement × Supplement dose | 0.37 | 0.21 | 0.29 | 0.84 |
abcThe means with different superscripts within the same columns are significantly different (P < 0.05).
T1: (Basal diet (BD); control); T2:(BD+ PBLM 2g/kg); T3:(BD+ PBLM 4g/kg); T4:(BD+ PBLM 8g/kg); T5: (BD+ POLM 2g/kg); T6: (BD+ POLM 4g/kg); T7: (BD+ POLM 8g/kg).
Data were analysed as a 3 × 2 factorial arrangement, excluding control group.
4. Conclusions
The findings of the current study revealed that the dietary supplementation with Piper betle leaf meal and Persicaria odorata leaf meal enhanced the biological production of broiler chickens. The broiler chickens fed on dietary supplementation PBLM 4 g/ kg and POLM 8 g/kg of feed getting maximum BWG, positive modulation of gut structure and attained superior feed efficiency. Based on the results of the current study, the dietary supplementation of PBLM 4 g/kg and POLM 8 g/kg would be the appropriate doses and could be the potential candidates as natural alternative growth promoters in poultry production. Further studies are warranted to explore the comparative efficacy of PBLM and POLM with AGPs in broilers ration.
5. Authors’ contributions
MAB, AAK, LTC, SAA, and AS contributed in the study design and carried out statistical analysis. MAB involved in feeding trial. MAB, AAK, UK, and SBI conducted sample collection. MAB, AAK and UK performed laboratory analysis. MAB, AAK, LTC, SAA, AS, UK, and SBI contributed to the preparation of the manuscript. The final manuscript was reviewed and approved by all authors.
Acknowledgement
This research project was supported by a grant (Grant Code: GP/2018/9616700), Geran Putra, Universiti Putra Malaysia, Malaysia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Muhammad Abdul Basit, Email: drbasit17@bzu.edu.pk.
Abdul Kadir Arifah, Email: arifah@upm.edu.my.
Teck Chwen Loh, Email: tcloh@upm.edu.my.
Abdul Aziz Saleha, Email: saleha@upm.edu.my.
Annas Salleh, Email: annas@upm.edu.my.
References
- Abudabos A.M., Alyemni A.H., Dafalla Y.M., Khan R.U. Effect of organic acid blend and bacillus subtilis alone or in combination on growth traits, blood biochemical and antioxidant status in broilers exposed to Salmonella typhimurium challenge during the starter phase. J. Appl. Anim. Res. 2017;45:538–542. doi: 10.1080/09712119.2016.1219665. [DOI] [Google Scholar]
- Abudabos A.M., Alyemni A.H., Dafalla Y.M., Khan R.U. The effect of phytogenic feed additives to substitute in-feed antibiotics on growth traits and blood biochemical parameters in broiler chicks challenged with Salmonella typhimurium. Environ. Sci. Pollut. Res. 2016;23:24151–24157. doi: 10.1007/s11356-016-7665-2. [DOI] [PubMed] [Google Scholar]
- Ahsan, U., Kuter, E., Raza, I., Köksal, B.H., Cengiz, Yıldız, M., Kızanlık, P.K., Kaya, M., Tatlı, O., Sevim, 2018. Dietary supplementation of different levels of phytogenic feed additive in broiler diets: The dynamics of growth performance, caecal microbiota, and intestinal morphometry. Rev. Bras. Cienc. Avic. 20, 737–746. https://doi.org/10.1590/1806-9061-2017-0698.
- Al-Abd N.M., Mohamed Nor Z., Mansor M., Azhar F., Hasan M.S., Kassim M. Antioxidant, antibacterial activity, and phytochemical characterization of Melaleuca cajuputi extract. BMC Complement. Altern. Med. 2015;15:385. doi: 10.1186/s12906-015-0914-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Kenganora M., Manjula S.N. Health benefits of morinda citrifolia (Noni): a review. Pharmacogn. J. 2016;8:321–334. doi: 10.5530/pj.2016.4.4. [DOI] [Google Scholar]
- Alshelmani, M.I., Loh, T.C., Foo, H.L., Sazili, A.Q., Lau, W.H., 2017. Effect of solid-state fermentation on nutrient content and ileal amino acids digestibility of palm kernel cake in broiler chickens. Indian J. Anim. Sci, 87, 1135-1140. [Google Scholar].
- Alshelmani M.I., Loh T.C., Foo H.L., Sazili A.Q., Lau W.H. Effect of feeding different levels of palm kernel cake fermented by Paenibacillus polymyxa ATCC 842 on nutrient digestibility, intestinal morphology, and gut microflora in broiler chickens. Anim. Feed Sci. Technol. 2016;216:216–224. doi: 10.1016/j.anifeedsci.2016.03.019. [DOI] [Google Scholar]
- AOAC Association of Official Analytical Chemist. 1995. Official method of analysis of AOAC International. 16th ed. Arlington: Association of Official Analytical Chemist.
- Aroche R., Martínez Y., Ruan Z., Guan G., Waititu S., Nyachoti C.M., Más D., Lan S. Dietary inclusion of a mixed powder of medicinal plant leaves enhances the feed efficiency and immune function in broiler chickens. J. Chem. 2018;2018:1–6. doi: 10.1155/2018/4073068. [DOI] [Google Scholar]
- Atiya A., Sinha B.N., Ranjan Lal U. New chemical constituents from the Piper betle Linn. (Piperaceae) Nat. Prod. Res. 2018;32:1080–1087. doi: 10.1080/14786419.2017.1380018. [DOI] [PubMed] [Google Scholar]
- Bee G., Silacci P., Ampuero-Kragten S., Čandek-Potokar M., Wealleans A.L., Litten-Brown J., Salminen J.P., Mueller-Harvey I. Hydrolysable tannin-based diet rich in gallotannins has a minimal impact on pig performance but significantly reduces salivary and bulbourethral gland size. Animal. 2017;11:1617–1625. doi: 10.1017/S1751731116002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus M., Dolinšek J., Cencič A., Škorjanc D. Effect of chestnut (Castanea sativa Mill.) wood tannins and organic acids on growth performance and faecal microbiota of pigs from 23 to 127 days of age. Bulg. J. Agric. Sci. 2013;19:841–847. https://www.agrojournal.org/19/04-35.html [Google Scholar]
- Christapher P.V., Parasuraman S., Vasanth Raj P., Saghir S.A.M., Asmawi M.Z., Vikneswaran M. Influence of extracting solvent on pharmacological activity and cytotoxicity of Polygonum minus, a commonly consumed herb in Southeast Asia. Pharmacogn. Mag. 2016;12:S424–S430. doi: 10.4103/0973-1296.191451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christapher P., Parasuraman S., Christina J., Asmawi M.Z., Vikneswaran M. Review on Polygonum minus, Huds, a commonly used food additive in Southeast Asia. Pharmacognosy Res. 2015;7:1–6. doi: 10.4103/0974-8490.147125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosby D.E., Cox N.A., Harrison M.A., Wilson J.L., Jeff Buhr R., Fedorka-Cray P.J. Salmonella and antimicrobial resistance in broilers: A review. J. Appl. Poult. Res. 2015;24:408–426. doi: 10.3382/japr/pfv038. [DOI] [Google Scholar]
- Debnath B., Choudhary K.B., Ravikanth K., Thakur A., Maini S. (Av / Agp / 10) with antibiotic growth promoter on overall growth performance and intestinal morphometry in broiler birds. Int. J. Pharm. Sci. Heal. Care. 2014;2:155–168. http://www.rspublication.com/ijphc/2014/april14/april14.htm [Google Scholar]
- Farahat M.H., Abdallah F.M., Ali H.A., Hernandez-Santana A. Effect of dietary supplementation of grape seed extract on the growth performance, lipid profile, antioxidant status & immune response of broiler chickens. Animal. 2017;11:771–777. doi: 10.1017/S1751731116002251. [DOI] [PubMed] [Google Scholar]
- Hassan H.M.A., Youssef A.W., Ali H.M., Mohamed M.A. Adding phytogenic material and/or organic acids to broilar diets: Effect on performance, nutrient digestibility and net profit. Asian J. Poult. Sci. 2015;9:97–105. doi: 10.3923/ajpsaj.2015.97.105. [DOI] [Google Scholar]
- Huang Q., Liu X., Zhao G., Hu T., Wang Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018;4:137–150. doi: 10.1016/j.aninu.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S.G., Patel M., Saxena D.K., Naik S.N. Antioxidant properties of Piper Betel (L) leaf extracts from six different geographical domain of India. J. Bioresour. Eng. Technol. 2014;2:12–20. [Google Scholar]
- Kamboh A.A., Arain M.A., Mughal M.J., Zaman A., Arain Z.M., Soomro A.H. Flavonoids: Health Promoting Phytochemicals for Animal Production-a Review. J. Anim. health Prod. 2015;11:369–373. doi: 10.14737/journal.jahp/2015/3.1.6.13. [DOI] [Google Scholar]
- Kamboh A.A., Khan M.A., Kaka U., Awad E.A., Memon A.M., Saeed M., Korejo N.A., Bakhetgul M., Kumar C. Effect of dietary supplementation of phytochemicals on immunity and haematology of growing broiler chickens. Ital. J. Anim. Sci. 2018;17:1038–1043. doi: 10.1080/1828051X.2018.1438854. [DOI] [Google Scholar]
- Karangiya V.K., Savsani H.H., Patil S.S., Garg D.D., Murthy K.S., Ribadiya N.K., Vekariya S.J. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Vet. World. 2016;9:245–250. doi: 10.14202/vetworld.2016.245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R.U., Naz S., Nikousefat Z., Tufarelli V., Javdani M., Qureshi M.S., Laudadio V. Potential applications of ginger (Zingiber officinale) in poultry diets. Worlds. Poult. Sci. J. 2012;68:245–252. doi: 10.1017/S004393391200030X. [DOI] [Google Scholar]
- Khan I., Zaneb H., Masood S., Yousaf M.S., Rehman H.F., Rehman H. Effect of Moringa oleifera leaf powder supplementation on growth performance and intestinal morphology in broiler chickens. J. Anim. Physiol. Anim. Nutr. (Berl) 2017;101:114–121. doi: 10.1111/jpn.12634. [DOI] [PubMed] [Google Scholar]
- Kim D.K., Lillehoj H.S., Lee S.H., Jang S.I., Park M.S., Min W., Lillehoj E.P., Bravo D. Immune effects of dietary anethole on Eimeria acervulina infection. Poult. Sci. 2013;92:2625–2634. doi: 10.3382/ps.2013-03092. [DOI] [PubMed] [Google Scholar]
- Kim D., Hong E., Kim J., Bang H., Choi J., Ji S. Effects of dietary quercetin on growth performance, blood biochemical parameter, immunologlobulin and blood antioxidant activity in broiler chickens. Kor J. Poult. Sci. 2015;42:33–40. doi: 10.5536/KJPS.2014.42.1.33. [DOI] [Google Scholar]
- Kumar M., Kumar V., Roy D., Kushwaha R., Vaswani S. Application of Herbal Feed Additives in Animal Nutrition – A Review. Int. J. Livest. Res. 2014;4:1–8. doi: 10.5455/ijlr.20141205105218. [DOI] [Google Scholar]
- Ma J.Q., Li Z., Xie W.R., Liu C.M., Liu S.S. Quercetin protects mouse liver against CCl4-induced inflammation by the TLR2/4 and MAPK/NF-κB pathway. Int. Immunopharmacol. 2015;28:531–539. doi: 10.1016/j.intimp.2015.06.036. [DOI] [PubMed] [Google Scholar]
- Manafi M. Comparison study of a natural non-antibiotic growth promoter and a commercial probiotic on growth performance, immune response and biochemical parameters of broiler chicks. J. Poult. Sci. 2015;52:274–281. doi: 10.2141/jpsa.0150027. [DOI] [Google Scholar]
- Mansoori B., Rogiewicz A., Slominski B.A. The effect of canola meal tannins on the intestinal absorption capacity of broilers using a D-xylose test. J. Anim. Physiol. Anim. Nutr. (Berl) 2015;99:1084–1093. doi: 10.1111/jpn.12320. [DOI] [PubMed] [Google Scholar]
- Mpofu D.A., Marume U., Mlambo V., Hugo A. The effects of Lippia javanica dietary inclusion on growth performance, carcass characteristics and fatty acid profiles of broiler chickens. Anim. Nutr. 2016;2:160–167. doi: 10.1016/j.aninu.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan G.R., Gabler N.K., Persia M.E. Effects of direct-fed microbial supplementation on broiler performance, intestinal nutrient transport and integrity under experimental conditions with increased microbial challenge. Br. Poult. Sci. 2014;55:89–97. doi: 10.1080/00071668.2013.865834. [DOI] [PubMed] [Google Scholar]
- Murugesan G.R., Syed B., Haldar S., Pender C. Phytogenic feed additives as an alternative to antibiotic growth promoters in broiler chickens. Front. Vet. Sci. 2015;2:1–6. doi: 10.3389/fvets.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhulu G., Reddy K.K., Mohamed J. The genus Polygonum (Polygonaceae): An ethnopharmacological and phytochemical perspectives - Review. Int. J. Pharm. Pharm. Sci. 2014;6:21–45. [Google Scholar]
- Oso A.O., Suganthi R.U., Reddy G.B.M., Malik P.K., Thirumalaisamy G., Awachat V.B., Selvaraju S., Arangasamy A., Bhatta R. Effect of dietary supplementation with phytogenic blend on growth performance, apparent ileal digestibility of nutrients, intestinal morphology, and cecal microflora of broiler chickens. Poult. Sci. 2019;98:4755–4766. doi: 10.3382/ps/pez191. [DOI] [PubMed] [Google Scholar]
- Ouyang K., Xu M., Jiang Y., Wang W. Effects of alfalfa flavonoids on broiler performance, meat quality, and gene expression. Can. J. Anim. Sci. 2016;96:332–341. doi: 10.1139/cjas-2015-0132. [DOI] [Google Scholar]
- Paraskeuas V., Fegeros K., Palamidi I., Hunger C., Mountzouris K.C. Growth performance, nutrient digestibility, antioxidant capacity, blood biochemical biomarkers and cytokines expression in broiler chickens fed different phytogenic levels. Anim. Nutr. 2017;3:114–120. doi: 10.1016/j.aninu.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra B., Das M.T., Kumar Dey S., Das T. A review on Piper betle L. J. Med. Plants Stud. 2016 [Google Scholar]
- Perez-Gregorio M.R., Mateus N., De Freitas V. Rapid screening and identification of new soluble tannin-salivary protein aggregates in saliva by mass spectrometry (MALDI-TOF-TOF and FIA-ESI-MS) Langmuir. 2014;30:8528–8537. doi: 10.1021/la502184f. [DOI] [PubMed] [Google Scholar]
- Pradhan D., Biswasroy P., Suri K.A. Variation in the percentage content of hydroxychavicol in different extracts of Piper betle L. by altering the extraction parameters. Int. J. Adv. Sci. Tech. Res. 2014;2:517–530. [Google Scholar]
- Saeed M., Naveed M., Arain M.A., Arif M., Abd El-Hack M.E., Alagawany M., Siyal F.A., Soomro R.N., Sun C. Quercetin: nutritional and beneficial effects in poultry. Worlds. Poult. Sci. J. 2017;73:355–364. doi: 10.1017/S004393391700023X. [DOI] [Google Scholar]
- Salami S.A., Majoka M.A., Saha S., Garber A., Gabarrou J.F. Efficacy of dietary antioxidants on broiler oxidative stress, performance and meat quality: Science and market. Avian Biol. Res. 2015;8:65–78. doi: 10.3184/175815515X14291701859483. [DOI] [Google Scholar]
- SAS. 2012. User’s Guide. 9.4 ed. Cary, NC: SAS Institute, Inc.
- Sav́on, L., I. Scull and M. Martinez, 2006. “Integral foliage meal for poultry feeding. Chemical composition, physical properties and phytochemical screening. Cuba. J. Agric. Sci. 41:359-369. http://agris.fao.org/agris-search/search.do?recordID=CU2010700069.
- Shah, S.K., Garg, G., Jhade, D., Patel, N., 2016. Piper betle. Int. J. Pharm. Sci. Rev. Res. Int. J. Pharm. Sci. Rev. Res. 38, 181–189. [Google Scholar].
- Short F.J., Gorton P., Wiseman J., Boorman K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996;59:215–221. doi: 10.1016/0377-8401(95)00916-7. [DOI] [Google Scholar]
- Standard Malaysia. 2009. Halal food-production, preparation, handling and storage general guidelines (second revision). Department of Standards, Selangor Darul Ehsan, Malaysia, MS1500:2009.
- Starčević K., Krstulović L., Brozić D., Maurić M., Stojević Z., Mikulec Ž., Bajić M., Mašek T. Production performance, meat composition and oxidative susceptibility in broiler chicken fed with different phenolic compounds. J. Sci. Food Agric. 2015;95:1172–1178. doi: 10.1002/jsfa.6805. [DOI] [PubMed] [Google Scholar]
- Syahidah A., Saad C.R., Hassan M.D., Rukayadi Y., Norazian M.H., Kamarudin M.S. Phytochemical analysis, identification and quantification of antibacterial active compounds in Betel leaves, Piper betle Methanolic Extract. Pakistan. J. Biol. Sci. 2017;20:70–81. doi: 10.3923/pjbs.2017.70.81. [DOI] [PubMed] [Google Scholar]
- Toghyani M., Toghyani M., Gheisari A., Ghalamkari G., Eghbalsaied S. Evaluation of cinnamon and garlic as antibiotic growth promoter substitutions on performance, immune responses, serum biochemical and haematological parameters in broiler chicks. Livest. Sci. 2011;138:167–173. doi: 10.1016/j.livsci.2010.12.018. [DOI] [Google Scholar]
- Touchette K.J.J.A., Carroll G.L., Allee R.L., Matteri C.J., Dyer L.A., Beausang M.E.Z. Effect of spray-dried plasma and lipopolysaccharide exposure on weaned pigs: II. effects on the hypothalamic-pituitary-adrenal axis of weaned pigs. J. Anim. Sci. 2002;80:502–509. doi: 10.2527/2002.802502x. [DOI] [PubMed] [Google Scholar]
- Vikram P., Chiruvella K.K., Ripain I.H.A., Arifullah M. A recent review on phytochemical constituents and medicinal properties of kesum (Polygonum minus Huds.) Asian Pac. J. Trop. Biomed. 2014;4:430–435. doi: 10.12980/APJTB.4.2014C1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros A., Chamorro S., Pizarro M., Arija I., Centeno C., Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011;90:566–578. doi: 10.3382/ps.2010-00889. [DOI] [PubMed] [Google Scholar]
- Yang C., Chowdhury M.A.K., Hou Y., Gong J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens. 2015;4:137–156. doi: 10.3390/pathogens4010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W., Liu W., Yang B., Wu L., Yang J., Zou T., Liu F., Xia L., Zhang D. Quercetin protects against perfluorooctanoic acid-induced liver injury by attenuating oxidative stress and inflammatory response in mice. Int. Immunopharmacol. 2015;28:129–135. doi: 10.1016/j.intimp.2015.05.043. [DOI] [PubMed] [Google Scholar]