Abstract
Objective
To evaluate the rate of developmental coordination disorder (DCD) and its correlation to cognition and self-experienced health-related quality of life (HRQoL) in children born very preterm.
Design
Prospective follow-up study.
Setting
Regional population of children born very preterm in Turku University Hospital, Finland, in 2001–2006.
Patients
A total of 170 children born very preterm were followed up until 11 years of age.
Main outcome measures
Motor and cognitive outcomes were evaluated using the Movement Assessment Battery for Children - Second Edition (Movement ABC-2) and the Wechsler Intelligence Scale for Children - Fourth Edition, respectively, and HRQoL using the 17-Dimensional Illustrated Questionnaire (17D). The Touwen neurological examination was performed to exclude other neurological conditions affecting the motor outcome.
Results
Eighteen children born very preterm (17 boys) (11.3%) had DCD, defined as Movement ABC-2 total test score ≤5th percentile. A positive correlation between motor and cognitive outcome (r=0.22, p=0.006) was found. Children born very preterm with DCD had lower cognitive scores than those without DCD (Full-Scale IQ mean 76.8 vs 91.6, p=0.001). Moreover, children born very preterm with DCD reported lower HRQoL than children born very preterm without motor impairment (17D mean 0.93 vs 0.96, p=0.03). However, HRQoL was higher in this group of children born very preterm compared with population-based normative test results (p<0.001).
Conclusions
DCD was still common at 11 years of age in children born very preterm in 2000s. DCD associated with adverse cognitive development and lower self-experienced HRQoL. However, this group of children born very preterm reported better HRQoL in comparison with Finnish norms.
Keywords: neurodevelopment, outcomes research, neonatology
What is known about the subject?
The incidence of cerebral palsy has decreased in children born very preterm.
Children born very preterm have an increased risk for developmental coordination disorder (DCD).
DCD may co-occur with cognitive dysfunction and lower health-related quality of life (HRQoL).
What this study adds?
DCD was still common in 11-year-old children born very preterm in 2000s.
Children born very preterm with DCD had adverse cognitive development and lower self-experienced HRQoL compared with children born very preterm without motor impairment.
Introduction
The incidence of cerebral palsy (CP) has decreased among children born very preterm.1–6 However, the rate of non-CP motor impairments such as developmental coordination disorder (DCD) has not decreased,7 and children born preterm are still at increased risk for cognitive impairment compared with term peers.8–11
DCD is defined as motor problems interfering with academic achievement or activities of daily living which cannot be explained by medical, neurological or cognitive impairment.12 The aetiology of DCD is multifactorial, and neuroimaging studies have shown alterations in the brain development and functioning in children with DCD.13–16 The prevalence of DCD has been shown to vary from 5% to 6% in school-aged children and from 8% to 51% in those born preterm.8 12 17–19 DCD has been shown to co-occur with developmental disorders such as social, behavioural and attention problems, and learning difficulties.12 17 20–22 Nevertheless, data on the relationship between DCD and cognitive development in early adolescence are limited.22
Severe neurodevelopmental impairments such as CP, cognitive impairment, and hearing and visual impairment have been reported to associate with poorer self-experienced health-related quality of life (HRQoL) in school-aged children born preterm, while those without these morbidities have reported HRQoL equal to peers.23 24 The effect of preterm birth on HRQoL seems to be most significant in younger years and seems to decrease over time.25 The impact of motor impairments such as DCD on HRQoL is not well known. Dewey and Volkovinskaia26 have found no differences in total HRQoL scores between adolescents with DCD and/or attention deficit hyperactivity disorder (ADHD) and typically developing adolescents. However, they found that adolescents with DCD and ADHD had lower HRQoL on the mood and emotions subscale and school environment subscale. Their additional comparisons indicated that on both these subscales adolescents with DCD and ADHD had significantly lower scores than adolescents with DCD only. Karras et al27 have found that children with DCD reported significantly lower scores in 4 out of 10 HRQoL subscales: psychological well-being, mood and emotions, parent relations and home life, and school environment.
The aims of this study were to evaluate the rate of DCD and to study the correlation between motor and cognitive development at 11 years of age in children born very preterm and/or with very low birth weight in 2000s, and to study the effect of DCD on self-experienced HRQoL. We hypothesised that DCD is still common in children born very preterm and/or with very low birth weight, that poorer motor outcome correlates with adverse cognitive performance, and that DCD correlates with lower perceived HRQoL as compared with children born very preterm and/or with very low birth weight without motor impairment.
Methods
Participants
This prospective study is part of the PIPARI (Pienipainoisten riskilasten käyttäytyminen ja toimintakyky imeväisiästä kouluikään; The Development and Functioning of Very Low Birth Weight Infants from Infancy to School Age) study of infants born very preterm.28 29 The participants were born to Finnish-speaking or Swedish-speaking families from January 2001 to December 2006 in Turku University Hospital, Finland, which is one of the five level III hospitals in Finland. From 2001 to 2003 the inclusion criteria were birth weight ≤1500 g and prematurity (<37 gestational weeks). From 2004, the inclusion criteria were broadened to all infants born <32 weeks of gestational age irrespective of birth weight. The exclusion criteria were severe congenital anomalies or diagnosed syndrome affecting cognitive development. The flow chart of the participants is shown in figure 1. Written informed consent for this follow-up study was provided by parents and children.
Figure 1.
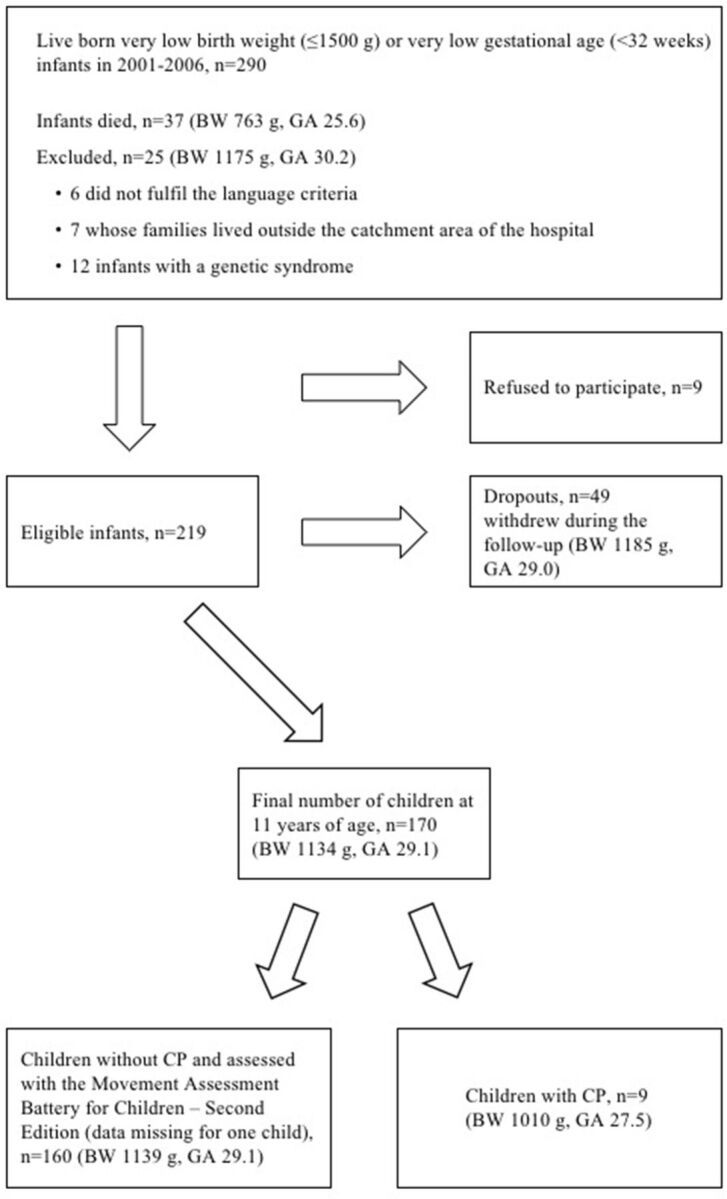
Flow chart of the participants, mean of gestational age (GA) in weeks and birth weight (BW). CP, cerebral palsy.
Patient involvement
This study was done without patient and public involvement.
Motor outcome
The diagnosis of CP was confirmed based on the classification proposed by Himmelmann et al30 after a systematic clinical follow-up by 2 years of corrected age by an experienced child neurologist. The motor outcome of the children born very preterm without CP was evaluated at 11 years of age by one of the three physicians using the Movement Assessment Battery for Children - Second Edition (Movement ABC-2).31 32 The three physicians performing the motor and neurological assessments were PhD students of the PIPARI study group. The raw scores were converted into total standard scores and percentile scores according to the test manual, using age band 3 (11–16 years) and the norms for 11-year-old children. A total test score >15th percentile indicated no movement difficulty, >5th to 15th percentile indicated risk of movement difficulties, and ≤5th percentile indicated DCD.32 The Touwen neurological examination was used to confirm that there were no other neurological conditions such as muscle diseases affecting the motor development.32–34 All the Movement ABC-2 assessments and Touwen neurological examinations were video-recorded. In case of any hesitation regarding the assessments, the videos were reassessed by one experienced child neurologist (LH).
Cognitive outcome
The cognitive development of children born very preterm at 11 years of age was assessed with the Wechsler Intelligence Scale for Children - Fourth Edition (WISC-IV), Finnish translation.35 36 The assessments were performed either in Finnish or Swedish according to the child’s native language. Finnish assessments were performed by one of the two psychologists, who were PhD students of the PIPARI study group. Swedish-speaking children were assessed by a native Swedish-speaking psychologist. General intelligence was measured with Full-Scale IQ, which consisted of the Verbal Comprehension Index, the Perceptual Reasoning Index, the Working Memory Index and the Processing Speed Index. The classification was based on the test manual.35 36 The scores were classified as average if the Full-Scale IQ was ≥90, low average 80–89 and borderline 70–79. A Full-Scale IQ <70 was classified as severe cognitive impairment.
Health-related quality of life
The self-experienced HRQoL of children born very preterm at 11 years of age was evaluated using a generic self-assessment measure, the 17-Dimensional Illustrated Questionnaire (17D).37 It consisted of 17 multiple-choice questions of health and function. The domains were mobility, vision, hearing, breathing, sleeping, eating, speech, excretion, school and hobbies, learning and memory, discomfort and symptoms, depression, distress, vitality, appearance, friends, and concentration. Each domain had a five-level tick box functioning scale alternating from a perfect level to a severe dysfunction. The children completed the questionnaire by themselves before the motor assessment, except for one child who was not able to read and was interviewed by the physician before the assessment. The relative weights of each dimension were defined in the instrument’s home page.38 The overall HRQoL was calculated from the health state descriptive system using population-based preference or utility weights for 11-year-old healthy Finnish school children. The HRQoL score varied from 0 (worst score, equal to death) to 1 (best score, equal to complete health).37
Statistical analysis
Differences in continuous background characteristics between the study children born very preterm and the children who withdrew were studied using a two-sample t-test or a Wilcoxon two-sample test. For categorical background characteristics, χ2 test or Fisher’s exact test was used. Correlations between the percentiles for the total scores of the Movement ABC-2 and Full-Scale IQ, and between the percentiles for the total scores of the Movement ABC-2 and WISC-IV indexes, were calculated using Pearson correlations. Associations between DCD and background characteristics were studied using logistic regression analysis. Differences in the Full-Scale IQ and indexes between children born very preterm with and without DCD were studied using two-sample t-test. The associations between motor outcome (children born very preterm with and without DCD), cognitive outcome (Full-Scale IQ and indexes) and background characteristics (birth weight, gestational age, and mother’s and father’s education) were studied using multiple linear regression model. The background characteristics were chosen a priori.
If up to three dimensions were missing from the 17D, multiple imputation was used to replace missing values with one value, as suggested by the instrument’s home page (http://www.15d-instrument.net/15d/replacing-missing-data/), in order to calculate the 17D total score. If more than three dimensions were missing, the questionnaire was not used in the analyses. Differences in the 17D scores between the groups of (1) children with and without DCD, (2) with and without CP, and (3) with and without severe cognitive impairment were studied using Mann-Whitney U test. The correlations between the Movement ABC-2 and the 17D as well as the Full-Scale IQ and the 17D were studied using Spearman’s correlation. The differences in 17D scores in the study children born very preterm compared with the Finnish population-based normative results were studied using Mann-Whitney U test. Statistical analyses were carried out using SAS V.9.4 for Windows. P values <0.05 were considered statistically significant.
Results
A total of 170 children born very preterm were followed until 11 years of age. The follow-up rate was 77.6% (out of 219 participants). The background characteristics of the study children and the children who withdrew are shown in table 1. The rate of CP did not differ between the study children and the children who withdrew (p=0.5). The mothers of the children who withdrew had lower educational level compared with the mothers of the study children (53% vs 36% with ≤12 years of education; p=0.04). No other differences in background characteristics shown in table 1 were found between the study children and the children who withdrew.
Table 1.
Background characteristics of the 11-year-old children (n=170) born at very low gestational age (<32 weeks) or with a very low birth weight (≤1500 g)
| Characteristics | Study children, n=170 | Children who withdrew, n=49 | P value |
| Gestational age, mean (SD) (minimum, maximum), weeks | 29.1 (2.7) (23.0, 35.9) | 29.0 (2.7) (23.7, 34.1) | 0.9 |
| Birth weight, mean (SD) (minimum, maximum), g | 1134.4 (315.3) (400.0, 2120.0) | 1184.6 (374.5) (565.0, 1970.0) | 0.3 |
| Small for gestational age (<−2 SD), n (%) | 56 (32.9) | 11 (22.5) | 0.2 |
| Male, n (%) | 94 (55.3) | 30 (61.2) | 0.5 |
| Caesarean section, n (%) | 101 (59.4) | 32 (65.3) | 0.5 |
| Bronchopulmonary dysplasia, n (%) | 22 (12.9) | 7 (14.3) | 0.8 |
| Operated necrotising enterocolitis, n (%) | 7 (4.2) | 3/48 (6.3) | 0.7 |
| Sepsis, n (%) | 30 (17.7) | 7 (14.3) | 0.6 |
| Laser-treated retinopathy of prematurity, n (%) | 4 (2.4) | 3/47 (6.4) | 0.2 |
| Brain MRI at term age, n (%)* | 0.9 | ||
| Normal findings | 96/165 (58.2) | 29/48 (60.4) | |
| Minor pathologies | 27/165 (16.4) | 7/48 (14.6) | |
| Major pathologies | 42/165 (25.5) | 12/48 (25.0) | |
| Mother’s education, n (%) | 0.04 | ||
| ≤12 years | 61/168 (36.3) | 24/45 (53.3) | |
| >12 years | 107/168 (63.7) | 21/45 (46.7) | |
| Father’s education, n (%) | 0.4 | ||
| ≤12 years | 110/166 (66.3) | 32/44 (72.7) | |
| >12 years | 56/166 (33.7) | 12/44 (27.3) |
*The specific MRI protocol and details about the classification of the findings have been previously described by Setänen et al.40
Motor development
All children born very preterm, including those with Full-Scale IQ <70, were able to follow the given instructions without any adaptations of test items and completed the Movement ABC-2. Accordingly, children born very preterm with Full-Scale IQ <70 were included in the analyses regarding DCD, as suggested by the recent European Academy of Childhood Disability recommendations.12 There were nine (5.3%) children born very preterm with CP who were assessed at 11 years of age; they were excluded from the analyses regarding the Movement ABC-2.
Of all the 161 children born very preterm without CP, one did not complete the Movement ABC-2. A total of 142 (88.8%) had a total test score >5th percentile. Of these children born very preterm, 12 (8.5%) had their score between the 5th and 15th percentile in the Movement ABC-2, indicating a risk for motor problems. There were 18 children born very preterm (11.3%) with a total test score ≤5th percentile in the Movement ABC-2; these children were denoted to have DCD after confirming with the Touwen neurological examination that they did not have such neurological findings or other neurological disorders which could explain their poor performance. Twelve of the children born very preterm with DCD were born extremely preterm (<28 gestational weeks) and/or with extremely low birth weight (≤1000 g), representing 18.2% of all (n=66) extremely preterm and/or extremely low birthweight children. All but one of the children born very preterm with DCD were boys. Of the other background characteristics shown in table 1, lower gestational age (p=0.04), bronchopulmonary dysplasia (p=0.04), sepsis (p=0.04) and major brain pathologies on MRI at term age (p=0.02) were associated with DCD.
Cognitive development
The mean value (SD (minimum, maximum)) of the Full-Scale IQ for the whole very preterm study cohort (n=170) was 88.3 (17.0 (40.0, 131.0)). The mean value for the verbal comprehension was 90.3 (14.8 (46.0, 122.0)), for the perceptual reasoning 92.0 (17.1 (40.0, 122.0)), for the working memory 92.6 (16.3 (46.0, 133.0)) and for the processing speed 93.9 (17.4 (47.0, 153.0)). Of all the 161 children born very preterm without CP, 89 (55.3%) performed within the average range (Full-Scale IQ ≥90), 34 (21.1%) had low average performance (Full-Scale IQ ≥80–89), 25 (15.5%) had borderline cognitive development (Full-Scale IQ ≥70–79) and 13 (8.1%) had severe cognitive impairment (Full-Scale IQ <70). The mean values of the Full-Scale IQ and its four indexes are shown by categories of motor outcome in table 2.
Table 2.
Cognitive outcome shown in 11-year-old children born very preterm with CP and according to the performance in the Movement Assessment Battery for Children in children without CP
| CP, n=9 | DCD, ≤5th percentile, n=18 | Children without motor impairment, >5th percentile, n=142 |
P value | |
| Full-Scale IQ | 62.4 (22.8) (40.0, 97.0) |
76.8 (18.2) (40.0, 100.0) |
91.6 (14.3) (52.0, 131.0) |
<0.001* |
| Verbal comprehension | 75.1 (21.1) (46.0, 98.0) |
83.8 (16.3) (46.0, 108.0) |
92.1 (13.4) (60.0, 122.0) |
0.02* |
| Perceptual reasoning | 64.8 (23.1) (40.0, 100.0) |
85.7 (17.9) (51.0, 109.0) |
94.8 (14.6) (62.0, 122.0) |
0.02* |
| Working memory | 77.0 (20.5) (46.0, 109.0) |
79.2 (13.9) (46.0, 97.0) |
95.4 (15.0) (55.0, 133.0) |
<0.001* |
| Processing speed | 72.4 (21.4) (47.0, 106.0) |
83.3 (19.4) (47.0, 118.0) |
96.5 (15.5) (56.0, 153.0) |
0.001* |
The mean values (SD) (minimum, maximum) of Full-Scale IQ and its four indexes are shown.
The outcomes are compared between children with DCD and children without motor impairment (two-sample t-test).
*The results remained statistically significant after adjusting for birth weight, gestational age, and mother’s and father’s education.
CP, cerebral palsy; DCD, developmental coordination disorder.
The Movement ABC-2 scores of children born very preterm without CP correlated positively with Full-Scale IQ (r=0.2, p=0.006), working memory (r=0.3, p<0.001), processing speed (r=0.2, p=0.03) and perceptual reasoning (r=0.2, p=0.03). The scatter plot of the Full-Scale IQ and the Movement ABC-2 is shown in figure 2. Children born very preterm with DCD had lower Full-Scale IQ than children born very preterm without motor impairment (mean 76.8 vs 91.6) (p<0.001). Similarly, children born very preterm with DCD scored lower than children born very preterm without motor impairment in all indexes, as shown in table 2. The results remained statistically significant after adjusting for birth weight, gestational age, and mother’s and father’s education.
Figure 2.
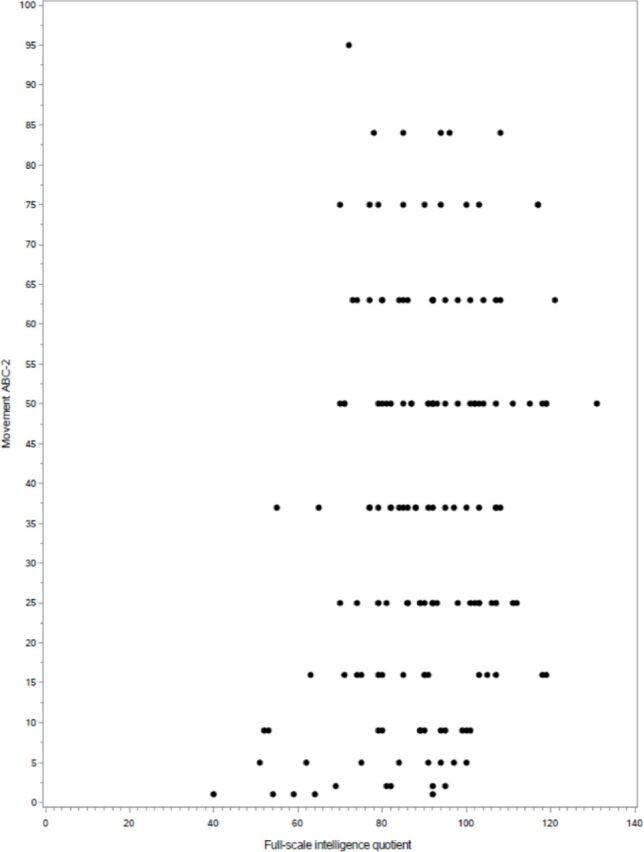
The scatter plot of the Full-Scale IQ and percentiles for the total scores of the Movement Assessment Battery for Children - Second Edition (Movement ABC-2) at 11 years of age in children born very low birth weight (≤1500 g) or at very low gestational age (<32 weeks).
Health-related quality of life
A total of 167 (98.2%) of the followed children born very preterm completed the 17D questionnaire. There were no statistically significant correlations between the Movement ABC-2 and the 17D questionnaire (r=0.1, p=0.06), nor between the Full-Scale IQ and the 17D questionnaire (r=0.07, p=0.4). However, children born very preterm with DCD had lower self-experienced HRQoL compared with children born very preterm without DCD (0.93 vs 0.96, p=0.03). Children born very preterm with DCD showed more problems than children born very preterm without DCD on the dimensions considering vision (0.96 vs 0.99, p=0.008), hearing (0.92 vs 0.98, p=0.01) and speech (0.96 vs 0.99, p=0.007). The HRQoL of children born very preterm with CP did not differ from the HRQoL of children born very preterm without CP (0.94 vs 0.96, p=0.6), nor did the HRQoL in children born very preterm with severe cognitive impairment (Full-Scale IQ <70) from the children born very preterm without cognitive impairment (0.93 vs 0.96, p=0.2). This cohort of children born very preterm reported better self-experienced HRQoL compared with Finnish population-based normative results37(p<0.001). Children born very preterm showed less problems than the normative population on the dimensions considering sleeping (p=0.02), discomfort and symptoms (p<0.001), depression (p<0.001), vitality (p=0.02), appearance (p=0.03), friends (p=0.01), and concentration (p=0.001).
Discussion
This study showed that DCD is still common in 11-year-old children born very preterm in 2000s. Children born very preterm with DCD had worse cognitive development than children born very preterm without motor impairment. Moreover, children born very preterm with DCD reported lower HRQoL than children born very preterm without DCD. However, the HRQoL was higher in this study cohort of children born very preterm than in Finnish norm population.
The finding of a high rate of DCD in children born very preterm in early adolescence is parallel to the recently reported rising trend of non-CP motor impairments in children born extremely preterm at the age of 6.5 and 8 years.7 21 The rate of DCD in children born extremely preterm of this PIPARI study cohort was 18%, while two recent studies from Sweden and Australia have reported non-CP motor impairment rates of 26%–37% in extremely preterm populations.7 21 The Swedish study reported that the rate of non-CP motor impairment was 37% when they used a cut-off based on their control group, but if the normative cut-offs32 had been used the rate would have been 12.5%. Some studies have reported the prevalence of non-CP motor impairment as being higher in boys,7 12 while others have shown no significant difference in the prevalence in boys and girls.21 In the present study, all but one of the children with DCD were boys. However, the small number of children with DCD did not enable reliable statistical analysis regarding sex.
A positive correlation between motor outcome and cognitive development in children born very preterm was found even if the correlations were not strong in magnitude. Children born very preterm with DCD had lower mean scores in the Full-Scale IQ and in all indexes compared with children born very preterm without motor impairment. The differences were clinically significant in magnitude, that is, 15 points for Full-Scale IQ, 8 points for verbal comprehension, 9 for perceptual reasoning, 16 for working memory and 13 for processing speed, all in favour of children without DCD. This is in line with previous studies that have reported lower Full-Scale IQ and processing speed in children born very preterm with DCD at 5 years of age22 and lower perceptual reasoning and processing speed in children born extremely preterm with DCD at 6.5 years of age.21 However, as WISC-IV has some items in the processing speed and the perceptual reasoning index subtests requiring fine motor control (eg, holding a pen, drawing in a small space and manipulating blocks), it is possible that motor impairment may have an effect on the child’s performance in these subtests. According to our results, DCD might also indicate problems in cognitive development at 11 years of age in children born very preterm. Lower motor scores accumulated among boys born very preterm in the present study. Future research may expand current findings about possible mechanisms leading to vulnerability according to sex.
This study showed lower self-experienced HRQoL in children born very preterm with DCD compared with children born very preterm without motor impairment in early adolescence. The affected domains were vision, hearing and speech. The absolute differences in HRQoL results between the groups were minor since the scoring system ranges from 0 to 1. Whether these statistically significant differences have clinical importance is not definite. A previous review using various instruments suggested difficulties in fine motor skills (and causing difficulty, eg, with brushing teeth, washing hair, dressing up and using knife and fork) and in social skills (causing, eg, loneliness and spending more time alone).39 Nevertheless, comparing different instruments should be treated with caution. Self-experienced HRQoL at 11 years of age was better in our study cohort of children born very preterm compared with the test normative at the same age in the Finnish population. This is an unexpected finding as children born very preterm have many impairments potentially lowering their HRQoL. The mothers of the children who withdrew from the study had lower educational level compared with the mothers of the study children. This may have influenced the results and may offer one explanation why the study cohort of children born very preterm reported their HRQoL better compared with the norms. However, we are not aware of differences in general health outcomes in the 1990s and 2000s. In any case, good HRQoL in children born very preterm at 11 years of age is a reassuring information for families with a preterm infant.
The strength of this study was its relatively high follow-up rate (78%) from birth to 11 years of age. The examinations were performed with the latest version of the Movement ABC-2, and a thorough Touwen neurological examination was used to support the definition of DCD. A possible limitation was that the motor assessments were not done repeatedly as suggested by the latest European Academy of Childhood Disability recommendations.12 However, these new guidelines were not available during the data collection. We also chose to use the strict cut-off of fifth percentile to define clinically significant non-CP motor impairment. There was no possibility to compare the rate of DCD with peers born at term due to lack of a control group. To assess cognitive development we used WISC-IV, which is a validated and widely used tool in Finland, and the national cut-offs are precise and up-to-date. Regarding HRQoL, the results the Finnish normative of the same age population were available, although these were based on data collection before 1996. Although the sample size of the whole study group was satisfactory, the total number of children born very preterm with DCD and CP was small, which restricts the power of the statistical analysis concerning these groups and the generalisability of the results.
Conclusions
This study supports previous findings that, even though more preterm born infants survive without CP, they still have an increased risk for DCD. Children born very preterm with DCD showed lower cognitive performance than children born very preterm without DCD. It is important to recognise motor problems early to provide interventions and support services needed and to provide cognitive assessments with a low threshold. The HRQoL of children born very preterm was to a large extent good, but did differ between children born very preterm with DCD and those without motor impairment.
Supplementary Material
Acknowledgments
The PIPARI Study Group: Mikael Ekblad, MD, PhD; Satu Ekblad, RN; Eeva Ekholm, MD, PhD; Annika Eurola, MD; Leena Haataja, MD, PhD; Mira Huhtala, MD, PhD; Jere Jaakkola, BM; Pentti Kero, MD, PhD; Riikka Korja, PhD; Katri Lahti, MD; Helena Lapinleimu, MD, PhD; Liisa Lehtonen, MD, PhD; Tuomo Lehtonen, MD; Marika Leppänen, MD, PhD; Annika Lind, PhD; Jaakko Matomäki, MSc; Jonna Maunu, MD, PhD; Petriina Munck, PhD; Laura Määttänen, MD; Pekka Niemi, PhD; Anna Nyman, PhD; Pertti Palo, MD, PhD; Riitta Parkkola, MD, PhD; Liisi Ripatti, MD, PhD; Päivi Rautava, MD, PhD; Katriina Saarinen, Physiotherapist; Virva Saunavaara, PhD; Sirkku Setänen, MD, PhD; Matti Sillanpää, MD, PhD; Suvi Stolt, PhD; Sanna Sutinen, BM; Päivi Tuomikoski-Koiranen, RN; Timo Tuovinen, BA; Karoliina Uusitalo, MD; Anniina Väliaho, MA; Milla Ylijoki, MD, PhD.
Footnotes
Collaborators: For collaboration regarding the 17D questionnaire: Marjo Apajasalo and Harri Sintonen.
Contributors: KU collected the data, drafted the initial manuscript and revised the manuscript. LH and LL conceptualised and designed the study, designed the data collection instrument, supervised the data collection, revised the manuscript, and critically reviewed the study for intellectual content. AN collected the data and revised the manuscript. MH, LR and PTR revised the manuscript and critically reviewed the study for intellectual content. Moreover, they were the specialists on HRQoL testing. RP supervised the data collection, revised the manuscript and critically reviewed the study for intellectual content. KL collected the data and reviewed the manuscript. MK performed the statistical analysis, and revised and reviewed the manuscript. SS designed the study, collected the data, revised the manuscript and critically reviewed the study for intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding: All phases of this study were supported by a grant from the Arvo and Lea Ylppö Foundation, the Emil Aaltonen Foundation, the Finnish Medical Foundation, the Finnish Cultural Foundation, the South-Western Finnish Foundation of Neonatal Research, the Turku University Research Foundation, and the Yrjö Jahnsson Foundation.
Disclaimer: The authors have no financial relationships relevant to this article to disclose.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The Ethics Review Committee of the Hospital District of Southwest Finland approved the study protocol in 2000 and in 2012.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. LL (ORCID identifier: https://orcid.org/0000-0001-8925-2594https://orcid.org/0000-0001-8925-2594) is the data manager, and the Hospital District of Southwest Finland is the data holder. Reuse of data is possible only with the permission of the data holder.
References
- 1.Platt MJ, Cans C, Johnson A, et al. Trends in cerebral palsy among infants of very low birthweight (<1500 g) or born prematurely (<32 weeks) in 16 European centres: a database study. Lancet 2007;369:43–50. 10.1016/S0140-6736(07)60030-0 [DOI] [PubMed] [Google Scholar]
- 2.Groenendaal F, Termote JUM, van der Heide-Jalving M, et al. Complications affecting preterm neonates from 1991 to 2006: what have we gained? Acta Paediatr 2010;99:354–8. 10.1111/j.1651-2227.2009.01648.x [DOI] [PubMed] [Google Scholar]
- 3.Sellier E, Platt MJ, Andersen GL, et al. Decreasing prevalence in cerebral palsy: a multi-site European population-based study, 1980 to 2003. Dev Med Child Neurol 2016;58:85–92. 10.1111/dmcn.12865 [DOI] [PubMed] [Google Scholar]
- 4.Fawke J. Neurological outcomes following preterm birth. Semin Fetal Neonatal Med 2007;12:374–82. 10.1016/j.siny.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 5.Guerrot A-M, Chadie A, Torre S, et al. Compared outcomes of very preterm infants born in 2000 and 2005. Acta Paediatr 2012;101:731–5. 10.1111/j.1651-2227.2012.02678.x [DOI] [PubMed] [Google Scholar]
- 6.Holsti A, Adamsson M, Serenius F, et al. Two-Thirds of adolescents who received active perinatal care after extremely preterm birth had mild or no disabilities. Acta Paediatr 2016;105:1288–97. 10.1111/apa.13499 [DOI] [PubMed] [Google Scholar]
- 7.Spittle AJ, Cameron K, Doyle LW, et al. Motor impairment trends in extremely preterm children: 1991-2005. Pediatrics 2018;141. 10.1542/peds.2017-3410 [DOI] [PubMed] [Google Scholar]
- 8.Pascal A, Govaert P, Oostra A, et al. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev Med Child Neurol 2018;60:342-355. 10.1111/dmcn.13675 [DOI] [PubMed] [Google Scholar]
- 9.Marlow N, Wolke D, Bracewell MA, et al. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med 2005;352:9–19. 10.1056/NEJMoa041367 [DOI] [PubMed] [Google Scholar]
- 10.Anderson PJ. Neuropsychological outcomes of children born very preterm. Semin Fetal Neonatal Med 2014;19:90–6. 10.1016/j.siny.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 11.Nyman A, Korhonen T, Munck P, et al. Factors affecting the cognitive profile of 11-year-old children born very preterm. Pediatr Res 2017;82:324–32. 10.1038/pr.2017.64 [DOI] [PubMed] [Google Scholar]
- 12.Blank R, Barnett AL, Cairney J, et al. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev Med Child Neurol 2019;61:242–85. 10.1111/dmcn.14132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown-Lum M, Zwicker JG. Brain imaging increases our understanding of developmental coordination disorder: a review of literature and future directions. Curr Dev Disord Rep 2015;2:131–40. 10.1007/s40474-015-0046-6 [DOI] [Google Scholar]
- 14.Biotteau M, Chaix Y, Blais M, et al. Neural signature of dcd: a critical review of MRI neuroimaging studies. Front Neurol 2016;7:227. 10.3389/fneur.2016.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahti K, Saunavaara V, Munck P, et al. Diffusion tensor imaging is associated with motor outcomes of very preterm born children at 11 years of age. Acta Paediatr 2020;109:1–8. 10.1111/apa.15004 [DOI] [PubMed] [Google Scholar]
- 16.Dewey D, Thompson DK, Kelly CE, et al. Very preterm children at risk for developmental coordination disorder have brain alterations in motor areas. Acta Paediatr 2019;108:1649–60. 10.1111/apa.14786 [DOI] [PubMed] [Google Scholar]
- 17.Zwicker JG, Missiuna C, Harris SR, et al. Developmental coordination disorder: a review and update. Eur J Paediatr Neurol 2012;16:573–81. 10.1016/j.ejpn.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 18.Ferrari F, Gallo C, Pugliese M, et al. Preterm birth and developmental problems in the preschool age. Part I: minor motor problems. J Matern Fetal Neonatal Med 2012;25:2154-9. 10.3109/14767058.2012.696164 [DOI] [PubMed] [Google Scholar]
- 19.Setänen S, Lehtonen L, Parkkola R, et al. The motor profile of preterm infants at 11 Y of age. Pediatr Res 2016;80:389–94. 10.1038/pr.2016.90 [DOI] [PubMed] [Google Scholar]
- 20.Harrowell I, Hollén L, Lingam R, et al. The impact of developmental coordination disorder on educational achievement in secondary school. Res Dev Disabil 2018;72:13–22. 10.1016/j.ridd.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolk J, Farooqi A, Hafström M, et al. Developmental coordination disorder and its association with developmental comorbidities at 6.5 years in apparently healthy children born extremely preterm. JAMA Pediatr 2018;172:765–74. 10.1001/jamapediatrics.2018.1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Hus JW, Potharst ES, Jeukens-Visser M, et al. Motor impairment in very preterm-born children: links with other developmental deficits at 5 years of age. Dev Med Child Neurol 2014;56:587–94. 10.1111/dmcn.12295 [DOI] [PubMed] [Google Scholar]
- 23.Huhtala M, Korja R, Rautava L, et al. Health-Related quality of life in very low birth weight children at nearly eight years of age. Acta Paediatr 2016;105:53–9. 10.1111/apa.13241 [DOI] [PubMed] [Google Scholar]
- 24.Natalucci G, Bucher HU, Von Rhein M, et al. Population based report on health related quality of life in adolescents born very preterm. Early Hum Dev 2017;104:7–12. 10.1016/j.earlhumdev.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 25.Zwicker JG, Harris SR. Quality of life of formerly preterm and very low birth weight infants from preschool age to adulthood: a systematic review. Pediatrics 2008;121:e366–76. 10.1542/peds.2007-0169 [DOI] [PubMed] [Google Scholar]
- 26.Dewey D, Volkovinskaia A. Health-Related quality of life and peer relationships in adolescents with developmental coordination disorder and attention-deficit-hyperactivity disorder. Dev Med Child Neurol 2018;60:711–8. 10.1111/dmcn.13753 [DOI] [PubMed] [Google Scholar]
- 27.Karras HC, Morin DN, Gill K, et al. Health-Related quality of life of children with developmental coordination disorder. Res Dev Disabil 2017;2018:1–11. [DOI] [PubMed] [Google Scholar]
- 28.Pipari Development and functioning of very low birth weight infants from infancy to school age. Available: https://sites.utu.fi/pipari/en/
- 29.Setänen S, Lehtonen L, Lapinleimu H, et al. Lessons learnt about the long-term neurodevelopment in very preterm born children in the PIPARI study. Lääketieteellinen Aikakausk Duodecim 2018;134:118–25. [Google Scholar]
- 30.Himmelmann K, Hagberg G, Beckung E, et al. The changing panorama of cerebral palsy in Sweden. IX. prevalence and origin in the birth-year period 1995-1998. Acta Paediatr 2005;94:287-94. 10.1111/j.1651-2227.2005.tb03071.x [DOI] [PubMed] [Google Scholar]
- 31.Henderson S, Sugden D. The movement assessment battery for children. Phys Ther 1992;23:286–94. [DOI] [PubMed] [Google Scholar]
- 32.Henderson S, Sugden D, Barnett A. Movement Assessment Battery for Children (Examiner’s Manual. London: Pearson Assessment, 2007. [Google Scholar]
- 33.Touwen BCL. The examination of the child with minor neurological dysfunction, 1979. [Google Scholar]
- 34.Hadders-Algra M. The neurological examination of the child with minor neurological dysfunction. 3rd Edn London: Mac Keith Press, 2010. [Google Scholar]
- 35.Wechsler D. Wechsler intelligence scale for children -IV. Käsikirja I. Esitys JA -Pisteytysohjeet (Handbook I. administration and scoring. Jyväskylä Psykologien Kustannus, 2011. [Google Scholar]
- 36.Wechsler D. Wechsler intelligence scale for children -IV. Käsikirja II. Teoriatausta, Standardointi JA Tulkinta (Handbook II. theoretical background, standardization and interpretation. Jyväskylä Psykologien Kustannus 2011, 2011. [Google Scholar]
- 37.Apajasalo M, Rautonen J, Holmberg C, et al. Quality of life in pre-adolescence: a 17-dimensional health-related measure (17D). Qual Life Res 1996;5:532–8. 10.1007/bf00439227 [DOI] [PubMed] [Google Scholar]
- 38.Apajasalo M, Rautonen J, Holmberg C, et al. 17D. Available: http://www.15d-instrument.net/16d-and-17d/17d/
- 39.Zwicker JG, Harris SR, Klassen AF. Quality of life domains affected in children with developmental coordination disorder: a systematic review. Child Care Health Dev 2013;39:562-80. 10.1111/j.1365-2214.2012.01379.x [DOI] [PubMed] [Google Scholar]
- 40.Setänen S, Haataja L, Parkkola R, et al. Predictive value of neonatal brain MRI on the neurodevelopmental outcome of preterm infants by 5 years of age. Acta Paediatr 2013;102:492–7. 10.1111/apa.12191 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


