Abstract
Background
Patients with biliary tract cancer (BTC) have poor prognosis with few treatment options. Bintrafusp alfa, a first-in-class bifunctional fusion protein composed of the extracellular domain of the transforming growth factor (TGF)-βRII receptor (a TGF-β ‘trap’) fused to a human IgG1 antibody blocking programmed death ligand 1 (PD-L1), has shown clinical efficacy in multiple solid tumors.
Methods
In this phase I, open-label trial expansion cohort, Asian patients with BTC whose disease progressed after first-line chemotherapy received bintrafusp alfa 1200 mg every 2 weeks until disease progression, unacceptable toxicity, or withdrawal. The primary endpoint is safety/tolerability, while the secondary endpoints include best overall response per Response Evaluation Criteria in Solid Tumors version 1.1.
Results
As of August 24, 2018, 30 patients have received bintrafusp alfa for a median of 8.9 (IQR 5.7–32.1) weeks; 3 patients remained on treatment for >59.7 weeks. Nineteen (63%) patients experienced treatment-related adverse events (TRAEs), most commonly rash (17%), maculopapular rash and fever (13% each), and increased lipase (10%). Eleven (37%) patients had grade ≥3 TRAEs; three patients had grade 5 events (septic shock due to bacteremia, n=1; interstitial lung disease (reported term: interstitial pneumonitis), n=2). The objective response rate was 20% (95% CI 8 to 39) per independent review committee (IRC), with five of six responses ongoing (12.5+ to 14.5+ months) at data cut-off. Two additional patients with durable stable disease had a partial response per investigator. Median progression-free survival assessed by IRC and overall survival were 2.5 months (95% CI 1.3 to 5.6) and 12.7 months (95% CI 6.7 to 15.7), respectively. Clinical activity was observed irrespective of PD-L1 expression and microsatellite instability-high status.
Conclusions
Bintrafusp alfa had clinical activity in Asian patients with pretreated BTC, with durable responses. Based on these results, bintrafusp alfa is under further investigation in patients with BTC (NCT03833661 and NCT04066491).
Trial registration number
Keywords: programmed cell death 1 receptor, tumor microenvironment, immunotherapy, gastrointestinal neoplasms
Background
Biliary tract cancer (BTC) is a rare, lethal, heterogeneous gastrointestinal disease further subclassified into intrahepatic cholangiocarcinoma (IHCC), extrahepatic cholangiocarcinoma (EHCC), gallbladder cancer, and ampullary cancer.1 BTC causes 2.3 deaths per 100,000 population globally and is most common in Asia—in particular China, Japan, the Republic of Korea, and Thailand—and Latin America, although its incidence varies geographically and depending on the subtype.1–3 Due to the initially asymptomatic nature of BTC, many patients present with locally advanced or metastatic disease at initial diagnosis and have poor prognosis.1 4
The current recommended first-line standard of care for unresectable, locally advanced, or metastatic disease is the combination of gemcitabine and cisplatin.4–6 There is currently no globally accepted standard-of-care treatment for locally advanced/metastatic BTC for which standard chemotherapy has failed; however, the treatment landscape is evolving.7 8 According to a meta-analysis of second-line chemotherapy, objective response rates in prospective studies are <10% (with short response durations), and the median progression-free survival (mPFS) and overall survival (mOS) are <3 and <7 months, respectively.7 The ABC-06 phase III trial in UK patients showed clinical benefit versus active symptom control, with mOS of 6.2 months with oxaliplatin and 5-fluorouracil (mFOLFOX) vs 5.3 months with active symptom control.9 Preliminary results from the ClarIDHy phase III trial in patients with cholangiocarcinoma and mutations in the isocitrate dehydrogenase 1 (IDH1) gene showed ivosidenib versus placebo improved mPFS (2.7 vs 1.4 months) and OS (10.8 vs 9.7 months), with 46% of patients reporting grade 3–4 treatment-related adverse events (TRAEs).10 Despite positive outcomes with targeted agents,10–13 there remains an urgent unmet need in patients with BTC for which standard chemotherapy has failed.
One potential way to address this need is by focusing and enhancing the antitumor activity of the immune system.14 Because tumor infiltration by certain cellular mediators of the adaptive immune system has been correlated with improved outcomes in BTC,15 immunotherapy has emerged as a promising treatment strategy. Indeed, high mutation rates in programmed death protein 1 (PD-1) and its ligand programmed death ligand 1 (PD-L1) have been observed,16 suggesting an important role of the PD-(L)1 pathway in BTC pathogenesis. While the anti-PD-1 inhibitor, pembrolizumab, is approved in Japan for advanced, microsatellite instability-high (MSI-H) solid tumors that progressed on chemotherapy, there are limited clinical data available to assess the efficacy of immunotherapies in BTC and no indication-specific approvals.17–21
An additional mechanism shown to have a potentially important role in this cancer type is the transforming growth factor β (TGF-β) pathway. TGF-β signaling can promote tumor growth by exerting regulatory effects on both cancer cells and immune cells present in the tumor microenvironment (TME) and by inducing angiogenesis, fibrosis, and epithelial-mesenchymal transition, all of which are commonly associated with alterations of the TME and increased invasiveness in BTCs.22–24 Clinical data support the importance of TGF-β, as alterations within this pathway are frequently observed in BTC.25 26 Therefore, removing TGF-β from the TME while simultaneously inhibiting the PD-(L)1 pathway—a non-redundant and complementary immunostimulatory mechanism—may provide a novel treatment approach in BTC.
Bintrafusp alfa (M7824) is a first-in-class bifunctional fusion protein composed of the extracellular domain of the human TGF-β receptor II (TGF-βRII or TGF-β ‘trap’) fused via a flexible linker to the C-terminus of each heavy chain of an IgG1 antibody blocking PD-L1 (anti-PD-L1). Data from two phase I trials of bintrafusp alfa (NCT02517398 and NCT02699515) have shown a manageable safety profile and encouraging early signs of clinical activity in patients with heavily pretreated advanced solid tumors.27–31 We report results from an expansion cohort of the phase I study designed to investigate the safety and efficacy of bintrafusp alfa in Asian patients with BTC unselected for PD-L1 expression and for which standard chemotherapy has failed.
Methods
Study design and participants
This report describes an expansion cohort of a phase I, open-label trial of bintrafusp alfa in Asian (Japan, Korea, Taiwan) patients with metastatic or locally advanced solid tumors. Eligible patients had histologically or cytologically confirmed BTC, with at least one lesion measurable by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, in patients who had failed prior chemotherapy (including in the neoadjuvant or adjuvant setting). Patients were aged 20 years or older; had a life expectancy of at least 12 weeks; an Eastern Cooperative Oncology Group performance status ≤1; and adequate hepatic, renal, and hematological function. In addition, the availability of tumor (primary or metastatic) archival material or fresh biopsies within 28 days before the first administration of bintrafusp alfa was mandatory. Patient selection was not based on tumor expression of PD-L1 or other biomarkers. Exclusion criteria included prior therapy with inhibitors of T cell coregulatory proteins or with any antibody/drug targeting TGF-β/TGF-β receptor, significant acute or chronic infections, an active autoimmune disease, and rapidly progressive disease (PD; which, in the opinion of the investigator, may predispose to inability to tolerate treatment or trial procedures). Full details on exclusion/inclusion criteria are available in the online supplementary information.
jitc-2020-000564supp001.pdf (39.2MB, pdf)
Each patient provided written informed consent before study enrollment.
Procedures
Bintrafusp alfa was administered at 1200 mg via intravenous infusion over 1 hour once every 2 weeks until confirmed PD, unacceptable toxicity, or trial withdrawal. Dosing modifications, such as changes in infusion rate, and dose delays were allowed; however, dose reductions were not permitted. To mitigate potential infusion-related reactions, premedication with an antihistamine and acetaminophen 30–60 min prior to each dose of bintrafusp alfa was mandatory. Steroids as premedication were not allowed. PD-L1 immunohistochemistry (IHC) was performed by using antibody clone 73-10 (Dako PD-L1 IHC 73-10 pharmDx; Dako, Carpinteria, California, USA) on fresh or archival tissue; tumors were categorized based on the proportion of tumor cells expressing PD-L1 according to a threshold of 1% as positive (≥1%) or negative (<1%). To assess the correlation between specific TGF-β-related or immune-related biomarkers and patient response to bintrafusp alfa treatment, gene expression analysis was done on pretreatment tumor samples. Full details on biomarker analyses can be found in the online supplementary information.
Outcomes
The primary endpoints of this trial are the number, severity, and duration of TRAEs according to National Cancer Institute Common Terminology Criteria for Adverse (NCI-CTCAE) version 4.03. Safety was assessed and documented at every visit, which included monitoring for adverse events, evaluation of performance status, physical examination, and clinical laboratory tests. Immune-related adverse events (irAEs) were identified using a list of preselected Medical Dictionary for Regulatory Activities (MedDRA) terms and must have begun after the first administration of bintrafusp alfa and no more than 90 days after the last treatment dose. Also, irAEs must have been treated with corticosteroids, immunosuppressants, or hormonal therapy and have no clear etiology. Secondary endpoints of this expansion cohort include best overall response (BOR) per independent review committee (IRC) and investigator assessment, duration of response, disease control rate, PFS per IRC assessment, and OS. The results described in this report include efficacy as assessed by the investigator and IRC. Tumor response was evaluated by CT or MRI and confirmed by repeated imaging assessment ≥4 weeks from the first documentation of response. Assessments were performed every 6 weeks during the treatment period, then every 12 weeks.
Statistical analysis
Analysis of safety and efficacy was performed in all patients who received at least one dose of bintrafusp alfa. Along with proportions of patients with objective responses, corresponding exact two-sided 95% CIs were calculated using the Clopper-Pearson method. Duration of tumor response, PFS, and OS were analyzed using the Kaplan-Meier method.
Results
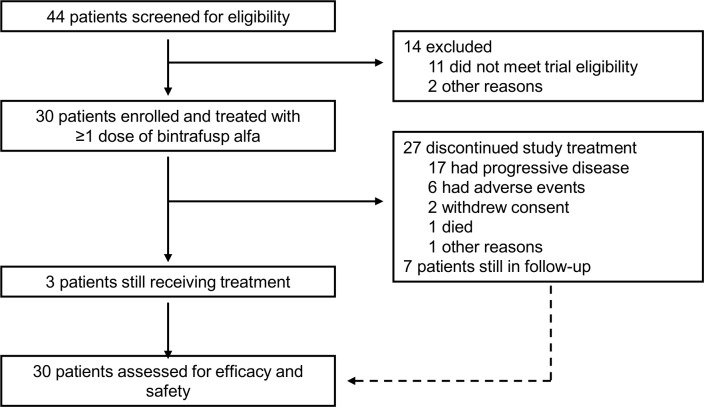
From February 1, 2017 to May 30, 2017, 30 Asian patients with pretreated BTC were enrolled (figure 1). The median age was 67 (IQR 58–69) years, and most (n=19, 63%) were male (table 1). In terms of disease site, 12 (40%) patients had gallbladder cancer, 10 (33%) had IHCC, 7 (23%) had EHCC, and 1 (3%) had ampullary cancer. Sixteen (53%) patients had positive PD-L1 expression, as defined by ≥1% tumor cell PD-L1 expression. At first diagnosis, 12 (40%) patients presented with distant metastatic disease. All 30 patients had received prior anticancer therapy, including platinum-based therapies in 25 (83%) patients.
Figure 1.
Trial profile.
Table 1.
Baseline patient and disease characteristics
| N=30 | |
| Sex | |
| Male | 19 (63) |
| Female | 11 (37) |
| Age, years | |
| Median (IQR) | 67 (58–69) |
| ECOG performance status | |
| 0 | 8 (27) |
| 1 | 22 (73) |
| Biliary tract cancer classification | |
| Gallbladder cancer | 12 (40) |
| Intrahepatic cholangiocarcinoma | 10 (33) |
| Extrahepatic cholangiocarcinoma | 7 (23) |
| Ampullary cancer | 1 (3) |
| Number of prior anticancer therapies* | |
| 1 | 26 (87) |
| 2 | 3 (10) |
| 3 | 1 (3) |
| PD-L1 expression† | |
| Positive | 16 (53) |
| Negative | 13 (43) |
| Not evaluable | 1 (3) |
| HBV/HCV positivity | |
| HBsAg | 1 (3) |
| HBsAb | 8 (27) |
| HBcAb | 13 (43) |
| HBV-DNA | 0 |
| HCV Ab | 0 |
| Immune phenotype status | |
| Immune-desert | 3 (10) |
| Immune-excluded | 23 (77) |
| Inflamed | 2 (7) |
| Not evaluable | 2 (7) |
Data are n (%) unless otherwise specified.
Positive: PD-L1 expression in ≥1% of tumor cells; negative: PD-L1 expression in <1% of tumor cells.
*Include neoadjuvant and adjuvant therapies.
†Defined as the proportion of tumor cells showing membranous PD-L1 staining.
HCV Ab, antibody [NOTE: THIS IS PUTTING HCV Ab IN PROOF. SHOULD BE: "Ab, antibody"]; ECOG, Eastern Cooperative Oncology Group; HBcAb, hepatitis B virus core antibody; HBsAb, hepatitis B virus surface antibody; HBsAg, hepatitis B virus surface antigen; HBV, hepatitis B virus; HBV-DNA, hepatitis B virus DNA; HCV, hepatitis C virus; PD-L1, programmed death ligand 1.
As of the database cut-off (August 24, 2018), the median Kaplan-Meier estimate of follow-up was 15.3 months. The median duration of therapy was 8.9 (IQR 5.7–32.1) weeks, and three patients remained on active treatment (for >59.7 weeks). In the 27 patients who discontinued treatment with bintrafusp alfa, the most common reason was PD (n=17).
Among the 30 patients who received bintrafusp alfa, the most common TRAEs occurring at any grade were rash (17%), fever and maculopapular rash (13% each), and lipase increased (10%) (table 2). Eleven (37%) patients experienced grade ≥3 TRAEs; six patients experienced at least one TRAE of maximum grade 3, and TRAEs of maximum grade 4 occurred in two patients (amylase increased and lipase increased; amylase increased, aspartate aminotransferase increased, and lipase increased).
Table 2.
Treatment-related adverse events occurring in ≥5% of patients or of grade 3–4 severity
| N=30 | ||
| Any grade | Grade 3–4 | |
| Any treatment-related adverse event | 19 (63) | 11 (37) |
| Rash | 5 (17) | 4 (13) |
| Fever | 4 (13) | 0 |
| Maculopapular rash | 4 (13) | 0 |
| Lipase increased | 3 (10) | 3 (10) |
| Anemia | 2 (7) | 1 (3) |
| Fatigue | 2 (7) | 0 |
| Hypothyroidism | 2 (7) | 0 |
| Alanine aminotransferase increased | 2 (7) | 1 (3) |
| Amylase increased | 2 (7) | 2 (7) |
| Aspartate aminotransferase increased | 2 (7) | 1 (3) |
| Blood alkaline phosphatase increased | 2 (7) | 0 |
| Gamma-glutamyltransferase increased | 2 (7) | 2 (7) |
| Infusion-related reaction | 2 (7) | 0 |
| Eczema | 1 (3) | 1 (3) |
| Lichenoid dermatitis | 1 (3) | 1 (3) |
| Lip squamous cell carcinoma | 1 (3) | 1 (3) |
| Seborrheic keratosis | 1 (3) | 1 (3) |
| Skin lesions* | 2 (7) | 0 |
| Keratoacanthoma | 2 (7) | 0 |
Data are n (%).
*Include MedDRA V.21.0 preferred terms squamous cell carcinoma of the skin, basal cell carcinoma, keratoacanthoma, hyperkeratosis, and actinic keratosis.
Three patients died due to toxicities. The first patient was from Taiwan and had a medical history of ichthyosis vulgaris; the patient developed symptoms of an infection before an emergency room visit and had taken amoxicillin/clavulanic acid without a physician visit, in addition to receiving local and systemic steroids for treatment-related lichenoid dermatitis over 4 months. Subsequently, the patient suffered from septic shock, which was attributed by the investigator to Staphylococcus aureus bacteremia, a secondary infection of an underlying skin condition, which ultimately led to death on day 249 (14 days after the last dose of bintrafusp alfa). The second patient was from Japan and had grade 3 interstitial lung disease (ILD; reported term: interstitial pneumonitis) after three doses of bintrafusp alfa, which improved to grade 1 on treatment with prednisolone, but ultimately led to discontinuation of bintrafusp alfa. The patient subsequently initiated chemotherapy due to PD, and 6 months after initial ILD diagnosis and 6 months and 4 days after last bintrafusp alfa administration, the ILD intensified to grade 4 and led to death. The third patient was from Japan and was hospitalized for grade 2 nausea, vomiting, and appetite loss on day 33. Grade 3 ILD (reported term: interstitial pneumonitis) developed in hospital on day 45—after three doses of bintrafusp alfa and 17 days after the last dose—which intensified to grade 4 after 3 days despite treatment with prednisolone, tazobactam-piperacillin, and sulfamethoxazole-trimethoprim, and ultimately led to death. Information on the results of an infectious blood panel was not provided by the hospital.
Treatment discontinuation due to a TRAE was observed in six patients (anemia (n=1), ILD (n=1; described above), alanine aminotransferase increased and aspartate aminotransferase increased (n=1), amylase increased and lipase increased (n=1), gamma-glutamyltransferase increased (n=1), and septic shock (n=1; described above)). Maculopapular rash (n=4) was the only irAE that occurred in >2 patients (online supplementary table S1). No grade ≥3 infusion-related adverse events were observed. Two patients had potentially TGF-β-mediated skin lesions (keratoacanthoma).
jitc-2020-000564supp002.pdf (78.8KB, pdf)
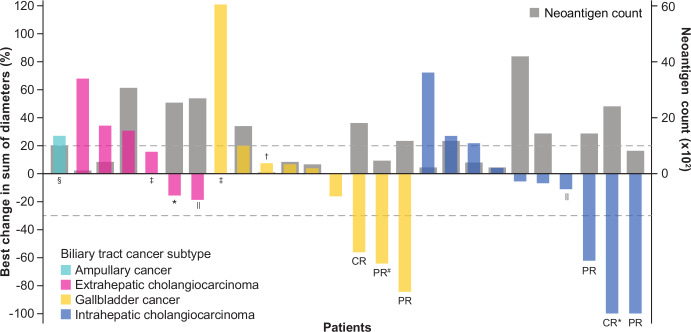
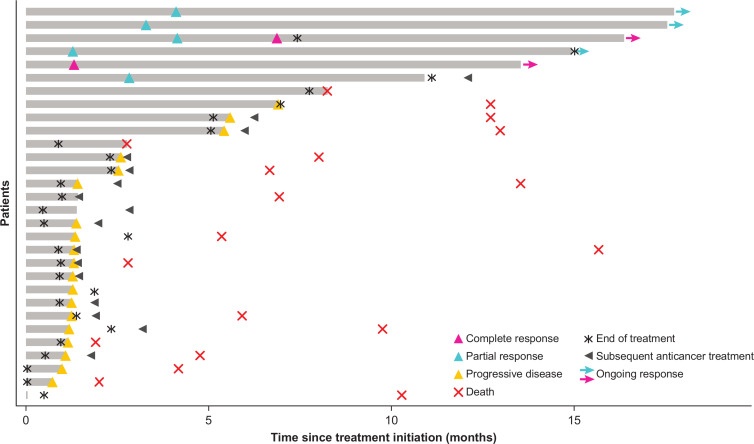
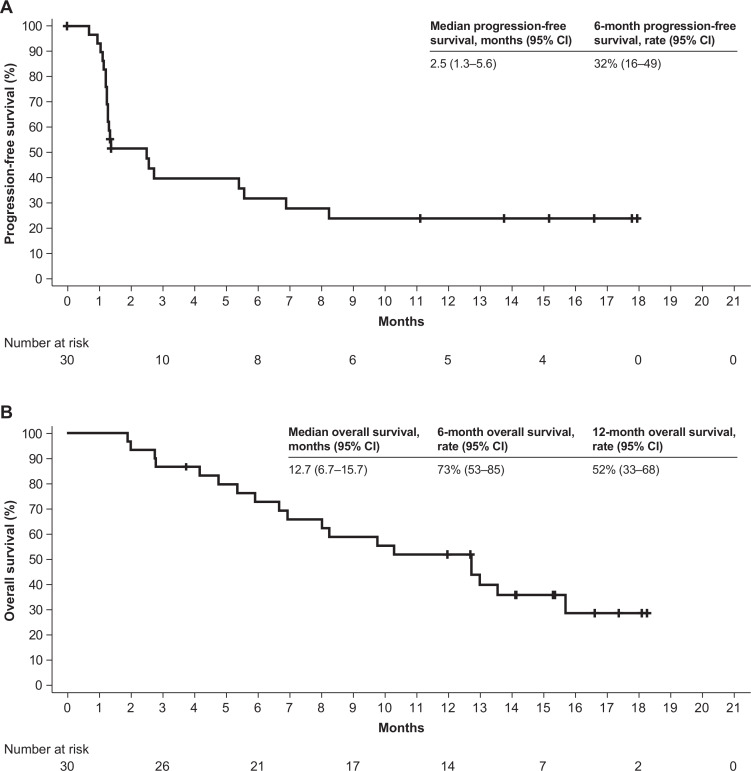
Objective responses were confirmed in six patients as adjudicated by the IRC, for an objective response rate of 20% (95% CI 8 to 39) according to RECIST version 1.1 (figure 2, table 3). Two patients had a complete response (CR), each with a response duration of 12.5+ months. Among the four patients with a partial response (PR), three had a response that was ongoing at the time of database cut-off, with response durations of 13.8+, 13.9+, and 14.5+ months. The fourth patient with a PR had a response duration of 8.3 months per IRC, which was considered ongoing as of the last assessment, and an investigator-assessed duration of response of 9.7 months before disease progression (figure 3). One of the patients with a PR per IRC and BTC subtype of gallbladder cancer had, as assessed by the investigator, initial pseudoprogression on the first evaluation visit, followed by a PR that was ongoing for 14.5+ months and tumor regression of 65% from baseline as of the cut-off date. At the time of this writing, this patient’s response was near CR and was ongoing (26+ months). Six patients had a BOR of stable disease per IRC, for a disease control rate of 40%. In addition to the six patients with a confirmed response per IRC, two patients with a BOR of stable disease per IRC (time to progression, 6.9 and 8.2 months) had a confirmed PR for 2.8 and 5.6 months per investigator (investigator-assessed objective response rate, 23%; table 3, online supplementary figure S1), and one patient with ampullary cancer had shrinkage of lung and liver non-target lesions after having stopped study treatment and receiving radiotherapy (Gamma Knife radiosurgery; Elekta, Stockholm, Sweden) for a brain lesion, for a total clinical response rate (objective response rate + delayed response after initial progression) of 30% (9 of 30) (figure 2, online supplementary figure S1). The mPFS was 2.5 months (95% CI 1.3 to 5.6), with 6-month and 12-month PFS rates of 32% and 24%, respectively. Furthermore, an mOS of 12.7 months (95% CI 6.7 to 15.7) and 6-month and 12-month OS rates of 73% and 52%, respectively, were observed (figure 4).
Figure 2.
Change in target lesions from baseline as adjudicated by the IRC. Responses were assessed in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The upper dotted line represents progression at 20% increase in size of target lesions, and the lower dotted line represents the RECIST boundary for complete response or partial response at 30% decrease in size of target lesions. Patients with no postbaseline assessment (n=2) or no target lesions identified by the IRC before first dose (n=2) are not displayed. *Patients with MSI-H phenotype. †MSI phenotype not available due to no leftover sample. ‡Patients with unavailable tumor mutation count data. §Patient with poststudy tumor shrinkage of non-target lesions. ‖Patients with an investigator-assessed best overall response of partial response. #Patient with a partial response following pseudoprogression per investigator assessment (best overall response per investigator, progressive disease). CR, complete response; MSI-H, microsatellite instability-high; PR, partial response.
Table 3.
Efficacy outcomes
| By investigator (N=30) |
By IRC (N=30) |
|
| Best overall response | ||
| Complete response | 1 (3) | 2 (7) |
| Partial response | 6 (20) | 4 (13) |
| Stable disease | 4 (13) | 6 (20) |
| Progressive disease | 17 (57) | 16 (53) |
| Not evaluable | 2 (7) | 2 (7) |
| Objective response rate | 7 (23%, 95% CI 10 to 42) | 6 (20%, 95% CI 8 to 39) |
| Objective response by biliary tract cancer subtype | ||
| Ampullary cancer | 0/1 (0) | 0/1 (0) |
| Extrahepatic cholangiocarcinoma | 1/7 (14) | 0/7 (0) |
| Gallbladder cancer | 2/12 (17)* | 3/12 (25) |
| Intrahepatic cholangiocarcinoma | 4/10 (40%) | 3/10 (30%) |
| Disease control rate | 11 (37%, 95% CI 20 to 56) | 12 (40%, 95% CI 23 to 59) |
| Median duration of response (range), months | 9.7 (2.8–12.5) | NE (8.3–14.5) |
| Median progression-free survival (95% CI), months | 2.5 (1.3 to 4.0) | 2.5 (1.3 to 5.6) |
| Median overall survival (95% CI), months | 12.7 (6.7 to 15.7) | |
Data are n (%) unless otherwise specified.
Responses were assessed in accordance with Response Evaluation Criteria in Solid Tumors version 1.1.
Only confirmed responses are included.
*One patient with gallbladder cancer had a partial response per IRC and initial pseudoprogression on the first evaluation visit, followed by a partial response as assessed by the investigator (investigator-assessed best overall response, progressive disease).
NE, not evaluable.
Figure 3.
Time to and duration of response as adjudicated by the IRC. Responses were assessed in accordance with Response Evaluation Criteria in Solid Tumors version 1.1.
Figure 4.
Kaplan-Meier analysis of progression-free survival as adjudicated by the IRC (A) and overall survival (B).
jitc-2020-000564supp003.pdf (111KB, pdf)
The objective response rates by BTC subtype were 30% and 25% in patients with IHCC and gallbladder cancer, respectively, as adjudicated by IRC. Complete responses were recorded in one patient with gallbladder cancer and one patient with IHCC; no objective response was recorded in patients with ampullary cancer or EHCC. Of the two additional patients with a confirmed PR per investigator assessment (described above), one patient had IHCC and one had EHCC (figure 2).
Among patients with PD-L1-positive (expression on ≥1% of tumor cells) and PD-L1-negative tumors, the confirmed objective response rates per IRC were 19% and 23%, respectively (online supplementary figure S2). Responses were observed across the range of expression levels of various biomarkers relevant to the mechanism of action of bintrafusp alfa, including markers of CD8+ T cells, cytokines (IFNG and TGFB1), and mesenchymal markers (VIM and TWIST1) (online supplementary figure S3A-F). Similarly, there was no apparent correlation between response and tumor mutation count (figure 2). Two patients were identified as having MSI-H status, with one patient having stable disease and one patient achieving a CR (figure 2). Furthermore, immune phenotype was evaluable in tumor samples from 28 patients, and responses were observed in patients with immune-desert tumors (one PR; defined as <1% of the tumor stroma area populated by lymphocytes, no dense immune cell infiltrates, and no contact of immune cells with tumor cells) and in those with immune-excluded tumors (three PRs and two CRs; defined as ≥1% of the tumor stroma area populated by lymphocytes, immune cells possibly located in the immediate vicinity of tumor cells but not efficiently infiltrate tumor cell clusters, and very infrequent physical contact between lymphocytes and tumor cells) (online supplementary figure S3G).
jitc-2020-000564supp004.pdf (123.7KB, pdf)
jitc-2020-000564supp005.pdf (131.8KB, pdf)
Discussion
Bintrafusp alfa demonstrated clinical activity in this cohort of Asian patients with BTC whose disease has progressed with standard chemotherapy, consistent with findings from the dose-escalation phase28 30 and other expansion cohorts of the present trial27 and the global, phase I study (NCT02517398).29 31 Indeed, the objective response rate was 20% per independent read, with durable responses observed. Additionally, the response rate per investigator was 23%, and the total clinical response (objective response rate + delayed response after initial progression) was 30%. Furthermore, second-line treatment with bintrafusp alfa showed an mOS of 12.7 months in the overall population. These efficacy results compare favorably with historical data in pretreated patients receiving second-line or later treatment, where most chemotherapy regimens have an mOS <1 year,7 as well as with results observed with immune cell inhibitor monotherapy in a Japanese phase I study of nivolumab (JapicCTI-153098), a phase II study of pembrolizumab (NCT02628067), and the phase III study (NCT01926236) of mFOLFOX (objective response rates of 3%, 6%, and 5%, respectively).9 18 19
Clinical activity was observed across BTC subtypes and irrespective of PD-L1 expression on tumor cells or in the TME (data not shown). Previous studies have suggested a link between response to anti-PD-L1 therapy and immune phenotype.32 However, responses observed in this study were recorded in patients with immune-desert and immune-excluded phenotypes, with most (five of six) occurring in the latter. Additionally, no correlation was observed between response and tumor mutation count, a surrogate for tumor mutational burden. Finally, only one of the six responders had an MSI-H phenotype. Overall, the lack of a discrete, single biomarker associated with response to bintrafusp alfa in this small cohort of patients underscores the value of dual inhibition of both the TGF-β and PD-L1 pathways as a promising therapeutic option for patients with locally advanced/metastatic BTC.
Incidence, severity and type of irAEs observed with bintrafusp alfa were comparable with those seen with immune checkpoint inhibitors.33 In addition to one septic shock event due to bacteremia that led to death, two ILD (interstitial pneumonitis) events led to death in this cohort, one of which occurred 6 months after the last bintrafusp alfa dose, which was followed by chemotherapy treatment and PD. Both were the only ILD events resulting in death observed in the entire phase I bintrafusp alfa program. Across both phase I trials investigating bintrafusp alfa (NCT02699515 and NCT02517398; combined N=689 as of August 24, 2019), the overall incidence of ILD occurring at any grade, grade ≥3, and grade 5 was 3%, 1%, and <1%, respectively. Furthermore, the occurrence of ILD in Japanese patients reported in this study is consistent with the higher incidence of drug-induced ILD among Japanese patients than that observed among the non-Japanese populations in other studies. Although the incidence of drug-induced ILD has alarmingly increased over the past decade in Japan, little is known about its cause, and no specific risk factors for ILD have been determined to date.34 Ongoing phase II/III trials of bintrafusp alfa will offer greater insights into the full safety and efficacy profile of bintrafusp alfa, as well as evaluate potential predictive biomarkers.
This study has certain limitations. The lack of a comparator group and the small number of patients enrolled in this study preclude any direct comparison between bintrafusp alfa and available therapies for this patient population. Additionally, the lack of non-Asian patients may represent a further limitation of this study. Nevertheless, this cohort highlights the efficacy of a novel anticancer agent in patients from a region with a high incidence of BTC.
Conclusions
Given the biological link between BTC and both TGF-β and PD-(L)1 signaling,16 25 26 35 36 the concomitant inhibition of these two non-redundant, protumorigenic, immunosuppressive pathways with bintrafusp alfa may provide enhanced clinical benefit and thus represents a potential therapy for BTC. Indeed, in this study of Asian patients with heavily pretreated BTC who had limited treatment options, bintrafusp alfa demonstrated promising early signs of clinical activity irrespective of PD-L1 expression, tumor mutation count, MSI-H status, and primary tumor location, with an objective response rate of 20% per IRC, overall antitumor activity in 30% of patients (including two patients with a PR as assessed by the investigator and one patient with poststudy tumor shrinkage of non-target lesions), and mOS of 12.7 months. Based on these findings, further study of bintrafusp alfa treatment in patients with BTC is warranted, and is ongoing in a phase II trial as monotherapy in second-line BTC (NCT03833661) and a phase II/III trial in combination with gemcitabine and cisplatin (NCT04066491).
Acknowledgments
The authors thank the patients and their families, investigators, and coinvestigators, and the study teams at each of the participating centers and at Merck KGaA, Darmstadt, Germany, and EMD Serono Research & Development Institute, Billerica, Massachusetts, USA, a business of Merck KGaA. The authors also thank Christian Ihling of Merck KGaA for his substantial contribution to the immune phenotype analysis. This study is part of an alliance between Merck KGaA and GlaxoSmithKline. Medical writing support was provided by Marjorie Rummelt, PhD, of ClinicalThinking, New Jersey, USA, which was also funded by Merck KGaA and GlaxoSmithKline in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Footnotes
Contributors: CY, D-YO, HJC, MK, MU, SK, L-TC, and MI collected and assembled the data. MO, CH, and ID analyzed and interpreted the data. All authors were involved in writing the report and approved the final version of the report.
Funding: Merck KGaA provided the study drug and worked with investigators on the trial design and plan, collection and analysis of data, and interpretation of results. The study was funded by Merck KGaA. Funding for a professional medical writer with access to the data was provided by Merck KGaA and GlaxoSmithKline.
Competing interests: CY reports honorarium from Merck Serono, Tokyo, Japan, an affiliate of Merck KGaA, Darmstadt, Germany. MO is an employee of Merck Biopharma, Tokyo, Japan, an affiliate of Merck KGaA. CH is an employee of Merck KGaA. ID is an employee of EMD Serono, Billerica, Massachusetts, USA, a business of Merck KGaA. MI reports research funding from Merck Serono, an affiliate of Merck KGaA. All other authors declare no competing interests.
Patient consent for publication: Not required.
Ethics approval: This study was conducted following international standards consistent with the International Council for Harmonisation E6 Guideline for Good Clinical Practice. The study protocol was approved by the ethics committees at all participating institutions.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck’s Data Sharing Policy. All requests should be submitted in writing to Merck’s data sharing portal (https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html). When Merck has a coresearch, codevelopment, or comarketing or copromotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.
References
- 1.Randi G, Malvezzi M, Levi F, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol 2009;20:146–59. 10.1093/annonc/mdn533 [DOI] [PubMed] [Google Scholar]
- 2.Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet 2015;385:117–71. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261–80. 10.1038/nrgastro.2016.51 [DOI] [PubMed] [Google Scholar]
- 4.Miyazaki M, Yoshitomi H, Miyakawa S, et al. Clinical practice guidelines for the management of biliary tract cancers 2015: the 2nd English edition. J Hepatobiliary Pancreat Sci 2015;22:249–73. 10.1002/jhbp.233 [DOI] [PubMed] [Google Scholar]
- 5.Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469–74. 10.1038/sj.bjc.6605779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273–81. 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 7.Lamarca A, Hubner RA, David Ryder W, et al. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 2014;25:2328–38. 10.1093/annonc/mdu162 [DOI] [PubMed] [Google Scholar]
- 8.Valle JW, Lamarca A, Goyal L, et al. New horizons for precision medicine in biliary tract cancers. Cancer Discov 2017;7:943–62. 10.1158/2159-8290.CD-17-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamarca A, Palmer DH, Wasan HS, et al. ABC-06 | a randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin / 5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced / metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol 2019;37:4003 10.1200/JCO.2019.37.15_suppl.4003 [DOI] [Google Scholar]
- 10.Abou-Alfa GK, Macarulla Mercade T, Javle M, et al. ClarIDHy: a global, phase 3, randomized, double-blind study of ivosidenib (ivo) vs placebo in patients with advanced cholangiocarcinoma (CC) with an isocitrate dehydrogenase 1 (IDH1) mutation. Barcelona, Spain: ESMO, 2019. [Google Scholar]
- 11.Javle M, Lowery M, Shroff RT, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol 2018;36:276–82. 10.1200/JCO.2017.75.5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogel A, Sahai V, Hollebecque A, et al. FIGHT-202: a phase 2 study of pemigatinib in patients (pts) with previously treated locally advanced or metastatic cholangiocarcinoma (CCA). ESMO; Barcelona, Spain: Ann Oncol, 2019: v851–934. [Google Scholar]
- 13.Droz M, Braun S, El-Rayes B, et al. Efficacy of derazantinib (DZB) in patients (pts) with intrahepatic cholangiocarcinoma (iCCA) expressing FGFR2-fusion or FGFR2 mutations/amplifications. ESMO. Barcelona, Spain: Ann Oncol, 2019. [Google Scholar]
- 14.Marks EI, Yee NS. Immunotherapeutic approaches in biliary tract carcinoma: current status and emerging strategies. World J Gastrointest Oncol 2015;7:338–46. 10.4251/wjgo.v7.i11.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goeppert B, Frauenschuh L, Zucknick M, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer 2013;109:2665–74. 10.1038/bjc.2013.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holcombe RF, Xiu J, Pishvaian MJ, et al. Tumor profiling of biliary tract carcinomas to reveal distinct molecular alterations and potential therapeutic targets. J Clin Oncol 2015;33:285 10.1200/jco.2015.33.3_suppl.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merck, Co I Merck’s KEYTRUDA® (pembrolizumab) receives five new approvals in Japan, including in advanced non-small cell lung cancer (NSCLC), as adjuvant therapy for melanoma, and in advanced microsatellite instability-high (MSI-H) tumors 2019. Available: https://www.mrknewsroom.com/news-release/oncology/mercks-keytruda-pembrolizumab-receives-five-new-approvals-japan-including-adva
- 18.Ueno M, Ikeda M, Morizane C, et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol 2019;4:611–21. 10.1016/S2468-1253(19)30086-X [DOI] [PubMed] [Google Scholar]
- 19.Ueno M, Chung HC, Nagrial A, et al. Pembrolizumab for advanced biliary adenocarcinoma: results from the multicohort, phase II KEYNOTE-158 study. Ann Oncol 2018;29:viii210 10.1093/annonc/mdy282.009 [DOI] [Google Scholar]
- 20.Xie C, Duffy AG, Mabry-Hrones D, et al. Tremelimumab in combination with microwave ablation in patients with refractory biliary tract cancer. Hepatology 2019;69:2048–60. 10.1002/hep.30482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gou M, Zhang Y, Si H, et al. Efficacy and safety of nivolumab for metastatic biliary tract cancer. Onco Targets Ther 2019;12:861–7. 10.2147/OTT.S195537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov 2012;11:790–811. 10.1038/nrd3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcano-Bonilla L, Mohamed EA, Mounajjed T, et al. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol 2016;5:61. 10.21037/cco.2016.10.09 [DOI] [PubMed] [Google Scholar]
- 24.Lustri AM, Di Matteo S, Fraveto A, et al. TGF-β signaling is an effective target to impair survival and induce apoptosis of human cholangiocarcinoma cells: A study on human primary cell cultures. PLoS One 2017;12:e0183932. 10.1371/journal.pone.0183932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Ma L, He Q, et al. TGF-β1 expression is associated with invasion and metastasis of intrahepatic cholangiocarcinoma. Biol Res 2015;48:26. 10.1186/s40659-015-0016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015;47:1003–10. 10.1038/ng.3375 [DOI] [PubMed] [Google Scholar]
- 27.Bang Y-J, Doi T, Kondo S, et al. Updated results from a phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-β, in patients with pretreated recurrent or refractory gastric cancer. Ann Oncol 2018;29:viii222–3. 10.1093/annonc/mdy282.045 [DOI] [Google Scholar]
- 28.Fujiwara Y, Koyama T, Helwig C, et al. M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-β, in Asian patients with advanced solid tumors. J Clin Oncol 2018;36:762 10.1200/JCO.2018.36.4_suppl.762 [DOI] [Google Scholar]
- 29.Paz-Ares L, Kim TM, Vicente D, et al. Updated results of M7824 (MSB0011359C): a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line (2L) NSCLC. Ann Oncol 2018;29:viii529 10.1093/annonc/mdy292.085 [DOI] [Google Scholar]
- 30.Strauss J, Heery CR, Schlom J, et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clin Cancer Res 2018;24:1287–95. 10.1158/1078-0432.CCR-17-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strauss J, Gatti-Mays ME, Cho BC, et al. Phase 1 evaluation of bintrafusp alfa (M7824), a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus (HPV)–associated malignancies. Cancer Res 2019;79:CT075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544–8. 10.1038/nature25501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khoja L, Day D, Wei-Wu Chen T, et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 2017;28:2377–85. 10.1093/annonc/mdx286 [DOI] [PubMed] [Google Scholar]
- 34.Handa T, Yonezawa A, Azuma A. Epidemiology and risk factors of drug-induced lung disease: what are the prevalence and risk factors of DILD? : Drug-Induced lung injury. Singapore: Springer, 2018: 13–26. [Google Scholar]
- 35.Huang C-K, Aihara A, Iwagami Y, et al. Expression of transforming growth factor β1 promotes cholangiocarcinoma development and progression. Cancer Lett 2016;380:153–62. 10.1016/j.canlet.2016.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zen Y, Harada K, Sasaki M, et al. Intrahepatic cholangiocarcinoma escapes from growth inhibitory effect of transforming growth factor-beta1 by overexpression of cyclin D1. Lab Invest 2005;85:572–81. 10.1038/labinvest.3700236 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-000564supp001.pdf (39.2MB, pdf)
jitc-2020-000564supp002.pdf (78.8KB, pdf)
jitc-2020-000564supp003.pdf (111KB, pdf)
jitc-2020-000564supp004.pdf (123.7KB, pdf)
jitc-2020-000564supp005.pdf (131.8KB, pdf)