Key Points
Question
Which interventions are cost-effective for the prevention and treatment of schizophrenia?
Findings
In this decision analytical model using a simulated cohort of 200 000 individuals, the following interventions were found to be cost-effective: practice as usual plus cognitive behavioral therapy for individuals at clinical high risk of psychosis; a mix of hospital admission and crisis resolution and home treatment team for individuals with acute psychosis; receipt of amisulpride, risperidone, or olanzapine combined with family intervention for individuals with first-episode psychosis; and receipt of clozapine for individuals with treatment-resistant schizophrenia.
Meaning
The results of this study suggest that cost savings and/or additional quality-adjusted life years may be gained by replacing current interventions with more cost-effective interventions.
This decision analytical model develops a whole-disease model for schizophrenia and uses it to inform resource allocation decisions across the entire care pathway for schizophrenia in the UK.
Abstract
Importance
The existing economic models for schizophrenia often have 3 limitations; namely, they do not cover nonpharmacologic interventions, they report inconsistent conclusions for antipsychotics, and they have poor methodologic quality.
Objectives
To develop a whole-disease model for schizophrenia and use it to inform resource allocation decisions across the entire care pathway for schizophrenia in the UK.
Design, Setting, and Participants
This decision analytical model used a whole-disease model to simulate the entire disease and treatment pathway among a simulated cohort of 200 000 individuals at clinical high risk of psychoses or with a diagnosis of psychosis or schizophrenia being treated in primary, secondary, and tertiary care in the UK. Data were collected March 2016 to December 2018 and analyzed December 2018 to April 2019.
Exposures
The whole-disease model used discrete event simulation; its structure and input data were informed by published literature and expert opinion. Analyses were conducted from the perspective of the National Health Service and Personal Social Services over a lifetime horizon. Key interventions assessed included cognitive behavioral therapy, antipsychotic medication, family intervention, inpatient care, and crisis resolution and home treatment team.
Main Outcomes and Measures
Life-time costs and quality-adjusted life-years.
Results
In the simulated cohort of 200 000 individuals (mean [SD] age, 23.5 [5.1] years; 120 800 [60.4%] men), 66 400 (33.2%) were not at risk of psychosis, 69 800 (34.9%) were at clinical high risk of psychosis, and 63 800 (31.9%) had psychosis. The results of the whole-disease model suggest the following interventions are likely to be cost-effective at a willingness-to-pay threshold of £20 000 ($25 552) per quality-adjusted life-year: practice as usual plus cognitive behavioral therapy for individuals at clinical high risk of psychosis (probability vs practice as usual alone, 0.96); a mix of hospital admission and crisis resolution and home treatment team for individuals with acute psychosis (probability vs hospital admission alone, 0.99); amisulpride (probability vs all other antipsychotics, 0.39), risperidone (probability vs all other antipsychotics, 0.30), or olanzapine (probability vs all other antipsychotics, 0.17) combined with family intervention for individuals with first-episode psychosis (probability vs family intervention or medication alone, 0.58); and clozapine for individuals with treatment-resistant schizophrenia (probability vs other medications, 0.81).
Conclusions and Relevance
The results of this study suggest that the current schizophrenia service configuration is not optimal. Cost savings and/or additional quality-adjusted life-years may be gained by replacing current interventions with more cost-effective interventions.
Introduction
Economic models have increasingly been used to inform decision-making regarding health care, as they provide an explicit way of synthesizing all available data to simulate the likely costs and consequences of using alternative interventions under scenarios that cannot be directly observed in the real world.1 A 2020 systematic review found several limitations of existing economic models for schizophrenia.2 Most existing models (83%) focused on antipsychotic medications, while there was a lack of models for nonpharmacologic interventions, such as cognitive behavioral therapy (CBT), family intervention, and crisis resolution and home treatment team (CRHT). Second, no antipsychotic medication was shown to be clearly cost-effective compared with the others because of inconsistent or even contradictory conclusions reported by different studies. Third, the quality of existing models was considered low. This systematic review highlighted issues relating to inconsistent assumptions and uses of evidence, which negatively affect the quality of existing economic studies in schizophrenia. Greater consistency could be achieved through the development of generic models that have been agreed on by key stakeholders. A whole-disease model (WDM) represents a type of generic model that is unique in that it can be used to inform multiple resource allocation decisions across the entire care pathway.
Whole-disease models are large-scale models that involve simulating whole disease and treatment pathways, thereby allowing for the economic evaluation of options for the prevention, early identification, diagnosis, treatment, and follow-up of a given disease using a single consistent model.3 This type of system-level modeling approach has been successfully applied to a number of disease areas, including cancer, metabolic diseases, and cardiovascular diseases.4 However, such an approach has not been applied to any mental health disorders.4 The aim of this study was to develop a WDM for schizophrenia services and use it to assess the cost-effectiveness of a range of interventions in the UK.
Methods
This study was reported according to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline for reporting health economic evaluations.5 Per the Common Rule, ethical approval and informed patient consent were not required given that this is a modeling study with no direct patient contact or influence on patient care directly related to this work.
The methods for developing the schizophrenia WDM were mainly informed by the methodologic framework set out by Tappenden et al.3 To help with the development and validation of the WDM, a group of 13 multidisciplinary stakeholders was convened though snowball sampling. The background of the 13 stakeholders included health care professionals practicing in the National Health Service (9 [69.2%]), academic researchers with expertise in mental health economic evaluation (12 [92.3%]), commissioners of mental health services (5 [38.5%]), and service users (2 [15.4%]).
Population
The target population for the model was individuals referred to secondary care mental health services in the UK because of psychotic symptoms, with a mean (SD) age of 23.5 (5.1) years and a sex ratio of 1.5:1 (men to women).6 Of those referred, 33.2% were not at risk of psychosis, 34.9% were at clinical high risk of psychosis (CHR-P), and 31.9% were individuals with psychosis.6 Those not at risk of psychosis were included because they also use resources associated with schizophrenia services (eg, specialist assessments).
Decision Problems
The decision problems to be addressed by the WDM were identified from the scope of the schizophrenia clinical guidelines developed by the National Institute for Health and Care Excellence (NICE).7,8 A total of 5 topics were identified (Table 1), which span most of the breadth of the schizophrenia pathway, ranging from the use of CBT for individuals at CHR-P to antipsychotic medication for individuals with treatment-resistant schizophrenia (TRS).
Table 1. Decision Problems Addressed Using the Schizophrenia Whole-Disease Model.
| Topic | Interventions and comparators |
|---|---|
| Interventions for patients at CHR-P | Practice as usual |
| Practice as usual plus 16 sessions of CBT | |
| Interventions for individuals with acute psychosis | Hospital admission alone |
| Mix of hospital admission and CRHT | |
| First-line oral antipsychotic medication for FEP | Amisulpride |
| Aripiprazole | |
| Haloperidol | |
| Olanzapine | |
| Placebo | |
| Quetiapine | |
| Risperidone | |
| Family intervention for FEP | Antipsychotic medication alone |
| Family intervention alone | |
| Antipsychotic medication plus 20 sessions of family intervention | |
| First-line oral antipsychotic medication for TRS | Clozapine |
| Haloperidol | |
| Olanzapine | |
| Quetiapine | |
| Risperidone |
Abbreviations: CHR-P, clinical high risk of psychosis; CRHT, crisis resolution and home treatment team; CBT, cognitive behavioral therapy; FEP, first-episode psychosis; TRS, treatment-resistant schizophrenia.
Outcomes and Cost Perspective
In accordance with the NICE Reference Case for economic evaluations,1 outcomes were valued in terms of quality-adjusted life-years (QALYs), which are the product of health-related quality of life and quantity of life lived (ie, survival). Costs included those relevant to the National Health Service and Personal Social Services. Costs were reported in 2016 to 2017 UK pounds. Both costs and QALYs were discounted at an annual rate of 3.5%.1
Statistical Analysis
Model Design and Implementation
For each intervention under assessment, the consequences of treatment were grouped into the following 4 categories: clinical benefits (eg, preventing relapse), clinical harms (eg, adverse effects), costs (eg, cost of providing the intervention and treating its adverse effects), and cost savings (eg, reduced cost of treating relapse). Not all consequences of interventions were included; common reasons for exclusion were that the treatment was not expected to affect patient outcomes and there was a lack of evidence. For example, clinical harms were only modeled for antipsychotic medications, not for psychosocial interventions because of a lack of adverse event data for nonpharmacologic interventions. The key consequences of all interventions included in the schizophrenia WDM are summarized in eAppendix 1 in the Supplement.
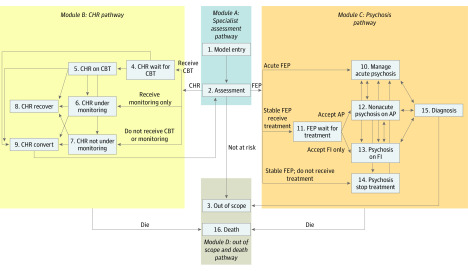
A WDM with a lifetime horizon was implemented using discrete event simulation in SIMUL8 2019 software (Simul8 Corp). Discrete event simulation is an individual-level modeling approach in which the clinical course of individual patients through the system is determined according to their characteristics, previous events, and chance. The probability of events may be based on patient history (eg, number of previous relapses) within the model and demographic characteristics (eg, age and sex). The implemented WDM covers 16 components (Figure), grouped into the following 4 modules: module A, initial assessment pathway; module B, CHR pathway; module C, psychosis pathway; and module D, out-of-scope and death pathway. The overall model logic is described in detail in eAppendix 2 in the Supplement. A list of key assumptions and simplifications of the model is presented in eAppendix 3 in the Supplement.
Figure. Model Structure.
Abbreviations: AP, antipsychotic medication; CBT, cognitive behavioral therapy; CHR, clinical high risk of psychosis; FEP, first-episode psychosis; and FI, family intervention.
Under the traditional piecewise framework for economic evaluation, the assessment of each decision problem listed in Table 1 would only involve running 1 part of the entire WDM (eg, assessment of topic 1 would only involve running the CHR pathway). However, because the underlying rationale of using a WDM approach is that all interventions are interrelated (ie, changes made to 1 intervention might affect the others), the entire WDM was run repeatedly for each decision problems listed in Table 1.
Input Data
In general, health economic models require 4 types of evidence, as follows: clinical evidence (baseline event risks, treatment effects and adverse events); health-related quality of life estimates (preference-based utility values); health care resource use; and costs. Model parameters were informed by numerous evidence sources, including systematic reviews and meta-analyses, clinical trials, clinical audits, observational studies, resource use surveys, costing studies, health valuation studies, and expert opinion. Evidence was mainly obtained from the meta-analyses conducted by the NICE schizophrenia Guideline Development Groups,7,8 supplemented with new evidence identified from rapid reviews of the literature conducted by the authors. A lack of evidence was identified for many parameters, including the long-term clinical effectiveness and adverse effects of interventions (eg, CBT, family intervention, and antipsychotic medications) and up-to-date costs of managing patients with schizophrenia in remission. When no relevant data were identified, expert opinion was used to inform the required model parameters, with alternative plausible values tested in sensitivity analyses. A summary of the key model parameters is reported in Table 2; a complete list of all model parameters, including data sources, is reported in eAppendix 4 in the Supplement.
Table 2. Summary of Key Parameters Used in the Schizophrenia Whole-Disease Modela.
| Parameter | Baseline value | Distribution |
|---|---|---|
| Epidemiologic data, % | ||
| Age, mean, y | 23.52 | Normal (SE, 2.85) |
| Men | 60.40 | Beta (α = 665.61; β = 436.39) |
| Starting disease status | ||
| Not at risk of psychosis | 33.21 | Dirichlet (n = 276, N = 831) |
| At CHR-P | 34.90 | Dirichlet (n = 290; N = 831) |
| FEP | 31.89 | Dirichlet (n = 265; N = 831) |
| Service provision data, % | ||
| CBT | ||
| Provision | 41.01 | Beta (α = 1011; β = 1454) |
| Take up | 51.00 | Beta (α = 510; β = 490) |
| Family intervention | ||
| Provision | 30.98 | Beta (α = 589; β = 1312) |
| Take up | 38.49 | Beta (α = 224; β = 358) |
| Antipsychotic | ||
| Provision | 100.00 | Assumed fixed |
| Take up for patients with FEP | 97.38 | Beta (α = 484; β = 13) |
| Delay in initiation of clozapine, y | 3.98 | Gamma (α = 137.25; β = 0.023) |
| Clinical effectiveness data: nonpharmacologic interventions, RR | ||
| Transition to psychosis for CBT vs practice as usual | 0.41 | Log normal (ln[SE], 0.29) |
| Relapse for family intervention vs standard care or other control | 0.63 | Log normal (ln[SE], 0.16) |
| Clinical effectiveness data: antipsychotic medication for individuals with FEP, OR | ||
| Annual probability of all-cause discontinuation for patients on placebo | 0.82 | Beta (α = 4949.54; β = 1079.87) |
| Amisulpride vs placebo | 0.18 | Log normal (ln[SE], 0.49) |
| Aripiprazole vs placebo | 0.24 | Log normal (ln[SE], 0.51) |
| Haloperidol vs placebo | 0.21 | Log normal (ln[SE], 0.34) |
| Olanzapine vs placebo | 0.11 | Log normal (ln[SE], 0.31) |
| Quetiapine vs placebo | 0.21 | Log normal (ln[SE], 0.32) |
| Risperidone vs placebo | 0.15 | Log normal (ln[SE], 0.40) |
| Haloperidol LAI vs placebo | 0.15 | Log normal (ln[SE], 0.45) |
| Paliperidone LAI vs placebo | 0.19 | Log normal (ln[SE], 0.53) |
| Clinical effectiveness data: antipsychotics for individuals with TRS, OR | ||
| Annual probability of discontinuing clozapine because of inefficacy | 0.02 | Beta (α = 4.98; β = 310.02) |
| Haloperidol vs clozapine | 5.56 | Log normal (ln[SE], 0.35) |
| Olanzapine vs clozapine | 1.37 | Log normal (ln[SE], 0.34) |
| Quetiapine vs clozapine | 4.35 | Log normal (ln[SE], 0.69) |
| Risperidone vs clozapine | 2.27 | Log normal (ln[SE], 0.40) |
| Health-related quality of life data | ||
| Individuals | ||
| At CHR-P | 0.71 | Beta (α = 100.22; β = 40.78) |
| With psychosis in remission | 0.80 | Normal (SE, 0.04) |
| With psychosis in relapse | 0.67 | Normal (SE, 0.06) |
| Disutility | ||
| Weight gain | 0.03 | Normal (SE, 0.01) |
| EPS | 0.07 | Normal (SE, 0.01) |
| Diabetes | 0.09 | Normal (SE, 0.05) |
| Cost data, £b | ||
| CBT | ||
| Cost per session | 97.00 | Gamma (α = 44.44; β = 2.18) |
| Sessions, No. | 16 | Assumed fixed |
| Family intervention | ||
| Cost per session | 112.00 | Gamma (α = 44.44; β = 2.52) |
| Sessions, No. | 20 | Assumed fixed |
| Oral antipsychotic, per d | ||
| Amisulpride | 0.47 | Gamma (α = 22.68; β = 0.02) |
| Aripiprazole | 4.08 | Gamma (α = 23.80; β = 0.17) |
| Haloperidol | 0.37 | Gamma (α = 30.86; β = 0.01) |
| Olanzapine | 0.13 | Gamma (α = 13.72; β = 0.01) |
| Quetiapine | 1.24 | Gamma (α = 6.25; β = 0.20) |
| Risperidone | 0.36 | Gamma (α = 5.41; β = 0.07) |
| Clozapine | 1.56 | Gamma (α = 156.25; β = 0.01) |
| LAI antipsychotic | ||
| Haloperidol, 28 d | 6.56 | Gamma (α = 13.72; β = 0.48) |
| Paliperidone, 30 d | 334.45 | Gamma (α = 82.64; β = 4.05) |
| Attendance at clozapine clinic | 16.40 | Gamma (α = 44.44; β = 0.37) |
| Managing patients with nonrelapsed schizophrenia, per y | 14 983.45 | Gamma (α = 2.04; β = 7341.89) |
| Assessing an acute episode of psychosis | 507.00 | Gamma (α = 348.55; β = 1.45) |
| CRHT team, per contact | 197.45 | Gamma (α = 44.44; β = 4.44) |
| Contacts with CRHT team, mean, No. | 16.3 | Gamma (α = 78.32; β = 0.21) |
| Hospital bed-day | 379.00 | Gamma (α = 44.44; β = 8.52) |
| Bed-days during 1 relapse, mean, No. | 138.90 | Weibull (α = 0.65; β = 0.61) |
| Cost of adverse events | ||
| Weight gain | ||
| Year 1 | 97.20 | Gamma (α = 44.44; β = 2.19) |
| Year 2 onwards | 309.68 | Gamma (α = 3.77; β = 6755.56) |
| Acute EPS, per episode | 51.95 | Gamma (α = 44.44; β = 1.17) |
| Diabetes, per y | 1336.31 | Gamma (α = 124 044.44; β = 0.01) |
| Neutropenia, per episode | 469.48 | Gamma (α = 92 802.96; β = 0.01) |
Abbreviations: CBT, cognitive behavioral therapy; CHR-P, clinical high risk of psychosis; CRHT, crisis resolution and home treatment team; EPS, extrapyramidal symptoms; FEP, first-episode psychosis; LAI, long-acting injectable; ln, natural log; OR, odds ratio; RR, relative risk; TRS, treatment-resistant schizophrenia.
A complete list of all parameters used in the model and their data sources are reported in eAppendix 3 in the Supplement.
To convert to US dollars, multiply by 1.2776.
Model Checking
Extensive model verification and validation activities were undertaken, including white-box tests (scrutinizing the programming code) and black-box tests (testing the behavior of the model),9 checking results with stakeholders and comparing results with published literature. The details of white-box and black-box tests conducted are reported in eAppendix 5 in the Supplement. The overall model behavior was checked by 1 of us (P.T.). In addition, 7 members of a service user advisory group affiliated with the Maudsley Biomedical Research Centre, London, commented on the model structure and key assumptions.
Model Evaluation Methods
In accordance with the lower end of the cost-effectiveness threshold range used by NICE, interventions with an incremental cost-effectiveness ratio lower than £20 000 ($25 552) per QALY were considered cost-effective.1 The incremental cost-effectiveness ratio was defined as the difference in the expected cost of 2 interventions, divided by the difference in the expected effects of the 2 interventions.
Extensive sensitivity analyses were undertaken to test the robustness of the results of the base case analyses to different sets of assumptions and using different input data, including 1-way and multiway sensitivity analysis to assess the consequences of uncertainty regarding the value of a single or multiple parameter(s); structural sensitivity analysis to assess the consequences of uncertainty regarding the structural assumptions of the model (eg, whether CBT can prevent psychosis or just delay the transition to psychosis); and probabilistic sensitivity analyses that examine the consequences of joint uncertainty of multiple parameters simultaneously.
Following published guidance,10 a cohort of 200 000 patients was adopted for deterministic analyses and 1000 samples were used for probabilistic sensitivity analysis. The stability of results was tested to different numbers of patients and probabilistic sensitivity analysis runs. No prespecified level of statistical significance was set.
Results
The base case and probabilistic sensitivity analysis results are presented in Table 3 and summarized in this section. The simulated cohort had a mean (SD) age of 23.5 (5.1) years, with 120 800 (60.4%) men, 66 400 (33.2%) not at risk of psychosis, 69 800 (34.9%) at CHR-P, and 63 800 (31.9%) with psychosis.
Table 3. Deterministic and Probabilistic Results of Cost-effectiveness Analysis.
| Intervention | Deterministic results | PSA results, per QALYa | |||||
|---|---|---|---|---|---|---|---|
| Discounted mean | Incremental | ICER | WTP, £20 000b | WTP, £30 000b | |||
| Cost per person, £b | QALYs per person | Cost, £b | QALY | ||||
| Interventions for patients at CHR-P | |||||||
| PAU plus CBT | 167 452 | 19.1904 | −1243 | 0.0000 | Dominating | 0.95 | 0.95 |
| PAU alone | 168 695 | 19.1904 | NA | NA | Dominated | 0.05 | 0.05 |
| Interventions for individuals with acute psychosis | |||||||
| Mix of hospital admission and CRHT | 168 078 | 19.1904 | −3655 | 0.000 | Dominating | 1.00 | 1.00 |
| Hospital admission alone | 171 733 | 19.1904 | NA | NA | Dominated | 0.00 | 0.00 |
| First-line oral antipsychotic medication for FEP | |||||||
| Quetiapine | 168 539 | 19.2005 | 1670 | 0.0071 | 235 211 | 0.06 | 0.06 |
| Haloperidol | 168 538 | 19.1981 | NA | NA | Extendedly dominated | 0.06 | 0.06 |
| Aripiprazole | 171 340 | 19.1977 | NA | NA | Dominated | 0.01 | 0.02 |
| Risperidone | 166 869 | 19.1934 | 1056 | 0.0112 | 94 286 | 0.30 | 0.30 |
| Placebo | 174 128 | 19.1931 | NA | NA | Dominated | 0.00 | 0.00 |
| Amisulpride | 165 813 | 19.1822 | NA | NA | NA | 0.39 | 0.39 |
| Olanzapine | 167 455 | 19.1794 | NA | NA | Dominated | 0.17 | 0.17 |
| Family intervention for FEP | |||||||
| Antipsychotic medication plus family intervention | 167 905 | 19.2033 | NA | NA | Dominating | 0.58 | 0.62 |
| Family intervention alone | 175 065 | 19.1987 | NA | NA | Dominated | 0.09 | 0.10 |
| Antipsychotic medication alone | 168 261 | 19.1849 | NA | NA | Dominated | 0.33 | 0.28 |
| First-line oral antipsychotic medication for TRS | |||||||
| Clozapine | 162 215 | 19.1977 | NA | NA | Dominating | 0.81 | 0.81 |
| Olanzapine | 165 444 | 19.1925 | NA | NA | Dominated | 0.16 | 0.16 |
| Risperidone | 169 324 | 19.1889 | NA | NA | Dominated | 0.03 | 0.03 |
| Haloperidol | 170 008 | 19.1883 | NA | NA | Dominated | 0.00 | 0.01 |
| Quetiapine | 172 043 | 19.1867 | NA | NA | Dominated | 0.00 | 0.00 |
Abbreviations: CBT, cognitive behavioral therapy; CHR-P, clinical high risk of psychosis; CRHT, crisis resolution and home treatment team; FEP, first-episode psychosis; ICER, incremental cost-effectiveness ratio; NA, not applicable; PAU, practice as usual; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life-year; TRS, treatment-resistant schizophrenia; WTP, willingness to pay.
Probability for the intervention to be most cost-effective within each topic.
To convert to US dollars, multiply by 1.2776.
Interventions for Patients at CHR-P
The base case analysis suggests that practice as usual plus CBT dominates practice as usual alone. The cost savings of CBT are substantial (£1243 [$1588] per person), likely because the evidence used to inform the WDM suggests that CBT can delay the transition from CHR-P to psychosis, and the treatment cost for individuals with psychosis or schizophrenia is much higher than the treatment cost for individuals at CHR-P. On the other hand, the QALY gains of CBT are marginal (5.19 × 10−5 per person), likely because evidence used in the WDM suggests that the utility for individuals at CHR-P is similar to individuals with psychosis. Assuming a willingness-to-pay (WTP) threshold of £20 000 ($25 552) per QALY gained, the probability that practice as usual plus CBT is cost-effective compared to practice as usual alone was estimated to be 0.96.
Interventions for Individuals With Acute Psychosis
The base case analysis suggests that a mix of CRHT and hospital admission produces the same QALY gains and additional cost savings (£3655 [$4670] per person) than hospital admission alone. This is because the evidence used to inform the WDM suggests equivalent effectiveness between hospital admission and CRHT,11 and the cost of hospital admission is much higher than the cost of CRHT services. Assuming a WTP threshold of £20 000 ($25 552) per QALY gained, the probability that a mix of CRHT and hospital admission is cost-effective compared with hospital admission alone was estimated to be 0.99.
First-Line Oral Antipsychotic Medication for Individuals With FEP
The base case analysis suggests that, of the 7 interventions assessed, amisulpride was the most cost-effective option, followed by risperidone and olanzapine. This is because the evidence used to inform the WDM suggests that these antipsychotic medications are associated with the lowest probability of all-cause drug discontinuation. Assuming a WTP threshold of £20 000 ($25 552) per QALY gained, amisulpride is most likely to be cost-effective (0.39), followed by risperidone (0.30) and olanzapine (0.17). The probability of any antipsychotic medication being the most cost-effective option was less than 0.05.
Family Intervention for Individuals With FEP
The base case analysis suggests that antipsychotic medication plus family intervention dominates both antipsychotic alone and family intervention alone. This is because the evidence used to inform the WDM suggests that family intervention can prevent relapse of psychosis, and the cost of treating relapse is much higher than the cost of family intervention. Assuming a WTP threshold of £20 000 ($25 552) per QALY gained, the probability that antipsychotic medication plus family intervention is the most cost-effective option compared with medication or family intervention alone was estimated to be 0.58.
First-Line Oral Antipsychotic Medications for Individuals With TRS
The base case analysis suggests that clozapine dominates all other antipsychotic medications. This is because the evidence used in the WDM suggests that, of the 5 antipsychotics assessed for individuals with TRS, clozapine was associated with the lowest all-cause discontinuation rate (including discontinuation due to inefficacy, intolerability, and nonadherence) and was less likely to cause acute extrapyramidal symptoms. Assuming a WTP threshold of £20 000 ($25 552) per QALY gained, the probability that clozapine is the most cost-effective option compared with other medications was estimated to be 0.81.
Summary of Base Case Results
Assuming a WTP pay threshold of £20 000 ($25 552) per QALY, the most cost-effective interventions were practice as usual plus 16 sessions of CBT for individuals with CHR-P. A mix of CRHT and hospital admission was most cost-effective for individuals with acute psychosis; amisulpride, risperidone, or olanzapine combined with 20 sessions of family intervention was most cost-effective for individuals with FEP; and clozapine was most cost-effective for individuals with TRS.
Sensitivity Analyses
The results of the sensitivity analyses are summarized in eAppendix 6 in the Supplement. They suggest that the conclusions for interventions for patients at CHR-P, for individuals with acute psychosis, and for first-line oral antipsychotic medication for those with FEP and TRS were robust to all types of sensitivity analyses conducted. The conclusion for family intervention for individuals with FEP was robust to all types of sensitivity analyses except the following: changes in the choice of first-line antipsychotic; effectiveness of family intervention in preventing relapse; and number of family intervention sessions provided. Antipsychotic medication alone was the most cost-effective intervention when amisulpride was used as the first-line antipsychotic medications for individuals with FEP; when the relative risk of family intervention in preventing relapse was increased from 0.63 to 0.83; or when a brief (ie, number of sessions and effectiveness halved) version of family intervention was assumed.
Discussion
Comparing Results With Published Literature
Comparison of our results with published literature by individual topic is detailed in eAppendix 7 in the Supplement and summarized in this section. Our findings regarding interventions for patients at CHR-P and with acute psychosis and for first-line oral antipsychotic medication for individuals with FEP and with TRS are consistent with published literature.12,13,14,15,16,17,18,19 For first-line oral antipsychotic medication for individuals with FEP, both the schizophrenia WDM and the model developed by the NICE schizophrenia Guideline Development Group7 found that no antipsychotic medication can be considered clearly more cost-effective than the other options. However, the schizophrenia WDM found amisulpride to be the most cost-effective option, while the NICE model suggests amisulpride was the least cost-effective option. This is likely because of differences in the input data used. The systematic review conducted by the NICE schizophrenia Guideline Development Group in 20087 found amisulpride to be associated with the second highest probability of relapse (second only to haloperidol), while the latest systematic reviews,20,21 which included additional trials, showed amisulpride to be associated with among the lowest probabilities of all-cause discontinuation and relapse rate.
Implications for Clinical Practice
The results of our analyses suggest that adoption of the following interventions could result in cost savings compared with the current service: practice as usual plus 16 sessions of CBT for individuals at CHR-P; a mix of hospital admission and CRHT for individuals with acute psychosis; antipsychotic medication (amisulpride, risperidone, or olanzapine) combined with 20 sessions of family intervention for individuals with FEP; and clozapine for individuals with TRS. Adoption of clozapine for individuals with TRS also resulted in additional QALYs. The results suggest that a brief family intervention (ie, 10 sessions) would not be cost-effective for individuals with FEP.
Strengths and Limitations
There are 3 key strengths of this study. First, it fills the evidence gap by presenting the first model-based economic analysis of the following interventions: CBT for individuals with CHR-P, CRHT for individuals with acute psychosis, family intervention for individuals with FEP, and clozapine and other atypical antipsychotics for individuals with TRS. There has been an increased interest in investment in brief family intervention because of resource constraints.22 However, our study showed that while a 20-session family intervention was cost-effective for individuals with FEP, a 10-session family intervention was not. This finding might change the current practice of providing family interventions. Second, this study provides an up-to-date assessment of antipsychotic medication for individuals with FEP based on the results of the latest network meta-analyses20,21 and modeled the cost and health consequences of 5 adverse effects of antipsychotics, including extrapyramidal symptoms, weight gain, glucose intolerance, diabetes, and neutropenia. Our analysis found amisulpride to be among the most cost-effective antipsychotics because of its therapeutic superiority in preventing relapse. Considering the current market share of amisulpride in the UK (ie, 1.39%),23 our findings may change the current clinical practice of prescribing antipsychotics. Third, all results presented within this study were based on a well-documented WDM, which is populated with input data carefully selected from high-quality literature. Extensive validation activities were undertaken to ensure the quality of the schizophrenia WDM. To our knowledge, this is the first WDM developed for the economic evaluation of a mental health disorder.
This study also has limitations. There are 2 major limitations of the schizophrenia WDM developed within this study. First, owing to resource constraints, input data for the WDM were obtained from published systematic reviews reported in the NICE schizophrenia guidelines,7,8 supplemented with new evidence identified from rapid reviews, rather than by undertaking our own de novo systematic reviews. As such, it is possible that newer high-quality evidence has not been included in the model. Second, as with any health economic model, the credibility of the schizophrenia WDM and its results are largely dependent on the quantity and quality of the evidence used to inform it. While searching for input data for the WDM, a lack of evidence was identified for many parameters, such as long-term clinical effectiveness and adverse effects of interventions (eg, CBT, family intervention, and antipsychotics) and up-to-date costs of managing patients with schizophrenia in remission. Even when evidence was available, it often had certain limitations, such as variation in criteria for relapse, relatively short follow-up periods, unclear masking, incomplete outcome data, and selective reporting. However, the model was designed to be adapted and reused, and thus, results can be updated as new or better-quality evidence is identified.
Conclusions
The results of this study suggested that the following interventions are likely to be cost-effective: CBT for individuals at CHR-P; a mix of hospital admission and CRHT for individuals with acute psychosis; amisulpride, risperidone, or olanzapine combined with family intervention for individuals with FEP; and clozapine for individuals with TRS. Cost savings and additional quality-adjusted life-years may be gained by replacing current interventions with more cost-effective interventions.
eAppendix 1. Key Consequences of Interventions Considered in the Model
eAppendix 2. Description of the Design-Oriented Model
eAppendix 3. List of Assumptions and Simplifications of the Model
eAppendix 4. Summary of Key Input Data
eAppendix 5. White-Box and Black-Box Tests Conducted
eAppendix 6. Results of Sensitivity Analysis
eAppendix 7. Comparing Results With Published Literature
eReferences.
Reference
- 1.National Institute for Health and Care Excellence Guide to the Methods of Technology Appraisal. National Institute for Health and Care Excellence; 2013. [PubMed] [Google Scholar]
- 2.Jin H, Tappenden P, Robinson S, et al. A systematic review of economic models across the entire schizophrenia pathway. Pharmacoeconomics. 2020. doi: 10.1007/s40273-020-00895-6 [DOI] [PubMed] [Google Scholar]
- 3.Tappenden P, Chilcott J, Brennan A, Squires H, Stevenson M. Whole disease modeling to inform resource allocation decisions in cancer: a methodological framework. Value Health. 2012;15(8):1127-1136. doi: 10.1016/j.jval.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 4.Jin H. Using Whole Disease Modelling to Inform Resource Allocation Decisions in Schizophrenia Services. Thesis. King’s College London; 2019.
- 5.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Pharmacoeconomics. 2013;31(5):361-367. doi: 10.1007/s40273-013-0032-y [DOI] [PubMed] [Google Scholar]
- 6.Fusar-Poli P, Byrne M, Badger S, Valmaggia LR, McGuire PK. Outreach and support in south London (OASIS), 2001-2011: ten years of early diagnosis and treatment for young individuals at high clinical risk for psychosis. Eur Psychiatry. 2013;28(5):315-326. doi: 10.1016/j.eurpsy.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 7.National Institute of Health and Care Excellence Psychosis and schizophrenia in adults: prevention and management. Updated March 1, 2014. Accessed April 29, 2020. https://www.nice.org.uk/guidance/cg178 [PubMed]
- 8.National Institute of Health and Care Excellence Psychosis and schizophrenia in children and young people: recognition and management. Updated October 26, 2016. Accessed April 29, 2020. https://www.nice.org.uk/guidance/cg155 [PubMed]
- 9.Pidd M. Computer Simulation in Management Science. 5th ed John Wiley and Sons, Inc; 2006. [Google Scholar]
- 10.Davis S, Stevenson M, Tappenden P, Wailoo AJ NICE DSU Technical Support Document 15: cost-effectiveness modelling using patient-level simulation. Published April 2014. Accessed September 9, 2018. http://nicedsu.org.uk/wp-content/uploads/2016/03/TSD15_Patient-level_simulation.pdf [PubMed]
- 11.Murphy SM, Irving CB, Adams CE, Waqar M. Crisis intervention for people with severe mental illnesses. Cochrane Database Syst Rev. 2015;(12):CD001087. doi: 10.1002/14651858.CD001087.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ising HK, Lokkerbol J, Rietdijk J, et al. Four-year cost-effectiveness of cognitive behavior therapy for preventing first-episode psychosis: the Dutch Early Detection Intervention Evaluation (EDIE-NL) Trial. Schizophr Bull. 2017;43(2):365-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCrone P, Johnson S, Nolan F, et al. Economic evaluation of a crisis resolution service: a randomised controlled trial. Epidemiol Psichiatr Soc. 2009;18(1):54-58. doi: 10.1017/S1121189X00001469 [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez-Recacha P, Chisholm D, Haro JM, Salvador-Carulla L, Ayuso-Mateos JL. Cost-effectiveness of different clinical interventions for reducing the burden of schizophrenia in Spain. Acta Psychiatr Scand Suppl. 2006;432(432):29-38. doi: 10.1111/j.1600-0447.2006.00917.x [DOI] [PubMed] [Google Scholar]
- 15.Phanthunane P, Vos T, Whiteford H, Bertram M. Cost-effectiveness of pharmacological and psychosocial interventions for schizophrenia. Cost Eff Resour Alloc. 2011;9(6):6. doi: 10.1186/1478-7547-9-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anh NQ, Linh BN, Ha NT, Phanthunane P, Huong NT. Schizophrenia interventions in Vietnam: primary results from a cost-effectiveness study. Glob Public Health. 2015;10(Supppl 1):S21-S39. doi: 10.1080/17441692.2014.986158 [DOI] [PubMed] [Google Scholar]
- 17.Davies LM, Drummond MF. Assessment of costs and benefits of drug therapy for treatment-resistant schizophrenia in the United Kingdom. Br J Psychiatry. 1993;162:38-42. doi: 10.1192/bjp.162.1.38 [DOI] [PubMed] [Google Scholar]
- 18.Glennie J. Pharmacoeconomic evaluations of clozapine in treatment-resistant schizophrenia and risperidone in chronic schizophrenia. Updated January 1, 1997. Accessed April 29, 2020. https://www.cadth.ca/pharmacoeconomic-evaluations-clozapine-treatment-resistant-schizophrenia-and-risperidone-chronic-0
- 19.Oh PI, Iskedjian M, Addis A, Lanctôt K, Einarson TR. Pharmacoeconomic evaluation of clozapine in treatment-resistant schizophrenia: a cost-utility analysis. Can J Clin Pharmacol. 2001;8(4):199-206. [PubMed] [Google Scholar]
- 20.Zhao YJ, Lin L, Teng M, et al. Long-term antipsychotic treatment in schizophrenia: systematic review and network meta-analysis of randomised controlled trials. BJPsych Open. 2016;2(1):59-66. doi: 10.1192/bjpo.bp.115.002576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. doi: 10.1016/S0140-6736(13)60733-3 [DOI] [PubMed] [Google Scholar]
- 22.Okpokoro U, Adams CE, Sampson S. Family intervention (brief) for schizophrenia. Cochrane Database Syst Rev. 2014(3):CD009802. doi: 10.1002/14651858.CD009802.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NHS Digital Prescription Cost Analysis: England, 2016. Published March 30, 2017. Accessed March 3, 2016. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/prescription-cost-analysis-england-2016
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Key Consequences of Interventions Considered in the Model
eAppendix 2. Description of the Design-Oriented Model
eAppendix 3. List of Assumptions and Simplifications of the Model
eAppendix 4. Summary of Key Input Data
eAppendix 5. White-Box and Black-Box Tests Conducted
eAppendix 6. Results of Sensitivity Analysis
eAppendix 7. Comparing Results With Published Literature
eReferences.