Key Points
Question
How often do commercially insured patients obtain follow-up treatment for opioid use disorder after a nonfatal opioid overdose?
Findings
In this cohort study of national commercial insurance claims for 6451 patients, 16.6% of patients obtained follow-up treatment after a nonfatal opioid overdose. Among those who had not received treatment for opioid use disorder before the overdose, patients of older age, female sex, black race, and Hispanic ethnicity were less likely to obtain follow-up.
Meaning
Timely treatment for opioid use disorder following overdose appears to be low among commercially insured patients, with race/ethnicity, sex, and age disparities.
Abstract
Importance
Timely initiation and referral to treatment for patients with opioid use disorder seen in the emergency department is associated with reduced mortality. It is not known how often commercially insured adults obtain follow-up treatment after nonfatal opioid overdose.
Objective
To investigate the incidence of follow-up treatment following emergency department discharge after nonfatal opioid overdose and patient characteristics associated with receipt of follow-up treatment.
Design, Setting, and Participants
A retrospective cohort study was conducted using an administrative claims database for a large US commercial insurer, from October 1, 2011, to September 30, 2016. Data analysis was performed from May 1, 2019, to September 26, 2019. Adult patients discharged from the emergency department after an index opioid overdose (no overdose in the preceding 90 days) were included. Patients with cancer and without continuous insurance enrollment were excluded.
Main Outcomes and Measures
The primary outcome was follow-up treatment in the 90 days following overdose, defined as a combined outcome of claims for treatment encounters or medications for opioid use disorder (buprenorphine and naltrexone). Analysis was stratified by whether patients received treatment for opioid use disorder in the 90 days before the overdose. Logistic regression models were used to identify patient characteristics associated with receipt of follow-up treatment. Marginal effects were used to report the average adjusted probability and absolute risk differences (ARDs) in follow-up for different patient characteristics.
Results
A total of 6451 patients were identified with nonfatal opioid overdose; the mean (SD) age was 45.0 (19.3) years, 3267 were women (50.6%), and 4676 patients (72.5%) reported their race as non-Hispanic white. A total of 1069 patients (16.6%; 95% CI, 15.7%-17.5%) obtained follow-up treatment within 90 days after the overdose. In adjusted analysis of patients who did not receive treatment before the overdose, black patients were half as likely to obtain follow-up compared with non-Hispanic white patients (ARD, −5.9%; 95% CI, −8.6% to −3.6%). Women (ARD, −1.7%; 95% CI, −3.3% to −0.5%) and Hispanic patients (ARD, −3.5%; 95% CI, −6.1% to −0.9%) were also less likely to obtain follow-up. For each additional year of age, patients were 0.2% less likely to obtain follow-up (95% CI, −0.3% to −0.1%).
Conclusions and Relevance
Efforts to improve the low rate of timely follow-up treatment following opioid overdose may seek to address sex, race/ethnicity, and age disparities.
This cohort study examines the use of follow-up treatment by patients who experience heroin or prescription opioid overdose.
Introduction
Each year, the emergency department (ED) provides care for an increasing number of patients who present with opioid overdose as well as medical complications of opioid use disorder (OUD).1,2,3 The ED serves as an essential touchpoint for patients seeking care for withdrawal and addiction.4,5,6,7 A key strategy in secondary prevention of opioid overdose deaths is the engagement of patients with OUD in treatment following discharge.8,9,10,11
However, few patients successfully transition to treatment following nonfatal overdose.12,13,14 In evidence from 2 states, less than 5% of Medicaid patients initiated treatment with medication for opioid use disorder (MOUD) following overdose.13,14 For patients who are ready to engage in treatment, care coordination can help to overcome barriers to access.4,9 Yet hospitals have few incentives and capacity to provide resource-intensive care navigation after ED visits.5,8,15,16,17,18
Patients have high risk of death in the days immediately following opioid overdose.19,20 The initiation of MOUD during or after emergency care is associated with improvements in a variety of patient outcomes, including all-cause mortality and engagement in outpatient treatment, and other hospital-based interventions have been developed.12,21,22,23,24 As a consequence, policy makers have identified the transition of patients from emergency care to sustained treatment (termed warm handoffs) as an urgent priority.25,26,27,28
In this study, we sought to examine the rate of follow-up treatment after discharge from the ED following overdose in a national population of commercially insured adults. Previous studies have focused on single states, the Medicaid population, and MOUD treatment.12,13,14,29 To our knowledge, no previous studies have included the full scope of treatment services available to patients.
We also sought to examine patient-level characteristics associated with timely receipt of follow-up care. Evidence suggests that significant treatment disparities on the basis of race, sex, and geography have emerged as the opioid epidemic has evolved, possibly owing to differences in health insurance coverage.30,31,32,33,34,35,36,37 We hypothesized that these treatment disparities by race and sex would persist within a commercially insured population.
Methods
Data Sources, Study Population, and Outcomes
We conducted a retrospective cohort study of adult patients who were discharged from the ED following treatment for opioid overdose between October 1, 2011, and September 30, 2016. We used an administrative claims database, the Optum Clinformatics Data Mart (Optum).38,39 The Optum database includes all inpatient, ED, outpatient, and pharmacy claims from a large national health insurance company that enrolled between 15 million and 18 million unique patients each year during the study period. Data analysis was performed from May 1, 2019, to September 26, 2019. The institutional review board at the University of Pennsylvania determined that this study was exempt from review because data are deidentified. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.40
Selection of Patient Cohort
We identified ED encounters for opioid overdose in the study period for patients with commercial insurance coverage (eFigure 1 in the Supplement). To do so, we used previously validated International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes before and after October 1, 2015, respectively (eTable 1 in the Supplement).41,42,43,44 We used Current Procedural Terminology codes to specifically identify ED encounters (eTable 1 in the Supplement).45
We excluded encounters for patients who did not have continuous insurance enrollment for 90 days before and after the date of the overdose, to provide a sufficient window to measure patient exposures and outcomes and exclude fatal overdoses. We excluded patients with age younger than 18 years.
We then limited the cohort to encounters for an index opioid overdose, defined as an encounter for opioid overdose with no ED encounter or hospital admission for opioid overdose in the preceding 90 days. We excluded encounters resulting in inpatient hospital admission to obtain a cohort of patients stable for ED discharge and likely to not have disability or sequelae from the overdose. In addition, we excluded encounters for patients with diagnosis of cancer based on treatment claims ICD-9-CM and ICD-10 diagnosis codes in the preceding 90 days (eTable 1 in the Supplement).12,46,47 Patients with pain related to active cancer diagnoses represent a separate population and may be prescribed high doses of prescription opioids.29 Of the remaining encounters, we included only the first index opioid overdose for any individual patient during the study period (eFigure 1 in the Supplement).
Outcomes
The primary outcome was whether the patient obtained follow-up treatment in the 90 days following the index opioid overdose. We defined follow-up treatment as the presence of either 1 pharmacy claim for MOUD or 1 medical claim for an outpatient or inpatient opioid treatment encounter. For pharmacy claims, we identified National Drug Codes for all formulations of buprenorphine, buprenorphine with naloxone, or naltrexone (eTable 2 in the Supplement).48,49,50 Methadone maintenance therapy was not covered by insurance for this population during the study period and was not included in this study. Medical claims for treatment encounters had an ICD-9-CM or ICD-10-CM diagnosis code for opioid use disorder in any position (eTable 3 in the Supplement) and Current Procedural Terminology or Healthcare Common Procedure Coding System codes for a variety of services including outpatient clinic visits, psychiatric services, inpatient and outpatient behavioral health services, outpatient treatment programs, and case management (eTable 3 in the Supplement).50 Repeated ED or inpatient hospital encounters were not included as follow-up treatment.
Supplemental analyses were performed for the purpose of hypothesis generation. These included secondary outcomes that were the receipt of MOUD independently from treatment encounters within 90 days of the index overdose. We also examined the number of days from the index overdose to follow-up treatment. To address the absence of mortality data, we determined the date of service for the last insurance claim for all patients in the cohort. We performed a sensitivity analysis excluding patients for whom there was no claim beyond the 90-day follow-up period. Although the absence of claims does not indicate death, we could not ensure survival to the end of the follow-up period for those patients.
Covariates
We examined patient-level characteristics as covariates that we hypothesized could be associated with access to follow-up treatment, including patient age, sex, and race/ethnicity. Optum uses data on race/ethnicity that is self-reported or derived from administrative data sources. We also included geographic location, according to 4 United States Census Regions (Northeast, South, Midwest, West).51 Year of the index overdose was included given the increasing overdose incidence over the study period.52 We examined the type of overdose (heroin or prescription opioid) based on diagnosis codes.41 Prescription opioid refers to medications available by prescription but does not mean that the patient received a prescription for the medication.
We also included exposures to treatment for behavioral health conditions in the 90 days preceding the index overdose. We included the presence of claims for anxiety or depression based on ICD-9-CM or ICD-10-CM diagnosis codes (eTable 1 in the Supplement) due to potential association with overdose.50 We also included claims for prescription opioid medications and benzodiazepines in the 90 days preceding the index overdose using American Hospital Formulary Service Pharmacologic-Therapeutic Classification codes.53 In addition, we determined whether patients had pharmacy claims for MOUD or medical claims for treatment encounters in the 90 days preceding the index overdose.
Statistical Analysis
First, we described the patient cohort, stratified by overdose type. We used 2-sided χ2 tests and t tests to describe differences in the cohort between overdose type. Next, we summarized patient outcomes, stratified by overdose type and treatment for OUD in the 90 days preceding the overdose.
We then used multivariable logistic regression models to examine the association between patient characteristics, as described in the first paragraph of the Covariates section, and the binary primary outcome. Given that patients were hypothesized to more likely access follow-up treatment if they had received recent treatment before the overdose, we stratified the analyses based on whether patients had received OUD treatment in the 90 days before the overdose. For ease of interpretation, we used predictive margins to report average adjusted probability and absolute risk differences (ARDs), with 95% CIs.54,55 For categorical variables, ARD represents the difference in adjusted probability of follow-up treatment between patients with a given characteristic and the reference value.
In addition to the primary analysis, we investigated potential interactions between race/ethnicity and overdose type by including an interaction term in the logistic regression model. Also, we used multivariable logistic regression models to examine the association between patient characteristics and the secondary outcome of MOUD treatment alone. In addition, we used Kaplan-Meier failure analysis to examine days to receipt of follow-up treatment, stratified by overdose type. Data analysis was conducted from June 1, 2019, to September 1, 2019. Analyses were performed using Stata software, version 15.1 (StataCorp LP).
Results
The total cohort consisted of 6451 patients, of whom 1896 (29.4%) overdosed from heroin and 4555 (70.6%) overdosed from prescription opioids (Table 1). Further delineation of the type of opioid overdose is reported in eTable 7 in the Supplement. The mean (SD) age was 45.0 (19.3) years and there were 3267 (50.6%) women. A total of 4676 patients (72.5%) reported their race as non-Hispanic white, 601 patients (9.3%) reported their race as black, and 536 patients (8.3%) who reported Hispanic ethnicity. Only 682 patients (10.6%) received treatment for opioid use disorder in the 90 days preceding the overdose, including 320 (5.0%) with pharmacy claims for MOUD. Patients with heroin overdose significantly differed across all patient characteristics compared with those with prescription opioid overdose.
Table 1. Characteristics of Patient Cohort, Stratified by Overdose Typea.
| Characteristic | No. (%) | ||
|---|---|---|---|
| All patients (n = 6451) | Overdose | ||
| Heroin (n = 1896) | Prescription opioid (n = 4555) | ||
| Age, mean (SD), y | 45.0 (19.3) | 31.0 (13.2) | 50.8 (18.4) |
| Sex | |||
| Male | 3184 (49.4) | 1291 (68.1) | 1893 (41.6) |
| Female | 3267 (50.6) | 605 (31.9) | 2662 (58.4) |
| Race/ethnicity | |||
| Non-Hispanic white | 4676 (72.5) | 1450 (76.5) | 3226 (70.8) |
| Black | 601 (9.3) | 148 (7.8) | 453 (9.9) |
| Hispanic | 536 (8.3) | 135 (7.1) | 401 (8.8) |
| Asian | 78 (1.2) | 9 (0.5) | 69 (1.5) |
| Unknown | 560 (8.7) | 154 (8.1) | 406 (8.9) |
| Year | |||
| 2011, quarter 4 | 229 (3.5) | 40 (2.1) | 189 (4.1) |
| 2012 | 1099 (17.1) | 239 (12.6) | 860 (18.9) |
| 2013 | 1164 (18.1) | 276 (14.6) | 888 (19.5) |
| 2014 | 1248 (19.3) | 362 (19.1) | 886 (19.5) |
| 2015 | 1387 (21.5) | 475 (25.1) | 912 (20.0) |
| 2016, quarters 1-3 | 1324 (20.5) | 504 (26.6) | 820 (18.0) |
| Region | |||
| Northeast | 659 (10.2) | 316 (16.7) | 343 (7.5) |
| South | 2627 (40.7) | 617 (32.5) | 2010 (44.1) |
| Midwest | 1619 (25.1) | 703 (37.1) | 916 (20.1) |
| West | 1546 (24.0) | 260 (13.7) | 1286 (28.2) |
| 90 d Before overdose | |||
| Anxiety treatment | 1625 (25.2) | 403 (21.3) | 1222 (26.8) |
| Depression treatment | 1416 (22.0) | 322 (17.0) | 1094 (24.0) |
| Prescription opioid claim | 3266 (50.6) | 317 (16.7) | 2949 (64.7) |
| Benzodiazepine claim | 2009 (31.1) | 373 (19.7) | 1636 (35.9) |
| MOUD claim | 320 (5.0) | 201 (10.6) | 119 (2.6) |
| Buprenorphine | 278 (4.3) | 168 (8.9) | 110 (2.4) |
| Naltrexone | 42 (0.7) | 33 (1.7) | 9 (0.2) |
| Treatment encounter for OUD | 539 (8.4) | 347 (18.3) | 192 (4.2) |
Abbreviations: MOUD, medication for opioid use disorder; OUD, opioid use disorder.
Two-sided t test and χ2 tests were performed; P < .001 for all patient characteristics.
Primary Analysis
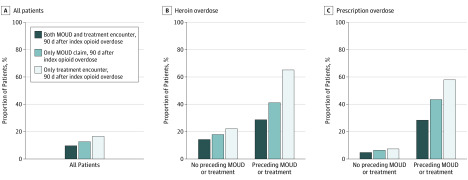
For all patients in the study cohort, 1069 individuals (16.6%; 95% CI, 15.7%-17.5%) obtained follow-up treatment in the 90 days following overdose (Figure 1; eTable 8 in the Supplement). Among the 5769 patients who did not receive treatment for OUD in the 90 days before the overdose, 643 (11.1%; 95% CI, 10.3%-12.0%) obtained follow-up treatment. Among the 682 patients who received treatment before the overdose, 426 individuals (62.5%; 95% CI, 58.7%-66.1%) patients obtained follow-up.
Figure 1. Patient Outcomes Stratified by Overdose Type and Treatment Status Before Overdose.
Data shown for status at 90 days before overdose for all patients (A), heroin overdose (B), and prescription opioid overdose (C). MOUD indicates medication for opioid use disorder.
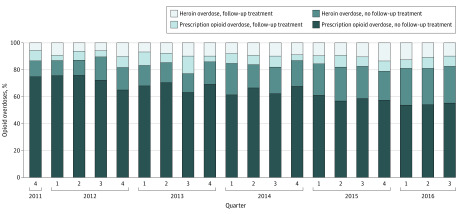
In the adjusted analysis for patients who did not receive treatment before the overdose, patients with prescription opioid overdose were less likely to obtain follow-up compared with heroin overdose (Table 2) (ARD, −8.8%; 95% CI, −11.2% to −6.5%). Compared with patients of non-Hispanic white race, black (ARD, −5.9%; 95% CI, −8.6% to −3.6%) and Hispanic (ARD, −3.5%; 95% CI, −6.1% to −0.9%) patients were less likely to obtain follow-up. Women were less likely to obtain follow-up than men (ARD, −1.7%; 95% CI, −3.3% to −0.5%). For each additional year of age, patients were 0.2% less likely to obtain follow-up (95% CI, −0.3% to −0.1%). However, patients with recent treatment for anxiety, including a treatment encounter for anxiety (ARD, 3.4%, 95% CI, 1.1%-5.8%) or prescription for a benzodiazepine (ARD, 2.8%; 95% CI, 0.7%-5.0%), were more likely to obtain follow-up. In this adjusted analysis, there was no statistically significant change with regard to the rate of patients obtaining follow-up treatment over the 5 years of the study (Figure 2).
Table 2. Adjusted Probability of Follow-up Treatment After Opioid Overdose, for Patients Not Treated Before Overdosea.
| Patient characteristics | Average adjusted probability, % (95% CI)b | P valuec |
|---|---|---|
| Overdose type | ||
| Prescription opioid | 8.3 (7.3- 9.2) | [Reference] |
| Heroin | 17.1 (15.1-19.2) | <.001 |
| Age, at mean, yd | 9.9 (9.1-10.7) | <.001 |
| Sex | ||
| Male | 11.9 (10.9-13.0) | [Reference] |
| Female | 10.1 (9.1-11.3) | .04 |
| Race/ethnicity | ||
| Non-Hispanic white | 12.1 (11.1-13.0) | [Reference] |
| Black | 6.1 (4.0-8.3) | <.001 |
| Hispanic | 8.5 (6.1-11.0) | .009 |
| Asian | 10.2 (2.8-17.5) | .62 |
| Unknown | 10.1 (7.4-12.8) | .18 |
| Year | ||
| 2011, quarter 4 | 12.2 (7.9-16.6) | [Reference] |
| 2012 | 9.3 (7.6-11.3) | .22 |
| 2013 | 11.5 (9.6-13.5) | .75 |
| 2014 | 10.0 (8.3-11.7) | .32 |
| 2015 | 12.9 (11.1-14.6) | .82 |
| 2016, quarters 1-3 | 11.1 (9.5-13.0) | .64 |
| Region | ||
| Northeast | 14.0 (11.6-16.6) | [Reference] |
| South | 10.4 (9.1-11.4) | .01 |
| Midwest | 11.1 (9.7-12.7) | .07 |
| West | 11.0 (9.3-12.8) | .06 |
| 90 d Before overdose | ||
| Anxiety treatment | ||
| No | 10.3 (9.4-11.2) | [Reference] |
| Yes | 13.8 (11.7-15.8) | .004 |
| Depression treatment | ||
| No | 10.9 (10.1-11.9) | [Reference] |
| Yes | 11.6 (9.7-13.5) | .64 |
| Prescription opioid claim | ||
| No | 11.0 (9.9-12.1) | [Reference] |
| Yes | 11.2 (9.8-12.7) | .84 |
| Benzodiazepine claim | ||
| No | 10.3 (9.4-11.2) | [Reference] |
| Yes | 13.2 (11.4-15.0) | .009 |
Results are given for patients who did not receive treatment for 90 days before the index opioid overdose, defined as either a pharmacy claim for medication for opioid use disorder or medical claim for opioid use disorder treatment encounter.
Estimated with logistic regression model using predictive margins. Average adjusted probability is the adjusted rate, holding covariates at their actual values, at which patients obtain follow-up treatment within 90 days after the index opioid overdose, defined as either a pharmacy claim for medication for opioid use disorder or medical claim for opioid use disorder treatment encounter.
P values are given for average marginal effects, which represent the difference in adjusted probability between a given characteristic and the reference group.
Average adjusted probability for continuous variable (age) is given for the mean patient age (46.3 years).
Figure 2. Proportion of Index Opioid Overdoses by Quarter, Stratified by Overdose Type and Receipt of Follow-up Treatment.
These associations were not present for patients who received treatment in the 90 days before overdose, apart from a decreased rate of follow-up for patients in the South (ARD, −15.0%; 95% CI, −25.9% to −4.1% and the West (ARD, −20.1%; 95% CI, −32.% to −7.6%), compared with the Northeast (eTable 4 in the Supplement).
Supplemental Analyses
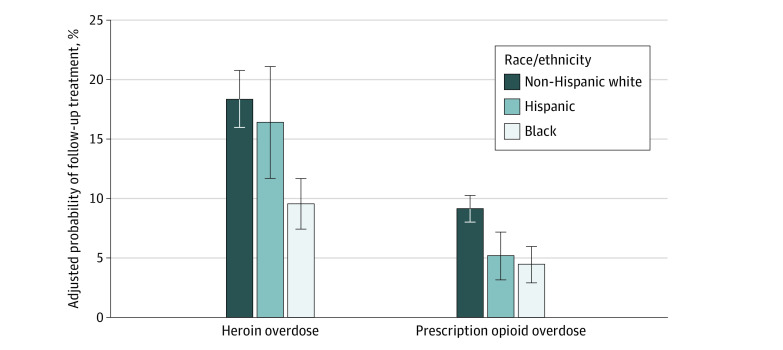
In supplemental analyses, differences in the adjusted probability of follow-up rate persisted across overdose type for black patients compared with non-Hispanic white patients (Figure 3). Among patients who did not receive treatment before overdose, black patients were less likely to obtain follow-up treatment than non-Hispanic white patients whether the index overdose was due to heroin (ARD, −8.8%; 95% CI, −11.5% to −6.1%) or prescription opioids (ARD, −4.7%; 95% CI, −5.7% to −3.7%). For Hispanic patients compared with patients of non-Hispanic white race, the difference in adjusted follow-up rate was significant only for patients with prescription opioid overdose (ARD, −4.0%; 95% CI, −5.% to 2.8%).
Figure 3. Average Adjusted Probability of Follow-up Treatment After Opioid Overdose, by Overdose Type and Race/Ethnicity.
Estimated from logistic regression model with interaction term for overdose type and race/ethnicity. Error bars denote 95% confidence intervals for average adjusted probability. Results shown only for patients who had not received treatment for opioid use disorder in the 90 days before the index opioid overdose. Race/ethnicity was self-reported or derived from other administrative data sources.
We investigated the secondary outcome of MOUD treatment alone. Among the 6131 patients who did not file an MOUD claim in the 90 days before the index overdose, 280 individuals (4.6%) had a claim for MOUD following the overdose. In adjusted analyses, patients who were older, women, black race, and experienced a prescription opioid overdose were less likely to obtain MOUD treatment, while patients with a prescription for a benzodiazepine or treatment encounters for OUD were more likely (eTable 5 in the Supplement).
We examined the timing of follow-up treatment following the index overdose, with results of the Kaplan-Meier failure analysis shown in eFigure 2 in the Supplement. Among all 1069 patients who obtained follow-up treatment, 318 individuals (29.7%) did so in 7 or fewer days after the overdose. In addition, we performed a sensitivity analysis excluding 233 patients (3.6%) who did not have claims beyond the 90-day follow-up period, which demonstrated equivalent outcomes to the primary analysis (eTable 6 in the Supplement).
Discussion
We analyzed commercial insurance claims to determine how often patients obtained treatment for OUD in the 90 days following ED presentation for nonfatal opioid overdose. Most had not received OUD treatment immediately preceding the overdose. Among that group, we found that only 11.1% of patients obtained follow-up treatment through an encounter in the outpatient setting, inpatient treatment, or filled prescriptions for a buprenorphine or naltrexone. The few patients that recently received treatment had a higher incidence of follow-up treatment. Despite the increasing number of overdoses across the years of this study, there was no significant change in the proportion of patients receiving follow-up treatment. Given that patients with commercial insurance likely have a superior ability to access care compared with patients who have public insurance, this persistently low rate suggests an opportunity for improvement.
Disparities in the receipt of follow-up treatment with regard to race/ethnicity, age, and age persisted within this cohort. In particular, black patients were half as likely to obtain treatment following overdose compared with non-Hispanic white patients. This disparity was present regardless of whether the overdose was due to heroin or prescription opioids. To our knowledge, these disparities in treatment following opioid overdose have not been previously documented. However, our findings are consistent with emerging evidence that there are disparities in buprenorphine treatment with regard to race/ethnicity and sex.30,32,33,36 Although this study cannot determine whether these disparities are associated with patient preferences, barriers to access, implicit or explicit bias, or other causes, it is important to better understand and account for these factors when designing systems that seek to improve engagement and equity in treatment.
Previous studies have examined changes in treatment rates before and after opioid overdose using data from individual states.12,13,14 These studies primarily focused on medication treatment, with only one study including a limited range of treatment encounters. Our study included a range of possible treatments, from outpatient clinic visits to inpatient residential treatment. In general, we found that fewer than half of patients who obtained follow-up treatment received medication. Treatment with opioid agonists has been associated with reduced risk of relapse by 50% compared with behavioral treatment alone.56 Better understanding of current treatment and referral patterns may help inform efforts to expand evidence-based practices.57,58
We hypothesized that the rate of follow-up treatment would be higher for patients with commercial insurance, given potentially greater resources and access to care. While we cannot directly compare across studies, the rate of OUD treatment in this cohort did not appear to be appreciably higher in this cohort than that described in other populations. Not all patients can be expected to engage in treatment after overdose.4 Higher rates of treatment engagement have been observed in experimental settings, often with screening of patients for substance use disorder.22,59,60,61,62 While the optimal rate of follow-up treatment may be difficult to estimate, there is still need for widely implemented interventions that may help patients overcome the many pervasive barriers to accessing care.4,15
We intentionally examined outcomes for a short time following the overdose. Recent evidence suggests that risk of death is high immediately following overdose, with nearly 5% of deaths occurring within 2 days of discharge from the ED.19 In a secondary analysis, only 30% of patients who obtained follow-up did so within 7 days. Patients may benefit from rapid linkage to treatment, potentially through recovery specialists who can provide navigation and harm reduction counseling regardless of the client’s willingness to engage in treatment.3,4,5
Limitations
This study has several limitations. First, we cannot account for patients who pay for OUD treatment out-of-pocket. Although treatment services, including MOUD, were covered by the insurer during the study period, some patients may have elected to pursue alternative options. Second, this study did not include patients who obtain methadone maintenance therapy. Methadone is an important treatment modality for many patients with opioid use disorder. However, methadone was not covered for this indication by the insurer during the study period. It is possible that patients in this cohort obtained methadone through self-pay or other mechanisms, although this rate cannot be estimated from these data and is difficult to extrapolate from other sources.63,64,65 Third, these data do not specifically account for patient deaths in the days following the index overdose. However, additional analysis that only included patients known to have survived to the end of the follow-up period showed similar results.
Fourth, the use of administrative claims data in this study limits our ability to ascertain the reasons that patients obtain or do not obtain follow-up treatment. It is not known whether patients do not receive appropriate referrals, lack treatment facilities in their communities, or may be unwilling to engage in treatment. A corollary limitation is that patients may have received prescriptions for MOUD but not filled those prescriptions. Fifth, this cohort likely includes patients who may not have OUD, which may explain differential rates in follow-up treatment for patients with heroin and prescription opioid overdose. Regardless, patients with accidental prescription opioid overdose also should obtain timely follow-up for reevaluation, medication adjustment, and discussion of the long-term risks associated with opioid use.
Conclusions
Engagement of patients into treatment following opioid overdose is necessary to prevent subsequent opioid overdose death and other harm. Among commercially insured patients who were not receiving active addiction treatment, only 11.1% received follow-up treatment after an overdose. We showed apparent disparities in treatment with regard to race/ethnicity (eg, black patients were half as likely to obtain follow-up compared with non-Hispanic white patients), sex, and age. Research is needed to better understand the mechanisms behind these disparities. As health professionals adopt evidence-based practices for initiating medications for treatment of OUD and linking patients to sustained treatment, payers and policy makers should implement strategies to overcome systemic barriers to ensure that patients are given the best opportunity to access timely treatment. These interventions must account for disparities to ensure expanded and equitable access to life-saving treatment following overdose.
eFigure 1. Flowchart for Selection of Patient Cohort
eTable 1. ICD-9-CM, ICD-10, CPT, and AHFS Codes for Selection of Patient Cohort and Patient Characteristics
eTable 2. National Drug Codes for Medications for Opioid Use Disorder
eTable 3. CPT, HCPCS, ICD-9-CM, and ICD-10-CM Codes for Treatment Encounters
eTable 4. Adjusted Probability of Follow-up Treatment After Opioid Overdose for Patients Treated Prior to Overdose
eTable 5. Adjusted Probability of MOUD Treatment After Opioid Overdose, Stratified by Treatment Status Prior to Overdose
eFigure 2. Kaplan-Meier Failure Curve for Days to First Follow up Treatment Following Index ED Overdose
eTable 6. Adjusted Probability of Follow-up Treatment After Opioid Overdose, Excluding Patients Without Known Claims Beyond 90-Day Follow-up Period (Sensitivity Analysis to Address Potential Mortality During Follow-up Period)
eTable 7. Index Opioid Overdoses by specific ICD-9 or ICD-10 Diagnosis Code, With Number and Frequency for Each Diagnosis Code
eTable 8. Patient Cohort and Unadjusted Outcomes, Stratified by Overdose Type and Treatment Status Before Overdose
References
- 1.Vivolo-Kantor AM, Seth P, Gladden RM, et al. Vital signs: trends in emergency department visits for suspected opioid overdoses—United States, July 2016-September 2017. MMWR Morb Mortal Wkly Rep. 2018;67(9):279-285. doi: 10.15585/mmwr.mm6709e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tedesco D, Asch SM, Curtin C, et al. Opioid abuse and poisoning: trends in inpatient and emergency department discharges. Health Aff (Millwood). 2017;36(10):1748-1753. doi: 10.1377/hlthaff.2017.0260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin A, Mitchell A, Wakeman S, White B, Raja A. Emergency department treatment of opioid addiction: an opportunity to lead. Acad Emerg Med. 2018;25(5):601-604. doi: 10.1111/acem.13367 [DOI] [PubMed] [Google Scholar]
- 4.Doran KM, Raja AS, Samuels EA. Opioid overdose protocols in the emergency department: are we asking the right questions? Ann Emerg Med. 2018;72(1):12-15. doi: 10.1016/j.annemergmed.2018.05.024 [DOI] [PubMed] [Google Scholar]
- 5.Samuels EA, D’Onofrio G, Huntley K, et al. A quality framework for emergency department treatment of opioid use disorder. Ann Emerg Med. 2019;73(3):237-247. doi: 10.1016/j.annemergmed.2018.08.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herring AA, Perrone J, Nelson LS. Managing opioid withdrawal in the emergency department with buprenorphine. Ann Emerg Med. 2019;73(5):481-487. doi: 10.1016/j.annemergmed.2018.11.032 [DOI] [PubMed] [Google Scholar]
- 7.Larochelle MR, Bernstein R, Bernson D, et al. Touchpoints—opportunities to predict and prevent opioid overdose: a cohort study. Drug Alcohol Depend. 2019;204:107537. doi: 10.1016/j.drugalcdep.2019.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Onofrio G, McCormack RP, Hawk K. Emergency departments: a 24/7/365 option for combating the opioid crisis. N Engl J Med. 2018;379(26):2487-2490. doi: 10.1056/NEJMp1811988 [DOI] [PubMed] [Google Scholar]
- 9.Houry DE, Haegerich TM, Vivolo-Kantor A. Opportunities for prevention and intervention of opioid overdose in the emergency department. Ann Emerg Med. 2018;71(6):688-690. doi: 10.1016/j.annemergmed.2018.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winetsky D, Weinrieb RM, Perrone J. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018;13(1):62-64. doi: 10.12788/jhm.2861 [DOI] [PubMed] [Google Scholar]
- 11.Naeger S, Mutter R, Ali MM, Mark T, Hughey L. Post-discharge treatment engagement among patients with an opioid-use disorder. J Subst Abuse Treat. 2016;69:64-71. doi: 10.1016/j.jsat.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 12.Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. doi: 10.7326/M17-3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frazier W, Cochran G, Lo-Ciganic WH, et al. Medication-assisted treatment and opioid use before and after overdose in Pennsylvania Medicaid. JAMA. 2017;318(8):750-752. doi: 10.1001/jama.2017.7818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyawala N, Landis R, Barry CL, Stein BD, Saloner B. Changes in outpatient services and medication use following a non-fatal opioid overdose in the West Virginia Medicaid program. J Gen Intern Med. 2019;34(6):789-791. doi: 10.1007/s11606-018-4817-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Onofrio G, Edelman EJ, Hawk KF, et al. Implementation facilitation to promote emergency department-initiated buprenorphine for opioid use disorder: protocol for a hybrid type III effectiveness-implementation study (Project ED HEALTH). Implement Sci. 2019;14(1):48. doi: 10.1186/s13012-019-0891-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz EB, Carrier ER, Umscheid CA, Pines JM. Comparative effectiveness of care coordination interventions in the emergency department: a systematic review. Ann Emerg Med. 2012;60(1):12-23.e1. doi: 10.1016/j.annemergmed.2012.02.025 [DOI] [PubMed] [Google Scholar]
- 17.Carrier EYT, Holzwart RA Coordination between Emergency and Primary Care Physicians. National Institute for Health Care Reform; February 2011. [Google Scholar]
- 18.Medford-Davis L, Marcozzi D, Agrawal S, Carr BG, Carrier E. Value-based approaches for emergency care in a new era. Ann Emerg Med. 2017;69(6):675-683. doi: 10.1016/j.annemergmed.2016.10.031 [DOI] [PubMed] [Google Scholar]
- 19.Weiner SG, Baker O, Bernson D, Schuur JD. One-year mortality of patients after emergency department treatment for nonfatal opioid overdose. Ann Emerg Med. 2020;75(1):13-17. doi: 10.1016/j.annemergmed.2019.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olfson M, Crystal S, Wall M, Wang S, Liu SM, Blanco C. Causes of death after nonfatal opioid overdose. JAMA Psychiatry. 2018;75(8):820-827. doi: 10.1001/jamapsychiatry.2018.1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department–initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636-1644. doi: 10.1001/jama.2015.3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Onofrio G, Chawarski MC, O’Connor PG, et al. Emergency department–initiated buprenorphine for opioid dependence with continuation in primary care: outcomes during and after intervention. J Gen Intern Med. 2017;32(6):660-666. doi: 10.1007/s11606-017-3993-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Busch SH, Fiellin DA, Chawarski MC, et al. Cost-effectiveness of emergency department-initiated treatment for opioid dependence. Addiction. 2017;112(11):2002-2010. doi: 10.1111/add.13900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu T, Snider-Adler M, Nijmeh L, Pyle A. Buprenorphine/naloxone induction in a Canadian emergency department with rapid access to community-based addictions providers. CJEM. 2019;21(4):492-498. doi: 10.1017/cem.2019.24 [DOI] [PubMed] [Google Scholar]
- 25.Kilaru AS, Perrone J, Kelley D, et al. Participation in a hospital incentive program for follow-up treatment for opioid use disorder. JAMA Netw Open. 2020;3(1):e1918511. doi: 10.1001/jamanetworkopen.2019.18511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitt AL, Humphreys K, Brandeau ML. Modeling health benefits and harms of public policy responses to the US opioid epidemic. Am J Public Health. 2018;108(10):1394-1400. doi: 10.2105/AJPH.2018.304590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed OM, Mao JA, Holt SR, et al. A scalable, automated warm handoff from the emergency department to community sites offering continued medication for opioid use disorder: lessons learned from the EMBED trial stakeholders. J Subst Abuse Treat. 2019;102:47-52. doi: 10.1016/j.jsat.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Governor Baker Signs Second Major Piece of Legislation to Address Opioid Epidemic in Massachusetts Published August 14, 2018. Accessed September 1, 2018. https://www.mass.gov/news/governor-baker-signs-second-major-piece-of-legislation-to-address-opioid-epidemic-in
- 29.Larochelle MR, Liebschutz JM, Zhang F, Ross-Degnan D, Wharam JF. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9. doi: 10.7326/M15-0038 [DOI] [PubMed] [Google Scholar]
- 30.Lagisetty PA, Ross R, Bohnert A, Clay M, Maust DT. Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiatry. 2019;76(9):979-981. doi: 10.1001/jamapsychiatry.2019.0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen B, Nolan ML, Kunins HV, Paone D. Racial differences in opioid overdose deaths in New York City, 2017. JAMA Intern Med. 2019;179(4):576-578. doi: 10.1001/jamainternmed.2018.7700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadland SE, Wharam JF, Schuster MA, Zhang F, Samet JH, Larochelle MR. Trends in receipt of buprenorphine and naltrexone for opioid use disorder among adolescents and young adults, 2001-2014. JAMA Pediatr. 2017;171(8):747-755. doi: 10.1001/jamapediatrics.2017.0745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiels MS, Freedman ND, Thomas D, Berrington de Gonzalez A. Trends in US drug overdose deaths in non-Hispanic black, Hispanic, and non-Hispanic white persons, 2000-2015. Ann Intern Med. 2018;168(6):453-455. doi: 10.7326/M17-1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saloner B, Karthikeyan S. Changes in substance abuse treatment use among individuals with opioid use disorders in the United States, 2004-2013. JAMA. 2015;314(14):1515-1517. doi: 10.1001/jama.2015.10345 [DOI] [PubMed] [Google Scholar]
- 35.Santoro TN, Santoro JD. Racial bias in the US opioid epidemic: a review of the history of systemic bias and implications for care. Cureus. 2018;10(12):e3733. doi: 10.7759/cureus.3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krawczyk N, Feder KA, Fingerhood MI, Saloner B. Racial and ethnic differences in opioid agonist treatment for opioid use disorder in a US national sample. Drug Alcohol Depend. 2017;178:512-518. doi: 10.1016/j.drugalcdep.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haffajee RL, Lin LA, Bohnert ASB, Goldstick JE. Characteristics of US counties with high opioid overdose mortality and low capacity to deliver medications for opioid use disorder. JAMA Netw Open. 2019;2(6):e196373. doi: 10.1001/jamanetworkopen.2019.6373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Optum Claims Data Accessed May 1, 2019. https://www.optum.com/solutions/data-analytics/data/real-world-data-analytics-a-cpl/claims-data.html
- 39.Sanghavi DAA, Hane C, Bleicher P. Optum Opioid Data, Health Affairs Blog.pdf. Health Affairs Blog; 2017. [Google Scholar]
- 40.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 41.Safe States Injury Surveillance Workgroup (ISW7) Consensus recommendations for national and state poisoning surveillance. Published April 2012. Accessed May 1, 2019. https://cdn.ymaws.com/www.safestates.org/resource/resmgr/imported/ISW7%20Full%20Report_3.pdf
- 42.Green CA, Perrin NA, Janoff SL, Campbell CI, Chilcoat HD, Coplan PM. Assessing the accuracy of opioid overdose and poisoning codes in diagnostic information from electronic health records, claims data, and death records. Pharmacoepidemiol Drug Saf. 2017;26(5):509-517. doi: 10.1002/pds.4157 [DOI] [PubMed] [Google Scholar]
- 43.Reardon JM, Harmon KJ, Schult GC, Staton CA, Waller AE. Use of diagnosis codes for detection of clinically significant opioid poisoning in the emergency department: a retrospective analysis of a surveillance case definition. BMC Emerg Med. 2016;16:11. doi: 10.1186/s12873-016-0075-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowe C, Vittinghoff E, Santos GM, Behar E, Turner C, Coffin PO. Performance measures of diagnostic codes for detecting opioid overdose in the emergency department. Acad Emerg Med. 2017;24(4):475-483. doi: 10.1111/acem.13121 [DOI] [PubMed] [Google Scholar]
- 45.American Medical Association CPT (Current Procedural Terminology). 2019. Accessed May 1, 2019. https://www.ama-assn.org/amaone/cpt-current-procedural-terminology
- 46.Centers for Disease Control and Prevention. International Classification of Diseases, Tenth Revision, Clinical Modification. Accessed May 1, 2019. https://www.cdc.gov/nchs/icd/icd10cm.htm
- 47.Centers for Disease Control and Prevention. ICD-9-CM Addenda, Conversion Table, and Guidelines. Accessed May 1, 2019. https://www.cdc.gov/nchs/icd/icd9cm_addenda_guidelines.htm
- 48.Saloner B, Levin J, Chang HY, Jones C, Alexander GC. Changes in buprenorphine-naloxone and opioid pain reliever prescriptions after the Affordable Care Act Medicaid Expansion. JAMA Netw Open. 2018;1(4):e181588. doi: 10.1001/jamanetworkopen.2018.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention Analyzing prescription data and morphine milligram equivalents (MME). Updated October 23, 2019. Accessed August 1, 2019. https://www.cdc.gov/drugoverdose/resources/data.html
- 50.Centers for Medicare and Medicaid Services Chronic Conditions Data Warehouse—opioid use disorder. 2019. Accessed May 1, 2019. https://www2.ccwdata.org/web/guest/condition-categories
- 51.United States Census Bureau Geography program. Accessed May 1, 2019. https://www.census.gov/programs-surveys/geography.html
- 52.National Institute on Drug Abuse Overdose death rates. Accessed February 28, 2020. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates
- 53.AHFS Clinical Drug Information. AHFS pharmacologic therapeutic classification Accessed May 1, 2019. https://www.ahfsdruginformation.com/ahfs-pharmacologic-therapeutic-classification/
- 54.Norton EC, Dowd BE, Maciejewski ML. Odds ratios—current best practice and use. JAMA. 2018;320(1):84-85. doi: 10.1001/jama.2018.6971 [DOI] [PubMed] [Google Scholar]
- 55.Norton EC, Dowd BE, Maciejewski ML. Marginal effects—quantifying the effect of changes in risk factors in logistic regression models. JAMA. 2019;321(13):1304-1305. doi: 10.1001/jama.2019.1954 [DOI] [PubMed] [Google Scholar]
- 56.Clark RE, Baxter JD, Aweh G, O’Connell E, Fisher WH, Barton BA. Risk factors for relapse and higher costs among Medicaid members with opioid dependence or abuse: opioid agonists, comorbidities, and treatment history. J Subst Abuse Treat. 2015;57:75-80. doi: 10.1016/j.jsat.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mojtabai R, Mauro C, Wall MM, Barry CL, Olfson M. Medication treatment for opioid use disorders in substance use treatment facilities. Health Aff (Millwood). 2019;38(1):14-23. doi: 10.1377/hlthaff.2018.05162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharfstein J, Meisel ZF Low-value treatment for opioid addiction: what is to be done? JAMA Forum. Published July 25, 2019. Accessed November 7, 2019. https://newsatjama.jama.com/2019/07/25/jama-forum-low-value-treatment-for-opioid-addiction-what-is-to-be-done/
- 59.Hawk K, D’Onofrio G. Emergency department screening and interventions for substance use disorders. Addict Sci Clin Pract. 2018;13(1):18. doi: 10.1186/s13722-018-0117-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D’Onofrio G, Degutis LC. Integrating Project ASSERT: a screening, intervention, and referral to treatment program for unhealthy alcohol and drug use into an urban emergency department. Acad Emerg Med. 2010;17(8):903-911. doi: 10.1111/j.1553-2712.2010.00824.x [DOI] [PubMed] [Google Scholar]
- 61.Bogenschutz MP, Donovan DM, Mandler RN, et al. Brief intervention for patients with problematic drug use presenting in emergency departments: a randomized clinical trial. JAMA Intern Med. 2014;174(11):1736-1745. doi: 10.1001/jamainternmed.2014.4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edwards FJ, Wicelinski R, Gallagher N, McKinzie A, White R, Domingos A. Treating opioid withdrawal with buprenorphine in a community hospital emergency department: an outreach program. Ann Emerg Med. 2020;75(1):49-56. doi: 10.1016/j.annemergmed.2019.08.420 [DOI] [PubMed] [Google Scholar]
- 63.Polsky D, Arsenault S, Azocar F. Private Coverage of methadone in outpatient treatment programs. Psychiatr Serv. 2020;71(3):303-306. doi: 10.1176/appi.ps.201900373 [DOI] [PubMed] [Google Scholar]
- 64.Reif S, Creedon TB, Horgan CM, Stewart MT, Garnick DW. Commercial health plan coverage of selected treatments for opioid use disorders from 2003 to 2014. J Psychoactive Drugs. 2017;49(2):102-110. doi: 10.1080/02791072.2017.1300360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fullerton CA, Kim M, Thomas CP, et al. Medication-assisted treatment with methadone: assessing the evidence. Psychiatr Serv. 2014;65(2):146-157. doi: 10.1176/appi.ps.201300235 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flowchart for Selection of Patient Cohort
eTable 1. ICD-9-CM, ICD-10, CPT, and AHFS Codes for Selection of Patient Cohort and Patient Characteristics
eTable 2. National Drug Codes for Medications for Opioid Use Disorder
eTable 3. CPT, HCPCS, ICD-9-CM, and ICD-10-CM Codes for Treatment Encounters
eTable 4. Adjusted Probability of Follow-up Treatment After Opioid Overdose for Patients Treated Prior to Overdose
eTable 5. Adjusted Probability of MOUD Treatment After Opioid Overdose, Stratified by Treatment Status Prior to Overdose
eFigure 2. Kaplan-Meier Failure Curve for Days to First Follow up Treatment Following Index ED Overdose
eTable 6. Adjusted Probability of Follow-up Treatment After Opioid Overdose, Excluding Patients Without Known Claims Beyond 90-Day Follow-up Period (Sensitivity Analysis to Address Potential Mortality During Follow-up Period)
eTable 7. Index Opioid Overdoses by specific ICD-9 or ICD-10 Diagnosis Code, With Number and Frequency for Each Diagnosis Code
eTable 8. Patient Cohort and Unadjusted Outcomes, Stratified by Overdose Type and Treatment Status Before Overdose