Abstract
The study compared the effect of 12-week multimodal training programme performed twice a week at the regular exercise facility (REF) with the 12-week multimodal training programme performed three times per week as a part of the research programme (EX). Additionally, the study analysed how the experimental training programme affect the physical performance of cognitive healthy and mild cognitive impaired elderly (MCI). The REF training group included 19 elderly (65.00±3.62 years). The experimental training programme combined cognitively healthy (EXH: n=16; 66.3±6.42 years) and age-matched individuals with MCI (EXMCI: n=14; 66.00±4.79 years). 10m maximal walking speed (10mMWS), Five Times Sit-to-Stand Test (FTSS), maximal and relative voluntary contraction (MVC & rel. MVC) were analysed. The REF group improved in 10mMWS (t=2.431, p=.026), the MVC (t=-3.528, p=.002) and relative MVC (t=3.553, p=.002). The EXH group improved in FTSS (t=5.210, P=.000), MVC (t=2.771, p=.018) and relative MVC (t=-3.793, p=.004). EXMCI improved in FTSS (t=2.936, p=.012) and MVC (t=-2.276, p=.040). According to results, both training programmes sufficiently improved walking speed and muscle strength in cognitively healthy elderly. Moreover, the experimental training programme improved muscle strength in MCI elderly.
Key Words: older adults, real-life implementation, multimodal exercise training, intention-to-treat strategy
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Ageing is associated with a progressive decline in physical performance, which is a powerful and independent risk factor for premature mortality.1 Physical performance is characterized as an ability to independently perform daily living activities without any difficulties.2 Logically lower physical performance is accompanied with a higher rate of sarcopenia, body fat, impairment of cardiovascular and metabolic systems, and neurocognitive functions, which results in a general decrease of overall quality of life in elderly.3-6 This progressive deterioration of physical performance and neurocognitive functions is even more significant in the elderly with cognitive impairment and early stages of dementia. Therefore, the increase of muscle mass, strength, flexibility, endurance and cardiorespiratory fitness, as essential components of physical performance, is very important, since when reduced, they can significantly contribute to the limited mobility, physical performance and cognitive state.1,2,9 There is good evidence that regular exercise improves physical performance in cognitively healthy elderly and individuals with mild cognitive impairment (MCI). Based on large research evidence regular exercise improves not only physical performance but also slows down or stabilizes the cognitive decline and improves the activation of structures in the brain, which are responsible for tasks requiring executive functions, attention, processing speed and memory in patients with MCI.2,7,10,11 However, the exercise type, dosing and length of the intervention are crucial of how long these effects would last.11 The American College of Sports Medicine and American Heart Association guidelines recommend resistance training with the intensity at 60-70% of 1 repetition maximum (1RM) for 8–12 repetitions on 2 or 3 non-consecutive days per week.11,12,13 The effective threshold for endurance exercise represents 150–300 minutes per week at moderate intensity or 75–150 minutes per week at a vigorous intensity, at least three days a week.12,14 Besides, coordination training is prescribed 2-3 times per week in duration for 45–60 minutes, with the most challenging tasks performed 2–3 times per session. However, the evidence is largely based on expert opinion rather than research evidence.14,15 However, many elderly might find it challenging to comply with this exercise frequency. Thus, the combination of exercises mentioned above in two or three sessions per week might be a feasible and effective alternative for creating a suitable multimodal exercise program. Nevertheless, it is unknown if the minimum frequency of multimodal training sessions twice per week is still sufficient to enhance the physical performance in the elderly who have had no previous experience with the regular exercise and are cognitively healthy or diagnosed with MCI.2,16 Within the study, we mainly aimed to compare the effectiveness of the 12-week multimodal, performed at the regular exercise facility (REF) twice per week, with the 12-week experimental multimodal training program which is a part of our research programs (EX) and is performed three times per week. The secondary aim was to compare the effectiveness of the experimental multimodal training between cognitively healthy elderly and age-matched elderly with MCI and how this training affects the indicators of physical performance such as walking speed and muscle strength.
Materials and Methods
Ethical statement
The study was approved by the Ethics Committees of University Hospital Bratislava, the Faculty of Physical Education and Sports Comenius University in Bratislava, and the Ethics Committee of the Bratislava Region Office.
The study protocol conforms to the ethical guidelines of the Declaration of Helsinki 2000, and its later amendments from 2013. All participants provided witnessed written informed consent prior to entering the study and their capacity to undergo exercise intervention was assessed by a cardiologist. The study was registered under the Clinical trials gov registration number: NCT03330470.
Participants
In total, forty-nine active, cognitively healthy elderly and their counterparts with MCI were recruited (sex: 12M/37F; age: 65.71±4.39 years; weight: 79.82 ±17.28 kg). No previous history of exercise within the past five years was recorded. The exclusion criteria were the presence of severe mobility impairments, cardiovascular, neurological and other uncontrolled chronic diseases that might interfere with the training programme
Study design
Training programs were fully supervised by professional coaches with previous experience of exercising with elderly and supervising research training programmes. Cognitively healthy elderly were stratified into REF and EX groups and compared to age and body weight-matched individuals with MCI. They took part in two different 12-week multimodal resistance, endurance and aerobic-coordination training programmes, one of them emphasizing the Intention to Treat Strategy (REF) and the other performed within the Research Facility (EX). Physical performance tests included measurements of performance in 10m maximal walking speed test (MWS), five times sit-to-stand test (FTSS) and measurements of maximal and relative voluntary contraction (MVC & rel. MVC). The elderly were tested 14 days before and within two weeks after the last session of the exercise intervention. Both testing sessions were in similar time-points, and the order of tests was strictly set. The tests were performed under the supervision of two or three researchers with previous testing experience. The baseline characteristics of participants are shown in Table 1. Additionally, we included the group of MCI elderly according to 1) Mini-Mental State Examination (MMSE, score 20-24) and 2) Montreal cognitive assessment, MoCA (score 22-25), a standard diagnostic test for MCI.17
Table 1.
Baseline characteristics of participants
| Group | n | Sex | Age (years) | Weight (kg) |
|---|---|---|---|---|
| REF | 19 | 5M/14F | 65.00 ± 3.62 | 84.27 ± 15.93 |
| EXH | 16 | 4M/12F | 66.3 ± 6.42 | 78.79 ± 19.66 |
| EXMCI | 14 | 3M/11F | 66.00 ± 4.79 | 74.42 ± 16.45 |
REF - multimodal training group at the regular exercise facility; EXH - training group of cognitively healthy individuals at the research facility; EXMCI - training group of individuals with mild cognitive impairment at the research facility; Data are mean ± standard deviation.
Fig 1.
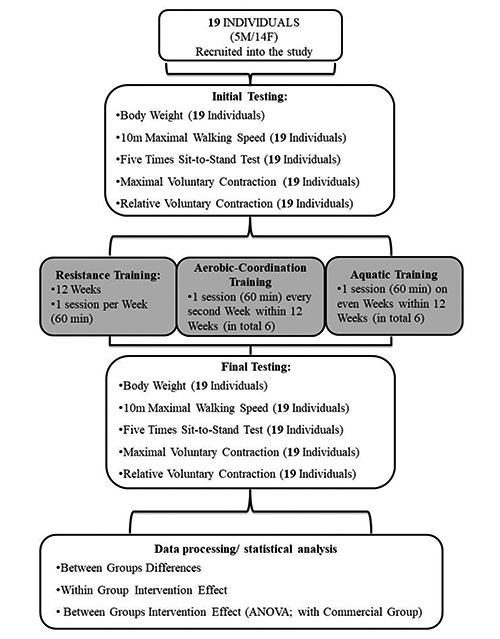
Study Design – Flow Chart: Multimodal training program performed at the Regular Exercise Facility
Training protocols
12-Week Multimodal Training Programme, performed at The Regular Exercise Facility
The multimodal training program, performed at the regular exercise facility (REF) using the intention to treat strategy, lasted 12 weeks and included resistance training sessions performed once per week, aerobic-coordination training session performed once every second week (week: 1, 3, 5, 7, 9, 11) and aquatic training session performed once every even week (week: 2, 4, 6, 8, 10, 12). Each training session was performed under the supervision of professional and previously experienced coaches. In addition, the REF was, contrary to the experimental multimodal training program cover by the participants.
Fig. 2.
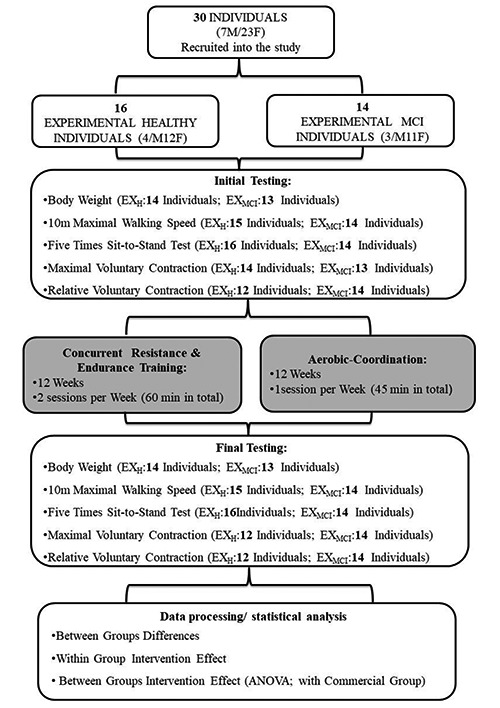
Study Design – Flow Chart: Multimodal training program performed at the Regular Exercise Facility. The dropouts in the post tests were due to non-training relates issues
The Resistance Training Session
The resistance training session lasted 60 minutes and consisted of 6 exercises, focused on the development of large muscle groups, in 2 (first two weeks) and 3 (following 9 weeks) sets with a load of 10-12 repetition maximums (RM). The tempo of exercises was 2/0/2/1.
Inter-set rest interval was 90 seconds. All training sessions included warm-up (10 minutes), the main activity (40 minutes) and cool down (10 minutes). Progressive overload of adding loads (2-5%of estimated 1RM) was used on a weekly basis according to the individual´s ability to perform the current workload. A progression was documented in the exercise diaries.2
Aerobic-Coordination Training Session
The aerobic-coordination training sessions lasted 60 minutes each and subjects performed coordination and low-intensity aerobic exercises. The sessions were led in group form. Some exercises included swiss balls, soft gym overballs and steppers as balance equipment. The intensity of exercise represented the aerobic training zone (RPE 13-16 Borg scale).2
The Aquatic Training Session
The aquatic training sessions were performed in a pool with water temperature 28°C, and depth between central chest area and shoulders (impact level 1 and 2). Each training session included shallow water endurance exercises for large muscle groups and aerobic exercises, using aquafitness equipment. The moderate-intensity load with the rating of perceived exertion at the aerobic training zone (RPE 13-16 Borg scale) was used.2
12-Week Experimental Multimodal Training Programme, performed at the Research Facility
The multimodal training program, performed at the research facility, consisted of concurrent resistance-endurance training sessions and aerobic-coordination training sessions. The concurrent resistance-endurance training sessions were performed twice per week in a duration of 60 minutes. The aerobic-coordination training session was performed once per week and lasted 60 minutes too. Within week one and two, subjects performed only one concurrent resistance – endurance and one aerobic-coordination session per week. Following nine weeks, another concurrent resistance – endurance training session was added. Thus, participants took part in two concurrent resistance – endurance training sessions and one aerobic-coordination session per week.2,18 Professional coaches supervised the training process. All sessions included warm-up (10 minutes), the resistance exercises (approximately 25 minutes), Nordic Walking (18-22 minutes) cool down (approximately 10 minutes), when elderly walked one lap (380m) by their preferred walking speed to cool down.10,18
Resistance Part
A resistance part of the resistance – endurance training session involved four exercises, focused on large muscle groups, in 2 (first 2 weeks) and 3 sets (following 9 weeks) with the load of 10-12 RM. The tempo of exercises was 2/0/2/1 Inter-set rest interval was 120 seconds. The first session we used for setting individual baseline training loads. Progressive overload of adding loads (2-5% of estimated 1RM) was used on a weekly basis according to the individual´s ability to perform the current workload. A progression was documented in the exercise diaries.2,10,18
Endurance Part
Within the endurance part of resistance – endurance training session, elderly performed Nordic Walking (distance: 1750-2100m) with the intensity of 90–95% of pace measured in pre-intervention Rockport Walk test. Progressive intensity increase according to individual´s walking speed, was used in the fifth and ninth week (Week 1.–4.: 1700m, 90% pace of Rockport test; Week 5.-8.: 2100m 90% pace of Rockport test; Week 9.–12.: 2100m, 95% pace of Rockport test).10,18
The Aerobic-Coordination Training Session
Each aerobic-coordination session lasted 60 minutes and elderly performed exercises including low aerobics. The sessions were led in group form. Only a stepper was used as balance equipment. The exercise intensity was in a range of aerobic training zone (RPE 13-16 Borg scale). The aerobic-coordination component primarily included changes in direction as well as alternations of left and right side within the performed aerobic composition.2,10,18
Measurements
10 Meters Test for Maximal Walking Speed
Volunteers were asked to walk as fast as they can without any assistance. Time to walk over 10 meters was measured electronically by a wireless photocell timekeeping system (FiTRO Light Gates, FiTRONIC, Slovakia) followed by walking speed (m/s) calculation. MWS was evaluated by performing three trials and the best of performed trials was used for further analyses. The walking technique was controlled visually by researchers and participants were instructed that one foot must always be in touch with the ground. Subjects were not verbally encouraged within the performance of the test.19
5 Times Sit-to-Stand Test
A standardized testing chair with a straight back and without armrest (43 cm seat height, regardless of the height of tested subject), was placed against the wall for safety. Volunteers started from a sitting position, with knees bent at 90°–degree angle, upper backs against the back of the chair and arms crossed over their chest, to a complete stand-up position and return to sitting position. The time (s) of 5 following repetitions within a trial was measured.20 The FTSS was performed twice, and the best result was used. Within the performance of the test, the elderly were not verbally encouraged.
The Maximal and Relative Voluntary Contraction in Isometric Knee Extension
MVC in an isometric extension of the knee joint was tested on an adjustable isometric dynamometric chair (S2P Ltd., Ljubljana, Slovenia) adapted for individual anthropometrical parameters of the subjects. Three trials of the bilateral isometric extension were performed. The rest interval was set at 1 minute between each trial. A trial with the highest produced MVC was used. Participants were verbally encouraged within the whole duration of the test. A trial with the highest force produced was taken for further analyses using ARS (Analysis & Reporting Software, S2P Ltd., Ljubljana, Slovenia). The maximal MVC of the participant was divided by his or her actual weight to get the relative MVC.
Table 2.
Pre to Post induced values within groups and main effect of time
| Within-group changes | Main Effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| REF | EXH | EXMCI | Time | |||||||
| Pre | Post | (P-value) | Pre | Post | (P-value) | Pre | Post | (P-value) | (P-value) | |
| 10m MWS (m/s) | 2.16 ± 0.26 | 2.36 ± 0.32* | .026 | 1.99 ± 0.33 | 2.14 ± 0.37 | .090 | 2.07 ± 0.28 | 2.16 ± 0.23 | .061 | .001 |
| FTSS (s) | 9.98 ± 2.12 | 9.13 ± 2.68 | .104 | 10.28 ± 1.31 | 8.85 ± 1.38*** | .000 | 9.91 ± 1.69 | 8.63 ± 1.24* | .012 | .000 |
| MVC (N/m) | 263.66 ± 80.38 | 309.88 ± 83.39** | .002 | 202.62 ± 42.64 | 226.41 ± 47.39* | .018 | 220.40 ± 54.94 | 243.23 ± 45.84* | .040 | .000 |
| rel. MVC (N/m.kg-2) | 3.18 ± 0.91 | 3.76 ± 0.86** | .002 | 2.93 ± 0.90 | 3.42 ± 1.10** | .004 | 3.13 ± 0.90 | 3.54 ± 0.83 | .055 | .000 |
REF - multimodal training group at the regular exercise facility; EXH - training group of cognitively healthy individuals at the research facility; EXMCI -training group of individuals with mild cognitive impairment at the research facility; 10m MWS - 10 meters maximal walking speed; FTSS (s) - five time sit-to-stand test; MVC (N/m) - maximal voluntary contraction; rel. MVC relative voluntary contraction;
Data are presented as mean ± standard deviation
** indicates significance at p<0.01 within group.
Statistical Analyses
All statistical analyses were realized in SPSS for Windows (SPSS version 23.0; IBM Corp., Armonk, NY). Conventional statistical methods were used to obtain means, standard deviation (SD). The Kolmogorov-Smirnov test and Shapiro-Wilk test were used to test normality and Levene’s test was used to analyses homogeneity of variance. Possible baseline between-group differences were assessed for three groups using a one-way analysis of variance (ANOVA). ANOVA with repeated measures (RMANOVA) was applied to analyze the intervention effects using a 3 group (REF, EXH, EXMCI,) × 2 time (PRE, POST) design. Any significant main effect was assessed by Bonferroni post hoc tests for within-group differences. Significance was defined as p<.05.22
Results
10 Meters Maximal Walking Speed
There was a significant main effect of time for 10mMWS (F=11.970, p=.001). Within-group changes, statistically significant increase of walking speed was observed only in the REF group (from 2.16±0.26 m/s to 2.36 ±0.32 m/s, t=-2.431, p=.026) (Table 2.). According to delta changes, no statistically significant changes were observed in any group (Table 3).
5 Times Sit to Stand Test
The significant main effect of time was observed in FTSS (F= 19.866, p=.000). Within-group changes, statistically significant decrease of time for FTSS test was observed in EXH group (from 10.28±1.31s to 8.85±1.38s, t=5.201, p=.001) and EXMCI group (from 9.40±1.37s to 8.63±1.24s, t=2.936; p=.012) (Table 2.). According to delta changes, statistically significant changes were observed in no group (Table 3.).
Maximal and Relative Voluntary Contraction
Maximal and relative MVC demonstrated significant main effects for time (MVC: F=20.148, p=.001; rel. MVC: F=22.734, p=.001). Within group changes, the significant increase of MVC was observed in the REF group (from 263.66±80.38 N/m to 309.88±83.39N/m, t=3.528, p=.002), EXH group (from 202.52±42.84N/m to 226.41±47.39N/m, t=-2.771, p=.018) and EXMCI group (from 220.40±54.94 84 N/m to 243.3 ± 45.84 N/m, t = 2.276, p= .040). Further, the relative MVC increased in the REF group (from 3.18±0.91 N/m.kg-2 to 3.76±0.86 N/m.kg-2, t=-3.553, p=.002) and the EXH group (from 2.93±0.90 N/m.kg-2 to 3.42±1.10 N/m.kg-2, t=-3.793, p=.004) (Table 2). According to delta changes, statistically significant changes were not observed in any training group (Table 3). The improvements in the 10mMWS, FTSS, MVC and relative MVC, as indicators of physical performance, indicate better low limbs strength, more stable balance, mobility and neuromuscular function in cognitively healthy elderly. Moreover, the improvement in the FTSS test and absolute MVC in patients with MCI, indicating stronger lower limbs, better coordination and neuromuscular function, might affect the cognitive performance and brain health.19,20,23
Table 3.
Delta change induced values among groups
| Training Groups | ||||
|---|---|---|---|---|
| REF | EXH | EXMCI | (P-value) | |
| ⌂10m MWS (m/s) | 0.20 ± 0.36 | 0.15 ± 0.31 | 0.13 ± 0.26 | .820 |
| ⌂ FTSS (s) | ´-0.85 ± 2.17 | ´-1.43 ± 1.10 | ´-0.77 ± 0.98 | .451 |
| ⌂ MVC (N/m) | 46.22 ± 57.11 | 34.29 ± 60.26 | 22.84 ± 37.54 | .463 |
| ⌂ rel. MVC (N/m.kg-2) | 0.57 ± 0.70 | 1.13 ± 1.33 | 0.56 ± 0.87 | .221 |
REF - multimodal training group at the regular exercise facility; EXH - training group of cognitively healthy individuals at the research facility; EXMCI - training group of individuals with mild cognitive impairment at the research facility; ⌂10m MWS - pre to post induced change in maximal walking speed for 10 meters; ⌂ FTSS - pre to post induced change in five times sit-to-stand-test; ⌂ MVC - pre to post induced change in maximal voluntary contraction, ⌂ rel. MVC - pre to post induced change in relative voluntary contraction
Data are presented as mean ± standard deviation.
Discussion
In this work, we primarily aimed to compare the effectiveness of the multimodal training that was applied in real life with the Intention-To-Treat strategy and the multimodal training applied at a research center to previously untrained cognitively healthy elderly. A secondary aim was to identify any specific differences in the training outcomes on walking speed, muscle strength and agility (defining some of the aspects of physical performance) in cognitively healthy elderly and MCI patients According to the results, both types of multimodal training programs sufficiently improved indicators of physical performance in the elderly. Moreover, the experimental training program was effective to generate benefits for both cognitively healthy elderly and patients with MCI.
Specificity of multimodal exercise interventions
Based on strong scientific evidence, it may be assumed that regular multimodal exercise combining many components from resistance, endurance, coordination, flexibility and functional training for around 60 minutes a day, 2 to 3 days per week is sufficient to improve various aspects of physical functioning such as lower limb strength, mobility, balance or walking endurance.1,2,7,8 According to overall evidence, the duration of multimodal training programs varies from 8 weeks to 15 months. However, the most significant effect on the physical performance may be obtained between the ninth and sixteenth week.7 According to the duration of training sessions, no apparent differences have been identified in these parameters.2,7
For general strength, some trials suggest that 30 minutes of specific resistance training in 12RM could lead to significant strength improvement.24 However, among the other trials that incorporated multimodal training, those that reported no significant increase tended to use a shorter total training duration per session (non-significant: 30–60 minutes; significant: 75–120 minutes).7, 25, 26
Both our training protocols partly follow the outcomes, as mentioned earlier. The length of each multimodal training program was twelve weeks, thus long enough to obtain the noticeable efficacy of the physical performance aspects in previously untrained healthy and MCI elderly. Furthermore, the overall duration of each training session was 60 minutes, and they were performed on two and three non-consecutive days per week. In REF group, the resistance training session lasted only 60 minutes in total and was performed once a week, in 2 to 3 series with the load 10–12 RM. The endurance training session was also conducted once a week in 60 minutes alternating the aerobic-coordination and aquatic training every week. However, the time of the session was sufficient to improve aspects of physical performance such and strength of lower limbs Similar results have also been obtained in both cognitively healthy and MCI groups where the multimodal, concurrent resistance - endurance training session consisted of 30 minutes of four resistance exercises in 2-3 series with the load of 10-12 RM and 20 minutes of Nordic Walking as an endurance component. This type of training session was performed twice a week. In addition, there was an additional aerobic-coordination training session lasting 45 minutes.2,10,18 Here, also significant improvements in the five-times-sit-to-stand test, maximal and voluntary contraction in cognitive healthy elderly and patients with mild cognitive impairment occurred.
Effects of multimodal training on physical performance indicators in healthy and MCI elderly
Several studies have shown beneficial effects of physical exercise on mobility and physical performance in MCI elderly.7,27,28 Moreover, exercises benefits executive, cognitive and memory in elderly with cognitive decline.7 However, most of the studies focused on the improvement of physical performance and mobility compared only to individuals with Alzheimer disease or other types of dementia.27,28 Baker et al. have reviewed the potential benefits of multimodal training by including 15 randomized controlled trials, totaling 2149 subjects (mean cohort age range from 67±8 years to 84±3 years).29 The authors concluded that multimodal training had a positive effect on the prevention of falls and improving balance. However, limited data available suggested a small effect on physical, functional outcomes and quality of life.7,27-30 Also our findings show that the frequency and structure of both presented multimodal training could improve in different range the indicators of physical performance. Also, changes in the physical performance predict brain plasticity changes, but they may not always translate to cognitive functions. However, to elicit the detectable cognitive effects, they should last longer than three months (>6 months).7 Although, the further research must be done to explore the sufficient duration, dosage and intensity of exercise that are needed to improve the cognitive function in MCI elderly. According to our study, de Oliviera Silva et al. has shown similar outcomes to our study. 12-week multimodal training program performed twice a week in MCI elderly significantly improved mobility and executive functions.30 The elderly MCI improved in the simple task 8–feet up and go test compared to controls (t=2.28; p=.03). Our findings confirm these outcomes and show that 12-week experimental multimodal training program performed three times per week is sufficient to improve parameter of physical performance such as maximal walking speed, and maximal relative voluntary contraction in patients with MCI. The EXMCI training group significantly improved in five-times-sit-to-stand test and maximal voluntary contraction. There was also observed improvement in relative voluntary contraction. Moreover, there were no significant differences between healthy and MCI elderly after finishing the training program. Thus we may conclude that the 12-week experimental multimodal training is effective in previously untrained MCI elderly, as well as in cognitively healthy elderly. The strength of the study is identifying and comparing the effectiveness of the intention-to-treat or real-life intervention strategy performed twice per week (one resistance session and one aerobic-coordination session and alternating aquatic session) with the experimental protocol, performed three times per week (two resistance–endurance sessions and one aerobic-coordination session) as a part of the research program, while the various aspects defining physical performance were determined. Moreover, an identification of the experimental multimodal training´s effectiveness on various aspects of physical performance in patients with MCI is of additional value. There are also some limitations to the study. The general limitations are the variability in design, possible differences in subjects’ motivation (paid vs free-of-charge program) small samples of participants, especially in the experimental groups as well as the slight statistical heterogeneity of the groups. Moreover, providing MCI patients with the real-life Intention-To-Treat intervention would also be very interesting in the future. From a practical point of view, our findings could be useful for coaches and medical practitioners who work with the elderly. The results demonstrated that there is no difference in the efficacy between multimodal training performed twice a week with the real-life Intention-To-Treat strategy and similar experimental training with the frequency three times per week provided as a part of the experimental research project. Both training strategies were sufficient to improve indicators of physical performance in cognitively healthy elderly, thus may indirectly improve overall health and quality of life for the elderly.7,19,20,23 If we compare the efficiency, the REF multimodal training performed twice a week might be a more suitable choice for both the business and clinical sphere. Moreover, experimental multimodal training can increase physical performance in patients with MCI. We might hypothesize that this type of multimodal training benefits not only for physical performance but the neurocognitive performance and brain health of patients with MCI due to the improvement of mobility and muscle strength, which are associated with changes in brain structures.13 In conclusion, both modes of multimodal training might be a key factor to make the elderly more active and support their adherence to the active lifestyle and their healthy ageing. However, further research should be done to explore how multimodal training affects physical performance and brain health in patients with MCI.
Acknowledgments
We want to thank all volunteers who took part in the study, the entire research team and the coaches who helped us.
List of acronyms
- 10mMWS
10 meters maximal walking speed
- EX
experimental training program
- EXH
experimental healthy group
- EXMCI
experimental mild cognitive impaired group
- FTSS
five times sit-to-stand test
- MCI
mild cognitive impairment
- MMSE
mini-mental state examination
- MoCA
Montreal cognitive assessment
- MVC
maximal voluntary contraction
- REF
regular exercise facility
- RM
repetition maximum
- RPE
rating of perceived exertion
- SD
standard deviation
Funding Statement
Funding: The study was supported by Slovak Research & Development Agency SRDA15-0253 (BU), Grant Agency of the Slovak Academy of Sciences VEGA 2/0107/18 (BU), Comenius University students grants UK/336/2018, UK/402/2019 and via the international cooperation scheme INTEREG V-A Slovakia – Austria Centre of Active Ageing - competency-based center for physical activity, prevention and health promotion of seniors (acronym CAA, ITMS2014+ 305041X157) funded by European Regional Development Fund
Contributor Information
Ľudmila Oreská, Email: ludmila.oreska@uniba.sk.
Lucia Slobodová, Email: lslobodova77@gmail.com.
Matej Vajda, Email: matej.vajda@uniba.sk.
Adriana Kaplánová, Email: adriana.kaplanova@gmail.com.
Veronika Tirpáková, Email: veronika.tirpakova@uniba.sk.
Ján Cvečka, Email: myofitsk@gmail.com.
Gabriel Buzgó, Email: gabriel.buzgo@uniba.sk.
Jozef Ukropec, Email: jozef.ukropec@savba.sk.
Barbara Ukropcová, Email: barbara.ukropcova@savba.sk.
References
- 1.Sbardelotto ML, Costa RR, Malysz KA, et al. Improvement in muscular strength and aerobic capacities in elderly people occurs independently of physical training type or exercise model. Clinics (Sao Paulo). 2019;74:1-9. doi:10.6061/clinics/2019/e833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sousa N, Mendes R, Abrantes C, et al. Effectiveness of combined exercise training to improve functional fitness in older adults: A randomized controlled trial. Geriatr Gerontol Int 2014;14:892–8. doi: 10.1111/ggi.12188. [DOI] [PubMed] [Google Scholar]
- 3.Izquierdo M, Häkkinen K, Ibañez J, et al. Effects of strength training on muscle power and serum hormones in middle-aged and older men. J. Appl. Physiol. 2001;90:1497–1507. doi: 10.1152/ jappl.2001.90.4.1497. [DOI] [PubMed] [Google Scholar]
- 4.Landi F, Onder G, Carpenter I, et al. Physical activity prevented functional decline among frail community-living elderly subjects in an international observational study. J Clin Epidemiol 2007;60:518–24. doi:10.1016/j.jclinepi.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Lopez P, Pinto RS, Radaelli R, et al. Benefits of resistance training in physically frail elderly: A systematic review. Aging Clin Exp Res 2018;30: 889–899. doi: 10.1007/s40520-017-0863-z. [DOI] [PubMed] [Google Scholar]
- 6.Peterson MD, Rhea MR, Sen A, et al. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev 2010;9:226–37. doi: 10.1016/j.arr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam FMH, Huang MZ, Liao LR, et al. Physical exercise improves strength, balance, mobility, and endurance in people with cognitive impairment and dementia: A systematic review. J Physiother 2018;64:4–15. doi: 10.1016/j.jphys.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Pitkälä K, Savikko N, Poysti M, et al. Efficacy of physical exercise intervention on mobility and physical functioning in older people with dementia: a systematic review. Exp Gerontol 2013;48:85–93. doi: 10.1016/j. [DOI] [PubMed] [Google Scholar]
- 9.Furtado HL, Sousa N, Simão R, et al. Physical exercise and functional fitness in independently living vs institutionalized elderly women: a comparison of 60- to 79-year-old city dwellers. Clin Interv Aging 2015;10:795–801. doi:10.2147/CIA.S80895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Máderová D, Krumpolec P, Slobodová L, et al. Acute and regular exercise distinctly modulate serum, plasma and skeletal muscle BDNF in the elderly. Neuropeptides 2019;78101961. [DOI] [PubMed] [Google Scholar]
- 11.Sanders LMJ, Hortobágyi T, la Bastide-van Gemert S, et al. Dose-response relationship between exercise and cognitive function in older adults with and without cognitive impairment: A systematic review and meta-analysis. PLoS One 2019;14:e 0210036. doi:10.1371/journal.pone.0210036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007;39:1423-34. doi: 10.1249 /mss.0b013e3180616b27 [DOI] [PubMed] [Google Scholar]
- 13.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007;39:1435-45. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 14.Farlie M.K, Robins L, Haas R, et al. Programme frequency, type, time and duration do not explain the effects of balance exercise in older adults: a systematic review with a meta-regression analysis. Br J Sports Med 2018;53:996–1002. 10.1136/bjsports-2016-096874 [DOI] [PubMed] [Google Scholar]
- 15.Phillips EM, Kennedy MA. The exercise prescription: A tool to improve physical activity. PM R 2012;4:818–25. [DOI] [PubMed] [Google Scholar]
- 16.Bouaziz W, Lang PO, Schmitt E, et al. Health benefits of multicomponent training programmes in seniors: a systematic review. International Journal of Clinical Practice 2016;70:520–36. doi: 10.1111/ijcp.12822. [DOI] [PubMed] [Google Scholar]
- 17.Schon M, Slobodova L, Tirpakova V, et al. A Link Between Cognitive Function and Physical Activity: the Impact of Aerobic-Strength Exercise in Seniors With Mild Cognitive Impairment and/or Impaired Glucose Metabolism. Alzheimer’s & Dementia, 2018;14 P1030. [Google Scholar]
- 18.Krumpolec P, Vallova S, Slobodova L, et al. Aerobic-Strength Exercise Improves Metabolism and Clinical State in Parkinson's Disease Patients. Front Neurol 2017;8:698. doi:10.3389/fneur.2017.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DH, Kim CA, Placide S, et al. Preoperative Frailty Assessment and Outcomes at 6 Months or Later in Older Adults Undergoing Cardiac Surgical Procedures: A Systematic Review. Ann Intern Med 2016;165:650–60. doi:10.7326/M16-0652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makizako H, Shimada H, Doi T, et al. Predictive Cutoff Values of the Five-Times Sit-to-Stand Test and the Timed "Up & Go" Test for Disability Incidence in Older People Dwelling in the Community. Physical therapy 2017;97:417–24. doi: 10.2522/ptj.20150665 [DOI] [PubMed] [Google Scholar]
- 21.Sarabon N, Rosker J, Fruhmann H, et al. Reliability of maximal voluntary contraction related parameters measured by a novel portable isometric knee dynamometer. Phys Med Rehab Kuror 2013;23: 22–7. doi: 10.1055/s-0032-1331190 [Google Scholar]
- 22.Ihalainen JK, Inglis A, Mäkinen T, et al. Strength Training Improves Metabolic Health Markers in Older Individual Regardless of Training Frequency. Front Physiol 2019;10:32. doi:10.3389/fphys.2019.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guadagnin EC, Priario LAA, Carpes FP, Vaz MA. Correlation between lower limb isometric strength and muscle structure with normal and challenged gait performance in older adults. Gait and Posture 2019;73:101–7. doi: 10.1016/j.gaitpost.2019.07.131. [DOI] [PubMed] [Google Scholar]
- 24.Bossers WJ, van der Woude LH, Boersma F, et al. A 9-week aerobic and strength training program improves cognitive and motor function in patients with dementia: a randomized, controlled trial. Am J Geriatr Psychiatry 2015;23:1106–16. doi: 10.1016/j.jagp.2014.12.191. [DOI] [PubMed] [Google Scholar]
- 25.Arcoverde C, Deslandes A, Moraes H, et al. Treadmill training as an augmentation treatment for Alzheimer’s disease: a pilot randomized controlled study. Arq Neuropsiquiatr 2014;72:190–6. doi: 10.1590/0004-282X20130231. [DOI] [PubMed] [Google Scholar]
- 26.Netz Y, Axelrad S, Argov E. Group physical activity for demented older adults feasibility and effectiveness. Clin Rehabil 2007;21:977–86. doi: 10.1177/0269215507078318. [DOI] [PubMed] [Google Scholar]
- 27.Liang JH, Xu Y, Lin L, et al. Comparison of multiple interventions for older adults with Alzheimer disease or mild cognitive impairment: A PRISMA-compliant network meta-analysis. Medicine (Baltimore). 2018;97:e10744. doi:10. 1097/MD.0000000000010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Northey JM, Cherbuin N, Pumpa KL, et al. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med 2018;52:154–60. doi: 10.1136/bjsports-2016-096587. [DOI] [PubMed] [Google Scholar]
- 29.Baker M, Kennedy D, Bohle P, et al. Efficacy and feasibility of a novel tri-modal robust exercise prescription in a retirement community: a randomized, controlled trial. J Am Geriatr Soc 2007;55:1-10. [DOI] [PubMed] [Google Scholar]
- 30.de Oliveira Silva F, Ferreira JV, Plácido J, et al. Three months of multimodal training contributes to mobility and executive function in elderly individuals with mild cognitive impairment, but not in those with Alzheimer’s disease: A randomized controlled trial. Maturitas 2019;126:28–33. doi: 10.1016/j.maturitas.2019.04.217. [DOI] [PubMed] [Google Scholar]


