This case-control study assesses multiple aggregated yellow-white globules as a diagnostic feature for basal cell carcinoma.
Key Points
Question
What is the utility of multiple aggregated yellow-white globules, a newly described dermoscopic feature, for the diagnosis of basal cell carcinoma?
Findings
In this case-control study of 656 lesions in 643 patients (291 basal cell carcinomas in 278 patients [cases] and 365 other diagnoses in 365 patients [controls]), the presence of multiple aggregated yellow-white globules was associated with a diagnosis of nonpigmented basal cell carcinoma and high-risk histologic subtypes of basal cell carcinoma.
Meaning
The presence of multiple aggregated yellow-white globules may aid the diagnosis of nonpigmented basal cell carcinoma, particularly high-risk subtypes on the head and neck.
Abstract
Importance
Basal cell carcinoma (BCC) is the most common skin cancer. Dermoscopic imaging has improved diagnostic accuracy; however, diagnosis of nonpigmented BCC remains limited to arborizing vessels, ulceration, and shiny white structures.
Objective
To assess multiple aggregated yellow-white (MAY) globules as a diagnostic feature for BCC.
Design, Setting, and Participants
In this retrospective, single-center, case-control study, nonpigmented skin tumors, determined clinically, were identified from a database of lesions consecutively biopsied during a 7-year period (January 1, 2009, to December 31, 2015). A subset of tumors was prospectively diagnosed, and reflectance confocal microscopy, optical coherence tomography, and histopathologic correlation were performed. Data analysis was conducted from July 1 to September 31, 2019.
Exposures
Investigators evaluated for the presence or absence of known dermoscopic criteria. MAY globules were defined as aggregated, white-yellow structures visualized in polarized and nonpolarized light.
Main Outcomes and Measures
The primary outcome was the diagnostic accuracy of MAY globules for the diagnosis of BCC. Secondary objectives included the association with BCC location and subtype. Interrater agreement was estimated.
Results
A total of 656 nonpigmented lesions from 643 patients (mean [SD] age, 63.1 [14.9] years; 381 [58.1%] male) were included. In all, 194 lesions (29.6%) were located on the head and neck. A total of 291 (44.4%) were BCCs. MAY globules were seen in 61 of 291 BCC cases (21.0%) and in 3 of 365 other diagnoses (0.8%) (P < .001). The odds ratio for diagnosis of BCC was 32.0 (96% CI, 9.9-103.2). The presence of MAY globules was associated with a diagnosis of histologic high-risk BCC (odds ratio, 6.5; 95% CI, 3.1-14.3). The structure was never seen in cases of superficial BCCs.
Conclusions and Relevance
The findings suggest that MAY globules may have utility as a new BCC dermoscopic criterion with a high specificity. MAY globules were negatively associated with superficial BCC and positively associated with deeper-seated, histologic, higher-grade tumor subtypes.
Introduction
Basal cell carcinoma (BCC) is the most common skin cancer worldwide.1 BCC incidence is increasing, with more than 2 million cases of BCC diagnosed annually in the United States.2,3,4,5 Dermoscopic features of BCC were first described by Menzies et al6 in 2000 and included large blue-gray ovoid nests, multiple nonaggregated blue-gray globules, ulceration, arborizing telangiectasia, spoke-wheel structures, and leaflike areas. More recently, shiny white structures, specifically blotches and strands, were added as a new BCC dermoscopic criterion.7 All these criteria have been confirmed to afford a high diagnostic accuracy for the diagnosis of BCC, with an overall sensitivity of 91.2% and a specificity of 95.0%, according to a recent meta-analysis.8 However, the sensitivity and specificity of these dermoscopic features for nonpigmented BCC are lower (84.3% sensitivity and 73.2% specificity).8 Given that most of the BCC criteria were defined for pigmented BCCs6 and that nonpigmented BCCs may be difficult to differentiate from other nonpigmented tumors,7,8,9,10 evaluation of new dermoscopic clues that may aid in the diagnosis of nonpigmented BCC and its differential diagnosis is needed.10
Some BCCs display multiple aggregated yellow-white (MAY) globules. This dermoscopic feature differs from previously described milialike cysts and from shiny white structures based on morphologic features and polarized vs nonpolarized light patterns of visualization. In 2014, Bellucci et al11 described the presence of yellow structures in 10% of BCCs; however, they were regarded mainly as milialike cysts. Yellow-orange structures were also described by Bañuls et al,12 but they were not characterized in extent. To explore the prevalence and diagnostic accuracy of this newly characterized structure for the diagnosis of BCC, we performed a retrospective assessment of clinical and dermoscopic images of lesions that could be included in the differential diagnosis of nonpigmented BCCs.
Methods
This retrospective case-control study was performed from July 1, 2017, to July 1, 2019. All images originated from a deidentified database of lesions consecutively seen in a single dermatology practice from January 1, 2009, to December 31, 2015, in Plantation, Florida. Given the relatively low frequency of amelanotic melanomas in the data set, we screened 2169 melanomas from the International Skin Imaging Collaboration (ISIC) archive, a publicly available image database. Twenty-two amelanotic melanomas were found and included. This study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center. The images analyzed were only of close-up magnified dermoscopy images, and patient identifiers did not appear on the images. Therefore, the Memorial Sloan Kettering Cancer Center Institutional Review Board deemed that consent was not required for these magnified images of individual lesions.
Three of us (C.N.-D., K.L, and A.R.) screened the clinical images of consecutive cases and included only those that had histopathologic results and were clinically nonpigmented. We excluded recurrent tumors, collision tumors, and cases with poor-quality images or lacking both polarized and nonpolarized modes. Cases included BCC of any subtype. Controls included lesions that are typically included in the differential diagnosis of nonpigmented BCC: squamous cell carcinoma (SCC), intradermal nevus (IDN), amelanotic melanoma, lichen planus–like keratosis, desmoplastic trichoepithelioma (DT), adnexal tumors (eg, fibrofolliculoma), and inflammatory diseases (eg, dermatitis and psoriasis). Patient age, sex, location of lesion, diagnosis, and predominant tumor subtype (if available) were recorded. Location was documented as specific anatomical locations and dichotomized into head and neck vs non–head and neck.
Clinical and Dermoscopic Images
Clinical and dermoscopic images were captured with a Nikon 1 camera (Nikon USA Inc) using Dermlite DL2 pro HR for polarized images and Dermlite fluid for nonpolarized images at 10 × magnification (3Gen). Images were taken in nonpolarized and polarized modes; the 2 modes were sequentially analyzed for each patient. Dermoscopic images were evaluated by 3 of us (C.N.-D., K.L., and A.R.), who were blinded to diagnosis, for consensus. A fourth reviewer (A.A.M.) resolved disagreement when consensus was not achieved. Dermoscopic images were analyzed for criteria based on the latest dermoscopic consensus by Kittler et al.13 We specifically analyzed BCC-specific criteria,6,14 including blotches and strands.7 The main dermoscopic structure analyzed consisted of MAY globules, defined by us as multiple, aggregated, white-to-yellowish globules arranged in clusters. This structure is visible in polarized and nonpolarized light, differentiating it from shiny white structures (blotches and strands) and from milialike cysts, respectively (Figure 1). We evaluated dermoscopic images for the presence or absence of MAY globules.
Figure 1. Morpheaform Basal Cell Carcinoma (BCC) and Infiltrative BCC Showing Multiple Aggregated Yellow-White Globules.
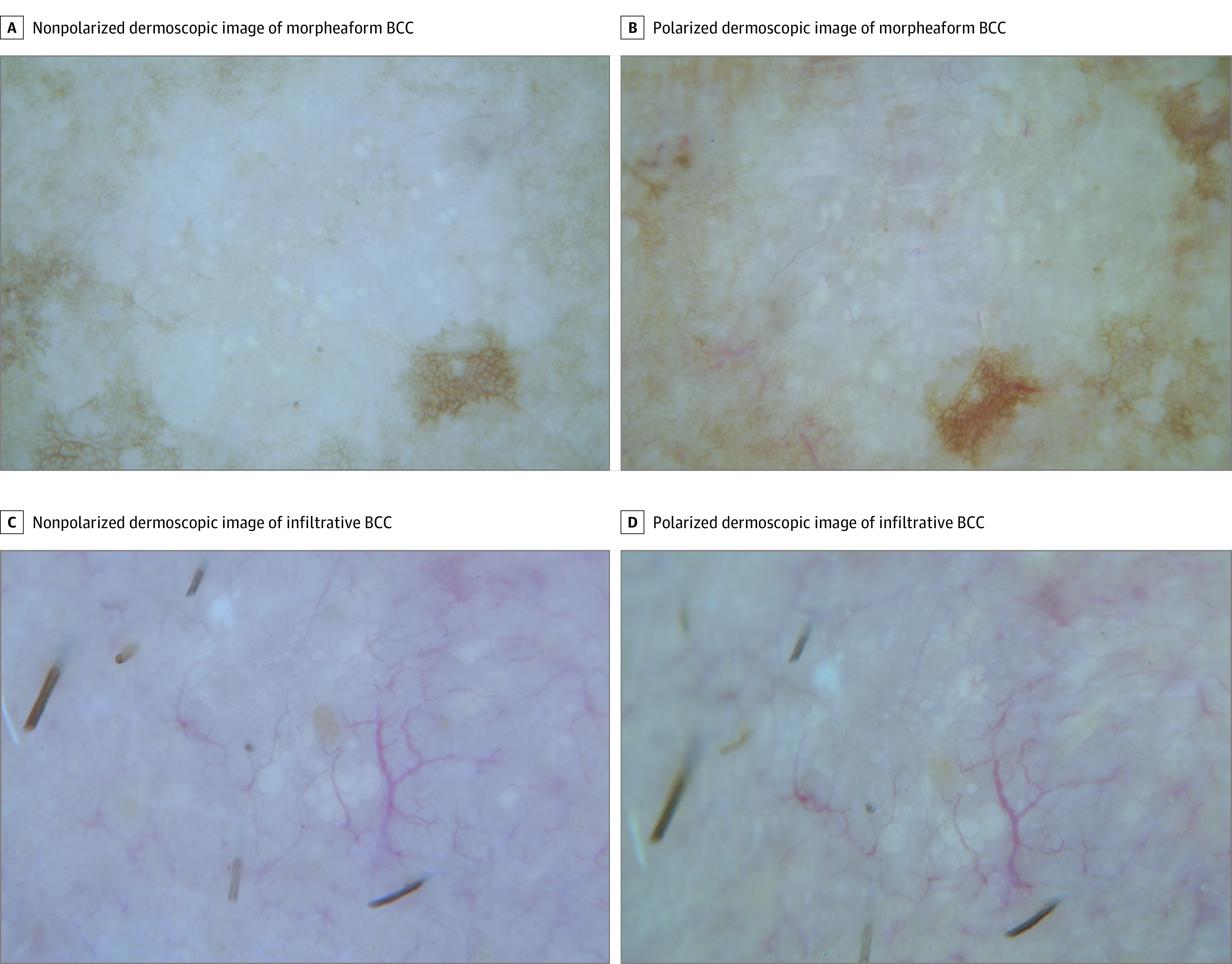
A and C, Nonpolarized images. B and D, Polarized images. Note that the structure is seen in both modes (original magnification ×10).
To evaluate for interrater agreement in classifying MAY globules in a subset of 150 consecutive lesions, we evaluated images independently blinded to the final diagnosis. This analysis was performed by the same 3 of us (C.N.-D., K.L., and A.R.).
Reflectance Confocal Microscopy, Optical Coherence Tomography, and Histopathologic Correlation
In a subgroup of prospectively diagnosed cases displaying MAY globules seen at a single dermatologic practice in Hauppauge, New York, reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) images were obtained before biopsy. Images were obtained with an arm-mounted RCM and/or a handheld RCM device (VivaScope 1500 and/or 3000; Caliber ID). For OCT images, we used a recently designed RCM-OCT probe.15 RCM criteria used were those described in a recent systematic review.16 OCT criteria were those used in a recent study.15 In these cases, a histopathologic correlation was performed using the precision biopsy technique, as previously described.17 In brief, precision biopsy enables 1-to-1 correlation with en face histopathologic images were evaluated by a Mohs micrographic surgeon (C.-C.J.C.) on frozen sections. Formalin-fixed, paraffin-embedded samples were evaluated by a dermatopathologist (K.J.B.).
Main Outcomes
Our primary outcome was the distribution of the presence or absence of clustered yellow globules for the diagnosis of BCC compared with all other diagnoses combined. Secondary outcomes were the distribution of MAY globules by BCC subtype and the distribution of MAY globules by anatomical location of the BCC. To analyze for different histologic BCC subtypes, we divided them into high risk (morpheaform and infiltrative) and low risk (superficial and nodular) BCCs.
Statistical Analysis
Distribution of participant and lesion characteristics was evaluated by histologic diagnosis of the study lesions. Descriptive statistics and graphical methods were used to describe the study participants and the characteristics of the individual lesions. The relative proportion of dermoscopic characteristics along with exact binomial 95% CIs were estimated. Interrater agreement was estimated using multirater κ along with binomial interpolations of the 95% CI. The κ estimates were interpreted per the guidelines of Landis and Koch.18 To assess the association between dermoscopic criteria and BCC type, logistic regression was performed with the dichotomous dependent variable being BCC vs other diagnosis and the independent variables being dermoscopic criteria. All analyses were 2-sided with an α level of 5%. P < .05 was considered to be statistically significant. Data analyses were conducted from July 1 to September 31, 2019. All analyses were performed with Stata, version 14.0 (StataCorp).
Results
A database review of 2555 lesions revealed 643 potential study lesions in 621 patients with clinically nonpigmented tumors; 9 cases were excluded (no biopsies performed in 4, collision tumors in 4, and polarized images not available in 1). Final analysis included 656 lesions (634 lesions from the database plus 22 amelanotic melanomas from the ISIC archive) in 643 patients. The mean (SD) age of the total cohort was 63.1 (14.9) years, and 381 (58.1%) were male. Of all 656 lesions, 194 (29.6%) were located on the head and neck. A total of 278 lesions in 291 patients (44.4%; mean [SD] age, 61.9 [14.9] years; 190 [64.3%] male) were BCCs (cases), and 365 lesions (55.6%) in 365 patients (mean [SD] patient age, 63.9 [14.9] years; 191 [53.1%] male) corresponded to other diagnoses (controls). The mean (SD) tumor size in the whole cohort was 7.6 (4.9) mm, with a mean (SD) tumor size of 6.8 (4.8) mm in the BCC group and 8.2 (4.9) mm in the other diagnosis group (P < .001). Patient diagnoses and BCC subtypes are given in Table 1.
Table 1. Diagnoses and BCC Subtypes.
| Characteristic | No. (%) of total cases (N = 656) |
|---|---|
| Diagnosis | |
| BCC | 291 (44.4) |
| SCC | 114 (17.4) |
| Actinic keratosis | 42 (6.4) |
| LPLK | 37 (5.6) |
| Amelanotic or hypomelanotic melanoma | 31 (4.7) |
| Seborrheic keratosis | 29 (4.4) |
| Bowen disease | 13 (2.0) |
| Keratoacanthoma | 12 (1.8) |
| Intradermal nevus | 12 (1.8) |
| Dermatitis | 11 (1.7) |
| Sebaceous hyperplasia | 6 (0.9) |
| Dermatofibroma | 5 (0.8) |
| Desmoplastic trichoepithelioma | 4 (0.7) |
| Psoriasis | 4 (0.7) |
| Molluscum contagiosum | 2 (0.3) |
| Othera | 43 (6.6) |
| BCC subtypeb | |
| Nodular | 224 (76.9) |
| Superficial | 27 (9.3) |
| Infiltrative | 24 (8.2) |
| Morpheaform or sclerosing | 10 (3.4) |
| Keratotic | 2 (0.7) |
| Basosquamous | 1 (0.3) |
| Pinkus | 1 (0.3) |
Abbreviations: BCC, basal cell carcinoma; LPLK, lichen planus–like keratosis; SCC, squamous cell carcinoma.
Other includes angiomas, clear cell acanthoma, cutaneous metastasis, cysts, folliculitis, foreign body granuloma, halo nevus, hydrocystoma, inverted follicular keratosis, lymphangioma, mucinosis, neurofibroma, pilar sheath acanthoma, pilomatrixoma, porokeratosis, prurigo nodularis, scar, steatocystoma, trichofolliculoma, and warts.
BCC subtype was missing in 2 cases.
Basal cell carcinomas were located in the head and neck in 124 patients (42.6%) and the trunk and extremities in 167 (57.4%). For other diagnoses, tumors were located in the head and neck in 70 patients (19.5%) and the trunk and extremities in 289 patients (79.2%) (P < .001). Basal cell carcinoma subtype distribution was nodular for 224 lesions (76.7%), superficial for 27 (9.2%), infiltrative for 24 (8.2%), morpheaform for 8 (2.7%), sclerosing for 2 (0.7%), keratotic for 2 (0.7%), Pinkus for 1 (0.3%), and basosquamous for 1 (0.3%). In 2 cases, subtyping data were not available.
Diagnostic Features
MAY globules were found in 64 of 656 cases (9.8%; 95% CI, 7.6%-12.3%). The structure was seen in 61 of 291 BCC cases (21.0%; 95% CI, 16.4%-26.1%) and in 3 of 365 cases with other diagnoses (0.8%; 95% CI, 0%-2.3%) (P < .001). The presence of MAY globules in BCCs was associated with a sensitivity of 20.9% (95% CI, 16.4%-26.1%), a specificity of 99.2% (95% CI, 97.6%-99.8%), a positive predictive value of 95.3% (95% CI, 86.6%-94.5%), and a negative predictive value of 61.0% (95% CI, 59.6%-62.4%). The odds ratio for diagnosis of BCC was 32.0 (96% CI, 9.9-103.2). The positive likelihood ratio was 25.4 (96% CI, 8.0-80.0), and the negative likelihood ratio was 0.8 (96% CI, 0.7-0.8).
Anatomical Location
When evaluating the presence of MAY globules restricted to head and neck lesions (n = 194), 51 patients (26.3%) presented with this structure. A total of 124 of 194 head and neck lesions (63.9%) corresponded to BCCs. Of the BCCs located on the head and neck, 48 of 124 (38.7%) manifested with MAY globules compared with 3 of 70 cases (4.2%) with other diagnosis (P < .001). The odds ratio for diagnosis of BCC when the structure was present was 14.1 (95% CI, 4.2-47.4) for head and neck lesions. The 3 lesions that presented with MAY globules other than BCC corresponded to DT (n = 2) and SCC (n = 1).
Subtype Analysis
MAY globules were observed in 18 of 32 high-risk BCCs (56.2%) (ie, infiltrative and morpheaform) and 41 of 210 low-risk BCCs (19.5%) (P < .001) (Figure 1, Figure 2A, and Figure 3A). MAY globules were 6.5 times more likely to be observed in higher-risk than lower-risk BCCs (odds ratio, 6.5; 95% CI, 3.1-14.3). The structure was not seen in any of the 27 superficial BCCs (Table 2).
Figure 2. Basal Cell Carcinoma With Multiple Aggregated Yellow-White (MAY) Globules.
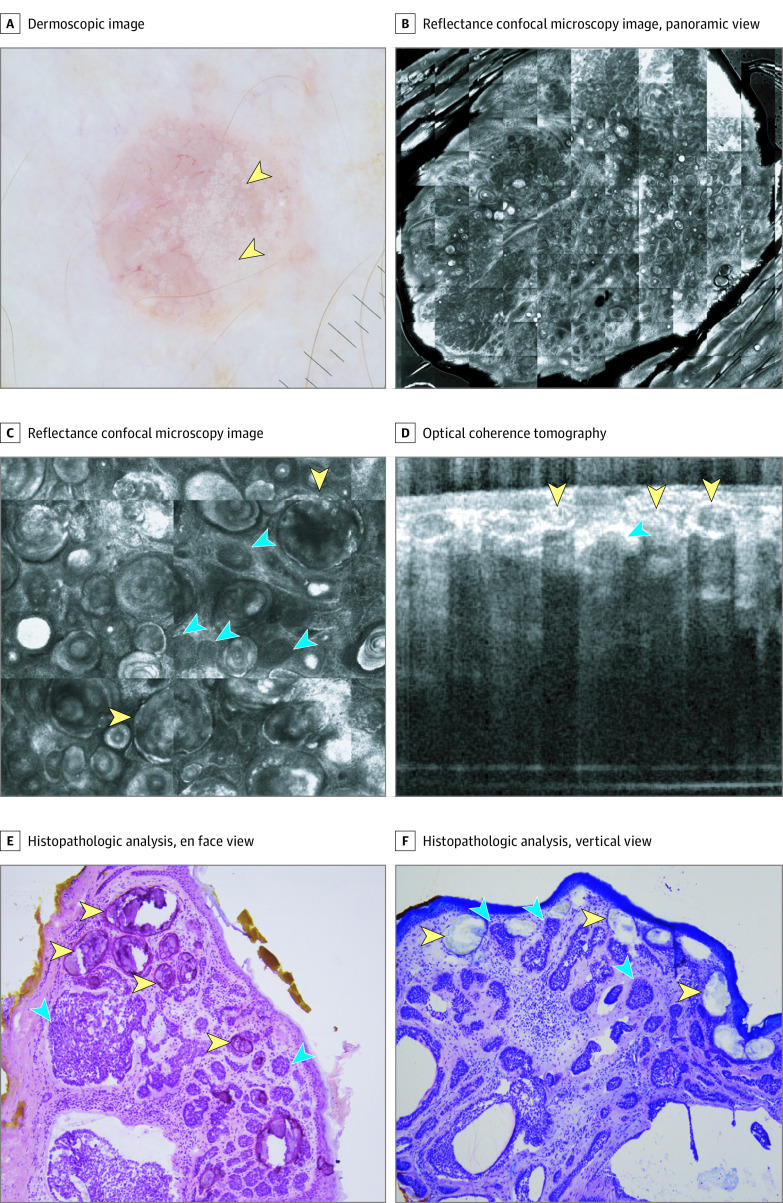
A, Dermoscopic appearance of MAY globules (yellow arrowheads). B, Reflectance confocal microscopy (RCM) panoramic view shows a well-defined tumor with hyperreflective amorphous areas (8 × 8 mm). C, RCM shows tumor nodules (blue arrowheads) and hyperreflective amorphous areas (yellow arrowheads) (750 × 750 μm). D, Optical coherence tomography shows hyperreflective structures with acoustic shadow (yellow arrowheads) and hyporeflective nodules (blue arrowheads). E, Histopathologic analysis, en face view, shows tumor islands with palisading and clefting (blue arrowheads) and calcium deposits (yellow arrowheads) (hematoxylin-eosin, original magnification ×10). F, Histopathologic analysis, vertical view, shows tumor islands with palisading and clefting (blue arrows) and subepidermal calcium deposits (yellow arrowheads) (hematoxylin-eosin, original magnification ×10).
Figure 3. Basal Cell Carcinoma With Multiple Aggregated Yellow-White (MAY) Globules.
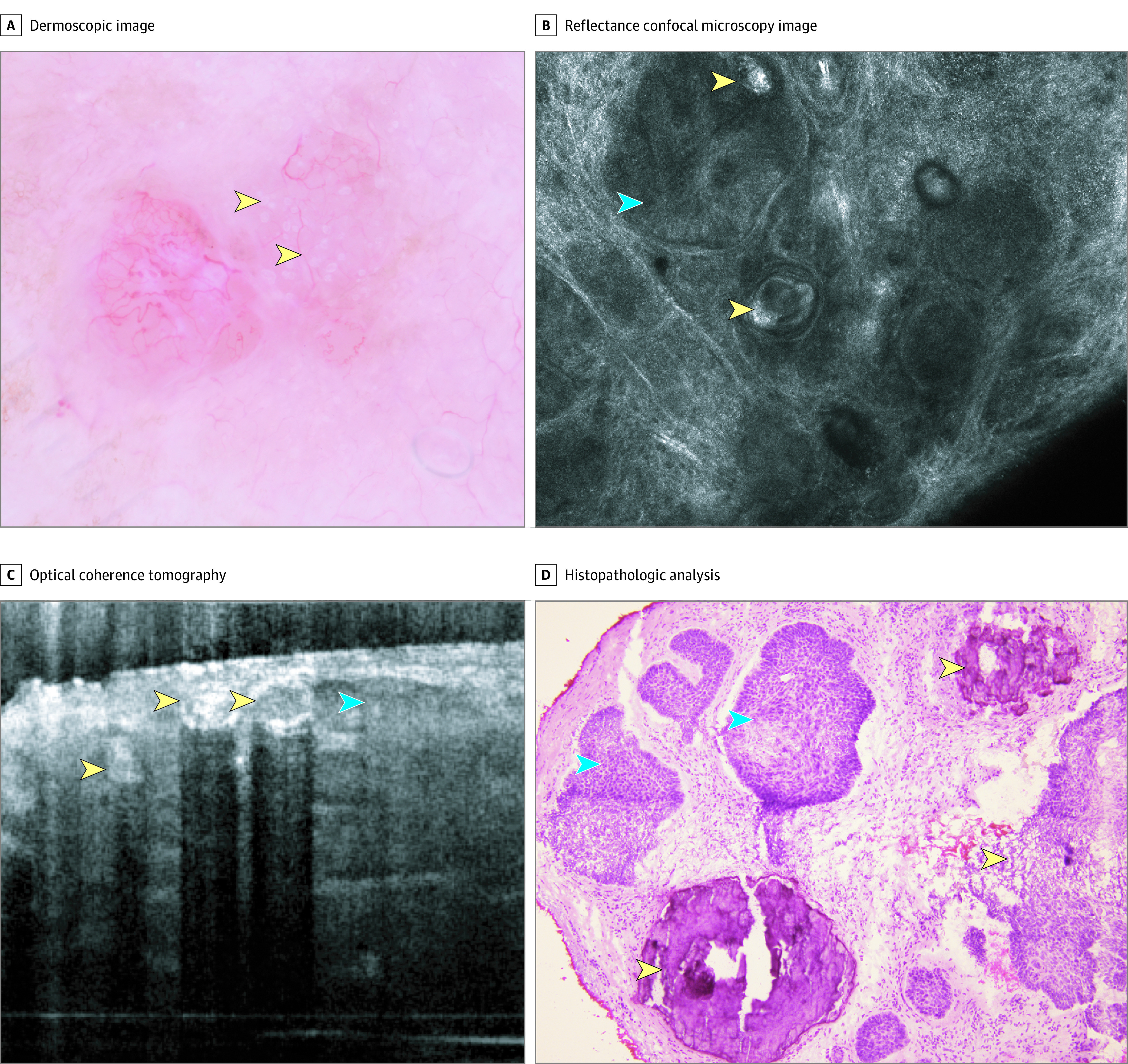
A, Dermoscopic appearance showing MAY globules (yellow arrowheads). B, Reflectance confocal microscopy shows tumor nodules (blue arrowheads) and hyperreflective amorphous areas (yellow arrowheads). C, Optical coherence tomography shows hyperreflective structures with acoustic shadow (yellow arrowheads) and hyporeflective nodules (blue arrowheads). D, Histopathologic analysis shows tumor islands with palisading and clefting (blue arrowheads) and areas of calcium deposits (yellow arrowheads).
Table 2. Dermoscopic Characteristics.
| Characteristic | Cases, No. (%) | OR (95% CI) | κ (95% CI) | ||
|---|---|---|---|---|---|
| BCC (n = 291) | Other diagnoses (n = 365) | Total (N = 656) | |||
| Multiple aggregated yellow-white globules | 61 (21.0) | 3 (0.8) | 64 (9.8) | 32.0 (9.9 to 103.2) | 0.895 (0.753 to 0.937) |
| Ulcerationa | 50 (17.2) | 60 (16.4) | 110 (16.8) | 1.1 (0.7 to 1.6) | 0.7261 (0.664 to 0.768) |
| Arborizing telangiectasiaa | 139 (47.8) | 22 (6.0) | 161 (24.5) | 14.3 (8.7 to 23.2) | 0.935 (0.935 to 0.966) |
| Ovoid nesta | 16 (5.5) | 0 (0) | 16 (2.4) | NR | −0.008 (0.017 to 0.003) |
| Blue-gray globulesa | 24 (8.3) | 0 (0) | 24 (3.7) | NR | 0.189 (0.126 to 0.494) |
| Blotches and strandsa | 214 (73.5) | 70 (19.2) | 284 (43.3) | 11.7 (8.1 to 16.9) | 0.827 (0.806 to 0.904) |
| Spoke-wheel structuresa | 4 (1.4) | 0 (0) | 4 (0.6) | NR | 0.328 (0.189 to 0.497) |
| Leaflike areasa | 36 (12.4) | 1 (0.3) | 37 (5.6) | 51.4 (7.0 to 377.2) | 0.747 (0.747 to 1.0) |
| Concentric structuresa | 15 (5.2) | 0 (0) | 15 (2.3) | NR | 0.272 (−0.008 to 0.392) |
| Short-fine telangiectasiaa | 115 (39.5) | 25 (6.9) | 140 (21.3) | 8.9 (5.6 to 14.2) | 0.484 (0.398 to 0.592) |
| In-focus dotsa | 75 (25.8) | 6 (1.6) | 81 (12.4) | 20.8 (8.9 to 48.5) | 0.782 (0.629 to 0.835) |
| Multiple small erosionsa | 23 (7.9) | 3 (0.8) | 26 (4.0) | 10.4 (3.1 to 34.8) | - |
| Serpentine vessels | 1 (0.3) | 16 (4.4) | 17 (2.6) | 0.1 (0 to 0.6) | −0.014 (−0.017 to −0.008) |
| Milialike cysts | 15 (5.2) | 16 (4.4) | 31 (4.7) | 1.2 (0.6 to 2.4) | 0.601 (0.477 to 0.791) |
| Polymorphous vessels | 11 (3.8) | 46 (12.6) | 57 (8.7) | 0.3 (0.1 to 0.5) | 0.669 (0.507 to 0.742) |
| Shiny white lines | 3 (1.0) | 28 (7.7) | 31 (4.7) | 0.1 (0 to 0.4) | 0.851 (0.658 to 1.0) |
| Rosettes | 17 (5.8) | 51 (14.0) | 68 (10.4) | 0.4 (0.2 to 0.7) | 0.741 (0.676 to 0.747) |
| Peppering | 2 (0.7) | 7 (1.9) | 9 (1.4) | 0.4 (0.1 to 1.7) | −0.005 (−0.005 to −0.003) |
| White circles | 3 (1.0) | 35 (9.6) | 38 (5.8) | 0.1 (0 to 0.3) | 0.767 (0.719 to 0.82) |
| Scale | 15 (5.2) | 179 (49.0) | 194 (29.6) | 0.1 (0 to 0.1) | 0.807 (0.782 to 0.824) |
| Glomerular vessels | 11 (3.8) | 115 (31.5) | 126 (19.2) | 0.1 (0 to 0.2) | 0.650 (0.501 to 0.697) |
| Hairpin vessels | 10 (3.4) | 41 (11.2) | 51 (7.8) | 0.3 (0.1 to 0.6) | 0.556 (0.332 to 0.63) |
| Orange color | 5 (1.7) | 50 (13.7) | 55 (8.4) | 0.1 (0 to 0.3) | 0.647 (0.538 to 0.779) |
Abbreviations: BCC, basal cell carcinoma; NR, not reported (odds ratios cannot be estimated because observations were below the thresholds).
BCC-specific criteria.
Interrater Agreement
We observed almost perfect interrater agreement for the presence of MAY globules (κ = 0.89; 95% CI, 0.75-0.94). The interrater agreements were 0.94 (95% CI, 0.94-0.97) for arborizing vessels, 0.83 (95% CI, 0.81-0.90) for shiny white structures (blotches and strands), 0.78 (95% CI, 0.63-0.84) for in-focus dots, and 0.73 (95% CI, 0.66-0.77) for ulceration (Table 2).
RCM and OCT Features
Under RCM, all 4 examined cases had hyperreflective amorphous areas (Figure 2B and C and Figure 3B) in addition to classic BCC-specific features (tumor nests with palisading and clefting). OCT was available for 2 cases. Lesions with these dermoscopic structures had hyperreflective areas, producing an optical shadow (Figure 2D and Figure 3C).
Histopathologic Correlation
In the 4 cases examined with a precision biopsy, aggregated yellow globules correlated with isolated, round areas of dystrophic calcification in or around tumor nodules and with the presence of calcified keratocysts (Figure 2E and F and Figure 3D). In addition, 2 cases were analyzed in formalin-fixed, paraffin-embedded tissue, showing small calcific deposits in the superficial dermis in association with small keratocysts.
Discussion
In this retrospective case-control study of 656 lesions in 643 patients with nonpigmented tumors, we found that the presence of MAY globules was associated with the diagnosis of BCC. In addition, the presence of MAY globules was associated with high-risk histologic subtypes. Although tumors other than BCC may display milialike cysts and/or shiny white structures,7 we observed that the presence of MAY globules was almost exclusively seen in BCCs. Although this dermoscopic feature was seen in only 21.0% of the nonpigmented BCCs evaluated, its frequency is within the range of other BCC-specific criteria, such as spoke-wheel structures, concentric structures, and leaf-like areas, with reported prevalence ranging from 8% to 20%.6,14 When present, however, MAY globules were highly associated with BCC, specifically with high-risk histologic subtypes.
Dermoscopy has improved the diagnostic accuracy of pink lesions by providing visualization of structures and clues not visible to the naked eye, thereby improving the diagnostic accuracy for SCC, amelanotic melanoma, and BCC, among others.10 However, hypomelanotic and amelanotic lesions are still challenging to diagnose with dermoscopy alone.10,19 Other subgroups of lesions that are difficult to diagnose on clinical and dermoscopic grounds alone are the recently described BCCs that present as white papules on chronic sun-damaged skin.9 The presence of MAY globules may be an important clue toward the diagnosis of BCC in this group of lesions by narrowing the differential diagnosis toward BCC. Another scenario in which the presence of MAY globules might emerge as an important dermoscopic feature is in the differentiation of BCC from IDN on the face, which can be challenging.14,20,21 If MAY globules are seen, one can rule out an IDN, prompting a biopsy.
Besides improving the diagnostic accuracy of BCC,8 dermoscopy has also improved the ability to identify BCC subtypes (ie, superficial vs nodular), which in turn can assist in real-time, bedside, management decisions.22,23 In the present study, a relevant finding was that MAY globules were not seen in superficial BCC subtypes. More importantly, if MAY globules were present, there was a higher odds of a high-risk BCC. A histologic high-risk BCC could be missed because of (1) sampling errors at the time of partial biopsy, (2) superficial shave biopsies, and (3) a deeper-seated or mixed-type BCC.24 If a pathology report indicates a superficial BCC in a lesion displaying MAY globules, one might request additional sectioning, or it may be prudent to avoid topical therapies and resort to more aggressive forms of treatment, such as surgical excision. In addition, the findings of MAY globules in BCCs can also guide the physician in selecting the most appropriate biopsy type (eg, tangential vs punch vs incisional).
On precision biopsy, we found that 4 cases of MAY globules were correlated with dystrophic calcification on histopathologic analysis. Two additional cases evaluated in formalin-fixed, paraffin-embedded samples confirmed that MAY globules were correlated with calcifications. Previous studies25,26 have found that calcifications are seen in 11% to 21% of all BCCs on histopathologic analysis. Furthermore, Slowdkoska et al25 found that these calcifications were more common in high-risk histologic subtypes (44% in infiltrative or morpheaform) than in low-risk histologic subtypes (22%). The dermoscopic findings in our study mirror these histopathologic findings, with 56% of histologic high-risk subtypes revealing MAY globules vs 19% in low-risk subtypes (superficial and nodular). In addition, Slowdkoska et al25 reported that BCC with calcifications may display keratocyst formation, which we observed in the proximity of calcified nodules as well. Wortsman et al27 described 7 or more hyperechoic dots on ultrasonography to be associated with high-risk histologic subtypes. The authors correlated hyperecoic dots with calcifications, necrosis, and micronodular subtypes. Some of these hyperechoic dots could correspond to the MAY globules described herein. The ultrasonographic findings could also mirror the OCT findings in this study (Figure 2 and Figure 3).
MAY globules were seen in 3 cases other than BCC. Two of these cases corresponded to DT and 1 to SCC. DT is a known mimicker of BCC clinically, dermoscopically, histopathologically, and under RCM. In this study, 2 of 3 DTs showed the presence of MAY globules. The limited number of DTs included in our study was too small to allow for any conclusions.28,29,30 DT is a potential diagnostic pitfall of MAY globules under dermoscopy and should be considered as part of the differential diagnosis. A previous study31 found that calcification is a common finding in trichoepitheliomas and seen in up to 30% of cases. SCC has also been reported to show calcification on histopathologic analysis in 3% of cases and explains the presence of MAY globules in 1 case of SCC in our series.26 Other tumors that can also display MAY globules include microcystic adnexal carcinoma.32,33 Dermoscopy of microcystic adnexal carcinoma is limited to only single case reports; analysis of 1 case32 showed MAY globules as well as calcification on histopathologic analysis. The authors discussed the difficulties in distinguishing microcystic adnexal carcinoma from an infiltrative BCC even with histopathologic analysis.32
MAY globules should be included in the differential diagnosis of other white-yellow structures seen with dermatoscopy.7,34,35,36,37,38,39,40,41,42 The main mimicker is milialike cysts. Milialike cysts are typically seen in seborrheic keratosis and congenital nevus, but any tumor can manifest with milialike cysts, including BCCs. Milialike cysts are typically visualized under nonpolarized light. In contrast, MAY globules are seen in both nonpolarized and polarized light. In addition, white or yellow globules seen in balloon cell nevus can potentially mimic MAY globules. In contrast, yellow globules of balloon cell nevus are seen in the context of a melanocytic lesion.34,35 Also, yellowish polylobular globules popcorn-like appearance seen in sebaceous hyperplasia tends to be blurry.36 Shiny white structures, which have been described as a BCC diagnostic criterion, do not form globules and are only seen under polarized light.7
Limitations
Limitations of this study include its retrospective, single-center design and the low number of cases of benign lesions known to present with yellowish structures as controls (eg, sebaceus hyperplasias, molluscum contagiosum, and pilomatrixomas). These lesions are usually multiple and/or easy to diagnose on clinical and dermoscopic grounds alone and are not typically part of the differential diagnosis of equivocal lesions and are infrequently biopsied. In addition, to enrich the non-BCC tumor data set, we obtained a subset of lesions from the ISIC archive; thus, the real-life frequency of MAY globules in non-BCC tumors could not be quantified with confidence. An additional limitation is that the population was mainly composed of white patients with intense sun exposure (residents of Florida). Findings might vary based on different sun-exposure backgrounds and skin types, and these results should be validated in other populations.
Conclusions
The findings suggest that MAY globules may have utility as a new BCC dermoscopic criterion to aid in diagnosis and help in the identification of high-risk BCC histologic subtypes. These structures may be associated with calcifications. Validation of our results is needed with data sets from other centers that include lesions not routinely biopsied, such as sebaceous hyperplasia and molluscum contagiosum.
References
- 1.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069-1080. doi: 10.1111/j.1365-2133.2012.10830.x [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151(10):1081-1086. doi: 10.1001/jamadermatol.2015.1187 [DOI] [PubMed] [Google Scholar]
- 3.Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379(4):363-374. doi: 10.1056/NEJMra1708701 [DOI] [PubMed] [Google Scholar]
- 4.Wu S, Han J, Li WQ, Li T, Qureshi AA. Basal-cell carcinoma incidence and associated risk factors in U.S. women and men. Am J Epidemiol. 2013;178(6):890-897. doi: 10.1093/aje/kwt073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asgari MM, Moffet HH, Ray GT, Quesenberry CP. Trends in basal cell carcinoma incidence and identification of high-risk subgroups, 1998-2012. JAMA Dermatol. 2015;151(9):976-981. doi: 10.1001/jamadermatol.2015.1188 [DOI] [PubMed] [Google Scholar]
- 6.Menzies SW, Westerhoff K, Rabinovitz H, Kopf AW, McCarthy WH, Katz B. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136(8):1012-1016. doi: 10.1001/archderm.136.8.1012 [DOI] [PubMed] [Google Scholar]
- 7.Navarrete-Dechent C, Bajaj S, Marchetti MA, Rabinovitz H, Dusza SW, Marghoob AA. Association of shiny white blotches and strands with nonpigmented basal cell carcinoma: evaluation of an additional dermoscopic diagnostic criterion. JAMA Dermatol. 2016;152(5):546-552. doi: 10.1001/jamadermatol.2015.5731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiter O, Mimouni I, Gdalevich M, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;80(5):1380-1388. doi: 10.1016/j.jaad.2018.12.026 [DOI] [PubMed] [Google Scholar]
- 9.Liopyris K, Navarrete-Dechent C, Yélamos O, Marchetti MA, Rabinovitz H, Marghoob AA. Clinical, dermoscopic and reflectance confocal microscopy characterization of facial basal cell carcinomas presenting as small white lesions on sun-damaged skin. Br J Dermatol. 2019;180(1):229-230. doi: 10.1111/bjd.17241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinz C, Tschandl P, Rosendahl C, et al. Accuracy of dermatoscopy for the diagnosis of nonpigmented cancers of the skin. J Am Acad Dermatol. 2017;77(6):1100-1109. doi: 10.1016/j.jaad.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 11.Bellucci C, Arginelli F, Bassoli S, Magnoni C, Seidenari S. Dermoscopic yellow structures in basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28(5):651-654. doi: 10.1111/jdv.12092 [DOI] [PubMed] [Google Scholar]
- 12.Bañuls J, Arribas P, Berbegal L, DeLeón FJ, Francés L, Zaballos P. Yellow and orange in cutaneous lesions: clinical and dermoscopic data. J Eur Acad Dermatol Venereol. 2015;29(12):2317-2325. doi: 10.1111/jdv.13249 [DOI] [PubMed] [Google Scholar]
- 13.Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74(6):1093-1106. doi: 10.1016/j.jaad.2015.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62(1):67-75. doi: 10.1016/j.jaad.2009.05.035 [DOI] [PubMed] [Google Scholar]
- 15.Sahu A, Yélamos O, Iftimia N, et al. Evaluation of a combined reflectance confocal microscopy-optical coherence tomography device for detection and depth assessment of basal cell carcinoma. JAMA Dermatol. 2018;154(10):1175-1183. doi: 10.1001/jamadermatol.2018.2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarrete-Dechent C, DeRosa AP, Longo C, et al. Reflectance confocal microscopy terminology glossary for nonmelanocytic skin lesions: a systematic review. J Am Acad Dermatol. 2019;80(5):1414-1427.e3. doi: 10.1016/j.jaad.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarrete-Dechent C, Liopyris K, Cordova M, Busam KJ, Marghoob AA, Chen CJ. Reflectance confocal microscopic and en face histopathologic correlation of the dermoscopic “circle within a circle” in lentigo maligna. JAMA Dermatol. 2018;154(9):1092-1094. doi: 10.1001/jamadermatol.2018.2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174. doi: 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 19.Liopyris K, Navarrete-Dechent C, Dusza SW, et al. Clinical and dermoscopic features associated with lichen planus-like keratoses that undergo skin biopsy: a single-center, observational study. Australas J Dermatol. 2019;60(2):e119-e126. doi: 10.1111/ajd.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamo R, Floristan U, Pampín A, Caro D, Pinedo F, López-Estebaranz JL. Usefulness of confocal microscopy in distinguishing between basal cell carcinoma and intradermal melanocytic nevus on the face. Actas Dermosifiliogr. 2015;106(8):e41-e44. doi: 10.1016/j.ad.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 21.Hoogedoorn L, Peppelman M, Blokx WA, van Erp PE, Gerritsen MJ. Prospective differentiation of clinically difficult to distinguish nodular basal cell carcinomas and intradermal nevi by non-invasive reflectance confocal microscopy: a case series study. J Eur Acad Dermatol Venereol. 2015;29(2):330-336. doi: 10.1111/jdv.12548 [DOI] [PubMed] [Google Scholar]
- 22.Longo C, Lallas A, Kyrgidis A, et al. Classifying distinct basal cell carcinoma subtype by means of dermatoscopy and reflectance confocal microscopy. J Am Acad Dermatol. 2014;71(4):716-724.e1. doi: 10.1016/j.jaad.2014.04.067 [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Elkin EB, Jason Chen CS, Marghoob A. Traditional versus streamlined management of basal cell carcinoma (BCC): a cost analysis. J Am Acad Dermatol. 2015;73(5):791-798. doi: 10.1016/j.jaad.2015.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haws AL, Rojano R, Tahan SR, Phung TL. Accuracy of biopsy sampling for subtyping basal cell carcinoma. J Am Acad Dermatol. 2012;66(1):106-111. doi: 10.1016/j.jaad.2011.02.042 [DOI] [PubMed] [Google Scholar]
- 25.Slodkowska EA, Cribier B, Peltre B, Jones DM, Carlson JA. Calcifications associated with basal cell carcinoma: prevalence, characteristics, and correlations. Am J Dermatopathol. 2010;32(6):557-564. doi: 10.1097/DAD.0b013e3181ca65e2 [DOI] [PubMed] [Google Scholar]
- 26.Walsh JS, Perniciaro C, Randle HW. Calcifying basal cell carcinomas. Dermatol Surg. 1999;25(1):49-51. doi: 10.1046/j.1524-4725.1999.08141.x [DOI] [PubMed] [Google Scholar]
- 27.Wortsman X, Vergara P, Castro A, et al. Ultrasound as predictor of histologic subtypes linked to recurrence in basal cell carcinoma of the skin. J Eur Acad Dermatol Venereol. 2015;29(4):702-707. doi: 10.1111/jdv.12660 [DOI] [PubMed] [Google Scholar]
- 28.Navarrete-Dechent C, Bajaj S, Marghoob AA, González S, Muñoz D. Multiple familial trichoepithelioma: confirmation via dermoscopy. Dermatol Pract Concept. 2016;6(3):51-54. doi: 10.5826/dpc.0603a10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ardigo M, Zieff J, Scope A, et al. Dermoscopic and reflectance confocal microscope findings of trichoepithelioma. Dermatology. 2007;215(4):354-358. doi: 10.1159/000107631 [DOI] [PubMed] [Google Scholar]
- 30.Khelifa E, Masouyé I, Kaya G, Le Gal FA. Dermoscopy of desmoplastic trichoepithelioma reveals other criteria to distinguish it from basal cell carcinoma. Dermatology. 2013;226(2):101-104. doi: 10.1159/000346246 [DOI] [PubMed] [Google Scholar]
- 31.Bettencourt MS, Prieto VG, Shea CR. Trichoepithelioma: a 19-year clinicopathologic re-evaluation. J Cutan Pathol. 1999;26(8):398-404. doi: 10.1111/j.1600-0560.1999.tb01864.x [DOI] [PubMed] [Google Scholar]
- 32.Inskip M, Magee J. Microcystic adnexal carcinoma of the cheek-a case report with dermatoscopy and dermatopathology. Dermatol Pract Concept. 2015;5(1):43-46. doi: 10.5826/dpc.0501a07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinohara R, Ansai S, Ogita A, Matsuda H, Saeki H, Tanaka M. Dermoscopic findings of microcystic adnexal carcinoma. Eur J Dermatol. 2015;25(5):516-518. doi: 10.1684/ejd.2015.2618 [DOI] [PubMed] [Google Scholar]
- 34.Jaimes N, Braun RP, Stolz W, Busam KJ, Marghoob AA. White globules correlate with balloon cell nevi nests. J Am Acad Dermatol. 2011;65(4):e119-e120. doi: 10.1016/j.jaad.2011.03.018 [DOI] [PubMed] [Google Scholar]
- 35.Cinotti E, Perrot JL, Labeille B, Douchet C, Thuret G, Cambazard F. Yellow globules in balloon cell naevus. Australas J Dermatol. 2013;54(4):268-270. doi: 10.1111/ajd.12006 [DOI] [PubMed] [Google Scholar]
- 36.Zaballos P, Ara M, Puig S, Malvehy J. Dermoscopy of sebaceous hyperplasia. Arch Dermatol. 2005;141(6):808. doi: 10.1001/archderm.141.6.808 [DOI] [PubMed] [Google Scholar]
- 37.Morales A, Puig S, Malvehy J, Zaballos P. Dermoscopy of molluscum contagiosum. Arch Dermatol. 2005;141(12):1644. doi: 10.1001/archderm.141.12.1644 [DOI] [PubMed] [Google Scholar]
- 38.Jaimes N, Zalaudek I, Braun RP, Tan BH, Busam KJ, Marghoob AA. Pearls of keratinizing tumors. Arch Dermatol. 2012;148(8):976. doi: 10.1001/archdermatol.2011.3475 [DOI] [PubMed] [Google Scholar]
- 39.Zaballos P, Llambrich A, Puig S, Malvehy J. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217(3):225-230. doi: 10.1159/000148248 [DOI] [PubMed] [Google Scholar]
- 40.Balagula Y, Braun RP, Rabinovitz HS, et al. The significance of crystalline/chrysalis structures in the diagnosis of melanocytic and nonmelanocytic lesions. J Am Acad Dermatol. 2012;67(2):194.e1-194.e8. doi: 10.1016/j.jaad.2011.04.039 [DOI] [PubMed] [Google Scholar]
- 41.Pampena R, Borsari S, Piana S, Longo C. Broadening the list of basal cell carcinoma mimickers: dermoscopic features of trichoadenoma. Dermatol Pract Concept. 2019;9(2):160-161. doi: 10.5826/dpc.0902a17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lupu M, Tebeica T, Voiculescu VM, Ardigo M. Tubular apocrine adenoma: dermoscopic and in vivo reflectance confocal microscopic aspects. Int J Dermatol. 2019;58(11):e210-e211. doi: 10.1111/ijd.14579 [DOI] [PubMed] [Google Scholar]