This randomized clinical trial assesses the effectiveness of an online, self-help program to prevent depression among patients with persistent back pain.
Key Points
Question
Can major depression be prevented in routine health care for patients with persistent back pain?
Findings
In this randomized clinical trial of 295 adults with persistent back pain, participants who received a guided, web-based, self-help intervention as an adjunct to treatment as usual showed a significant reduction in the incidence of major depressive episodes over a 12-month period compared with participants who received treatment as usual only.
Meaning
This finding suggests that a proactive, web-based self-help intervention is an appropriate, low-threshold therapy in routine health care that can reduce the incidence of major depressive episodes in patients with persistent back pain.
Abstract
Importance
Depression is a frequent comorbid condition in patients with persistent back pain and is associated with substantial adverse consequences, including the risk of developing opioid use disorders. Shifting the focus from depression treatment to preventing depression might be a viable way to reduce the disease burden.
Objective
To evaluate the effectiveness of a web-based self-help intervention to reduce the incidence of major depressive episode (MDE) in patients with persistent back pain.
Design, Setting, and Participants
Prevention of Depression in Back Pain Patients (PROD-BP) was a pragmatic, observer-blinded randomized clinical trial with a parallel design conducted in Germany. Eligible adults with a diagnosis of persistent back pain and subclinical depressive symptoms, but who were depression free, were recruited either on-site or after discharge from 82 orthopedic clinics between October 1, 2015, and July 31, 2017. All analyses were conducted according to the intention-to-treat principle from October 31, 2018, to April 30, 2019.
Interventions
The intervention group received an e-coach–guided, web-based self-help intervention that was based on cognitive behavioral therapy and tailored to the needs of patients with persistent back pain. The intervention included 6 obligatory modules and 3 optional modules to be completed by participants as well as feedback from e-coaches. Both the intervention and control groups had unrestricted access to treatment as usual.
Main Outcomes and Measures
Primary outcome was time to onset of an MDE over a 12-month period as assessed by blinded diagnostic raters using the Structured Clinical Interview for DSM-5. Secondary outcomes included depression severity, quality of life, pain intensity, pain-related disability, pain self-efficacy, work capacity, and user satisfaction assessed with a variety of instruments.
Results
A total of 295 participants (mean [SD] age, 52.8 [7.7] years; 184 women [62.4%]) were recruited and randomized to either the intervention group (n = 149) or control group (n = 146). The intervention reduced the risk of MDE onset by 52% (hazard ratio, 0.48; 95% CI, 0.28-0.81; P < .001). Twenty-one participants (14.1%) in the intervention group and 41 participants (28.1%) in the control group experienced an MDE over the 12-month period. The number needed to treat to prevent 1 new case of MDE was 2.84 (95% CI, 1.79-9.44).
Conclusions and Relevance
Results of this trial showed that among patients with persistent back pain, depression can be prevented by a guided web-based self-help intervention in addition to treatment as usual. This finding suggests that using a scalable digital approach to integrate psychological treatment into routine pain management is feasible.
Trial Registration
German Clinical Trials Register Identifier: DRKS00007960
Introduction
Persistent back pain and major depression are among the leading causes of global disease burden, accompanied by various adverse outcomes such as decreased quality of life, work absence, mortality, and substantial economic costs.1,2,3,4 Both conditions frequently occur concurrently and lead to substantial adverse effects on pain management,5,6,7 including poor treatment adherence as well as increased symptom burden, medical complications, and pain-related disability.7,8,9,10,11,12 Even more alarming given the opioid-overdose crisis is how major depression contributes to opioid use in individuals with persistent pain, which highlights the urgency of novel evidence-based measures in pain management.13,14,15,16
Precluding the development of major depression, thus preventing the associated disease burden, is the most desirable outcome.17,18,19,20,21 Evidence suggests that depression can be prevented through psychological interventions.21,22,23,24 However, proactive interventions for depression are not part of the routine health care provided to patients with pain.25,26,27,28 One reason for this lack of integration may be a fragmented health care system in which physical and behavioral health care are separated, thus ignoring the interconnectedness of health conditions.29,30,31,32 Digital or web-based solutions have been implemented to help overcome this fragmentation and improve continuity of care by increasing the reach, scalability, and affordability of psychological interventions.33,34,35 With regard to pain management, web-based services might be particularly beneficial in locations without readily available health care practitioners who are trained in treating comorbid mental conditions. Furthermore, the ease of prescription might be a pivotal advantage of web-based interventions vs on-site treatment in the medical field.36,37
A previous randomized clinical trial reported on the potential of a guided, web-based self-help intervention to reduce the risk of a major depressive disorder or recurrence in a general population sample (hazard ratio [HR], 0.59; 95% CI, 0.42-0.82; P = .002).38 This promising result raises the question of whether these outcomes could be achieved in patients with a somatic disorder who are treated in routine health care settings (which in Germany includes pain management). Clinicians are challenged to provide care to patients with complex multimorbidity who often are older and have less education.39,40,41 As a response to this challenge, we conducted a pragmatic, observer-blinded randomized clinical trial with a parallel design called Prevention of Depression in Back Pain Patients (PROD-BP) to evaluate whether a guided, web-based, self-help intervention (eSano BackCare-DP) along with treatment as usual would reduce the incidence of major depressive episode (MDE) compared with treatment as usual alone over a 12-month follow-up period.42
Methods
Setting and Patients
The PROD-BP trial was conducted according to the trial protocol (Supplement 1)42; the amendments to the protocol are listed in the eMethods and eResults in Supplement 2. The local ethics committee at the University of Freiburg and the data security committee of the German Pension Insurance approved the trial. All participants provided written informed consent. We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
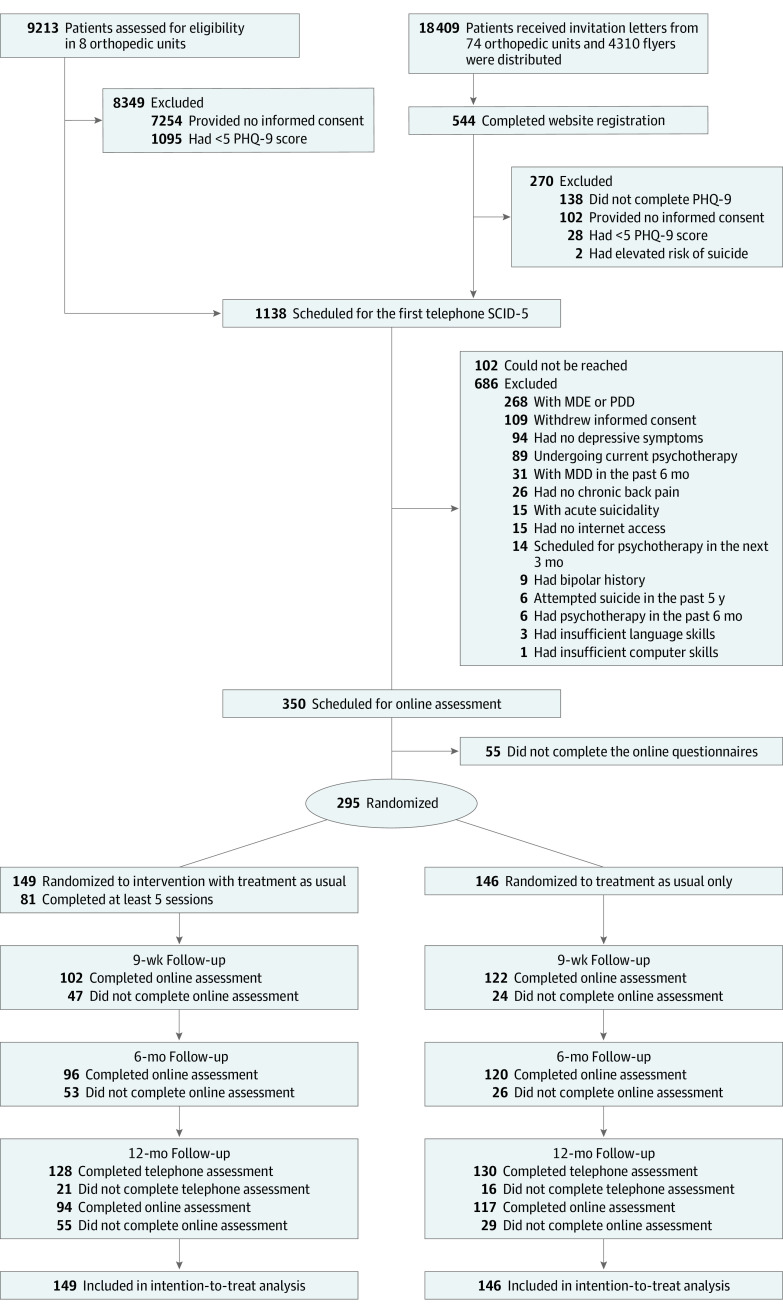
Recruitment was conducted in 82 orthopedic clinics across Germany from October 1, 2015, to July 31, 2017. The last follow-up assessment was completed in October 2018. Research staff informed and invited patients in person in 8 clinics, whereas recruitment after patient discharge was carried out in 74 clinics using information letters and flyers. A total of 18 409 patients received invitation letters, and 4310 flyers were distributed, which generated 544 website registrations, of which 274 individuals met the criteria for the telephone interview. Patients were included if they (1) were aged 18 years or older; (2) had a physician-confirmed diagnosis of persistent back pain (regardless of intensity) with self-reported chronicity of at least 6 months; (3) had sufficient German language skills; (4) had internet access; and (5) reported persistent subthreshold depressive symptoms in the past 3 months, with 2 of 3 positive results on the Patient Health Questionnaire-9 (PHQ-9) screening consisting of a score of 5 or higher (PHQ-9 total score range: 0-27, with 0-4 indicating minimal depression, 5-9 indicating mild depression, 10-14 indicating moderate depression, 15-19 indicating moderately severe depression, 20-27 indicating severe depression).43 We excluded persons who met the DSM-544 criteria for (1) a current MDE or an MDE within the past 6 months (based on the study by Kupfer45), (2) a current persistent depressive disorder, or (3) a bipolar disorder, all of which were assessed through the Structured Clinical Interview for DSM-5 (SCID-5).46 Additional exclusion criteria were (4) ongoing psychotherapy, psychotherapy in the previous 6 months, or being on a waiting list for psychotherapy, and (5) current suicidal ideation or suicidal attempts within the past 5 years (item 9 of the PHQ-9 and SCID-5).43,46
Randomization and Blinding
Eligible participants were randomized 1:1 (stratified by clinic, with recruitment by letter processed as 1 clinic) to either the intervention group or the control group (Figure 1). We used an automatic randomization software (Sealed Envelope; Sealed Envelope Ltd), applying permuted block randomization and variable to randomly arranged block sizes of 4, 6, and 8. One of us (S.S.), who was blinded to all intervention processes, organized the randomization and treatment randomization. Those of us who carried out the recruitment, screened for eligibility, and/or conducted outcome assessments were unaware of the group randomization. Blinding was discontinued at the end of the 12-month follow-up telephone assessment, before the adverse events assessment. Blinding breakdown was documented. Participants and e-coaches could not be blinded. One of us (Y.T.), who was blinded to the group randomization, conducted the statistical analyses.
Figure 1. CONSORT Diagram.
MDD indicates major depressive disorder; MDE, major depressive episode; PDD, persistent depressive disorder; PHQ-9, Patient Health Questionnaire-9; SCID-5, Structured Clinical Interview for DSM-5.
Interventions
The intervention group received a guided, web-based self-help intervention plus treatment as usual, whereas the control group received treatment as usual only. Despite national guidelines on standard treatment as usual for back pain,47 treatment as usual after orthopedic care varies. In Germany, treatment as usual for subclinical depression might consist of visits to a primary care physician but may not entail treatment by a mental health care specialist. To assess the variations in treatment as usual, we recorded the health care utilization of all included participants using the Trimbos/iMTA Questionnaire for Costs Associated with Psychiatric Illness.48
The intervention (eSano BackCare-DP), which is described further in the study protocol (Supplement 1),42 is a guided self-help program with 6 obligatory modules and 3 optional modules (mean [SD] completion time, 43 [32] minutes per module), that is based on cognitive behavioral therapy principles. Participants could choose to receive automated motivational text messages in addition to the online program, entailing brief exercises in daily life depending on treatment progress. During the intervention, e-coaches (trained and supervised psychologists) guided the participants by giving written feedback within 24 hours after each completed module and by answering queries. The mean (SD) guidance time was 64.8 (47) minutes per completed treatment. The intervention was password-protected and accessible on a secure platform maintained by a company that specializes in web-based interventions (Minddistrict).
Outcomes
Outcomes were assessed by telephone (SCID-5; Hamilton Depression Rating Scale [total score range: 0-51, with 0-7 indicating no depression, 8-13 indicating mild depression, 14-18 indicating moderate depression, 19-22 indicating severe depression, ≥23 indicating very severe depression]; Quick Inventory of Depressive Symptomatology [total score range: 0-27, with 0-5 indicating no depression, 6-10 indicating mild depression, 11-15 indicating moderate depression, 16-20 indicating severe depression, 21-27 indicating very severe depression])46,49,50 at baseline and 12 months after randomization as well as by online self-report at baseline and 9 weeks, 6 months, and 12 months after randomization.
The primary outcome was time to onset of an MDE within the 12-month follow-up period. Major depressive episode was diagnosed on the telephone by trained, supervised, and blinded clinicians using the depression-related modules of the SCID-5.46 The life chart method was used to reduce a potential recall bias.51 Interobserver reliability between clinicians and their supervisors was calculated on the basis of a minimum of 4 interviews per clinician.
Secondary outcomes included clinician-rated depression severity (assessed with the Hamilton Depression Rating Scale49 and Quick Inventory of Depressive Symptomatology50) and self-rated depression severity (the PHQ-943). Other outcomes included quality of life (Assessment of Quality of Life total score range: 20-99, with higher scores indicating reduced quality of life),52 pain intensity (Numerical Rating Scale total score range: 0 [no pain] to 10 [worst pain imaginable] in the past week and a rating scale with 4 categories from none to severe),53 pain-related disability (Oswestry Disability Index total score range: 0-100, with higher scores indicating greater disability),54 pain self-efficacy (Pain Self-Efficacy Questionnaire total score range: 0-60, with higher scores indicating increased functional levels),55 work capacity (Subjective Prognostic Employment Scale total score range: 0-3, with higher scores indicating lower perception of prognostic employment),56 and user satisfaction (Client Satisfaction with Online Interventions Questionnaire-8 total score range: 8-32, with higher scores indicating greater client satisfaction).57
Adverse Events
Adverse events were monitored in 4 ways. First, after lifting the randomization blinding in the 12-month follow-up telephone assessment, we used a standardized questionnaire to ask the participants about adverse events they experienced during the trial and to seek their opinion regarding a possible link to the trial regimen. Second, participants in the intervention group reported weekly possible adverse events within the online intervention, which was monitored within a maximum of 48 hours by the e-coaches. Third, the adverse effects of psychotherapy were assessed using the Inventory for Assessing Negative Effects of Psychotherapy.58 Fourth, the reliable symptom deterioration rate was calculated for all depression outcomes and follow-up points. An independent Data and Safety Monitoring Board for this trial was informed of any adverse event.
Statistical Analysis
All analyses were conducted according to the intention-to-treat principle. Missing data were imputed using multivariate imputation by chained equations with 20 generated complete data sets. The imputation method was predictive mean matching, and distance-aided donor selection was applied to improve predictive mean matching. Each imputed data set was analyzed and then pooled using the Rubin rule. We used R packages (R Foundation for Statistical Computing) for all analyses (eMethods and eResults in Supplement 2).
For the primary outcome, Kaplan-Meier curves and Cox proportional hazards regression models were used to determine the difference in time to onset of an MDE in weeks over a 12-month period between the intervention group and control group. The proportional hazards assumption was tested with a weighted residual test. Group differences in Kaplan-Meier curves were evaluated by log-rank test for equal survival functions and in Cox proportional hazards regression model by a likelihood ratio test. Furthermore, we examined the number needed to treat (NNT) to avoid 1 additional MDE (NNT = [1 + HR] / [1 – HR]). Group differences in secondary outcomes were investigated with linear regression models for continuous outcomes and with logistic regression models for dichotomous outcomes. Group and baseline values were used as covariates. We examined the close to symptom-free status for all depression scales according to these definitions: Hamilton Depression Rating Scale score of 7 or lower, Quick Inventory of Depressive Symptomatology score of 5 or lower, and PHQ-9 score lower than 5.43,49,50,59 The reliable change (improvement and deterioration) and corresponding 95% CI were calculated on an individual level for all depression outcomes, with a reliable change index of at least 1.96.43,60
All statistical tests used were 2-sided. P ≤ .05 indicated statistical significance. Data were analyzed from October 31, 2018, to April 30, 2019.
Post Hoc Analyses
To address the limitations of a previous trial,38 we investigated the influence of the number of lifetime MDEs and prior experiences with psychotherapy in moderator analyses. Therefore, 2 extended Cox proportional hazards regression models were used for the respective main effect and interaction with group.
Results
Study Flow
Between October 1, 2015, and July 31, 2017, a total of 9757 individuals were recruited from 82 orthopedic clinics, of which 1138 people were scheduled for the baseline telephone assessment (Figure 1). Of the 350 individuals eligible for inclusion and online assessment, 295 were enrolled and randomized: 149 to the intervention group and 146 to the control group. A total of 258 participants (87.5%) completed the 12-month telephone assessment. The interobserver reliability between clinicians and their supervisors was excellent (κ = 0.96; 95% CI, 0.95- 0.97; P < .001), based on 79 interviews and 17 assessors.
Demographic and clinical characteristics of participants were similar in both groups (Table 1). Among the 295 participants, 184 (62.4%) were women, the mean (SD) age was 52.8 (7.7) years, and 207 (70.2%) reported a low educational level according to the International Standard Classification of Education.61 Two hundred fourteen participants (72.5%) had no prior experience with psychotherapy. On-site recruitment in the orthopedic clinics vs recruitment by letter resulted in a sample comprising more male participants (90 [40.5%] vs 21 [28.8%]) (eTable 1 in Supplement 2).
Table 1. Baseline Characteristicsa.
| Variable | No. (%) | ||
|---|---|---|---|
| Intervention group (n = 149) | Control group (n = 146) | All (n = 295) | |
| Patient characteristics | |||
| Age, mean (SD), y | 51.7 (8.5) | 53.9 (6.7) | 52.8 (7.7) |
| Sex | |||
| Men | 60 (40.3) | 51 (34.9) | 111 (37.6) |
| Women | 89 (59.7) | 95 (65.1) | 184 (62.4) |
| Marital status | |||
| Single | 15 (10.1) | 13 (8.9) | 28 (9.5) |
| Relationship/married | 107 (71.8) | 111 (76.0) | 218 (73.9) |
| Divorced or separated | 21 (14.1) | 16 (11.0) | 37 (12.5) |
| Widowed and single | 4 (2.7) | 6 (4.1) | 10 (3.4) |
| Widowed and in relationship/married | 2 (1.3) | 0 | 2 (0.7) |
| Children, yes | 117 (78.5) | 115 (78.8) | 232 (78.6) |
| Educational levelb | |||
| Low | 102 (68.5) | 105 (71.9) | 207 (70.2) |
| Middle | 25 (16.8) | 18 (12.3) | 43 (14.6) |
| High | 22 (14.8) | 23 (15.8) | 45 (15.3) |
| Social support | |||
| None | 4 (2.7) | 3 (2.1) | 7 (2.37) |
| Low | 31 (20.8) | 42 (28.8) | 73 (24.8) |
| Sufficient | 50 (33.6) | 37 (25.3) | 87 (29.5) |
| High | 46 (30.9) | 45 (30.8) | 91 (30.9) |
| Very high | 18 (12.1) | 19 (13.0) | 37 (12.5) |
| Previous therapy experience | |||
| Only psychotherapy | 21 (11.1) | 17 (11.6) | 38 (12.9) |
| Only medication | 12 (8.1) | 11 (7.5) | 23 (7.8) |
| Psychotherapy and medication | 20 (13.4) | 23 (15.8) | 43 (14.6) |
| IAS, mean (SD)c | 8.99 (3.79) | 8.63 (3.56) | 8.81 (3.67) |
| Depressive symptoms, mean (SD) | |||
| HAM-D scored | 7.39 (4.34) | 7.60 (4.44) | 7.50 (4.38) |
| QIDS scoree | 5.34 (3.14) | 5.38 (3.16) | 5.36 (3.15) |
| PHQ-9 scoref | 8.02 (3.43) | 7.98 (3.78) | 8.00 (3.60) |
| No. of previous MDEsg | |||
| 0 | 53 (35.6) | 65 (44.5) | 118 (40.0) |
| 1 | 46 (30.9) | 47 (32.2) | 93 (31.5) |
| 2 | 30 (20.1) | 24 (16.4) | 54 (18.3) |
| 3 | 14 (9.4) | 5 (3.4) | 19 (6.4) |
| ≥4 | 6 (4.0) | 5 (3.4) | 11 (3.7) |
Abbreviations: HAM-D, Hamilton Depression Rating Scale; IAS, Internet Affinity Scale; MDE, major depressive episode; PHQ-9, Patient Health Questionnaire-9; QIDS, Quick Inventory of Depressive Symptomatology.
Information is based on observed data unless otherwise indicated.
Educational level is based on the International Standard Classification of Education (low: level 1-2, medium: level 3-4, high: level 5+).
Based on imputed data. IAS total score range: 0-20, with higher scores indicating high internet affinity.
Based on imputed data. HAM-D total score range: 0-51, with 0-7 indicating no depression, 8-13 indicating mild depression, 14-18 indicating moderate depression, 19-22 indicating severe depression, ≥23 indicating very severe depression.
Based on imputed data. QIDS total score range: 0-27, with 0-5 indicating no depression, 6-10 indicating mild depression, 11-15 indicating moderate depression, 16-20 indicating severe depression, 21-27 indicating very severe depression.
Based on imputed data. PHQ-9 total score range: 0-27, with 0-4 indicating minimal depression, 5-9 indicating mild depression, 10-14 indicating moderate depression, 15-19 indicating moderately severe depression, 20-27 indicating severe depression.
Assessed via SCID-5 (Structured Clinical Interview for DSM-5).
Effectiveness of Intervention
Cox proportional hazards regression analysis revealed that the intervention reduced the risk of MDE onset by 52% (HR, 0.48; 95% CI, 0.28-0.81; P < .001), favoring the intervention group (Table 2). No evidence of nonproportionality of HRs was found: a global test of nonproportionality yielded χ2 = 2.02 and df = 1 (P = .16).
Table 2. Primary and Secondary Outcomes.
| Variable | Mean (SD) | Intention-to-treat analysis (95% CI)a | P valueb | |
|---|---|---|---|---|
| Intervention group (n = 149) | Control group (n = 146) | |||
| Primary outcome | ||||
| Depression onset, No. (%) | 21 (14.1) | 41 (28.1) | HR, 0.48 (0.28 to 0.81)c | <.001 |
| Secondary outcomes | ||||
| Depression | ||||
| HAM-D scored | ||||
| Baseline | 7.39 (4.34) | 7.60 (4.44) | NA | NA |
| 12 mo | 5.63 (3.88) | 7.24 (5.38) | −0.32 (−0.54 to −0.11) | NA |
| QIDS scoree | ||||
| Baseline | 5.34 (3.14) | 5.38 (3.16) | NA | NA |
| 12 mo | 3.74 (2.80) | 4.83 (3.76) | −0.32 (−0.54 to −0.10) | NA |
| PHQ-9 scoref | ||||
| Baseline | 8.02 (3.43) | 7.98 (3.78) | NA | NA |
| 9 wk | 6.28 (3.45) | 7.86 (3.99) | −0.43 (−0.62 to −0.23) | NA |
| 6 mo | 6.47 (3.39) | 7.50 (4.05) | −0.28 (−0.49 to −0.07) | NA |
| 12 mo | 5.91 (3.53) | 7.43 (4.00) | −0.40 (−0.61 to −0.19) | NA |
| Pain | ||||
| Intensity, Numeric Rating Scale scoreg | ||||
| Baseline | 1.59 (0.68) | 1.62 (0.66) | NA | NA |
| 9 wk | 1.39 (0.68) | 1.58 (0.72) | −0.25 (−0.47 to 0.02) | NA |
| 6 mo | 1.42 (0.69) | 1.47 (0.70) | −0.06 (−0.30 to 0.18) | NA |
| 12 mo | 1.39 (0.74) | 1.53 (0.68) | −0.18 (−0.45 to 0.09) | NA |
| Disability, Oswestry Disability Indexh | ||||
| Baseline | 27.34 (12.41) | 26.77 (13.14) | NA | NA |
| 9 wk | 23.42 (11.72) | 26.03 (12.98) | −0.24 (−0.42 to −0.05) | NA |
| 6 mo | 22.00 (11.28) | 24.29 (12.92) | −0.31 (−0.50 to −0.12) | NA |
| 12 mo | 20.17 (10.62) | 23.60 (13.17) | −0.31 (−0.50 to −0.12) | NA |
| Self-efficacy, PSEQ scorei | ||||
| Baseline | 39.75 (11.07) | 38.22 (11.79) | NA | NA |
| 9 wk | 42.88 (9.88) | 40.36 (10.96) | 0.17 (0.03 to 0.37) | NA |
| 6 mo | 41.91 (10.41) | 39.46 (11.82) | 0.33 (0.13 to 0.53) | NA |
| 12 mo | 44.76 (9.74) | 40.53 (11.15) | 0.33 (0.12 to 0.53) | NA |
| Quality of life | ||||
| AQoL-6D scorej | ||||
| Baseline | 46.15 (7.80) | 45.96 (7.46) | NA | NA |
| 9 wk | 43.18 (8.10) | 45.15 (8.70) | −0.25 (−0.42 to −0.08) | NA |
| 6 mo | 42.59 (7.83) | 44.42 (8.80) | −0.24 (−0.42 to −0.05) | NA |
| 12 mo | 40.96 (7.49) | 44.39 (8.68) | −0.43 (−0.61 to −0.25) | NA |
| Work capacity | ||||
| SPE scorek | ||||
| Baseline | 1.41 (1.08) | 1.42 (1.13) | NA | NA |
| 9 wk | 1.34 (1.11) | 1.26 (1.15) | 0.08 (−0.11 to 0.27) | NA |
| 6 mo | 1.27 (1.18) | 1.29 (1.14) | −0.02 (−0.26 to 0.23) | NA |
| 12 mo | 1.20 (1.13) | 1.36 (1.18) | −0.13 (−0.35 to 0.09) | NA |
Abbreviations: AQoL-6D, Assessment of Quality of Life; HAM-D, Hamilton Depression Rating Scale; HR, hazard ratio; NA, not applicable; PHQ-9, Patient Health Questionnaire-9; PSEQ, Pain Self-Efficacy Questionnaire; QIDS, Quick Inventory of Depressive Symptomatology; SPE, Subjective Prognostic Employment Scale.
Standardized and covariate-adjusted regression estimate for group difference. Primary outcome is HR (95% CI), and secondary outcomes are standardized regression estimates or β (95% CI). 95% CIs are based on robust SEs.
P < .05 is statistically significant.
Based on Cox proportional hazards regression model controlled for baseline severity (HAM-D score).
HAM-D total score range: 0 to 51 (0-7, no depression; 8-13, mild depression; 14-18, moderate depression; 19-22, severe depression; ≥23, very severe depression).
QIDS total score range: 0 to 27 (0-5, no depression; 6-10, mild depression; 11-15, moderate depression; 16-20, severe depression; 21-27, very severe depression).
PHQ-9 total score range: 0 to 27 (0-4, minimal depression; 5-9, mild depression; 10-14, moderate depression; 15-19, moderately severe depression; 20-27, severe depression).
NRS total score range: 0 to 10 (higher scores indicate higher pain intensity).
ODI total score range: 0 to 100 (higher scores indicate greater disability).
PSEQ total score range: 0 to 60 (higher scores indicate increased functional levels).
AQoL-6D total score range: 20 to 99 (higher scores indicate reduced quality of life).
SPE total score range: 0 to 3 (higher scores indicate lower perception of prognostic employment).
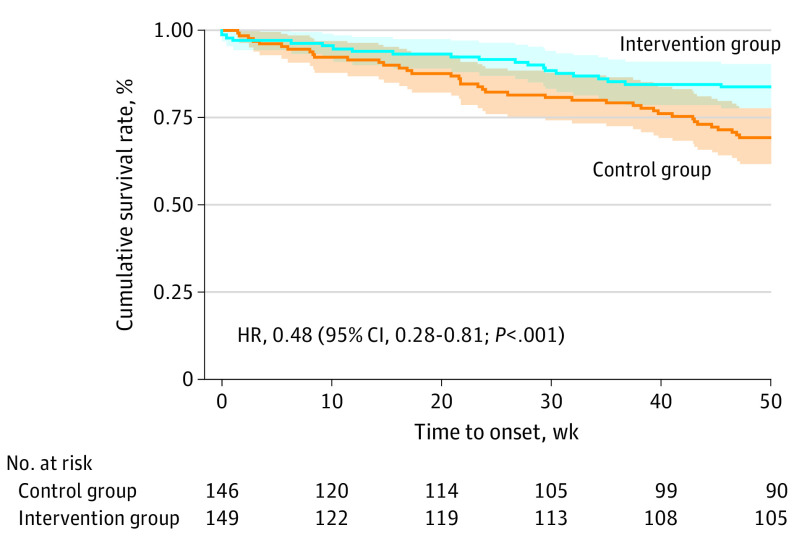
The time to onset of an MDE within a 12-month period differed between the groups. The Kaplan-Meier estimates of the cumulative survival rates (no MDE) at 12 months (52 weeks) were 83.77% (95% CI, 77.65%-90.39%) for the intervention group and 68.38% (95% CI, 60.82%-76.88%) for the control group (Figure 2); 231 participants (78.3%) were right-censored.
Figure 2. Kaplan-Meier Survival Curve of Time to Onset of Major Depressive Disorder.
Shading indicates 95% CI.
Incidence of MDE differed between the groups, with 21 participants (14.1%) in the intervention group and 41 participants (28.1%) in the control group (Δ = 14.67%; 95% CI, 5.56%-24.02%). The NNT to prevent 1 additional case of MDE was 2.84 (95% CI, 1.79-9.44).
Intervention adherence is displayed in the eFigure in Supplement 2. Post hoc moderator analyses, including the number of lifetime MDE as a predictor in the Cox proportional hazards regression model, yielded a decrease of effectiveness with each additional lifetime MDE of 1.49 (95% CI, 1.04-2.15). Prior psychotherapy experiences led to a decrease in effectiveness by a factor of 3.63 (95% CI, 1.22-10.77). Detailed results of the post hoc moderator analyses are reported in the eMethods and eResults in Supplement 2.
Secondary Outcomes
Table 2 shows standardized and baseline-adjusted regression estimates for secondary outcomes for all follow-up assessments based on the intention-to-treat principle. Small to medium effect sizes in favor of the intervention group were observed for all outcomes except for work capacity (Subjective Prognostic Employment Scale mean score [SD]: 1.27 [1.18]) and pain intensity (Numerical Rating Scale mean score [SD]: 1.42 [0.69]) at the 6-month follow-up. eTable 4 in Supplement 2 displays reliable change indexes and close to symptom-free status for all continuous depression outcomes. Odds ratios for reliable improvement ranged between 1.40 (95% CI, 0.79-2.48) for the Quick Inventory of Depressive Symptomatology and 1.90 (95% CI, 1.01- 3.57) for the PHQ-9 at the 12-month follow-up in favor of the intervention group.
We did not find differences in health care utilization between the 2 groups (Table 3).62 For example, pain medication prescription was only slightly lower in the intervention group at the 12-month follow-up (5.58%; 95% CI, −10.72% to 22.41%), and visits to primary care physicians were slightly higher in the intervention group (−4.58%; 95% CI, −17.21% to 7.20%). Satisfaction with the intervention was high (Client Satisfaction with Online Interventions Questionnaire-8 mean score [SD]: 22.87 [4.92]).
Table 3. Health Care Service Use During the 12-Month Follow-up.
| Service | No. (%) | Difference between groups, % (95% CI)a | ||||
|---|---|---|---|---|---|---|
| Control group | Intervention group | |||||
| 6-mo Follow-up (n = 111)b | 12-mo Follow-up (n = 73)c | 6-mo Follow-up (n = 76)b | 12-mo Follow-up (n = 59)c | 6-mo Follow-upb | 12-mo Follow-upc | |
| Primary care physician | 91 (82.0) | 61 (83.6) | 60 (79.0) | 52 (88.1) | 3.03 (−9.03 to 14.24) | −4.58 (−17.21 to 7.20) |
| Psychotherapist | 10 (9.0) | 6 (8.2) | 3 (4.0) | 7 (11.9) | 5.06 (−2.20 to 13.17) | −3.64 (−14.11 to 7.89) |
| Psychiatrist | 0 | 1 (1.4) | 4 (5.3) | 1 (1.7) | −5.26 (−9.89 to 2.24) | −0.32 (−6.48 to 7.06) |
| Neurologist | 12 (10.8) | 8 (11.0) | 12 (15.8) | 7 (11.9) | −4.98 (−14.65 to 5.82) | −0.09 (−11.89 to 11.00) |
| Psychosomatic medicine specialist | 6 (5.4) | 2 (2.7) | 3 (4.0) | 3 (5.1) | 1.46 (−4.98 to 9.06) | −2.34 (−9.84 to 6.71) |
| Prescription | ||||||
| Antidepressant | 9 (8.1) | 12 (16.4) | 10 (13.2) | 10 (17.0) | −5.05 (−13.86 to 5.08) | −0.51 (−13.10 to 12.85) |
| Pain medication | ||||||
| All | 43 (38.7) | 35 (48.0) | 39 (51.3) | 25 (42.4) | −12.58 (−27.00 to 1.27) | 5.58 (−10.72 to 22.41) |
| Opioid | 10 (9.1) | 6 (8.2) | 10 (12.7) | 5 (8.5) | −4.15 (−13.12 to 6.08) | −0.26 (−10.08 to 10.56) |
Abbreviation: TiC-P, Trimbos/iMTA Questionnaire for Costs Associated with Psychiatric Illness.
Based on Newcombe.62
Six-month follow-up covering the previous 3 months as measured with the TiC-P.
Twelve-month follow-up covering the previous 3 months as measured with the TiC-P.
Adverse Events and Safety
In the telephone assessments, a total of 129 participants reported adverse events during the trial, with comparable proportions of adverse events in the intervention group (n = 63 [48.8%]) and control group (n = 66 [51.2%]). A total of 31 participants in the intervention group reported adverse events within the online intervention or directly to their e-coaches. None of these events was associated with the intervention. No serious adverse event (ie, psychiatric hospitalization, suicide attempt, or suicide) occurred. The results of the Inventory for Assessing Negative Effects of Psychotherapy are displayed in eTables 2 and 3 in Supplement 2. Often reported adverse effects assumed to be associated with participation in the intervention were as follows: increased suffering from past experiences (n = 7 [4.7%]) and increased conflicts in relationships (n = 7 [4.7%]).
Discussion
Findings of the PROD-BP trial demonstrated that, for patients with subthreshold depressive symptoms and persistent back pain, a guided web-based intervention implemented into routine orthopedic aftercare significantly reduced the incidence of MDE over a 12-month follow-up period (HR, 0.48; 95% CI, 0.28-0.81; P < .001). The magnitude of this preventive effect was at the upper end of the estimated effects of depression prevention measures in high-risk target groups.63 The effect was even slightly larger than that reported in a previous web-based depression prevention trial in the general population (HR, 0.59; 95% CI, 0.42-0.82), although the 95% CIs overlapped.38 We believe that this result is encouraging and indicates that preventing MDE is an achievable goal in a routine health care setting for patients who are persistently physically ill.
However, when considering the dissemination of the web-based intervention, certain aspects need to be taken into account. First, not all participants may benefit equally from the intervention. Post hoc moderator analyses indicated that the intervention was less effective in patients with a lifetime history of depression or those with previous psychotherapy experience. Second, the guidance element of the intervention, amounting to approximately 1 hour of therapist time per complete treatment, might have been a substantial component. Despite mixed evidence of the role of human support in the effectiveness of and adherence to web-based interventions, guided programs have been found to be cost-effective, depending on society’s willingness to pay.64,65,66
The PROD-BP trial has several implications for clinical practice and research. The study sample was composed of a representative group of patients with persistent back pain (a mean age of 53 years, 62% women, and mainly low to medium levels of educational attainment), contributing to a high generalizability of the results.5,67 Both men and older adults are frequently underrepresented in behavioral health care studies68,69 and online intervention trials.38,70,71 The successful use of 2 different recruitment strategies in this trial suggests that personal contact helps in recruiting hard-to-reach participants. Furthermore, the results corroborate that it is feasible to recruit people with low internet affinity into a web-based intervention trial.71,72
The intervention was implemented as an aftercare program tailored to the patients’ needs. As such, it aimed to bridge 2 gaps: the gap between inpatient care and aftercare, and the gap between 2 separate health care sectors (orthopedics and behavioral health). The value of this transactional, low-threshold treatment approach is illustrated by the finding that 72.5% of the participants attended outpatient professional psychological help for the first time. This finding shows the potential of web-based interventions to extend access to psychological treatments to individuals who previously had not sought, had declined to receive, or had not been able to attend behavioral health services. In light of the current opioid drug use crisis, expanded access has been established as a major goal in pain management.13 However, pain medication prescription was only slightly lower in the intervention group at the 12-month follow-up (5.58%). Secondary outcomes indicate improvements of the intervention group in pain-related functioning and quality of life but not pain intensity. These findings should be examined in future confirmatory studies.
The current form of the web-based intervention is ready for use in clinical practice and can be translated into other languages and adapted for other chronic diseases. However, poor internet availability and reduced knowledge about web-based self-help interventions might limit its large-scale implementation. Future studies should investigate the possible barriers of such programs and how to overcome them in routine health care.
Certain methodological features define this trial. The control group received treatment as usual, the outcomes were clinician rated (observer blinded), and the intervention was integrated into the routine health care setting. These methodological factors are commonly associated with lower effect sizes compared with wait list control groups, self-report measurements, and highly standardized study procedures geared toward efficacy.73,74 The promising results from efficacy trials of web-based interventions for depression usually turn out to be too optimistic when implemented in the routine health care setting.35,72 However, this was not the case in this pragmatic trial. One possible explanation for the high difference in MDE incidence between intervention group and control group in this trial could be that the baseline sample was already close to the MDE threshold. The relatively high incidence of MDE in the control group supports this assumption.
Limitations
This study has several limitations. First, blinding participants to their assigned treatment condition to avoid expectancy effects was not possible. Although this problem is inherent in all psychotherapy trials, it remains a possible risk of bias. However, expectancy effects are also common in routine health care; therefore, they are not generally unwanted effects in pragmatic trials.75,76 Second, blinding was lifted at the end of the telephone assessments before the adverse event assessments because the participants were asked to state whether they attributed a potential adverse event to the intervention or to other circumstances. This timing might have biased the adverse event assessment. Third, variations in treatment as usual might have biased the results. However, randomization was stratified by recruitment clinic, and no differences in health care utilization between the clinics were observed (Table 3).
Conclusions
The PROD-BP trial demonstrated that MDE onset or recurrence can be prevented in routine health care for patients with persistent back pain. We believe that the web-based intervention described herein can help integrate psychological treatment as a rapid and accessible therapy into pain management. Clinicians could prescribe online self-help programs for patients who report subclinical depressive symptoms in routine screening.
Trial Protocol
eMethods and eResults
eTable 1. Baseline Participant Characteristics by Recruitment Strategy
eTable 2. Side Effects (INEP) at 9-Weeks Follow-up Assessment
eTable 3. Side Effects (INEP) at 6-Months Follow-up Assessment
eTable 4. Reliable Improvement, Reliable Deterioration, and Close to Symptom-Free Status in Depressive Symptoms
eFigure. Intervention Adherence
eReferences
Data Sharing Statement
References
- 1.Institute for Health Metrics and Evaluation (IHME) Findings from the Global Burden of Disease Study 2017 Published January 4, 2019. Accessed May 1, 2019. http://www.healthdata.org/policy-report/findings-global-burden-disease-study-2017
- 2.Hoy D, March L, Woolf A, et al. The global burden of neck pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1309-1315. doi: 10.1136/annrheumdis-2013-204431 [DOI] [PubMed] [Google Scholar]
- 3.Broadhead WE, Blazer DG, George LK, Tse CK. Depression, disability days, and days lost from work in a prospective epidemiologic survey. JAMA. 1990;264(19):2524-2528. doi: 10.1001/jama.1990.03450190056028 [DOI] [PubMed] [Google Scholar]
- 4.Rice ASC, Smith BH, Blyth FM. Pain and the global burden of disease. Pain. 2016;157(4):791-796. doi: 10.1097/j.pain.0000000000000454 [DOI] [PubMed] [Google Scholar]
- 5.Von Korff M, Crane P, Lane M, et al. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain. 2005;113(3):331-339. doi: 10.1016/j.pain.2004.11.010 [DOI] [PubMed] [Google Scholar]
- 6.Costa L da CM, Maher CG, McAuley JH, et al. Prognosis for patients with chronic low back pain: inception cohort study. BMJ. 2009;339:b3829. doi: 10.1136/bmj.b3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433-2445. doi: 10.1001/archinte.163.20.2433 [DOI] [PubMed] [Google Scholar]
- 8.Linton SJ, Bergbom S. Understanding the link between depression and pain. Scand J Pain. 2011;2(2):47-54. doi: 10.1016/j.sjpain.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 9.Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976). 2002;27(5):E109-E120. doi: 10.1097/00007632-200203010-00017 [DOI] [PubMed] [Google Scholar]
- 10.Arnow BA, Blasey CM, Lee J, et al. Relationships among depression, chronic pain, chronic disabling pain, and medical costs. Psychiatr Serv. 2009;60(3):344-350. doi: 10.1176/ps.2009.60.3.344 [DOI] [PubMed] [Google Scholar]
- 11.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain. 2016;17(9 suppl):T70-T92. doi: 10.1016/j.jpain.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg D. The detection and treatment of depression in the physically ill. World Psychiatry. 2010;9(1):16-20. doi: 10.1002/j.2051-5545.2010.tb00256.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volkow ND, Icaza MEM, Poznyak V, Saxena S, Gerra G; UNODC-WHO Informal Scientific Network . Addressing the opioid crisis globally. World Psychiatry. 2019;18(2):231-232. doi: 10.1002/wps.20633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253-1263. doi: 10.1056/NEJMra1507771 [DOI] [PubMed] [Google Scholar]
- 15.Cheatle MD. Depression, chronic pain, and suicide by overdose: on the edge. Pain Med. 2011;12(suppl 2):S43-S48. doi: 10.1111/j.1526-4637.2011.01131.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA. 2016;315(15):1624-1645. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuijpers P, Beekman ATF, Reynolds CF III. Preventing depression: a global priority. JAMA. 2012;307(10):1033-1034. doi: 10.1001/jama.2012.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muñoz RF, Bunge EL. Prevention of depression worldwide: a wake-up call. Lancet Psychiatry. 2016;3(4):306-307. doi: 10.1016/S2215-0366(15)00555-6 [DOI] [PubMed] [Google Scholar]
- 19.Thapar A, Collishaw S, Potter R, Thapar AK. Managing and preventing depression in adolescents. BMJ. 2010;340:c209. doi: 10.1136/bmj.c209 [DOI] [PubMed] [Google Scholar]
- 20.Ebert DD, Cuijpers P. It is time to invest in the prevention of depression. JAMA Netw Open. 2018;1(2):e180335. doi: 10.1001/jamanetworkopen.2018.0335 [DOI] [PubMed] [Google Scholar]
- 21.Curry SJ, Krist AH, Owens DK, et al. ; US Preventive Services Task Force . Interventions to prevent perinatal depression: US Preventive Services Task Force recommendation statement. JAMA. 2019;321(6):580-587. doi: 10.1001/jama.2019.0007 [DOI] [PubMed] [Google Scholar]
- 22.van Zoonen K, Buntrock C, Ebert DD, et al. Preventing the onset of major depressive disorder: a meta-analytic review of psychological interventions. Int J Epidemiol. 2014;43(2):318-329. doi: 10.1093/ije/dyt175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merry SN, Hetrick SE, Cox GR, Brudevold-Iversen T, Bir JJ, McDowell H. Psychological and educational interventions for preventing depression in children and adolescents. Cochrane Database Syst Rev. 2011;(12):CD003380. doi: 10.1002/14651858.CD003380.pub3 [DOI] [PubMed] [Google Scholar]
- 24.Brent DA, Brunwasser SM, Hollon SD, et al. Effect of a cognitive-behavioral prevention program on depression 6 years after implementation among at-risk adolescents: a randomized clinical trial. JAMA Psychiatry. 2015;72(11):1110-1118. doi: 10.1001/jamapsychiatry.2015.1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ormel J, Cuijpers P, Jorm AF, Schoevers R. Prevention of depression will only succeed when it is structurally embedded and targets big determinants. World Psychiatry. 2019;18(1):111-112. doi: 10.1002/wps.20580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arango C, Díaz-Caneja CM, McGorry PD, et al. Preventive strategies for mental health. Lancet Psychiatry. 2018;5(7):591-604. doi: 10.1016/S2215-0366(18)30057-9 [DOI] [PubMed] [Google Scholar]
- 27.Foster NE, Anema JR, Cherkin D, et al. ; Lancet Low Back Pain Series Working Group . Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018;391(10137):2368-2383. doi: 10.1016/S0140-6736(18)30489-6 [DOI] [PubMed] [Google Scholar]
- 28.Narasimhan M, Allotey P, Hardon A. Self care interventions to advance health and wellbeing: a conceptual framework to inform normative guidance. BMJ. 2019;365:l688. doi: 10.1136/bmj.l688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fineberg HV. Shattuck lecture. A successful and sustainable health system—how to get there from here. N Engl J Med. 2012;366(11):1020-1027. doi: 10.1056/NEJMsa1114777 [DOI] [PubMed] [Google Scholar]
- 30.Becker AE, Kleinman A. Mental health and the global agenda. N Engl J Med. 2013;369(1):66-73. doi: 10.1056/NEJMra1110827 [DOI] [PubMed] [Google Scholar]
- 31.Das P, Naylor C, Majeed A. Bringing together physical and mental health within primary care: a new frontier for integrated care. J R Soc Med. 2016;109(10):364-366. doi: 10.1177/0141076816665270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. doi: 10.1001/jama.2019.13978 [DOI] [PubMed] [Google Scholar]
- 33.Hedman E. Therapist guided internet delivered cognitive behavioural therapy. BMJ. 2014;348:g1977. doi: 10.1136/bmj.g1977 [DOI] [PubMed] [Google Scholar]
- 34.Singla DR, Raviola G, Patel V. Scaling up psychological treatments for common mental disorders: a call to action. World Psychiatry. 2018;17(2):226-227. doi: 10.1002/wps.20532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersson G, Titov N, Dear BF, Rozental A, Carlbring P. Internet-delivered psychological treatments: from innovation to implementation. World Psychiatry. 2019;18(1):20-28. doi: 10.1002/wps.20610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Titov N, Hadjistavropoulos HD, Nielssen O, Mohr DC, Andersson G, Dear BF. From research to practice: ten lessons in delivering digital mental health services. J Clin Med. 2019;8(8):E1239. doi: 10.3390/jcm8081239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrews G, Hobbs MJ. Pragmatic treatment options for depression and anxiety disorders are needed. World Psychiatry. 2016;15(3):241-242. doi: 10.1002/wps.20364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buntrock C, Ebert DD, Lehr D, et al. Effect of a web-based guided self-help intervention for prevention of major depression in adults with subthreshold depression: a randomized clinical trial. JAMA. 2016;315(17):1854-1863. doi: 10.1001/jama.2016.4326 [DOI] [PubMed] [Google Scholar]
- 39.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37-43. doi: 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 40.Weiss CO, Boyd CM, Yu Q, Wolff JL, Leff B. Patterns of prevalent major chronic disease among older adults in the United States. JAMA. 2007;298(10):1160-1162. doi: 10.1001/jama.298.10.1160-b [DOI] [PubMed] [Google Scholar]
- 41.Makris UE, Abrams RC, Gurland B, Reid MC. Management of persistent pain in the older patient: a clinical review. JAMA. 2014;312(8):825-836. doi: 10.1001/jama.2014.9405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sander L, Paganini S, Lin J, et al. Effectiveness and cost-effectiveness of a guided Internet- and mobile-based intervention for the indicated prevention of major depression in patients with chronic back pain-study protocol of the PROD-BP multicenter pragmatic RCT. BMC Psychiatry. 2017;17(1):36. doi: 10.1186/s12888-017-1193-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Löwe B, Kroenke K, Herzog W, Gräfe K, Gräfe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9). J Affect Disord. 2004;81(1):61-66. doi: 10.1016/S0165-0327(03)00198-8 [DOI] [PubMed] [Google Scholar]
- 44.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed American Psychiatric Association; 2013. [Google Scholar]
- 45.Kupfer DJ. Long-term treatment of depression. J Clin Psychiatry. 1991;52(suppl):28-34. [PubMed] [Google Scholar]
- 46.First M, Williams J, Karg R, Spitzer R. Structured Clinical Interview for DSM-5: Research Version (SCID-5-RV). American Psychiatric Association; 2015. [Google Scholar]
- 47.German Medical Association, National Association of Statutory Health Insurance Physicians, Association of Scientific Medical Societies National Disease Management Guideline: low back pain, short version. 1st ed. Version 5. Amended October 2015. Accessed May 1, 2019. https://www.leitlinien.de/mdb/downloads/nvl/kreuzschmerz/archiv/kreuzschmerz-1aufl-vers5-short.pdf
- 48.Bouwmans C, De Jong K, Timman R, et al. Feasibility, reliability and validity of a questionnaire on healthcare consumption and productivity loss in patients with a psychiatric disorder (TiC-P). BMC Health Serv Res. 2013;13(1):217. doi: 10.1186/1472-6963-13-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. doi: 10.1016/S0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- 51.Lyketsos C, Nestadt G, Cwi J, Heithoff K, Eaton W. The life chart interview: a standardized method to describe the course of psychopathology. Int J Methods Psychiatr Res. 1994;4:143-155. [Google Scholar]
- 52.Richardson JR, Peacock SJ, Hawthorne G, Iezzi A, Elsworth G, Day NA. Construction of the descriptive system for the Assessment of Quality of Life AQoL-6D utility instrument. Health Qual Life Outcomes. 2012;10(1):38-46. doi: 10.1186/1477-7525-10-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. doi: 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 54.Mannion AF, Junge A, Grob D, Dvorak J, Fairbank JCT. Development of a German version of the Oswestry Disability Index, part 2: sensitivity to change after spinal surgery. Eur Spine J. 2006;15(1):66-73. doi: 10.1007/s00586-004-0816-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain. 2007;11(2):153-163. doi: 10.1016/j.ejpain.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 56.Mittag O, Raspe H. A brief scale for measuring subjective prognosis of gainful employment: findings of a study of 4279 statutory pension insurees concerning reliability (Guttman scaling) and validity of the scale [in German]. Rehabilitation (Stuttg). 2003;42(3):169-174. doi: 10.1055/s-2003-40095 [DOI] [PubMed] [Google Scholar]
- 57.Boß L, Lehr D, Reis D, et al. Reliability and validity of assessing user satisfaction with web-based health interventions. J Med internet Res. 2016;18(8):e234. doi: 10.2196/jmir.5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ladwig I, Rief W, Nestoriuc Y. What are the risks and side effects of psychotherapy? development of an Inventory for the Assessment of Negative Effects of Psychotherapy (INEP). Verhaltenstherapie. 2014;24(4):252-264. doi: 10.1159/000367928 [DOI] [Google Scholar]
- 59.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990-996. doi: 10.1016/j.biopsych.2005.09.014 [DOI] [PubMed] [Google Scholar]
- 60.Trajković G, Starčević V, Latas M, et al. Reliability of the Hamilton Rating Scale for Depression: a meta-analysis over a period of 49 years. Psychiatry Res. 2011;189(1):1-9. doi: 10.1016/j.psychres.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 61.International Standard Classification of Education Education Statistics (EdStats). Accessed April 20, 2020. https://datatopics.worldbank.org/education/wRsc/classification
- 62.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17(8):873-890. doi: [DOI] [PubMed] [Google Scholar]
- 63.Beekman AT, Smit F, Stek ML, Reynolds CF III, Cuijpers PC. Preventing depression in high-risk groups. Curr Opin Psychiatry. 2010;23(1):8-11. doi: 10.1097/YCO.0b013e328333e17f [DOI] [PubMed] [Google Scholar]
- 64.Donker T, Blankers M, Hedman E, Ljótsson B, Petrie K, Christensen H. Economic evaluations of internet interventions for mental health: a systematic review. Psychol Med. 2015;45(16):3357-3376. doi: 10.1017/S0033291715001427 [DOI] [PubMed] [Google Scholar]
- 65.Paganini S, Teigelkötter W, Buntrock C, Baumeister H. Economic evaluations of internet- and mobile-based interventions for the treatment and prevention of depression: a systematic review. J Affect Disord. 2018;225:733-755. doi: 10.1016/j.jad.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 66.Shim M, Mahaffey B, Bleidistel M, Gonzalez A. A scoping review of human-support factors in the context of Internet-based Psychological Interventions (IPIs) for depression and anxiety disorders. Clin Psychol Rev. 2017;57:129-140. doi: 10.1016/j.cpr.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 67.Dionne CE, Von Korff M, Koepsell TD, Deyo RA, Barlow WE, Checkoway H. Formal education and back pain: a review. J Epidemiol Community Health. 2001;55(7):455-468. doi: 10.1136/jech.55.7.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kessler RC, Demler O, Frank RG, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352(24):2515-2523. doi: 10.1056/NEJMsa043266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, Kessler RC. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):629-640. doi: 10.1001/archpsyc.62.6.629 [DOI] [PubMed] [Google Scholar]
- 70.Karyotaki E, Riper H, Twisk J, et al. Efficacy of self-guided internet-based cognitive behavioral therapy in the treatment of depressive symptoms: a meta-analysis of individual participant data. JAMA Psychiatry. 2017;74(4):351-359. doi: 10.1001/jamapsychiatry.2017.0044 [DOI] [PubMed] [Google Scholar]
- 71.Andersson G, Titov N. Advantages and limitations of internet-based interventions for common mental disorders. World Psychiatry. 2014;13(1):4-11. doi: 10.1002/wps.20083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gilbody S, Littlewood E, Hewitt C, et al. ; REEACT Team . Computerised Cognitive Behaviour Therapy (cCBT) as treatment for depression in primary care (REEACT trial): large scale pragmatic randomised controlled trial. BMJ. 2015;351:h5627. doi: 10.1136/bmj.h5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cuijpers P, van Straten A, Bohlmeijer E, Hollon SD, Andersson G. The effects of psychotherapy for adult depression are overestimated: a meta-analysis of study quality and effect size. Psychol Med. 2010;40(2):211-223. doi: 10.1017/S0033291709006114 [DOI] [PubMed] [Google Scholar]
- 74.Hempel S, Suttorp MJ, Miles JN, et al. Empirical Evidence of Associations Between Trial Quality and Effect Size. Agency for Healthcare Research and Quality; 2011. [PubMed] [Google Scholar]
- 75.Rutherford BR, Wager TD, Roose SP. Expectancy and the treatment of depression: a review of experimental methodology and effects on patient outcome. Curr Psychiatry Rev. 2010;6(1):1-10. doi: 10.2174/157340010790596571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greenberg RP, Constantino MJ, Bruce N. Are patient expectations still relevant for psychotherapy process and outcome? Clin Psychol Rev. 2006;26(6):657-678. doi: 10.1016/j.cpr.2005.03.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods and eResults
eTable 1. Baseline Participant Characteristics by Recruitment Strategy
eTable 2. Side Effects (INEP) at 9-Weeks Follow-up Assessment
eTable 3. Side Effects (INEP) at 6-Months Follow-up Assessment
eTable 4. Reliable Improvement, Reliable Deterioration, and Close to Symptom-Free Status in Depressive Symptoms
eFigure. Intervention Adherence
eReferences
Data Sharing Statement