Key Points
Question
Do patients with primary degenerative mitral valve prolapse, severe mitral regurgitation, and ventricular ectopy have evidence of occult myocardial inflammation or ischemia in addition to characteristic myocardial fibrosis?
Findings
In this study of 20 patients, most patients with severe degenerative mitral regurgitation and ventricular ectopy exhibited myocardial fluorine 18–labeled fluorodeoxyglucose uptake, a surrogate marker of myocardial inflammation. Additionally, the segments of fluorine 18–labeled fluorodeoxyglucose uptake frequently coexisted with areas of myocardial fibrosis, even in asymptomatic patients with normal left ventricular function and dimensions.
Meaning
These findings suggest that patients with degenerative mitral valve prolapse and ventricular ectopy have evidence of subclinical myocardial inflammation or ischemia.
Abstract
Importance
Myocardial replacement fibrosis has been reported to occur in one-third of patients with mitral valve prolapse (MVP) and significant mitral regurgitation (MR). However, it remains unknown whether there are detectable changes in myocardial metabolism suggestive of inflammation or ischemia that accompany the development of fibrosis.
Objectives
To characterize the burden and distribution of fluorine 18–labeled (18F) fluorodeoxyglucose (FDG) uptake and late gadolinium enhancement (LGE) in patients with degenerative MVP and ventricular ectopy.
Design, Setting, and Participants
Prospective observational study of 20 patients with MVP and significant primary degenerative MR who were referred for mitral valve repair and underwent hybrid positron emission tomography/magnetic resonance imaging (PET/MRI). Ventricular arrhythmias were categorized as either complex (n = 12) or minor (n = 8). Coregistered hybrid 18F FDG-PET and MRI LGE images were assessed and categorized. Recruitment occurred in the new patient clinic of a mitral valve repair reference center. This study was conducted from January 11, 2018, to June 26, 2019.
Exposures
Simultaneous cardiac 18F FDG-PET and MRI with LGE imaging on a hybrid PET/MRI system and ambulatory rhythm monitoring.
Main Outcomes and Measures
Patients were categorized by the presence and pattern of FDG uptake and LGE, the severity of ventricular arrhythmias, and the indication for mitral valve surgery.
Results
In the cohort of 20 patients, the median age was 59.5 years (interquartile range, 52.5-63.2 years). Focal, or focal-on-diffuse uptake, of 18F-FDG (PET positive) was detected in 17 of 20 patients (85%). The FDG uptake coexisted with areas of LGE (PET/MRI positive) in 14 patients (70%). Of the 5 asymptomatic patients with normal ventricular indices and absence of any surgical indications, all were PET/MRI positive.
Conclusions and Relevance
In this pilot study, we demonstrate a novel association between degenerative MVP and FDG uptake, a surrogate for myocardial inflammation and/or ischemia. Such evidence of myocardial injury, even in asymptomatic patients, suggests an ongoing subclinical disease process. These findings warrant further investigation into whether imaging for myocardial inflammation, ischemia, and scar has a role in arrhythmic risk stratification and whether it provides incremental prognostic value in patients with chronic severe mitral regurgitation undergoing active surveillance.
This study characterizes the burden and distribution of fluorine 18–labeled fluorodeoxyglucose uptake and late gadolinium enhancement in patients with degenerative mitral valve prolapse and ventricular ectopy.
Introduction
A subset of patients with mitral valve prolapse (MVP) may develop ventricular arrhythmias (VA) and/or sudden cardiac death at a young age, prior to any perceptible stigmata of myocardial dysfunction or symptoms.1 Left ventricular (LV) fibrosis identified by cardiac magnetic resonance imaging (MRI) has been described as a consistent feature of MVP-related sustained VAs.2 It has been hypothesized that repetitive traction by excess leaflet motion is the inciting stress stimulus that prompts activation of fibroblasts and recruitment of inflammatory cells, which leads to myocardial fibrosis.2 Altered myocardial architecture (fibrosis) and electrotonic interactions between cardiomyocytes and myofibroblasts is thought to form the substrate for triggered and reentrant VAs.3 In addition to replacement fibrosis, subclinical myocardial inflammation or ischemia may be an additional proarrhythmic mechanism in patients with MVP.4,5 We hypothesized that patients with significant degenerative mitral regurgitation (MR) owing to MVP and a history of ventricular ectopy have evidence of occult inflammation in addition to myocardial fibrosis, as detected by integrated fluorine 18–labeled fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) and MRI (hybrid PET/MRI), an imaging modality that allows for simultaneous detection and quantification of functional, anatomic, and metabolic information.6
Methods
Study Population and Participating Center
Patients with chronic degenerative MR were prospectively enrolled and underwent hybrid PET/MRI. Recruitment occurred in the Department of Cardiovascular Surgery at the Mount Sinai Medical Center, and all the patients had at least 3 to 4 MR and were referred for mitral valve surgery. The study was approved by the Mount Sinai institutional review board, and all patients gave written informed consent. Mitral valve prolapse was confirmed by transthoracic echocardiography, as previously defined.7
Echocardiography, Clinical Variables, and Arrhythmia Monitoring
Patients were stratified according to American College of Cardiology/American Heart Association (ACC/AHA) guidelines for severe primary MR and assigned in 1 of 3 indications for surgery: class I: clinical symptoms and LV ejection fraction less than 60% and LV end-systolic dimension greater than 40 mm; class IIa: asymptomatic with new onset of atrial fibrillation or/and pulmonary hypertension (pulmonary artery systolic pressure greater than 50 mm Hg); and lastly class IIb: early surgery, assigned to patients with severe MR without any of the 4 objective class I or IIa indications.8 Patients without recent (<6 months) ambulatory rhythm monitoring underwent a 24-hour Holter monitor followed by ambulatory event monitor (≥7 days) (n = 16). If patients had a recent monitor performed elsewhere, the prior monitoring results were analyzed (n = 3). One patient had exercised-induced nonsustained ventricular tachycardia and did not undergo subsequent ambulatory monitoring. Ventricular arrhythmias were categorized as either complex (c-VA) or minor (m-VA), according to the Lown and Wolf classification (Table).9
Table. Baseline Demographicsa.
| Clinical and echocardiographic variables | Median (IQR) | |
|---|---|---|
| Complex ventricular arrhythmia (n = 12) | Minor ventricular arrhythmia (n = 8) | |
| Age, y | 59.5 (54.7-62.25) | 58.5 (45.7-64.2) |
| Female, No. (%) | 6 (50) | 3 (37.5) |
| Bileaflet MV prolapse, No. (%) | 5 (41.6) | 4 (50) |
| LVEF, % | 59 (55-63) | 62.5 (60-66.2) |
| LVESD | 35.5 (33.2-39.2) | 40.5 (36-54.2) |
| LVEDD | 53.5 (51-57) | 59.5 (53-64.7) |
| Severe (4+) MR, No. (%) | 6 (50) | 3 (37.5) |
| Moderate-severe (3+) MR, No. (%) | 6 (50) | 5 (62.5) |
| Mitral annular disjunction, No. (%) | 9 (75) | 4 (50) |
| Pickelhaube sign, No. (%) | 3 (25) | 1 (12.5) |
| Electrocardiographic and arrhythmic variables | ||
| Inverted/biphasic T waves in inferior leads, No. (%) | 5 (41.6) | 2 (25) |
| Corrected QT interval, ms | 413.5 (400.2-432) | 431 (418.5-440.5) |
| History of atrial fibrillation, No. (%) | 4 (33.3) | 1 (12.5) |
| Syncope, No. (%) | 1 (8.3) | 1 (12.5) |
| PVC burden, % | 4.8 (0.5-17.7) | 0.5 (0.1-1) |
| Pleomorphic PVCs, No. (%) | 10 (83.3) | 0 |
| Ventricular couplets, No. (%) | 8 (66.6) | 0 |
| Nonsustained ventricular tachycardia, No. (%) | 11 (91.6) | 0 |
| Classification of ventricular arrhythmias (Lown), No. (%) | ||
| Grade | ||
| 1 | 0 | 7 (87.5) |
| 2 | 0 | 1 (12.5) |
| 3 | 0 | 0 |
| 4A | 2 (16.6) | 0 |
| 4B | 10 (83.3) | 0 |
| Complex ventricular arrhythmias | 12 (100) | 0 |
| Indications for mitral valve surgery, No (%) | ||
| Class I | 9 (75) | 5 (62.5) |
| Class IIa | 3 (25) | 3 (37.5) |
| Surgical intervention, No (%) | 8 | 6 |
| MV repair only | 1 (8.33) | 0 |
| MV repair + TV repair | 4 (33.3) | 4 (50) |
| MV repair + TV repair + Cryomaze + LAA exclusion | 3 (25) | 2 (25) |
Abbreviations: LAA, left atrial appendage; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricularend-systolic diameter; MV, mitral valve; PVC, premature ventricular contraction; TV, tricuspid valve.
Lown and Wolf classification of ventricular arrhythmias: isolated unifocal PVCs (grade 0,1, or 2), pleomorphic PVCs (grade 3), couplets (grade 4A), and triplets/nonsustained TV (grade 4B). Continuous variables are presented as median and interquartile range (25th to 75th percentile: quartile 1 and quartile 3).
Hybrid PET-MRI
Each patient underwent simultaneous cardiac 18F FDG-PET and MRI with late gadolinium enhancement (LGE) imaging on a hybrid PET/MRI system (Biograph-mMR; Siemens Healthineers), as previously described.10 Patients were categorized into 5 groups: (1) PET positive and MRI positive (PET+/MRI+) when a pattern of increased focal or focal-on-diffuse 18F-FDG uptake coexisted with a pattern of LGE (Figure 1); (2) PET+ and MRI negative (MRI−) if there was increased focal or focal-on-diffuse 18F-FDG uptake without underlying LGE; (3) PET negative (PET−)/MRI+ ; (4) PET−/MRI−; and (5) physiologic 18F-FDG uptake. Additional imaging methods are provided in the eMethods of the Supplement.
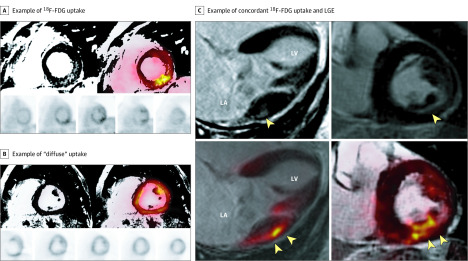
Figure 1. Hybrid Positron Emission Tomography/Magnetic Resonance Imaging (PET/MRI) of 3 Different Patients.
A, Example of fluorine 18–labeled fluorodeoxyglucose (18F-FDG) uptake that colocalizes with regions of late gadolinium enhancement (LGE) (PET+/MRI+) in a focal-on-diffuse pattern (maximum standardized uptake value [SUVmax], 3.5; maximum tissue-to-background ratio [TBRmax], 1.8; target-normal-myocardium ratio [TNMR], 1.8). These images were obtained from a man in his early 60s with asymptomatic degenerative mitral valve prolapse, significant mitral regurgitation (3+), and complex ventricular ectopy (nonsustained ventricular tachycardia and pleomorphic premature ventricular contractions) but without any objective surgical indications. The patient is being followed up with active surveillance. B, Example of diffuse uptake (SUVmax, 9.6; TBRmax, 4.2; TNMR, 1.3), which was interpreted as physiological or nonspecific uptake. C, Example of concordant 18F-FDG uptake and LGE in an asymptomatic patient with chronic severe degenerative mitral regurgitation and absent left ventricular remodeling (left ventricular ejection fraction, 60% and end-systolic dimension, 38 mm). The top 2 images are LGE imaging sequences and the bottom 2 images are 18F-FDG imaging. The yellow arrowheads indicate areas of either LGE or FDG uptake, respectively. LA indicates left atrial; LV, left ventricular.
Statistics
Descriptive statistics were used to characterize the study cohort. Continuous variables are presented as median and interquartile range (25th to 75th percentile: quartile 1 and quartile 3). Calculations were performed by using SPSS, version 12.0 (SPSS Inc).
Results
Baseline Characteristics and Surgical Indications
The baseline demographics were numerically comparable (Table). Nine of 12 patients in the c-VA cohort (75%) and 5 of 8 in the m-VA cohort (62.5%) had a class I indication for surgery. In the c-VA cohort, there was 1 asymptomatic patient with normal LV indices who had atrial fibrillation. The remaining 5 patients were referred for early surgery (class IIa, asymptomatic, and no objective indications).
Hybrid PET/MRI Assessment
All Patients
Image analysis demonstrated that 17 patients (85%) exhibited focal or focal-on-diffuse uptake of 18F-FDG (PET+) (eTable in the Supplement), and 14 patients (70%) exhibited both focal uptake of 18F-FDG and LGE (PET+/MRI+), while 3 (15%) exhibited focal FDG uptake in the absence of any LGE (PET+/MRI−), 1 (5%) had LGE but no FDG uptake (PET−/MRI+), and 1 (5%) had neither FDG uptake nor LGE (PET−/MRI−). Physiologic 18F-FDG uptake was identified in 1 patient (5%). Of the 5 patients with no symptoms, normal LV indices, and absence of any objective surgical indications (early surgery), all were classified as PET+/MRI+ (Figure 1).
The basal-mid inferolateral and basal-mid anterolateral segments were the most commonly involved FDG+ segments, while the basal-mid inferolateral segments were most frequently LGE+ locations (eFigure in the Supplement).
Complex Ventricular Ectopy vs Minor Ventricular Ectopy
There was a trend toward a higher mean (SD) LGE burden in those patients with c-VAs (5.9% [3.9%]) compared with those patients with m-VAs (3.3% [2.3%]) (eTable in the Supplement). There was less FDG uptake in the c-VA cohort compared with the m-VA cohort (mean [SD] maximum standard uptake value, 3.5 [1.2] vs 6.9 [4.2]; and maximum tissue-to-background ratio, 2.1 [0.9] vs 4.0 [2.4], respectively). Because of the small sample sizes, meaningful statistical comparisons between the 2 groups were precluded.
Discussion
In this pilot study, we demonstrated that most patients (85%) with MVP, severe degenerative MR, and ventricular ectopy exhibit myocardial FDG uptake (PET+), a potential surrogate marker of myocardial inflammation. The segments of FDG uptake frequently (70%) coexisted with areas of myocardial fibrosis (LGE+), even in asymptomatic patients with normal LV function and dimensions (Figure 1). The distribution of FDG uptake matched that of LGE, and the PET+ and LGE+ segments tended to be adjacent as well as concordant, raising the question of whether there is an inflammatory component of the disease process that is prodromal to the development of LV fibrosis. The prognostic importance of inflammation, as assessed by 18F-FDG uptake, has been demonstrated in other forms of cardiomyopathy, whereby the presence of myocardial inflammation is associated with higher rates of malignant VAs.4,11 However, it should be noted that regional myocardial 18F-FDG uptake can also occur as a result of myocardial ischemia.6,12 Additional studies are needed to determine whether the observed FDG uptake does in fact equate to myocardial inflammation, increased metabolic demand or myocardial ischemia and whether an association exists between the degree of FDG uptake and the burden/severity of VAs in patients with MVP. Further study is also needed to determine whether the distribution and burden of FDG uptake affects overall prognosis. In this series of patients, there appeared to be 2 basic patterns of 18F-FDG uptake: (1) papillary muscle and/or the inferolateral and basal inferior segments of the LV, locations typically associated with fibrosis in MVP, and (2) a multifocal pattern (Figure 2).2 While we do not know the clinical significance of these patterns, future studies should test whether degree/pattern of FDG is associated with risk for sudden death.4
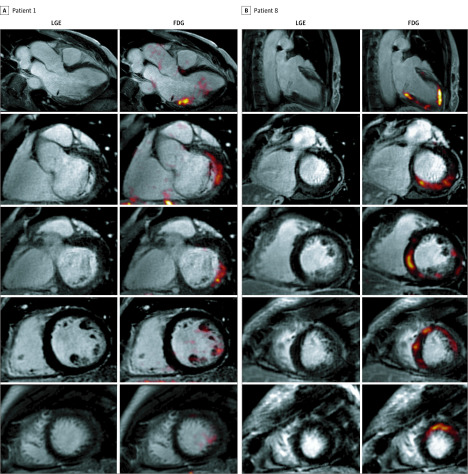
Figure 2. Representative Images of 2 Different Patterns of Fluorodeoxyglucose (FDG) Uptake on Hybrid Positron Emission Tomography/Magnetic Resonance Imaging (PET/MRI) From 2 Different Patients.
A, Focal FDG uptake and late gadolinium enhancement (LGE) in the inferolateral and basal inferior segments of the left ventricle in a man in his mid 50s with severe mitral regurgitation and posterior-leaflet prolapse who was asymptomatic with normal ventricular indices (left ventricular ejection fraction, 65%; left ventricular end-systolic dimension, 34 mm) and no objective indications for surgery. He developed episodes of nonsustained ventricular tachycardia (>15 beats) at peak exercise. B, Multifocal pattern of FDG uptake and LGE, in this case involving the septum, inferior, and lateral walls, in a woman in her mid 60s with symptomatic severe mitral regurgitation and bileaflet prolapse. She had infrequent premature ventricular contractions (burden = 0.5%), but they were pleomorphic (at least 3 different morphologies).
It remains unclear whether there is an association between the severity of VAs and the burden of FDG uptake or LGE in patients with MVP with severe MR. While small sample sizes preclude the use of statistical analysis to compare the imaging results between cohorts, there was a trend toward a higher burden of scar (based on mean percentage LGE) in the patients with c-VA, while the patients with m-VAs exhibited higher FDG uptake (based on mean maximum standard uptake value) (eTable in the Supplement). This raises the intriguing question of whether VA burden could represent different stages of a progressive disease process, whereby those with rare/isolated PVCs identify an earlier stage of the disease characterized by more inflammation and less replacement fibrosis, and those with more complex VAs represent a later, scarred out, stage of the disease.13 This would need to be assessed in future larger studies.
Limitations
This study has several limitations, including a small number of patients and selection bias, because all patients had significant MR and were recruited from a reference surgical center. These results of this study cannot be extrapolated to patients with the most serious arrhythmic complications (sustained VAs) because they were not included. The VA classification system we used was validated for ischemic heart disease and has not been tested in patients with MVP. While 18F-FDG is standard tracer for imaging myocardial inflammation, increased 18F-FDG uptake is not a direct measure of inflammation nor does it distinguish the cell type absorbing the tracer,14,15 and FDG is also taken up in ischemic and postischemic myocardium. Also, we did not perform histopathological analysis. It should also be noted that if the FDG uptake does reflect inflammation, we do not know whether MVP is the cause or the sequelae.
Conclusions
The findings of this pilot study indicate that myocardial inflammation may occur in patients with MVP and degenerative MR and suggests a potential link to myocardial fibrosis. Importantly, these surrogates for myocardial inflammation and fibrosis were found in asymptomatic patients with significant MR who did not have any objective indications for surgery. Future studies will help define whether imaging for inflammation and myocardial scar can identify those patients with MVP who are at higher risk for malignant ventricular arrhythmias and whether such imaging is warranted in patients with severe MR under active surveillance.
eTable. Quantitative PET/MRI Analysis
eFigure. Distribution of Late Gadolinium Enhancement (LGE) and 18F-fluorodeoxyglucose (18F-FDG) Uptake in a 17-Segment Model
eMethods.
References
- 1.Hourdain J, Clavel MA, Deharo JC, et al. . Common phenotype in patients with mitral valve prolapse who experienced sudden cardiac death. Circulation. 2018;138(10):1067-1069. doi: 10.1161/CIRCULATIONAHA.118.033488 [DOI] [PubMed] [Google Scholar]
- 2.Kitkungvan D, Nabi F, Kim RJ, et al. . Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol. 2018;72(8):823-834. doi: 10.1016/j.jacc.2018.06.048 [DOI] [PubMed] [Google Scholar]
- 3.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: the fibroblast awakens. Circ Res. 2016;118(6):1021-1040. doi: 10.1161/CIRCRESAHA.115.306565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muser D, Castro SA, Alavi A, Santangeli P. Potential role of PET in assessing ventricular arrhythmias. PET Clin. 2019;14(2):281-291. doi: 10.1016/j.cpet.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 5.Tung R, Bauer B, Schelbert H, et al. . Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: the potential role of occult inflammation in arrhythmogenesis. Heart Rhythm. 2015;12(12):2488-2498. doi: 10.1016/j.hrthm.2015.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nensa F, Bamberg F, Rischpler C, et al. ; European Society of Cardiovascular Radiology (ESCR); European Association of Nuclear Medicine (EANM) Cardiovascular Committee . Hybrid cardiac imaging using PET/MRI: a joint position statement by the European Society of Cardiovascular Radiology (ESCR) and the European Association of Nuclear Medicine (EANM). Eur Radiol. 2018;28(10):4086-4101. doi: 10.1007/s00330-017-5008-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoghbi WA, Adams D, Bonow RO, et al. . Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303-371. doi: 10.1016/j.echo.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 8.Nishimura RA, Otto CM, Bonow RO, et al. . 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70(2):252-289. doi: 10.1016/j.jacc.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 9.Lown B, Wolf M. Approaches to sudden death from coronary heart disease. Circulation. 1971;44(1):130-142. doi: 10.1161/01.CIR.44.1.130 [DOI] [PubMed] [Google Scholar]
- 10.Dweck MR, Abgral R, Trivieri MG, et al. . Hybrid magnetic resonance imaging and positron emission tomography with fluorodeoxyglucose to diagnose active cardiac sarcoidosis. JACC Cardiovasc Imaging. 2018;11(1):94-107. doi: 10.1016/j.jcmg.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wicks EC, Menezes LJ, Barnes A, et al. . Diagnostic accuracy and prognostic value of simultaneous hybrid 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging. 2018;19(7):757-767. doi: 10.1093/ehjci/jex340 [DOI] [PubMed] [Google Scholar]
- 12.Nazir MS, Ismail TF, Reyes E, Chiribiri A, Kaufmann PA, Plein S. Hybrid positron emission tomography-magnetic resonance of the heart: current state of the art and future applications. Eur Heart J Cardiovasc Imaging. 2018;19(9):962-974. doi: 10.1093/ehjci/jey090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindner D, Zietsch C, Tank J, et al. . Cardiac fibroblasts support cardiac inflammation in heart failure. Basic Res Cardiol. 2014;109(5):428. doi: 10.1007/s00395-014-0428-7 [DOI] [PubMed] [Google Scholar]
- 14.Haroon A, Zumla A, Bomanji J. Role of fluorine 18 fluorodeoxyglucose positron emission tomography-computed tomography in focal and generalized infectious and inflammatory disorders. Clin Infect Dis. 2012;54(9):1333-1341. doi: 10.1093/cid/cis193 [DOI] [PubMed] [Google Scholar]
- 15.Al-Mashhadi RH, Tolbod LP, Bloch LO, et al. . 18Fluorodeoxyglucose accumulation in arterial tissues determined by PET signal analysis. J Am Coll Cardiol. 2019;74(9):1220-1232. doi: 10.1016/j.jacc.2019.06.057 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Quantitative PET/MRI Analysis
eFigure. Distribution of Late Gadolinium Enhancement (LGE) and 18F-fluorodeoxyglucose (18F-FDG) Uptake in a 17-Segment Model
eMethods.