This case series evaluates the clinical response to treatment with the interleukin 17 antagonist secukinumab in patients with Netherton syndrome.
Key Points
Question
Is inhibition of the interleukin 17 signaling pathway an effective therapeutic strategy for patients with Netherton syndrome, a severe and difficult-to-treat genetic condition with high disease burden?
Findings
In this case series, 4 patients with Netherton syndrome were treated with secukinumab, followed by reductions in redness and scaling, relief of pruritus, and improvement in quality of life, particularly in those with the erythrodermic phenotype.
Meaning
This study suggests that anti–interleukin 17 therapy may be a promising option for Netherton syndrome.
Abstract
Importance
Netherton syndrome (NS) is a rare, severe genetic disorder of cornification with high morbidity. Treatment for NS has been notoriously difficult. Recent studies showed an upregulated helper T cell (TH) 17/interleukin 23 (IL-23) pathway in NS, suggesting the possibility of treatment strategies that target IL-17.
Objective
To evaluate the clinical response of NS to treatment with the IL-17 antagonist secukinumab.
Design, Setting, and Participants
This case series study reports the experience of compassionate use therapy with secukinumab in 4 patients with severe NS, including 2 children, from December 1, 2018, to December 1, 2019, with 3 patients still undergoing treatment at the time of final analysis. Data were analyzed from December 1, 2018, to December 1, 2019.
Main Outcomes and Measures
Expression of IL-17 in the skin was evaluated by immunohistochemical analysis, and serum cytokine concentrations were measured using a commercially available assay. Treatment response was assessed using the Ichthyosis Area and Severity Index (IASI) total score, including measures of erythema and scaling, the Dermatology Life Quality Index (DLQI), and the 5-D itch scale.
Results
In all 4 patients (age range, 9-27 years; 3 male and 1 female), immunostaining with an IL-17A antibody showed an increased number of positive cells in lesional skin. Cytokine assessment in serum samples revealed increased levels of CCL20. Treatment duration with secukinumab was 3 to 12 months at the time of this report. After 3 months of therapy, IASI scores were reduced by 44% to 88%, DLQI scores were reduced by 40% to 76%, and 5-D itch scale scores were reduced by 27% to 62%. This outcome was sustained at the 6-month follow-up. Two patients with an erythrodermic phenotype showed marked improvement of all parameters. A refractory palmoplantar eczematous eruption occurred in 2 patients, and a candidal nail infection developed in 2 patients. No severe adverse events were reported.
Conclusions and Relevance
This initial case series reporting the use of anti–IL-17 therapy in NS demonstrated marked cutaneous improvement, particularly in 2 pediatric patients with erythrodermic phenotypes. Further studies are needed to evaluate the long-term benefit of this potential treatment modality.
Introduction
Netherton syndrome (NS) is a rare autosomal recessive skin disorder caused by mutations in SPINK5 (OMIM 605010), leading to severely impaired skin barrier function. Patients are at risk for complications such as hypernatremic dehydration, impaired thermoregulation, failure to thrive, and sepsis. Skin infections are common, as are allergies, asthma, and increased levels of circulating eosinophils and total IgE.1,2 Affected individuals have lifelong ichthyosis lineariz circumflexa, associated with erythroderma, and pruritus.3 Treatment of NS is difficult, with the mainstay being bland emollients. Topical corticosteroids and calcineurin inhibitors may provide short-term benefit, but the former have a high risk of increased transcutaneous absorption and the latter have a high risk of resultant Cushing syndrome and immune suppression. As for systemic agents, low-dose retinoids, infliximab, and intravenous immunoglobulins have been tried with variable success.4,5,6,7 Recent studies showed that NS shares an immune profile similar to psoriasis with helper T cell (TH) 17/interleukin 23 (IL-23) and IL-17/tumor necrosis factor (TNF) synergistic activation.8,9,10 Herein we report our experience using the IL-17A antagonist secukinumab in patients with NS.
Methods
Four patients with severe NS were included in this case series. Expression of IL-17 in the skin was evaluated by immunohistochemical analysis, and serum cytokine concentrations were measured by Luminex assay (Biorad) (eMethods in the Supplement). Compassionate use of secukinumab was initiated from December 1, 2018, on an individual basis after obtaining written informed consent from patients or their parents in accordance with the institutional ethical standards of Children’s Research Center, University Children’s Hospital Zurich, and guarantee of cost reimbursement by the local health care authorities aiming for best clinical care. This study followed the appropriate reporting guideline for case series.
Data were collected until December 1, 2019. Secukinumab was administered in a weight-adapted dosing regimen equivalent to that used in clinical trials for psoriasis11: 75 mg for less than 25 kg, 150 mg for 25 to 50 kg, and 300 mg for greater than 50 kg at baseline and weeks 1, 2, 3, and 4 and monthly thereafter. Patients continued their skin care routine and used topical corticosteroids only as a rescue medication. The patients were evaluated 3 and 6 months after treatment initiation, including laboratory monitoring of complete blood cell count, liver enzyme levels, and kidney function tests. Treatment response was assessed by the following instruments: (1) the Ichthyosis Area and Severity Index (IASI) total score, including measures of erythema (IASI-E) and scaling (IASI-S)8; (2) the Dermatology Life Quality Index (DLQI) and the Children’s DLQI; and (3) the 5-D itch scale.12
Data were analyzed from December 1, 2018, to December 1, 2019. Data were collected in Excel (2013; Microsoft Corporation). Data were expressed as median and range as appropriate, with number and percentage for categorical variables.
Results
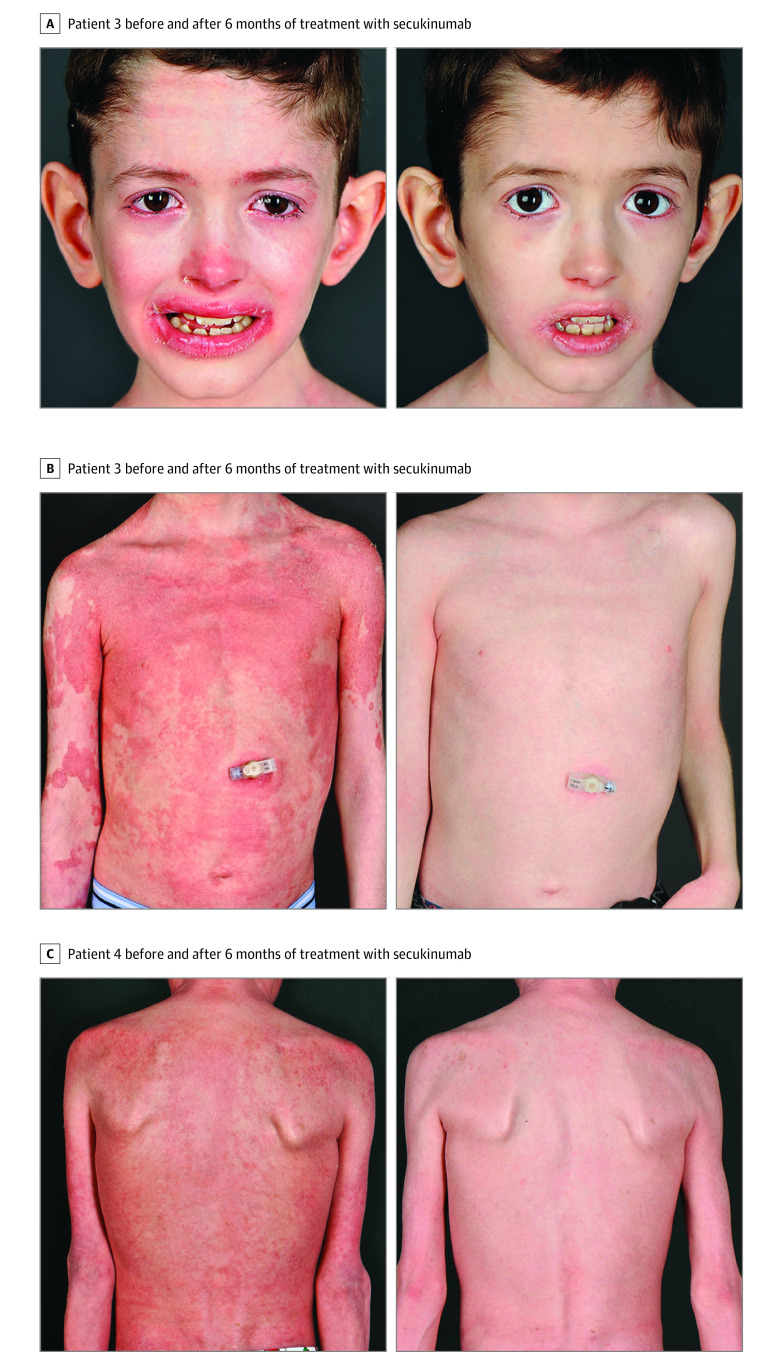
Characteristics of the 4 patients (3 male and 1 female; age range, 9-27 years) are summarized in the Table. The 2 adult patients presented with ichthyosis lineariz circumflexa, whereas the 2 pediatric patients had the ichthyosiform erythroderma phenotype (Figure 1 and eFigure 1 in the Supplement). Immunostaining with the IL-17A antibody showed an increased number of positive cells in lesional skin (eFigure 2 in the Supplement). Analysis of serum cytokine concentrations showed a 1.5-fold increase of CCL20 compared with healthy controls, reflecting the activation of the IL-17 pathway (eFigure 3 in the Supplement). After initiation of secukinumab treatment, cutaneous improvement was observed in all 4 patients, most remarkably in the 2 children (Figure 1 and eFigure 1 in the Supplement). This response occurred after only 2 doses of secukinumab and was sustained during follow-up. Treatment duration was 3 to 12 months at the time of this report. After 3 months of therapy, IASI scores were decreased by 44% to 88%, DLQI and Children’s DLQI scores were decreased by 40% to 76%, and 5-D itch scale scores were decreased by 27% to 62% (Figure 2 and eFigure 4 in the Supplement). After 6 months of treatment, greater decreases of 55% to 88% occurred for the IASI, 69% to 88% for the DLQI and Children’s DLQI, and 38% to 63% for the 5-D itch scale. The reductions of the IASI measures for erythema and scaling after 6 months were 48% to 91% and 31% to 81%, respectively. Patient 1 decided to stop therapy after 3 months because he believed his overall benefit was insufficient. Both pediatric patients had chronic blepharitis, which improved greatly with treatment. Recurrent otitis externa (once per month) in patient 3 and painful skin infections (twice per month) in patient 4 resolved. The use of topical corticosteroids decreased from 2 to 3 times per week to 1 or 2 times per month. The reduction of pruritus was accompanied by improved night sleep. We observed continued weight gain along the third percentile for patients 3 and 4 as in the 2 years before treatment. The growth rate of patient 3 was 4.7 cm/y (10th to 25th percentiles) during the 2 years before therapy; for patient 4, 4.3 cm/y (third to tenth percentile). These rates changed to 5.5 cm/y (50th to 75th percentile) for patient 3 and 4.6 cm/y (25th to 50th percentile) for patient 4 during the 6 months of treatment.
Table. Patient Characteristics and Previous Treatments.
| Patient No./age/sex | SPINK5 mutations | Phenotype | Atopy | Complications | IgE level, mg/dL | Prior therapies (duration in mo) |
|---|---|---|---|---|---|---|
| 1/21/M | Heterozygous, c.1302 + 4A>T (intron 14), c.2314-2A>T (intron 24)a | Ichthyosis lineariz circumflexa | Food allergy, allergic rhinitis | NA | 0.5 | Topical corticosteroids and calcineurin inhibitors |
| 2/27/F | Heterozygous, c.891C>T (p.Cys297Cys) (exon 11), c.1431-12G>A intron 15b | Ichthyosis lineariz circumflexa | Allergic asthma | Failure to thrive | 0.3 | Topical corticosteroids and calcineurin inhibitors, NB–UV-B (3), adalimumab (12), alitretinoin (37) |
| 3/9/M | Heterozygous, c.153delT (p.Gln52Lysfs*6) (exon 3), c.891C>T (p.Cys297Cys) (exon 11)b | Erythrodermic | Food allergy | Failure to thrive, recurrent infections | 0.9 | Topical corticosteroids and calcineurin inhibitors, IVIG (108), acitretin (36), alitretinoin (8) |
| 4/9/M | Homozygous, c.1431-12G>A intron 15 | Erythrodermic | Food allergy | Failure to thrive, recurrent infections | 1.2 | Topical corticosteroids and calcineurin inhibitors, IVIG (108) |
Abbreviations: IVIG, intravenous immunoglobulin; NA, not applicable; NB, narrowband.
To convert IgE to mg/L, multiply by 10.
Indicates new SPINK5 mutation not reported in the Human Gene Mutation Database.
Previously reported synonymous mutation causing out-of-frame skipping of exon 11.
Figure 1. Clinical Response Following Secukinumab Therapy in the 2 Pediatric Patients With Netherton Syndrome .
Figure 2. Ichthyosis Area and Severity Index (IASI) Score, 5-D Itch Scale, and Dermatology Life Quality Index (DLQI) Before and 3 and 6 Months After Treatment With Secukinumab.
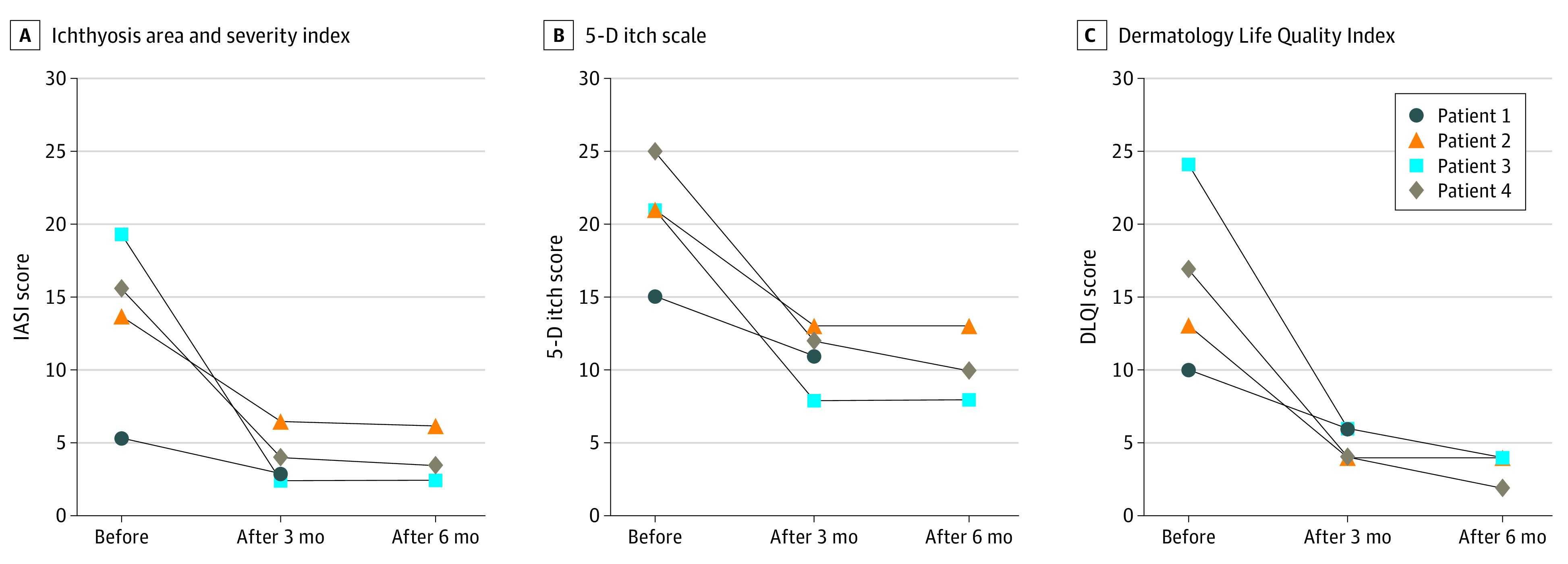
The IASI scores range from 0 to 48, with higher scores indicating maximum erythema and scaling; for the 5-D itch scale, from 5 to 25, with higher scores indicating maximum pruritus; and for the DLQI, from 0 to 30, with higher scores indicating maximum impairment.
Onychomycosis due to Candida albicans occurred in 2 patients who previously never had fungal infections, and common viral warts occurred in 1 patient during treatment. The candidal nail infection was treated with systemic fluconazole, leading to slow resolution. Patients 3 and 4 developed an acute pruritic palmoplantar eczematous reaction, which was refractory to topical corticosteroids (eFigure 5 in the Supplement). Results of a skin biopsy of patient 3 showed spongiotic dermatitis with psoriasiform acanthosis and hyperparakeratosis; fungal infection was excluded (eFigure 6 in the Supplement). At last follow-up, patient 2 had been treated with secukinumab for 12 months; patient 3, for 8 months; and patient 4, for 7 months. They all wished to continue with their therapy.
Discussion
In this case series, we report the use of anti–IL-17 therapy in NS, based on the IL-17 pathway activation in the patients’ skin and serum. Marked cutaneous improvement was demonstrated, particularly in 2 pediatric patients with erythrodermic phenotype, with significant decrease of pruritus and life-changing benefits.
The disease burden of NS has been highlighted by De Palma et al,13 who reported the highest degree of itch in patients with NS compared with other ichthyoses. This finding is associated with significant impairment of quality of life and sleep, overprotection by parents, stigmatization, and isolation.3 In 2017, Paller et al8 reported that several disorders of keratinization are defined by an IL-17–dominant immune profile, and Malik et al10 reported that patients with NS had the highest induction of TH17/IL-23 pathway genes. Targeted therapy has been tried with the anti-TNF agent infliximab in 2 adult patients with NS, resulting in cutaneous improvement.4,5 In this case series, the 2 pediatric patients with erythrodermic phenotype showed the most impressive response to secukinumab, with a life-changing decrease of pruritus and skin discomfort. They were able to limit their use of topical corticosteroids and reduce the risk of Cushing syndrome and growth retardation.
Secukinumab was approved by the US Food and Drug Administration in January 2015 for the treatment of moderate-to-severe psoriasis with good tolerability.14,15 Nasopharyngitis and upper respiratory tract infection are the most common adverse events but were not observed in our patients.15 Blockade of IL-17 increases the risk of mucocutaneous candidiasis, and 2 patients developed onychomycosis by C albicans. We believe that the acute palmoplantar dermatitis in 2 of our patients with pruritus as a dominant symptom and the histopathological findings remarkable for spongiosis and retained granular layer favors an eczematous reaction. However, we appreciate that the clinical and histopathological features of patient 3 may also be interpreted as a psoriasiform palmoplantar reaction as sometimes seen during TNF-blocking therapy. We hypothesize that blocking IL-17A may lead to a compensatory increased TH2 response, resulting in this pruritic eczematous reaction.
Limitations
Immunohistofluorescence analysis in lesional skin using the goat anti–IL-17A polyclonal antibody (AF-317-NA; R&D Systems) had positive results; however, we are aware of the potential low specificity of this antibody, which may cross-react with myeloperoxidase as previously reported.16 We tried repeating immunostaining of IL-17A with a different antibody (ab79056; Abcam), but this was only possible in 2 samples with no conclusive results. However, the increased serum levels of CCL20, a major downstream cytokine of IL-17A pathway activation, supported the use of secukinumab. This report lacks monitoring of serum cytokine levels or repeated immunostaining of skin sections during treatment. The small number of patients and short follow-up time prevents us from drawing conclusions regarding long-term response and safety and the effects of secukinumab on growth rate, allergies, and hair growth in NS.
Conclusions
Our findings demonstrate a good to excellent therapeutic effect of IL-17 inhibition with secukinumab in 4 patients with NS. Further studies are needed to investigate the immune profile of patients with NS and to elaborate the long-term benefit of new targeted therapies.
eMethods. Immunohistofluorescence Analyses and Assessment of Serum Cytokine Profiles by Luminex
eFigure 1. Clinical Response to Secukinumab Therapy in the 2 Adult Patients With Netherton Syndrome
eFigure 2. Immunostaining of Lesional Skin
eFigure 3. Cytokine Concentrations of CCL20, MDC and TARC in the Serum Samples of NS
eFigure 4. IASI Total Score in Comparison With IASI- E and IASI-S Score Before and After 3 and 6 Months of Treatment With Secukinumab
eFigure 5. Palmoplantar Eczematous Reaction Under Secukinumab Therapy in Patients 3 and 4
eFigure 6. Histopathologic Findings of a Skin Biopsy of the Palmoplantar Eczematous Eruption in Patient 3
References
- 1.Chavanas S, Bodemer C, Rochat A, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25(2):141-142. doi: 10.1038/75977 [DOI] [PubMed] [Google Scholar]
- 2.Furio L, Hovnanian A. Netherton syndrome: defective kallikrein inhibition in the skin leads to skin inflammation and allergy. Biol Chem. 2014;395(9):945-958. doi: 10.1515/hsz-2014-0137 [DOI] [PubMed] [Google Scholar]
- 3.Versteegh JJ, Dulfer K, Stuvel K, Pasmans SG, Utens EM. Netherton syndrome; neuropsychological and psychosocial functioning of child and adult patients and their parents. J Health Psychol. 2018;21:1359105318790052. doi: 10.1177/1359105318790052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roda Â, Mendonça-Sanches M, Travassos AR, Soares-de-Almeida L, Metze D. Infliximab therapy for Netherton syndrome: a case report. JAAD Case Rep. 2017;3(6):550-552. doi: 10.1016/j.jdcr.2017.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontao L, Laffitte E, Briot A, et al. Infliximab infusions for Netherton syndrome: sustained clinical improvement correlates with a reduction of thymic stromal lymphopoietin levels in the skin. J Invest Dermatol. 2011;131(9):1947-1950. doi: 10.1038/jid.2011.124 [DOI] [PubMed] [Google Scholar]
- 6.Hartschuh W, Hausser I, Petzoldt D. Successful retinoid therapy of Netherton syndrome [in German]. Hautarzt. 1989;40(7):430-433. [PubMed] [Google Scholar]
- 7.Renner ED, Hartl D, Rylaarsdam S, et al. Comèl-Netherton syndrome defined as primary immunodeficiency. J Allergy Clin Immunol. 2009;124(3):536-543. doi: 10.1016/j.jaci.2009.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paller AS, Renert-Yuval Y, Suprun M, et al. An IL-17–dominant immune profile is shared across the major orphan forms of ichthyosis. J Allergy Clin Immunol. 2017;139(1):152-165. doi: 10.1016/j.jaci.2016.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czarnowicki T, He H, Leonard A, et al. The major orphan forms of ichthyosis are characterized by systemic T-cell activation and Th-17/Tc-17/Th-22/Tc-22 polarization in blood. J Invest Dermatol. 2018;138(10):2157-2167. doi: 10.1016/j.jid.2018.03.1523 [DOI] [PubMed] [Google Scholar]
- 10.Malik K, He H, Huynh TN, et al. Ichthyosis molecular fingerprinting shows profound TH17 skewing and a unique barrier genomic signature. J Allergy Clin Immunol. 2019;143(2):604-618. doi: 10.1016/j.jaci.2018.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pediatric study in children and adolescents with severe plaque psoriasis. ClinicalTrials.gov identifier: NCT02471144. Updated March 13, 2020. Accessed April 21, 2020. https://clinicaltrials.gov/ct2/show/NCT02471144
- 12.Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5-D itch scale: a new measure of pruritus. Br J Dermatol. 2010;162(3):587-593. doi: 10.1111/j.1365-2133.2009.09586.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Palma AM, Mazereeuw-Hautier J, Giehl K, et al. Burden of itch in ichthyosis: a multicentre study in 94 patients. J Eur Acad Dermatol Venereol. 2019;33(11):2095-2100. doi: 10.1111/jdv.15613 [DOI] [PubMed] [Google Scholar]
- 14.Langley RG, Elewski BE, Lebwohl M, et al. ; ERASURE Study Group; FIXTURE Study Group . Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326-338. doi: 10.1056/NEJMoa1314258 [DOI] [PubMed] [Google Scholar]
- 15.Bissonnette R, Luger T, Thaçi D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177(4):1033-1042. doi: 10.1111/bjd.15706 [DOI] [PubMed] [Google Scholar]
- 16.Tamarozzi F, Wright HL, Thomas HB, Edwards SW, Taylor MJ. A lack of confirmation with alternative assays questions the validity of IL-17A expression in human neutrophils using immunohistochemistry. Immunol Lett. 2014;162(2 Pt B):194-198. doi: 10.1016/j.imlet.2014.10.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Immunohistofluorescence Analyses and Assessment of Serum Cytokine Profiles by Luminex
eFigure 1. Clinical Response to Secukinumab Therapy in the 2 Adult Patients With Netherton Syndrome
eFigure 2. Immunostaining of Lesional Skin
eFigure 3. Cytokine Concentrations of CCL20, MDC and TARC in the Serum Samples of NS
eFigure 4. IASI Total Score in Comparison With IASI- E and IASI-S Score Before and After 3 and 6 Months of Treatment With Secukinumab
eFigure 5. Palmoplantar Eczematous Reaction Under Secukinumab Therapy in Patients 3 and 4
eFigure 6. Histopathologic Findings of a Skin Biopsy of the Palmoplantar Eczematous Eruption in Patient 3