Abstract
Background
Oral submucous fibrosis (OSMF) is a premalignant condition mainly caused by areca nut chewing and is characterized by progressive fibrosis of submucosal tissues and epithelial atrophy. Activation of transforming growth factor beta (TGF-β) signaling is considered main causative event for increased collagen production and fibrosis. In this study, molecular pathogenesis of OSMF was investigated based on the expression of the TGF-β genes in OSMF tissues compared to normal controls.
Methods
A total of 33 OSMF and 10 normal tissues were collected from patients and their clinic-epidemiological data was recorded. The expression of TGF-β isoform genes- TGF β1, TGF β2, TGF β3 and its receptor TGF βR1, TGF βR2 was studied by real time polymerase chain reaction (PCR). Comparison of the expression of these genes among normal controls and OSMF patients was done. The PCR results were confirmed by histopathological and immunohistochemical staining.
Results
The histological changes included atrophic epithelium, loss of rete ridges, presence of inflammatory cells and dense collagen bundles in connective tissue. PCR showed statistically significant upregulation of TGF-β isoforms in OSMF as compared to normal tissues. Of the three isoforms, maximum fold change was observed in TGF-β1. Similarly, both TGF-βR1 and TGF-βR2 were found to be elevated in OSMF tissues compared to normal. The semi-quantitative analysis by immunohistochemical staining revealed statistically significant difference between normal and OSMF tissues.
Conclusion
TGF-β signaling plays a major role in the molecular pathogenesis of OSMF as shown by increased mRNA expression of all the three TGF-β isotypes and their receptors.
Keywords: Oral submucous fibrosis, Transforming growth factor beta, TGF-β, TGF-β isoforms, TGF-β receptors, Polymerase chain reaction
1. Introduction
Oral submucous fibrosis (OSMF) is a chronic, debilitating potentially malignant disorder characterized by epithelial dystrophy and fibrosis of the submucosa.1 It is primarily caused by areca nut chewing present in smokeless tobacco products consumed by 25.9% of Indians.2 Epidemiological studies suggest a rapid rise of OSMF cases in India, occurring predominantly in males with increasingly younger populations affected by this disorder.3 Treatment of OSMF is very challenging, as signs and symptoms continue to progress despite attempted treatments. Malignant transformation to oral squamous cell carcinoma (OSCC) varies from 2.3 to 10%.4,5
Transforming growth factor beta (TGF-β) comes under superfamily of related growth factors.6 Three separate TGF-β isoforms (TGF-β1, TGF-β2 and TGF-β3) have been recognized and are initially secreted as inactive peptides by cells into the extra cellular matrix.7 These three isoforms bind to TGF-β receptor 2 (TGFR2) which then recruits and activates TGF-β receptor 1 (TGFR1) to activate receptor signalling.8 The latent TGF-β once activated binds to its receptor and the intracellular transduction pathways following this involve either the Smad pathways (canonical pathways) or the Non Smad (non-canonical) signaling pathways.9
The current study stems from lacunae in the understanding of molecular mechanisms of OSMF. The mechanism of induction of fibrosis by areca nut remains uncertain. Activation of the transforming growth factor beta (TGF-β) signaling has been postulated as the main causative event for the increased collagen production and fibrosis. Some studies have reported role of TGF-β in OSMF.10, 11, 12, 13 It is postulated that there is an activation of TGF-β signaling in epithelial cells by areca nut components which influence neighboring fibroblasts and activate the canonical downstream SMAD signaling pathway leading to fibrosis via epithelial mesenchymal interaction.14 In a recent report, involvement of JNK/ATF2/Jun axis in the activation of TGF-β pathway has been demonstrated.15 However, majority of these studies have been carried out in cell lines or animal models. Secondly, dramatic variation in the induction dose of areca nut among patients for developing OSMF and variations in response towards the same treatment protocol has been observed suggesting a genetic predisposition.16
Regardless of the convincing evidence for the effects of TGF-β on the oral mucosa, the distribution of TGF-β family members and their functions in OSMF development are still poorly understood. In this study, we propose to study the expression of genes associated with TGF-β in OSMF at molecular level and validate it with histopathological and immunohistochemical staining (IHC). Such a clinical-histopathological-molecular correlation in OSMF will generate robust evidence and improve our understanding of this disease.
2. Materials & methods
2.1. Study population
The patients reporting to the Outpatient Department of Oral Medicine and Radiology of the Faculty of Dentistry, Jamia Milia Islamia, New Delhi were assessed for eligibility. Of the 53 OSMF patients assessed for eligibility, 33 were enrolled in the study between January 2018 and April 2019. All patients or their relatives gave written informed consent to participate in this study. Approval from Internal Research Review Committee and Institutional Ethics Committee were taken before the study protocol was initiated.
2.2. Sample collection
Tissue samples were collected from 33 OSMF patients and 10 normal subjects. In this study, both males and females above 15 years of age were included. Only those OSMF patients who satisfied all the inclusion criteria were enrolled: use of areca nut with/without smokeless tobacco (both current and previous users were included); OSMF patients with symptoms like burning sensation of the mouth specially due to hot and spicy food and/or difficulty in swallowing and chewing; presence of clinical features like restricted mouth opening, vertical fibrous bands, stiffness, blanching and/or circumoral bands. Those patients with a significant medical condition interfering with the safety of the protocol; pregnant females; patients already undertaking OSMF treatment; and patients having any other dental condition leading to trismus such as pericoronitis were excluded from the study. Incisional biopsy samples were collected from all the OSMF patients. Normal oral tissues were obtained from age and sex matched patients undergoing 3rd molar surgery, having no adverse habits (of tobacco, areca nut and alcohol use) and no signs of oral potentially malignant disorders or oral cancer. The cases were graded as per Kerr et al.17
Demographic details of all OSMF patients were recorded in a clinical proforma designed for this study. A detailed history of areca nut and/or tobacco use in any form was asked from the patient encompassing fields like duration of habit, frequency of use, type of tobacco product, duration of use per day, spitting/swallowing of the product. The clinical features of OSMF and other potentially malignant oral disorders were assessed using a standard examination protocol. Incisional biopsy was done under recommended doses of local anesthesia from right or left buccal mucosa. Immediately following incisional biopsy, the tissue obtained was divided in to 2 parts. One part was fixed in formalin and used for histopathological evaluation. The second part was immersed in RNA stabilizing solution.(RNA later) and stored at −80 °C and used for real time PCR assay.
2.3. Hematoxylin & Eosin staining
Normal and OSMF tissues samples after collection were put into 4% formalin. The samples were processed within 24 h and paraffin blocks were prepared. Tissue sectioning was done by microtomy and 5 μm thick sections were put on glass slides. Hematoxylin & Eosin staining of the prepared tissue slides was done as described by us previously.18 Three samples out of the 33 collected from OSMF patients showed presence of Oral squamous cell carcinoma (Grade 5 of the Kerr et al. classification17). These patients were referred separately for further management and their tissue samples were not considered for further analysis in this study.
2.4. Quantitative polymerase chain reaction analysis
RNA was isolated from 10 normal and 30 OSMF patients using Qiagen RNA isolation kit (Hilden, Germany, Cat No. 74104). The concentration and purity of the isolated RNA were checked using nanodrop. From the isolated RNA, 2 μg was used for cDNA synthesis by Qiagen cDNA synthesis kit (Cat No. 205311). The cDNA was prepared using Oligo (dT) cDNA synthesis method. For quantitative (q) PCR analysis the prepared cDNA was used along with the primers specific to the TGF-β ligands (β1, β2 and β3) and their receptors (βR1, βR2). Beta actin was used as the reference gene. Samples were run in the Rotor-Gene Q (Qiagen, Model No. 219119) and the PCR reaction was performed as described by us previously.19
Sample analysis was done by Q-Rex Software and Ct values were used for further analysis and to calculate ΔΔCt. From ΔΔCt values, fold change gene expression was obtained by normalizing the data with the normal control samples. All the kit protocols were followed as per the manufacturer's instructions. Primers were designed using NCBI-primer design tool. The primer sequence of the respective genes is provided in Table 1.
Table 1.
Shows the primer sequence of the respective genes used for quantitative PCR analysis in this study.
| GENE | Forward primer 5′-3′ sequence | Reverse primer 5′-3′ sequence |
|---|---|---|
| TGFβ1 | TCCGAGAAGCGGTACCTGAA | TGCTGTCACAGGAGCAGTGG |
| TGFβ2 | AGTGCCTGAACAACGGAT | GTACAAAAGTGCAGCAGG |
| TGFβ3 | GCGTGAGTGGCTGTTGAGA | CCAAGTTGCGGAAGCAGTA |
| TGFβRI | TACAGCTTTGCCTGAACTCT | CACGACAGAGTTACCTAAAG |
| TGFβRII | CTGCACATCGTCCTGTGGA | CTGCACCGTTGTTGTCAGTG |
| GAPDH | CAATGACCCCTTCATTGACC | GATCTCGCTCCTGGAAGATG |
2.5. Immunohistochemistry
Tissue immunohistochemistry was performed on formalin fixed paraffin sections with 5 μm thick sections. Protocol was followed as described by us previously.18 Rabbit primary antibody against anti-TGFβ1 (abcam, ab92486) was used at 1:1000 dilution and the tissue sections were incubated overnight at 4 °C. Following incubation and washing, tissue slides were incubated with anti-rabbit horseradish peroxidase (HRP) conjugated secondary antibody for 1 h at room temperature. DAB (3,3′-diaminobenzedine) was used as a substrate and the color formation was monitored until the sections turned light brown. Same incubation time was followed in all the slides. Isotype control antibody was used to ensure specific staining. Images were taken at 20× magnification (Nikon Eclipse Ti, Japan), and images were analyzed using ImageJ. Semi-quantitative analysis of normal and OSMF samples was done.
3. Results
3.1. Demographic data of the OSMF patients
A total of 53 OSMF patients were assessed for eligibility and 33 were enrolled in the study. Out of these, 3 were discontinued from this study as histopathological impression revealed squamous cell carcinoma in these patients. The demographic details of 30 patients are shown in Table 2. The mean age of the patients was 31.96 years (SD ± 9.72) with male:female ratio as 9:1.
Table 2.
Shows demographic distribution of patients included in this study.
| S.No. | Age group (years) | Males | Females | Total |
|---|---|---|---|---|
| 1 | ≤20 | 2 | 0 | 2 |
| 2 | 21–30 | 17 | 1 | 18 |
| 3 | 31–40 | 2 | 1 | 3 |
| 4 | >40 | 6 | 1 | 7 |
| Total | 27 | 3 | 30 |
3.2. Baseline tobacco characteristics of OSMF patients
In this study, 14 patients reported with smokeless tobacco habits and 15 patients reported with combined habit of smoking and chewing smokeless tobacco. A total of 4 patients had areca nut chewing habit, of which one patient reported with no other adverse habits.
Among the smokeless tobacco users, majority of the OSMF patients (80%) had habit of Gutkha chewing (a preparation of crushed areca nut, tobacco, catechu, paraffin wax, slaked lime and sweet or savory flavorings). Others reported with khaini (16.6%), sada pan (13%), tobacco pan (10%) and pan masala (3.3%) habits.
3.3. Grading of the OSMF patients
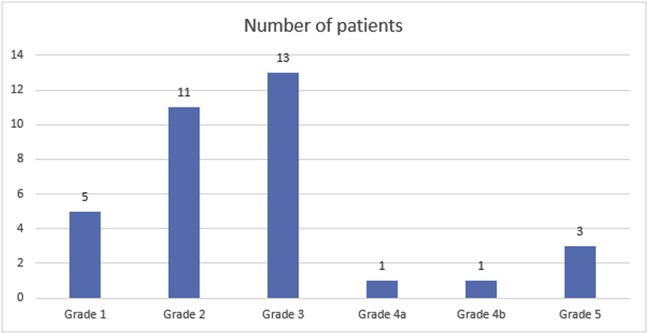
The OSMF patients enrolled in this study were graded based on the grading system proposed by Kerr et al.17 The OSMF patients were categorized in 5 grades based on their signs and symptoms and histopathological impression as shown in Fig. 1.
Fig. 1.
Histogram distribution plot of patients based on their signs and symptoms and histopathological impression into five grades based on classification system of Kerr et al.
3.4. Histology
The H & E stained section as shown in Fig. 2A showed epithelium overlying the connective tissue stroma. The epithelium was hyperkeratinized stratified squamous type showing atrophy with absence of rete ridges in few cases while short rete ridges in others. There was evidence of dysplasia in only one case in the epithelial cells. The underlying connective tissue consisted of bundles of collagen fibres arranged in a haphazard manner along with few dilated and constricted blood vessels and numerous fibroblasts. The inflammatory cells seen were mainly lymphocytes and plasma cells.
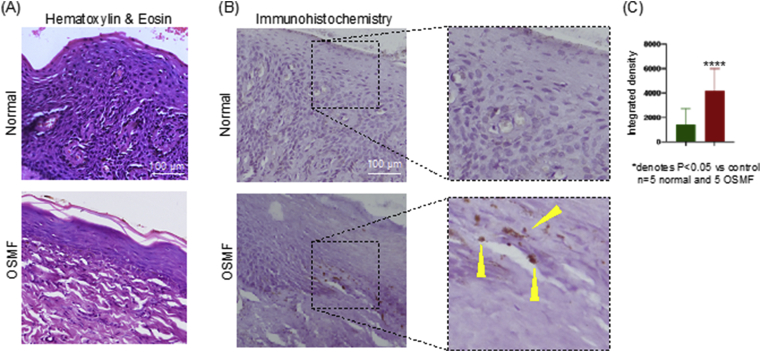
Fig. 2.
OSMF disease condition is associated with epithelial atrophy and elevated TGF-β expression:
(A) Representative H&E images showing normal oral mucosa and OSMF tissue. OSMF section shows thin atrophic epithelium with dense bundles of collagen fibres in the connective tissue. All the images were taken at 20×
magnification. Scale bar: 100 μm
(B) Representative images of TGF-β stained IHC section showing negative expression in the normal oral mucosa and positively stained cells in the basal cell layer of oral epithelium. All the images were taken at 20× magnification. Scale bar: 100 μm
(C) Semi-quantitative analysis of IHC using ImageJ software showing statistically significant difference between normal and OSMF tissues. Integrated density, which reflects the expression level of TGF-β based on the brown color was calculated for the respective tissue images.
In the OSMF cases of early stage, large numbers of neutrophils and lymphocytes were seen in subepithelial, connective tissue zone along with myxedematous changes. In the intermediate stage OSMF cases, hyalinization of subepithelial zone was noticed with compression of blood vessels by fibrous bundles.
In few cases of advanced stage, inflammatory cell infiltration was hardly seen. Marked fibrous areas with hyaline changes extend from subepithelial to superficial muscle layers. Number of blood vessels was dramatically low in subepithelial zone. Moreover, atrophic degenerative changes were observed in muscle fibers.
3.5. Quantitative PCR
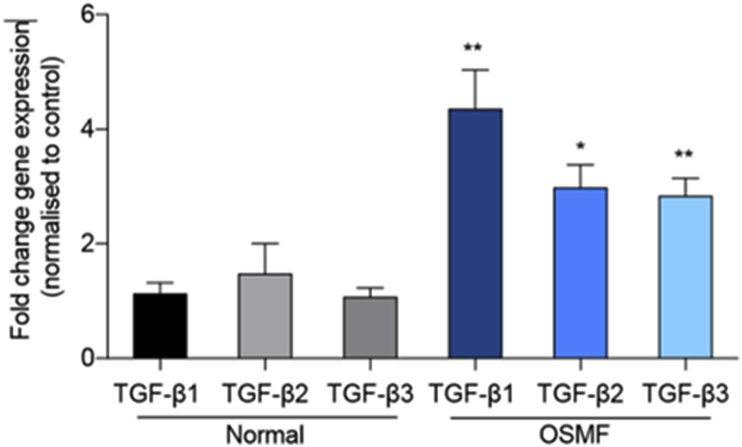
In order to provide clues on the role of TGF-β in the pathogenesis of OSMF, we quantified the three isoforms by qPCR. Most studies on the role of TGF-β in OSMF were performed using the TGF-β1 isoform. In this study, there was expression of all the TGF-β isoforms in both control and OSMF samples. The mean difference in gene expression between OSMF tissues compared to their normal counterpart was high in all isoforms; TGF-β1 (3.225 ± 1.080 SEM, P < 0.0061), TGF-β2 (1.503 ± 0.7037 SEM, P < 0.0422), and TGF-β3 (1.760 ± 0.4959 SEM, P < 0.0015). Highly significant differences were observed in mRNA expression of TGF-β1 and 3 and statistically significant differences were observed in relation to TGF-β2 between normal control and OSMF patients as shown in Fig. 3. The expression of TGF-β1 was the highest of the three members of the TGF-β family.
Fig. 3.
OSMF tissue samples have increased RNA expression of various TGF-β isoforms: Quantitative PCR (qPCR) analysis of TGFβ1, TGFβ2, and TGFβ3 mRNA was done. As shown, fold change gene expression of respective isoforms was calculated ad presented as the histogram. TGF-β1 levels were significantly higher in OSMF as compared to normal. Similarly, TGF-β2 and TGF-β3 expression were also moderately increased in OSMF.
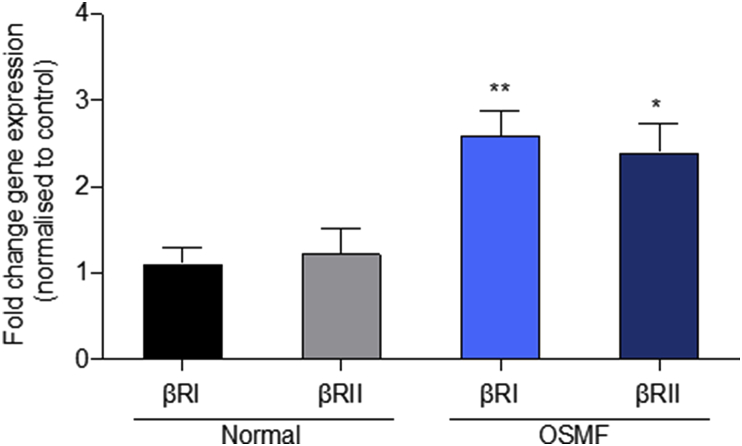
Members of the TGF-β family bind to TGF-β receptor (TβR) types I and II, which leads to phosphorylation and activation of the Smad proteins. We found a significant increase in TGF-βRI and TGF-βRII with mean difference (1.489 ± 0.4687 SEM, P > 0.0038 for TGF-βRI and 1.176 ± 0.5682 SEM, P > 0.0486 for TGF-βRII) in gene expression between OSMF tissues compared to their normal counterpart. Based on fold change gene expression, highly significant differences were observed between normal and OSMF tissues in relation to TGF-βR1 and significant differences were observed for TGF-β R2 (Fig. 4).
Fig. 4.
RNA levels of TGF-β receptor isoforms are increased in OSMF tissue samples:
qPCR analysis of two TGFβ isoforms (TGF-βRI and TGF-βRII) was done. The histogram data shows the fold change gene expression of βRI and βRII isoforms. The mRNA levels of both isoforms were modestly increased in tissue samples obtained from OSMF as compared to normal.
3.6. Immunohistochemistry
In most of the TGF-β stained OSMF sections, positively stained cells were present in the basal cell layer of oral epithelium (Fig. 2B). In addition, in few cases the positive expression was also observed in the superficial layers of epithelium. Moreover, in some cases, the expression is also found positive in the cells of superficial and deeper layers of connective tissue. These features were indicative of the fact that there is an activation of TGF-β signaling in epithelial cells as well the activation of the downstream signaling pathway leading to fibrosis via epithelial mesenchymal interaction.
The semi-quantitative analysis of IHC revealed a statistically significant (p < 0.05) difference between normal and OSMF tissue samples based on the integrated density of TGF-β staining (Fig. 2C).
4. Discussion
OSMF is a potentially malignant disorder which is caused mainly due to areca nut chewing. It is characterized by burning sensation on having hot and spicy food, restricted mouth opening and blanching of the oral mucosa. Pathogenesis of this chronic, insidious disease is considered to be primarily a collagen metabolic disorder characterized by increased collagen synthesis, reduced collagen degradation and it's abnormal cross-linking. The collagen synthesis is controlled by the delicate balance of pro and anti fibrogenic cytokines such as TGF-β, Connective tissue growth factor (CTGF), Endothelin-1 and bone morphogenetic protein 4 and 7.20
TGF-β is a peptide cytokine expressed by several cell types and regulates various cellular processes whereas its uncontrolled signaling is postulated in many fibrotic reactions. There have been reports of involvement of TGF-β signaling in OSMF. Kale et al. aimed to correlate the role of TGF-β with loss of adipose tissue in OSMF by studying TGF-β staining of epithelium, fibroblast, macrophages and inflammatory cells. In their study, early cases showed more intense staining than the advanced cases.21 Kamath and colleagues correlated the expressions of TGF-β1 and TGF-β2 immunohistochemically on paraffin sections of various stages of OSMF.22 They observed positivity for both the markers in epithelium, around the blood vessels, in the areas of inflammatory infiltrate, fibroblasts and in muscles. TGF-β1 expression was found to be higher and more intense than that of TGF-β2 in all the cases. TGF-β2 was restricted in its expression to submucosal area with minimal involvement of the epithelium and the deeper muscle tissue. Maria and co-workers induced OSMF in Sprague-Dawley rats by injections with solutions of areca nut and pan masala extracts.23 The tissues were then analyzed for the TGF-β1 gene by PCR. Quantitative real-time PCR showed a significant up regulation of TGF-β1.
In the present study, increased expression of all the TGF-β isoforms were observed in OSMF tissues compared to normal controls. TGF-β1 was found to be the dominant isoform among the three. Also, the expression of both TGF-βRI and TGF-βR2II were found to be elevated.
In summary, while we found all three TGF-β isoforms to be expressed and biologically active in OSMF, we also showed that they exhibit differential expression as well as co-expression with their receptors. These distinct expression patterns may, in turn, dictate different roles, prognosis values, and disease outcomes between the isoforms, according to the stage and grade of the disease. Further investigation of the precise role and function of these isoforms in the different grades of OSMF will help refine more precise therapeutic options by targeting specific isoforms in a subgrade specific manner and lead to improved patient response and outcome.
Declaration of competing interest
None declared.
Acknowledgement
Authors are thankful to the Dean, Faculty of Dentistry, Jamia Milia Islamia and Director, CIRBSc, Jamia Milia Islamia for providing institutional support. We are also thankful to our patients for their enthusiastic participation in this study. Dr. Arpita Rai has received Extra mural research project from Ministry of AYUSH (Z.28015/60/2018-HPC (EMR)- AYUSH-C; Principal Investigator-Dr. Arpita Rai). Dr. Sher Ali is grateful for financial support from DBT (BT/PR12828/AAQ/1/622/2015), New Delhi and to SERB-DST For the Award of J C Bose National Fellowship (SR/S2/JCB-49/2011).
References
- 1.Le P.V., Gornitsky M. Oral stent as treatment adjunct for oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:148–150. doi: 10.1016/s1079-2104(96)80404-5. [DOI] [PubMed] [Google Scholar]
- 2.Bhawna G. Burden of smoked and smokeless tobacco consumption in India - results from the Global adult Tobacco Survey India (GATS-India)- 2009-201. Asian Pac J Cancer Prev APJCP. 2013;14:3323–3329. doi: 10.7314/apjcp.2013.14.5.3323. [DOI] [PubMed] [Google Scholar]
- 3.Hazarey V.K., Erlewad D.M., Mundhe K.A., Ughade S.N. Oral submucous fibrosis: study of 1000 cases from central India: OSF: study of 1000 cases. J Oral Pathol Med. 2006;36:12–17. doi: 10.1111/j.1600-0714.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- 4.Angadi P.V., Rao S.S. Areca nut in pathogenesis of oral submucous fibrosis: revisited. Oral Maxillofac Surg. 2011;15:1–9. doi: 10.1007/s10006-010-0219-8. [DOI] [PubMed] [Google Scholar]
- 5.Lian I.B., Tseng Y.T., Su C.C., Tsai K.Y. Progression of precancerous lesions to oral cancer: results based on the Taiwan National Health Insurance Database. Oral Oncol. 2013;49:427–430. doi: 10.1016/j.oraloncology.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Meng X.M., Nikolic-Paterson D.J., Lan H.Y. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 7.Yu L., Border W.A., Huang Y., Noble N.A. TGF-beta isoforms in renal fibrogenesis. Kidney Int. 2003;64:844–856. doi: 10.1046/j.1523-1755.2003.00162.x. [DOI] [PubMed] [Google Scholar]
- 8.Xu P., Liu J., Derynck R. Post-translational regulation of TGF-β receptor and Smad signaling. FEBS Lett. 2012;586:1871–1884. doi: 10.1016/j.febslet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson I.B., Horiguchi M., Zilberberg L., Dabovic B., Hadjiolova K., Rifkin D.B. Latent TGF-β-binding proteins. Matrix Biol. 2015;47:44–53. doi: 10.1016/j.matbio.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haque M.F., Harris M., Meghji S., Barrett A.W. Immunolocalization of cytokines and growth factors in oral submucous fibrosis. Cytokine. 1998;10:713–719. doi: 10.1006/cyto.1997.0342. [DOI] [PubMed] [Google Scholar]
- 11.Moutasim K.A., Jenei V., Sapienza K. Betel-derived alkaloid up-regulates keratinocyte alphavbeta6 integrin expression and promotes oral submucous fibrosis. J Pathol. 2011;223:366–377. doi: 10.1002/path.2786. [DOI] [PubMed] [Google Scholar]
- 12.Khan I., Agarwal P., Thangjam G.S., Radhesh R., Rao S.G., Kondaiah P. Role of TGF-β and BMP7 in the pathogenesis of oral submucous fibrosis. Growth Factors. 2011;29:119–127. doi: 10.3109/08977194.2011.582839. [DOI] [PubMed] [Google Scholar]
- 13.Khan I., Kumar N., Pant I., Narra S., Kondaiah P. Activation of TGF-β pathway by areca nut constituents: a possible cause of oral submucous fibrosis. PloS One. 2012;7 doi: 10.1371/journal.pone.0051806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pant I., Kumar N., Khan I., Rao S.G., Kondaiah P. Role of areca nut induced TGF-β and epithelial-mesenchymal interaction in the pathogenesis of oral submucous fibrosis. PloS One. 2015;10 doi: 10.1371/journal.pone.0129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PantI Rao SG., Kondaiah P. Role of areca nut induced JNK/ATF2/Jun axis in the activation of TGF-β pathway in precancerous Oral Submucous Fibrosis. Sci Rep. 2016;6:34314. doi: 10.1038/srep34314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Y., Ling T., Wu H. Expression of transforming growth factor beta 1 in keratinocytes of oral submucous fibrosis tissue. Zhonghua Kou Qiang Yi Xue Za Zhi. 1997;32:239–241. [PubMed] [Google Scholar]
- 17.Kerr A.R., Warnakulasuriya S., Mighell A.J. A systematic review of medical interventions for oral submucous fibrosis and future research opportunities. Oral Dis. 2011;17(1):42–57. doi: 10.1111/j.1601-0825.2011.01791.x. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad T., Mukherjee S., Pattnaik B. Miro 1 regulates intercellular mitochondrial transport and enhances mesenchymal stem cell rescue efficiency. EMBO J. 2014;9:994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad T., Sundar I.K., Lerner C.A. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence. Faseb J. 2015;7:2912–2929. doi: 10.1096/fj.14-268276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wollina U., Verma S.B., Ali F.M., Patil K. Oral submucous fibrosis: an update. Clin Cosmet Invest Dermatol. 2015;8:193–204. doi: 10.2147/CCID.S80576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kale A.D., Mane D.R., Shukla D. Expression of transforming growth factor β and its correlation with lipodystrophy in oral submucous fibrosis: an immunohistochemical study. Med Oral Patol Oral Cir Bucal. 2013;18:e12–18. doi: 10.4317/medoral.18226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamath V.V., Krishnamurthy S., Satelur K.P., Rajkumar K. Transforming growth factor-β1 and TGF-β2 act synergistically in the fibrotic pathway in oral submucous fibrosis: an immunohistochemical observation. Indian J Med Paediatr Oncol. 2015;36:111–116. doi: 10.4103/0971-5851.158842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maria S., Kamath V.V., Satelur K., Rajkumar K. Evaluation of transforming growth factor beta 1 gene in oral submucous fibrosis induced in Sprague-Dawley rats by injections of areca nut and pan masala (commercial areca nut product) extracts. J Canc Res Therapeut. 2016;12:379–385. doi: 10.4103/0973-1482.148729. [DOI] [PubMed] [Google Scholar]