Abstract
Osteogenic differentiation of Mesenchymal stem cells (MSCs) on scaffold is crucial for bone tissue engineering. Alkaline phosphatase (ALP) assay is an important method of assessing osteogenesis. Here, a very simple and innovative procedure is being described for quantification of osteogenic differentiation of MSCs in presence of scaffold using ALP assay. Different concentrations of the scaffold particles with the same number of MSCs were assayed for alkaline phosphatase activity using p-NPP as substrate for ALP activity. G-bone scaffold was used in concentrations of 5, 20, 60 and 100 mg/ml and same number of MSCs were seeded. Any scaffold which can be grind and weighed may be used. It was found that100 mg/ml G-bone graft was most useful for promoting osteogenesis and addition of growth factors further promoted. So, we were able to ascertain the concentration of scaffold which promotes osteogenesis the most.
Keywords: Mesenchymal stem cells, Osteogenesis, Alkaline phosphatase, Scaffold
1. Introduction
Alkaline phosphatase (ALP) is considered to be an initial marker of osteoblast differentiation. An increase in level of ALP suggests active bone formation, as ALP is a by-product of osteoblast activity. Tissue non specific ALP has also been suggested as a potential marker of cells that are predisposed to differentiate along a mineralizing phenotype.1
Mesenchymal stem cells (MSCs) are extensively studied and useful cells for osteogenesis having clinical implications in dental and orthopedic fields. The osteogenic differentiation of MSCs may vary in the presence of scaffold and also with growth factors.2,3
Here, we are describing a very simple and innovative method for quantification of osteogenic differentiation of MSCs in the presence of scaffold. Different concentrations of the scaffold particles with the same number of MSCs were assayed for alkaline phosphatase activity using p-NPP as substrate for ALP activity.
The scaffold studied is G-bone synthetic multiphasic hydroxyapatite. It is made up of multiphasic calcium hydroxyapatite (HA) in low crystalline form. It is a mixture of HA, tricalcium phosphate (TCP) and other forms of calcium like calcium carbonate and bicalcium phosphate. It is a widely accepted and clinically useful bone graft substitute.
Our protocol can be used to measure the osteogenic potential of any scaffold in a quantitative manner.
2. Material and methods
2.1. Reagents
The Osteogenic media consisted of complete culture media, that is, α-Minimum essential media (α-MEM), nonessential amino acids (Hi-media laboratories, India) with 10% fetal bovine serum (Gibco, Life Technologies) 100 U/mL penicillin, 0.2 mg/mL streptomycin; supplemented with 100 nM dexamethasone, 50 μM ascorbic acid and 10 mM β –glycerophosphate (Sigma-Aldrich, USA)
The Wash buffer was 0.05% tween 20 in Calcium-magnesium free Phosphate buffer saline (PBS).BCIP/NBT Tablet (Sigma Aldrich, USA) was dissolved in 10 ml distilled water [Note: Keep in dark as it is light sensitive].10% Formalin was used to fix the cells.
-
A.
Quantitative measurement of ALP activity
To determine how the osteogenic capacity of mesenchymal stem cells varies in the presence of the scaffold we have used a novel method. The scaffold used here is G-Bone scaffold (Surgiwear, India). One could use any scaffold that can be grind and weighed. Alkaline phosphatase activities were evaluated with p-NPP as ALP substrate.
2.2. Procedure
Coat the 96 well plate with 200 μl different concentrations of the G-Bone scaffold i.e. 5, 20, 60and 100 mg/ml in culture media. Centrifuge the plates at 2000 rpm for 2 min at RT and remove 100 μl media by multichannel pipets.3 X 103/100 μl MSCs were seeded on them with and without differentiation media. Incubate the MSCs with media change at 3, 7, and 11 days.Wash the cultured cells with PBS twice and cellular membranes were lysed by 200 μl lysis buffer (Tris-HCl 25 mM,TritonX-100 0.5%) at 4 °C for 2 h. After complete lysis, 50 μl lysate was aliquoted into another 96 well plate and 50 mmol/l p-nitrophenylphosphate (p-NPP) in a sodium carbonate buffer at pH 10.4 was added to cell lysate, and the mixture was incubated at 37 °C for 30 min in an incubator. The amount of released p-nitrophenylphosphate was estimated by measuring the absorbance at 405 nm by spectrophotometric method. The quantity of p-nitrophenol (pNP) in each well was determined using a standard curve established using p-NPP and purified ALP enzyme.
-
B.
Quantitative measurement of ALP activity after adding cytokines and growth factors
Coat the 96 well plate with 200 μl different concentrations of the G- Bone scaffold i.e. 5, 20, 60 and 100 mg/ml in culture media. Centrifuge the plates at 2000 rpm for 2 min at RT and removed 100 μl media by multichannel pipets.3 X 103/100 μl MSCs were seeded on them with and without differentiation media along with growth factors or cytokine like IGF(50 ng/ml), FGF(1 ng/ml), and PDGF (5 ng/ml).Incubate the MSCs with media change with growth factors and cytokines for 3, 7, and 11 days. Wash the cultured cells with PBS twice and cellular membranes were lysed by 200 μl lysis buffer (Tris-HCl 25 mM, Triton X-100 0.5%) at 4 °C for 2 h. After complete lysis, 50 μl lysate was aliquoted into another 96 well plate and 50 mmol/l p-nitrophenylphosphate (p-NPP) in a sodium carbonate buffer at pH 10.4 was added to cell lysate, and the mixture was incubated at 37 °C for 30 min in an incubator. The amount of released p-nitrophenylphosphate was estimated by measuring the absorbance at 405 nm by spectrophotometric method. The quantity of p-nitrophenol (pNP) in each well was determined using a standard curve established using p-NPP and purified ALP enzyme.
2.3. Interpretation
Two dimensional cell cultures (culture of cells on a flask or petri dish) is a simple and inexpensive way of studying the cells and performing various physiological tests like ALP assay. Conducting the same investigations in a three dimensional culture becomes challenging. A three dimensional culture more closely mimics the natural environment and the cell behavior in vivo can be predicted more closely in it.4,5
Three dimensional cell culture is a basic prerequisite for tissue engineering so that the cells used can replace the desired tissue or organ in the body. Though it is of utmost importance to promote osteogenesis on scaffolds clinically, osteogenic support or MSC differentiation of a given scaffold is difficult to determine in vitro. It is because, it is not easy to grow MSCs on scaffold, being a three-dimensional culture. Further, staining with dye cannot be detected under microscope as the scaffolds used are opaque. So, we proceeded by grinding the scaffold and adding it in growth media in a definite concentration and adding to culture plates. This process makes a layer of scaffold and MSCs can be cultured on it and after differentiation can be lysed and equal amount of lysate can be used for ALP assay.
Considering the importance of ALP assay, various methods have been used for evaluation of ALP but researchers still aim to achieve the desired level of accuracy and sensitivity.6 With the present technique, a quantification could be done with the ALP assay itself.
Wang et al. (2013)7 conducted a study to assess the osteogenic differentiation of umbilical cord human mesenchymal stem cells (UCMSCs) on nano-hydroxyapatite/chitosan/poly(lactide-co-glycolide) (nHA/CS/PLGA) scaffold. For in vivo evaluation, the 14 day culture of UCMSCs on scaffold was implanted on mice. 14 days after implantation, the constructs were taken out and osteoid tissue was observed under microscope. Another in vivo study was done in dogs to compare the quality and quantity of regenerated bone using Bio-Oss, Hydroxyapatite/Tricalcium phosphate (HA/TCP) and MSC loaded HA/TCP scaffolds. Histological and histomorphometric evaluations were done 6 weeks after implantation.8
Our method may be used as a valuable intermediate step in such in vivo studies, as it would help to ascertain the concentration of scaffold which promotes osteogenesis the most and that concentration may be studied further.
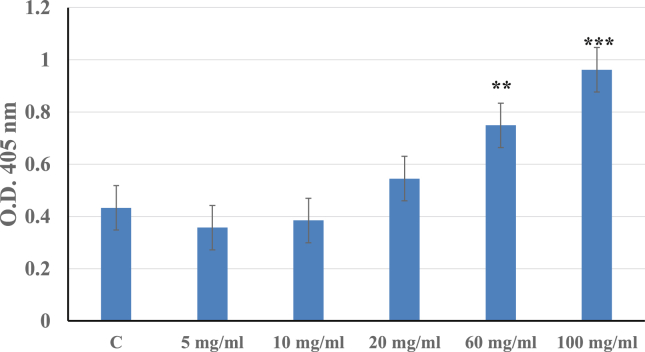
Scaffolds offer a three-dimensional space for the cells to form into new tissues with appropriate structure and function, and also may facilitate the delivery of cell released factors, which helps in regulating cellular function in desired sites of the body. The scaffold (G-Bone) used for the present experiment is clinically acceptable and no toxicity to cells has been reported with it. It was observed that as the concentration of the scaffold increase, higher differentiation was observed. A significantly higher osteogenic differentiation was observed at 60 mg/ml and 100 mg/ml concentrations. Osteogenic differentiation of MSCs has increased with increasing concentration of G-bone scaffold and at 100 mg/ml G-bone scaffold osteogenic differentiation was maximum (Fig. 1).
Fig. 1.
Quantification of osteogenic differentiation of MSCs in presence of G-Bone scaffold using Alkaline phosphatase assay. Data shown as mean± S.E.M.; n = 3; ***p ≤ 0.001; **p ≤ 0.01; *p ≤ 0.05compared with control.
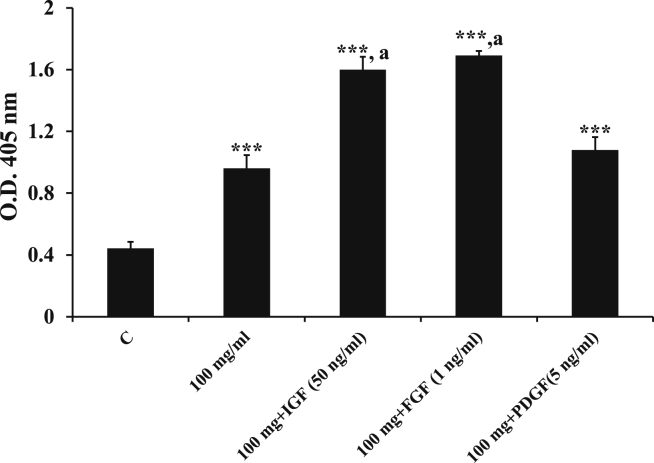
It has been proved by several studies that growth factors promote osteogenesis.9,10 In concordance with these studies, adding growth factors or cytokines like IGF (50 ng/ml), FGF(1 ng/ml), and PDGF (5 ng/ml)significantly increased the osteogenic differentiation in our experiment. The effects of adding IGF and FGF were statistically significant when compared to the scaffold without growth factors. For PDGF, though there was an increase in osteogenic differentiation, it did not reach significance (Fig. 2).
Fig. 2.
Additive effect of growth factors was measured on MSCs at 100 mg/ml G-bone scaffold with FGF2 (1 ng/ml), IGF1 (50 ng/ml) and PDGF (5 ng/ml) for two weeks. Data shown as mean± S.E.M.; n = 3; ***p ≤ 0.001; **p ≤ 0.01; *p ≤ 0.05 compared with control.
ap≤ 0.001 as compared to 100 mg/ml scaffold.
So, it was concluded that for the present graft material, 100 mg/ml G-bone graft was most useful for promoting osteogenesis and addition of growth factors further promoted it. The protocol used here may have a clinical as well as material development implications also as it helps in identification of the most suitable concentration of the graft material to be used.
Declaration of competing interest
The authors have no conflicts of interest to declared.
Acknowledgements
Funding: The study was partially funded by Department of Health Research (DHR), Faculty of Dental Sciences, King George's Medical University, Lucknow, India.
Contributor Information
Divya Mehrotra, Email: divyamehrotra@kgmcindia.edu.
Satyendra Kumar Singh, Email: satyendraks@kgmcindia.edu.
References
- 1.Tomlinson M.J., Dennis C., Yang X.B., Kirkham J. Tissue non-specific alkaline phosphatase production by human dental pulp stromal cells is enhanced by high density cell culture. Cell Tissue Res. 2015;361(2):529–540. doi: 10.1007/s00441-014-2106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du J., Lu Y., Song M. Effects of ERK/p38 MAPKs signaling pathways on MTA-mediated osteo/odontogenic differentiation of stem cells from apical papilla: a vitro study. BMC Oral Health. 2020;20:50. doi: 10.1186/s12903-020-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Gendy R., Yang X.B., Newby P.J., Boccaccini A.R., Kirkham J. Osteogenic differentiation of human dental pulp stromal cells on 45S5 Bioglass® based scaffolds in vitro and in vivo. Tissue Eng Part A. 2013;19(5-6):707–715. doi: 10.1089/ten.tea.2012.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapałczyńska M., Kolenda T., Przybyła W. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14(4):910–919. doi: 10.5114/aoms.2016.63743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duval K., Grover H., Han L.H. Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda) 2017;32(4):266–277. doi: 10.1152/physiol.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 6.Balbaied T., Moore E. Overview of optical and electrochemical alkaline phosphatase biosensors: recent approaches in cells culture techniques. Biosensors. 2019;9(3):102. doi: 10.3390/bios9030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang F., Zhang Y.C., Zhou H., Guo Y.C., Su X.X. Evaluation of in vitro and in vivo osteogenic differentiation of nano-hydroxyapatite/chitosan/poly(lactide-co-glycolide) scaffolds with human umbilical cord mesenchymal stem cells. J Biomed Mater Res. 2014;102(3):760–768. doi: 10.1002/jbm.a.34747. [DOI] [PubMed] [Google Scholar]
- 8.Vahabi S., Amirizadeh N., Shokrgozar M.A. A comparison between the efficacy of BioOss,hydroxyapatite tricalcium phosphate and combination of mesenchymal stem cells in inducing bone regeneration. Chang Gung Med J. 2012;35:28–37. doi: 10.4103/2319-4170.106169. [DOI] [PubMed] [Google Scholar]
- 9.Suliman S., Ali H.R.W., Karlsen T.A. Impact of humanised isolation and culture conditions on stemness and osteogenic potential of bone marrow derived mesenchymal stromal cells. Sci Rep. 2019;9:16031. doi: 10.1038/s41598-019-52442-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reible B., Schmidmaier G., Moghaddam A. Insulin like growth factor-1 as a possible alternative to bone morphogenetic protein-7 to induce osteogenic differentiation onhuman mesenchymal stem cells in vitro. Int J Mol Sci. 2018;19(6):1674. doi: 10.3390/ijms19061674. [DOI] [PMC free article] [PubMed] [Google Scholar]