Abstract
Introduction
One of the leading causes of death worldwide is heart failure. Despite advances in the treatment and prevention of heart failure, the number of affected patients continues to increase. We have recently developed 3D bioprinted biomaterial-free cardiac tissue that has the potential to improve cardiac function. This study aims to evaluate the in vivo regenerative potential of these 3D bioprinted cardiac patches.
Methods
The cardiac patches were generated using 3D bioprinting technology in conjunction with cellular spheroids created from a co-culture of human IPSC derived cardiomyocytes, fibroblasts, and endothelial cells. Once printed and cultured, the cardiac patches were implanted into a rat myocardial infarction model (n=6). A control group (n=6) without the implantation of cardiac tissue patches was used for comparison. The potential for regeneration was measured 4 weeks after the surgery with histology and echocardiography.
Results
At 4 weeks after surgery, the survival rates were 100% and 83% in the experimental and the control group respectively. In the cardiac patch group, the average vessel counts within the infarcted area were higher than those of the control group. The scar area in the cardiac patch group was significantly smaller than that of the control group. Echocardiography showed a trend of improvement of cardiac function for the experimental group, and this trend correlated with increased patch production of extracellular vesicles (EVs).
Conclusion
3D bioprinted cardiac patches have the potential to improve the regeneration of cardiac tissue and promote angiogenesis in the infarcted tissues and reduce the scar tissue formation.
Keywords: Tissue engineering, 3D bioprinting, cardiac patch, biomaterial-free, vascularization, heart failure
Introduction
Heart failure remains one of the leading causes of death worldwide despite improvements in management through mechanical and surgical therapeutic strategies (Heidenreich et al. 2013; Jessup and Brozena 2003). For end-stage heart failure, a heart transplant is the standard curative therapy. However, the shortage of donor hearts limits the application of this procedure that also requires lifelong immunosuppression and impacts the recipient’s quality of life (Stehlik et al. 2012). Other surgical interventions, including the implantation of mechanical ventricular assist devices, have high associated medical costs and a limited number of eligible patients (Miller, Guglin, and Rogers 2013). Currently, heart failure treatments are primarily palliative in that they alleviate symptoms and retard the speed of deterioration of the heart, but do not counter the loss of functional cardiac tissue.
Cardiac stem cell therapy, in which pluripotent stem cells are injected into the myocardium, has recently been developed and extensively investigated as an alternative therapeutic strategy for heart failure. Several studies described improved cardiac function in damaged hearts and regeneration of tissue using various cell sources. However, many significant issues remain unaddressed with this technique, such as the low retention rate of the cells, insufficient nutritional supply in the tissue, and lack of proper integration with the host tissue (Wang and Guan 2010).
To address the limitations of stem cell-based therapy, a tissue engineering approach has been developed as an alternative treatment. A general strategy for cardiac tissue engineering is to create a stable cardiac tissue through three-dimensional (3-D) printing with a scaffold for implantation therapy. However, these scaffold-based approaches contain several drawbacks, such as immunogenicity of the scaffold, suboptimal mechanical properties, and fast degradation of the scaffold (Jawad et al. 2008).
We previously published a novel biomaterial-free 3D bioprinting method for the creation of cardiac patches in order to develop a functional tissue for cardiac cell retention and tissue transplantation (Ong, Fukunishi, Zhang, et al. 2017). The objective of this study is to evaluate the in vivo regenerative potential of the biomaterial-free 3D bioprinted cardiac tissue in a rat model. We describe an in vivo study of the transplantation of the novel biomaterial-free cardiac tissue engineered patch in rats, which has shown long term retention and yields functional and vascularized improvement in the rat model.
Methods
All procedures and protocols involving animals were approved by the Johns Hopkins University Institutional Animal Care and Use Committee and performed in accordance with relevant institutional and federal guidelines and regulations for the care and use of laboratory animals.
Generation of spheroids
We used human induced pluripotent stem cells (iPSCs) derived from cardiomyocytes (hiPSC-CM) by modulation of Wnt signaling using small molecules (CHIR99021, Tocris, R&D Systems, Cat. No. 4423, and IWR-1, Sigma-Aldrich, Cat. No. I0161) as previously described (Ong, Fukunishi, Zhang, et al. 2017). Human adult ventricular cardiac fibroblasts (FB) (Sciencell, Cat. No. 6310) and human umbilical vein endothelial cell line (HUVEC) (Lonza, Cat. No. CC-2935) were cultured with as previously described (Ong, Fukunishi, Nashed, et al. 2017). Co-culture of fixed proportion of three cell lines (hiPSC-CM: FBs: HUVECs, 70:15:15) were done in an ultra-low attachment 96-well U-bottom plates (Sigma-Aldrich, No.CLS700724EA) at 200 μL per well, or 33,000 cells per well. The 96-well plates were incubated for 3 days (37°C, 5% carbon dioxide, 95% humidity). The presence of mixed cell multicellular spheroids at the center and bottom of individual wells was confirmed under light microscopy.
Characterization of spheroids
The spheroids were characterized via immunostaining as described previously.(Ong, Fukunishi, Zhang, et al. 2017) The spheroids were fixed and embedded for sectioning. Slides were incubated with rabbit anti-CD31 antibody (anti-CD31 antibody, Abcam, USA), then were labeled with corresponding fluorescently conjugated secondary antibodies (Alexa Fluor, Abcam, USA), and cell nuclei were stained with 4’,6-Diamidino-2-Phenylindole (DAPI).
3D bioprinting of cardiac patches
We have previously described and published our protocol for 3D bioprinting of monolayer cardiac patches of size ~ 3.6 × 3.6 mm using only cardiospheres, without the use of biomaterials(Ong, Fukunishi, Zhang, et al. 2017). The thickness of the mono layer patch in this study was about 350–400 um. In brief, a 3D bioprinter (Regenova, Cyfuse Biomedical K.K., Tokyo, Japan) was used to create biomaterial-free cardiac patches. The predetermined 3D geometry (one single layer of spheroids) was designed using the software of the 3D bioprinter. In a sterile environment, the 3D bioprinter identified the locations of the spheroids within the wells of the ultra-low adhesion 96-well tissue culture plate by automated software detection, a robotic arm then used vacuum suction to pick up, transfer and load the cell aggregates individually onto a needle array in exact spatial coordinates according to the 3D design. After bioprinting, the cardiac patch was allowed to mature for 3 days (72 hours) culture within the needle array, while placed on a shaker (Compact Digital Microplate Shaker, Thermo Scientific, Waltham, MA) at 150 rounds per minute (rpm) in the incubator. During this time, the spheroids fuse to form a cardiac patch with synchronous beating function. The needle array was removed (decannulation) and the cardiac patches were cultured for 7–10 days before implantation surgery.
In vivo implantation
Implantations of the cardiac patches were performed with female Lewis nude rat (8–10 weeks) which was T-cell deficient and with depleted cell in thymus-dependent areas of peripheral lymphoid organs. The experimental myocardial infarction (MI) was induced as described previously (Takagawa et al. 2007). Briefly, animals were anesthetized with inhaled 1.5–2% isoflurane, intubated, and ventilated with a respirator and supplemental oxygen. The heart was then exposed via a median sternotomy. A 8–0 suture (Ethicon, Inc., Johnson and Johnson, Somerville, NJ) was placed to occlude the left anterior descending artery (LAD). Myocardial infarction was confirmed by visual inspection of myocardial blanching. The patch implantation surgery was done after the visualization of successful LAD ligation. The sternum incision and the skin incision was closed after patch implantation surgery. The omentum patch was used to cover cardiac patch for the prevention of cardiac patch dislocation. The diaphragm was opened from pericardial cavity at the middle about 2mm, and omentum was translocated to intra-pericardial space using cotton swab. The omentum was covered with cardiac patch and fixed at 4 corner surrounding the patch by 7–0 prolene suture. The endotracheal tube was removed after spontaneous breathing was observed. In the control group, only omentum patch was implanted.
The experimental treatments were administered immediately after occlusion: one 3D printed cardiac patch (CP) and a native omentum patch (OP) were secured over the free wall of the left ventricle with surgical glue and 6–0 suture respectively in animals from the MI+CP group (n=6). Animals in the Control group (n=6) underwent all surgical procedures for MI induction with only OP treatment, which was sutured over the infarcted site. After treatment administration, the incision closed and endotracheal tube removed as above under aseptic technique. Standard post-operative care, including analgesia, was administered until the animals ate normally and became active.
Echocardiography
All transthoracic echocardiography (Vevo 2100, 40-MHz mechanical transducer, VisualSonics) was performed in conscious rats four weeks after surgery. The animals were held in a supine position. The heart was imaged in two-dimensional mode in the parasternal short axis view. An M-mode pointer was positioned perpendicular to the interventricular septum and the LV posterior wall at the level of the papillary muscles. To assess their cardiac function, including left ventricular diastolic/systolic dimension (Left ventricular internal diameter end diastole and end systole (LVID;s/d), Interventricular septal end diastole(IVS;d), Left ventricular posterior wall thickness at end-diastole(LVPD;d)) and high-frame rate color Doppler imaging was performed was measured three times for each mouse from a frozen M-mode tracing. Ejection fraction was calculated from these parameters as previously described (Cihakova et al. 2008).
Histological assessments
The left ventricular free wall was cut horizontally into 3 rings, each ring was sequentially cut into 2 samples (i.e. from the site of CP application) that were embedded for sectioning. Each sample was deparaffinized with xylenes, alcohol and distilled water. The samples were then incubated with primary antibodies (mouse anti-human specific nuclear antigen [HNA, Abcam, USA], rabbit anti-human specific cardiac Troponin T [hcTnT, Abcam, USA], and then stained with corresponding fluorescent secondary antibodies (Alexa Fluor, Abcam, USA). Engrafted hiPSC-ECs were identified by CD31 (anti-CD31 antibody, Abcam, USA) expression and engrafted hiPSC-CMs were identified by the co-expression of HNA and TnT. Vascular density was evaluated by counting the number of vascular structures that were positive for CD31 expression. Infarct size was measured as previously described (Takagawa et al. 2007) via stained samples with Masson’s Trichrome staining and presented as the ratio of scar surface area to the total LV surface area. Apoptotic programmed cell death of the cardiac tissue in the cardiac patch was determined using a TUNEL assay (DeadEnd™ Fluorometric TUNEL System, Promega, Madison, WI). Live and dead cell counts were counted using ImageJ software on representative images.
Extracellular vesicle analysis
Extracellular vesicles (EVs) were isolated as previously described (Lamichhane, Raiker, and Jay 2015). Briefly, patches were incubated in serum free medium (RPMI 1640 Medium, Thermo Fisher Scientific, Waltham, MA) for the indicated time periods, (one week and four weeks after patch fabrication) supernatant was collected and EVs were isolated via differential centrifugation. EV concentration was assessed by NanoTracking Analysis (NTA) software following imaging with a NanoSight LM10 as described previously (Lamichhane et al. 2017). Samples were analyzed with 60-second exposure at camera level 16 and threshold level 8.
Statistical analysis
For all experiments, data were represented graphically as bar or line charts with error bars, representing the mean with the standard error of the mean, using Prism 7 (GraphPad, La Jolla, CA). Statistical analysis of echocardiography, scar area measurement and vessel counting was performed with unpaired two-tailed t-tests. A p-value less than 0.05 was considered statistically significant.
Results
Differentiation and characterization of hiPSC-CMs and spheroids
Human iPSCs were differentiated into cardiomyocytes (Figure 1A). Spontaneous beating was typically observed in hiPSC-CMs on the eighth day after differentiation, and the density of beating cells usually increased up to day 15. In our experiment, only beating hiPSC-CMs were used to create spheroids. The cell ratios and total cell numbers and cell types were optimized to create spheroids amenable to 3D bioprinting as previously described (Figure 1B) (Ong, Fukunishi, Zhang, et al. 2017). In the presence of endothelial cells (ECs) and fibroblasts (FBs), spheroids formed within 24 hours and started beating spontaneously by 48 hours. The spheroid beating rate was about 29 beats per minute as we described previously.(Ong, Fukunishi, Zhang, et al. 2017) Spheroids fixed on day 3 after formation expressed endothelial cell specific marker (CD31) and cardiac cell specific marker (Troponin T) (Figure 1 C & D).
Figure 1.
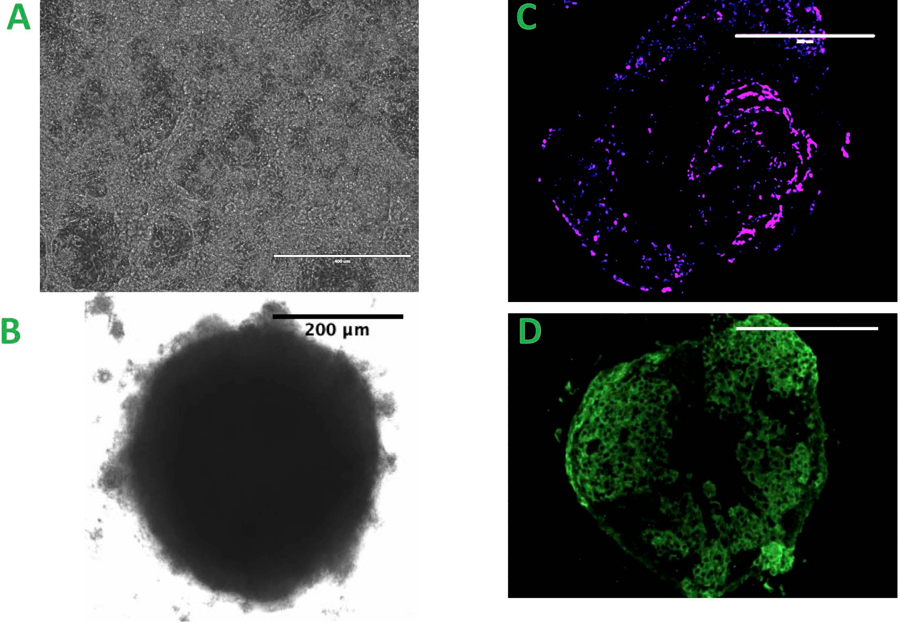
A. iPSC differentiated Cardiomyocyte, scale bar = 400μm B. The spheroid created from the co-culture of three types of cells, scale bar = 200 μm C&D. Spheroids with CD31 positive staining C. The magenta: the presence of endothelial cell, scale bar = 200 μm D. The green: Troponin T staining of individual spheroid, scale bar = 200 μm
Fabrication and in vitro characterization of human cardiac patches
Cardiac patches of 3.6mm × 3.6mm size from human iPSCs were bioprinted in vitro using the biomaterial-free bioprinting method described previously (Figure 2A). For each patch, 1.8 million hiPSC-CMs, 0.4 million human cardiac fibroblasts and 0.4 million human umbilical vein endothelial cells were used to create a monolayer cardiac patch (Figure 2B). As previously reported (Ong, Fukunishi, Zhang, et al. 2017), patches can be incubated in vitro for at least one week with greater than 90% cell viability. The cell viability of patches 4 weeks post-implantation was estimated as 94.7 +/− 2.78 % with the detection of apoptotic programmed cell death by TUNEL staining (Figure 2C). We assessed EV production by patches after 1 week and 4 weeks in vitro to evaluate the cellular viability of the patch. The data reveal a ~5-fold increase in net EV secretion by patches after 4 weeks compared to 1 week incubation (Supplemental Figure 2). One of the possibilities is that the exosomes might play a role in enhancing cell viability.
Figure 2.
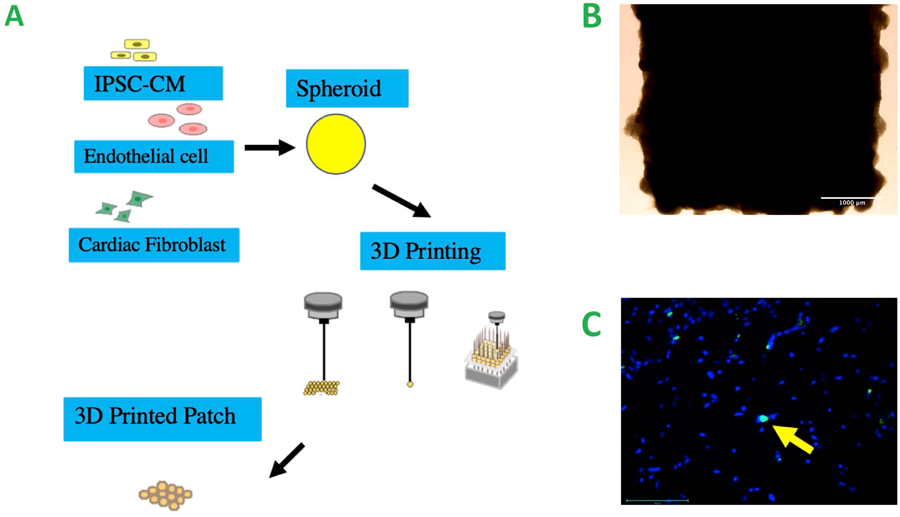
A. Schematic of bioprinting, co-cultures of the cells for the creation of spheroids, 3D bioprinting of the cardiac patch from the spheroids B. Cardiac patch from the bioprinting prior to implantation, scale bar = 1000 μm C. TUNEL staining of the 3D printed cardiac patch 4 weeks after fabrication, Yellow arrow: apoptotic cells. scale bar = 100 μm
In vivo characterization and assessment of hiPSC-derived cardiac patch impact on a rat model with myocardial infarction
The regenerative potential of cardiac patch was evaluated in a rat MI model. Infarction was surgically induced by occluding the distal left anterior descending coronary artery (Figure 3A), then one monolayer cardiac patch and one omentum patch were sutured over the site of infarction in animals from the patch group (n=6) (Figure 3B). One omentum patch was sutured over the injury site in animals from the control group (n=6) (Figure 3C), while animals in the MI group recovered without either experimental treatment. After four weeks, the survival rate of patch group and control group were 100% and 83.3% respectively. Human Nuclear Antigen (HNA) and human Troponin T positive staining were noted in the histological assessment 4 weeks after surgery (Figure 3D–E: HNA, F-G: Troponin T). The scar areas of the infarction in both groups were evaluated as previously described (Takagawa et al. 2007). The average percentage of scar area in the patch group was significantly lower than that of control group (mean ± SD of 10.6 ± 5.1% and 19.39 ± 8.1% respectively, p = 0.048) (Figure 4 A–C). The vascularization of the infarcted area was also evaluated four weeks after surgery. The average vessel count of the patch group was significantly higher than that of control group (mean ± SD of 15.13 ±2.82 and 7.17 ± 2.85 respectively, p = 0.001) (Figure 5A–C). Finally, cardiac function was evaluated 4 weeks after injury via echocardiography. Measurements of left ventricular ejection fraction (EF) and cardiac output (CO) were shown to be better in the patch group than in the control group (EF(%): 50.0 ± 18.9, 40.1 ± 14.4 respectively, p = 0.36) (CO: 104.6 ± 45.5, 68.6 ± 16.4 respectively, p = 0.1). Thus, the patch group induced a trend towards improvement in cardiac function via preservation of the EF and CO (Figure 6A & B).
Figure 3.
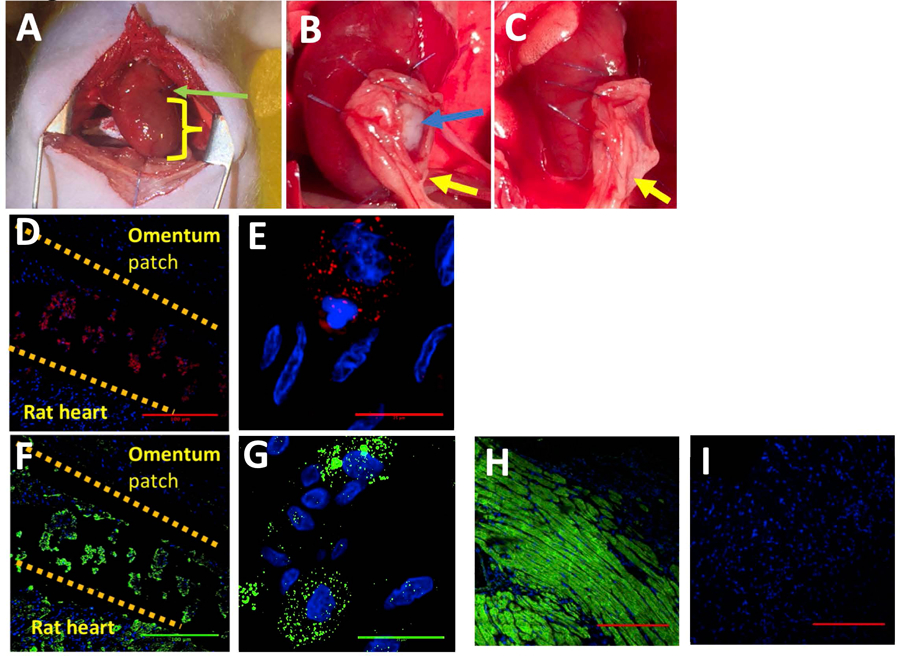
A. Occlusion of the distal left anterior descending coronary artery, green arrow: suture yellow: the infarcted area B. Blue arrow: the cardiac patch, yellow arrow: the omentum patch implanted into the experiment group (n = 6) C. The omentum patch implanted in the control group (n = 6) D-G. Evidence of retention of the cells in rat 4 weeks after surgery. Top: the omentum, middle: the patch area, bottom: the rat heart. D-E. HNA (red), DAPI (blue). Red in the patch area: the presence of human nuclear antigen from the stem cell. D: scale bar = 100 μm, E: scale bar = 25 μm F-G. Troponin T (green), DAPI (blue). Green: the presence of troponin T in both the rat heart and patch area. F: scale bar = 100 μm, G: scale bar = 25 μm H-I: Positive and Negative control of the Troponin T staining, H: positive control, I: Negative control, scale bar = 100 μm
Figure 4.
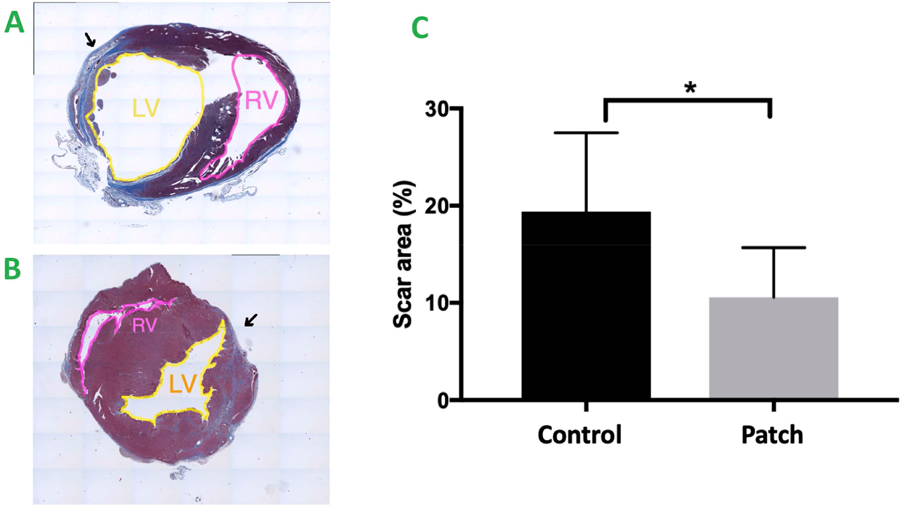
A&B Masson Trichrome staining of the rat heart 4 weeks after surgery. Blue: the area of fibrosis, Red: represents normal myocardium. Yellow: Left Ventricle (LV), Pink: Right Ventricle (RV), Black Arrow: Scar Area.
A. The control group. B. The patch group. C. Statistical analysis of scar area (percentage = infarcted area / total area),* indicates p<0.05. Patch group vs control group (10.6 ± 5.1% vs. 19.39 ± 8.1% (Mean ± SD) respectively, p = 0.048)
Figure 5.
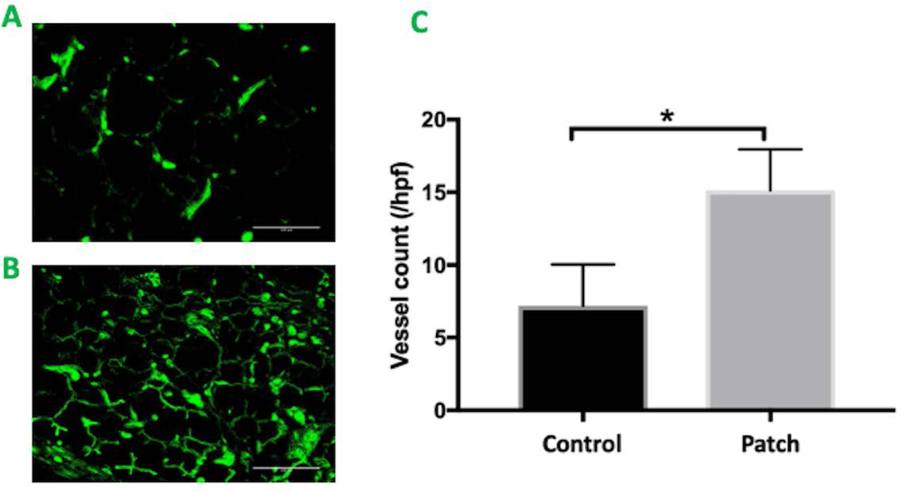
A&B. CD 31 staining of the infarcted area. Green: positive staining of the endothelial cell. A. The control group. B. The patch group. scale bar = 100 μm C. Statistical analysis of vessel counting * indicates p<0.05. Patch group vs control group (15.13 ± 2.82 vs. 7.17 ± 2.85 (Mean +/ SD) respectively, p = 0.001)
Figure 6.
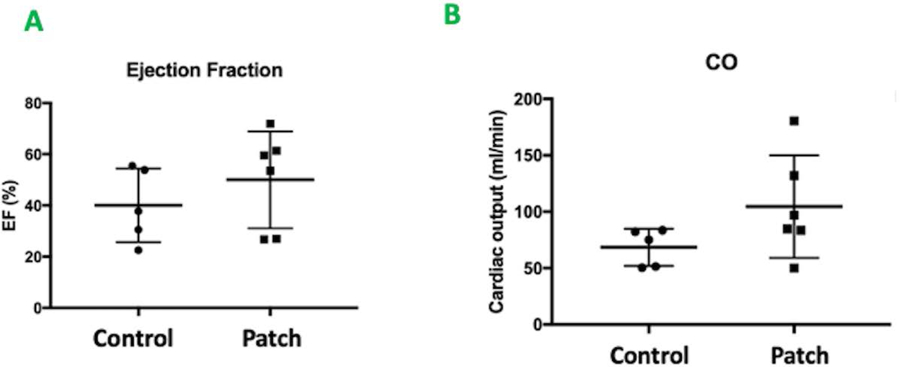
A&B. Echocardiography performed 4 weeks after implantation. A. Left Ventricular Ejection Fraction. Patch group vs. control group (EF(%): 50.0 ± 18.9 vs. 40.1 ± 14.4, p = 0.36) B. Cardiac Output (ml/min). Patch group vs control group (CO: 104.6 ± 45.5 vs. 68.6 ± 16.4, p =0.1)
Comment
In this study, we created a biomaterial-free 3D printed contractile cardiac patch with potential clinical use. A number of recent studies using engineered tissue and cell injection into a heart failure model have demonstrated some improvement in cardiac function (Alcon, Cagavi Bozkulak, and Qyang 2012; Langer and Vacanti 1993). However, these studies have several limitations, including poor retention of stem cells, grafts with low cell density and excessive cell death in the infarcted area (Wang and Guan 2010; Zimmermann et al. 2006; Jawad et al. 2008).
To address the shortcomings of previous studies, we employ the biomaterial-free patch creation method we have previously described. The design of this patch is based on our previous study that the cardiomyocyte: fibroblast: endothelial cell in the ratio of 70:15:15, with organized electrical activity and regular beating behavior observed. (Ong, Fukunishi, Zhang, et al. 2017). Furthermore, according to Baudino et al., fibroblasts accelerate the process of cell aggregation and endothelial cells enhance the vasculogenesis process (Baudino et al. 2006). Thus, in this study we used cardiac spheroids containing 70% induced pluripotent stem cell derived cardiomyocytes, 15% fibroblasts, and 15% human umbilical vein endothelial cells in this study. Using this 70:15:15 ratio of cells, our study demonstrates the successful creation of contractile cardiac tissue patches and the occurrence of vascularization within cardiac spheroids 8 days after spheroid creation (Figure 3). Further, this patch configuration was shown to induce increased net EV secretion over time in culture, which could contribute to therapeutic efficacy. Given the reported efficacy of EVs such as exosomes in cardiac repair applications (Arslan et al. 2013; Ibrahim, Cheng, and Marbán 2014; Khan et al. 2015; Gallet et al. 2017; Agarwal et al. 2017), this potential mechanism represents an interesting area of future study. The biomaterial-free strategy employed here reduces the potential for an inflammatory response and the risk of infection induced by scaffold materials.
Critically, cell retention within implanted patches 4 weeks after surgery was found to be much greater compared with prior studies in which more than 85% of cells are lost the first day after cell injection therapy (Kraehenbuehl et al. 2011). The presence of endothelial cells in the microenvironment may contribute to the superior survival duration of cardiomyocytes demonstrated within our engineered cardiac patch (Gao et al. 2017). This aligns with previous studies that have described co-culture of different types of cells resulting in improved maturation of cells, neovessel formation, and cell viability (Mironov et al. 2009; Nakayama 2013; Narmoneva et al. 2004; Jain 2003; Koike et al. 2004). We also found that the enhancement of vasculogenesis in the infarcted area in the patch group may be related to the reduction of the scar area.
Additionally, we demonstrated that there is an improving trend of cardiac function in the patch group over time via an echocardiography study. In addition to prolonged paracrine effects mediated by enhanced cell survival with the patches, another possible explanation for this trend is provided by Gao et al. (Gao et al. 2017), who demonstrated that cardiac patches engineered from human induced-pluripotent stem-cell derived cardiac cells are associated with increased cTnI phosphorylation, which in turn counteracts the contractile dysfunction which is normally contributed to by a decline in cTnI phosphorylation after myocardial infarction or during end-stage heart failure (van der Velden et al. 2004; Messer, Jacques, and Marston 2007). Since the size of cardiac patches in our study (3.6mm × 3.6mm × 0.04mm) is not large enough to cover the entire infarcted site in the rat heart (average rat heart size ~ 1 × 1 × 1cm, length × width × thickness of the whole heart), the predicted cytoprotective mechanism of our engineered patch was not prominent enough to demonstrate a significant difference in cardiac function. However, the improving trend of cardiac function may suggest that our engineered cardiac patch has potential in boosting the cardiac function in an infarcted heart.
We now list some limitations to our study that should be taken into consideration in future investigations. Firstly, the heart rate of the rat was different from the contraction rate of the iPSC-cardiac patch which consists of a mixture of atrial, nodal and ventricular cardiomyocytes (Yamamoto et al. 2016). The contraction of the cardiac was mainly mediated through Calcium channel, whereas the ventricular cell of the rat heart was mainly mediated by Sodium channel (Sakakibara et al. 1993). Secondly, the follow-up period of our study should be extended to evaluate the long-term regenerative potential and effects of the cardiac patch in rat myocardium. Thirdly, engineered tissue of larger dimensions should be fabricated to cover the entire infarcted area in order investigate augmentation of contractile function of the infarcted heart. Finally, integration of the cardiac patch into the native heart needs to be demonstrated. Further studies with larger cardiac patches and longer follow-up periods would be beneficial for better understanding the potential regenerative mechanism of engineered cardiac tissue on a damaged heart. In conclusion, our biomaterial-free 3D bioprinted tissue engineering technique for cardiac stem cell-based therapy has promising regenerative therapeutic potential. We look forward to the development of this technology into a versatile strategy for the management of end-stage heart failure.
Supplementary Material
Supplemental Figure 1: Masson Trichrome staining of the rat heart 4 weeks after surgery. Blue: the area of fibrosis, red: represents normal myocardium. Top Row. The control group. Bottom Row. The patch group
Supplemental Figure 2: Quantification of EV concentration, the EV secretion from the patch increased over 4 weeks after fabrication
Acknowledgements
The authors acknowledge the following funding sources: Magic That Matters Fund for Cardiovascular Research (Johns Hopkins Hospital to NH), National Institutes of Health (HL141611 to SMJ), Pharmaceutical Research and Manufacturers of America (Predoctoral Fellowship to AJ)
Footnotes
Disclosures
The authors have nothing to disclose.
Reference:
- Agarwal U, George A, Bhutani S, Ghosh-Choudhary S, Maxwell JT, Brown ME, Mehta Y, Platt MO, Liang Y, Sahoo S, and Davis ME 2017. ‘Experimental, Systems, and Computational Approaches to Understanding the MicroRNA-Mediated Reparative Potential of Cardiac Progenitor Cell-Derived Exosomes From Pediatric Patients’, Circ Res, 120: 701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcon A, Cagavi Bozkulak E, and Qyang Y 2012. ‘Regenerating functional heart tissue for myocardial repair’, Cell Mol Life Sci, 69: 2635–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, and de Kleijn DP 2013. ‘Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury’, Stem Cell Res, 10: 301–12. [DOI] [PubMed] [Google Scholar]
- Baudino TA, Carver W, Giles W, and Borg TK 2006. ‘Cardiac fibroblasts: friend or foe?’, Am J Physiol Heart Circ Physiol, 291: H1015–26. [DOI] [PubMed] [Google Scholar]
- Cihakova D, Barin JG, Afanasyeva M, Kimura M, Fairweather D, Berg M, Talor MV, Baldeviano GC, Frisancho S, Gabrielson K, Bedja D, and Rose NR 2008. ‘Interleukin-13 protects against experimental autoimmune myocarditis by regulating macrophage differentiation’, Am J Pathol, 172: 1195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marban L, Ghaleh B, and Marban E 2017. ‘Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction’, Eur Heart J, 38: 201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Gregorich ZR, Zhu W, Mattapally S, Oduk Y, Lou X, Kannappan R, Borovjagin AV, Walcott GP, Pollard AE, Fast VG, Hu X, Lloyd SG, Ge Y, and Zhang J 2017. ‘Large Cardiac-Muscle Patches Engineered from Human Induced-Pluripotent Stem-Cell-Derived Cardiac Cells Improve Recovery from Myocardial Infarction in Swine’, Circulation. [DOI] [PMC free article] [PubMed]
- Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG, Committee American Heart Association Advocacy Coordinating, Thrombosis Council on Arteriosclerosis, Biology Vascular, Radiology Council on Cardiovascular, Intervention, Cardiology Council on Clinical, Epidemiology Council on, Prevention, and Council Stroke. 2013. ‘Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association’, Circ Heart Fail, 6: 606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim, Ahmed Gamal-Eldin, Ke Cheng, and Eduardo Marbán. 2014. ‘Exosomes as Critical Agents of Cardiac Regeneration Triggered by Cell Therapy’, Stem Cell Reports, 2: 606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK 2003. ‘Molecular regulation of vessel maturation’, Nat Med, 9: 685–93. [DOI] [PubMed] [Google Scholar]
- Jawad H, Lyon AR, Harding SE, Ali NN, and Boccaccini AR 2008. ‘Myocardial tissue engineering’, Br Med Bull, 87: 31–47. [DOI] [PubMed] [Google Scholar]
- Jessup, Mariell, and Brozena Susan 2003. ‘Heart Failure’, New England Journal of Medicine, 348: 2007–18. [DOI] [PubMed] [Google Scholar]
- Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, and Kishore R 2015. ‘Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction’, Circ Res, 117: 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike N, Fukumura D, Gralla O, Au P, Schechner JS, and Jain RK 2004. ‘Tissue engineering: creation of long-lasting blood vessels’, Nature, 428: 138–9. [DOI] [PubMed] [Google Scholar]
- Kraehenbuehl TP, Ferreira LS, Hayward AM, Nahrendorf M, van der Vlies AJ, Vasile E, Weissleder R, Langer R, and Hubbell JA 2011. ‘Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction’, Biomaterials, 32: 1102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane TN, Leung CA, Douti LY, and Jay SM 2017. ‘Ethanol Induces Enhanced Vascularization Bioactivity of Endothelial Cell-Derived Extracellular Vesicles via Regulation of MicroRNAs and Long Non-Coding RNAs’, Sci Rep, 7: 13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane Tek N., Raiker Rahul S., and Jay Steven M. 2015. ‘Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery’, Molecular pharmaceutics, 12: 3650–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R, and Vacanti JP 1993. ‘Tissue engineering’, Science, 260: 920–6. [DOI] [PubMed] [Google Scholar]
- Messer AE, Jacques AM, and Marston SB 2007. ‘Troponin phosphorylation and regulatory function in human heart muscle: dephosphorylation of Ser23/24 on troponin I could account for the contractile defect in end-stage heart failure’, J Mol Cell Cardiol, 42: 247–59. [DOI] [PubMed] [Google Scholar]
- Miller LW, Guglin M, and Rogers J 2013. ‘Cost of ventricular assist devices: can we afford the progress?’, Circulation, 127: 743–8. [DOI] [PubMed] [Google Scholar]
- Mironov Vladimir, Visconti Richard P., Kasyanov Vladimir, Forgacs Gabor, Drake Christopher J., and Markwald Roger R. 2009. ‘Organ printing: Tissue spheroids as building blocks’, Biomaterials, 30: 2164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Koichi. 2013. ‘Chapter 1 - In Vitro Biofabrication of Tissues and Organs’ in, Biofabrication (William Andrew Publishing: Boston: ). [Google Scholar]
- Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, and Lee RT 2004. ‘Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration’, Circulation, 110: 962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CS, Fukunishi T, Nashed A, Blazeski A, Zhang H, Hardy S, DiSilvestre D, Vricella L, Conte J, Tung L, Tomaselli G, and Hibino N 2017. ‘Creation of Cardiac Tissue Exhibiting Mechanical Integration of Spheroids Using 3D Bioprinting’, J Vis Exp. [DOI] [PMC free article] [PubMed]
- Ong CS, Fukunishi T, Zhang H, Huang CY, Nashed A, Blazeski A, DiSilvestre D, Vricella L, Conte J, Tung L, Tomaselli GF, and Hibino N 2017. ‘Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes’, Sci Rep, 7: 4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y, Furukawa T, Singer DH, Jia H, Backer CL, Arentzen CE, and Wasserstrom JA 1993. ‘Sodium current in isolated human ventricular myocytes’, Am J Physiol, 265: H1301–9. [DOI] [PubMed] [Google Scholar]
- Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Kirk R, Rahmel AO, Hertz MI, Heart International Society of, and Transplantation Lung. 2012. ‘The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report−-2012’, J Heart Lung Transplant, 31: 1052–64. [DOI] [PubMed] [Google Scholar]
- Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, and Springer ML 2007. ‘Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches’, J Appl Physiol (1985), 102: 2104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden J, Merkus D, Klarenbeek BR, James AT, Boontje NM, Dekkers DH, Stienen GJ, Lamers JM, and Duncker DJ 2004. ‘Alterations in myofilament function contribute to left ventricular dysfunction in pigs early after myocardial infarction’, Circ Res, 95: e85–95. [DOI] [PubMed] [Google Scholar]
- Wang F, and Guan J 2010. ‘Cellular cardiomyoplasty and cardiac tissue engineering for myocardial therapy’, Adv Drug Deliv Rev, 62: 784–97. [DOI] [PubMed] [Google Scholar]
- Yamamoto W, Asakura K, Ando H, Taniguchi T, Ojima A, Uda T, Osada T, Hayashi S, Kasai C, Miyamoto N, Tashibu H, Yoshinaga T, Yamazaki D, Sugiyama A, Kanda Y, Sawada K, and Sekino Y 2016. ‘Electrophysiological Characteristics of Human iPSC-Derived Cardiomyocytes for the Assessment of Drug-Induced Proarrhythmic Potential’, PLoS One, 11: e0167348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, and Eschenhagen T 2006. ‘Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts’, Nat Med, 12: 452–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Masson Trichrome staining of the rat heart 4 weeks after surgery. Blue: the area of fibrosis, red: represents normal myocardium. Top Row. The control group. Bottom Row. The patch group
Supplemental Figure 2: Quantification of EV concentration, the EV secretion from the patch increased over 4 weeks after fabrication


