Abstract
The respiratory system, including the peripheral lungs, large airways and trachea, is one of the most recently evolved adaptations to terrestrial life. To support the exchange of respiratory gases, the respiratory system is interconnected with the cardiovascular system, and this interconnective nature requires a complex interplay between a myriad of cell types. Until recently, this complexity has hampered our understanding of how the respiratory system develops and responds to postnatal injury to maintain homeostasis. The advent of new single-cell sequencing technologies, developments in cellular and tissue imaging and advances in cell lineage tracing have begun to fill this gap. The view that emerges from these studies is that cellular and functional heterogeneity of the respiratory system is even greater than expected and also highly adaptive. In this Review, we explore the cellular crosstalk that coordinates the development and regeneration of the respiratory system. We discuss both the classic cell and developmental biology studies and recent single-cell analysis to provide an integrated understanding of the cellular niches that control how the respiratory system develops, interacts with the external environment and responds to injury.
When animals moved from a water-based environment to land, they had to evolve a system to derive oxygen from the air and dispose of waste gases such as carbon dioxide. The respiratory system in mammals is generally divided into the upper respiratory tract and the lower respiratory tract. The upper respiratory tract encompasses the nose or nostrils, nasal cavity, mouth and throat (pharynx) and terminates at the voice box (larynx). The lower respiratory tract consists of the trachea and the lungs, which are further subdivided into terminal and respiratory airways and the peripheral alveoli, where gas exchange occurs (Fig. 1a). Lungs are found in most terrestrial animals, including amphibians, birds, reptiles and mammals. Although lung structure differs between species, gas exchange with the cardiovascular system remains its core function. Coordinating the development and homeostasis of an organ as complex as the lungs requires critical cell-intrinsic changes and cell-cell interactions. To achieve this complexity, the mammalian lung undergoes a progressive developmental process starting with specification of the endoderm progenitors in the anterior foregut followed by postnatal maturation of the gas exchange interface. These processes are further defined by specific cellular and tissue developmental events such as proximal-distal patterning of the endoderm and mesenchyme to specify large and small airways, branching morphogenesis of the airways, which expands the respiratory tree, and postnatal alveologenesis, which subdivides the peripheral alveoli into a larger surface area for optimal gas exchange. To maintain its functions throughout animal life, the respiratory system is able to counter insults and injuries by mounting a regenerative response and repopulate the tissue with the different cell types. Defects in any of these processes can result in a severe reduction in respiratory function and can lead to various respiratory diseases (Supplementary Table 1). All of these compartments within the respiratory system contain distinct stem cell lineages critical for tissue regeneration after injury and in the context of chronic diseases.
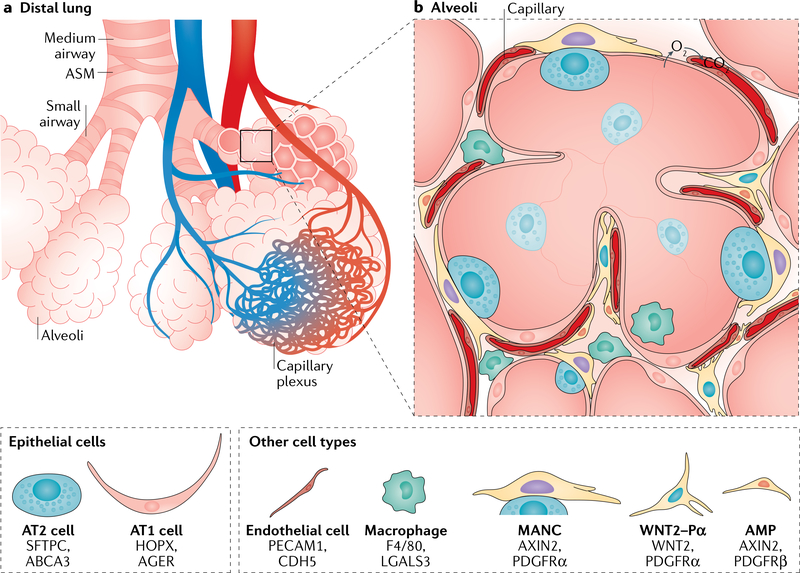
Fig. 1 |. Cellular composition of airways.
a | The proximal region of the lower respiratory tract consists of the trachea, which proceeds distally to generate branching large and small airways, which terminate in alveoli. These airway tubes are wrapped by smooth muscle. b,c | Respiratory epithelium in the mouse and the human consists of many distinct cell types: goblet cells secrete mucus to entrap inhaled particulates; multiciliated cells expel the mucus together with the entrapped particulates; club cells are secretory cells that produce various factors with protective and immunomodulatory functions and also serve in detoxification of harmful substances; basal cells are progenitor cells for the airway epithelia; pulmonary neuroendocrine cells (PNECs; solitary as well as in clusters known as neuroendocrine bodies (NEBs)) probe the microenvironment to influence smooth muscle tone as well as regulate immune responses. There are also rare epithelial cell types, including brush (tuft) cells, which may serve an important role in regulating allergen driven type 2 immune responses, and the recently identified ionocytes, which appear to be a main source of cystic fibrosis transmembrane conductance regulator (CFTR) activity, thereby regulating mucus production (with a potential role in cystic fibrosis). Stromal cells such as airway smooth muscle (ASM), fibroblasts (sonic hedgehog responsive (Gli1 positive) and WNT responsive (Axin2 positive)) provide ligands and extracellular matrix that modulate airway epithelial cell turnover and restrict airway tube diameter. The tracheal region and large airways in the human respiratory tract and the trachea in mice harbour cartilaginous rings and submucosal glands. The latter contain mucous cells, serous cells and myoepithelial cells and, together with goblet cells, are responsible for the production of luminal mucus together with goblet cells (panel b). Unlike in the human, mouse small airways do not contain basal or goblet cells (panel c). Key markers expressed in each cell type shown in panels b and c are indicated.
In this Review, we focus on the development and regeneration of the spatially complex cellular micro-environments (niches) within the respiratory system, focusing on the lower respiratory tract, including the trachea and lungs. We explore how cellular crosstalk helps to define and generate the complex architecture of these niches in development and after injury. Recent technical innovations are highlighted and certain differences between the mouse and human respiratory systems are discussed to underscore where future investigations are needed to begin to address the lack of targeted therapies for lung disease.
Foregut.
The portion of the gut tube that gives rise to several organs, including the oesophagus, stomach and the entire respiratory tract.
Mesenchyme.
Mesoderm-derived cells and tissue.
Branching morphogenesis.
The developmental process that generates the branched ductal tree-like structures observed in multiple organ systems, including lung, pancreas, kidney, liver, prostate gland, mammary gland and circulatory system. The process is characteristically marked by tube outgrowth and tip branching.
Pseudostratified epithelium.
A single layer of epithelial cells all with contacts to the basal laminal surface but with different positions of nuclei, giving the appearance of stratified layers.
Cellular composition
The respiratory system in its totality is composed of multiple branched tissue systems, including the airways and blood vessels. While the branching patterns of these tissues differ between species, the general tissue organization is remarkably similar. The entire mammalian respiratory system is lined with epithelial cells. The larger airways of the respiratory system, including the trachea, are underlaid with bands of smooth muscle and in larger mammals, including humans and animals such as pigs and sheep, cartilaginous rings, both of which provide support and tone to airway flow. The branched structure of the airways is paralleled by the pulmonary vasculature, which eventually intersects with cells in the alveolus to form the gas diffusible interface.
Main epithelial cell types
The trachea and proximal airways in humans as well as in the mouse consist of a pseudostratified epithelium, which contains multiple cell lineages, including multiciliated cells, secretory cells, goblet cells and basal stem/progenitor cells (BSCs) (Fig. 1b,c). Functionally, multiciliated cells have cilia on the apical surface that shuttle inhaled particulates and mucus out of the airways in a retrograde manner. Mucus-producing goblet and secretory cells are a critical first line of defence for trapping inhaled particulates and microorganisms. BSCs act as resident stem cells for the trachea and proximal airways of the human respiratory system and the trachea and main stem bronchi of the mouse respiratory system, and they are capable of repopulating the pseudostratified epithelium during homeostasis and after injury. The trachea and airways are underlined with heterogeneous mesenchymal cell lineages, including cartilage around the trachea, smooth muscle and interstitial fibroblasts. These cells can act as niche-supporting cells to regulate the regenerative response of airway epithelium after injury1,2 (see later for further details).
Surfactant.
A mixture of lipids and proteins that is secreted into the alveolar space to reduce surface tension and prevent lung collapse.
The alveoli are distinct in structure and development from the more proximal airways of the mammalian lung. The two major epithelial lineages within the alveoli are alveolar type 1 (AT1) and AT2 epithelial cells (also known as alveolar epithelial cells or pneumocytes; Fig. 2). AT1 cells are flattened and squamous. They comprise more than 95% of the gas exchange surface of the lung and intimately associate with the underlying endothelial capillary plexus (Fig. 2a) to form the thin gas diffusible interface. AT2 cells are cuboidal and produce pulmonary surfactant, which reduces surface tension to prevent alveolar collapse during respiration. Furthermore, AT2 cells can act as progenitors for AT1 cells in the adult lung and to a lesser extent during early postnatal lung maturation, when AT2 cells exhibit a final burst of proliferation3,4. The underlying endothelial capillary plexus develops in the alveolar region through intussusceptive angio- genesis5. This process involves the spreading of a thin endothelial cell extension in which holes or pores form to bridge connections to other endothelial cells, resulting in a large web-like structure. In addition to epithelial and endothelial cells, the lung alveolus contains several mesenchymal cell lineages with distinct markers that support alveolar homeostasis and repair after injury1,3,6–8 (Fig. 2b; see also later).
Fig. 2 |. Cellular composition of alveoli.
a | The small airways terminate in alveolar sacs, where the inhaled air is circulated and oxygen-carbon dioxide gas exchange occurs within the capillary plexus. b | The alveolar niche is composed of alveolar type 1 (AT1) cells and alveolar type 2 (AT2) cells. AT1 cells are thin and elongated and cover the gas exchange surface area, remaining in close contact with the capillary plexus. AT2 cells produce pulmonary surfactant (stored in the lamellar bodies in the cytoplasm), which preserves surface tension of the alveolus to prevent collapse during breathing. Interstitial fibroblasts consisting of Axin2-positive myogenic precursors (AMPs), Wnt2-expressing platelet-derived growth factor-α (PDGFRa)-positive cells (WNT2-Pa) and mesenchymal alveolar niche cells (MANCs) make up most of the alveolar mesenchyme. These cells largely support alveolar structure by producing extracellular matrix as well as expressing ligands that support epithelial cell proliferation and differentiation (see also Fig. 5). Additionally, there are sentinel immune cells such as alveolar and interstitial macrophages that constantly survey the alveolar microenvironment for harmful pathogens or particulates. ASM, airway smooth muscle.
The entire respiratory system is encased by a layer of mesothelium, which is related to tissues such as the epicardium, which lines the external surface of the heart. The mesothelium can generate certain populations of mesenchymal cells during development and is also a rich source of paracrine growth factors, including fibroblast growth factors (FGFs) and WNTs, which have an important role in patterning the airways and promoting mesenchymal cell proliferation and differentiation during respiratory system development9–12. The role of mesothelium in adult respiratory system regeneration remains unclear.
Rare and novel epithelial cell types
The airway epithelium also contains a number of highly specialized and/or rare cell types, including the pulmonary neuroendocrine cells (PNECs), goblet cells (which are considered rare in the mouse but are abundant in the human airways), brush cells (also known as tuft cells) and ionocytes. Although the presence of some of these cell types was documented through histological techniques, newer cell-specific markers and genetic reporters have been essential in elucidating their functional significance.
PNECs appear throughout the large and small airways and are marked by expression of ASCL1, DLL1 and PGP9.5. In mice, PNECs are generally found in clusters at bronchiolar branch junctions, forming neuroepithelial bodies or as solitary cells in the upper airways and trachea (Fig. 1b,c). Unlike other epithelial cells, PNECs are innervated and can act as sensory cells within the airways. PNECs harbour secretory granules loaded with a variety of neurotransmitters in the form of amines and peptides, including calcitonin-gene-related peptide and Y-aminobutyric acid13,14, and thus they are capable of connecting environmental sensation to neuropeptide and neurotransmitter outputs15. PNECs have been shown to critically modulate immune response to allergens and influence elevated mucus production13,16.
More recently, single-cell RNA sequencing (see Box 1) has allowed further characterization of other airway cell lineages17,18. Brush cells have a microtubule network extending to apical microvilli on the airway surface and express numerous G protein-coupled receptors, including taste receptors18,19. Brush cells are also present in other mucosal tissues, including the intestine, where they act as a critical first-line innate immune defence against parasite infection, producing cytokines such as IL-25 (reFs20,21).
Box 1 |. Advancements in technologies to study the lung.
New technologies that increase the resolution for dissecting differences on a cellular, transcriptional and epigenetic level will have a dramatic impact on our understanding of lung biology. moreover, studies aimed at identifying and confirming rare or unique cell types are becoming more abundant. New genetic mouse models are using combinatorial DNA recombination techniques, such as simultaneously using Cre and Flp recombinases to lineage trace rare cells that are uniquely identifiable by two marker genes55. The use of single- cell RNA sequencing (scRNA-seq) techniques has identified multiple new epithelial and mesenchymal cell types in the mouse lung and the human lung. RNA sequencing of single cells derived from mouse lung mesenchyme demonstrated that there are at least five transcriptionally unique populations1,8,129. Recent work using scRNA-seq shows that the human and mouse lung mesenchyme has conserved subsets that are anatomically distinct, residing near the proximal airways or distal alveoli130. Furthermore, scRNA-seq has uncovered novel airway epithelial cell types such as ionocytes in the human and the mouse17,18. Comparative scRNA-seq of normal and diseased lung tissue from transplant donors and transplant recipients with pulmonary fibrosis revealed unique transcriptional signatures in macrophages and epithelial cells131. There are now several initiatives, from the National Institutes of Health as well as private foundations, that support the procurement and sharing of lung-specific scRNA-seq datasets (lungmAP project) or whole organism scRNA-seq datasets (Tabula muris, a Chan Zuckerberg Initiative Mouse Atlas), which have become important resources for routine enquiry to generate hypotheses for future experiments132,133. With the vast amount of data derived from scRNA-seq experiments, computational software has become necessary to process and analyse these data (to reduce their dimensionality) and these methods are constantly evolving134. Computational methods can identify transitional cells using a pseudotemporal ordering of gene expression, thus revealing possible stem/progenitor cells and their lineage trajectories within an organ. These analyses would normally require more traditional experiments such as genetic lineage tracing, which are both labour-intensive and time-intensive. Given the lack of robust genetic techniques that can be applied to study human tissue, such new technologies will have a profound impact on our understanding of the unique characteristics of the human lung (see also Box 3).
Progress in imaging techniques has also been instrumental in increasing our understanding of lung cell biology. Higher-resolution and whole-mount imaging have demonstrated morphological changes in cells such as alveolar type 1 cells, revealing that they can span multiple alveoli in the adult lung76. live imaging in lung explant cultures and intravital imaging by multiphoton microscopy have yielded new insights into the differentiation and morphology of early alveolar progenitors56. Furthermore, the development of intravital imaging techniques for adult mice has demonstrated an important role of dendritic cells in air surveillance in the alveoli and implicated the lung as a critical reservoir for platelet production135,136.
Recently, two independent studies identified a rare cell type in the adult murine and human tracheal and proximal airways and termed these cells ‘iono-cytes’. This cell type expresses high levels of the cystic fibrosis transmembrane conductance regulator gene (CFTR) transcript and derives from the basal cell line- age17,18. Mutations in CFTR cause cystic fibrosis, which is associated with alterations in the viscosity of the airway mucus lining that lead to a predisposition for infections22. Owing to the unique characteristics of the pulmonary ionocytes, these new findings suggest that this cell type may be a major cellular player in cystic fibrosis. Furthermore, these studies likely represent just the beginning of a new era of mapping the cell lineages within the complex respiratory system of mammals.
In addition to the pseudostratified epithelium, airways feature unique structures known as submucosal glands (SMGs), which are tubule-acinar invaginations situated throughout the cartilaginous airways in the human respiratory system (in the mouse, SMGs are restricted to the uppermost part of the trachea). The SMGs consist of collecting ducts, ciliated ducts, myoepithelial cells and acini with serous cells and mucus- producing cells. Accordingly, by producing glandular fluids, SMGs are a companion to mucus-producing goblet cells. Myoepithelial cells are functionally required for the excretion of the glandular fluids into the airway by providing a contractile force that squeezes the acini. Notably, recent work demonstrated that myoepithelial cells can also proliferate, migrate to the airway surface and generate non-SMG luminal epithelial cells within the large airways in several models of proximal airway epithelial injury23,24. These new observations suggest that the SMGs harbour reserve progenitor cells that can be evoked after severe airway injury to aid tissue repair.
Respiratory system development
Development of the respiratory tract is complex and involves concerted morphogenesis of the epithelium and the development of the mesenchyme (Fig. 3a,b). The lower respiratory tract is specified early in mammalian development, but the critical steps of alveolar maturation occur postnatally.
Fig. 3 |. Respiratory tract development and endoderm-mesoderm interactions.
a | By embryonic day 12.5 in mouse the NKX2.1-postive endoderm expresses SOX2 and SOX9 in proximal and distal cells, respectively. At this point branching morphogenesis has begun and the endoderm projects outward towards the FGF10-expressing mesoderm (dashed outline; see panel b). b | The respiratory mesoderm, which is derived from cardiopulmonary progenitors and expresses TBX4, is shown encapsulated by mesothelium. Mesodermal cells express FGF10 in the regions adjacent to the distal tip endoderm. Primitive airway smooth muscle (ASM), tracheal cartilage and the vascular tree are being specified during this time. c | The distal tip endoderm expresses SOX9 and ID2. These cells respond to FGF10 and WNTs derived from the mesoderm, which together stimulate their outgrowth. In a feedback response, the endoderm expresses ligands, such as sonic hedgehog (SHH) and WNT7b, that stimulate the differentiation of the mesoderm to ASM. d,e | Alveologenesis starts with the formation of primitive alveoli, which are circular and bulb-like (saccular stage; from embryonic day 17.5 to postnatal day 5 in the mouse). These primitive alveoli form at the termini of the bronchial tree and harbour flattened alveolar type 1 (AT1) cells as well as alveolar type 2 (AT2) cells, which remain cuboidal. During the alveolar stage (postnatal days 5 to 30 in the mouse) the formation of secondary septae occurs. This remodelling process increases the total surface area of the lung alveolus and is thought to be driven by mesenchymal cells, such as the secondary crest myofibroblasts (SCMFs). SCMFs are stimulated by epithelium-derived PDGFA and SHH. It is postulated that the contractile activity of the SCMFs physically shapes the alveolus. Additionally, WNT-responsive AT2 cells (AT2Axin2 cells) proliferate during this time (receiving WNT ligands from mesenchymal cells) and increase production of pulmonary surfactant, which is critical for the transition to air breathing. Furthermore, the capillary plexus becomes more closely aligned with AT1 cells during alveologenesis.
Lateral plate mesoderm.
A portion of the primary germ layers of the embryo that resides on the periphery of the embryo and gives rise to the primordial mesoderm surrounding early lung endoderm.
β-Catenin.
A critical protein in the WNT signalling cascade that is retained in the cytoplasm in unstimulated cells. on WNT- ligand stimulation, β-catenin translocates to the cell nucleus, where it cooperates with TCF/LeF proteins to promote WNT-target gene transcription.
Development of the lung epithelium during branching morphogenesis
The lung endoderm is specified in the ventral anterior foregut endoderm (AFE) at embryonic day 9.0 (E9.0) in the mouse and at approximately 4–5 weeks’ gestation in the human. This specification step is noted by expression of the transcription factor NKX2.1 in the early endoderm progenitors on the ventral side of the foregut. Conversely, SOX2 expression, which is present throughout the foregut endoderm before respiratory system specification, becomes restricted to the dorsal AFE. Several studies have pointed to the necessity of WNT signalling for specification of lung endoderm progenitors within the AFE. WNT2 and WNT2b are expressed in the anterior lateral plate mesoderm and act redundantly to initiate the expression of NKX2.1 on the ventral side of the AFE through β-catenin activity25,26. The proper expression of WNTs in the lateral plate mesoderm is regulated by the transcription factors OSR1, OSR2 and TBX5 (reFs27,28). The early NKX2.1-positive respiratory primordium pushes ventrally and begins to pinch off and elongate into the future tracheal tube. Concomitantly, signalling pathways activated by growth factors such as FGF10 derived from the pulmonary mesenchyme promote its early outgrowth and differ- entiation29–32 (see also later). Rapidly after the formation of a distinct tracheal tube, the process of branching morphogenesis is initiated to create the extensive arborized network of airways, which is supported by the key morphogen sonic hedgehog (SHH), which is expressed in the respiratory endoderm. It has been shown that a ligand-receptor-based Turing model together with tissue geometry effects (influenced by the presence of the mesenchyme and tissue-specific expression of signalling ligands and receptors) can explain the formation of a distinct branching pattern downstream of signalling modules controlled by FGF10 and SHH33.
In mice, the proximal-distal patterning of the airway endoderm is marked by distinct expression of SOX2 in the proximal airway epithelium and SOX9 and ID2 in the distal epithelium (Fig. 3a,c). By contrast, in the developing human lungs, SOX2 and SOX9 are coexpressed in the distal endoderm progenitors34.
Cellular differentiation of the airway epithelium spans the developmental time between E9.5 and E16.5 in the mouse. Although the different epithelial lineages of the airways are not easily distinguished by morphology during early respiratory tract development, their presence can be detected through expression of distinct marker genes. As recently shown in mice, TRP63-positive basal cells are present early in development (E10.5). These cells are multipotent and capable of generating both proximal and distal epithelial line- ages35. Secretory cell lineage — marked by the expression of Scgb3a2 — emerges in mice as early as E11.5 (reFs36,37), whereas the presence of the multiciliated cell lineage marked by expression of the transcription factor FOXJ1 and the cilium protein β-tubulin is observed by E14.5 (reF.38). This is a brief overview of these early events in airway development, and readers are encouraged to explore more comprehensive reviews on this subject39–43.
Turing model.
A mathematical model of diffusion-driven instability that has been applied to biologically relevant simulations of ligand-driven morphogenetic events.
Chromatin remodelling complex.
A complex of proteins that control accessibility of chromatin through modification of histones or DNA.
Multiple molecular pathways regulate cellular differentiation in the respiratory tract. SOX2 is essential for airway epithelial differentiation, and loss of its expression leads to failure of secretory and multiciliated cell differentiation44–46. Notch signalling has a central role in balancing the differentiation of multiciliated versus secretory cell lineages within the developing airways. Notch signalling is required for secretory cell differentiation, and loss of Notch leads to airways populated by multiciliated cells and the absence of secretory cells47. Conversely, activation of Notch leads to mucous cell metaplasia in the proximal airways of mice, which is associated with dramatic increases in the numbers of mucus-producing secretory cells48. FOXJ1 is essential for differentiation of multiciliated epithelium in the airways49.
There is now evidence that airway epithelial development is tightly coupled to epigenetic changes in chromatin. Loss of histone deacetylase 1 (HDAC1) and HDAC2 during mouse respiratory endoderm development leads to a loss of proximal airway epithelial differentiation, in part through activation of the morphogen BMP4, which promotes differentiation of the distal tip endoderm progenitors (destined for the peripheral lung) over proximal endoderm progenitors (destined for proximal airways)50,51. A similar phenotype is observed on loss of the transcription regulatory protein SIN3A52. Since HDAC1, HDAC2 and SIN3A are all components of the NuRD chromatin remodelling complex, these studies suggest that epigenetic chromatin remodelling by the NuRD complex is critical for proximal endoderm progenitor differentiation during lung development. Furthermore, inactivation of the Polycomb repressor complex gene Ezh2 during development leads to ectopic differentiation of TRP63-positive basal cells in the proximal airway epithelium50,51.
Progression of branching morphogenesis terminates with the formation of distal alveoli. Alveolar epithelial lineages emerge from the Sox9-positive-Id2-positive distal tip endoderm in mice. Previous work using lineage tracing of Id2-positive cells during development indicated that before E13.5 these cells are multipotent and can give rise to both proximal and distal epithelial cell types53. At E16.5, the differentiation of Id2-positive cells is restricted to distal alveolar cell fates. In mice, AT1 and AT2 cells mature just before birth, but the exact timing of AT1 and AT2 cell fate specification and their lineage relationship are active areas of study. Data from lineage tracing experiments with Id2-positive cells suggest a progressive lineage restriction model of alveolar epithelial cell fate commitment, whereby progenitors give rise to AT2 cells, some of which later differentiate into AT1 cells53. However, another study demonstrated the presence of a bipotent ‘alveolar epithelial progenitor’ (AEP) in late lung development at E18.3 that was capable of simultaneous generation of AT1 and AT2 cells54. Recently, a study combining detailed single-cell RNA sequencing analysis along with novel lineage tracing models (Box 1) was used to show that AT1 and AT2 cells commit to their respective fates early in lung development55. This period of AT1 and AT2 cell specification runs concurrently with other processes, such as branching morphogenesis and proximal-distal patterning of the airway endoderm, indicating that cell fate decisions in the lung endoderm occur far earlier than previously thought55. In addition, there is evidence that mechanical cues have an important role in AT1 versus AT2 cell lineage specification during lung development. An important source of such forces is the inhalation of amniotic fluid, which occurs as a consequence of fetal breathing movements that start at around E16.5 in mice. This amniotic fluid inhalation was found to be required for proper AT1 cell fate specification56. By contrast, differentiation into the AT2 lineage was shown to depend on cell protrusion out of the airway tubules towards the mesenchyme. This cell protrusion was associated with actomyosin-regulated reduction of the apical surface area of protruding cells, which protected the protruding cells from experiencing mechanical forces exerted by the inhaled amniotic fluid, thereby preventing acquisition of the AT1 cell fate56.
Development of the lung mesenchyme
The morphogenesis of the lung epithelium occurs in concert with the specification of mesenchymal lineages. As soon as the early lung bud emerges from the anterior foregut, it becomes surrounded by lateral plate mesoderm, which will develop into the various mesenchymal lineages within the lungs and trachea. How the mesenchyme differentiates into mature lineages and how it influences the adjoining epithelium and endothelium to provide the diverse architectural niches in the adult lung is still elusive.
Recent studies using inducible cell lineage tracing strategies have begun to elucidate the spatial and temporal differentiation of mesenchymal cells during development. It has been revealed that the lung mesenchyme originates from a multipotent pool of progenitors that can generate both pulmonary and cardiovascular mesenchymal lineages and are thus called ‘cardiopulmonary progenitors’57. As development progresses, cardiopulmonary progenitors lose their ability to contribute towards the cardiovascular lineages and ultimately differentiate into a unique mesenchymal lineage in anatomically distinct regions, associated with the conducting airways, large blood vessels or alveolar interstitium57,58. In contrast to the epithelial lineages, mesenchymal cell lineages are difficult to discern by histology alone, making our understanding of possible heterogeneity within this compartment unclear.
The mesenchyme provides signalling factors, including FGFs, WNTs, bone morphogenetic proteins (BMPs), retinoic acid and transforming growth factor-β (TGFβ), that promote branching morphogenesis, epithelial and endothelial differentiation and postnatal alveologenesis29,32,59–61. Early studies demonstrated that the mesenchyme surrounding the distal branching tips of the airways isolated from embryonic lung buds can stimulate lung branching62,63. This distal mesenchyme expresses high levels of FGF10, which is necessary for and sufficient to promote lung branching29–32,64 (Fig. 3b,c). FGF10 signalling functions in part by providing a key patterning and mitogenic signal to maintain distal endoderm expansion in the lung. Lineage mapping of FGF10-expressing cells demonstrates that at E11.5 they are capable of generating several mesenchymal cell types, including airway smooth muscle (ASM), vascular smooth muscle (VSM) and interstitial fibroblasts. However, by E15.5 they become committed to generating distal alveolar interstitial fibroblasts65,66.
Lamellar body.
A secretory lysosomal-related organelle in the alveolar type 2 cells that stores lung surfactant phospholipids and proteins.
As lung development proceeds, the ASM and VSM differentiate to surround the proximal airways and major blood vessels. ASM and VSM differentiation is controlled by endodermal expression of WNT7b and SHH in conjunction with an autocrine WNT2 sig- nal61,67,68 (Fig. 3c). Loss of WNT signalling in the lung mesenchyme results in impaired ASM differentiation69, whereas loss of lung endoderm-derived WNT-ligand secretion results in the loss of tracheal cartilage formation and defective vascular development70,71. Recent lineage tracing has implicated cells that respond to WNT and SHH but do not express FGF10 as primary ASM progenitors, showing that these cells are a different population from the one present in the distal tip mes- enchyme66. As the ASM enwraps the branching tips and stalk of the primordial airways, it has been suggested that localized ASM emergence regulates bifurcation points during branching morphogenesis72. However, in the absence of ASM, proximal-distal patterning of the epithelium and branching morphogenesis appear to proceed relatively normally73, indicating that other mechanisms for branching are at play.
Primordial lung mesenchyme also contributes to the establishment of the pulmonary vascular network, with cardiopulmonary progenitors giving rise to endothelial cells within the large blood vessels of the lungs57. Of note, the distal capillary plexus arises from a VE-cadherin- positive endothelial cell population that exists in the lateral mesoderm and grows into the lung very early in development. How these two different endothelial cells intermix to form the complex pulmonary vascular network is unclear. However, SHH signalling appears to have an important role as Shh−/− mouse mutants have defects in the vascular connection between the outflow tract of the heart and the pulmonary vasculature57.
Cellular maturation during lung alveologenesis
The final but pivotal period for development of the lung occurs primarily during postnatal life. This phase, termed ‘alveologenesis’, proceeds rapidly in mice, peaking within postnatal days 7–10 with a full resolution into a mature organ by 4–6 weeks (postnatal days 30–42) after birth. In humans, lung alveologenesis begins at late gestation (36 weeks of pregnancy) and persists through at least the first decade of life74,75. Alveologenesis involves extensive cellular and tissue remodelling that results in the creation of a large externalized gas exchange surface area76. These remodelling processes include a final burst of AT2 cell proliferation, an increase in pulmonary surfactant production, extensive AT1 cell flattening and considerable mesenchymal differentiation (Fig. 3d,e).
In the final stages of embryonic development, the alveolar epithelial cells begin to exhibit some of their hallmark cellular and morphological characteristics, including extensive flattening of AT1 cells and expression of pulmonary surfactant proteins and lamellar body formation in AT2 cells. While expansion of AT1 cell number ceases within the first few days after birth in the mouse, AT1 cells progressively expand their coverage of the alveolar surface area. AT1 cell flattening marks the formation of the primitive air saccules in the distal tip of the branched airways starting between E17.5 and E18.5 in the mouse. This is controlled, in part, by epigenetic factors such as HDAC3-dependent repression of the microRNA miR17–92 cluster, which results in increased TGFβ signalling to promote AT1 cell flattening77,78. Correlating with their important cellular alignment with the pulmonary vascular capillary plexus, AT1 cells appear to be an important source of vascular endothelial growth factor A (VEGFA)76. However, there is paucity of experimental data regarding which signals are necessary and sufficient to cause the vascular endothelial plexus to integrate closely with AT1 cells. Some insight into the molecular regulation of this process may be gleaned from findings showing that mutations in FOXF1 lead to alveolar capillary dysplasia, characterized by the abnormal location of the capillaries in the central portion of the alveolar septa79. Furthermore, in mice, endothelial cell-specific knockout of Foxf1 impaired the formation of embryonic vasculature, including the pulmonary vascular plexus, which was accompanied by a decrease in vascular endothelial growth factor receptor signalling activity80. In the gastrointestinal system Foxf1 has been shown to be a direct target of SHH signalling through the binding of GLI2, one of the downstream transcription factors of the SHH pathway, to the Foxf1 promoter81. Along with the role of SHH in regulating the early connection between the pulmonary vasculature and the outflow tract of the heart (see earlier), these data suggest that SHH is critical at multiple stages of lung development by coordinating formation of the blood-gas exchange niche.
During alveologenesis, AT2 cells produce the necessary components of the surfactant system as well as proliferate and increase in number. There is a dramatic increase in surfactant protein and lipid production starting at approximately E17.5 to prepare the lungs for postnatal respiration. Defects in this process can lead to immediate respiratory distress at birth and death. With use of a reporter mouse line for the WNT-target gene Axin2, it was shown that there is an increase in WNT responsiveness in the AT2 cell lineage after birth: at postnatal day 4 nearly half of all AT2 cells were shown to respond to WNT (AT2Axin2 cells)4. Stimulation of the WNT pathway increased AT2 cell proliferation and number, whereas loss of β-catenin resulted in aberrant differentiation of AT2Axin2 cells into AT1 cells and increase in AT1 cell number at the expense of AT2 cells4. These observations indicate that WNT signalling balances cell differentiation in the early postnatal lung, allowing production of AT2 cells required for alveolar maturation and homeostasis (Fig. 3e).
Defects in the development and proper formation of the lung alveoli lead to severe disease in humans, including bronchopulmonary dysplasia (BPD) (Supplementary Table 1). BPD can be caused by many different types of insult, including premature birth, and is typically treated with medications that increase airflow or reduce oedema and inflammation but also may require surfactant replacement or mechanical ventilation. Some common respiratory infections can also lead to BPD. Even if the immediate symptoms of BPD are alleviated, patients may exhibit long-term consequences, including respiratory insufficiency later in life. These observations suggest that the alveologenesis stage of lung development can be perturbed and the plasticity between AT1 and AT2 cell fate may be exploited to treat BPD and repair epithelial injury.
Apart from the specification and maturation of AT1 and AT2 cells, alveologenesis critically depends on the concerted remodelling of the pulmonary mesenchyme, which is required for alveolar maturation. The onset of alveologenesis triggers the appearance of a population of mesenchymal cells expressing elastin and alpha smooth muscle actin (ACTA2), termed ‘secondary crest myofibroblasts’ (SCMFs). Primitive alveoli are divided by primary septae, which are tissue ridges consisting of mesenchymal and endothelial cells lined with AT1 cells. Soon after birth in mice, the SCMFs are localized along these septal ridges. SCMFs help divide the individual alveoli and their activity promotes the formation of secondary septae, which divide the alveoli further and increase the overall surface area82. Genetic disruption of pathways critical for SCMF formation results in alveolar simplification, marked by a characteristic loss of secondary septae. Current models suggest that the extensive deposition of the extracellular protein elastin by mesenchymal cells and myosin-dependent force generation by SCMFs are responsible for producing secondary septae from the bulb-like primitive alveoli (Fig. 3e). The result of this remodelling is an abundance of secondary septae throughout the alveolus that are lined by AT1 epithelial cells and intermixed with AT2 cells.
Several studies have pointed to an important role for platelet-derived growth factor (PDGF) ligands expressed by the alveolar epithelium in promoting SCMF develop- ment83–86 (Fig. 3e). Global inactivation of Pdgfa in mice results in simplified alveoli, as indicated by a loss of alveolar secondary septae83. As shown in mice, during alveologenesis PDGF receptor-a (encoded by Pdgfra) is expressed in a subset of lung mesenchyme, including SCMF cells, and inactivation of this receptor or its ligand broadly in the mesenchyme and epithelium, respectively, resulted in simplified alveoli with perturbed SCMF formation82,84. Together, these data indicate that PDGF signalling is required for alveolarization owing to, at least in part, its role in promoting SCMF maturation. Additionally, lineage tracing studies have demonstrated that SCMF fate is dependent on epithelial cell-derived SHH87. As discussed earlier, SHH-responsive mesenchymal cells develop near the airways and major blood vessels, contributing to ASM, VSM and adjacent mes- enchyme66,87. However, during alveologenesis SHH- responsive cells were the primary contributors to SCMF fate87. Consistent with these observations, SHH ligand expression is enriched in AT1 epithelial cells in the postnatal lung88. Despite these insights, mechanistic data on how the cellular remodelling processes drive alveolo- genesis are still lacking, and further studies are needed to define the onset of mesenchymal cell heterogeneity as well as the critical signalling pathways involved in the communication between these cells and the adjacent epithelium and endothelium.
Homeostasis and regeneration
Compared with other barrier tissues exposed to the external environment with dedicated stem cells, such as the gastrointestinal tract and the skin, the airway and alveolar regions of the lung are relatively quiescent, exhibiting low cellular turnover and proliferation at homeostasis. However, upon tissue damage, several types of stem and/or progenitor cells are engaged that have the capacity to self-renew and differentiate into multiple cell lineages (Figs 4,5). Our understanding of the behaviour of these different stem/progenitor cell lineages is informed by the different injury models and specific lineage tracing techniques (Box 1). Finally, there is emerging evidence of the importance of the stromal cells that support the epithelial niche, including both mesenchymal and endothelial cells, in the regeneration of the respiratory system.
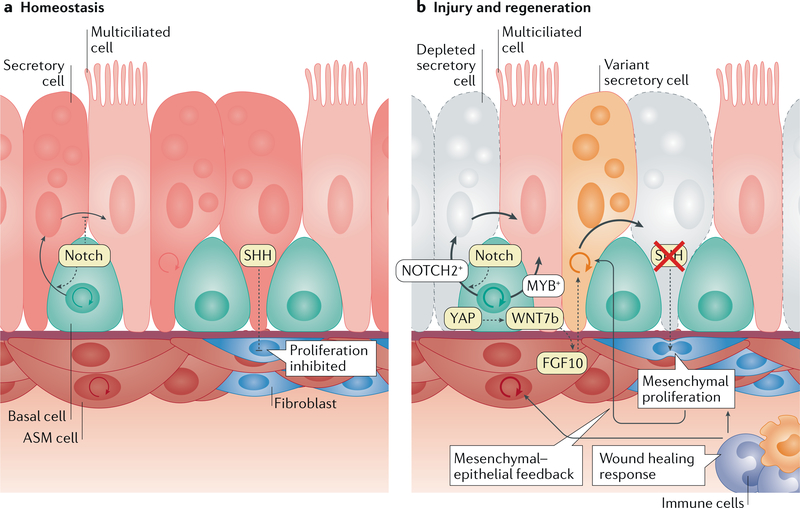
Fig. 4 |. The airway niche turnover at homeostasis and in response to injury.
a | The turnover of the airway epithelium is low during homeostasis. Nevertheless, basal cells continuously self-renew at a low rate and preferentially differentiate towards the secretory cell lineage over the multiciliated cell lineage, which is regulated by Notch signalling within the basal cell population. Secretory cells have the capability to trans differentiate into multiciliated cells, which is inhibited by Notch signalling. The surrounding airway smooth muscle (ASM) tissue harbours LGR6-expressing cells that exhibit some homeostatic turnover. The epithelium secretes sonic hedgehog (SHH) to actively suppress the proliferation of peribronchiolar mesenchyme. b | Airway injury stimulates multiple cell types to regenerate the tissue. In response to tissue injury, basal cells are able to give rise to both secretory and multiciliated cells, likely reflecting the heterogeneity in the lineage (NOTCH2-expressing versus MYB-expressing subpopulations). Loss of SHH signalling between the epithelium and mesenchyme also relieves the block on mesenchymal cell proliferation. This loss of mesenchymal quiescence was shown to feedback on the epithelium and increase epithelial cell proliferation. In naphthalene injury models that mainly kill secretory cells (greyed-out cells) the spared or uninjured secretory cells (also referred to as ‘variant secretory cells’) proliferate and repopulate the lost secretory cells. Downregulation of Hippo signalling after airway injury causes YAP- mediated increase in epithelial WNT7b expression, which promotes FGF10 expression in the adjoining ASM. ASM-derived FGF10 then stimulates secretory cell proliferation and differentiation. Epithelial cell death induced by injury can also trigger a wound healing response that leads to the recruitment of inflammatory cells and subsequent expansion of mesenchymal cell populations.
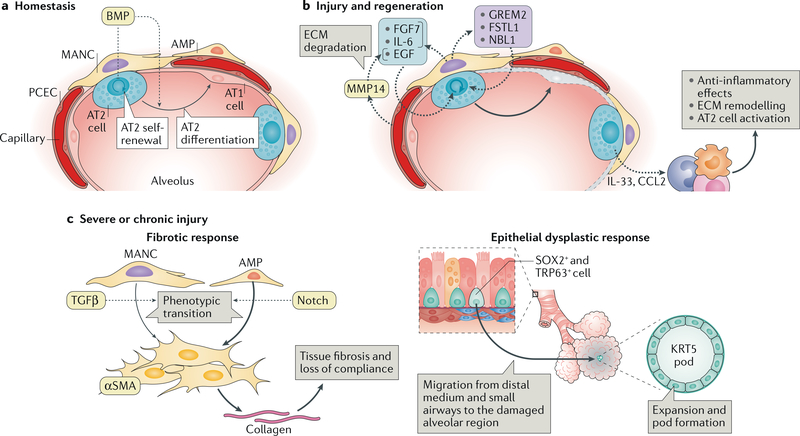
Fig. 5 |. Response of the alveolar niche to tissue injury.
a | The alveolar niche has two types of epithelial cell: alveolar type 1 (AT1) cells and alveolar type 2 (AT2) cells. The latter includes a WNT-responsive AT2 subset of cells that have been shown to serve as alveolar epithelial progenitors. At homeostasis the alveolar epithelial progenitors self-renew and differentiate into AT1 cells. These processes are regulated by bone morphogenetic protein (BMP) signalling, which restricts AT2 self-renewal, favouring AT1 differentiation. The rates of this homeostatic cell turnover are generally low. The stromal population comprises platelet-derived growth factor receptor- a-expressing fibroblasts, such as mesenchymal alveolar niche cells (MANCs), which are situated next to AT2 cells and Axin2-positive myofibrogenic precursors (AMPs), which are sparsely located throughout the alveoli and near blood vessels. b | In response to tissue injury, the alveolar niche upregulates ligands that promote AT2 cell proliferation and differentiation into AT1 cells (which are predominantly lost in response to insults; indicated in grey). Pulmonary capillary endothelial cells (PCECs) have been shown to respond to injury by upregulating matrix metalloproteinase 14 (MMP14), which by digesting the extracellular matrix (ECM) releases epidermal growth factor (EGF)-like ligands that stimulate epithelial cell growth. Injury- activated MANCs promote AT2 proliferation and self-renewal by producing FGF7 and IL-6 as well as BMP antagonists such as NBL1, GREM2 and FSTL1. AT2 cell-produced chemokines and cytokines, including CCL2 and IL-33, recruit monocytes (which differentiate into macrophages) and innate lymphoid cells (ILCs). Innate lymphoid cells support epithelial regeneration in part by dampening the acute inflammatory responses and by modulating the polarization of macrophages to the alternative (M2) lineage, which promotes AT2 cell proliferation likely via mechanisms involving ECM remodelling. c | Severe or chronic lung injury can result in dysplastic responses — fibrosis (left) and generation of abnormal cell clusters (right) — that if left unchecked will ultimately impair lung compliance and gas exchange. The fibrotic response is derived from fibroblasts — mostly AMPs and to a lesser extent MANCs — that proliferate on injury and transition into collagen-producing, alpha smooth muscle actin (αSMA)-positive myofibroblasts that deposit excessive amounts of ECM, which severely impairs lung compliance. A crucial signal for this myofibrogenic transition is provided by transforming growth factor-β(TGFβ), which is derived from the surrounding ECM. In the setting of severe alveolar epithelial cell destruction, such as that triggered by influenza infection, distally located SOX2-positive and TRP63-positive basal cells migrate out of the distal medium and/or small airways to regions of injury in the alveoli and expand as KRT5-expressing epithelial-like pods that radiate out of the tissue. The formation of these pods responds to hypoxia and is thought to be an epithelial cell-derived wound healing response that maintains the tissue barrier but ultimately does not generate functional alveolar epithelium.
The airway niche and its response to injury
The murine trachea and conducting airways harbour two distinct progenitor cell populations, BSCs for the trachea and proximal airways and secretory (or club) cells in the bronchiolar airways (Figs 2,4). In the trachea, BSCs are marked by expression of TRP63 and keratin 5 (KRT5), and have the ability to self-renew as well as generate multiciliated and secretory cells. In homeostasis BSC proliferation is limited, but on exposure to chemical injury such as from sulfur dioxide, which destroys the luminal tracheal epithelia, the remaining BSCs increase their proliferation rates: they increase in number within the first 24 h and subsequently differentiate into multiciliated and secretory cells. In the mouse lower respiratory system, BSCs are found exclusively in the trachea and main stem bronchi. However, in humans, TRP63-KRT5 double-positive BSCs are found throughout most of the airway tree and therefore likely have a more pronounced role in maintaining and repairing the airway epithelium.
Naphthalene.
An aromatic hydrocarbon that is metabolized to a toxic form by the airway epithelial club cell lineage in cells that express the enzyme CYP2F2. it is used to model club cell ablation.
Some airway injury models are tailored to kill specific cell types. For example, naphthalene treatment in mice results in the selective depletion of secretory cells, while multiciliated and neuroendocrine cells are mostly spared. In this model, rare surviving secretory cells that lack expression of the cytochrome P450 CYP2F2, which is required for processing naphthalene — referred to as ‘variant secretory cells’ — are capable of re-entering the cell cycle and repopulating the airways within several weeks89,90. Whether secretory cells within the human lung also have similar regenerative capacity is unknown, but given the presence of BSCs throughout most if not all of the airway tree, it is unclear whether this function is required in any biologically relevant context.
Hippo signalling.
A signalling pathway that controls nuclear translocation of the transcription co-activators YAP and TAZ and activation of their target genes that function as a key mechanoresponsive pathway.
The mechanisms that regulate the progenitor activity of basal and secretory cells are under active investigation. Homeostatic turnover of BSCs is regulated in part through steady-state signalling via FGF receptor 2 (FGFR2) and BMP91,92. Multiple studies have provided evidence that Notch signalling among BSCs regulates their differentiation favouring the acquisition of a secretory cell (club cell) fate (Fig. 4a). Accordingly, loss of Notch signalling leads to a shunting of BSCs into the multiciliated fate93,94. Antibody-mediated inhibition of the Notch ligands jagged 1 and jagged 2 can also cause trans differentiation of secretory cells into multiciliated epithelial cells95. Apart from Notch, the homeostatic patterning of the distal airways is also controlled by additional signalling inputs that are known to impact cell proliferation and growth, including FGF10, p53 and Hippo signalling2,96,97. Recently, by combining lineage-tracing and single-cell RNA sequencing (Box 1), it was demonstrated that the stem cell abilities of BSCs are different in homeostatic conditions versus regeneration after acute injury18. During homeostasis, BSCs differentiate into secretory cell progeny, which in a subsequent trans-differentiation step give rise to the multiciliated lineage18,98. However, after acute injury, BSCs can differentiate directly into either secretory or multiciliated lineages93,99. It is worth noting that studies point towards heterogeneity in the BSC population, with cells expressing NOTCH2 destined to generate the secretory lineage and cells expressing the transcriptional activator MYB giving rise to the multiciliated lineage93. This heterogeneity likely makes possible the observed flexibility of BSC differentiation potential in response to tissue damage.
The mesenchymal niche surrounding the airways has a critical role in both maintaining homeostasis and regeneration of airway epithelium. A recent study demonstrated that SHH produced in the adult lung epithelium at homeostasis maintains adjacent mesenchymal quiescence100. Accordingly, epithelial-specific deletion of SHH resulted in the loss of quiescence in the mesenchymal cell lineage (mostly fibroblasts). Notably, this loss of mesenchymal quiescence was accompanied by an increase in epithelial cell proliferation, indicating a feedback mechanism from the mesenchyme to the epithelium. Overall, these data suggest that perturbation of epithelial-mesenchymal crosstalk in the adult lung can disrupt the normal quiescent state of both compartments even in the absence of injury. After naphthalene injury in mouse adult lung, the peribronchial ASM cells induce the expression of FGF10, which in turn promotes secretory cell proliferation2. In the trachea, BSCs regulate this FGF10 secretion in a Hippo-YAP-mediated manner: downregulation of Hippo signalling after airway injury causes an increase in epithelial WNT7b expression, which acts in a paracrine fashion to increase FGF10 expression in the adjoining ASM101. These ASM cells were shown to express the marker Lgr6, and when these Lgr6-positive cells were ablated, secretory cell proliferation and re-epithelialization of the airway after naphthalene treatment were markedly impaired1. There is also evidence that in mice, injury-induced FGF10 allows the basal cell population to be extended to non-cartilaginous airways to promote regeneration of these regions35,101. These data substantiate the existence of an intricate crosstalk between epithelial and mesenchymal cells in airway regeneration (Fig. 4b). In some lung injury models, exposure of the epithelial basement membrane resulting from epithelial cell death can trigger a wound healing response that is associated with the recruitment of inflammatory cells and subsequent expansion of mesenchymal cell populations, such as SHH-responsive and WNT-responsive fibroblasts.
Basement membrane.
A thin layer of extracellular matrix that provides support and separates epithelia from the surrounding tissue.
Bleomycin.
A chemotherapeutic drug that elicits extensive DNA damage. it is used to model lung injury that is associated with severe damage to the epithelium accompanied by stimulation of a fibrotic response from the mesenchyme.
Diphtheria toxin.
An exotoxin derived from bacteria that when expressed in a cell-specific manner leads to the selective ablation of that cell type.
The alveolar niche and its response to injury
As the functional unit of the lung, the alveolar compartment and its associated niche are indispensable for terrestrial life. As described already, the development of the alveolar compartment requires input from multiple cell types. The thin diffusible gas exchange interface, which is primarily composed of AT1 cells, pulmonary capillary endothelial cells (PCECs) and mesenchymal cells, appears extremely fragile at first glance. However, the alveolar niche responds robustly to injury and can regenerate in its entirety within a few weeks. We are now beginning to understand the cellular and molecular mechanisms that stimulate the varied epithelial, mesenchymal and endothelial cell responses to injury.
Within the alveolar epithelium, lineage tracing studies have shown that the AT2 cell population self-renews and differentiates into AT1 cells3,102. This is true both in homeostasis and after injury, including lung regrowth after pneumonectomy as well as bleomycin- induced lung injury3. Similarly, specific ablation of AT2 cells via AT2-directed diphtheria toxin expression revealed that surviving AT2 cells can re-enter the cell cycle to repopulate lost AT2 cells3. A subset of AT1 cells also appear to be able to convert into the AT2 lineage after pneumonectomy in mice103. These studies suggest that AT1 and AT2 cells both retain some level of plasticity that could be leveraged to promote a regenerative response.
Two groups have reported that WNT-responsive AT2 cells (AT2Axin2 cells), which are important for alveolo- genesis as discussed earlier, are also present in the mature adult lung7,104. Influenza-mediated lung injury demonstrated that these AT2Axin2 cells proliferated in areas surrounding the most damaged regions of the lungs. They were also capable of generating a considerable number of AT1 cells after injury (Fig. 5a,b). Hence, these AT2Axin2 cells have been named ‘alveolar epithelial progenitors’ (AEPs)104. Importantly, the AEP subset accounted for almost all of the AT2 cell proliferation observed after influenza injury, indicating that it is the primary cell lineage contributing to alveolar repair after severe injury. On the basis of transcriptome and open chromatin architecture data, AEPs exhibit a unique transcriptional and epigenetic state compared with the AT2 population as a whole104. Additional data indicate that mesenchymally derived FGF7 and FGF10 as well as WNT signalling regulate AEP self-renewal and differentiation into mature AT2 and AT1 cells7,104 (Fig. 5b).
In recent years, new murine reporter lines and Cre drivers have markedly aided the effort to resolve the identity of stromal cells residing in the alveolar compartment. Alveolar interstitial fibroblasts, identified with use of a nuclear-GFP reporter expressed from the endogenous Pdgfra promoter, were found juxtaposed to AT2 cells in the mouse lung3,8. Historically, these alveolar mesenchymal cells have been characterized as lipid droplet-containing cells called lipofibro- blasts105–107. The Pdgfra-expressing mesenchyme was also shown to be important for AT2 growth in organoid assays (BOX 2) and has been referred to as PDGFRa-positive type 2-associated stromal cells (or TASCs)3.
Box 2 |. Lung organoids.
Organotypic cultures (organoids) are derived from primary tissue-resident progenitor cells, which are grown in a three-dimensional matrix allowing the cells to expand and differentiate into multiple cell types observed in the native tissues. The tracheal and lung epithelium rely on the progenitor cell activity of basal cells, secretory cells and alveolar type 2 (AT2) cell subsets such as alveolar epithelial progenitors to restore homeostasis after injury. Using lineage-specific reporter mice or specific antibody cocktails for human tissue, purified basal, secretory and AT2 cells can be isolated and propagated for several weeks and passages in cell culture3,98. For organoid generation, these purified stem/progenitor cells are embedded in Matrigel, where they self-replicate and differentiate into other airway or alveolar lineages. AT2 cells must be combined with mesenchymal support cells (such as platelet-derived growth factor receptor-a-positive fibroblasts) to proliferate and establish organoids. This is in contrast to basal cells, which can form spheroids in conditions that do not require mesenchymal cell support99. The actual structure of these organoids bears some resemblance to in vivo tissue organization, and certain cellular properties and functions can be recapitulated in these models. For instance, murine AT2 cells will self-renew and differentiate into flattened AT1 cells and the mesenchymal support cells will embed themselves within these organoids.
Lung organoids can be used for testing specific ligands and signalling regulators as well as the different niche-supporting cell types for their roles in modulating proliferation and differentiation of epithelial progenitor cells92. Future work should focus on further increasing the cellular complexity of organoids by incorporating other relevant cell types, such as lung macrophages and endothelial cells, to fully recapitulate aspects of the alveolar unit as it appears in vivo112. Whether the complexity and functionality observed in mouse alveolar organoids can be fully reproduced using primary human cell organoids remains to be determined. Currently, organoids using cells from diseased human tissue, such as pulmonary fibrosis or lung cancer, can be used to interrogate how diseased cells signal to drive the pathology. These disease-oriented organoids are promising platforms for high-throughput drug screening for lung disease.
More recent studies from multiple groups using a combination of lineage tracing, population-based RNA sequencing and single-cell RNA sequencing (Box 1) have resolved additional heterogeneity within the mesenchymal cell types in the lung. The Pdgfra-expressing mesenchyme is now demonstrated to comprise two distinct populations: a WNT-responsive (Axin2-reporter positive; referred to as Axin2-Pα) subpopulation and a Wnt2-expressing (referred to as Wnt2-Pα) subpopulation8. The Axin2-Pa cells are enriched with transcripts coding for regulators of extracellular matrix (ECM) production and for signalling molecules, including FGF7, IL-6 and BMP antagonists such as NBL1 and GREM2 (BMP — which derives from multiple cellular sources, including AT2 cells and mesenchymal cells6,8 — is known to inhibit self-renewal of AT2 cells at the expense of their differentiation towards the AT1 lineage). In addition, TASCs were shown to upregulate the BMP antagonist FSTL1 in response to pneumonectomy6.
Importantly, AT2 cells express the cognate receptors for FGF7 and IL-6. When tested in organoid cultures (Box 2), the Axin2-Pa cells but not the Wnt2-Pα cells preferentially promoted AT2 cell proliferation and differentiation into AT1 cells. Moreover, exogenous FGF7, IL-6 and GREM2 all promoted organoid formation from AT2 cells. On the basis of their unique functional phenotype and the close proximity to AT2 cells in situ, the Axin2-Pa mesenchymal cells were designated as mesenchymal alveolar niche cells (MANCs)8. In addition, it was shown that alveolar organoid formation from a mixed epithelial cell population composed of airway and alveolar cells was promoted by a mesenchymal cell population expressing LGR5 (reF.1). It is still unclear whether these alveolar LGR5-positive cells have any overlap with the PDGFRa-expressing mesenchymal subsets.
Matrigel.
A gelatinous mixture of extracellular protein matrix, including laminin, that is used for three-dimensional organoid cell cultures.
Damage- and pathogen-associated molecular patterns.
Cellular and microbial by-products that stimulate an inflammatory response.
Innate lymphoid cells.
A group of innate immune cells that do not express B or T cell receptors but that exhibit functions analogous to those of helper T cells. These cells are mainly found at mucosal surfaces, such as in the lungs, where they act as critical sentinel cells responding to pathogens and allergens.
M2-polarized macrophages.
Macrophage subpopulation that is considered to be more anti-inflammatory in activity and that is typically associated with wound healing responses.
As discussed earlier, AT1 cells remain in close association with endothelial cells of the pulmonary capillary plexus. Following lung injury, this endothelial-AT1 cell interface needs to be re-established for proper respiration. While AT2 cells proliferate and differentiate into AT1 cells after pneumonectomy, proliferation in PCECs is also markedly increased108. Ablation of the known PCEC mitogen receptors VEGFR2 and FGFR1 in endothelial cells resulted in a loss of PCEC proliferation as expected, but also led to concomitant reduction in AT2 cell proliferation108. PCECs were shown to produce matrix metalloproteinase 14, which can liberate growth factors that stimulate AT2 cells from the alveolar ECM108. Further work in ex vivo lung epithelial organoid co-cultures revealed that fetal lung endothelial cells could support the preferential outgrowth of distal epithelial organoids (Box 2), which is dependent on endothelial cell-produced thrombospondin 1 (reF.109). Conversely, in chronic injury such as caused by repetitive bleomycin instillation, endothelium has been shown to activate Notch signalling in the surrounding mesenchyme, resulting in increased fibrogenic potential and a deleterious effect on lung regeneration110. Because endothelium is a major cellular component in the alveolar niche, further work is necessary to decipher the heterogeneity of endothelial cells and their specific actions in an injury response.
The regenerative potential in the lung is also greatly influenced by inflammatory responses after tissue injury. The necrotic cell death or viral propagation observed after acute injury will release damage- and pathogen- associated molecular patterns (DAMPs and PAMPs), which stimulate resident immune cell types such as type 2 innate lymphoid cells (ILCs) and alveolar macrophages. In injury models such as bleomycin-induced damage or influenza infection, the inflammatory response is profound, involving multiple immune cell types, and is generally considered a necessary process to restore homeostasis by removal of cellular and microbial by-products. After influenza infection, epithelial cells produce IL-33, which then stimulates ILC-derived expression of an epidermal growth factor family member, amphiregulin, which may act by directly stimulating epithelial cells after injury and without which pulmonary function — measured as blood oxygen saturation — is severely diminished111. Furthermore, tissue damage, such as caused by pneumonectomy, induces recruitment of monocytes to the alveolus, which depends on the secretion of the AT2-derived chemokine CCL2. In the alveolus, monocytes respond to type 2 ILC-derived IL-13 and give rise to M2-polarized macrophages, and this axis supports AT2 proliferation likely via mechanisms involving macrophage-mediated ECM remodelling112. The response mediated by macrophages also has a direct effect on AT2 cell proliferation as well as growth in ex vivo organoid models. Together these studies underscore the importance of epithelial-immune cell crosstalk to coordinate a regenerative response in the lung.
Pathological tissue remodelling
Apart from physiological responses aimed at tissue regeneration, injury can also lead to a dysplastic cellular response in the mesenchyme and epithelium. Irreversible lung remodelling provoked by chronic injury and unchecked inflammation is considered to be a main driver of lung disease (Supplementary Table 1).
One notable pathological tissue remodelling occurring in the injured lung is tissue fibrosis. Fibrosis is marked by the accumulation of myofibroblasts (marked by the expression of ACTA2), which produce and remodel ECM, with consequent increases in collagen deposition. In the lung, unresolved fibrosis causes tissue scarring, thereby impairing lung compliance. One form of lung fibrosis in humans is idiopathic pulmonary fibrosis (IPF), which is a progressive disease of unknown cause. Owing to the lack of effective treatment, IPF has a high mortality rate.
Bleomycin-induced lung injury in mice has been used to evoke a transient fibrotic response that has some resemblance to the expansive fibrosis observed in IPF. However, it is important to note that most of the effects of the bleomycin-induced response resolve after a few months instead of progressing towards pathological fibrosis as seen in IPF. It is becoming increasingly apparent that epithelial dysfunction can result in a profound fibrotic response in the lung. As mentioned earlier, AT2 cells produce pulmonary surfactant, including surfactant lipoproteins such as the hydrophobic pulmonary surfactant-associated protein C (SP-C; encoded by SFTPC), which maintain the surface properties of surfactant. Expression of the Sftpc variant, SftpcI73T — which results in impaired intracellular trafficking of the SP-C pro-protein and overall reduction in mature SP-C in the surfactant — leads to epithelial dysfunction and an overt pulmonary fibrosis phenotype in mice113. This paradigm has also been demonstrated in epithelial cell- specific telomerase-deficient mouse models, which have increased susceptibility to bleomycin-induced fibrosis. In line with this, it has been observed that cells derived from patients with IPF have shorter telomeres114–116.
Lineage tracing in mice indicates that in the course of the fibrotic response in the lung the resident mesenchymal cells differentiate into myofibroblasts, and much work has been devoted to identifying the signalling pathways that trigger this switch102. The pathological myofibroblasts were traced back to SHH-responsive cells, which seem to originate from a perivascular mesenchymal cell type positive for PDGFRp117. WNT signalling also has a role in the fibrotic response, as WNT-responsive mesenchymal cells, marked by Axin2 expression and PDGFRp — referred to as ‘Axin2-positive myofibrogenic precursors’ or AMPs — generate most of the myofibroblasts in the lung after injury8 (Fig. 5c). In addition to Axin2-positive myofibrogenic precursors, PDGFRa-expressing mesenchymal cells, such as MANCs also proliferate and generate myofibroblasts, but their contribution to this lineage appears to be less pro- nounced118. It has been shown that PCECs also respond to injury by upregulating the expression of the Notch ligand jagged 1, thereby stimulating Notch signalling in perivascular fibroblasts to induce myofibroblast differentiation110. A critical regulator of differentiation of fibroblasts is TGFβ, which is activated by being released from its ECM-bound, latent form in the setting of injury. Activation of TGFβ is mediated in part by adjacent cells expressing integrin avβ6, and mice deficient in integrin avp6 fail to develop fibrosis in the bleomycin injury model119. In mice these fibrogenic responses are transient: the myofibroblasts are cleared and the fibrous tissue eventually resolves within 3 months of the initial injury120. By studying this resolution phase, it was found that nuclear receptor peroxisome proliferator-activated receptor-Y may be an exploitable target to limit the myogenic differentiation of mesenchymal cells with the use of peroxisome proliferator-activated receptor-Y agonists121.
While such a deleterious pathological fibrotic response mediated by mesenchymal cells has been appreciated in the lung for many decades, it has recently become clear that aberrant responses of the epithelium due to acute injury can also lead to dysplastic and dysfunctional repair. Acute influenza injury can cause a dramatic expansion of KRT5-expressing cells in regions of severe damage122–124. These KRT5-positive cells express other markers of BSCs, including TRP63 (see also earlier), and form clusters (pods) that appear to radiate out from the conducting airways and alveolar regions, thereby causing tissue keratinization and disturbing tissue architecture. Lineage tracing experiments revealed that these pods originate from a distal basal cell subset that expresses SOX2 and migrates from the more proximal airways to regions of massive epithelial destruction125 (Fig. 5c). Although early studies suggested that these KRT5-expressing cells may act as progenitors supporting alveolar epithelial regeneration, recent work using more accurate cell lineage tracing techniques has shown that these cells do not generate normal resident epithelial cells. Such KRT5-positive cell pods can also be found in injured and diseased human lungs, where they appear to persist for many years126,127. Mechanistically, formation of KRT5-positive pods was linked to hypoxia: severe lung injury leads to a loss of vasculature, which creates a hypoxic niche that supports the expansion of these cells128. Such an epithelial response could be interpreted as an emergency repair system to prevent loss of tissue integrity after severe injury since the normal regenerative effects may take longer to occur. In other words, the keratinized epithelium found in the lung after dramatic acute injury could represent a dysplastic response to occupy the large voids created by tissue loss. Such a dysplastic response may cause long-term respiratory dysfunction if keratinized regions are not replaced with normal alveolar and airway tissue. Future studies to characterize the KRT5-positive pods and understand their formation may direct efforts on how to suppress or reverse this response. One possibility is to therapeutically improve endothelial cell survival to prevent hypoxia.
Conclusions and perspectives
Because of the inherent architectural complexity and cellular heterogeneity, the respiratory system, and the lungs in particular, have been difficult to study. Importantly, single-cell techniques and imaging strategies that have emerged in recent years (Boxes 1–3) have greatly aided in expanding our understanding of this complex system on a cellular level. Findings within the last few years have revealed extensive cellular heterogeneity and have begun to provide a new road map of interactions between the different cell types and how they contribute to lung development and maintenance. Emerging single-cell technologies combined with computer algorithms that allow visualization of thousands of cellular transcriptomes will lead to many more exciting insights soon, including the ability to probe cellular heterogeneity as well as progenitor cell activity in human lung tissue (Box 3). This information will be highly valuable to gain a better understanding of complex diseases that are a consequence of a transcriptionally heterogeneous cellular milieu of the respiratory system. As these newer techniques and experimental designs bear fruit, our knowledge of this impressively complex organ will be further refined, opening up possibilities for efficient treatments of paediatric and adult lung diseases (Supplementary Table 1).
Box 3 |. Modelling the human lung.
For decades it has been known that the human respiratory system has unique structural differences from the mouse respiratory system. These differences are likely due to the physiological requirements to conduct gases to a vastly larger surface area in the human lungs than in the mouse lungs. It is hypothesized that these differences may underlie the pathophysiology of diseases such as chronic obstructive pulmonary disease, pulmonary hypertension and acute respiratory distress syndrome (Supplementary Table 1), none of which has a mouse model that captures the heterogeneous origin and phenotype of these conditions in the human lung. one notable difference in the architecture of the human distal airways is the presence of respiratory bronchioles. These structures do not exist in the rodent airways137,138. Respiratory bronchioles form the direct connection between the conducting portion of the respiratory system and the gas exchange alveolar region. The cellular constituents in the respiratory bronchioles remain poorly understood, but recent evidence has shown that this portion of the airway system is particularly susceptible to damage associated with chronic lung diseases such as chronic obstructive pulmonary disease139–141. While rodents are unlikely to provide an appropriate animal model to study structures such as respiratory bronchioles, other model animals, including ferrets, appear to contain these structures, providing a potential system to explore their development and function142.
Techniques such as RNA sequencing (both population-based and single-cell-based RNA sequencing; see also Box 1), generation of induced pluripotent stem cell (iPSC) models and the use of organoid cell culture (see also Box 2) will allow rigorous screening and validation of potential new cell types in the human respiratory tract.
For example, population RNA sequencing of mouse alveolar epithelial progenitors was used to identify cell-surface markers that were then used to isolate human alveolar epithelial progenitors, which displayed an enrichment for WNT responsiveness and unique capacity to form alveolar organoids104. Identification of new, specific markers for the isolation of distinct subsets of stromal cells will allow researchers to further interrogate the complexity of intercellular interactions using human organoid models.
Building upon the discoveries made from mouse lung development, protocols have now been developed that allow the generation of iPSC-derived NKX2–1-expressing human-specific respiratory tract endoderm cells143,144. Furthermore, the NKX2–1- expressing cells can be differentiated into cells that are phenotypically similar to distal alveolar type 2 cells as well as airway cells143,145. The assays using differentiation of iPSCs will be an invaluable tool for modelling human genetic disorders, testing pharmacologic agents and modelling niche environments. Together, these model systems will be powerful tools for translating many of the novel findings from mouse experiments to relevant human diseases.
Supplementary Material
Acknowledgements
The authors apologize for the omission of any references due to the space constraints of this review. The authors acknowledge important support from the National Institutes of Health.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Molecular Cell Biology thanks J. Rajagopal, and other, anonymous, reviewer(s), for their contribution to the peer review of this work.
Supplementary information
Supplementary information is available for this paper at
References
- 1.Lee JH et al. Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell 170, 1149–1163 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volckaert T et al. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J. Clin. Invest. 121, 4409–4419 (2011). Lee et al. (2014) and Volckaert et al. (2011) demonstrate the requirement of anatomically distinct mesenchymal cell types, such as LGR6- positive airway smooth muscle cells in promoting epithelial recovery after injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkauskas CE et al. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 123, 3025–3036 (2013).This study uses genetic lineage tracing techniques to show that AT2 cells contain a progenitor cell function in the adult lung and can differentiate into AT1 cells.
- 4.Frank DB et al. Emergence of a wave of Wnt signaling that regulates lung alveologenesis by controlling epithelial self-renewal and differentiation. Cell Rep. 17, 2312–2325 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caduff JH, Fischer LC & Burri PH Scanning electron microscope study of the developing microvasculature in the postnatal rat lung. Anat. Rec. 216, 154–164 (1986). [DOI] [PubMed] [Google Scholar]
- 6.Chung MI, Bujnis M, Barkauskas CE, Kobayashi Y & Hogan BLM Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cel proliferation and differentiation. Development 145, dev163014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA & Desai TJ Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 359, 1118–1123 (2018).This study identifies an Axin2-positive AT2 cell in the adult lung with progenitor cell activity that is maintained by a WNT-producing mesenchymal niche cell.
- 8.Zepp JA et al. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell 170, 1134–1148 (2017).This study reveals the cellular and functional complexity within the adult lung mesenchyme using single-cell RNA sequencing methods and identified mesenchymal cell types important for alveolar niche support and for generating deleterious myofibroblasts after injury.
- 9.Que J et al. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc. Natl Acad. Sci. USA 105, 16626–16630 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin Y, Wang F & Ornitz DM Mesothelial- and epithelial-derived FGF9 have distinct functions in the regulation of lung development. Development 138, 3169–3177 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixit R, Ai X & Fine A Derivation of lung mesenchymal lineages from the fetal mesothelium requires hedgehog signaling for mesothelial cell entry. Development 140, 4398–4406 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Gise A et al. Contribution of fetal, but not adult, pulmonary mesothelium to mesenchymal lineages in lung homeostasis and fibrosis. Am. J. Respir. Cell Mol. Biol. 54, 222–230 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrios J et al. Pulmonary neuroendocrine cells secrete GABA to induce goblet cell hyperplasia in primate models. Am. J. Respir. Cell Mol. Biol. 10.1165/rcmb.2018-0179OC (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song H et al. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc. Natl Acad. Sci. USA 109, 17531–17536 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branchfield K et al. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science 351, 707–710 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sui P et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science 360, eaan8546 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plasschaert LW et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560, 377–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montoro DT et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560, 319–324 (2018). Plasschaert et al. and Montoro et al. describe, by means of single-cell RNA sequencing, new epithelial cell lineages within the adult trachea and airways of mice and humans, including the ionocyte, which expresses high levels of CFTR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krasteva G & Kummer W “Tasting” the airway lining fluid. Histochem. Cell Biol. 138, 365–383 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Gerbe F & Jay P Intestinal tuft cells: epithelial sentinels linking luminal cues to the immune system. Mucosal Immunol 9, 1353–1359 (2016). [DOI] [PubMed] [Google Scholar]
- 21.von Moltke J, Ji M, Liang HE & Locksley RM Tuft-cell-derived IL-25 regulates an intestinal ILC2- epithelial response circuit. Nature 529, 221–225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratjen F et al. Cystic fibrosis. Nat. Rev. Dis. Primers 1, 15010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynch TJ et al. Submucosal gland myoepithelial cells are reserve stem cells that can regenerate mouse tracheal epithelium. Cell Stem Cell 22, 653–667 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tata A et al. Myoepithelial cells of submucosal glands can function as reserve stem cells to regenerate airways after injury. Cell Stem Cell 22, 668–683 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goss AM et al. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev. Cell 17, 290–298 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris-Johnson KS, Domyan ET, Vezina CM & Sun X p-Catenin promotes respiratory progenitor identity in mouse foregut. Proc. Natl Acad. Sci. USA 106, 16287–16292 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rankin SA, Gallas AL, Neto A, Gomez-Skarmeta JL & Zorn AM Suppression of Bmp4 signaling by the zinc-finger repressors Osr1 and Osr2 is required for Wnt/beta-catenin-mediated lung specification in Xenopus. Development 139, 3010–3020 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arora R, Metzger RJ & Papaioannou VE Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLOS Genet. 8, e1002866 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellusci S, Grindley J, Emoto H, Itoh N & Hogan BL Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124, 4867–4878 (1997). [DOI] [PubMed] [Google Scholar]
- 30.Ohuchi H et al. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem. Biophys. Res. Commun. 277, 643–649 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Sekine K et al. Fgf10 is essential for limb and lung formation. Nat. Genet. 21, 138–141 (1999). [DOI] [PubMed] [Google Scholar]
- 32.Weaver M, Dunn NR & Hogan BL Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development 127, 2695–2704 (2000).This seminal article reveals the signalling complexity that regulates branching morphogenesis within the developing lung, focusing on two critical signalling pathways: BMP and FGF10.
- 33.Menshykau D, Blanc P, Unal E, Sapin V & Iber D An interplay of geometry and signaling enables robust lung branching morphogenesis. Development 141, 4526–4536 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Nikolic MZ et al. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. eLife 6, e26575 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y et al. Spatial-temporal lineage restrictions of embryonic p63+ progenitors establish distinct stem cell pools in adult airways. Dev. Cell 44, 752–761 (2018).This study describes the early developmental potential of TRP63-positive basal cells in the lung and documents the presence of a small subset of these cells that respond to acute lung injury in the adult.
- 36.Guha A et al. Neuroepithelial body microenvironment is a niche for a distinct subset of Clara-like precursors in the developing airways. Proc. Natl Acad. Sci. USA 109, 12592–12597 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurotani R et al. Role of secretoglobin 3A2 in lung development. Am. J. Respir. Crit. Care Med. 178, 389–398 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rawlins EL, Ostrowski LE, Randell SH & Hogan BL Lung development and repair: contribution of the ciliated lineage. Proc. Natl Acad. Sci. USA 104, 410–417 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herriges M & Morrisey EE Lung development: orchestrating the generation and regeneration of a complex organ. Development 141, 502–513 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrisey EE et al. Molecular determinants of lung development. Ann. Am. Thorac Soc. 10, S12–S16 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrisey EE & Hogan BL Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell 18, 8–23 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Que J, Choi M, Ziel JW, Klingensmith J & Hogan BL Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation 74, 422–437 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Swarr DT & Morrisey EE Lung endoderm morphogenesis: gasping for form and function. Annu. Rev. Cell Dev. Biol. 31, 553–573 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Que J, Luo X, Schwartz RJ & Hogan BL Multiple roles for Sox2 in the developing and adult mouse trachea. Development 136, 1899–1907 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Que J et al. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134, 2521–2531 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tompkins DH et al. Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLOS ONE 4, e8248 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsao PN et al. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 136, 2297–2307 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guseh JS et al. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development 136, 1751–1759 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, Knowles HJ, Hebert JL & Hackett BP Mutation of the mouse hepatocyte nuclear factor/ forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J. Clin. Invest. 102, 1077–1082 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galvis LA et al. Repression of Igf1 expression by Ezh2 prevents basal cell differentiation in the developing lung. Development 142, 1458–1469 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snitow ME et al. Ezh2 represses the basal cell lineage during lung endoderm development. Development 142, 108–117 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao C et al. Sin3a regulates epithelial progenitor cell fate during lung development. Development 144, 2618–2628 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rawlins EL, Clark CP, Xue Y & Hogan BL The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development 136, 3741–3745 (2009).Using a novel genetic lineage tracing model, the authors of this study reveal the multipotent nature of the distal tip endoderm in the early lung and show that it is temporally restricted.
- 54.Desai TJ, Brownfield DG & Krasnow MA Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507, 190–194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frank DB et al. Early lineage specification defines alveolar epithelial ontogeny in the murine lung. Proc. Natl Acad. Sci. USA 116, 4362–4371 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J et al. The strength of mechanical forces determines the differentiation of alveolar epithelial cells. Dev. Cell 44, 297–312 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Peng T et al. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature 500, 589–592 (2013).Using multiple genetic lineage tracing models, the authors identify a novel mesoderm cell type that generates multiple lineages within the heart and lungs to drive their tissue integration during development.
- 58.Kumar ME et al. Mesenchymal cells. Defining a mesenchymal progenitor niche at single-cell resolution. Science 346, 1258810 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen F et al. A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J. Clin. Invest. 120, 2040–2048 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Desai TJ, Malpel S, Flentke GR, Smith SM & Cardoso WV Retinoic acid selectively regulates Fgf10 expression and maintains cell identity in the prospective lung field of the developing foregut. Dev. Biol. 273, 402–415 (2004). [DOI] [PubMed] [Google Scholar]
- 61.Goss AM et al. Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf-B and Fgf10 expression. Dev. Biol. 356, 541–552 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shannon JM, Nielsen LD, Gebb SA & Randell SH Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev. Dyn. 212, 482–494 (1998). [DOI] [PubMed] [Google Scholar]
- 63.Shannon JM Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev. Biol. 166, 600–614 (1994). [DOI] [PubMed] [Google Scholar]
- 64.Abler LL, Mansour SL & Sun X Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung. Dev. Dyn. 238, 1999–2013 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El Agha E et al. Characterization of a novel fibroblast growth factor 10 (Fgf10) knock-in mouse line to target mesenchymal progenitors during embryonic development. PLOS ONE 7, e38452 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moiseenko A et al. Origin and characterization of alpha smooth muscle actin-positive cells during murine lung development. Stem Cells 35, 1566–1578 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Miller LA et al. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev. Dyn. 231, 57–71 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Shu W, Jiang YQ, Lu MM & Morrisey EE Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development 129, 4831–4842 (2002). [DOI] [PubMed] [Google Scholar]
- 69.Yin Y et al. An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev. Biol. 319, 426–436 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cornett B et al. Wntless is required for peripheral lung differentiation and pulmonary vascular development. Dev. Biol. 379, 38–52 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Snowball J, Ambalavanan M, Whitsett J & Sinner D Endodermal Wnt signaling is required for tracheal cartilage formation. Dev. Biol. 405, 56–70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim HY et al. Localized smooth muscle differentiation is essential for epithelial bifurcation during branching morphogenesis of the mammalian lung. Dev. Cell 34, 719–726 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hines EA, Jones MK, Verheyden JM, Harvey JF & Sun X Establishment of smooth muscle and cartilage juxtaposition in the developing mouse upper airways. Proc. Natl Acad. Sci. USA 110, 19444–19449 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herring MJ, Putney LF, Wyatt G, Finkbeiner WE & Hyde DM Growth of alveoli during postnatal development in humans based on stereological estimation. Am. J. Physiol. Lung Cell. Mol. Physiol 307, L338–344 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Narayanan M et al. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. Am. J. Respir. Crit. Care Med. 185, 186–191 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J et al. The development and plasticity of alveolar type 1 cells. Development 143, 54–65 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu Y, Thomson JM, Wong HY, Hammond SM & Hogan BL Transgenic over-expression of the microRNA miR-17–92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev. Biol. 310, 442–453 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y et al. HDAC3-dependent epigenetic pathway controls lung alveolar epithelial cell remodeling and spreading via miR-17–92 and TGF-beta signaling regulation. Dev. Cell 36, 303–315 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stankiewicz P et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am. J. Hum. Genet. 84, 780–791 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ren X et al. FOXF1 transcription factor is required for formation of embryonic vasculature by regulating VEGF signaling in endothelial cells. Circ. Res. 115, 709–720 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Madison BB, McKenna LB, Dolson D, Epstein DJ & Kaestner KH FoxF1 and FoxL1 link hedgehog signaling and the control of epithelial proliferation in the developing stomach and intestine. J. Biol. Chem. 284, 5936–5944 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Branchfield K et al. A three-dimensional study of alveologenesis in mouse lung. Dev. Biol. 409, 429–441 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bostrom H et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 85, 863–873 (1996). [DOI] [PubMed] [Google Scholar]
- 84.Gouveia L, Betsholtz C & Andrae J PDGF-A signaling is required for secondary alveolar septation and controls epithelial proliferation in the developing lung. Development 145, dev161976 (2018). [DOI] [PubMed] [Google Scholar]
- 85.Lindahl P et al. Alveogenesis failure in PDGF- A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development 124, 3943–3953 (1997). [DOI] [PubMed] [Google Scholar]
- 86.Sun T et al. A human YAC transgene rescues craniofacial and neural tube development in PDGFRalpha knockout mice and uncovers a role for PDGFRalpha in prenatal lung growth. Development 127, 4519–4529 (2000). [DOI] [PubMed] [Google Scholar]
- 87.Li C et al. Progenitors of secondary crest myofibroblasts are developmentally committed in early lung mesoderm. Stem Cells 33, 999–1012 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kugler MC et al. Sonic Hedgehog signaling regulates myofibroblast function during alveolar septum formation in murine postnatal lung. Am. J. Respir. Cell. Mol. Biol. 57, 280–293 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Giangreco A, Reynolds SD & Stripp BR Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am. J. Pathol. 161, 173–182 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hong KU, Reynolds SD, Giangreco A, Hurley CM & Stripp BR Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am. J. Respir. Cell. Mol. Biol. 24, 671–681 (2001). [DOI] [PubMed] [Google Scholar]
- 91.Balasooriya GI, Goschorska M, Piddini E & Rawlins EL FGFR2 is required for airway basal cell self-renewal and terminal differentiation. Development 144, 1600–1606 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tadokoro T, Gao X, Hong CC, Hotten D & Hogan BL BMP signaling and cellular dynamics during regeneration of airway epithelium from basal progenitors. Development 143, 764–773 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pardo-Saganta A et al. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell 184–197 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mori M et al. Notch3-Jagged signaling controls the pool of undifferentiated airway progenitors. Development 142, 258–267 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lafkas D et al. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature 528, 127–131 (2015). [DOI] [PubMed] [Google Scholar]
- 96.Mahoney JE, Mori M, Szymaniak AD, Varelas X & Cardoso WV The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev. Cell 30, 137–150 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McConnell AM et al. p53 regulates progenitor cell quiescence and differentiation in the airway. Cell Rep. 2173–2182 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Rock JR et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl Acad. Sci. USA 106, 12771–12775 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mou H et al. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell 19, 217–231 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng T et al. Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature 526, 578–582 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Volckaert T et al. Fgf10-Hippo epithelial- mesenchymal crosstalk maintains and recruits lung basal stem cells. Dev. Cell 43, 48–59 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rock JR et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl Acad. Sci. USA 108, E1475–E1483 (2011).This study shows that an epithelial-mesenchymal transition process does not occur at a high level after lung injury.
- 103.Jain R et al. Plasticity of Hopx+ type I alveolar cells to regenerate type II cells in the lung. Nat. Commun. 6, 6727 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zacharias WJ et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 555, 251–255 (2018).This study identifies the alveolar epithelial progenitor cell, which can be identified by expression of Axin2, and is responsible for most of the alveolar epithelial regeneration after acute lung injury.
- 105.Chen L, Acciani T, Le Cras T, Lutzko C & Perl AK Dynamic regulation of platelet-derived growth factor receptor alpha expression in alveolar fibroblasts during realveolarization. Am. J. Respir. Cell. Mol. Biol. 47, 517–527 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaplan NB, Grant MM & Brody JS The lipid interstitial cell of the pulmonary alveolus. Age and species differences. Am. Rev. Respir. Dis. 132, 1307–1312 (1985). [DOI] [PubMed] [Google Scholar]
- 107.McGowan SE & Torday JS The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu. Rev. Physiol. 59, 43–62 (1997). [DOI] [PubMed] [Google Scholar]
- 108.Ding BS et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 147, 539–553 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee JH et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1- thrombospondin-1 axis. Cell 156, 440–455 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.This study reveals the importance of endothelial- epithelial signalling in regulating alveolar epithelial differentiation.
- 111.Cao Z et al. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat. Med. 22, 154–162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Monticelli LA et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 12, 1045–1054 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lechner AJ et al. Recruited monocytes and type 2 immunity promote lung regeneration following pneumonectomy. Cell Stem Cell 21, 120–134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nureki SI et al. Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis. J. Clin. Invest. 128, 4008–4024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alder JK et al. Telomere dysfunction causes alveolar stem cell failure. Proc. Natl Acad. Sci. USA 112, 5099–5104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alder JK et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc. Natl Acad. Sci. USA 105, 13051–13056 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Povedano JM, Martinez P, Flores JM, Mulero F & Blasco MA Mice with pulmonary fibrosis driven by telomere dysfunction. Cell Rep. 12, 286–299 (2015). [DOI] [PubMed] [Google Scholar]
- 118.Kramann R et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16, 51–66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li R et al. Pdgfra marks a cellular lineage with distinct contributions to myofibroblasts in lung maturation and injury response. eLife 7, e36865 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Munger JS et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319–328 (1999). [DOI] [PubMed] [Google Scholar]
- 121.Xie T et al. Transcription factor TBX4 regulates myofibroblast accumulation and lung fibrosis. J. Clin. Invest. 126, 3063–3079 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.El Agha E et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell 20, 571 (2017). [DOI] [PubMed] [Google Scholar]
- 123.Kumar PA et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147, 525–538 (2011).This study was the first to demonstrate the expansion of epithelial pods following severe lung injury mediated by influenza infection.
- 124.Vaughan AE et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517, 621–625 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zuo W et al. p63+Krt5+ distal airway stem cells are essential for lung regeneration. Nature 517, 616–620 (2015). Vaughan et al. and Zuo et al. along with Kumar et. al. (2011) describe the expansion of KRT5-positive basal-like cells after acute lung injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ray S et al. Rare SOX2+ airway progenitor cells generate KRT5+ cells that repopulate damaged alveolar parenchyma following influenza virus infection. Stem. Cell Rep. 7, 817–825 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Taylor MS et al. A conserved distal lung regenerative pathway in acute lung injury. Am. J. Pathol. 188, 1149–1160 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taylor MS et al. Delayed alveolar epithelialization: a distinct pathology in diffuse acute lung injury. Am. J. Respir. Crit. Care Med. 197, 522–524 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xi Y et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat. Cell Biol. 19, 904–914 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xie T et al. Single-cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Rep. 22, 3625–3640 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang C et al. Expansion of hedgehog disrupts mesenchymal identity and induces emphysema phenotype. J. Clin. Invest. 128, 4343–4358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reyfman PA et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 10.1164/rccm.201712-2410OC (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ardini-Poleske ME et al. LungMAP: the molecular atlas of lung development program. Am. J. Physiol. Lung Cell. Mol. Physiol 313, L733–L740 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tabula Muris C et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Becht E et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2018). [DOI] [PubMed] [Google Scholar]
- 136.Lefrancais E et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544, 105–109 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thornton EE et al. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J. Exp. Med. 209, 1183–1199 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boers JE, Ambergen AW & Thunnissen FB Number and proliferation of Clara cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med.159, 1585–1591 (1999). [DOI] [PubMed] [Google Scholar]
- 139.Boers JE, Ambergen AW & Thunnissen FB Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 157, 2000–2006 (1998). [DOI] [PubMed] [Google Scholar]
- 140.Bhatt SP et al. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med.194, 178–184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hogg JC, Pare PD & Hackett TL The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol. Rev.97, 529–552 (2017).This report, along with references therein, provides critical insight into how the structural differences between the human and mouse lung may contribute to disease processes in humans.
- 142.Koo HK et al. Small airways disease in mild and moderate chronic obstructive pulmonary disease: a cross-sectional study. Lancet Respir. Med. 6, 591–602 (2018). [DOI] [PubMed] [Google Scholar]
- 143.Vinegar A, Sinnett EE, Kosch PC & Miller ML Pulmonary physiology of the ferret and its potential as a model for inhalation toxicology. Lab Anim. Sci. 35, 246–250 (1985). [PubMed] [Google Scholar]
- 144.Jacob A et al. Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell 21, 472–488 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huang SX et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 32, 84–91 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McCauley KB et al. Single-cell transcriptomic profiling of pluripotent stem cell-derived SCGB3A2+ airway epithelium. Stem. Cell Rep. 10, 1579–1595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.