Abstract
The aim of this study was to investigate the effect of enhanced recovery after surgery (ERAS) on perioperative outcomes, with an emphasis on patient-reported outcomes (PROs) and functional recovery.
We compared the clinical outcomes in a cohort of 275 patients undergoing liver resection before and after the implementation of ERAS. The PROs were preoperatively and postoperatively compared until 14 days after surgery using the MD Anderson Symptom Inventory.
The patients in the ERAS group experienced fewer symptoms and a shorter functional recovery time than the patients in the non-ERAS group. The group × time interactions were different between the groups for pain (F = 4.70, P = .001) and walking (F = 2.75, P = .03). On the 3rd, 4th, and 5th days after surgery, the ERAS group experienced less pain and more walking than the non-ERAS group. The ERAS group experienced less fatigue (0.407 [95% confidence interval, CI: −0.795, −0.020], P = .035), less sleep interference (0.615 [95% CI: −1.215, −0.014], P = .045), a lower rate of reduced appetite (0.281 [95% CI: −0.442, −0.120], P = .001), and less abdominal distension (0.262 [95% CI: −0.504, −0.020], P = .034) than the non-ERAS group. Those in the ERAS group had a significantly shorter median time from surgery to mild fatigue (5.41 vs 6.87 days, P = .003), mild pain (4.45 vs 6.09 days, P = .001), mild interference when walking (3.85 vs 5.54 days, P < .001), and mild interference when sleeping (5.49 vs 7.43 days, P < .001). ERAS patients were more likely than non-ERAS patients to achieve a functional recovery (5.70 vs 6.79 days, P < .001) status in a shorter time period. The ERAS pathway, operation time, and the minimally invasive approach were independent predictors of functional recovery time.
In hepatocellular carcinoma liver resection patients, the primary mechanism of ERAS is to reduce the postoperative interference burden and promote rapid functional recovery.
Keywords: enhanced recovery after surgery, hepatectomy, patient-reported outcomes, perioperative care
1. Introduction
Hepatocellular carcinoma (HCC) is a worldwide health problem that is responsible for >250,000 deaths annually. Surgical resection is the optimal treatment for cure; however, HCC patients undergoing liver resection often suffer from various symptoms that are caused by both the disease itself and its treatments.[1,2]
Enhanced recovery after surgery (ERAS) is characterized by a series of optimization measures implemented during the perioperative period that are based on evidence-based medicine. ERAS is used to attenuate physical and psychological stress responses and complications, as well as to potentiate postoperative rehabilitation for patients who undergo a variety of surgical procedures.[3] Furthermore, the protocol has been shown to reduce morbidity and length of stay (LOS) following hepatic surgery.[4,5] However, many of the published studies mainly focus on outcomes consisting of concrete primary endpoints, including early return of bowel function, decreased complication rates, and/or reduced length of inpatient stay.[6,7] They have not captured crucial outcomes, such as symptom burden and functional recovery, from the patient perspective.
Patient-reported outcomes (PROs) refer to “any report of the status of a patient's health condition that comes directly from the patient, without interpretation of the patient's response by a clinician or anyone else.”[8] As patient-reported outcome measures (PROMs) increasingly become key outcome indicators in health care, there is growing interest in PROs in surgical practice for comparative efficacy research and its influence on clinical decision-making in the perioperative management period.[9,10]
Therefore, our objective was to compare perioperative outcomes with a focus on patient-reported symptoms and functional recovery before and after the implementation of an ERAS programme.
2. Materials and methods
The ERAS programme involved preoperative education, allowing the oral intake of clear fluids for up to 2 hours before the induction of anesthesia, fluid management, minimally invasive techniques, optimal pain control, avoidance of mechanical bowel preparation, and the early initiation of oral feeding and mobilization. The non-ERAS group received conventional care, including routine nasogastric tube drainage, a standard fluid regimen during surgery, tracheal intubation, general anesthesia, the and the postoperative use of intravenous patient-controlled analgesia or intravenous opioids.
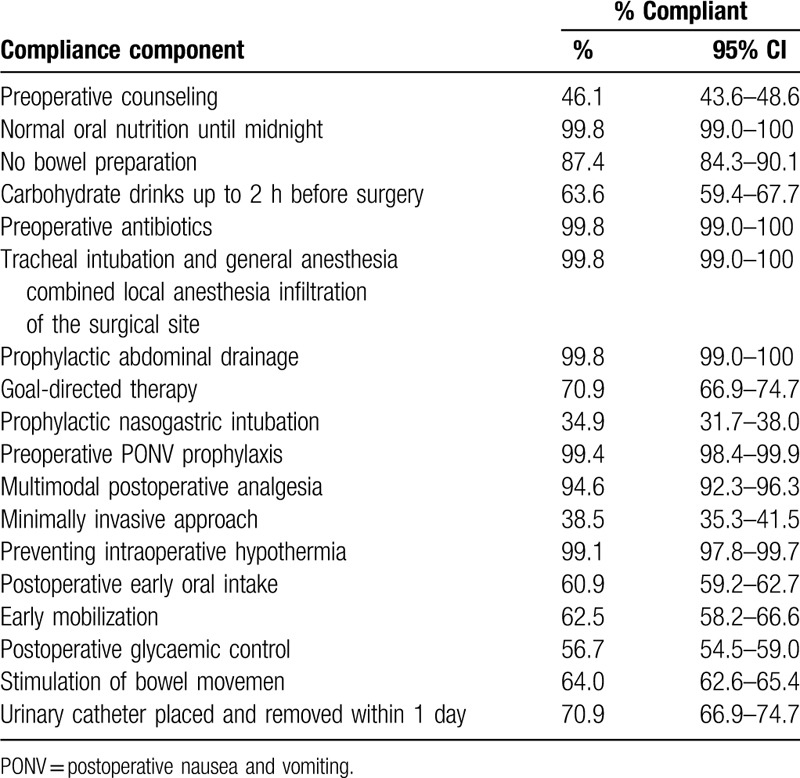
Compliance with the ERAS pathway was defined as adherence to the recommendations in the published guidelines. We estimated the percentage of patients who complied with each component of the compliance measures, with 95% exact binomial confidence intervals (CIs). Details on compliance with all individual elements are shown in Table 1.
Table 1.
Percentage of patients compliant with enhanced recovery after surgery components.
We launched our ERAS programme in May 2017 as part of a clinical quality improvement effort. Group 1 included all consecutive patients who underwent selective partial liver resection for HCC and followed our ERAS pathway between May 2017 and March 2018. Patients who underwent partial liver resection for HCC in the 8 months before the start of our ERAS programme (September 2016–May 2017) were considered historical controls or group 2. The study was approved by the Institutional Ethics Committees of the West China Hospital. A patient's decision to participate in the study was voluntary. Informed consent was obtained from each patient before surgery for the use of their data for research.
The patients experiencing symptoms were assessed using the M. D. Anderson Symptom Inventory (MDASI-Chinese).[11] The tool comprises 3 sections that include 13 core symptom questions, 6 questions particularly relating to HCC (abdominal distension, diarrhea, fever, itching, weight loss, and jaundice), and 6 questions that assess the impact of the symptoms on the patient's well-being (eg, activity, mood, walking, among others). The content validity index of the Symptom Inventory was 0.90, and Cronbach alpha was 0.88.
For the symptom inventory score, patients were asked to rank symptom severity during the previous 24 hours on a 0- to 10-point numeric rating scale, with 0 representing “not present or fully functional without life interference” and 10 representing “as bad as you can imagine or completely interferes with daily function.” Mild or no existing symptoms were defined as a score <4 in 2 consecutive assessments.
The baseline data were collected from medical records. The symptom burden was assessed at 3 time points: T1, before the operation (typically 1–3 days before surgery); T2, every day during hospitalization after surgery; and T3, 14 days after surgery. The definition of complications was decided according to the Dindo-Clavien classification.[12]
Grade I complications were defined as issues that did not require pharmacological treatment, surgery, endoscopy, or radiological interventions. Grade II complications required pharmacologic treatment with drugs other than those allowed for grade I complications. Grade III complications required surgical, endoscopic, or radiologic intervention. Grade IIIa complications required invasive intervention that was not performed under general anesthesia, and Grade IIIb complications required invasive intervention that was performed under general anesthesia. Grade IVa complications involved single organ dysfunction (including dialysis). Grade IVb complications involved multiorgan dysfunction. A grade V complication was death.
The criteria for functional recovery were as follows: good pain control with only oral analgesia; tolerance for solid food; no requirement for IV fluids; adequate passage of stool; and independent mobility at the preoperative level.[13]
2.1. Statistical analysis
The baseline characteristics were compared between the non-ERAS and ERAS groups using χ2 and t tests. Cronbach alphas were calculated to determine the internal consistency of the MDASI-C. χ2 tests were used to evaluate differences in the distribution of postoperative symptoms. Two-way repeated analysis of variances (ANOVAs) were used to compare the postoperative symptoms that occurred in the 2 groups of patients during their hospital stay. Kaplan-Meier analysis with one minus event probability was used to evaluate the time required for symptoms to return to a state of mild or no burden for fatigue, sleep, walking and functional recovery after surgery (from day of surgery to day 14 post surgery). Linear regression was performed to analyze the factors affecting patient functional recovery time. Data were analyzed using SPSS version 19.0 for Windows (SPSS Inc, Chicago, IL).
3. Results
3.1. Compliance of patients with ERAS programme elements
Details on compliance with all individual elements are shown in Table 1. Overall, 76.4% of the patients were compliant with at least 70% of the elements. Only 5 patients (1.8%) were compliant with ≤50% of the elements.
3.2. Study patient demographics and clinical profiles
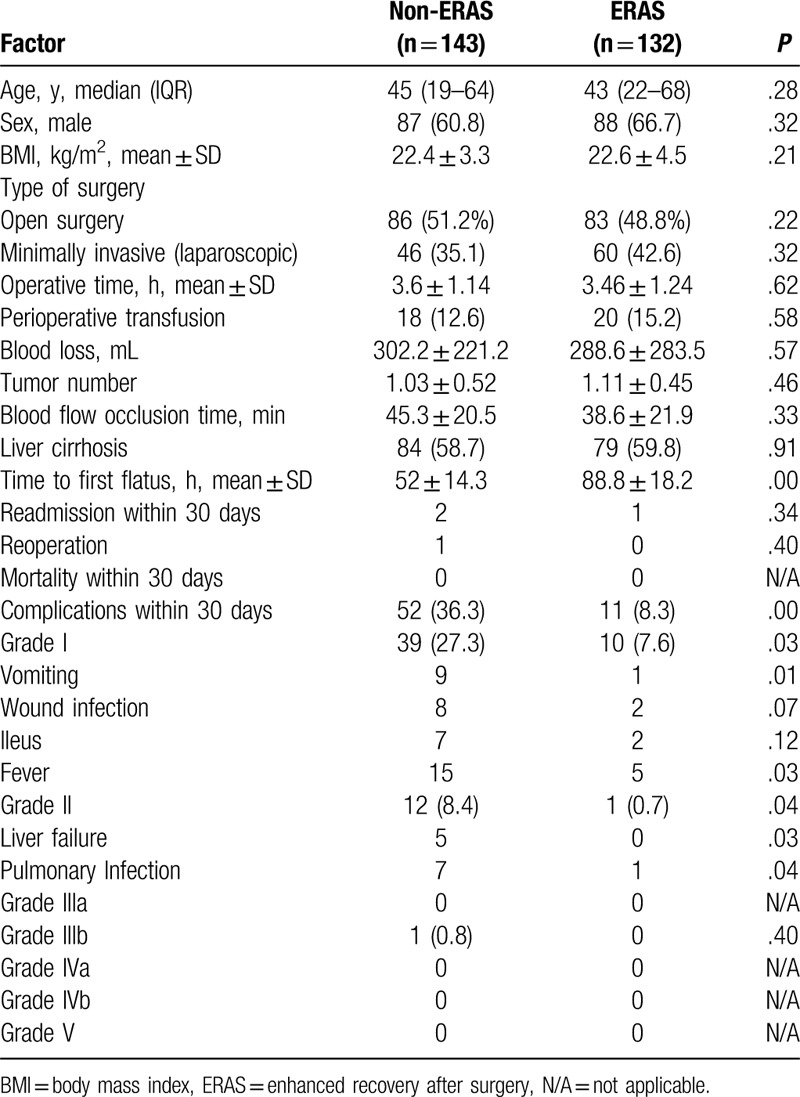
A total of 275 patients participated in this study. The median age was 44.6 years (interquartile range 19–68 years). A total of 175 patients (63.6%) were male, and 106 operations (39%) were minimally invasive. The composition of the surgical approach between the two groups (35.1% vs 42.6%, P = .32) was not significantly different. All other baseline characteristics (tumor number, body mass index, operative time, blood loss, and liver cirrhosis) in the non-ERAS and ERAS groups were not significantly different. There was no perioperative mortality, but there was 1 reoperation in the non-ERAS group. Using the Clavien-Dindo Classification of Surgical Complications, there were significantly more overall complications (36.3% vs 8.3%, P = .00) and grade I complications (27.3% vs 7.6%, P = .03) in the non-ERAS group than in the ERAS group. Grade I complications included vomiting, fever, ileus, and a wound infection, which are not traditionally regarded as surgical complications but are correlated with comfort measures in patients after surgery. Grade II complications included liver failure and pulmonary infection. There was greater statistical significance (8.4% vs 0.7%, P = .04) in the non-ERAS group than in the ERAS group. There were no grade IIIa or grade IIIb complications in the 2 groups (Table 2).
Table 2.
Demographics, clinical characteristics, and perioperative outcomes.
3.3. Patient-reported symptom burden during hospital stay
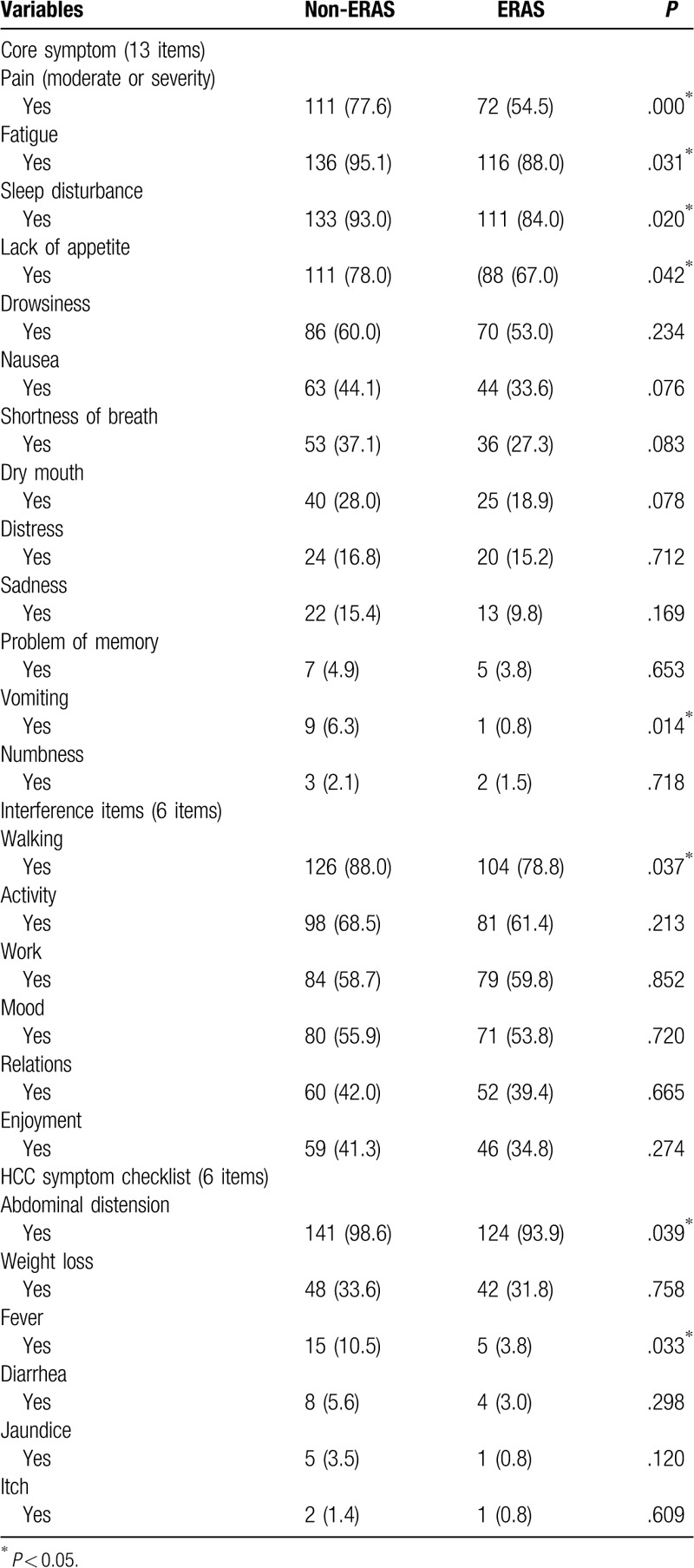
Differences in symptom burden distribution between the 2 groups during hospital stay are shown in Table 3. In the core symptom section, the distributions of pain, (P = .00), fatigue (P = .03), sleep disturbance (P = .02), lack of appetite (P = .04), and vomiting (P = .01) were lower in the ERAS group than in the non-ERAS group. In the symptom interference and HCC symptom section, the distribution of walking (P = .037), abdominal distension (P = .039), and fever (P = .03) were less in the ERAS group than in the non-ERAS group.
Table 3.
Differences in MDASI-C symptom burden between non-ERAS and ERAS.
3.4. Longitudinal assessments of PROs analyzed in the hospital and until 14 days after surgery
The variables with different symptom distributions in the two groups were included in the repeated measures ANOVA. Fever and vomiting were excluded because the sample size was too small (Table 3). The results of the two-way repeated measures ANOVA indicated that there were significant differences in group × time interactions between the groups in relation to pain (F = 4.70, P = 0.001) and walking (F = 2.75, P = 0.03). The main effect of the group was significant for pain (F = 26.3, P < 0.001), walking (F = 6.19, P = 0.015), fatigue (F = 4.59, P = 0.035), abdominal distension (F = 4.60, P = 0.034), lack of appetite (F = 11.98, P = 0.001) and sleeping (F = 4.14, P = 0.045). The main effect of time was significant (p < 0.05) for pain, walking, sleeping, fatigue, abdominal distension and lack of appetite ( Figs. 1 and 2).
The ERAS group experienced less pain (Fig. 1A) and walking interference (Fig. 1B) than the non-ERAS group, with significant differences on the third, fourth, and fifth day after surgery, respectively (P < .05). In regard to the pain score, on the third postoperative day, there was a statistically significant difference between the ERAS group (5.06 ± 1.50) and the non-ERAS group (5.58 ± 1.52) (0.523 [95% CI: −0.864, −0.181], P = .003). On the fourth postoperative day, there was a statistically significant difference between the ERAS group (4.45 ± 1.71) and the non-ERAS group (4.99 ± 1.59) (0.545 [95% CI: −0.919, −0.172], P = .005). On the fifth postoperative day, there was a statistically significant difference between the ERAS group (3.65 ± 1.81) and the non-ERAS group (4.61 ± 1.88) (0.962 [95% CI: −1.430, −0.494], P = .000). In terms of walking interference, on the third postoperative day, there was a statistically significant difference between the ERAS group (5.08 ± 1.49) and the non-ERAS group (5.58 ± 1.52) (0.508 [95% CI: −0.848, −0.167], P = .004). On the fourth postoperative day, there was a statistically significant difference between the ERAS group (4.65 ± 1.68) and the non-ERAS group (5.28 ± 1.44) (0.629 [95% CI: −0.969, −0.289], P = .000). On the fifth postoperative day, there was a statistically significant difference between the ERAS group (4.72 ± 1.32) and the non-ERAS group (5.40 ± 1.62), (0.682 [95% CI:−1.022, −0.341], P = .000).
Figure 1.
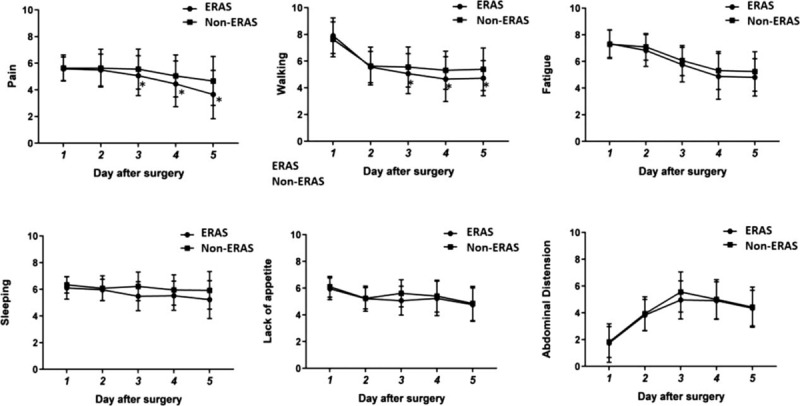
Trend map of symptoms during hospital. Error-bars indicate the standard deviation of the mean. The group × time interactions show difference in pain (A) and walking (B). ∗P < .05 (Difference between the two groups at different times). Compared with non-ERAS, the ERAS group experienced less fatigue (P = .035) (C), sleeping (P = .045) (D), lack of appetite (P = 0.001) (E), and abdominal distension (P = .034) (F). ERAS = enhanced recovery after surgery.
In the core symptom section, the ERAS group experienced less fatigue (0.407 [95% CI: −0.795, −0.020], P = .035) (Fig. 1C), sleeping interference (0.615 [95% CI: −1.215, −0.014], P = .045) (Fig. 1D) and lack of appetite (0.281 [95% CI: −0.442, −0.120], P = .001) (Fig. 1E) than the non-ERAS group. In the HCC symptom section, the ERAS group experienced less abdominal distension than the non-ERAS group (0.262 [95% CI: −0.504, −0.020], P = .034) (Fig. 1F).
In addition to less abdominal distension and lack of appetite, the ERAS group returned to a mild or no symptom interference status faster than the non-ERAS. The mean time to mild or no (scores <4) pain was a median of 4.45 days (95% CI 4.12–4.73 days) in the ERAS group compared with 6.09 days (95% CI 5.64–6.55 days, P = .001; Fig. 2A) in the non-ERAS group. The patients in the ERAS group had a significantly shorter median time to no or mild fatigue, at 5.41 days (95% CI 5.05–5.76 days) compared with 6.87 days (95% CI 6.42–7.31 days; P = .003; Fig. 2B). Similar results were observed in relation to walking and sleeping (Fig. 2C and D).
Figure 2.
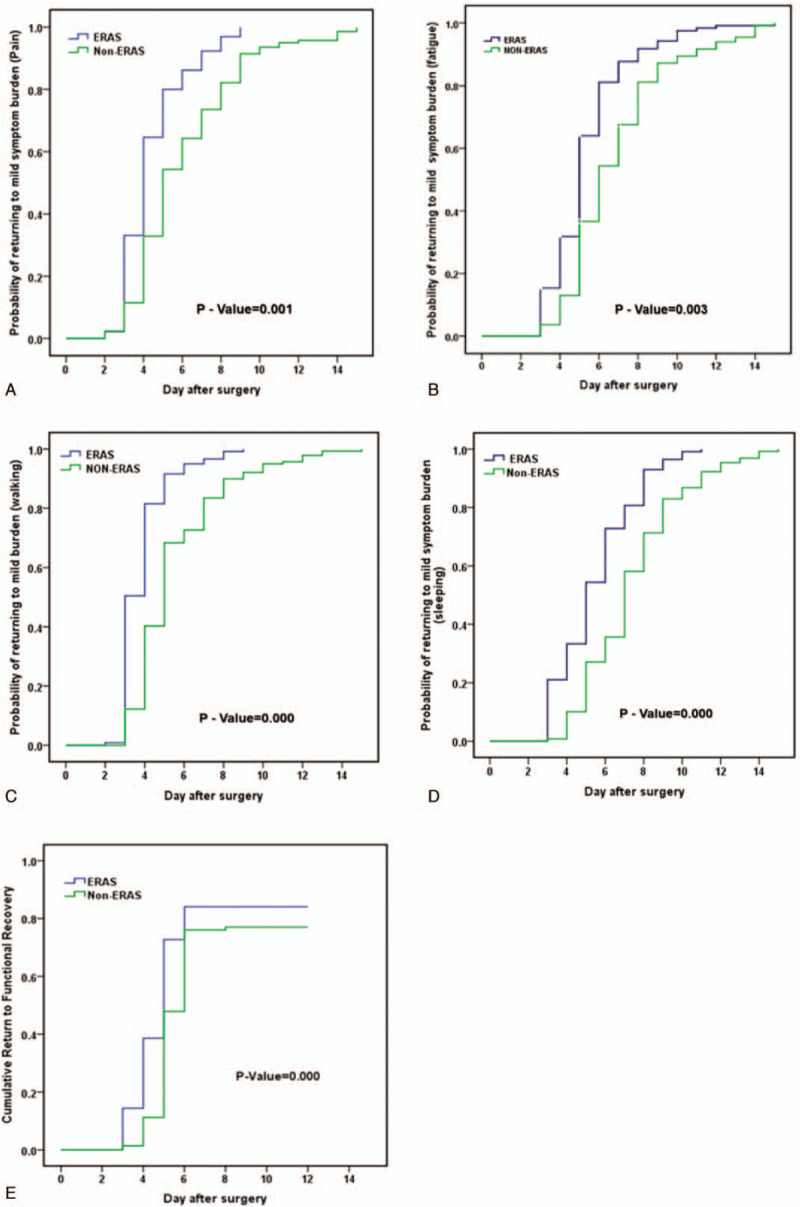
Time to functional recovery. (A) Return to mild Pain (<4) day after surgery (P = 0.001). (B) Return to mild fatigue (<4) (P = 0.003). (C) Return to mild (<4) for interference with walking after surgery (P < 0.001). (D) Return to mild or none (<4) for interference with Sleeping after surgery (P < 0.001). (E) Return to functional recovery time (P < 0.001) day after surgery.
3.5. Analysis of factors affecting patient functional recovery time
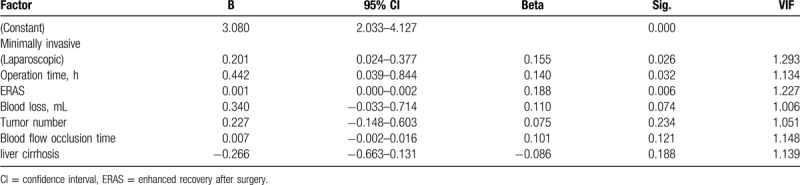
ERAS patients achieved functional recovery in a shorter period of time than non-ERAS patients, at 5.70 days (95% CI 5.21–6.19 days) in ERAS patients compared with 6.79 days (95% CI 6.31–7.27 days; P < .001; Fig. 2E) in non-ERAS patients. Multiple linear regression models determined that independent predictors of time to functional recovery were the ERAS pathway, operation time, and a minimally invasive approach for surgery (laparoscopic) (P < .05; Table 4).
Table 4.
Factors affecting functional recovery time.
4. Discussion
Kehlet et al considered that ERAS programmes should be based on multidisciplinary collaborations to promote multimodal care for rapid postoperative recovery, with a focus on reducing complications and LOS.[14] PROs can be included in clinical trials as primary or secondary endpoints and are increasingly recognized by regulators, clinicians, and patients as valuable tools to collect patient-centered data.[15,16] PROs provide unique information on the impact of a medical condition and its treatment from the patient perspective; therefore, PROs can be included in clinical trials to ensure the outcome of a trial intervention is comprehensively assessed. Our research suggests that ERAS shortens the functional recovery time and might also have the added benefits of reducing postoperative symptom burden and life interruptions. Patients in both pathways experienced similar symptom burdens. However, it seems that ERAS allows patients to return to their desired functional status quicker than conventional methods by minimizing the disruption of postoperative symptom burden to life activities and enjoyment.
Multiple studies have shown that ERAS improves patient outcomes by reducing stress, reducing complications, shortening hospital stays, and reducing hospitalization costs. Our findings of a reduced average hospital stay for 1 day without increases in complications or readmission rates were similar to other findings.[4,6] Our study contributes to the field, as it demonstrates the broader functional benefits of ERAS from a patient perspective, and it improves the outcomes reported by the patient, such as pain, sleep, fatigue, walking, and other disturbances. Clinically, we often only pay attention to bleeding, liver failure, infection, and thrombosis. However, we often overlook the face that patients who undergo hepatectomy also experience some common and serious symptoms such as pain, fatigue, sleep disorders, and so on. Unresolved symptoms may affect daily life, functional status, and quality of life.[17] The MD Anderson Symptom Scale is an indicator for assessing postoperative symptom-related dysfunction in cancer patients. This study provides postoperative symptom and interference information for patients undergoing hepatectomy for HCC. Our data show that the distributions of these symptoms were statistically different among the ERAS and non-ERAS groups. We also found that the ERAS group showed a significant improvement in the postoperative symptom burden and interference; they returned to a mild or asymptomatic state of pain, fatigue, sleep, and walking disturbances quickly. Pain and walking disturbances are sensitive markers of postoperative functional recovery.[18] Some studies also reported fatigue, insomnia, and pain as “sentinel” symptoms, which are likely to have a major effect on functional status and overall symptom burden.[19] Pain can directly or indirectly lead to sleep disorders and fatigue.[20] When patients experience pain, they also experience fatigue, sleep disturbances, decreased activity, loss of appetite, and abdominal distension. Therefore, doctors and nurses should pay increased attention to the comprehensive symptom assessments and management of patients with HCC undergoing hepatectomy.
It is worth noting that this analysis found a shortened functional recovery time after hepatectomy and rapid recovery. Furthermore, we identified a strong association between operation time and surgical methods that was independent of tumor number and cirrhosis.
The limitations of our study are primarily due to its retrospective and observational design. The sample size was small and consisted of the first 132 patients to whom we applied the protocol. We need to increase patient compliance with the protocol to improve our results. A high rate of or full implementation of the ERAS protocol could significantly improve short-term outcomes, and we will work very hard to achieve and analyze this goal. Long-term follow-up results are lacking, and the long-term benefits of ERAS cannot be evaluated. More rigorous experimental designs with large sample sizes are needed to assess the long-term utility of this programme.
In conclusion, this study demonstrates that avoiding severe symptom burden is the most important factor for rapid functional recovery in liver resection patients. This understanding can help to improve the quality of life of HCC patients by improving the information given before treatment, as well as by monitoring these outcomes during follow-up.
Author contributions
Conceptualization: Qiu Ping Ren.
Data curation: Feng Ming Xiao, Ze Rong Xie, Juan Wan.
Investigation: Ze Rong Xie.
Methodology: Ze Rong Xie, Meng-Hang Wu.
Project administration: Qiu Ping Ren, Yan Li Luo.
Writing – original draft: Qiu Ping Ren, Ze Rong Xie.
Writing – review & editing: Qiu Ping Ren, Yan Li Luo, Tian Fu Wen.
Footnotes
Abbreviations: ANOVA = analysis of variance, ERAS = enhanced recovery after surgery, HCC = hepatocellular carcinoma, LOS = length of stay, MDASI = M. D. Anderson Symptom Inventory, NRS = numeric rating scale, PROMs = patient-reported outcome measures, PROs = patient-reported outcomes.
How to cite this article: Ren QP, Luo YL, Xiao FM, Wen TF, Wu MH, Juan-Wan, Xie ZR. Effect of enhanced recovery after surgery program on patient-reported outcomes and function recovery in patients undergoing liver resection for hepatocellular carcinoma. Medicine. 2020;99:20(e20062).
The authors report no conflicts of interest.
This study was supported by grants from the enhanced recovery after surgery Program of West China hospital of Sichuan University (ZY2016204).
Availability of data and materials: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol 2017;34:153–9. [DOI] [PubMed] [Google Scholar]
- [2].Jiang JF, Lao YC, Yuan BH, et al. Treatment of hepatocellular carcinoma with portal vein tumor thrombus: advances and challenges. Oncotarget 2017;8:33911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ljungqvist O. ERAS—enhanced recovery after surgery: moving evidence-based perioperative care to practice. JPEN J Parenter Enteral Nutr 2014;38:559–66. [DOI] [PubMed] [Google Scholar]
- [4].Zhao Y, Qin H, Wu Y, et al. Enhanced recovery after surgery program reduces length of hospital stay and complications in liver resection: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine 2017;96:e7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Melloul E, Hubner M, Scott M, et al. Guidelines for perioperative care for liver surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg 2016;40:2425–40. [DOI] [PubMed] [Google Scholar]
- [6].Hughes MJ, McNally S, Wigmore SJ. Enhanced recovery following liver surgery: a systematic review and meta-analysis. HPB (Oxford) 2014;16:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rouxel P, Beloeil H. Enhanced recovery after hepatectomy: A systematic review. Anaesth Crit Care Pain Med 2019;38:29–34. [DOI] [PubMed] [Google Scholar]
- [8].Powell J, Powell S, Robson A. A systematic review of patient-reported outcome measures in paediatric otolaryngology. J Laryngol Otol 2018;132:2–7. [DOI] [PubMed] [Google Scholar]
- [9].Bouazza YB, Chiairi I, El Kharbouchi O, et al. Patient-reported outcome measures (PROMs) in the management of lung cancer: a systematic review. Lung Cancer (Amsterdam, Netherlands) 2017;113:140–51. [DOI] [PubMed] [Google Scholar]
- [10].Cottone F, Deliu N, Collins GS, et al. Modeling strategies to improve parameter estimates in prognostic factors analyses with patient-reported outcomes in oncology. Qual Life Res 2019;28:1315–25. [DOI] [PubMed] [Google Scholar]
- [11].Wang XS, Wang Y, Guo H, et al. Chinese version of the M. D. Anderson Symptom Inventory: validation and application of symptom measurement in cancer patients. Cancer 2004;101:1890–901. [DOI] [PubMed] [Google Scholar]
- [12].Grochowiecki T, Madej K, Galazka Z, et al. Usefulness of modified Dindo-Clavien scale to evaluate the correlation between the severity of surgical complications and complications related to the renal and pancreatic grafts after simultaneous kidney and pancreas transplantation. Transplant Proc 2016;48:1677–80. [DOI] [PubMed] [Google Scholar]
- [13].Coolsen MM, Wong-Lun-Hing EM, van Dam RM, et al. A systematic review of outcomes in patients undergoing liver surgery in an enhanced recovery after surgery pathways. HPB (Oxford) 2013;15:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630–41. [DOI] [PubMed] [Google Scholar]
- [15].Luckett T, King MT. Choosing patient-reported outcome measures for cancer clinical research—practical principles and an algorithm to assist non-specialist researchers. Eur J Cancer (Oxford, England: 1990) 2010;46:3149–57. [DOI] [PubMed] [Google Scholar]
- [16].Mercieca-Bebber R, Calvert M, Kyte D, et al. The administration of patient-reported outcome questionnaires in cancer trials: Interviews with trial coordinators regarding their roles, experiences, challenges and training. Contemp Clin Trials Commun 2018;9:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ryu E, Kim K, Cho MS, et al. Symptom clusters and quality of life in Korean patients with hepatocellular carcinoma. Cancer Nurs 2010;33:3–10. [DOI] [PubMed] [Google Scholar]
- [18].Shi Q, Wang XS, Vaporciyan AA, et al. Patient-reported symptom interference as a measure of postsurgery functional recovery in lung cancer. J Pain Symptom Manage 2016;52:822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Barsevick AM. The concept of symptom cluster. Semin Oncol Nurs 2007;23:89–98. [DOI] [PubMed] [Google Scholar]
- [20].Nguyen LT, Alexander K, Yates P. Psychoeducational intervention for symptom management of fatigue, pain, and sleep disturbance cluster among cancer patients: a pilot quasi-experimental study. J Pain Symptom Manage 2018;55:1459–72. [DOI] [PubMed] [Google Scholar]


