Opinion Statement:
Cellular immunotherapy has been rapidly evolving and increasingly utilized in the management of relapsed and refractory lymphoma. CD19-specific chimeric antigen receptor T cells (CARTs) have achieved impressive results in pivotal clinical trials. Although CART development continues, these products have fundamental limitations that may make them less desirable in particular settings. For example, CARTs can only target cell surface antigens and thus are incapable of targeting intracellular tumor-associated proteins. In contrast to CARTs, conventional T cell receptors (TCR) allow T cells to target any cellular antigen, including intracellular proteins, since they interact with peptides presented by MHC I and II molecules. T cells recognizing EBV antigens through native TCRs have been successfully employed for treatment and prophylaxis of EBV-associated lymphomas, including post-transplant lymphoproliferative disorder. Currently, transgenic TCR transduced T cells targeting non-viral tumor antigens remain experimental but, if successful, could become an invaluable cellular therapy option. Because the manufacturing process of autologous T cell products, including CARTs and other tumor-specific T cells, takes several weeks, patients often need bridging therapy to maintain disease control, which may be challenging. Novel cellular platforms, such as genetically modified NK and NKT cells, may be amenable to allogeneic use, and thus may allow production as a readily available, “off-the-shelf” product. As cellular therapies beyond CART continue to grow, available therapeutic options for relapsed and refractory lymphoma patients are expected to expand further.
Keywords: Lymphoma, cellular therapy, immunotherapy, EBV, EBVSTs, CART, T cells, NKT cells, DLI, CTL, tumor antigens, neoantigens, transgenic TCR, adoptive cell therapy, tumor-specific T cells
Introduction:
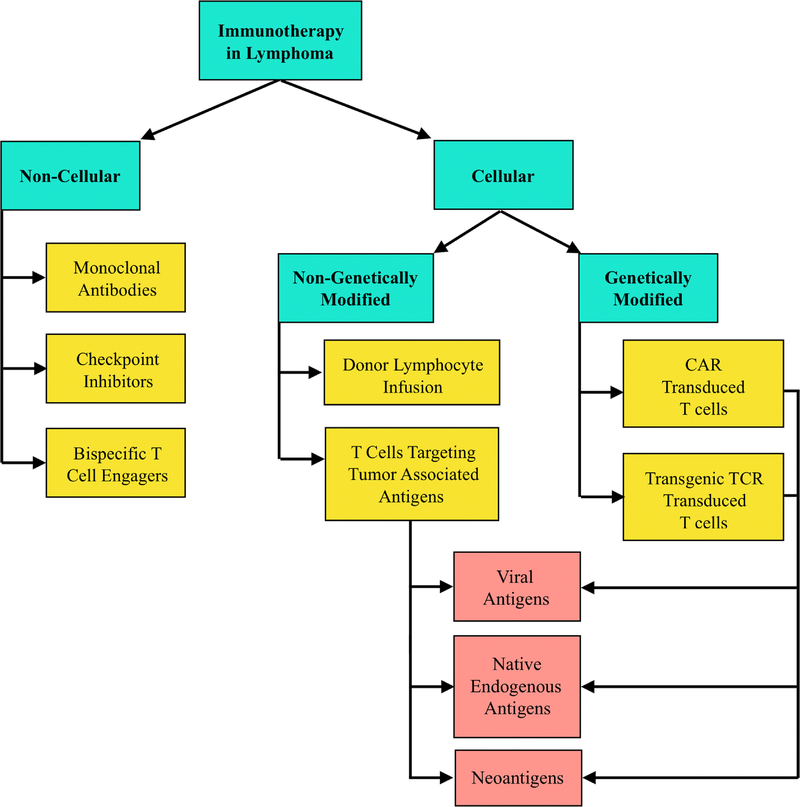
The field of immuno-oncology is rapidly evolving and has already significantly impacted outcomes in lymphoma. Various immunotherapeutic modalities have been pursued (Figure 1), including monoclonal antibodies, checkpoint inhibitors and cellular immunotherapy. The latter encompasses the use of diverse immune effectors either in their native state or as genetically modified forms (Figure 1 and Table 1), which are delivered as adoptive cell therapy (ACT). ACT refers to a process whereby immune effectors (usually T lymphocytes) are isolated and ex-vivo expanded (while potentially being modified or selected for particular specificities) before being infused into patients. The types of T lymphocytes transferred include cells that have not been genetically modified and thus express their native T-cell receptor (TCR); cells transduced with an alternative, transgenic TCR (tgTCR); and cells genetically modified to express an artificial, chimeric antigen receptor (CAR). We will refer to these three types of T lymphocyte products as native T cells, tgTCR-T cells, and CAR-T cells (CARTs), respectively. While other immune effectors, such as NKT and NK cells, can be employed in a similar fashion, T cells targeting tumor-associated antigens (TAA) have been at the forefront of immune cellular approaches used to treat patients with refractory lymphoma. In this review, we will provide an overview of cellular immunotherapies in lymphoma with focus on cellular modalities other than CART.
Figure 1. Overview of Approaches to Immunotherapy in Lymphoma.
Table 1.
Key terminologies referring to various kinds of cellular immunotherapy
| Description | |
|---|---|
| Adoptive Cell Therapy | A process whereby immune effector cells are isolated and expanded exvivo before being infused into patients. During expansion, these cells can be manipulated or selected to enhance specificity and potency. The immune cells transferred may be used without genetic modifications or they can be genetically modified. T cells are usually employed but other immune effector cells can similarly be used, such as NK or NKT cells. |
| Native T Cells | T cells bearing a native T cell receptor (TCR) and that have not been genetically modified. |
| Chimeric Antigen Receptor Transduced T Cells (CART) | T cells that are genetically modified in vitro via viral or non-viral transduction to express an artificial, chimeric antigen receptor (CAR). A CAR is composed of an extracellular antigen-binding domain (derived from a monoclonal antibody) and intracellular signaling domains (CD3ζ and CD28 or CD137) that drive T cell activation (Figure 2). |
| Transgenic TCR Transduced T Cells (tgTCR-T cells) | Genetically modified T cells where transgenic TCRs are transduced to recognize and target a single epitope of a specific tumor antigen presented via MHC I or II on antigen presenting cells or target cells (Figure 2). tgTCR-T cells can potentially be made to recognize any individual antigen expressed by target cells, including intracellular proteins. |
| Tumor-Specific T Cells (TSTs) | T cells that target a particular tumor-associated antigen (TAA). These may include viral antigens (e.g. EBV antigens) or endogenous antigens expressed by the tumor cells. |
| EBV-Specific T Cells (EBVSTs) | T cells that specifically target EBV antigens (e.g. LMP 1 and 2). |
1. CART Therapy
CART therapy (Table 1 and Figure 2) has achieved remarkable success in the management of aggressive non-Hodgkin lymphoma (NHL), as evidenced by the pivotal ZUMA1 and JULIET clinical trials [1, 2]. In ZUMA-1, an autologous CD19-specific CART product (axicabtagene ciloleucel) was used in 108 patients with relapsed or refractory aggressive NHL [1]. Updated analysis of the combined phase 1 and phase 2 portions showed 82% overall response rate (ORR), including 58% complete response (CR) rate, with 12-month overall survival (OS) and progression free survival (PFS) of 59% and 44%, respectively [1]. Comparable results were seen in the JULIET trial, in which another autologous CD19-specific CART product (tisagenlecleucel) was used in 93 patients with relapsed or refractory diffuse large B cell lymphoma (DLBCL) [2]. ORR was 52% ORR, including 40% CR rate, with 12-month OS and PFS of 49% and 83%, respectively [2]. The main adverse events in both trials were cytokine release syndrome (CRS) and neurotoxicity, both of which are the focus of active research. Based on those two key trials, both axicabtagene ciloleucel and tisagenlecleucel were granted breakthrough FDA approvals in patients with relapsed or refractory DLBCL after two or more prior lines of therapy.
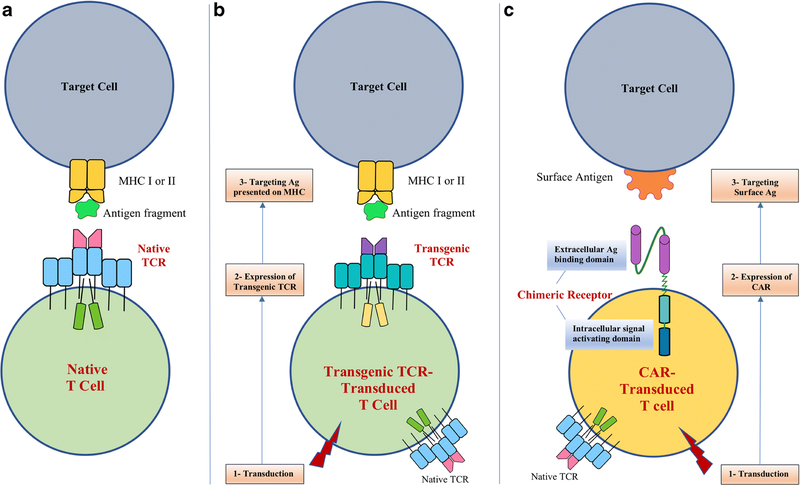
Figure 2. Native T cells, Transgenic TCR-Transduced T cells, and CAR-Transduced T cells.
A conventional TCR is composed of 2 polypeptide chains (α and β chains) that are assembled as membrane complex together with the proteins making up the CD3 antigen. All T cells express TCRs (a native TCR) (A), but they can be genetically modified to express another TCR (a transgenic TCR) (B), which is also encoded as 2 separate chains. A CAR (C) is an artificial construct that combines the antigen-recognizing moiety of a monoclonal antibody with elements of the T-cell signaling machinery, most frequently a portion of the TCR/CD3-associated ζ chain and part of a costimulatory molecule such as CD28 or CD137 (4–1BB). Like transgenic TCR-T cells, CAR-T cells are genetically engineered to express a CAR. A correctly assembled TCR recognizes epitopes presented by MHC molecules potentially representing every cellular protein. In contrast, a CAR can only bind whole surface antigens. (APC: Antigen presenting cell. MHC: Major histocompatibility complex. TCR: T cell receptor. Ag: Antigen)
The success of CD19-specific CART cell therapy has led to the development of other CART products targeting the same or alternative hematopoietic antigens. Table 2 lists some of the antigens being pursued in NHL and other lymphoproliferative disorders. Nonetheless, despite their remarkable activity in some settings, CARTs have several limitations, the most fundamental being that they can only target antigens that are expressed on the surface of tumor cells. Thus, intracellular proteins, which correspond to the majority of antigens expressed in a cell, are not targetable with CARTs. In contrast, T cells with native or transgenic TCRs have the potential to target any cellular antigen, including intracellular proteins, since their TCRs bind to major histocompatibility complex (MHC) molecules displaying fragments of every possible protein made in a cell (Figure 2). Thus, T cells whose activity depends on a conventional TCR may be preferable in some contexts.
Table 2.
CAR targets in lymphoproliferative disorders
| Antigen | Diseases |
|---|---|
| BCMA | Multiple Myeloma [39, 40] |
| Kappa light chain | Multiple Myeloma. NHL/CLL [41] |
| CD5 | T cell malignancies (T-ALL and T cell lymphoma)[42] |
| CD19 | NHL [2, 1], ALL[43, 44] |
| CD20 | B-cell lymphoma, mantle cell lymphoma [45] |
| CD22 | B-acute lymphoblastic leukemia (ALL)[46] |
| CD30 | Hodgkin Lymphoma[47, 48] |
| CD37 | B-cell and T-cell Lymphomas[49] |
2. Beyond CART Therapy
2.1. Donor Lymphocyte Infusion (DLI)
In a DLI, lymphocytes isolated from an allogeneic hematopoietic stem cell transplant (allo-HSCT) donor are infused into a recipient who has residual or relapsed disease after undergoing HSCT, with the intent of eliciting graft versus lymphoma (GVL) activity [3]. The original forms of DLI employed native (unmodified) T cells and were truly the first example of ACT in a broad sense for lymphoma [4, 5]. Importantly, the susceptibility to the GVL effect depends on the specific type of lymphoma. Follicular lymphoma (FL), for example, seems to be particularly sensitive to DLI after allo-HSCT [4, 5, 3, 6]. A retrospective study evaluated DLI in 28 patients with either low grade NHL (n=23, including 14 FL) or transformed FL (n=5) who had progressive disease (PD) or mixed chimerism (MC) post allo-HSCT. Cumulative response rates were 76.5% in patients with PD and 91.6% in those with MC.
The GVL effect associated with unmodified DLI occurs presumably against minor (or major, if the transplant is not fully HLA matched) antigens expressed in tumor cells that are not shared between recipient and donor. In virtually all cases, the identity of those antigens is unknown. Nevertheless, in specific cases, for instance if a tumor expresses viral antigens, these can presumably be targeted. This was the rationale for using unmodified DLI to treat EBVassociated post-transplant lymphoproliferative disorder (PTLD), a complication that can occur after allo-HSCT performed with strongly immunosuppressive conditioning regimens. In this setting, T cell immune surveillance is weakened, which allows unchecked proliferation of EBVinfected B cells. Reasoning that the immune system of healthy donors (who have no evidence of active EBV infection) should contain a population of effector cells active against EBV, Papadopoulos et al investigated using DLI in 5 patients who had developed EBV-associated PTLD after allo-HSCT [7]. Remarkably, complete clinical or pathological responses were achieved within 30 days in all 5 patients [7]. Notably, 3 patients had skin and oral mucocutaneous graft-versus-host disease (GVHD), and the other 2 patients had different degrees of pulmonary deterioration of unclear etiology [7]. Despite the small number of patients, this study showed that unmodified DLI can be used to treat EBV-associated PTLD after allo-HSCT, albeit with a significant risk of causing GVHD.
Further refinement of DLI can be achieved by modifying the lymphocyte product through depletion or enrichment of specific T cell populations. For example, regulatory (Treg) or naïve T cells can be depleted with the aim of selecting for the most potent antitumor T cells [8]. Selective depletion of Tregs prior to infusion enhances the product’s GVL effect [9]. In addition, because naïve T cells appear to have a central role in the development of severe GVHD, naïve T cell depletion has been employed to lessen this risk [10]. Studies showed that this approach reduces the incidence of chronic GVHD but does not affect acute GVHD [11, 10].
Since DLI infusion monotherapy is unlikely to induce appreciable sustained responses in a heavily treated population, researchers have looked into combining chemotherapy with DLI. For instance, a retrospective study evaluating DLI plus bendamustine was conducted in 18 patients with Hodgkin lymphoma (HL) who progressed after allo-HSCT [12]. Patients were treated with bendamustine followed by DLI and achieved an ORR of 55% (3 CR and 7 PR). Median OS was 11 months and PFS was 6 months. The OS at one year was 70% for patients who responded versus 16% for those who did not. These data suggest that DLI plus bendamustine can be a reasonable salvage option for HL patients who progress after allo-HSCT, although larger scale prospective studies are needed to confirm this.
2.2. T cells Targeting EBV-Associated Lymphoma Antigens via Native TCRs
The close association between EBV infection and the development of some lymphomas[13–18] prompted further strategies specifically targeting expressed EBV-encoded proteins. Although, as mentioned, DLI can be used as strategy to control PTLD, this treatment is associated with a very high risk of development of GVHD. To mitigate this risk, Rooney et al developed a method to selectively expand in-vitro EBV-specific T cells (EBVSTs) that are present in healthy donor peripheral blood lymphocytes [19]. Since these EBVSTs are specific for EBV-derived epitopes, they should not react against recipient antigens and thus the risk of GVHD should be virtually nil. Donor EBVSTs were used in 10 allo-HSCT recipients, 3 with evidence of EBV reactivation and the remaining being at high risk of reactivation. This therapy was tolerated well without significant complications [19]. In the 3 patients with EBV reactivation, EBVST therapy led to remarkable reduction of EBV DNA levels to reach control range within 4 weeks [19], including one patient who had resolution of immunoblastic lymphoma. None of the 7 patients who received EBVSTs as prophylaxis had any GVHD or EBV reactivation for a follow-up period of up to 11 months [19]. Genetic marking showed persistence of EBVSTs for a median of 10 weeks [19]. This key study showed that EBVSTs are safe and effective to control EBV-associated PTLD. Results with this approach were updated in a larger study by Heslop et al, who reported on the use of EBVSTs in 114 patients to treat or prevent EBV-related PTLD after allo-HSCT [20]. Impressively, none of the 101 patients who received EBVSTs as prophylaxis developed EBV-related PTLD. Of 13 patients who had active PTLD, 11 attained complete remission [20]. This therapy continued to be well tolerated with no major side effects, and genetic marking of EBVSTs documented their persistence up to 9 years [20]. These and similar studies [21] confirm that EBVSTs are effective and safe to treat or prevent EBVrelated PTLD post allo-HSCT.
Many patients with EBV-associated lymphoma, however, are not post allo-HSCT and have no donors identified. In those patients, autologous T cells need to be used as a platform for EBVST-mediated ACT. This approach poses some problems because any EBV/tumor-specific T cells are likely hyporesponsive to the patients’ tumors. Thus, further strategies have been developed to optimize the immune reactivity of autologous T cells against EBV-derived antigens, in particular focusing their activity against less immunogenic proteins such as latent membrane protein (LMP) 1, LMP2, and Epstein Barr nuclear antigen (EBNA) 1 (Figure 3). For example, a study utilized genetically modified antigen presenting cells (APCs) to expand autologous T cells specifically targeting LMP1 and LMP2 [22]. It included 50 patients with EBV-associated lymphoma (HL or NHL) who received EBVSTs directed against LMP2 or LMP1 and LMP2. Among them were 29 patients who received EBVSTs as adjuvant therapy after achieving remission following multiple lines of therapy and were at high risk for relapse. At two years, their event free survival (EFS) was 82% [22]. As to the 21 patients with active disease who received EBVSTs, 11 achieved CR and 2 attained PR [22]. These results indicate that autologous EBVSTs given as therapy for active disease or in an adjuvant setting can lead to sustained responses in EBV-associated lymphoma.
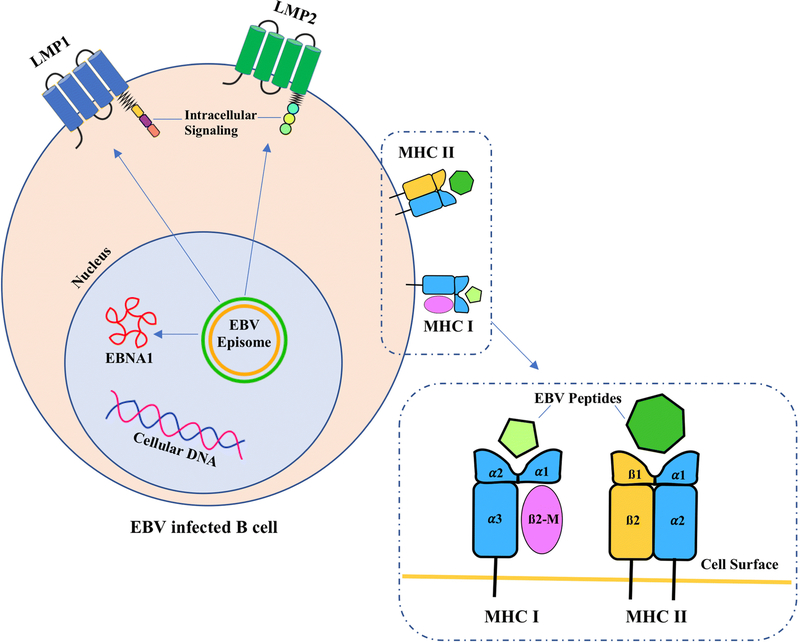
Figure 3. Examples of EBV Derived Proteins & Antigen Presentation.
EBV encoded proteins LMP1, LMP2 and EBNA1. LMP1 and LMP2 are expressed as cell surface antigens. EBNA1 resides in the nucleus. These EBV proteins are processed and presented on MHC I and MHC II molecules. All these antigens are targets for adoptive cellular therapy using native T cells or transgenic TCR T cells.
LMP1: Latent membrane protein 1
LMP2: Latent membrane protein 2
EBNA1: Epstein-Barr virus nuclear antigen 1
MHC I: Major histocompatibility complex class I (composed of an α chain with 3 domains [α1, α2 and α3] and a β−2 microglobulin (B2-M) molecule).
MHC II: Major histocompatibility complex class II (composed of 2 polypeptide chains [α and β], each with 2 domains).
A rare subtype of EBV-associated lymphoma is the extranodal NK/T cell lymphoma (ENKTL). Cho et al investigated using EBVSTs targeting LMP1/LMP2 as prevention against relapse in 10 patients with ENKTL after achieving remission by conventional means (e.g. chemotherapy, radiotherapy and HSCT) [23]. Results were encouraging with a 4-year OS of 100% and PFS of 90% (median follow-up of 55.5 months). Thus, this study suggests that EBVSTs may have an adjuvant role in preventing relapse in patients with ENKTL who achieve remission by conventional therapies [23].
To further improve the antitumor activity of EBVSTs, additional modifications of these T cells may be needed. One of the efforts to improve the efficacy of EBVSTs has been to protect them against deleterious cytokines released by tumor cells or their microenvironment.
Abundance of transforming growth factor-β (TGF-β) in the tumor microenvironment is known to enhance tumor immune evasion. Accordingly, a phase 1 study was conducted involving eight patients with EBV-associated HL (seven with relapsed disease and one in remission) to evaluate if inhibiting the TGF-β effects in T cells would lead to enhanced efficacy of EBVSTs and better outcomes [24]. The EBVSTs targeting LMP1 and LMP2 used in this study were further manipulated to express a dominant-negative TGF-β receptor type 2 (DNR2). Preclinical studies showed that DNR2-expressing EBVSTs were resistant to TGF-β in vitro and maintained their capacity to target tumor antigens effectively [24]. Among the 7 patients with refractory relapsed disease, 2 achieved CR (up to ≥ 4 years), 1 achieved PR for 19 months and 4 had stable disease (SD) for 4–13 months [24]. One patient received EBVSTs for prevention of relapse and remained in CR beyond 2 years [24]. The persistence of EBVSTs exceeded 4 years and there were no significant toxicities [24]. These promising results indicate that rendering EBVSTs resistant to the inhibitory effects of TGF-β can lead to better outcomes, although larger scale prospective studies are needed to confirm those findings.
Other potential approaches for improving T cell activity in hostile environments may be relevant in other settings. For example, PTLD has been described after solid organ transplant (SOT) as a consequence of the immune dysregulation that results from the immunosuppressive anti-rejection drugs used (e.g. tacrolimus). These same drugs would also be deleterious to adoptively transferred T cells, potentially precluding the use of autologous EBVSTs to treat PTLD. To address this problem, investigators have genetically engineered EBVSTs to make them resistant to tacrolimus by retroviral transfer of a calcineurin A mutant (CNA12) [25]. EBVSTs transduced with CNA12 (CNA12-EBVSTs) were used in immunodeficient mice bearing a B cell lymphoma xenograft in the presence or absence of tacrolimus. Mice treated with CNA12-EBVSTs had a remarkable response in the form of lymphoma regression that was not hindered by the presence of tacrolimus, and had longer survival compared to mice treated with control EBVSTs [25]. These results are encouraging and have potential clinical applications in patients with PTLD post SOT in whom discontinuation of tacrolimus may not be an option.
Despite encouraging results with autologous EBVSTs, some practical problems persist. Firstly, their manufacture takes at least one month and thus they cannot be urgently used. Furthermore, it is not possible to generate EBVSTs in all patients, often due to excessive prior therapy, an underlying immune dysfunction, and the immunosuppressive effects of active tumor, all of which compromise T cell function. All these issues lead to cell products of variable specificity and potency. To circumvent these problems, a strategy of creating banks of EBVSTs from healthy donors has been developed. These third-party EBVSTs are “off-the-shelf” products that can be readily used to treat patients with EBV-associated lymphoma, provided they are at least partially HLA-matched [26–28].
2.3. T cells Targeting Non-Viral Lymphoma-Associated-Antigens via Native TCRs
Although some lymphomas are associated with EBV infection and thus express foreign, viral antigens, the vast majority are not. Owing to that, efforts have been invested in developing tumor-specific T cells (TSTs) able to target non-viral lymphoma-associated antigens. Tumorassociated antigens (TAA) are potentially immunogenic proteins expressed by tumor cells. Unfortunately, almost all TAAs are not tumor-specific but at best only preferentially expressed by tumors. Except for the foreign, viral antigens expressed by virus-associated lymphoma, classic TAAs are usually endogenous proteins that are lineage restricted, are expressed at low levels in normal cells, or are expressed only during embryonic development or in immune privileged sites such as the testis. In contrast, neoantigens are tumor antigens that can potentially be truly tumor-specific and are expressed as a result of non-synonymous mutations and other genetic aberrations.
Both classic TAAs and neoantigens have been pursued as targets for cellular immunotherapy in lymphoma. Among the challenges faced with targeting classic TAAs is that many of those antigens are self-antigens and thus, in contrast with viral antigens, are weakly immunogenic. Protocols used to expand TSTs targeting lymphoma-associated antigens have been developed in a fashion similar to EBVSTs. Most utilize APCs that are infected by viruses that code for the specific TAA or that are loaded with peptide pools derived from TAAs [29].
Recently, the T cell leukemia/lymphoma 1 (TCL1) antigen has been identified as a TAA that can be targeted in B cell lymphomas [30]. Although TCL1 is expressed by normal B cells, there is significant overexpression in B cell lymphomas, including DLBCL and FL. Weng et al were able to develop and expand TSTs targeting TCL1 in vitro, with cytotoxicity assays showing that TCL1-TSTs were successful at targeting and lysing B cell lymphoma cells [30]. While these data are still preclinical, they hold the promise of translation into future cellular immunotherapy.
Moreover, Leen et al developed a protocol to expand autologous TSTs targeting multiple TAA, including PRAME, SSX2, MAGEA4, NY-ESO-1 and survivin, and investigated their use in 18 patients with different types of lymphoma (NHL, HL and composite lymphoma) [31]. Remarkably, 10 of 11 patients who received TSTs as adjuvant therapy continued to be in remission at 24 months. Of the 7 patients who received TSTs as therapy for active disease, 3 had CR (all had HL), 3 had SD at 3–6 months post therapy, and 1 had transient stabilization with subsequent progression [31]. These data are encouraging and expand the horizon for future research targeting multiple TAAs as a novel therapeutic modality.
2.4. T cells Targeting Neoantigens via Native TCRs
Mutations in cancer cells often lead to the expression of neoantigens, which distinctively set them apart from normal cells. Thus, they represent attractive targets since successful cellular immunotherapy would eliminate the cancer cells without affecting normal cells. One major challenge is that traditional techniques require personalized neoepitope detection and validation for every patient. A study by Khodadoust et al investigated neoantigen-based T cell cytotoxicity using samples from 17 patients with mantle cell lymphoma [32]. Neoantigens were identified using a novel methodology that involved MHC antigen profiling (via direct proteomic analysis) as well as genomic analysis of lymphoma cells. Interestingly, all neoantigens identified belonged to the lymphoma immunoglobulin (Ig) variable regions (heavy or light chain) and were mostly presented via MHC-II [32]. Subsequently, autologous T cells specifically targeting those neoantigens were isolated and expanded. When mixed with autologous lymphoma cells, T cell-mediated cytotoxicity against lymphoma cells occurred [32]. This identifies Ig neoantigens as possible targets for future cellular therapy in lymphoma and suggests that this methodology may be used to develop future cellular therapies targeting neoantigens.
In an effort to better understand and identify potential neoantigens, a large scale intensive analysis of different tumor samples (including some lymphomas) from 8705 patients was done with focus on alternative splicing [33]. Analysis of RNA and whole exome sequencing showed that cancer cells had up to 30% increase in alternative splicing compared to normal cells. In malignancies, peptides generated from RNA neojunctions, representing potential neoantigens, could be detected [33]. These tumor-specific splicing aberrations are thought to represent a large class of possible neoantigens. While this is still in a preclinical phase, it opens the door for future tumor vaccines as well as cellular immunotherapy targeting these neoantigens.
3. Tumor-Specific tgTCR-T cells
Instead of expanding in vitro T cell populations that react against specific antigens via their native TCRs, advances in cellular therapy have allowed genetic transfer of optimized tumor-specific TCRs, which here we refer to as transgenic TCR-T cells (tgTCR-T cells) (Figure 2). While tgTCR-T cells have shown appreciable success in clinical trials of other cancers such as melanoma [34], progress has lagged behind in lymphoma and this technology is currently still investigational. A critical hurdle to this approach is the limited number of well characterized tumor-associated epitopes for which a high affinity TCR is available. On the other hand, even if the gene for the tgTCR is successfully transduced in T cells, other problems can arise: if the native TCR is not ablated in the tgTCR-T cells, there is a potential for erroneous pairing of transgenic and endogenous chains (each TCR is composed of 2 independent polypeptide chains) with subsequent production of dysfunctional TCRs or TCRs targeting an unintended (such as a self) antigen. Moreover, even if the tgTCR is correctly expressed, a major limitation of this approach is that the recognition of tumor epitopes is HLA-restricted, and therefore a specific tgTCR can only be used to treat patients that share a compatible HLA allele. Finally, a single epitope is targeted, which may facilitate the emergence of escape mutants.
Examples of antigens being studied for this approach are MAGE-A and NY-ESO-1, which are expressed in about 40–50% of NHL [29]. However, caution still has to be exercised since a recent study showed lethal cardiotoxicity (due to unexpected cross-reactivity) when high affinity tgTCR-T cells targeting MAGE-A3 were used to treat myeloma and melanoma patients [35]. Thus, currently, this tool remains investigational and needs further refinement before larger scale clinical application is pursued in lymphoma. A critical element for future success is the ability to ensure that tgTCR-T cells consistently distinguish cancerous from healthy cells.
4. Beyond T Cells
Although T cells have been the focus of most ACT efforts, other immune effector cell populations may offer specific advantages, in particular a much lower risk of GVHD when used in a non-autologous setting, and thus a greater potential to manufacture “off-the-shelf” products (which can be readily available for treatment). NKT cells are a subset of innate-like lymphocytes that share properties of both T cells and NK cells, and utilize an almost universal TCR to react to antigens presented on the invariant CD1d molecule. Tian et al recently discovered that the CD62L+ NKT subset has prolonged in vivo persistence, setting the stage for the development of NKT cellular therapy in lymphoma using NKT cells transduced with a CAR [36]. Mouse models examining the use of NKT CD62L+ transduced with anti-CD19 CAR showed sustained regression in B cell lymphoma [36]. While this data is still preclinical, a clinical trial is currently planned to explore this approach[37]. If successful, NKT CAR therapy will carry the advantage of possible “off-the-shelf” allogeneic use, which will represent a breakthrough in terms of cost and time of preparation of the product.
Alternatively, NK cell-based cellular immunotherapies are also being pursued. Apart from being amenable to genetic modification that can endow them with specific antitumor activity, NK cells are known to have intrinsic anti-lymphoma cytotoxicity. For example, a recent phase 2 clinical trial investigated using haploidentical NK cell therapy in combination with rituximab and interleukin-2 in patients with refractory NHL [38]. Among 15 patients evaluated, 2 (13.3%) had CR for 3 and 9 months, and 4 (26.6%) achieved objective response at 2 months [38]. This therapy was well tolerated overall. There was no GVHD, CRS or neurotoxicity reported. Interestingly, patients who responded had low levels of immune-suppressor cells (Tregs and myeloid derived suppressor cells) compared to those who did not respond [38]. These results support haploidentical NK therapy as another option in the growing arsenal of cellular immunotherapy for patients with refractory lymphoma.
Conclusions and Future Directions:
Cellular immunotherapy has been rapidly evolving and increasingly utilized in the management of relapsed or refractory lymphomas, especially as a consequence of the impressive results obtained with CD19-specific CARTs. Pivotal clinical trials of two CART products, axicabtagene ciloleucel and tisagenlecleucel, have led to their breakthrough FDA approval. Given this success, additional CAR lymphoma targets are currently being explored.
While effective, current CART products have significant drawbacks, including that their activity is limited against a single antigen that has to be located on the surface of tumor cells. TCR-mediated antigen recognition allows potential targeting of a much larger array of tumor antigens because virtually every tumor protein (membrane-bound or intracellular) can be processed and presented by MHC molecules. On the other hand, in contrast with the single epitope targeted by a CAR or a transgenic TCR, antigen recognition mediated by diverse native TCRs targets multiple epitopes and thus decreases the chance of immune escape driven by tumor mutations. Nonetheless, few bona fide tumor-specific antigens have been identified.
Virus-associated lymphomas should in theory be amenable to targeting of tumor-specific antigens via recognition of viral proteins (provided they continue to be expressed after malignant transformation occurs). The clinical activity seen with EBVSTs supports this approach, even though targeting foreign viral antigens alone may not be sufficient for consistent responses. Indeed, further engineering of EBVSTs may be needed for optimal activity, as demonstrated by the improved activity of EBVSTs when rendered resistant to the deleterious effects of inhibitory cytokines.
Unfortunately, apart from viral antigens and neoantigens (which are challenging to identify), most known TAAs are not truly tumor-specific, but rather endogenous molecules that are either poorly immunogenic or problematic as targets because of their expression in healthy organs. In this setting, effective immune responses against tumor cells can potentially be associated with toxicity to normal tissues. Nonetheless, the encouraging results seen with strategies focusing on developing T cells reactive against classic TAAs show that this approach may be feasible.
Finally, one major limitation of current T cell therapies is that products need to be autologous because of potential risks of GVHD and product rejection when they are used in an allogeneic setting. A major disadvantage of an autologous product is the time needed for its manufacture. In addition, previous exposure of a patient to lymphotoxic agents likely compromises the potential efficacy of an autologous product. Therefore, a readily available, “offthe-shelf”, universal immune effector product derived from healthy donors is highly desirable. Genetic modification of healthy T cells to prevent GVHD and rejection, through ablation of native TCRs and MHC molecules for instance, is being investigated in this setting. Alternatively, specific immune effector populations such as NK and NKT cells are naturally less prone to cause GVHD and thus may be particularly suited for use as universal effectors instead of T cells.
All limitations notwithstanding, recent advances in immunology, genetics and cell processing, as well as improved understanding of tumor biology will likely expand far beyond CARTs the range of cellular immunotherapies available to treat lymphomas.
Acknowledgments
This work was supported in part by grants from the Leukemia and Lymphoma Society Specialized Center of Research (grant 7018) and the National Institutes of Health National Cancer Institute (grant 3P50CA126752). MG has research funding from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (5T32DK060445).
Carlos A. Ramos has received compensation from Novartis and Celgene for service on advisory boards, and has received research funding from Tessa Therapeutics.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Mahmoud R. Gaballa declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References:
* of importance
** of outstanding importance
- 1.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. 2017;377(26):2531–44. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. New England Journal of Medicine. 2018;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 3.Bloor AJ, Thomson K, Chowdhry N, Verfuerth S, Ings SJ, Chakraverty R et al. High response rate to donor lymphocyte infusion after allogeneic stem cell transplantation for indolent nonHodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14(1):50–8. doi: 10.1016/j.bbmt.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 4.van Besien KW, de Lima M, Giralt SA, Moore DF Jr., Khouri IF, Rondon G et al. Management of lymphoma recurrence after allogeneic transplantation: the relevance of graftversus-lymphoma effect. Bone Marrow Transplant. 1997;19(10):977–82. doi: 10.1038/sj.bmt.1700781. [DOI] [PubMed] [Google Scholar]
- 5.Mandigers CM, Verdonck LF, Meijerink JP, Dekker AW, Schattenberg AV, Raemaekers JM. Graft-versus-lymphoma effect of donor lymphocyte infusion in indolent lymphomas relapsed after allogeneic stem cell transplantation. Bone Marrow Transplant. 2003;32(12):1159–63. doi: 10.1038/sj.bmt.1704290. [DOI] [PubMed] [Google Scholar]
- 6.Thomson KJ, Morris EC, Milligan D, Parker AN, Hunter AE, Cook G et al. T-cell-depleted reduced-intensity transplantation followed by donor leukocyte infusions to promote graft-versuslymphoma activity results in excellent long-term survival in patients with multiply relapsed follicular lymphoma. J Clin Oncol. 2010;28(23):3695–700. doi: 10.1200/jco.2009.26.9100. [DOI] [PubMed] [Google Scholar]
- 7.Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH et al. Infusions of Donor Leukocytes to Treat Epstein-Barr Virus-Associated Lymphoproliferative Disorders after Allogeneic Bone Marrow Transplantation. New England Journal of Medicine. 1994;330(17):1185–91. doi: 10.1056/nejm199404283301703. [DOI] [PubMed] [Google Scholar]
- 8.Grant M, Bollard CM. Developing T-cell therapies for lymphoma without receptor engineering. Blood Adv. 2017;1(26):2579–90. doi: 10.1182/bloodadvances.2017009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikiforow S, Kim HT, Daley H, Reynolds C, Jones KT, Armand P et al. A phase I study of CD25/regulatory T-cell-depleted donor lymphocyte infusion for relapse after allogeneic stem cell transplantation. Haematologica. 2016;101(10):1251. doi: 10.3324/haematol.2015.141176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleakley M, Heimfeld S, Loeb KR, Jones LA, Chaney C, Seropian S et al. Outcomes of acute leukemia patients transplanted with naive T cell–depleted stem cell grafts. The Journal of Clinical Investigation. 2015;125(7):2677–89. doi: 10.1172/JCI81229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant ML, Bollard CM. Cell therapies for hematological malignancies: don’t forget nongene-modified t cells! Blood reviews. 2018;32(3):203–24. doi: 10.1016/j.blre.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sala E, Crocchiolo R, Gandolfi S, Bruno-Ventre M, Bramanti S, Peccatori J et al. Bendamustine Combined with Donor Lymphocytes Infusion in Hodgkin’s Lymphoma Relapsing after Allogeneic Hematopoietic Stem Cell Transplantation. Biology of Blood and Marrow Transplantation. 2014;20(9):1444–7. doi: 10.1016/j.bbmt.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Shannon-Lowe C, Rickinson AB, Bell AI. Epstein-Barr virus-associated lymphomas. Philos Trans R Soc Lond B Biol Sci. 2017;372(1732):20160271. doi: 10.1098/rstb.2016.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson LR, Nalesnik MA, Swerdlow SH. Impact of Epstein-Barr virus in monomorphic Bcell posttransplant lymphoproliferative disorders: a histogenetic study. Am J Surg Pathol. 2006;30(12):1604–12. doi: 10.1097/01.pas.0000213317.59176.d2. [DOI] [PubMed] [Google Scholar]
- 15.Walker RC, Marshall WF, Strickler JG, Wiesner RH, Velosa JA, Habermann TM et al. Pretransplantation assessment of the risk of lymphoproliferative disorder. Clin Infect Dis. 1995;20(5):1346–53. doi: 10.1093/clinids/20.5.1346. [DOI] [PubMed] [Google Scholar]
- 16.Aris RM, Maia DM, Neuringer IP, Gott K, Kiley S, Gertis K et al. Post-transplantation lymphoproliferative disorder in the Epstein-Barr virus-naive lung transplant recipient. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1712–7. doi: 10.1164/ajrccm.154.6.8970360. [DOI] [PubMed] [Google Scholar]
- 17.Shahinian VB, Muirhead N, Jevnikar AM, Leckie SH, Khakhar AK, Luke PP et al. EpsteinBarr virus seronegativity is a risk factor for late-onset posttransplant lymphoroliferative disorder in adult renal allograft recipients. Transplantation. 2003;75(6):851–6. doi: 10.1097/01.Tp.0000055098.96022.F7. [DOI] [PubMed] [Google Scholar]
- 18.Uhlin M, Wikell H, Sundin M, Blennow O, Maeurer M, Ringden O et al. Risk factors for Epstein-Barr virus-related post-transplant lymphoproliferative disease after allogeneic hematopoietic stem cell transplantation. Haematologica. 2014;99(2):346–52. doi: 10.3324/haematol.2013.087338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345(8941):9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 20.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–35. doi: 10.1182/blood-2009-08-239186.•• Key study showing EBVSTs are safe and effective in treating or preventing EBV-related PTLD after allo-HSCT.
- 21.Doubrovina E, Oflaz-Sozmen B, Prockop SE, Kernan NA, Abramson S, Teruya-Feldstein J et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119(11):2644–56. doi: 10.1182/blood-2011-08-371971.•• Study showing EBVSTs are effective in treating EBV related lymphoproliferative disease after allo-HSCT.
- 22.Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014;32(8):798–808. doi: 10.1200/jco.2013.51.5304.• Autologous EBVSTs targeting LMP2 or LMP1 and LMP2 antigens show sustained responses in EBV associated lymphoma.
- 23.Cho S-G, Kim N, Sohn H-J, Lee SK, Oh ST, Lee H-J et al. Long-term Outcome of Extranodal NK/T Cell Lymphoma Patients Treated With Postremission Therapy Using EBV LMP1 and LMP2a-specific CTLs. Molecular Therapy. 2015;23(8):1401–9. doi: 10.1038/mt.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bollard CM, Tripic T, Cruz CR, Dotti G, Gottschalk S, Torrano V et al. Tumor-Specific TCells Engineered to Overcome Tumor Immune Evasion Induce Clinical Responses in Patients With Relapsed Hodgkin Lymphoma. 2018;36(11):1128–39. doi: 10.1200/jco.2017.74.3179.• Enhanced antitumor activity of EBVSTs can be achieved by rendering them resistant to the inhibitory effects of TGF-β.
- 25.Ricciardelli I, Blundell MP, Brewin J, Thrasher A, Pule M, Amrolia PJ. Towards gene therapy for EBV-associated posttransplant lymphoma with genetically modified EBV-specific cytotoxic T cells. Blood. 2014;124(16):2514–22. doi: 10.1182/blood-2014-01-553362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallot G, Vollant S, Saiagh S, Clemenceau B, Vivien R, Cerato E et al. T-cell therapy using a bank of EBV-specific cytotoxic T cells: lessons from a phase I/II feasibility and safety study. J Immunother. 2014;37(3):170–9. doi: 10.1097/cji.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 27.Haque T, Wilkie GM, Jones MM, Higgins CD, Urquhart G, Wingate P et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110(4):1123–31. doi: 10.1182/blood2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 28.Rouce RH, Thakkar A, Sharma S, Shah N, Reyna A, Ramos CA et al. Use of highly characterized EBV-Specific T Cells outside of the immediate Post-Transplant setting. Cytotherapy. 2019;21(5, Supplement):e3. doi: 10.1016/j.jcyt.2019.04.010. [DOI] [Google Scholar]
- 29.Perna SK, Huye LE, Savoldo B. Management of patients with non-Hodgkin’s lymphoma: focus on adoptive T-cell therapy. ImmunoTargets and therapy. 2015;4:55–63. doi: 10.2147/itt.S31389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng J, Rawal S, Chu F, Park HJ, Sharma R, Delgado DA et al. TCL1: a shared tumorassociated antigen for immunotherapy against B-cell lymphomas. Blood. 2012;120(8):1613–23. doi: 10.1182/blood-2011-09-382838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leen A, Tzannou I, Bilgi M, Liu H, Vera JF, Gerdemann U et al. Immunotherapy for Lymphoma Using T Cells Targeting Multiple Tumor Associated Antigens. Blood. 2015;126(23):186. [Google Scholar]
- 32.Khodadoust MS, Olsson N, Wagar LE, Haabeth OA, Chen B, Swaminathan K et al. Antigen presentation profiling reveals recognition of lymphoma immunoglobulin neoantigens. Nature. 2017;543(7647):723–7. doi: 10.1038/nature21433.• Lymphoma Immunoglobulin neoantigens identified as possible targets fo future cellular therapy.
- 33.Kahles A, Lehmann K-V, Toussaint NC, Hüser M, Stark SG, Sachsenberg T et al. Comprehensive Analysis of Alternative Splicing Across Tumors from 8,705 Patients. Cancer Cell. 2018;34(2):211–24.e6. doi: 10.1016/j.ccell.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science (New York, NY). 2006;314(5796):126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122(6):863–71. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian G, Courtney AN, Jena B, Heczey A, Liu D, Marinova E et al. CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J Clin Invest. 2016;126(6):2341–55. doi: 10.1172/JCI83476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CD19.CAR Allogeneic NKT for Patients With Relapsed or Refractory B-Cell Malignancies (ANCHOR)-ClinicalTrials.gov Identifier: NCT03774654. 2019. https://clinicaltrials.gov/ct2/show/NCT03774654.
- 38.Bachanova V, Sarhan D, DeFor TE, Cooley S, Panoskaltsis-Mortari A, Blazar BR et al. Haploidentical natural killer cells induce remissions in non-Hodgkin lymphoma patients with low levels of immune-suppressor cells. Cancer Immunol Immunother. 2018;67(3):483–94. doi: 10.1007/s00262-017-2100-1.• Novel immune effector cells such as haploidentical NK cells can be used in patients with refractory NHL.
- 39.Tai Y-T, Anderson KC. B cell maturation antigen (BCMA)-based immunotherapy for multiple myeloma. Expert opinion on biological therapy. 2019:1–14. doi: 10.1080/14712598.2019.1641196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen AD, Garfall AL, Stadtmauer EA, Lacey SF, Lancaster E, Vogl DT et al. B-Cell Maturation Antigen (BCMA)-Specific Chimeric Antigen Receptor T Cells (CART-BCMA) for Multiple Myeloma (MM): Initial Safety and Efficacy from a Phase I Study. Blood. 2016;128(22):1147. [Google Scholar]
- 41.Ramos CA, Savoldo B, Torrano V, Ballard B, Zhang H, Dakhova O et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. The Journal of clinical investigation. 2016;126(7):2588–96. doi: 10.1172/JCI86000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mamonkin M, Rouce RH, Tashiro H, Brenner MK. A T cell-directed chimeric antigen receptor for the selective treatment of T cell malignancies. Blood. 2015:blood-2015–02-629527. doi: 10.1182/blood-2015-02-629527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. doi: 10.1056/NEJMoa121513410.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. doi:10.1126/scitranslmed.300593010.1126/scitranslmed.300593010.1126/scitranslmed.300593010.1126/scitranslmed.3005930 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4–1BB domains: pilot clinical trial results. Blood. 2012;119(17):3940–50. doi: 10.1182/blood-2011-1038796910.1182/blood-2011–10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan J, Niu Q, Deng B, Liu S, Wu T, Gao Z et al. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. 2019. doi: 10.1038/s41375-019-0488-7 10.1038/s41375–019-0488–7. [DOI] [PubMed] [Google Scholar]
- 47.Wang C-M, Wu Z-Q, Wang Y, Guo Y-L, Dai H-R, Wang X-H et al. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I Trial. Clinical Cancer Research. 2017;23(5):1156. doi: 10.1158/10780432.CCR-16-1365. [DOI] [PubMed] [Google Scholar]
- 48.Ramos CA, Ballard B, Zhang H, Dakhova O, Gee AP, Mei Z et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J Clin Invest. 2017;127(9):3462–71. doi: 10.1172/jci94306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scarfò I, Ormhøj M, Frigault MJ, Castano AP, Lorrey S, Bouffard AA et al. Anti-CD37 chimeric antigen receptor T cells are active against B-and T-cell lymphomas. Blood. 2018;132(14):1495. doi: 10.1182/blood-2018-04-842708. [DOI] [PMC free article] [PubMed] [Google Scholar]