Abstract
Loudness dependence of auditory evoked potentials (LDAEP) has long been considered to reflect central basal serotonin transmission. However, the relationship between LDAEP and individual serotonin receptors and transporters has not been fully explored in humans and may involve other neurotransmitter systems. To examine LDAEP’s relationship with the serotonin system, we performed PET using serotonin-1A (5-HT1A) imaging via [11C]CUMI-101 and serotonin transporter (5-HTT) imaging via [11C]DASB on a mixed sample of healthy controls (n=4: 4 females, 0 males), patients with unipolar (MDD, n=11: 4 females, 7 males) and bipolar depression (BD, n=8: 4 females, 4 males). On these same participants, we also performed electroencephalography (EEG) within a week of PET scanning, using 1000 Hz tones of varying intensity to evoke LDAEP. We then evaluated the relationship between LDAEP and 5-HT1A or 5-HTT binding in both the raphe (5-HT1A)/midbrain (5-HTT) areas and in the temporal cortex. We found that LDAEP was significantly correlated with 5-HT1A positively and with 5-HTT negatively in the temporal cortex (p<0.05), but not correlated with either in midbrain or raphe. In males only, exploratory analysis showed multiple regions in which LDAEP significantly correlated with 5-HT1A throughout the brain; we did not find this with 5-HTT. This multimodal study partially validates preclinical models of a serotonergic influence on LDAEP. Replication in larger samples is necessary to further clarify our understanding of the role of serotonin in perception of auditory tones.
Keywords: LDAEP, electroencephalography, auditory evoked potential, serotonin 1A, serotonin transporter, positron emission tomography
1. Introduction
Psychiatric disorders, including mood disorders such as major depressive disorder (MDD) and bipolar disorder (BD), cause immense global burden. In 2013, MDD and BD were the 2nd and 17th leading causes of years lost to disability, respectively (Collaborators G.B.o.D.S., 2015). A physiological understanding of these disorders is critical to providing effective care.
One of the principal leads into mood disorder pathophysiology is the serotonin system (Belmaker and Agam, 2008; Sobczak et al., 2002). Serotonin is understood to be involved in auditory perception—layer IV of the primary auditory cortex is richly innervated with both serotonergic fibers from the raphe nucleus (Lewis et al., 1986; Morrison and Foote, 1986) and ascending projections from the auditory pathway (Winer, 1984). Therefore, examining this region may provide a window into the serotonergic system. For example, hearing a tone results in an auditory evoked potential as measured by electroencephalography (EEG). If the volume of a tone is increased, it results in a proportional increase in evoked potential, called the loudness dependence of the auditory evoked potential (LDAEP). LDAEP is hypothesized to be linked to basal levels of central serotonergic transmission in the auditory cortex due to serotonin’s modulatory effects on the cortex (Hegerl and Juckel, 1993). Dipole source analyses have supported involvement of auditory cortex in generation of LDAEP (Hegerl et al., 1994).
This hypothesis has received some support using various methods. Notably, in cats, when serotonin transmission was blocked via serotonergic modulators applied directly to the brainstem, a larger LDAEP was measured, and when serotonin transmission was increased, a smaller LDAEP was measured (Juckel et al., 1999; Juckel et al., 1997). In humans, a reduced LDAEP with alcohol administration (which has serotonergic agonist activity) (Hegerl et al., 1996) and an inverse correlation of LDAEP with a serotonin syndrome scale have been observed (Hegerl et al., 1998). Moreover, LDAEP has been found to correlate with depressive symptoms in people with a history of childhood trauma, which is suggested to reduce central serotonin transmission (Lee and Park, 2016) and has also been found to vary with mood states in BD (Lee et al., 2012), suggesting that LDAEP may be connected to serotonergic process that cause SSRIs to carry a risk for mania. In addition, studies with single photon emission computed tomography (SPECT) suggest a correlation between serotonin transporter (5-HTT) binding in the midbrain and LDAEP, though findings have been mixed across radiotracers; two studies showed a negative correlation (Lee et al., 2011; Pogarell et al., 2008) while one showed a positive correlation (Pogarell et al., 2004). Pretreatment LDAEP has also been shown to be related to selective serotonin reuptake inhibitor (SSRI) treatment response in individuals with depression (Gallinat et al., 2000; Hegerl et al., 2001; Jaworska et al., 2013; Lee et al., 2015; Park et al., 2012) and in other disorders with agents such as lithium (Hegerl et al., 1988; Hegerl et al., 1992). These studies have consistently found an association between a large LDAEP, hypothesized to reflect low central serotonin, and successful response to treatment.
However, there has been evidence in humans that does not support the relationship between LDAEP and central serotonin transmission such as the lack of change in LDAEP with acute tryptophan depletion, which decreases central serotonin (O’Neill et al., 2008b) and the fact that LDAEP appears not to change with SSRI treatment (Gallinat et al., 2000; Guille et al., 2008). In addition, there may be other neurotransmitter systems involved in the LDAEP signal (O’Neill et al., 2007; O’Neill et al., 2008a); LDAEP has been found to correlate with illness duration in schizophrenia, which may involve dopamine (Park et al., 2015). Interestingly, LDAEP has also been positively associated with serum brain derived neurotrophic factor (BDNF) which has been posited to be altered in depression, and negatively associated with serum triglycerides which has been thought to be related to suicide risk (Park et al., 2014a; Park et al., 2014b).
Positron emission tomography (PET) imaging may provide a useful means to directly assess LDAEP’s relationship with the serotonergic system. PET has several advantages over SPECT imaging including increased sensitivity (Rahmim and Zaidi, 2008) and the ability to utilize 11C and 18F for radiolabeling, which are more chemically similar to endogenous compounds than the heavier nuclides required for SPECT imaging. Comparing quantitative PET signals with LDAEP can help clarify the relationship of LDAEP with serotonin receptor and transporter levels in humans. While this does not directly answer whether LDAEP reflects serotonin transmission, it allows exploration of which receptors or transporters are associated with the signal, which can provide valuable information about the underpinnings of this EEG signal.
In the present study, we performed both EEG and PET imaging with two radiotracers—one that binds to serotonin-1A receptors (5-HT1A) and one that binds to serotonin transporters (5-HTT)—to fully explore LDAEP’s relationship with the serotonergic system. We included healthy control participant data, as well as data from both patients with MDD and BD before and after lithium treatment, as these populations combined with the effect of treatment would be expected to yield a wide range of serotonin system states that could help assess the validity and generalizability of a relationship between 5-HT1A, transporter and LDAEP.
We examined two regions of interest (ROIs) per PET radiotracer: the raphe nucleus (5-HT1A) or midbrain (5-HTT), and the temporal cortex (both 5-HT1A and 5-HTT - which includes superficial structures (e.g. the primary auditory cortex), but excludes deep structures (e.g. the hippocampus and amygdala)—see Figure 1), based on evidence from previous studies in humans and cats of that indicate the temporal cortex as the likely source of LDAEP (Hegerl et al., 1994). Our inclusion of the raphe nucleus and midbrain stems from aforementioned studies in which serotonergic agents applied directly to the brainstem of cats caused changes in LDAEP (Juckel et al., 1999; Juckel et al., 1997). In addition, we have found in our own work greater raphe nucleus 5-HT1A density in individuals with MDD relative to controls (Miller et al., 2009; Parsey et al., 2010; Parsey et al., 2006b) and BD (Lan et al., 2013; Sullivan et al., 2009) (though we have not replicated a difference in BD in our most recent study (Ananth et al., Submitted), which could indicate an overall deficit in central serotonin transmission, as 5-HT1A autoreceptors reduce overall serotonin output from the raphe nucleus (Blier et al., 1998) (though other neurotransmitter systems could also be affected, such as the noradrenergic system (Szabo and Blier, 2001)). This may have a direct effect on LDAEP. For 5-HTT, we examined the midbrain, encompassing the superior and inferior colliculi, rather than just the raphe, as 5-HTT does not have the same somatodendritic distribution as 5-HT1A (Miller et al., 2013). Moreover, prior SPECT studies found a correlation of 5-HTT with LDAEP in the midbrain (Lee et al., 2011; Pogarell et al., 2008; Pogarell et al., 2004).
Figure 1:
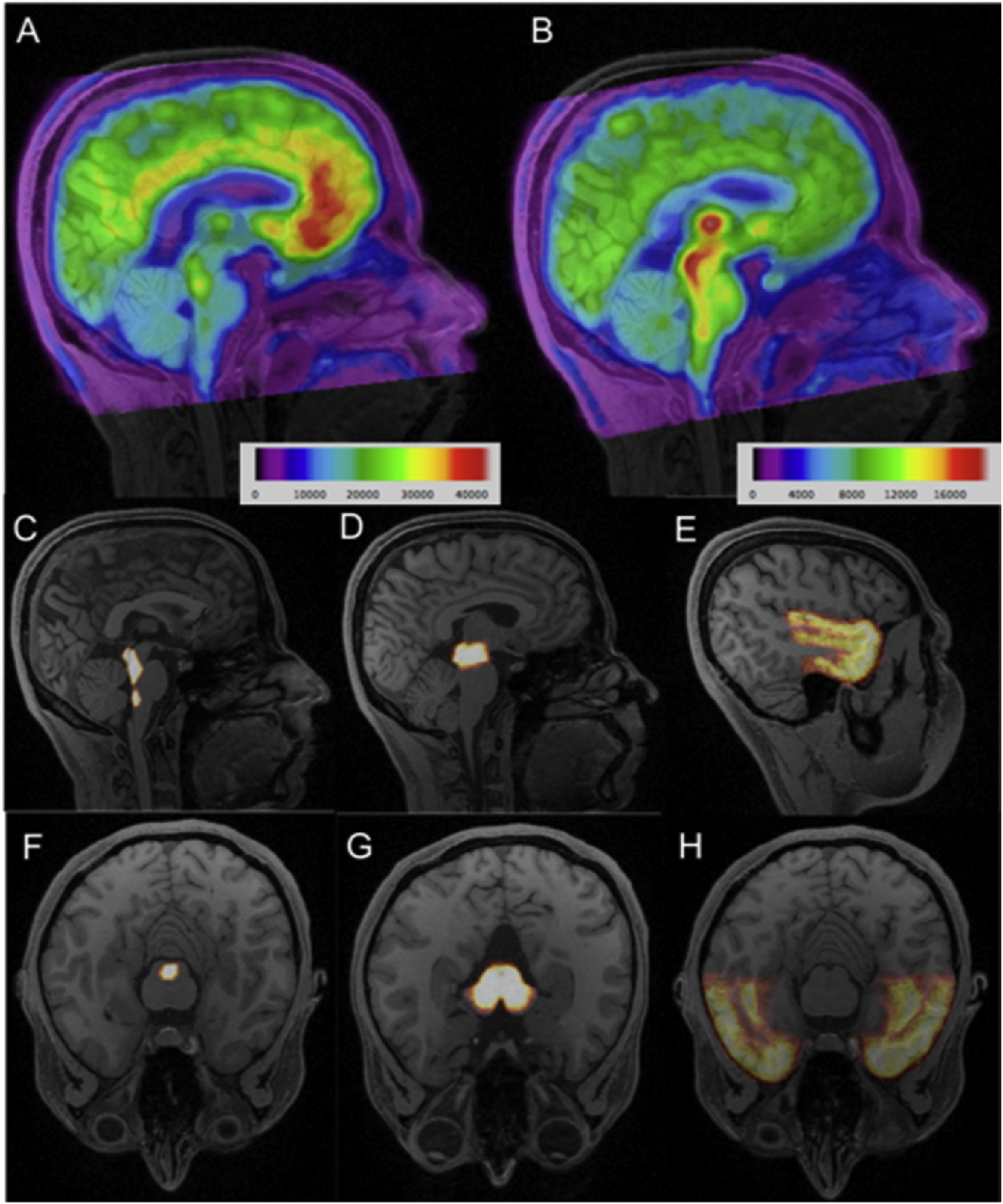
Top Row (A-B): Representative average [11C]CUMI-101 (A) and [11C]DASB (B) PET images for a single participant, overlaid on the participant’s MRI. Colorbar represents activity and is in microCuries. Center and Bottom Rows (C-H): Probabilistic regions of interest labels are shown in sagittal (center row: C-E) and axial (bottom row: F-H) views. For [11C]CUMI-101, the a priori regions were the raphe nucleus (C, F) and temporal cortex (E, H), while a priori regions for [11C]DASB were the midbrain (D, G) and temporal cortex (E, H). Color intensity corresponds to probability of voxel corresponding to region of interest.
We hypothesized that LDAEP would correlate positively with 5-HT1A in the raphe nucleus and temporal cortex, as greater 5-HT1A in the raphe nucleus will halt serotonin transmission, which would be reflected by post-synaptic 5-HT1A upregulation and a larger LDAEP in the temporal cortex. We also hypothesized that LDAEP would correlate negatively with 5-HTT in the midbrain and temporal cortex, as a greater LDAEP should correspond with reduced serotonin in the synaptic cleft and hence, less serotonin transporters for reuptake.
Given the known sex differences in MDD and BD (Arnold, 2003; Diflorio and Jones, 2010; Karanti et al., 2014), as well as in LDAEP (Oliva et al., 2011) it is also important to determine whether sex can influence the PET/EEG relationship. Further, sex differences have been seen in both 5-HTT and 5-HT1A binding (Cannon et al., 2013; Jovanovic et al., 2008), although conflicting evidence exists for sex effects in 5-HTT especially when considering PET outcome measure, radiotracer used, and population studied (Martinez et al., 2013; Miller et al., 2013). Further, a previous study examining the correlation between SPECT-derived 5-HTT binding and LDAEP found that the correlation was much stronger in females than in males (Pogarell et al., 2008), though no explanation was given for this finding. Similar lines of conflicting evidence exist for age effects in both 5-HTT/5-HT1A (Dillon et al., 1991; Karrer et al., 2019; Parsey et al., 2002; van Dyck et al., 2000). Finally, the temporal lobe has connections across the brain, including such areas as the cingulate cortex, frontal cortex, and occipital complex (Bajada et al., 2017)—while it is likely that LDAEP originates in temporal cortex, it is possible that serotonin receptor and transporter distribution elsewhere is associated with the signal due to these connections. Therefore, as an exploratory measure, we analyzed sex and age differences in the relationship between LDAEP and both 5-HT1A and 5-HTT across all regions of the brain.
2. Methods
2.1. Participants
The participants in this study are a subset of those acquired under a protocol aimed at elucidating lithium’s mechanism of action in BD (R01MH090276; PI: Ramin Parsey). Importantly, this study also included healthy control participants and participants with MDD. Therefore, this study utilizes a mixed-population sample with PET imaging pre- and post-treatment for MDD and BD participants.
The Institutional Review Boards of Columbia University Medical Center, the New York State Psychiatric Institute, Yale University, and Stony Brook Medicine approved the study. All participants provided informed written consent. Though 27 individuals received both PET and EEG (one participant was removed due to not receiving a PET scan), four were removed due to noisy EEG data (see section 1.2.5), in which evoked potential peaks could not be discerned. The resulting sample comprised 23 adults (see Table 1). Of these, 11 met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for MDD, 8 met DSM-IV criteria for BD, and 4 did not meet criteria for any axis I disorders. Diagnosis was made based on a structured clinical interview for DSM-IV Axis I Disorders (SCID IV), the 17-item Hamilton Depression Rating Scale (HDRS-17), and the Beck Depression Inventory (BDI). Inclusion criteria for participants with BD and MDD included: age 18–70 years, in a current depressive episode as defined by DSM IV criteria for MDD or BD, a score of at least 15 on the HDRS-17, able to tolerate a 3-week washout of current antidepressant treatment prior to the start of the study, able to stop all psychotropic and other drugs likely to interact with 5-HTT or 5-HT1A, and be medication free for 3 weeks (most medications, neuroleptics), 6 weeks (fluoxetine), or 3 months (serotonin depleting drugs such as reserpine) prior to the start of the study, and able to be off anti-coagulant treatment, with the exception of aspirin, for 10 days before PET imaging (short-acting benzodiazepines for distressing anxiety/insomnia allowed up to 24 hours prior to each PET scan). Exclusion criteria included: lack of capacity to consent, already on an effective medication for their depression, other major psychiatric disorders such as schizophrenia, drug or alcohol dependence within six months, anorexia or bulimia nervosa within a year (except for bulimia nervosa, non-purging type plus a normal BMI, which was not exclusionary), intravenous drug use within 5 years, or MDMA (ecstasy) use more than 15 times in the past 10 years or any MDMA use in the past month, a first degree relative with schizophrenia if the participant was less than 33 years old (mean age of onset of schizophrenia plus two standard deviations), significant physical illness that may affect the brain or serotonergic system, significant suicidal ideation with intent before or during medication washout, electroconvulsive therapy (ECT) within six months, pregnancy, currently lactating, or abortion within past two months, metal implants, pacemaker, shrapnel, metal prostheses, medicinal patch that cannot be removed (contraindicated for MRI, which is needed for regional delineation), neurological disease or loss of consciousness for more than a few minutes (asked of patients to screen for neurological damage), and hearing loss greater than 20 decibels (dB) or inter-aural hearing difference of 10 dB as measured by screening audiometry. Demographic data can be found in Table 1. Of the 23 participants with usable PET and EEG data, 2 participants with BD and 5 with MDD received post-treatment (lithium) PET and EEG, in addition to pre-treatment scans. These comprised 3 females and four males. These 7 extra scans were included, bringing the total number of PET/EEG scans to 30. Pre/post treatment status was accounted for in statistical analyses to include a larger range of serotonergic states, which would yield more information on the PET-LDAEP relationship.
Table 1:
Participant Demographics
| Diagnosis | MDD | BD | P-value* | Control |
|---|---|---|---|---|
| N N=23 participants (N=30 total scans**) |
11 | 8 | -- | 4 |
| Age (SD) | 34.5 (12.8) | 31.3 (14.5) | 0.63 | 22.0 (4.1) |
| Number Female/Male (% Female/% Male) |
4/7 (36%/64%) |
4/4 (50%/50%) |
0.55 | 4/0 (100%/0%) |
| Screening HDRS-17 (SD) | 23.6 (4.8) | 22.2 (3.7) | 0.45 | 1.5 (0.6) |
| HDRS-24 Pre-Treatment (SD) | 27.4 (6.5) | 28.6 (3.9) | 0.60 | -- |
| HDRS-24 Post-Treatment (SD) | 16.6 (12.1) | 16.4 (10.5) | 0.98 | -- |
MDD: major depressive disorder, BD: bipolar disorder, SD: standard deviation, HDRS: Hamilton Depression Rating Scale, 17 or 24 Item
Calculated by t-test for continuous variables, chi-square for categorical variables. P-values only computed across MDD and BD groups based on small sample (N=4) and different variables in control group.
2 participants with BD and 5 with MDD received pre- and post-treatment PET and EEG, making the total sample size 30 even though there were 23 total participants.
2.2. Experimental Protocol
Participants with MDD or BD who were on ineffective medication underwent a medication free period, as described above, following a washout from any antidepressant treatment. Following the medication free period, participants received a structural magnetic resonance imaging (MRI) scan, PET scans with both radiotracers on the same day, and EEG, all within a week of each other. Participants were then started on treatment with lithium and titrated to a therapeutic plasma level of 0.8 – 1.2 mEp/l. After 6–10 weeks of lithium therapy, participants received repeat MRI, PET, and EEG. As described above, seven PET/EEG studies in our sample were post-treatment scans.
2.3. Radiochemistry and Input Function Measures
23 participants (11 MDD, 8 BD, 4 control) received [11C]CUMI-101 scans, and 17 (7 MDD, 6 BD, 4 control) received [11C]DASB scans (see Figure 1). [11C]CUMI-101 and [11C]DASB were synthesized as previously described (Belanger et al., 2004; Kumar et al., 2007). For both radiotracers, arterial samples were collected automatically for the first 6 minutes and manually thereafter throughout the PET scans. Samples were measured for radioactivity using a 1480 Wizard 3M automatic gamma counter (Wallac, Turku, Finland). Six arterial samples were also analyzed using high-pressure liquid chromatography to assess the percentage of parent compound—these were fit to a Hill function for [11C]CUMI-101 or a bi-exponential function for [11C]DASB (Wu et al., 2007). Plasma radioactivity counts were multiplied by the percentage of parent compound to obtain the metabolite-corrected arterial input function, which was fit with a line before the peak and a sum of 3 exponentials after the peak (Milak et al., 2010; Ogden et al., 2007).
For 11 scans (5 MDD, 3 BD, 3 control), a less-invasive quantification method known as simultaneous estimation (SIME) was used. Briefly, this method works by estimating input function fit parameters, assumed to be common to all brain regions, simultaneously with kinetic parameters from several ROIs with varying kinetics (Ogden et al., 2010). A single blood sample, either arterial (1) or venous (10), was used to anchor the arterial input function. We initially validated this technique using a single arterial sample (Ogden et al., 2010), and have recently validated the use of a venous sample for both [11C]CUMI-101 and [11C]DASB (Bartlett et al., 2018)
2.4. Image Acquisition and Analysis
MRI was performed on either a Siemens Magnetom Skyra or Prisma scanner at Stony Brook University. The parameters are as follows: for the Skyra, relaxation time 2300 ms, echo time 2.98 ms, T1 900 ms, flip angle 9 degrees, slice thickness 1 mm, resolution 1mm × 1mm × 1mm; for the Prisma, relaxation time 2300 ms, echo time 3.05 ms, T1 900 ms, flip angle 9 degrees, slice thickness 0.87 mm, resolution 0.9mm × 0.9mm × 0.9mm. PET was performed with an ECAT EXACT HR+ (Siemens/CTI, Knoxville, Tennessee). [11C]CUMI-101 emission data were collected for 120 minutes after bolus radiotracer injection as 20 frames of increasing duration (3 × 20 sec, 3 × 1 min, 3 × 2 min, 2 × 5 min, 9 × 10 min). [11C]DASB emission data were collected for 100 minutes after bolus radiotracer injection as 19 frames of increasing duration (3 × 20 sec, 3 × 1 min, 3 × 2 min, 2 × 5 min, 8 × 10 min). Image analysis was performed using in-house Matlab 2012b software (The Mathworks, Natick, Massachusetts), with extensions to Functional Magnetic Resonance Imaging of the Brain’s (FMRIB’s) Linear Image Registration Tool (FLIRT v.5.2 (Jenkinson and Smith, 2001)), Brain Extraction Tool v1.2 (Smith, 2002), and Statistical Parametric Mapping (SPM5, (Ashburner and Friston, 2005)). Frame-by-frame rigid body registration was performed to a reference frame to correct for subject motion using FLIRT, followed by co-registration of the average PET image to the subject’s MRI, also using FLIRT, to generate eight possible coregistrations. The eight possible coregistrations were estimated as previously validated using different combinations of the PET frames (e.g. the mean over the early frames vs the late frames) and MRI image (e.g. skull-stripped vs whole field of view) as the sources and targets, respectively (DeLorenzo et al., 2009). Each option was visually inspected and the optimal coregistration was selected. As previously shown, PET data with limited whole-brain spatial information, resulting from varying biodistributions across PET radiotracers, can be difficult to accurately coregister and therefore, benefits from visual inspection/selection with a validated procedure (DeLorenzo et al., 2009).
As previously described, the temporal cortex and midbrain ROIs were previously hand drawn on a set of 18 template MRI scans based on brain atlases and published reports (Duvernoy, 1991; Killiany et al., 1997; Talairach and Tournoux, 1988). These templates were warped to each subjects’ MRI using the Automatic Registration Toolbox (ART) (Ardekani et al., 2005) to generate probabilistic temporal cortex and midbrain ROIs in subject-space. Because the raphe nucleus is not visible on MRI scans, a separate [11C]WAY100635 (which binds to serotonin-1A) study in 52 healthy controls was used to delineate the raphe nucleus ROI. In that study, voxel binding maps were calculated for these 52 participants and averaged in a standard space. A thresholding technique was used within the brainstem to identify the raphe nucleus ROI, which has higher binding than the background, in the standard space average image. This average image was associated with a high-resolution MRI image in the same space. The raphe nucleus ROI in standard space, which comprised 279 voxels (2232 mm3), was used as an atlas for the current study; it was warped to the individual subject’s PET image using Advanced Normalization Tools (ANTs) (Avants et al., 2008; Avants et al., 2011) through the individual’s MRI (Delorenzo et al., 2013). The activity within the raphe nucleus, midbrain, and temporal cortex ROIs were separately averaged within each PET frame to generate time activity curves for each region (see Figure 1 for ROI placement).
We have previously determined likelihood estimation in graphical analysis (LEGA) (Ogden, 2003) as an optimal modeling technique for both [11C]CUMI-101 (Milak et al., 2008) and [11C]DASB (Ogden et al., 2007) PET data. Using the time activity curves and arterial input function, either calculated directly or estimated using SIME as described above, we applied LEGA to generate binding estimates. As previously validated, BPF was the outcome measure used for [11C]CUMI-101, which is calculated as (VT – VND)/fP, where VT is the region of interest’s total distribution volume, VND is the reference region’s distribution volume (assumed to contain only nonspecific binding; cerebellar grey matter used here), and fP is the free fraction of radiotracer in the plasma (Innis et al., 2007). For [11C]DASB, we used the outcome measure VT/fP, as previously validated, because there is no accepted reference region to calculate VND for this tracer (Parsey et al., 2006a). A priori ROIs included the temporal cortex and raphe nucleus for [11C]CUMI-101 and the temporal cortex and midbrain for [11C]DASB. Standard errors for each PET measure were calculated using a bootstrapping technique that incorporates errors in fitting the brain data (Ogden and Tarpey, 2006) and were used for all statistical analyses.
2.5. Electroencephalography and Loudness Dependent Auditory Evoked Potentials
All EEG data were acquired by a single operator. EEG was acquired with a Biosemi ActiveTwo 32 channel system. Participants had their hearing tested and were then seated in a comfortable chair in a sound-attenuated room. Participants listened to 1000 Hz tones of 60, 70, 80, 90, and 100 dB in pseudorandomized order. Each tone lasted for 40 milliseconds (ms), with an interstimulus interval between 1500 and 2100 ms. Participants listened to five blocks of 100 tones each, for a total of 500 tones. EEG was collected using the 10–20 system via ActiView software and processed using BrainVision Analyzer 2. Data was rereferenced offline to the average activity recorded from the left and right mastoid bones, and band-pass filtered from 0.1 to 30 Hz, with a 24 dB/octave rolloff (Widmann et al., 2015). Ocular activity was recorded using electrodes above and below the right eye and at the outer canthi of both eyes—ocular artifacts were corrected using a regression-based approach (Gratton et al., 1983). EEG data were segmented into 1000 ms epochs and were further examined for artifacts using an automated procedure. Voltage greater than 50 μV between sample points, a voltage difference greater than 175 μV within a segment, or a maximum voltage of less than 0.50 μV within 100 ms intervals were counted as artifacts. One participant had 20 segments per sound level removed by artifact rejection in their post-treatment EEG; the rest had three or less segments removed.
EEG data at each sound intensity were averaged and baseline corrected for 200 ms before each epoch, yielding five waveforms for each participant. These waveforms were examined at the Cz position, as previous studies have shown this to be the most effective position for measuring LDAEP (Gallinat et al., 2000; Simmons et al., 2011). The largest negative peak between 50 and 200 ms was detected automatically and defined as N1, while the largest positive peak between 150 and 300 ms was detected automatically and defined as P2. LDAEP was defined as the slope of the linear regression of the difference between P2 and N1 at each sound intensity (see Figure 2). Averaged waveforms were manually inspected and removed if significant noise was still present and local peaks could not be discerned—six EEGs from four participants (including 2 post-treatment scans) were removed based on this criterion., as mentioned in section 1.2.1. Standard errors for LDAEP were used in all statistical analyses and were calculated as the error of the regression slope, i.e.:
where is the residual of an N1–P2 peak-to-peak amplitude from the regression line, n is the number of data points in the regression (i.e. 5 data points, one for each decibel level), and is the difference of each decibel level at each data point from the mean decibel level ( is equal to 80 dB in all cases).
Figure 2:
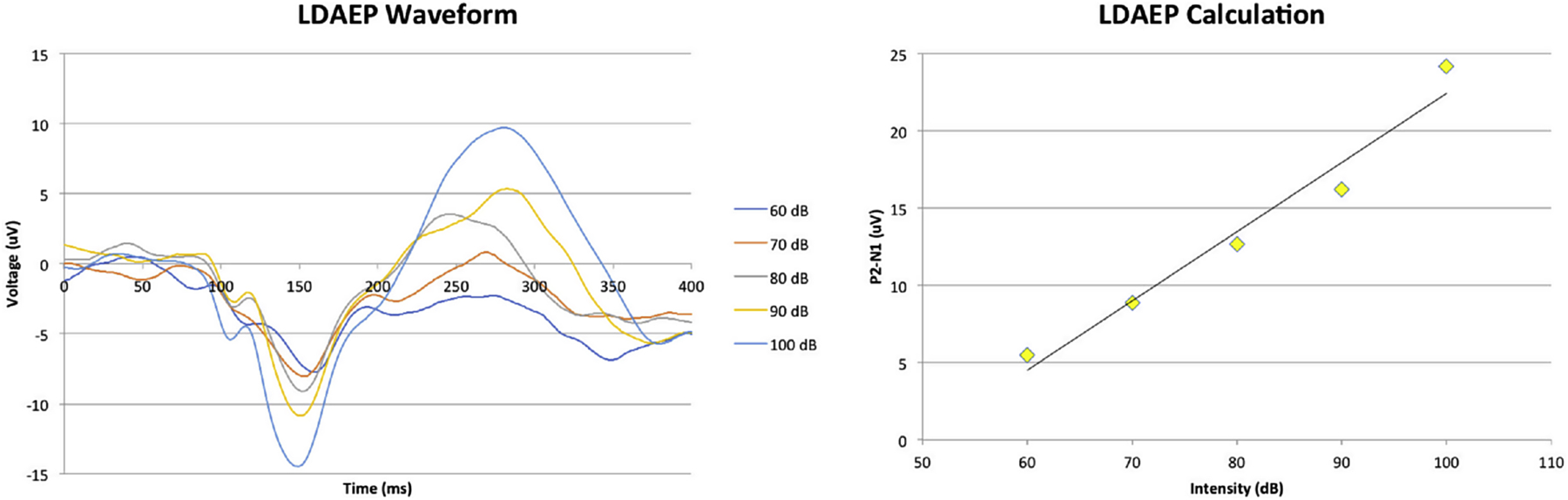
LDAEP calculation. A: Example EEG waveform in response to tones of varying loudness levels. B: N1–P2 peak-to-peak amplitude by decibel level. LDAEP is defined as the slope of this regression.
2.6.1. Statistical Analysis: A priori hypothesis testing
Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC). To determine the relationship between LDAEP and 5-HT1A/5-HTT binding, separate linear mixed models were constructed with either 5-HT1A or 5-HTT in the a priori regions (5-HT1A: raphe and temporal cortex and 5-HTT: midbrain and temporal cortex) as the model outcome and LDAEP and region as fixed factors. The interaction between LDAEP and region was also examined. Standard errors for both PET measures and LDAEP were included. To account for repeated measures (pre- and post-treatment scans in 7 of the participants), the relationship between pre- and post-treatment scans within participant was modeled as a random factor. LDAEP and PET measures were log-transformed to meet model assumptions in all statistics.
2.6.2. Statistical Analysis: Exploratory Analyses
The effects of sex, age, and pre/post treatment status on the relationships between 5-HT1A/5-HTT and LDAEP were examined with linear mixed models as above, but with independent linear mixed models fit for each region across all individual brain regions in our atlas: anterior cingulate cortex, amygdala, cerebellum, cingulate cortex, dorsal caudate, dorsolateral prefrontal cortex, dorsal putamen, entorhinal cortex, hippocampus, inferior anterior cingulate cortex, insula, medial prefrontal cortex, midbrain, occipital cortex, orbitofrontal cortex, parietal cortex, parahippocampal gyrus, raphe nucleus, superior anterior cingulate cortex, temporal cortex, thalamus, uncus, ventral striatum, and cerebellar white matter. Individual linear mixed models were fit for each region, rather than grouping regions together to avoid issues in fitting the variance-covariance structure for each of the 25 regions. Pre/post-treatment status was included as a fixed factor to control for any differences in PET binding pre- and post-treatment. Rather than an interaction with region as in the a priori models, an interaction with sex was considered, as well as age. Post-hoc analyses exploring this sex effect were further conducted (LDAEP-PET relationship in males, females, and comparing this relationship in males vs females).. The significance of main effects, interactions, and post-hocs in all models were determined via Type III F-tests based on the fitted linear mixed effect models. Bonferroni correction was performed for the 25 regions examined across both tracers (50 total linear mixed models tested), with a further penalization for the three post-hoc analyses conducted per linear mixed model (LDAEP-PET relationship in males, females, and males vs females), yielding a total correction for 150 exploratory statistical tests (Bonferroni correction threshold: p<0.0003).
3. Results
3.1. A priori hypothesis testing
LDAEP was not significantly related to 5-HT1A across the a priori regions temporal cortex and raphe nucleus (p=0.22). The correlation between 5-HT1A and LDAEP was not significantly different between the a priori regions (p=0.38). However, when examining post-hoc analyses, temporal cortex 5-HT1A binding was significantly positively associated with LDAEP (p=0.02, b=0.33), whereas raphe nucleus 5-HT1A was not significantly associated with LDAEP (p=0.69, b=0.11). The model accounted for 77.0% of the variance in 5-HT1A.
LDAEP was related to 5-HTT at a trend level across the a priori regions temporal cortex and midbrain (p=0.057). The correlation between 5-HTT and LDAEP was not significantly different between the a priori regions (p=0.40). However, when examining post-hoc analyses, temporal cortex 5-HTT binding was significantly negatively associated with LDAEP (p=0.01, b=−0.72), whereas midbrain 5-HTT was not significantly associated with LDAEP (p=0.18, b=−0.54). The model accounted for 68.7% of the variance in 5-HTT. P-values are reported uncorrected for a priori hypothesis testing (see Figure 3).
Figure 3:
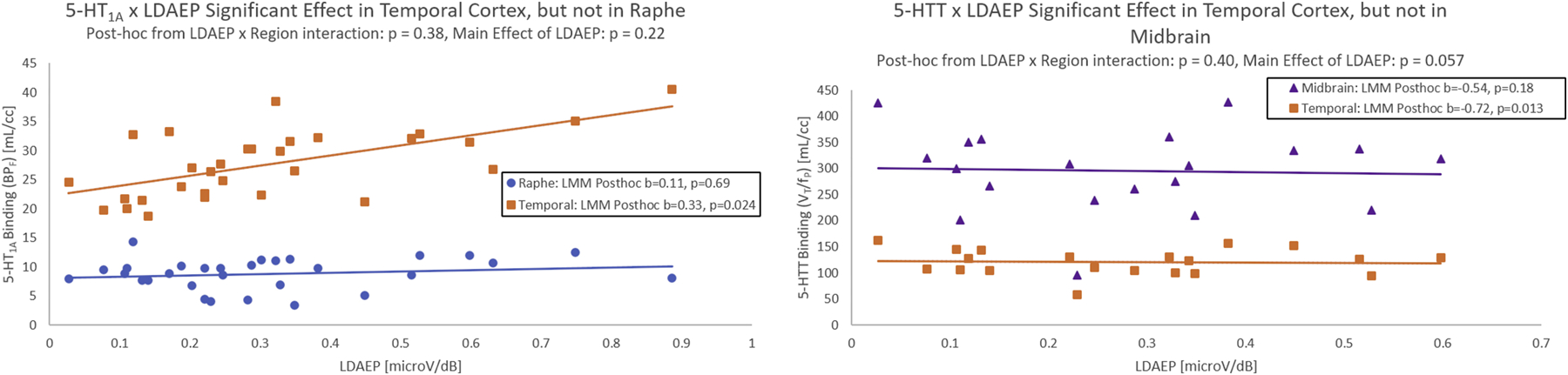
5-HT1A (LEFT) and 5-HTT (RIGHT) binding shown against LDAEP for all participants in the a priori raphe nucleus (5-HT1A), midbrain (5-HTT), and temporal cortex. Linear regression fits are shown between 5-HT1A/5-HTT and LDAEP for illustration purposes. All beta coefficients (b) and p-values reported are from the linear mixed models (LMM) fit that account for possible repeated measures.
3.2. Exploratory Analyses
For full results of the exploratory whole-brain analyses examining the sex- and age-specific relationships of LDAEP by 5-HT1A/5-HTT, see Supplemental Tables 1 and 2. In brief, the relationship between LDAEP and 5-HT1A/5-HTT did not significantly differ between males and females (p-values > 0.0003, Bonferroni threshold for the 150 = 25 regions × 2 tracers × 3 post-hoc tests). However, there was a significant relationship between LDAEP and 5-HT1A in males only for the brain regions: temporal cortex, anterior cingulate cortex, medial prefrontal cortex, occipital cortex, orbitofrontal cortex, and parietal cortex (p’s <= 0.0003; Supplemental Table 1). In females, LDAEP and 5-HT1A were not significantly related in any region (p’s>0.0003). These results indicate a global pattern of 5-HT1A and LDAEP correspondence in males (see Figure 4).
Figure 4:
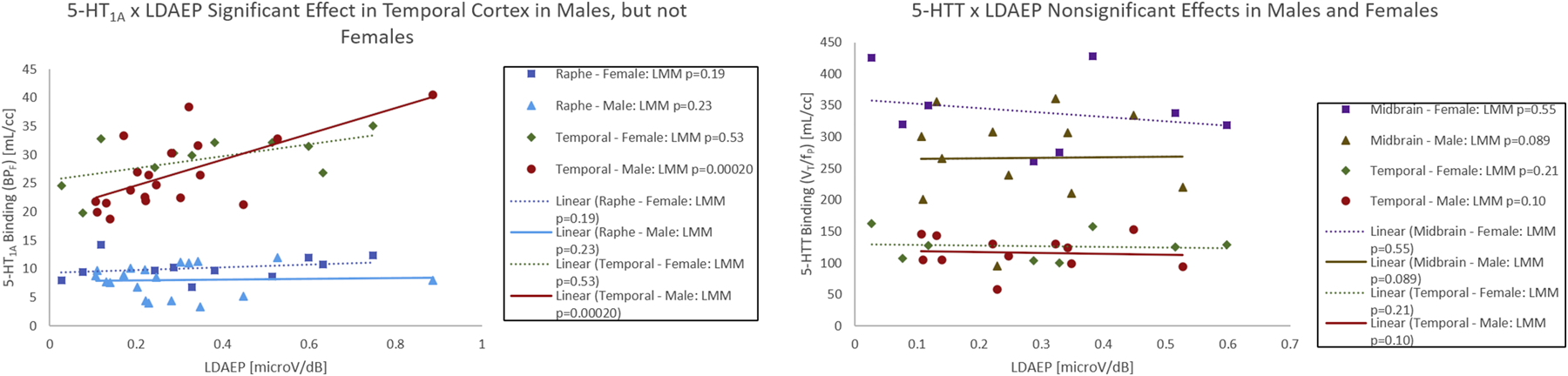
Results from exploratory analyses illustrated for 5-HT1A (LEFT) and 5-HTT (RIGHT) binding against LDAEP stratified by sex in the a priori raphe nucleus (5-HT1A), midbrain (5-HTT), and temporal cortex. Linear regression fits are shown between 5-HT1A/5-HTT and LDAEP for males and females for illustration purposes. All p-values reported are from the exploratory linear mixes models (LMM) fit that account for possible repeated measures and include age, sex, and pre/post treatment as main effects.
With 5-HTT, after Bonferroni correction, the relationship between LDAEP and 5-HTT was not significant in males or females in any region tested.
Across both 5-HT1A and 5-HTT, the main effects of pre/post-treatment status, sex, and age were not significant (p-values>0.0003).
To investigate the effect of diagnosis, the above exploratory models were repeated including a diagnosis (HC, MDD, BD) model factor, whereby the main effect of diagnosis was not significant (p’s = 0.09 to 0.96 for the 5-HT1A model and p’s = 0.13 to 0.61 for the 5-HTT model).
Because there were only n=4 healthy controls included and all healthy controls were female, we further repeated all a priori and exploratory analyses with only the MDD and BD participants. The a priori results were replicated where only post-hoc LDAEP by 5-HT1A/5-HTT relationships were significant in the temporal cortex (p=0.03 for 5-HT1A and p=0.02 for 5-HTT). As with all participants included, the relationship between 5-HT1A and LDAEP was significant in several regions of the brain in males only (see Supplemental Tables 3 and 4). The null findings for LDAEP by 5-HT1A in females, as well as for LDAEP by 5-HTT in males and females were replicated.
To avoid the additional statistical confounds that may result from repeated measures within participants, and to ensure the results would replicate, we repeated the analyses using only single, pre-treatment measurements for each participant (the n=7 post-treatment scans were excluded). Here, the a priori results were replicated where only post-hoc LDAEP by 5-HTT relationships were significant in the temporal cortex (p=0.02). However, the relationship between LDAEP and 5-HT1A in the temporal cortex was weakened with the removal of these scans (p=0.27), possibly due to a smaller sample size. The diffuse pattern of LDAEP × 5-HT1A positive correlation was strengthened relative to the initial model that included both pre- and post-treatment scans, and the null findings for LDAEP by 5-HT1A in females, as well as for LDAEP by 5-HTT in males and females were replicated (see Supplemental Tables 5 and 6).
One male participant had notably higher LDAEP and higher 5-HT1A binding than other male participants. Sex-specific results remained unchanged when this participant was excluded; for example, in the temporal lobe in males, the estimated slope between 5-HT1A and LDAEP decreased from 0.064 to 0.055, but the relationship was still significant (see Supplemental Table 7).
4. Discussion
The prospect of loudness dependence of auditory evoked potentials (LDAEP) as a marker of central serotonin transmission has existed for close to 30 years (Hegerl and Juckel, 1993). Evidence supporting the link between LDAEP and serotonin has been well established in animals (Juckel et al., 1999; Juckel et al., 1997). However, human studies have thus far been conflicting regarding LDAEP’s relationship with serotonin transmission. For example, acute tryptophan depletion (O’Neill et al., 2008b) and SSRI administration (Gallinat et al., 2000) have not significantly altered LDAEP in humans, whereas LDAEP has been shown to predict response to SSRIs (Gallinat et al., 2000; Hegerl et al., 2001; Jaworska et al., 2013; Park et al., 2012). These conflicting results have thus obfuscated our understanding of LDAEP (O’Neill et al., 2008a). Part of the difficulty in examining the serotonergic basis of LDAEP in humans is that it is impossible to target specific aspects of the serotonin system in the same manner as animals—one cannot, for example, directly apply a serotonin-1A antagonist to a person’s brainstem and observe the response. Compounding this difficulty is the sheer complexity of the serotonin system, which involves over 14 different receptors and 7 different subtypes (Pytliak et al., 2011). We cannot examine all receptors at once in vivo and thus, can only probe the relationship with individual receptors.
Our findings suggest that there is a correlation between LDAEP and serotonin receptor/transporter binding in the temporal cortex, but not in the midbrain/raphe nucleus. In addition, there is a sexually dimorphic pattern of correlation between LDAEP and serotonin receptor binding across many brain regions. Specifically, when including sex and age in our model, we found a sex dimorphism such that in males only, LDAEP showed significantly positive correlation with 5-HT1A binding in various areas of the brain after Bonferroni correcting for 150 tests (adjusted p-value significance threshold: < 0.0003). It is possible that in an a priori analysis without such strict multiple comparisons correction more regions would show a significant positive correlation. Of all the regions, the a priori regions had the largest model coefficients in males, with 0.67 for raphe nucleus and 0.96 for temporal cortex. This means that for every unit change of 1 for LDAEP, binding potential for 5-HT1A would change by 0.67 or 0.96, respectively. For the serotonin transporter, however, no significant relationships between LDAEP and 5-HTT were found in any brain region. Our findings were largely consistent when post-treatment scans and controls were removed from the analysis, which suggests that including multiple measures on the same participant did not bias the results.
Importantly, the correlations between LDAEP and the serotonergic system were chiefly present in males and not females. Our lab has previously found that differences in raphe nucleus 5-HT1A binding between people with and without MDD are significantly greater in males than in females (Kaufman et al., 2015; Pillai et al., 2018a). Moreover, we have found a high correlation of 5-HT1A receptor binding across different brain regions in males, which may explain the correlation across many regions here. While this collinearity between brain regions is greater in controls, it is still present in depression (Pillai et al., 2018b). Therefore, it can be inferred that 5-HT1A binding potential may not be directly related to LDAEP in areas outside the temporal cortex and raphe, but rather that LDAEP is associated with global changes in 5-HT1A binding that occurs proportionately in several regions of the brain.
However, our finding that 5-HTT in the midbrain does not significantly correlate with LDAEP contradicts previous SPECT studies (Lee et al., 2011; Pogarell et al., 2008). It is unlikely that this is due to properties of the radiotracer, as a comparative study between 5-HTT tracers, including [11C]DASB and [11C]ADAM found the main difference to be the more rapid kinetics of the former (Huang et al., 2002). A key difference between our studies is that the previous SPECT studies examined healthy controls exclusively (Lee et al., 2011; Pogarell et al., 2008), while our study used a mixed sample of healthy control, MDD, and BD participants. Further investigation with a larger sample size and with pharmacological blocking studies of LDAEP, with agonists or antagonists of various serotonin receptors, would be necessary to further untangle sex-specific and receptor-specific dependencies of LDAEP and the serotonergic system. In addition, previous studies did not explicitly examine temporal cortex, which is the hypothesized source of LDAEP.
The mechanism behind the sexual dimorphism in LDAEP/5-HT1A binding correlation is far from clear. While there have been sex differences documented in auditory evoked potentials (Bakos et al., 2016; Jalaei et al., 2019; Jalaei et al., 2017) and in serotonin receptor binding (Moses-Kolko et al., 2011), if 5-HT1A were solely involved in producing LDAEP, the correlation should hold in spite of these differences. One possible hypothesis is that serotonin receptors are only partly responsible for generating LDAEP; it may be influenced by other neurotransmitter systems, or even different serotonin receptors. In this case, hormonal differences may alter serotonin receptor binding distributions (as has been shown in gonadectomized rats (Zhang et al., 1999)) which can then alter the association between 5-HT1A and LDAEP.
Despite this, our exploratory finding of a significant correlation between 5-HT1A binding and LDAEP in males suggests that there may be a mechanism by which serotonergic activity contributes to auditory potentials. Hegerl and Juckel proposed that LDAEP is related to low-level tonic (that is, slow and sustained) neurotransmission of serotonin to the primary auditory cortex, a hypothesis bolstered by dipole source analysis and prior studies showing decreased LDAEP with administration of serotonin agonists (Hegerl and Juckel, 1993). High levels of tonic activation are thought to decrease the level of cortical response due to lateral inhibition (Grossberg, 1984), i.e. the ability of an activated neuron to lower the activity of surrounding neurons (Hartline and Ratliff, 1957). Therefore, greater basal serotonergic transmission would result in more lateral inhibition and thus, smaller amplitude changes in evoked potentials as sounds increase in loudness. Recent evidence supporting this has been found in mice: administration of the drug 5-Methoxy-N,N-demethyltryptamine (5-MeO-DMT), a nonselective 5-HT1A/5-HT2A agonist, was found to inhibit serotonin release and reduce low frequency cortical oscillations in the primary auditory, primary visual, and prefrontal cortices (Riga et al., 2016). This suggests that higher 5-HT1A across the brain is related to lower tonic activation of the auditory cortex and a higher LDAEP upon phasic stimulation.
Evidence from rodent studies indicates that both 5-HT1A and 5-HT2A have strong modulatory effects on brain reactivity (Basura et al., 2008; Papesh and Hurley, 2016) and GABAergic transmission (Garcia-Oscos et al., 2015). In fact, while administering 5-meO-DMT in rodents caused a reduction in basal low frequency oscillations in auditory, prefrontal, and primary visual cortex, knocking out the 5-HT2A receptors selectively prevented this reduction in auditory cortex, but not in prefrontal or primary visual cortices. This suggests that 5-HT2A may play a larger role for the auditory cortex and LDAEP in particular. In addition, a polymorphism in the 5-HT1B receptor has been linked to higher LDAEP on the left side of the brain (Juckel et al., 2008). A pharmacological blocking study of LDAEP, with agonists or antagonists of various serotonin receptors, would be necessary to determine if one or the other receptor contributed more to the signal. In addition, our findings do not rule out the influence of other neurotransmitter systems, such as dopamine, on LDAEP.
To consolidate our findings with the serotonergic hypothesis of LDAEP, we hypothesize the following. High levels of serotonin levels result in tonic activation and lower cortical reactivity. This is reflected by high 5-HTT levels, which increase in response to serotonin in the synaptic cleft. Conversely, 5-HT1A will be low, as large amounts of serotonin will cause a homeostatic decrease in post-synaptic receptor levels. Presynaptic 5-HT1A is also expected to be low, as decreased autoreceptor availability will disinhibit the raphe nucleus, resulting in greater serotonin release—however, given the cortical source of LDAEP the correlation is not as strong. Meanwhile, low levels of serotonin, reflected by low 5-HTT, result in a homeostatic increase in 5-HT1A and a corresponding increase in reactivity. It is important to note that, as stated above, there is evidence in preclinical models that 5-HT1A may in fact have a direct effect on tonic 5-HT transmission in addition to being reflective of 5-HT levels, though receptor blocking studies will be necessary to determine whether this is true in humans.
Sample size was the main limitation in this study. While 27 participants received both EEG and PET scans, only 23 participants’ data passed our quality control protocols, with 7 of these 23 participants receiving both pre and post-lithium treatment scans, bringing the final sample size for LDAEP/PET data to 30 scan sets. While limited in the number of post-treatment scans (n=7), post-treatment scans were included to represent a range within the serotonin system and provide further information on the nature of the relationship between LDAEP and serotonin receptor/transporter binding. That the relationship between 5-HT1A/5-HTT and LDAEP was preserved even when post-treatment and control participants were removed further supports its validity. Moreover, main effects of pre/post treatment status were not significant. Importantly, the linear mixed effects models controlled for the repeated measures with fixed and random effects to ensure the models were not biased. Another limitation was the limited number of healthy controls available for analysis with both LDAEP and PET data, and the fact that all of our controls were female. However, the results remained consistent when controls were removed from the analysis. Given our recent finding that the association between raphe nucleus 5-HT1A and post-synaptic 5-HT1A binding is reduced in MDD relative to healthy controls (Pillai et al., 2018b), however, it will be important to assess differences in 5-HT1A-LDAEP relationships across populations in a larger study. While a future study with a large control group would be invaluable, the association of LDAEP with 5-HT1A in a mixed sample such as this is still an important finding that can inform future studies. Finally, the small sample size limited our power to assess sex differences—while our exploratory analysis indicated diffuse correlations between LDAEP and 5-HT1A in males but not females, a larger study would be necessary to definitively affirm this finding.
A possible confound of this study was the cross-reactivity of [11C]CUMI-101 to both 5-HT1A and the noradrenergic a1 receptor (Shrestha et al., 2014). However, the temporal cortex is not richly innervated with this receptor (Zilles et al., 1993) as it is with 5-HT1A (Varnas et al., 2004), making it unlikely that adrenergic binding accounts the correlation we observed. Another potential limitation is that our thresholding method of delineating the raphe nucleus may have incorporated other regions into the ROI, such as the inferior and superior colliculi, which are known to have serotonin-1A receptors (Butt et al., 2002; Hurley, 2007). Regional heterogeneity, therefore, may have decreased the effect size for the raphe nucleus in this study. However, it should be noted that this is the delineation that we have found to have significantly different 5-HT1A binding between people with and without MDD. In addition, we did not use a dedicated auditory cortex ROI and instead used temporal cortex as a whole; it is possible that using auditory cortex only would yield greater effects. Ideally, PET and EEG data would have been acquired simultaneously to avoid the possibility of changes between the times of PET and EEG, though there are significant technical challenges to such methods including head movement and artifact.
Finally, we used a relatively novel method to recover an arterial input function in the case that only a single blood draw could be performed. It is possible that differences in quantification that resulted from this method influenced our results. However, this is unlikely given that PET binding outcome measures for [11C]CUMI-101 and [11C]DASB were not significantly different across the quantification methods (Bartlett et al., 2018).
5. Conclusions
By comparing PET and EEG, an exploratory analysis suggests that that loudness dependence of auditory evoked potentials (LDAEP) is diffusely correlated with 5-HT1A across the brain, specifically in males. This multimodal, in vivo, human study provides evidence supporting a long-established animal model of serotonin transmission. Should this study be replicated in a larger sample, it would add to our understanding of the role of serotonin transmission in sensory perception.
Supplementary Material
Acknowledgments
We acknowledge the biostatistical computation and support provided by the Biostatistical Consulting Core at School of Medicine, Stony Brook University, particularly the support provided by Dr. Mengru Zhang, Ph.D. This work was funded by NIMH grants F30MH109412 (PI: Pillai) and R01MH090276 (PI: Parsey). We would like to thank Emily Hale-Rude for EEG setup assistance and Dr. Craig Tenke for assistance in experimental design and for auditory stimuli.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- Ananth M, Bartlett E, DeLorenzo C, Lin X, Kunkel L, Vadhan NP, Perlman G, Godstrey M, Holzmacher D, Ogden RT, Parsey RV, Huang C, Submitted. Prediction of Lithium Treatment Response in Bipolar Depression using 5-HTT and 5-HT1A PET. [DOI] [PMC free article] [PubMed]
- Ardekani BA, Guckemus S, Bachman A, Hoptman MJ, Wojtaszek M, Nierenberg J, 2005. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods 142, 67–76. [DOI] [PubMed] [Google Scholar]
- Arnold LM, 2003. Gender differences in bipolar disorder. Psychiatr Clin North Am 26, 595–620. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ, 2005. Unified segmentation. Neuroimage 26, 839–851. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC, 2008. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12, 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC, 2011. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajada CJ, Haroon HA, Azadbakht H, Parker GJM, Lambon Ralph MA, Cloutman LL, 2017. The tract terminations in the temporal lobe: Their location and associated functions. Cortex 97, 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakos S, Tollner T, Trinkl M, Landes I, Bartling J, Grossheinrich N, Schulte-Korne G, Greimel E, 2016. Neurophysiological Mechanisms of Auditory Information Processing in Adolescence: A Study on Sex Differences. Dev Neuropsychol 41, 201–214. [DOI] [PubMed] [Google Scholar]
- Bartlett EA, Ananth M, Rossano S, Zhang M, Yang J, Lin SF, Nabulsi N, Huang Y, Zanderigo F, Parsey RV, DeLorenzo C, 2018. Quantification of Positron Emission Tomography Data Using Simultaneous Estimation of the Input Function: Validation with Venous Blood and Replication of Clinical Studies. Mol Imaging Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basura GJ, Abbas AI, O’Donohue H, Lauder JM, Roth BL, Walker PD, Manis PB, 2008. Ontogeny of serotonin and serotonin2A receptors in rat auditory cortex. Hear Res 244, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger MJ, Simpson NR, Wang T, Van Heertum RL, Mann JJ, Parsey RV, 2004. Biodistribution and radiation dosimetry of [11C]DASB in baboons. Nucl Med Biol 31, 1097–1102. [DOI] [PubMed] [Google Scholar]
- Belmaker R, Agam G, 2008. Major depressive disorder. New England Journal of Medicine 358, 55–68. [DOI] [PubMed] [Google Scholar]
- Blier P, Pineyro G, el Mansari M, Bergeron R, de Montigny C, 1998. Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann N Y Acad Sci 861, 204–216. [DOI] [PubMed] [Google Scholar]
- Butt CM, Zhao B, Duncan MJ, Debski EA, 2002. Sculpting the visual map: the distribution and function of serotonin-1A and serotonin-1B receptors in the optic tectum of the frog. Brain Res 931, 21–31. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Klaver JM, Klug SA, Carlson PJ, Luckenbaugh DA, Ichise M, Drevets WC, 2013. Gender-specific abnormalities in the serotonin transporter system in panic disorder. Int J Neuropsychopharmacol 16, 733–743. [DOI] [PubMed] [Google Scholar]
- Collaborators G.B.o.D.S., G.B.o.D.S., 2015. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorenzo C, Delaparte L, Thapa-Chhetry B, Miller JM, Mann JJ, Parsey RV, 2013. Prediction of selective serotonin reuptake inhibitor response using diffusion-weighted MRI. Front Psychiatry 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo C, Klein A, Mikhno A, Gray N, Zanderigo F, Mann JJ, Parsey RV, 2009. A new method for assessing PET-MRI coregistration. pp. 725920W-72592W–72598.
- Diflorio A, Jones I, 2010. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry 22, 437–452. [DOI] [PubMed] [Google Scholar]
- Dillon KA, Gross-Isseroff R, Israeli M, Biegon A, 1991. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: effects of age and alcohol. Brain Res 554, 56–64. [DOI] [PubMed] [Google Scholar]
- Duvernoy H, 1991. The Human Brain Surface, Three-Dimensional Sectional Anatomy and MRI. Sringer-Verlag Wien, New York. [Google Scholar]
- Gallinat J, Bottlender R, Juckel G, Munke-Puchner A, Stotz G, Kuss HJ, Mavrogiorgou P, Hegerl U, 2000. The loudness dependency of the auditory evoked N1/P2-component as a predictor of the acute SSRI response in depression. Psychopharmacology (Berl) 148, 404–411. [DOI] [PubMed] [Google Scholar]
- Garcia-Oscos F, Torres-Ramirez O, Dinh L, Galindo-Charles L, Perez Padilla EA, Pineda JC, Atzori M, Salgado H, 2015. Activation of 5-HT receptors inhibits GABAergic transmission by pre-and post-synaptic mechanisms in layer II/III of the juvenile rat auditory cortex. Synapse 69, 115–127. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E, 1983. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol 55, 468–484. [DOI] [PubMed] [Google Scholar]
- Grossberg S, 1984. Some normal and abnormal behavioral syndromes due to transmitter gating of opponent processes. Biol Psychiatry 19, 1075–1118. [PubMed] [Google Scholar]
- Guille V, Croft RJ, O’Neill BV, Illic S, Phan KL, Nathan PJ, 2008. An examination of acute changes in serotonergic neurotransmission using the loudness dependence measure of auditory cortex evoked activity: effects of citalopram, escitalopram and sertraline. Hum Psychopharmacol 23, 231–241. [DOI] [PubMed] [Google Scholar]
- Hartline HK, Ratliff F, 1957. Inhibitory interaction of receptor units in the eye of Limulus. J Gen Physiol 40, 357–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegerl U, Bottlender R, Gallinat J, Kuss HJ, Ackenheil M, Moller HJ, 1998. The serotonin syndrome scale: first results on validity. Eur Arch Psychiatry Clin Neurosci 248, 96–103. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Gallinat J, Juckel G, 2001. Event-related potentials. Do they reflect central serotonergic neurotransmission and do they predict clinical response to serotonin agonists? J Affect Disord 62, 93–100. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Gallinat J, Mrowinski D, 1994. Intensity dependence of auditory evoked dipole source activity. Int J Psychophysiol 17, 1–13. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Juckel G, 1993. Intensity dependence of auditory evoked potentials as an indicator of central serotonergic neurotransmission: a new hypothesis. Biol Psychiatry 33, 173–187. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Juckel G, Schmidt LG, Rommelspacher H, 1996. Serotonergic ethanol effects and auditory evoked dipole activity in alcoholic and healthy subjects. Psychiatry Res 63, 47–55. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Prochno I, Ulrich G, Muller-Oerlinghausen B, 1988. Are auditory evoked potentials suitable for predicting the response to lithium prophylaxis? A study on the effects of repeated measurement, age, gender, and personality on the amplitude/stimulus intensity function in healthy volunteers. Pharmacopsychiatry 21, 336–337. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Wulff H, Muller-Oerlinghausen B, 1992. Intensity dependence of auditory evoked potentials and clinical response to prophylactic lithium medication: a replication study. Psychiatry Res 44, 181–190. [DOI] [PubMed] [Google Scholar]
- Huang Y, Hwang D-R, Narendran R, Sudo Y, Chatterjee R, Bae S-A, Mawlawi O, Kegeles LS, Wilson AA, Kung HF, 2002. Comparative evaluation in nonhuman primates of five PET radiotracers for imaging the serotonin transporters: [11C] McN 5652,[11C] ADAM,[11C] DASB,[11C] DAPA, and [11C] AFM. Journal of Cerebral Blood Flow & Metabolism 22, 1377–1398. [DOI] [PubMed] [Google Scholar]
- Hurley LM, 2007. Activation of the serotonin 1A receptor alters the temporal characteristics of auditory responses in the inferior colliculus. Brain Res 1181, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE, 2007. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27, 1533–1539. [DOI] [PubMed] [Google Scholar]
- Jalaei B, Azmi M, Zakaria MN, 2019. Gender differences in binaural speech-evoked auditory brainstem response: are they clinically significant? Braz J Otorhinolaryngol 85, 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalaei B, Zakaria MN, Mohd Azmi MH, Nik Othman NA, Sidek D, 2017. Gender Disparities in Speech-evoked Auditory Brainstem Response in Healthy Adults. Ann Otol Rhinol Laryngol 126, 290–295. [DOI] [PubMed] [Google Scholar]
- Jaworska N, Blondeau C, Tessier P, Norris S, Fusee W, Blier P, Knott V, 2013. Response prediction to antidepressants using scalp and source-localized loudness dependence of auditory evoked potential (LDAEP) slopes. Prog Neuropsychopharmacol Biol Psychiatry 44, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S, 2001. A global optimisation method for robust affine registration of brain images. Med Image Anal 5, 143–156. [DOI] [PubMed] [Google Scholar]
- Jovanovic H, Lundberg J, Karlsson P, Cerin A, Saijo T, Varrone A, Halldin C, Nordstrom AL, 2008. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage 39, 1408–1419. [DOI] [PubMed] [Google Scholar]
- Juckel G, Hegerl U, Giegling I, Mavrogiorgou P, Wutzler A, Schuhmacher C, Uhl I, Brune M, Mulert C, Pogarell O, Rujescu D, 2008. Association of 5-HT1B receptor polymorphisms with the loudness dependence of auditory evoked potentials in a community-based sample of healthy volunteers. Am J Med Genet B Neuropsychiatr Genet 147b, 454–458. [DOI] [PubMed] [Google Scholar]
- Juckel G, Hegerl U, Molnar M, Csepe V, Karmos G, 1999. Auditory evoked potentials reflect serotonergic neuronal activity--a study in behaving cats administered drugs acting on 5-HT1A autoreceptors in the dorsal raphe nucleus. Neuropsychopharmacology 21, 710–716. [DOI] [PubMed] [Google Scholar]
- Juckel G, Molnar M, Hegerl U, Csepe V, Karmos G, 1997. Auditory-evoked potentials as indicator of brain serotonergic activity--first evidence in behaving cats. Biol Psychiatry 41, 1181–1195. [DOI] [PubMed] [Google Scholar]
- Karanti A, Bobeck C, Osterman M, Kardell M, Tidemalm D, Runeson B, Lichtenstein P, Landen M, 2014. Gender differences in the treatment of patients with bipolar disorder: A study of 7354 patients. J Affect Disord 174c, 303–309. [DOI] [PubMed] [Google Scholar]
- Karrer TM, McLaughlin CL, Guaglianone CP, Samanez-Larkin GR, 2019. Reduced serotonin receptors and transporters in normal aging adults: a meta-analysis of PET and SPECT imaging studies. Neurobiol Aging 80, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Sullivan GM, Yang J, Ogden RT, Miller JM, Oquendo MA, Mann JJ, Parsey RV, DeLorenzo C, 2015. Quantification of the Serotonin 1A Receptor Using PET: Identification of a Potential Biomarker of Major Depression in Males. Neuropsychopharmacology 40, 1692–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killiany RJ, Moss MB, Nicholson T, Jolesz F, Sandor T, 1997. An interactive procedure for extracting features of the brain from magnetic resonance images: the lobes. Hum Brain Mapp 5, 355–363. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Rami-Mark C, Kaufmann U, Baldinger P, Hahn A, Hoflich A, Savli M, Stein P, Wadsak W, Mitterhauser M, Winkler D, Lanzenberger R, Kasper S, 2014. Effects of hormone replacement therapy on cerebral serotonin-1A receptor binding in postmenopausal women examined with [carbonyl-(1)(1)C]WAY-100635. Psychoneuroendocrinology 45, 1–10. [DOI] [PubMed] [Google Scholar]
- Kumar JS, Prabhakaran J, Majo VJ, Milak MS, Hsiung SC, Tamir H, Simpson NR, Van Heertum RL, Mann JJ, Parsey RV, 2007. Synthesis and in vivo evaluation of a novel 5-HT1A receptor agonist radioligand [O-methyl- 11C]2-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine-3,5(2H,4H)dione in nonhuman primates. Eur J Nucl Med Mol Imaging 34, 1050–1060. [DOI] [PubMed] [Google Scholar]
- Lan MJ, Hesselgrave N, Ciarleglio A, Ogden RT, Sullivan GM, Mann JJ, Parsey RV, 2013. Higher pretreatment 5-HT1A receptor binding potential in bipolar disorder depression is associated with treatment remission: a naturalistic treatment pilot PET study. Synapse 67, 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Park YM, 2016. How Childhood Maltreatment Is Related to Suicidality, Bipolarity and Central Serotonergic Activity in Patients with Major Depressive Disorder: A Cross-Sectional Pilot Study. Psychiatry Investig 13, 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Park YM, Lee SH, Shim M, 2015. Prediction of Long-Term Treatment Response to Selective Serotonin Reuptake Inhibitors (SSRIs) Using Scalp and Source Loudness Dependence of Auditory Evoked Potentials (LDAEP) Analysis in Patients with Major Depressive Disorder. Int J Mol Sci 16, 6251–6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IH, Yang YK, Chen PS, Huang HC, Yeh TL, Lu RB, Chiu NT, Yao WJ, Lin SH, 2011. Loudness dependence of auditory evoked potentials (LDAEP) correlates with the availability of dopamine transporters and serotonin transporters in healthy volunteers-a two isotopes SPECT study. Psychopharmacology (Berl) 214, 617–624. [DOI] [PubMed] [Google Scholar]
- Lee KS, Park YM, Lee SH, 2012. Serotonergic dysfunction in patients with bipolar disorder assessed by the loudness dependence of the auditory evoked potential. Psychiatry Investig 9, 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Foote SL, Morrison JH, 1986. The monoaminergic innervation of primate neocortex. Hum Neurobiol 5, 181–188. [PubMed] [Google Scholar]
- Martinez A, Finegersh A, Cannon DM, Dustin I, Nugent A, Herscovitch P, Theodore WH, 2013. The 5-HT1A receptor and 5-HT transporter in temporal lobe epilepsy. Neurology 80, 1465–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak MS, DeLorenzo C, Zanderigo F, Prabhakaran J, Kumar JS, Majo VJ, Mann JJ, Parsey RV, 2010. In vivo quantification of human serotonin 1A receptor using 11C-CUMI-101, an agonist PET radiotracer. J Nucl Med 51, 1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak MS, Severance AJ, Ogden RT, Prabhakaran J, Kumar JS, Majo VJ, Mann JJ, Parsey RV, 2008. Modeling considerations for 11C-CUMI-101, an agonist radiotracer for imaging serotonin 1A receptor in vivo with PET. J Nucl Med 49, 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Brennan KG, Ogden TR, Oquendo MA, Sullivan GM, Mann JJ, Parsey RV, 2009. Elevated serotonin 1A binding in remitted major depressive disorder: evidence for a trait biological abnormality. Neuropsychopharmacology 34, 2275–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Hesselgrave N, Ogden RT, Sullivan GM, Oquendo MA, Mann JJ, Parsey RV, 2013. Positron emission tomography quantification of serotonin transporter in suicide attempters with major depressive disorder. Biological psychiatry 74, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Foote SL, 1986. Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J Comp Neurol 243, 117–138. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Price JC, Shah N, Berga S, Sereika SM, Fisher PM, Coleman R, Becker C, Mason NS, Loucks T, Meltzer CC, 2011. Age, sex, and reproductive hormone effects on brain serotonin-1A and serotonin-2A receptor binding in a healthy population. Neuropsychopharmacology 36, 2729–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill BV, Croft RJ, Leung S, Oliver C, Phan KL, Nathan PJ, 2007. High-dose glycine inhibits the loudness dependence of the auditory evoked potential (LDAEP) in healthy humans. Psychopharmacology (Berl) 195, 85–93. [DOI] [PubMed] [Google Scholar]
- O’Neill BV, Croft RJ, Nathan PJ, 2008a. The loudness dependence of the auditory evoked potential (LDAEP) as an in vivo biomarker of central serotonergic function in humans: rationale, evaluation and review of findings. Hum Psychopharmacol 23, 355–370. [DOI] [PubMed] [Google Scholar]
- O’Neill BV, Guille V, Croft RJ, Leung S, Scholes KE, Phan KL, Nathan PJ, 2008b. Effects of selective and combined serotonin and dopamine depletion on the loudness dependence of the auditory evoked potential (LDAEP) in humans. Hum Psychopharmacol 23, 301–312. [DOI] [PubMed] [Google Scholar]
- Ogden RT, 2003. Estimation of kinetic parameters in graphical analysis of PET imaging data. Stat Med 22, 3557–3568. [DOI] [PubMed] [Google Scholar]
- Ogden RT, Ojha A, Erlandsson K, Oquendo MA, Mann JJ, Parsey RV, 2007. In vivo quantification of serotonin transporters using [(11)C]DASB and positron emission tomography in humans: modeling considerations. J Cereb Blood Flow Metab 27, 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden RT, Tarpey T, 2006. Estimation in regression models with externally estimated parameters. Biostatistics 7, 115–129. [DOI] [PubMed] [Google Scholar]
- Ogden RT, Zanderigo F, Choy S, Mann JJ, Parsey RV, 2010. Simultaneous estimation of input functions: an empirical study. J Cereb Blood Flow Metab 30, 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva JL, Leung S, Croft RJ, O’Neill BV, Stout JC, Nathan PJ, 2011. Evidence for sex differences in the loudness dependence of the auditory evoked potential in humans. Hum Psychopharmacol 26, 172–176. [DOI] [PubMed] [Google Scholar]
- Papesh MA, Hurley LM, 2016. Modulation of auditory brainstem responses by serotonin and specific serotonin receptors. Hear Res 332, 121–136. [DOI] [PubMed] [Google Scholar]
- Park YM, Jung E, Kim HS, Hahn SW, Lee SH, 2015. Differences in central serotoninergic transmission among patients with recent onset, sub-chronic, and chronic schizophrenia as assessed by the loudness dependence of auditory evoked potentials. Schizophr Res. [DOI] [PubMed] [Google Scholar]
- Park YM, Lee BH, Lee SH, 2014a. The association between serum lipid levels, suicide ideation, and central serotonergic activity in patients with major depressive disorder. J Affect Disord 159, 62–65. [DOI] [PubMed] [Google Scholar]
- Park YM, Lee BH, Um TH, Kim S, 2014b. Serum BDNF levels in relation to illness severity, suicide attempts, and central serotonin activity in patients with major depressive disorder: a pilot study. PLoS One 9, e91061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YM, Lee SH, Park EJ, 2012. Usefulness of LDAEP to predict tolerability to SSRIs in major depressive disorder: a case report. Psychiatry Investig 9, 80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ, 2006a. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry 163, 52–58. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E, Mikhno A, Milak M, Zanderigo F, Sullivan GM, Oquendo MA, Mann JJ, 2010. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry 68, 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, Van Heertum RL, Arango V, Mann JJ, 2006b. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry 59, 106–113. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, Mann JJ, 2002. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res 954, 173–182. [DOI] [PubMed] [Google Scholar]
- Pillai RL, Zhang M, Yang J, Boldrini M, Mann JJ, Oquendo MA, Parsey RV, DeLorenzo C, 2018a. Will imaging individual raphe nuclei in males with major depressive disorder enhance diagnostic sensitivity and specificity? Depression and anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai RL, Zhang M, Yang J, Mann JJ, Oquendo MA, Parsey RV, DeLorenzo C, 2018b. Molecular connectivity disruptions in males with major depressive disorder. Journal of Cerebral Blood Flow & Metabolism 0, 0271678X18764053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogarell O, Koch W, Schaaff N, Popperl G, Mulert C, Juckel G, Moller HJ, Hegerl U, Tatsch K, 2008. [123I] ADAM brainstem binding correlates with the loudness dependence of auditory evoked potentials. Eur Arch Psychiatry Clin Neurosci 258, 40–47. [DOI] [PubMed] [Google Scholar]
- Pogarell O, Tatsch K, Juckel G, Hamann C, Mulert C, Popperl G, Folkerts M, Chouker M, Riedel M, Zaudig M, Moller HJ, Hegerl U, 2004. Serotonin and dopamine transporter availabilities correlate with the loudness dependence of auditory evoked potentials in patients with obsessive-compulsive disorder. Neuropsychopharmacology 29, 1910–1917. [DOI] [PubMed] [Google Scholar]
- Pytliak M, Vargova V, Mechirova V, Felsoci M, 2011. Serotonin receptors - from molecular biology to clinical applications. Physiol Res 60, 15–25. [DOI] [PubMed] [Google Scholar]
- Rahmim A, Zaidi H, 2008. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun 29, 193–207. [DOI] [PubMed] [Google Scholar]
- Riga MS, Bortolozzi A, Campa L, Artigas F, Celada P, 2016. The serotonergic hallucinogen 5-methoxy-N,N-dimethyltryptamine disrupts cortical activity in a regionally-selective manner via 5-HT(1A) and 5-HT(2A) receptors. Neuropharmacology 101, 370–378. [DOI] [PubMed] [Google Scholar]
- Shrestha SS, Liow JS, Lu S, Jenko K, Gladding RL, Svenningsson P, Morse CL, Zoghbi SS, Pike VW, Innis RB, 2014. (11)C-CUMI-101, a PET radioligand, behaves as a serotonin 1A receptor antagonist and also binds to alpha(1) adrenoceptors in brain. J Nucl Med 55, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JG, Nathan PJ, Berger G, Allen NB, 2011. Chronic modulation of serotonergic neurotransmission with sertraline attenuates the loudness dependence of the auditory evoked potential in healthy participants. Psychopharmacology (Berl) 217, 101–110. [DOI] [PubMed] [Google Scholar]
- Smith SM, 2002. Fast robust automated brain extraction. Hum Brain Mapp 17, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczak S, Honig A, van Duinen MA, Riedel WJ, 2002. Serotonergic dysregulation in bipolar disorders: a literature review of serotonergic challenge studies. Bipolar Disord 4, 347–356. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Ogden RT, Oquendo MA, Kumar JS, Simpson N, Huang YY, Mann JJ, Parsey RV, 2009. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol Psychiatry 66, 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo ST, Blier P, 2001. Serotonin (1A) receptor ligands act on norepinephrine neuron firing through excitatory amino acid and GABA(A) receptors: a microiontophoretic study in the rat locus coeruleus. Synapse 42, 203–212. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, 1988. Co-Planar Stereotactic Atlas of the Human Brain Three-Dimensional Proportional System: An Approach of Cerebral Imaging. Theime Medical Publisher, New York. [Google Scholar]
- van Dyck CH, Malison RT, Seibyl JP, Laruelle M, Klumpp H, Zoghbi SS, Baldwin RM, Innis RB, 2000. Age-related decline in central serotonin transporter availability with [(123)I]beta-CIT SPECT. Neurobiol Aging 21, 497–501. [DOI] [PubMed] [Google Scholar]
- Varnas K, Halldin C, Hall H, 2004. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp 22, 246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann A, Schroger E, Maess B, 2015. Digital filter design for electrophysiological data--a practical approach. J Neurosci Methods 250, 34–46. [DOI] [PubMed] [Google Scholar]
- Winer JA, 1984. Anatomy of layer IV in cat primary auditory cortex (AI). J Comp Neurol 224, 535–567. [DOI] [PubMed] [Google Scholar]
- Wu S, Ogden RT, Mann JJ, Parsey RV, 2007. Optimal metabolite curve fitting for kinetic modeling of 11C-WAY-100635. J Nucl Med 48, 926–931. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma W, Barker JL, Rubinow DR, 1999. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience 94, 251–259. [DOI] [PubMed] [Google Scholar]
- Zilles K, Qu M, Schleicher A, 1993. Regional distribution and heterogeneity of alpha-adrenoceptors in the rat and human central nervous system. J Hirnforsch 34, 123–132. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


