Supplemental Digital Content is available in the text
Keywords: ART failure, HIV, immune markers, sPD-L1, viral load
Abstract
Despite viral control, basal chronic inflammation and its related comorbidities remain unsolved problems among HIV-infected individuals. Soluble factors derived from myeloid cells have emerged as potent markers associated with HIV-related comorbidities and mortality. In the present report, we explored the relationship between soluble programmed death-ligand 1 (sPD-L1) and HIV-1 infection, antiretroviral therapy (ART), CD4/CD8 ratio, viral load (VL), and sexually transmitted coinfections.
A prospective observational study on 49 HIV-1 infected adults.
We found sPD-L1 levels were significantly higher in 49 HIV infected subjects than in 30 uninfected adults (1.05 ng/ml vs 0.52 ng/ml; P < .001). In this line, sPD-L1 levels were found to be elevated in 16 HIV infected subjects with undetectable VL compared with the uninfected subjects (0.75 ng/ml vs 0.52 ng/ml; P = .02). Thirteen ART-treated individuals with virological failure exhibited the highest sPDL1 levels, which were significantly higher than both 20 ART naïve infected individuals (1.68 ng/ml vs 0.87 ng/ml; P = .003) and the 16 ART-treated individuals with suppressed viremia (1.68 ng/ml vs 0.79 ng/ml; P = 002). Entire cohort data showed a statistically significant positive correlation between VL and sPD-L1 levels in plasma (r = 0.3; P = 036).
Our findings reveal sPDL-1 as a potential biomarker for HIV infection especially interesting in those individuals with virological failure.
1. Introduction
The considerable development of antiretroviral therapy (ART) in recent decades has led to the control of HIV infection in the vast majority of the individuals. However, a high rate of comorbidities has been observed among people living with HIV, despite HIV reaching undetectable levels after ART, with a lack of complete immune system restoration compared with the population not infected with HIV.[1,2] Chronic immune dysfunctions, particularly persistent inflammation, are likely contributors to the enhanced risk of morbidity and mortality among HIV-infected people.[3] Of all the immune markers, those of myeloid origin, such as soluble CD14 or CD163, have shown the greatest clinical impact due to their robustness in predicting morbidity and mortality.[4,5,6] Therefore, these markers are an interesting field of study in HIV infection.
Nearly 25% of individuals with HIV who began ART with a CD4 count < 200 cells/μl are immune non-responders, given they do not achieve > 500 CD4 cells/μl even after 7 years of treatment or longer resulting in an increased risk of morbidities.[2] Although some HIV related immune dysfunctions, such as basal chronic inflammation, have been explained by microbial translocation, the causes of suboptimal immune CD4 recovery are not completely understood. The most feasible explanation involves immune checkpoints, such as programmed cell death protein 1 (PD-1) and the observed T cell exhaustion phenotype. Along these lines, following ART, PD-1 overexpression on CD8 T cells has been associated with impaired CD4 T cell immune reconstitution.[7]
A possible link between basal chronic inflammation and HIV-associated T cell exhaustion could be the expression of inhibitory immune checkpoint ligands. One of the most important immunological checkpoint ligands is programmed death-ligand 1 (PD-L1). Anti-PD-L1 therapy has had great success in the field of cancer; however, its importance in the context of infectious diseases continues to be studied.[8] This molecule is mainly expressed on myeloid and antigen-presenting cells, such as dendritic cells, monocytes, and macrophages[9,10,11] leading to a reduction in antigen presentation and causing the T cell exhaustion.[12]
In this report, we quantified soluble PD-L1 (sPD-L1) in the plasma of 49 individuals with HIV and studied its relationship with ART success, viral load (VL), CD4/CD8 ratio, and sexually transmitted infections (STIs). We found increased sPD-L1 levels in individuals with HIV compared with those without HIV, and these increased levels were associated with VL. Moreover, we observed higher sPD-L1 levels in individuals with virological ART failure compared with both naïve infected individuals and those with undetectable VL. Altogether, these data suggest that sPD-L1 is an interesting immune marker in the HIV context, particularly for infected individuals with ART failure.
2. Methods
2.1. Study design
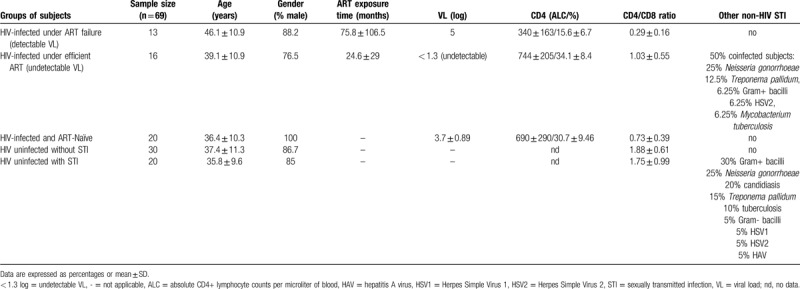
The study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by Ramón y Cajal Hospital Ethics Committee. All the participants provided written consent for the study. Individuals who fulfilled the diagnostic criteria for HIV infection were included in the study. Plasma samples from 79 individuals were collected between February and July of 2016 at 3 Madrid centers: Sandoval Health Center and Ramón, Cajal Hospital, and La Paz Hospital. A total of 49 individuals had HIV-1 (20 drug naïve and 29 undergoing ART), and 30 did not have HIV-1 or any other STI. Among those treated with ART, 13 had virological failure (>1000 HIV-1 RNA copies/ml) and 16 had suppressed VL (<1.3 log or < 20 HIV-1 RNA copies/ml); STIs reported in 8 of those16 cases, according to clinical reports. There were no statistically significant differences in age and sex between the groups. Age- and sex-paired HIV-uninfected subjects and individuals with other STIs were used as controls. For some of our analyses, individuals were classified according to certain clinical parameters. None of the women included in the study were pregnant. The clinical data of the participants included in the study are summarized in Table 1.
Table 1.
Participants characteristics.
2.2. Soluble PD-L1 measurement
The sPD-L1 levels in plasma from HIV infected subjects and healthy volunteers were quantified using an enzyme-linked immunosorbent assay (PDL1 ELISA Kit, Cloud-Clone Corp., USA).
2.3. Viral load determination
HIV-1 viremia was quantified using a COBAS AmpliPrep/COBAS TaqMan HIV-1 Test v2.0 in all HIV+ plasma samples, according to the manufacturers instructions.
2.4. 2.4 Statistical analysis
The statistical significance was calculated using a Mann–Whitney U test or one-way ANOVA, depending on the analysis. The correlations were assessed using Spearman rank–order correlation for non-normally distributed data. Statistical significance was set at P < .05. The analyses were conducted using Prism 6.0 software (GraphPad).
3. Results
Descriptive statistics for the study population are summarized in Table 1. The 49 participants with HIV exhibited significantly higher sPD-L1 levels than the 20 uninfected adults (1.05 ng/ml vs 0.46 ng/ml; P < .001) (Fig. 1a). Remarkably, comparing 16 infected individuals with undetectable VL with 20 uninfected, sPD-L1 levels were found to be elevated in the infected individuals, despite viremia control (0.75 ng/ml vs 0.46 ng/ml; P = .01) (Fig. 1b). These data suggest sPD-L1 is elevated by HIV infection and remains high despite control of the infection.
Figure 1.
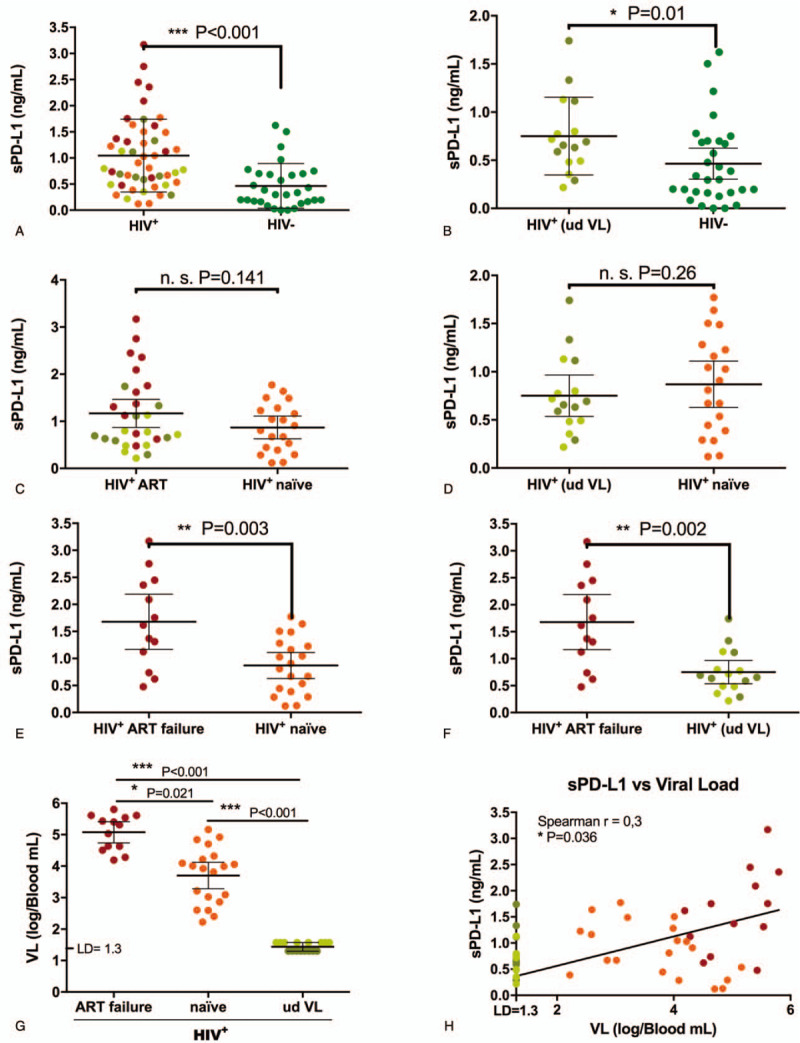
sPD-L1 levels in plasma from indiviuals with HIV-1. (a-f) sPD-L1 levels in plasma from both HIV -uninfected subjects and individuals with HIV-1 measured by enzyme-linked immunosorbent assay. Infected individuals were classified in different subgroups to compare sPD-L1 levels. Bars represent means with a 95% CI. ∗, P < .05, ∗∗, P < .01 and ∗∗∗, P < .001, calculated by the Mann–Whitney U test. (g) Viral load in individuals with HIV-1 classified according to their treatment. Bars represent means with a 95% CI. ∗, P < .05, calculated by the Kruskal–Wallis one way analysis of variance with Dunn multiple comparisons post-hoc-test. (h) Spearman correlation between sPD-L1 levels and viral load in plasma from individuals with HIV-1. HIV+, individuals with HIV; HIV-, individuals without HIV; VL, viral load; ud VL, undetectable viral load; ART, antiretroviral therapy; STI, sexual transmitted infection; n.s., nonsignificant; LD, limit of detection).
Next, we tested the possible effect of ART in sPD-L1. We found no statistically significant differences when comparing all 29 HIV-infected individuals under ART with 20 naïve individuals with HIV (1.17 ng/ml vs 0.88 ng/ml, P = .141) (Fig. 1c). Along these lines, ART exposure appeared to not affect sPD-L1 levels when comparing 16 individuals with HIV undergoing ART with undetectable viremia with 20 ART naïve untreated participants (0.75 ng/ml vs 0.87 ng/ml; P = .26) (Fig. 1d). We then examined what occurred in those participants with ART failure. Remarkably, we observed that the 13 ART-treated participants with virological failure exhibited the highest sPDL1 levels, which were significantly higher than both the naïve (1.68 ng/ml vs 0.87 ng/ml; P = .003) (Fig. 1e) and the 16 ART-treated individuals with suppressed viremia (1.68 ng/ml vs 0.79 ng/ml; P = .002) (Fig. 1f). The above results could be explained by differences in mean VL, given the individuals with ART failure also showed the highest VL (5.1 log on ART-failure, 3.7 log on naïve and < 1.6 log, on undetectable VL individuals) (Fig. 1g). Analysis of the entire cohort data showed a statistically significant positive correlation between VL and sPD-L1 levels in plasma (r = 0.3; P = .036) (Fig. 1h). Altogether, these data illustrate the association between VL and sPD-L1 levels.
We also studied the possible effects of other STIs on sPD-L1 levels. We found no statistically significant differences in the sPD-L1 levels of HIV-infected individuals with or without concomitant STIs (0.57 ng/ml vs 0.88 ng/ml, P = .22) (see Supplemental Fig. 1a, which demonstrates no differences in sPD-L1 plasma levels between groups). We then evaluated the effects of STIs in individuals without HIV. Here again, we found no statistically significant differences in sPD-L1 values when compared HIV uninfected individuals with other STI coinfections and those without other STIs coinfections (0.64 ng/ml vs 0.46 ng/ml, P = .33) (see Supplemental Fig. 1b, which demonstrates no differences in sPD-L1 plasma levels between groups).
Finally, we studied the possible impact of sPD-L1 on CD4 percentages and the CD4/CD8 ratio. We found an inverse correlation between sPD-L1 and both CD4 percentage and the CD4/CD8 ratio; however, these correlations were not statistically significant (see Supplemental Fig. 2a and 2b, which illustrates non- statistically significant correlations between sPD-L1 plasma levels and CD4 percentages and ratios). Although these data could suggest sPD-L1 was involved in the decreased CD4 percentage in HIV pathology, when we compared infected individuals with an inverted CD4/CD8 lymphocyte ratio (<1) with those with a normal CD4/CD8 ratio (≥1) we found no differences between groups in the entire cohort (see Supplemental Fig. 2c, which demonstrates no differences in sPD-L1 plasma levels between groups), nor in the naïve untreated participants (see Supplemental Fig. 2d, which demonstrates no differences in sPD-L1 plasma levels between groups). Therefore, the observed inverse correlation between sPD-L1 and the CD4/CD8 ratio could be explained mainly by the differences in the VL among groups (see Supplemental Fig. 2e, which demonstrates statistically significant differences in VL between groups).
4. Discussion
PD-L1 has represented a breakthrough for immunotherapy in the field of cancer; however, its importance and involvement in the context of infectious diseases continues to be studied. Some studies have reported an upregulation of the PD-1 receptor on T cells from individuals with HIV.[13] Nevertheless, their ligand PD-L1 has been much less studied, even though it could play a causal role in the phenomenon of the T cell exhaustion observed in HIV infection. The last is especially striking in regard to the remarkable results of the unique anti-PD-L1 clinical trial to date in HIV (NCT02028403), in which a single low dose of anti-PD-L1 monoclonal antibody enhanced HIV-1-specific immunity in a subset of participants.[14] In our study, we attempted to evaluate the significance of plasma sPD-L1 as an immune biomarker in HIV infection.
In a previous study, we had found sPD-L1 to be highly elevated in other infectious diseases, such as sepsis.[9] In the current study, we observed sPD-L1 to be increased in individuals with HIV; however, we could not find increased levels of sPD-L1 in individuals with non-HIV STIs. The difference in these data could be explained by the persistence of microbial translocation inducing chronic inflammation in all HIV-infected individuals, regardless of the levels of viremia.[15,16,17] In fact, we and others have previously reported that certain bacterial products such as lipopolysaccharide, a well-known inducer of immune activation, increases PD-L1 expression when translocated to peripheral blood.[9,18,19] In contrast, other non-HIV STIs normally do not display peripheral inflammation. In this line, abnormal levels of inflammation have been observed even in virologically controlled HIV-infected patients with undetectable viral load.[16,20] This data is similar to what we showed regarding sPD-L1 levels in the present report. For this reason, the association between the levels of sPD-L1 and the appearance of HIV-related comorbidities would be an interesting subject of study in the near future. Also, PD-L1 could play a role in low CD4 counts, given that PD-L1 causes T cell apoptosis in some contexts such as sepsis.[21]
In this study, we found a patent impact of VL on sPDL1 levels. Despite contemporary ARTs suppress HIV replication, they do not eliminate the latent reservoir, leading to a persistent viral replication even under suppressive ART.[22] Accordingly, HIV-infected individuals (even those with undetectable VL) showed higher levels of sPD-L1 than uninfected subjects, as was observed for other innate immune markers in HIV-infected subjects under ART and suppressed viraemia vs healthy controls.[23] Interestingly, we found no differences in sPD-L1 values when comparing treated HIV-infected individuals with undetectable VL and ART-naïve infected subjects with higher VL. The latter result together with ART failure individuals having the highest sPD-L1 levels, could be explained by the above-mentioned chronic basal inflammation due to the HIV persistence, the bacterial translocation or the infection time exposure (unknown data in this study), although this hypothesis would require further studies.[9,24]
Higher ART adherence has been associated with lower levels of other inflammation biomarkers, immune activation, and coagulopathy among Ugandans living with HIV who achieved viral suppression shortly after ART initiation.[25] These data suggest that successful ART could have biological consequences beyond viral suppression. Our data appear to indicate that infected individuals with virological ART failure may have higher sPD-L1 positively associated with their detectable VL. Nevertheless, a deeper and larger longitudinal study would be necessary to be able to conclude this definitively.
5. Conclusions
Our data propose sPD-L1 as an interesting immune marker in the HIV context, particularly for monitoring HIV-related inflammation in infected individuals with ART failure.
Acknowledgments
We thank the blood donor service from La Paz University Hospital for the recruitment of HIV uninfected subjects.
Author contributions
Conceptualization: Eduardo López-Collazo, José Avendaño-Ortiz and África Holguín.
Experimental assays: José Avendaño-Ortiz, Roberto Lozano-Rodríguez, Marina Rubio Garrido, Luis A Aguirre.
Samples recruitment: Jorge del Romero, Carmen Rodríguez
Writing the original draft: Eduardo López-Collazo and José Avendaño-Ortiz.
Critical review: Eduardo López-Collazo, Santiago Moreno and África Holguín
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ART = antiretroviral therapy, HIV = human immunodeficiency virus, PD-1 = programmed cell death protein 1, sPD-L1 = soluble programmed death-ligand 1, STIs = sexually transmitted infections, VL = viral load.
How to cite this article: Avendaño-Ortiz J, Rubio-Garrido M, Lozano-Rodríguez R, del Romero J, Rodríguez C, Moreno S, Aguirre LA, Holguín Á, López-Collazo E. Soluble PD-L1: a potential immune marker for HIV-1 infection and virological failure. Medicine. 2020;99:20(e20065).
José Avendaño-Ortiz and Marina Rubio-Garrido contributed equally to this work.
This study was supported by grants from Fondos de Investigación Sanitarias and Fondos FEDER (FIS PI14/01234, PI18/00148 and PIE15/00065) to ELC and (PI15/01005 and PI18/00904) to AH, Fundación Alonso to ELC, “Comunidad de Madrid” (PEJ15/BIO/AI-0021) to JAO and (PEJD-2017/PRE-BMD-4497) to MR.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Wilson EMP, Sereti I. Immune restoration after antiretroviral therapy: the pitfalls of hasty or incomplete repairs. Immunol Rev 2013;254:343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kelley CF, Kitchen CMR, Hunt PW, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 2009;48:787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Klatt NR, Chomont N, Douek DC, et al. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev 2013;254:326–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011;203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Knudsen TB, Ertner G, Petersen J, et al. Plasma soluble CD163 level independently predicts all-cause mortality in HIV-1-infected individuals. J Infect Dis 2016;214:1198–204. [DOI] [PubMed] [Google Scholar]
- [6].Krishnan S, Wilson EMP, Sheikh V, et al. Evidence for innate immune system activation in HIV type 1-infected elite controllers. J Infect Dis 2014;209:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shive CL, Clagett B, McCausland MR, et al. Inflammation perturbs the IL-7 axis, promoting senescence and exhaustion that broadly characterize immune failure in treated HIV infection. J Acquir Immune Defic Syndr 2016;71:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol 2018;18:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Avendaño-Ortiz J, Maroun-Eid C, Martín-Quirós A, et al. PD-L1 overexpression during endotoxin tolerance impairs the adaptive immune response in septic patients via HIF1. J Infect Dis 2018;217:393–404. [DOI] [PubMed] [Google Scholar]
- [10].Tang H, Liang Y, Anders RA, et al. PD-L1 on host cells is essential for PD-L1 blockade–mediated tumor regression. J Clin Invest 2018;128:580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003;9:562–7. [DOI] [PubMed] [Google Scholar]
- [12].Zarour HM. Reversing T-cell dysfunction and exhaustion in cancer. Clin Cancer Res 2016;22:1856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Evans VA, van der Sluis RM, Solomon A, et al. Programmed cell death-1 contributes to the establishment and maintenance of HIV-1 latency. AIDS 2018;32:1491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gay CL, Bosch RJ, Ritz J, et al. Clinical trial of the anti-PD-L1 antibody BMS-936559 in HIV-1 infected participants on suppressive antiretroviral therapy. J Infect Dis 2017;215:1725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Erlandson KM, Campbell TB. Inflammation in chronic HIV infection: what can we do? J Infect Dis 2015;212:339–42. [DOI] [PubMed] [Google Scholar]
- [16].Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015;29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hoenigl M, Kessler HH, Gianella S. Editorial: HIV-associated immune activation and persistent inflammation. Front Immunol 2019;10:2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Avendaño-Ortiz J, Llanos-González E, Toledano V, et al. Pseudomonas aeruginosa colonization causes PD-L1 overexpression on monocytes, impairing the adaptive immune response in patients with cystic fibrosis. J Cyst Fibros 2019;18:630–5. [DOI] [PubMed] [Google Scholar]
- [19].Huang G, Wen Q, Zhao Y, et al. NF-(B plays a key role in inducing CD274 expression in human monocytes after lipopolysaccharide treatment. PLoS One 2013;8:e61602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pérez PS, Romaniuk MA, Duette GA, et al. Extracellular vesicles and chronic inflammation during HIV infection. J Extracell Vesicles 2019;8:1687275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang Y, Zhou Y, Lou J, et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care 2010;14:R220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sarmati L, D’Ettorre G, Parisi SG, et al. HIV replication at low copy number and its correlation with the HIV reservoir: a clinical perspective. Curr HIV Res 2015;13:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Williams JC, Zhang X, Karki M, et al. Soluble CD14, CD163, and CD27 biomarkers distinguish ART-suppressed youth living with HIV from healthy controls. J Leukoc Biol 2018;103:671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bertucci F, Finetti P, Colpaert C, et al. PDL1 expression in inflammatory breast cancer is frequent and predicts for the pathological response to chemotherapy. Oncotarget 2015;6:13506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Castillo-Mancilla JR, Morrow M, Boum Y, et al. Brief report: higher ART adherence is associated with lower systemic inflammation in treatment-Naive Ugandans who achieve virologic suppression. J Acquir Immune Defic Syndr 2018;77:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


