Abstract
BACKGROUND
Recurrent myocardial infarction (MI) is common in patients with coronary artery disease and is associated with high mortality. Long-term reprogramming of myeloid progenitors occurs in response to inflammatory stimuli and alters the organism’s response to secondary inflammatory challenges.
OBJECTIVES
This study examined the effect of recurrent MI on bone marrow response and cardiac inflammation.
METHODS
The investigators developed a surgical mouse model in which 2 subsequent MIs affected different left ventricular regions in the same mouse. Recurrent MI was induced by ligating the left circumflex artery followed by the left anterior descending coronary artery branch. The study characterized the resulting ischemia by whole-heart fluorescent coronary angiography after optical organ clearing and by cardiac magnetic resonance imaging.
RESULTS
A first MI-induced bone marrow “memory” via a circulating signal, reducing hematopoietic maintenance factor expression in bone marrow macrophages. This dampened the organism’s reaction to subsequent events. Despite a similar extent of injury according to troponin levels, recurrent MI caused reduced emergency hematopoiesis and less leukocytosis than a first MI. Consequently, fewer leukocytes migrated to the ischemic myocardium. The hematopoietic response to lipopolysaccharide was also mitigated after a previous MI. The increase of white blood count in 28 patients was lower after recurrent MI compared with their first MI.
CONCLUSIONS
The data suggested that hematopoietic and innate immune responses are shaped by a preceding MI.
Keywords: bone marrow, emergency hematopoiesis, immune memory, inflammation, recurrent myocardial infarction
Recurrent myocardial infarction (MI) is a common clinical problem and a predictor of morbidity and mortality in patients (1,2). Even with contemporary primary percutaneous intervention, reinfarction still occurs in 1 of 10 patients. One-year mortality in patients with recurrent MI is higher than that in patients with a first infarct (38.3% vs. 10.3%), and 20% of patients with recurrent MI die within 7 days (1). The poor outcome after recurrent MI is attributed to additional loss of viable myocardium and heart failure, but studies investigating the underlying mechanisms are lacking.
A hallmark of the immune response to MI is massive recruitment of short-lived neutrophils and monocytes from hematopoietic organs, which increase myeloid cell production by a process called emergency hematopoiesis (3). Newly made leukocytes enter the heart and deploy a variety of molecular cues to enable myocardial healing. A sufficient inflammatory reaction is necessary for optimal healing after MI; however, unrestrained myeloid cell activity results in adverse cardiac remodeling and heart failure (4).
Recently, the concept of “trained immunity” reinvigorated interest in changes to innate immune cells’ functional programs. After inflammatory stimuli, more vigorous activity occurs in response to subsequent events, whereas states of tolerance occur after lipopolysaccharide (LPS) challenge (5). The initial findings obtained in macrophages have now been extended to bone marrow hematopoiesis (6,7). Within this framework, the innate immune response to MI may also depend on previous stimuli, yet it is currently unclear whether recurrent MI elicits a response identical to that observed after a first MI. Motivated by the importance of post-MI leukocyte recruitment for acceleration of atherosclerosis and heart failure development (3), we hypothesized that the inflammatory signals that induce emergency hematopoiesis after MI also have consequences for recurrent cardiovascular events.
Because there was no method available to test this hypothesis, we developed a model of recurrent MI. In the mouse, the major part of the left ventricle is supplied by the left coronary artery (8). Below the tip of the left atrial appendage, the artery divides into: 1) a branch that supplies blood to the posterolateral area of the heart (left circumflex [LCX] artery) (8); and 2) a branch that continues to the apex and provides blood to the anterior wall (left anterior descending [LAD] artery) (8). We first ligated the LCX and later the LAD branch to induce a second MI in the same mouse. We then tested the hypothesis that recurrent MI induces an immune response that diverges from a first infarct.
METHODS
PROCEDURES TO INDUCE MYOCARDIAL ISCHEMIA IN MICE
To ligate the LAD artery, we followed previously published protocols (9). To permanently ligate the LCX artery, the heart was exposed, and the first artery branching off the LAD was located. To induce recurrent MI, mice first underwent LCX ligation followed by LAD ligation 10 days later.
PATIENTS WITH RECURRENT MI
Human subject data were collected retrospectively from a patient cohort at Massachusetts General Hospital, using the Partners Healthcare Research Patient Data Registry, under a research protocol approved by the Partners Healthcare institutional review board. Troponin-T (TnT), and white blood cell counts (WBCs) were collected for all adult Massachusetts General Hospital patients who received at least 2 distinct diagnoses of MI within a 6-month period, from January 1, 2017 through March 31, 2018 (N = 701). For inclusion, patients were required to have had 2 successive MIs 7 to 180 days apart, with WBC values recorded in the 24 h before MI, and WBC and positive TnT values recorded at least once in the 96 h post-MI. We identified 32 patients who met these criteria. Four patients were excluded because the maximum TnT levels associated with their first and recurrent MIs differed by >10 U. The cohort was 57% (16 of 28 patients) male, 79% (22 of 28 patients) white, with a mean age of 68.2 ± 13.9 years, and a mean ± SD of 43.4 ± 44.1 days between the 2 MIs. Changes in WBC following MI were investigated by comparing the most recent WBC in the 24 h before the start of MI to the maximum WBC in the 96 h post-MI, controlling for the maximum TnT level recorded in the same period.
RESULTS
A MOUSE MODEL OF RECURRENT MI
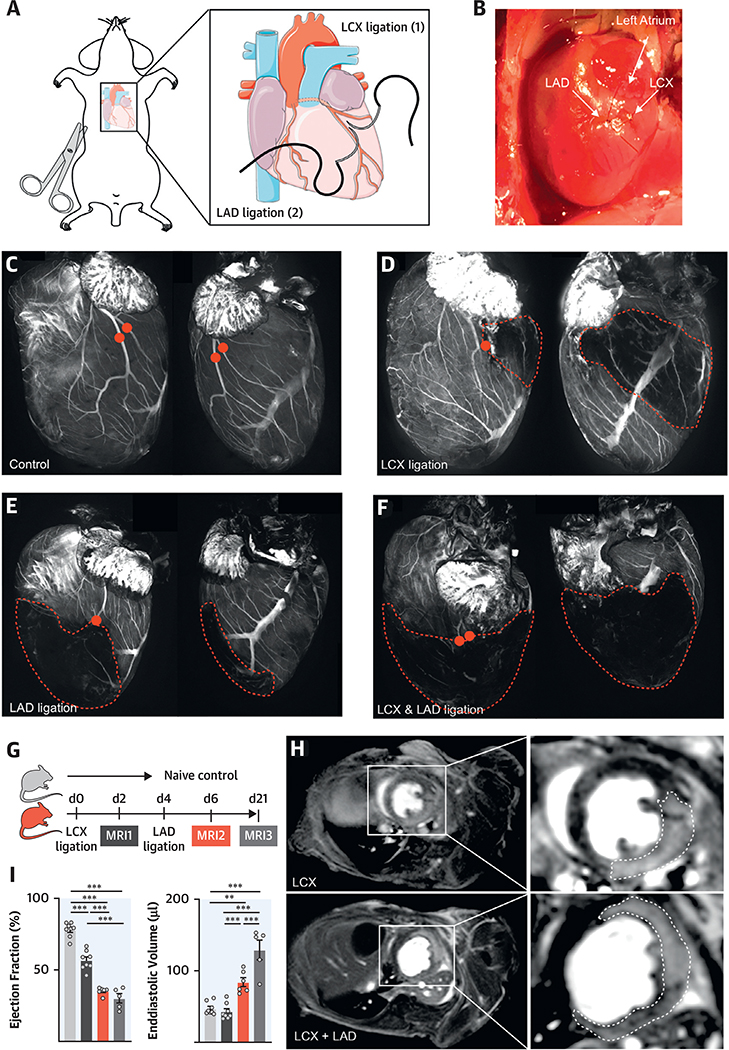
Investigating recurrent multifocal ischemia in mice requires a method that allows subsequent MIs in the same mouse. We devised a surgical model in which the LAD and LCX arteries were separately ligated distal of the left coronary artery’s first branching point (Figures 1A and 1B). To explore both outcomes of the surgeries, we performed whole heart fluorescent coronary angiography after optical tissue clearing (10,11) and in vivo labeling of the cardiac vasculature with a fluorescent dye. Clearing was done after injection of fluorescein-labeled albumin, which served as a vascular imaging agent and visualized the coronary arteries and their branches (Figure 1C). Ligation of the LCX induced a posterolateral infarct (Figure 1D, Online Figure 1), whereas LAD ligation affected the anterior wall, including the apex (Figure 1E). After combined ligations, a large infarcted area emerged (Figure 1F, Online Figures 1A and 1B).
FIGURE 1. Recurrent MI in Mice.
(A) Surgical approach. The left anterior descending (LAD) and left circumflex (LCX) are ligated separately. (B) Image of LAD and LCX ligations. (C) Confocal projections of the coronaries in naive mice, (D) mice with LCX, (E) LAD infarctions, and (F) after ligation of both vessels. Red dots indicate the ligation sites; the infarcted area is outlined. (G) Timeline. (H) Magnetic resonance imaging (MRI) of LCX myocardial infarction (MI) (top panel) and subsequent LAD MI (bottom panel). (I) Ejection fraction and end-diastolic volume by MRI, in naive mice and mice 2 days after 1 or2 days after recurrent MI and 3 weeks thereafter. **p < 0.01; ***p < 0.001; n = 5to8,1-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. Data are mean ± SEM.
We then examined the consequences of these surgeries with delayed gadolinium enhancement cardiac magnetic resonance imaging (Figure 1G). LCX ligation created posterolateral infarcts that reduced the ejection fraction (EF) 2 days after MI, whereas ventricular dimensions remained unchanged (Figures 1H and 1I, Online Video 1). Ligating the LAD in the same mouse 2 days later generated larger combined infarcts, reduced the EF, and caused dilation of the left ventricle (Figures 1H and 1I, Online Video 2). Twenty-one days after the first MI (17 days after the second), the left ventricle dilated further (Figure 1I). At this timepoint, EF was reduced and dilation of the left ventricle increased compared with mice that underwent LAD ligation only (Online Figures 1C to 1E). We ligated the LCX first because it is a more challenging procedure, especially if there are adhesions from the preceding surgery.
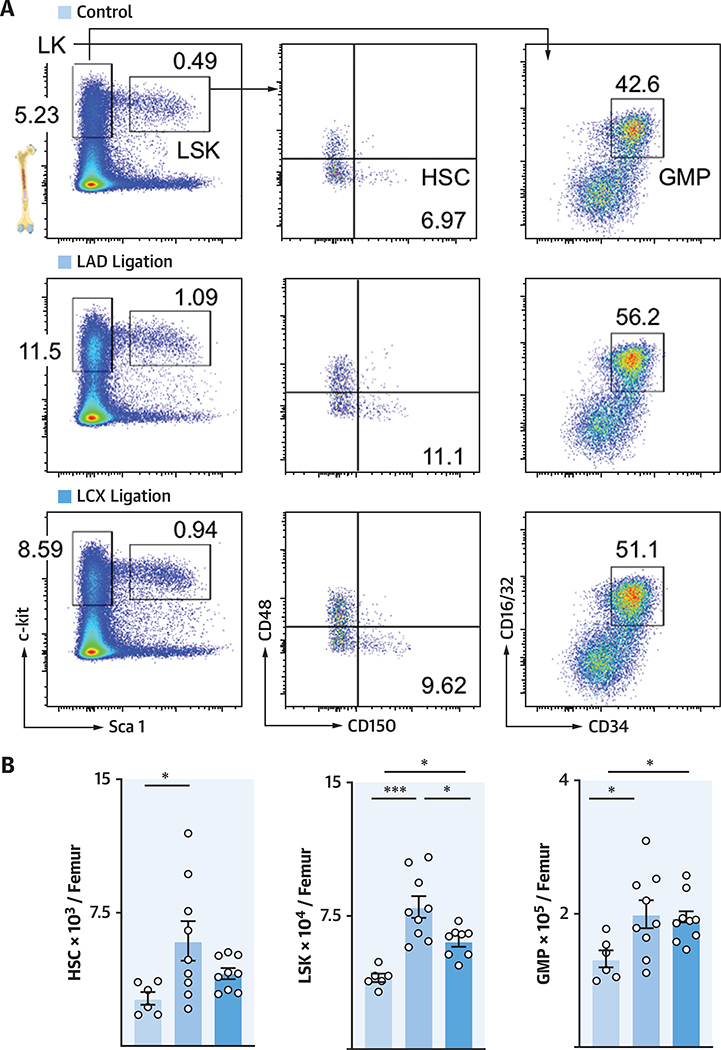
HEMATOPOIETIC RESPONSE TO A SINGLE LAD VERSUS A SINGLE LCX INFARCT
We first tested whether lateral infarcts after LCX ligation induced a bone marrow activation similar to the previously described LAD infarcts (12). We compared lineage− ckit+ sca-1+ (LSK) progenitor cells and most upstream CD150+ CD48+ hematopoietic stem cell (SLAM [signaling lymphocyte adhesion molecule]) numbers in the femur after LCX ligation versus LAD ligation or in naive control mice. We found that hematopoietic stem and progenitor cell (HSPC) numbers increased after LCX ligation relative to naive control mice, albeit not to the same extent as after LAD ligation (Figures 2A and 2B), likely because the induced LCX infarcts were smaller than LAD infarcts. The number of downstream granulocyte macrophage progenitors (GMPs) increased after either LCX or LAD ligation (Figure 2B). In a separate experiment, we created similarly sized LCX and LAD infarcts, which led to a comparable hematopoietic response (Online Figure 2). We concluded that the amplitude of emergency hematopoiesis was determined by infarct size rather than by anatomic location.
FIGURE 2. LCX and LAD Ligation Activate Bone Marrow.
(A) Dot plots and (B) quantification of hematopoietic stem and progenitor cells (LT-HSC, Lineage− ckit+ sca-1+ [LSK], and granulocyte macrophage progenitors [GMP]) in naive mice and mice 3 days after LAD or LCX Ligation. *p < 0.05; ***p < 0.001; n = 6to9,1-way ANOVA followed byTukey’s multiple comparisons test. Data are mean ± SEM. LT-HSC = Long-term hematopoietic stem cells; other abbreviations as in Figure 1.
RECURRENT MI ATTENUATES SYSTEMIC INFLAMMATION.
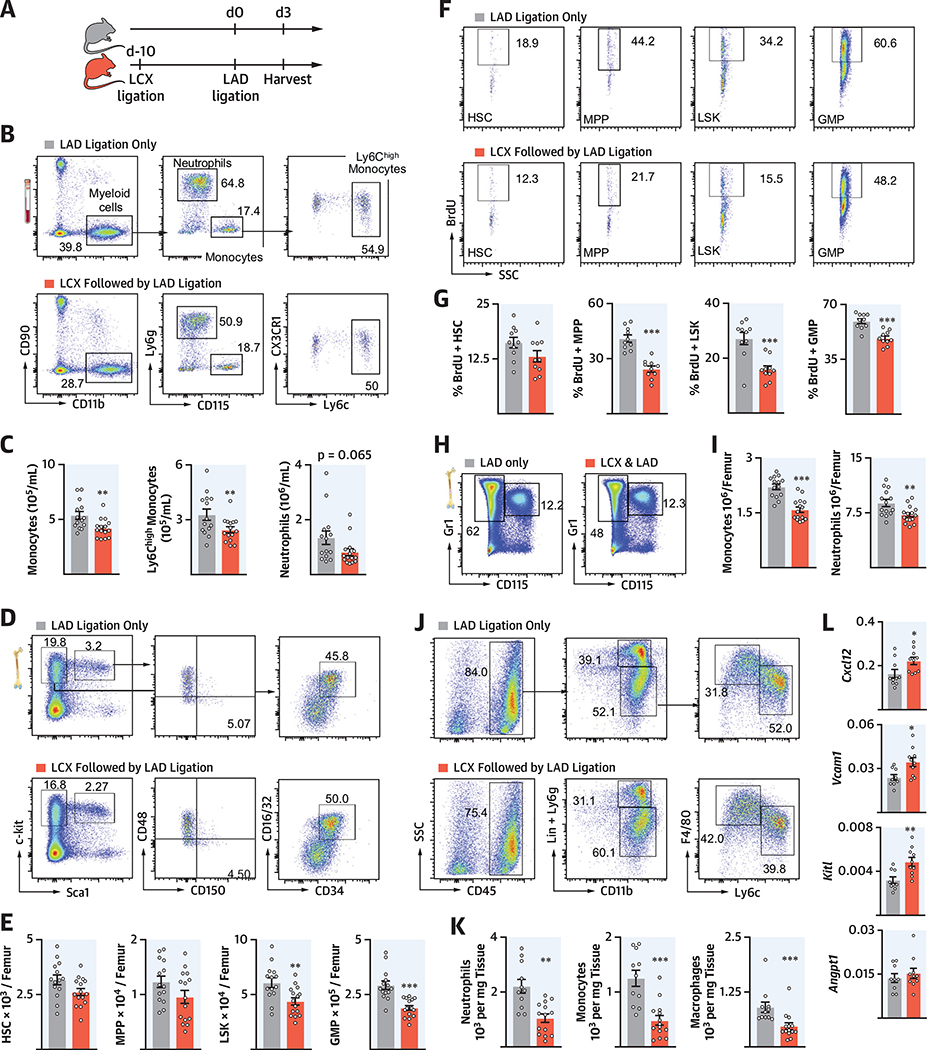
To investigate whether previous MI altered the hematopoietic response to a recurrent infarct, we compared the response after permanent LAD ligation in mice that either had or had not undergone LCX ligation 10 days before (Figure 3A). We first evaluated whether hematopoiesis had returned to steady state 10 days after MI (Online Figure 3A). At this time point, HSPC numbers, which expand significantly in the days after MI (12), dropped to normal levels (Online Figures 3B and 3C). Likewise, neutrophil and monocyte numbers in the bone marrow were comparable in naive mice and in mice 10 days after LAD ligation (Online Figure 3D). We thus settled on an experimental design in which a control cohort underwent LAD ligation only, whereas the experimental cohort received LAD ligation 10 days after a preceding LCX infarct (recurrent MI) (Figure 3A). Blood troponin levels indicated that myocardial necrosis after LAD ligation was similar in both cohorts (Online Figures 4A and 4B).
FIGURE 3. Attenuated Systemic Inflammation in Recurrent MI.
(A) Experimental outline. (B) Dot plots and (C) quantification of blood myeloid cells from mice with single MI compared with recurrent MI. (D) Flow plots and (E) quantification of hematopoietic stem and progenitor cells (HSPCs) in bone marrow. (F) Dot plots and (G) quantification of bromodeoxyuridine (BrdU) incorporation for HSPC proliferation. (H) Dot plots and (I) quantification of neutrophils and monocytes in bone marrow. (J) Dot plots and (K) quantification of cardiac leukocytes from mice with a single LAD infarct compared with a recurrent infarct. (L) Gene expression by quantitative polymerase chain reaction qPCR in marrow. mRNA levels were normalized to gapdh. *p < 0.05; **p < 0.01; ***p < 0.001; n = 9 to 15, Student’s t-test. Data are mean ± SEM. MPPs = multipotent progenitor cells; other abbreviations as in Figure 1.
We next compared innate immune cell numbers 3 days after a first MI (LAD only) to 3 days after recurrent MI (LAD with preceding LCX). Blood myeloid cell numbers were lower in mice with recurrent MI (Figures 3B and 3C). Accordingly, multipotent progenitor cells (MPPs), LSK, and GMP numbers were significantly lower on day 3 after recurrent MI (Figures 3D and 3E), when post-MI emergency hematopoiesis peaked (12). In alignment with these HSPC numbers, MPP, LSK, and GMP proliferation were lower in the femurs of mice with recurrent MI than after a first infarct (Figures 3F and 3G). This was accompanied by lower levels of neutrophils and monocytes in the bone marrow (Figures 3H and 3I). Perhaps most importantly, infarct leukocyte numbers were lower after recurrent MI (Figures 3J and 3K). In the spleen, which also serves as a monocyte source (13), leukocyte numbers were similar, whereas HSPC numbers increased after a recurrent MI (Online Figures 4C to 4F). Of note, sham surgery 10 days before LAD ligation did not elicit immune memory (Online Figure 5).
RECURRENT MI CHANGES THE HEMATOPOIETIC NICHE
In addition to growth factors and danger signals, HSPC proliferation and leukocyte production is governed by the hematopoietic niche, including proteins encoded by the genes Cxcl12, Angptl, Kitl, and Vcaml (14). Expression of these genes was significantly higher in mice with recurrent MI compared with those with a first infarct (Figure 3L). These data implicated the involvement of the bone marrow niche, which instructed HSPC activity in our observation of lower hematopoietic expansion after recurrent MI.
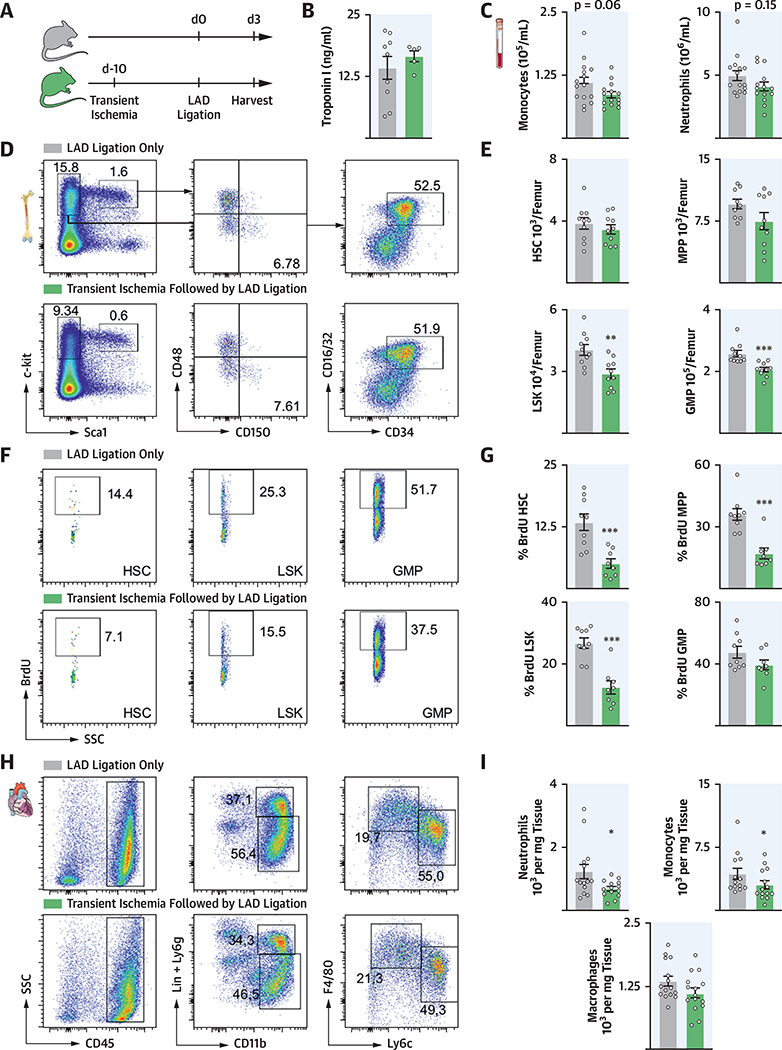
PRECEDING ISCHEMIA RESTRICTS POST-MI EMERGENCY HEMATOPOIESIS
Next, we extended the previous findings to a model of transient ischemia (15). We instigated ischemia by blocking LAD branch blood supply for 30 min, followed by reperfusion. Ten days later, mice underwent permanent ligation of the LAD, whereas control mice underwent LAD ligation without the preceding ischemia (Figure 4A). At this time point after transient ischemia, emergency hematopoiesis returned to steady state (Online Figure 6). Troponin levels in mice in which transient ischemia preceded MI were similar to those that only underwent LAD ligation (Figure 4B). Mice with previous transient ischemia showed a trend toward fewer circulating myeloid cells in the blood (Figure 4C) and had lower bone marrow LSK and GMP numbers 3 days after permanent LAD ligation (Figures 4D and 4E). Likewise, HSPC proliferation was significantly lower (Figures 4F and G). Infarct leukocyte recruitment was attenuated after transient ischemia (Figures 4H and 4I).
FIGURE 4. Transient Ischemia Restricts Marrow Activity After MI.
(A) Experimental design. (B) Troponin I values 24 h after LAD MI (n = 4to 9). (C) Myeloid cell numbers in the blood after transient ischemia followed by MI compared with MI only. (D) Flow plots and (E) quantification of HSPC in marrow. (F) Dot plots and (G) quantification of BrdU incorporation assay for HSPC. (H) Dot plots and (I) quantification ofcardiac leukocytes. *p < 0.05; **p < 0.01; ***p < 0.001; n = 8to15, Student’s t-test. Data are mean ± SEM. SSC = sidescatter; otherabbreviationsas in Figures 1 and 3.
A CIRCULATING FACTOR DAMPENS EMERGENCY HEMATOPOIESIS
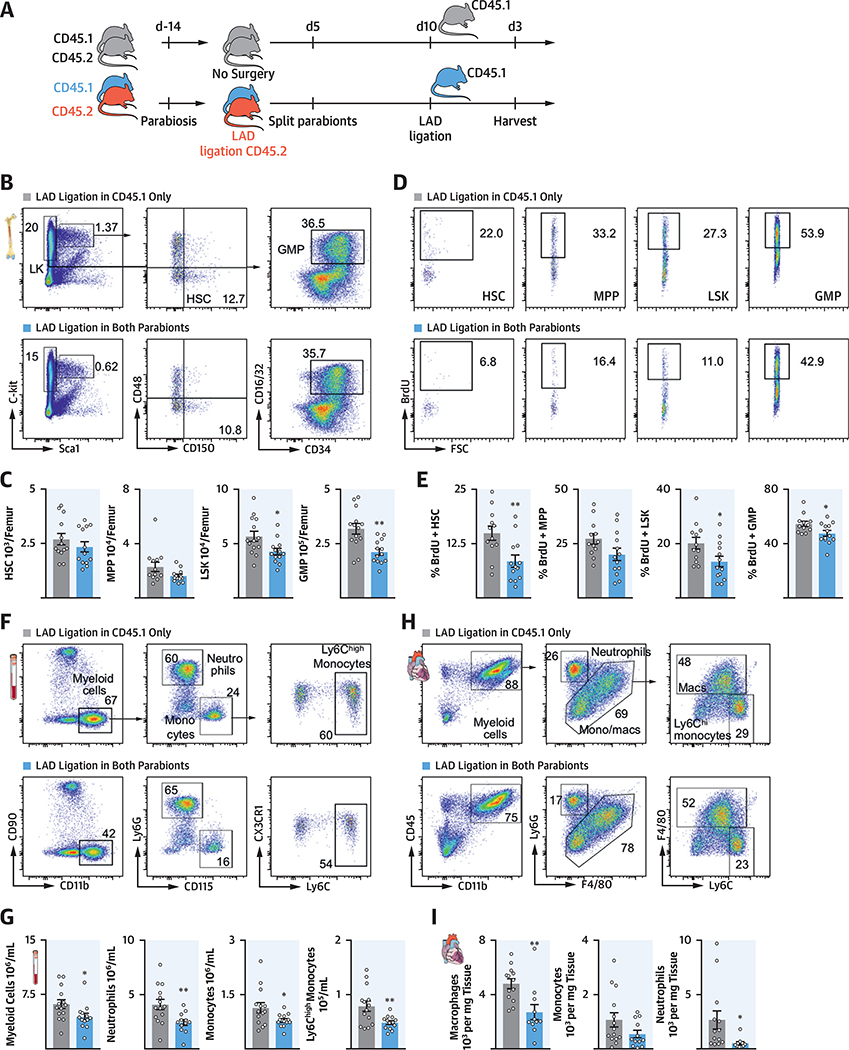
To test whether a circulating factor limited emergency hematopoiesis after recurrent MI, mice were joined in parabiosis to create a shared circulation. After 2 weeks of parabiosis (the time necessary to create equilibrium between both mice), 1 parabiont underwent LAD MI, whereas the other parabiont did not. Five days later, the mice were split, and the noninfarcted parabiont was subsequently subjected to an LAD MI. Mice in the experimental group were therefore exposed to the blood of the infarcted parabiont 10 days before MI. Mice joined in parabiosis without previous MI underwent LAD ligation and were used as control mice (Figure 5A).
FIGURE 5. A Circulating Factor Attenuates Progenitor Cell Expansion.
(A) Experimental design. (B) Dot plots and (C) quantification of HSPC. (D) Dot plots and (E) quantification of BrdU incorporation in HSPC. (F) Dot plots and (G) quantification of blood myeloid cells. (H) Gating and (I) quantification of leukocyte subsets from infarct. *p < 0.05; **p < 0.01;n = 12to14, Student’s t-test. Data are mean ± SEM. Abbreviations as in Figures 1 and 3.
We found that LSK and GMP numbers were lower in the bone marrow of mice in which a parabiont had previous MI surgery (Figures 5B and 5C). Accordingly, we found lower proliferation rates of long-term hematopoietic stem cells, MPP, LSK, and GMP in their bone marrow (Figures 5D and 5E), which resulted in decreased numbers of myeloid cells in the blood (Figures 5F and 5G). Fewer myeloid cells entered the infarcted myocardium in mice whose parabiont had experienced previous MI (Figures 5H and 5I). These data demonstrated that a soluble signal traveled from the infarcted parabiont to the noninfarcted parabiont’s bone marrow, where it dampened post-MI bone marrow activation.
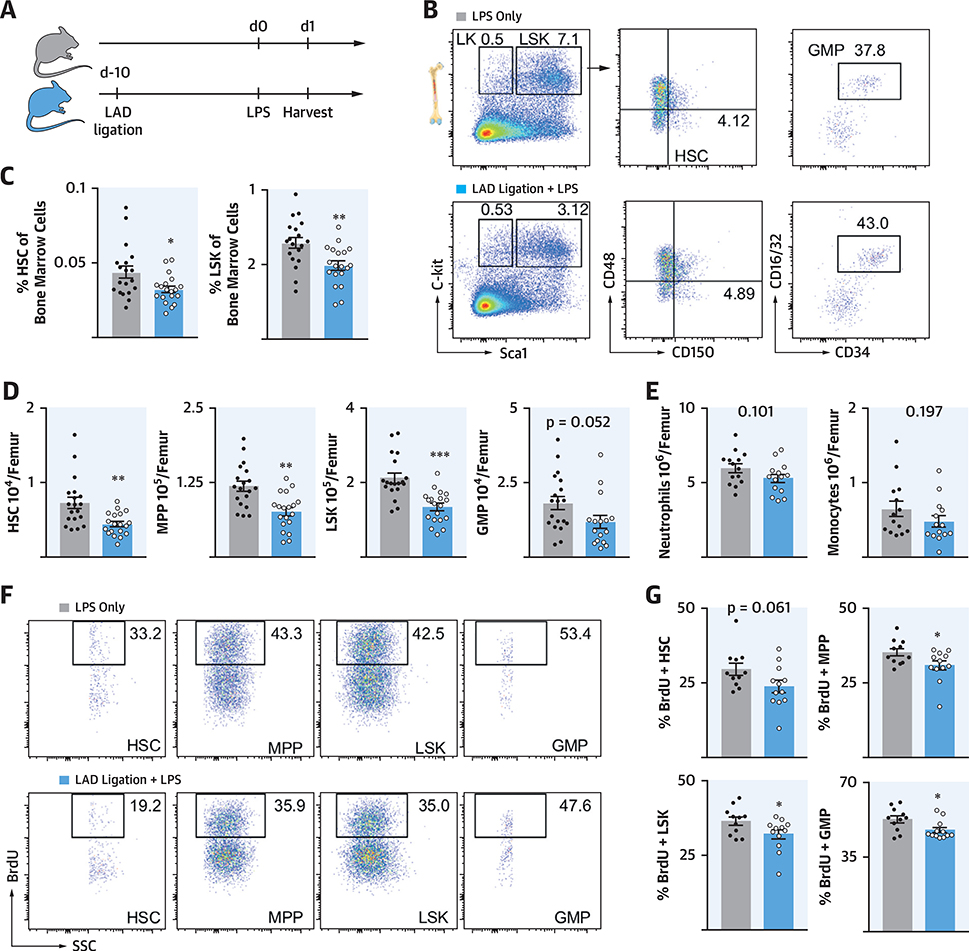
PREVIOUS MI ATTENUATES LPS-INDUCED EMERGENCY HEMATOPOIESIS
To test if these observations extended to nonischemic recall stimuli, we used the bacterial wall component LPS as a secondary challenge 10 days after MI (Figure 6A), following the design of classic trained immunity experiments (5,6). After LPS injection, we found fewer HSPCs in the bone marrow of mice with previous MI (Figures 6B to 6D). In addition, we observed a trend toward reduced neutrophil and monocyte numbers in the bone marrow of mice that underwent MI 10 days before LPS exposure (Figure 6E). As after recurrent MI, leukocyte production was diminished, indicated by lower bromodeoxyuridine (BrdU) incorporation in HSPCs of mice with MI before LPS injection (Figures 6F and 6G).
FIGURE 6. Prior MI Attenuates HSPC Expansion After LPS.
(A) Experimental design. (B) Flow plots, (C) frequencies, and (D) quantification of marrow HSPC. (E) Leukocytes in marrow. (F) Dot plots and (G) quantification of BrdU incorporation in marrow HSPC. *p < 0.05; **p < 0.01; ***p < 0.001; n = 12 to 14, Student’s t-test. Data are mean ± SEM. LPS = lipopolysaccharide; other abbreviations as in Figures 1 and 3.
BONE MARROW MACROPHAGES FACILITATE HEMATOPOIETIC TOLERANCE
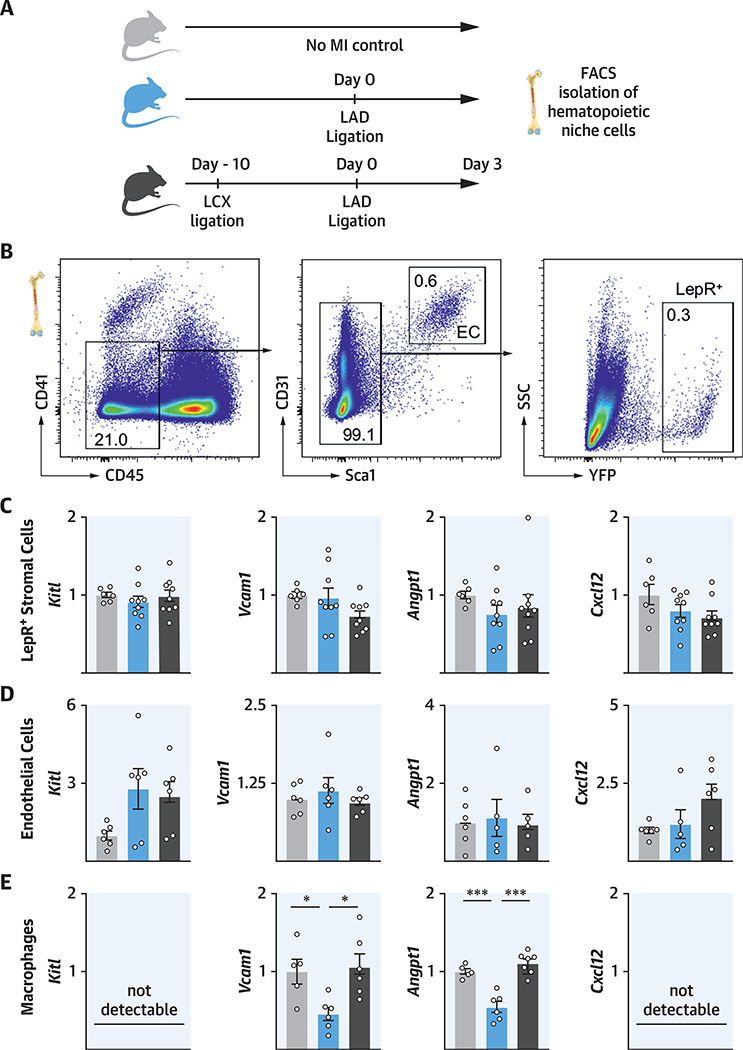
Macrophages are a component of the hematopoietic niche (16) that governs HSPC quiescence, maintenance, and proliferation. As previously described (16), we found macrophages to be MHCIIintermed Cx3cr1neg CD169high Vcam1high (Online Figure 7). Because of reports of trained immunity and tolerance (5), we hypothesized that bone marrow macrophages might be involved in retaining memory of MI. We isolated hematopoietic niche cells with fluorescence-activated cell sorting (FACS) in mice 3 days after MI and compared niche factor expression with naive control mice and with mice with recurrent MI (Figures 7A and 7B). There were no significant differences for Kitl, Vcaml, Angptl, and Cxcl12 expression in LepR+ stromal mesenchymal cells or bone marrow endothelial cells in mice with single MI versus recurrent MI (Figures 7C and 7D). However, in mice with recurrent MI, bone marrow macrophages expressed significantly more Vcaml and Angptl (Figure 7E), although their total number was slightly reduced (Online Figure 8). Because these factors regulate HSPC retention and quiescence (14), the data suggested that macrophages contributed to bone marrow tolerance in recurrent MI.
FIGURE 7. Macrophages Attenuate Myelopoiesis in Recurrent MI by Higher Expression of Quiescence- and Retention-Promoting Factors.
(A) Experimental design. Hematopoietic niche cells were isolated with fluorescence-activated cell sorting (FACS). (B) Gating for isolating bone marrow endothelial cells and LepR+ stromal cells. (C to E) Gene expression in LepR+ stromal cells, endothelial cells, and bone marrow macrophages. mRNA levels were normalized to gapdh. *p < 0.05; ***p < 0.001; n = 5 to 9,1-way ANOVA followed by Tukey’s multiple comparisons test. Data are mean ± SEM. Abbreviations as in Figure 1.
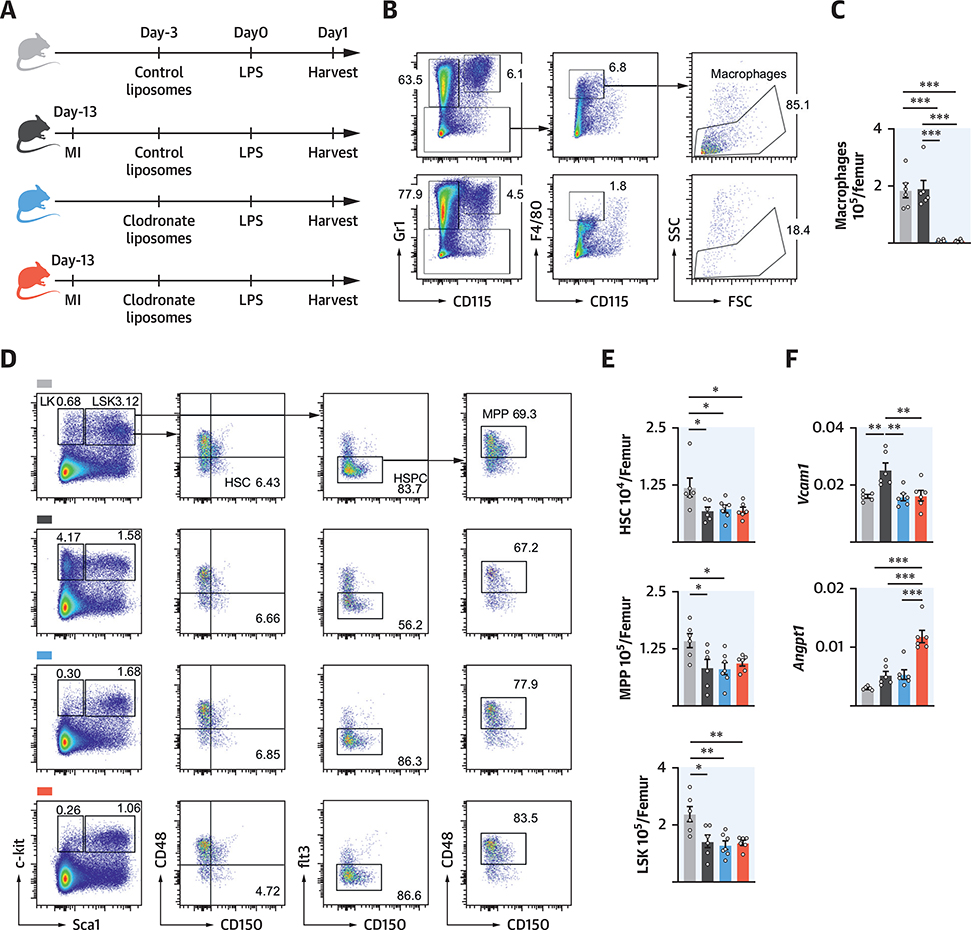
To test this hypothesis, we depleted macrophages with clodronate liposomes in mice with or without previous MI (Figures 8A to 8C) and examined the effect of LPS on HSPC expansion. We reproduced the previously described attenuated myeloid cell expansion in mice with previous MI that received LPS and control liposomes compared with mice without MI (Figures 8D and 8E). After macrophage ablation, HSPC numbers fell even in the absence of MI (Figures 8D and 8E), which was in accordance with previous reports that suggested that niche macrophages maintain HSPC via the adhesion molecule Vcam-1 (16). In the absence of macrophages, HSPC numbers after LPS challenge were similar irrespective of whether mice had a previous MI. We next measured expression of Vcaml and Angptl in whole bone marrow after macrophage depletion. After LPS stimulation, Vcaml levels were higher in mice with a previous MI, which was abrogated when macrophages were depleted (Figure 8F). Interestingly, Angptl levels rose after macrophage depletion. We speculate that other cell types contribute to post-MI hematopoietic memory (e.g., HSPC), which displayed trained immunity (6) and expressed Angptl (17).
FIGURE 8. Macrophage Ablation Abolishes Marrow Memory.
(A) Experimental design. (B) Dot plots and (C) quantification of marrow macrophages. (D) FACS plots and (E) cell numbers of marrow HSPC. (F) Gene expression by quantitative polymerase chain reaction in total bone marrow. mRNA levels were normalized to gapdh Ctvalues. *p < 0.05; **p < 0.01; ***p < 0.001; n = 5to8,1-way ANOVA followed by Tukey’s multiple comparisons test. Data are mean ± SEM. Abbreviations as in Figures 1, 3, and 7.
REDUCED BLOOD LEUKOCYTOSIS IN PATIENTS WITH RECURRENT MI
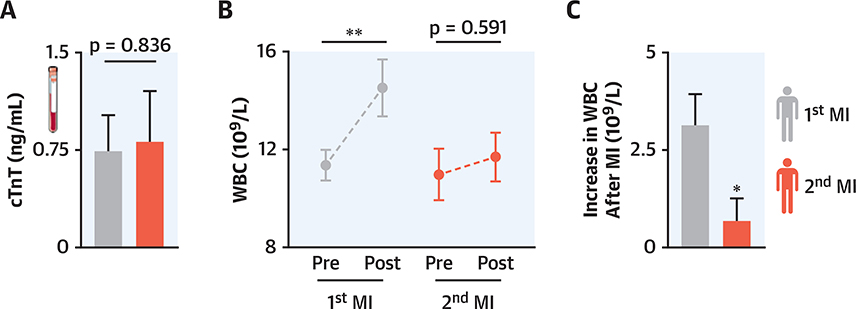
To explore the clinical relevance of our observations in mice, we retrospectively analyzed blood leukocyte kinetics in patients during their first MI versus recurrent MI. We screened 701 patients for whom troponin and WBCs were available and who received diagnoses of MI in a period during which the same troponin assay was employed. Twenty-eight patients were included in the study (please see Methods section for criteria). In this cohort, pre-MI leukocyte counts and troponin levels were similar for first and recurrent MI (Figures 9A and 9B). Interestingly, the WBC increased significantly only after the first and not after the recurrent MI (Figure 9B). The WBC increase after recurrent MI was significantly lower than that after the first MI (Figure 9C), which recapitulated our observations in mice.
FIGURE 9. Limited Increase in Blood Leukocytes in Patients With Recurrent MI.
(A) Maximal troponin levels of the same patients within the first 96 hofa first and second MI. (B) Maximal white blood cell (WBC) counts in the same patients 24 h before and within 96 h post-MI. (C) Pre-post WBC change in the same patients after a first and second infarct. *p < 0.05; **p < 0.001; n = 28. Significance of differences between cardiac troponin T (TnT) values was calculated using the Wilcoxon signed rank test. For pre- and post-MI WBCs, a 1-way ANOVA testwas used, and for the increase in WBC post-MI, a paired Student’s t-testwas used. Data are mean ± SEM. Abbreviations as in Figure 1.
DISCUSSION
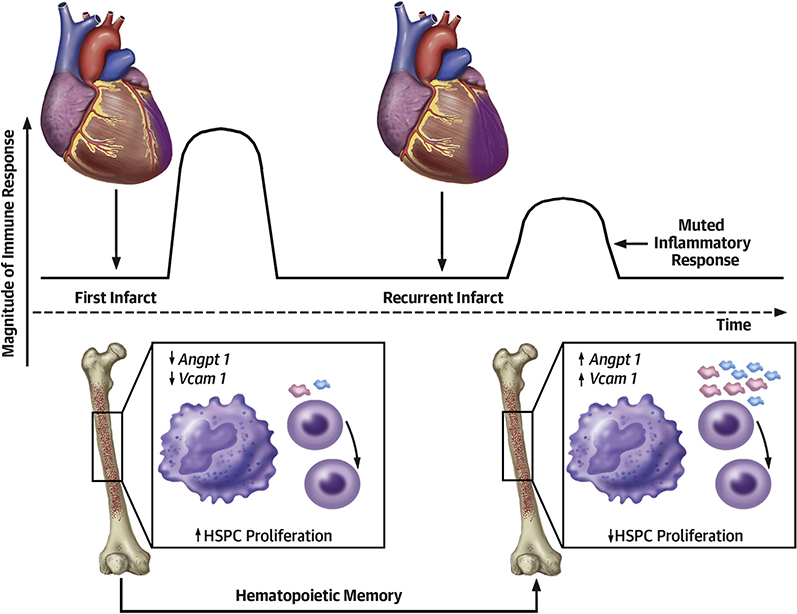
Innate immunity and cardiac inflammation have recently been linked to heart failure, a connection that may contribute to the dismal survival rate of patients with recurrent MI (1). There are no available systems for modeling inflammatory circuits in recurrent MI. We therefore designed a model that allowed the induction of 2 sequential infarctions in the same mouse. By studying the impact of recurrent MI on innate immunity and emergency hematopoiesis, we observed a muted response that was induced by a circulating signal released after the first infarct, which was possibly mediated by bone marrow macrophages in the hematopoietic niche (Central Illustration).
CENTRAL ILLUSTRATION.
Inflammation After Recurrent MI
The heart recruits millions of circulating myeloid cells to ischemic myocardium. Because these cells are short lived (18), the bone marrow augments leukocyte production to meet the organism’s need. After MI, the marrow is alerted by signals from the sympathetic nervous system (19) and from circulating mediators produced by the ischemic heart, such as interleukin- 1p and granulocyte-macrophage colony stimulating factor (20,21). This process controls HSPC quiescence, proliferation, and differentiation, and subsequently, blood leukocyte levels in response to myocardial injury.
Cardiovascular mortality correlates with blood leukocyte levels (22). Because patients with recurrent MI have lower survival rates and aggravated heart failure (1), we speculated that recurrent MI would solicit heightened systemic supply of myeloid cells maintained by higher HSPC proliferation rates in the bone marrow. However, our data indicated the opposite. Peripheral blood monocytosis and myeloid cell numbers in the heart were lower, and bone marrow activity was diminished after recurrent MI. Our retrospective clinical data demonstrated that leukocytosis was also lower in patients with recurrent MI. A changed cell subset repertoire might also occur after recurrent MI, which might affect recovery via an altered balance of pro- inflammatory and tissue repair functions.
The potential clinical implications of this work are manifold: if recurrent MI leads to an insufficient inflammatory response, then perhaps these patients should not receive anti-inflammatory therapeutics after MI. The potential of such therapeutic interventions is currently being discussed, especially in light of the recent CANTOS (Canakinumab Antiinflammatory Thrombosis Outcome Study) (23). Previous studies that explored anti-inflammatory therapeutics that neutralized tumor necrosis factor-a (24) or nonspecific drugs (e.g., colchicine) (25) returned discouraging results. Careful patient selection, including excluding recurrent MI, might help identify a population that benefits from therapeutically dampened hematopoiesis. If an infarct does not recruit myeloid cells sufficiently, wound healing is delayed because dying cardiomyocytes are not removed, which results in more necrotic tissue remnants, lack of granulation tissue, and an insufficient collagen matrix (4). Patients with leukocytosis and leukopenia have a higher mortality after acute MI (26). Thus, future studies should prospectively examine immune cell levels and hematopoiesis in patients with recurrent MI, and whether these parameters are associated with adverse outcomes.
Mechanistically, we identified altered expression of niche factors in bone marrow macrophages, which are known to influence HSPC retention, quiescence, and differentiation (16). Recurrent MI did not result in a reduction of retention factors observed after a first infarct, which lowered Cxcl12, Vcaml, Scf, and Angptl expression. Our parabiosis experiments indicated that a circulating, likely heart-derived factor was causally involved. Such signals, which have to be identified in future work, might act directly on macrophages in the bone marrow, where they could induce metabolic and epigenetic rewiring. In trained immunity, epigenetic modifications involve histone modifications (5) that prime myeloid cells and their progenitors for secondary challenges (6,27), enabling a higher response after re-stimulation. Therefore, it would be interesting to conduct epigenetic screens in bone marrow macrophages to compare the effects of first and recurrent MI. We expected an increased immune response in recurrent compared with first MI, partially because the ischemic myocardium produces interleukin-1p and granulocyte-macrophage colony stimulating factor (20,21), 2 cytokines that induce HSPC training rather than tolerance (6,7). Surprisingly, our data showed that MI induced tolerance.
STUDY LIMITATIONS
We examined young and healthy mice, which likely mount an optimal inflammatory response in response to myocardial injury. Increasing or lowering inflammation in these mice had negative effects on recovery (4). Patients with recurrent MI have atherosclerosis and comorbidities that cause low grade inflammation. Hyperlipidemia heightens the bone marrow’s inflammatory reactions to LPS (7). Accordingly, the effects of recurrent MI may differ if atherosclerosis and its risk factors are present. It is still unclear which events are responsible for the altered expression of hematopoietic quiescence factors after recurrent ischemia. The data obtained after macrophage depletion with clodronate liposomes implicated bone marrow macrophages; however, this cell depletion occurred sys- temically and not only in the marrow.
CONCLUSIONS
Our data suggest that MI alters the innate immune response to subsequent events (Central Illustration). The many preclinical questions arising from our observations can be tackled using the new mouse model of recurrent MI.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Kaley Joyes for editing the manuscript and the Partners Healthcare Research Patient Data Registry group for facilitating use of their database.
This work was funded in part by the National Institutes of Health (HL139598, HL125428, HL131495, HL131478, HL142494, HL230000, T32HL076136), the European Union’s Horizon 2020 Research and Innovation Program (Grant No 667837). Drs. Higgins and Brody were supported by a grant from the One Brave Idea Initiative. Drs. Cremer and Rohde were funded by Deutsche Forschungsgemeinschaft (CR603/1-1 and RO5071/1-1). Dr. Nahrendorf has received research funding and consulting fees from Novartis, IFM Therapeutics, and Verseau.
ABBREVIATIONS AND ACRONYMS
- BrdU
bromodeoxyuridine
- EF
ejection fraction
- FACS
fluorescence-activated cell sorting
- HSPC
hematopoietic stem and progenitor cells
- GMP
granulocyte macrophage progenitor
- LAD
left anterior descending coronary artery
- LCX
left circumflex coronary artery
- LSK
lineage− ckit+ sca-1+
- LPS
lipopolysaccharide
- MI
myocardial infarction
- MMPs
multipotent progenitor cells
- TnT
troponin T
- WBC
white blood count
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
COMPETENCY IN MEDICAL KNOWLEDGE: Recurrent MI causes less hematopoiesis and leukocytosis than a first MI by attenuating hematopoietic factor expression in bone marrow macrophages.
TRANSLATIONAL OUTLOOK: More research is needed to establish whether a muted inflammatory response to recurrent MI contributes to accelerated development of heart failure.
APPENDIX For an expanded Methods section and supplemental figures and videos, please see the online version of this paper.
REFERENCES
- 1.Thune JJ,Signorovitch JE, Kober L, et al. Predictors and prognostic impact of recurrent myocardial infarction in patients with left ventricular dysfunction, heart failure, orboth following a first myocardial infarction. EurJ HeartFail 2011;13:148–53. [DOI] [PubMed] [Google Scholar]
- 2.Stone SG, Serrao GW, Mehran R, et al. Incidence, predictors, and implications of reinfarction after primary percutaneous coronary intervention in ST-segment-elevation myocardial infarction: the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction Trial. Circ Cardiovasc Interv 2014;7:543–51. [DOI] [PubMed] [Google Scholar]
- 3.Nahrendorf M Myeloid cell contributions to cardiovascular health and disease. Nat Med 2018; 24:711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007;204:3037–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saeed S, Quintin J, Kerstens HH, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014;345:1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitroulis I, Ruppova K, Wang B, et al. Modulation of myelopoiesis progenitors is an integral compo-nentoftrained immunity. Cell 2018;172:147–61.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christ A, Günther P, Lauterbach MAR, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 2018;172:162–75.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández B, Durán AC, Fernández MC, Fer- nández-Gallego T, Icardo JM, Sans-Coma V. The coronary arteries of the C57BL/6 mouse strains: implications for comparison with mutant models. J Anat 2008;212:12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eberli FR, Sam F, Ngoy S, Apstein CS, Colucci WS. Left-ventricular structural and functional remodeling in the mouse after myocardial infarction: assessment with the isovolumetrically- contracting Langendorff heart. J Mol Cell Cardiol 1998;30:1443–7. [DOI] [PubMed] [Google Scholar]
- 10.Richardson DS, Lichtman JW. Clarifying tissue clearing. Cell 2015;162:246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Susaki EA, Tainaka K, Perrin D, et al. Whole- brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 2014;157:726–39. [DOI] [PubMed] [Google Scholar]
- 12.Dutta P, Sager HB, Stengel KR, et al. Myocardial infarction activates CCR2(+) hematopoietic stem and progenitorcells. Cell Stem Cell 2015;16:477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009;325:612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014; 505:327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsey ML, Bolli R, Canty JMJ, et al. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 2018;314:H812–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow A, Lucas D, Hidalgo A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 2011;208:261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou BO, Ding L, Morrison SJ. Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting angiopoietin-1. Elife 2015;4:e05521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013;38:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sager HB, Heidt T, Hulsmans M, et al. Targeting interleukin-1 p reduces leukocyte production after acute myocardial infarction. Circulation 2015;132:1880–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anzai A, Choi JL, He S, et al. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J Exp Med 2017;214:3293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engstrom G, Melander O, Hedblad B. Leukocyte count and incidence of hospitalizations due to heart failure. Circ Heart Fail 2009;2:217–22. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- 24.Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004;109:1594–602. [DOI] [PubMed] [Google Scholar]
- 25.Deftereos S, Giannopoulos G, Panagopoulou V, et al. Anti-inflammatory treatment with colchicine in stable chronic heart failure: a prospective, randomized study. J Am Coll Cardiol HF 2014;2:131–7. [DOI] [PubMed] [Google Scholar]
- 26.Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene. Arterioscler Thromb Vasc Biol 2005;25: 658–70. [DOI] [PubMed] [Google Scholar]
- 27.Cheng SC, Quintin J, Cramer RA, et al. mTOR- and HIF-1a-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014; 345:1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.