Short abstract
Objective
Postoperative delirium (POD) is a perennial and distressing complication in older patients, and it may be caused by various factors. This study aimed to determine whether serum melatonin levels are associated with POD in older patients who undergo major abdominal surgery.
Methods
We collected serum samples from patients undergoing major abdominal surgery. Melatonin was measured by enzyme-linked immunosorbent assay and delirium was assessed by the Confusion Assessment Method after surgery. We classified the patients into the POD group and non-POD group.
Results
A total of 120 patients met the inclusion criteria and 104 patients were included in the analysis. Postoperative serum melatonin levels were significantly lower than preoperative levels. Multivariate analyses showed that a longer duration of anesthesia (odds ratio [OR] = 1.29, 95% confidence interval [CI] = 1.02–1.64), advanced age (OR = 3.36, 95% CI = 1.37–8.22), and lower postoperative levels of melatonin (OR = 0.50, 95% CI = 0.25–1.00) were associated with POD.
Conclusions
Serum melatonin levels significantly decline after surgery and anesthesia. Significantly decreased serum melatonin concentrations postoperatively may be a biomarker for predicting POD in older patients undergoing major abdominal surgery with general anesthesia.
Keywords: Postoperative delirium, melatonin, abdominal surgery, anesthesia, older patient, cognitive status
Background
Delirium is a transient dysfunction of cerebral metabolism, and it clinically manifests through a wide range of neuropsychiatric abnormalities and is reversible.1 The incidence of postoperative delirium (POD), which leads to increased hospitalization period and costs,2 has been reported to be 44% in older patients without precautions.3 However, the mechanism of POD remains unclear. Previous studies have considered the occurrence of POD to be a consequence of a complex interrelationship among various predisposing factors, such as advanced age, opioid use, cerebral vascular diseases, pulmonary diseases,4 and anticholinergic drugs.5 Robinson et al.3 found that pre-existing cognitive dysfunction was the strongest predictor of development of POD. Luo et al.6 suggested that the risk of POD could be significantly reduced after monitoring the depth of anesthesia by the bispectral index (BIS) or auditory-evoked potentials. Recent reports have also indicated that the pathogenesis of POD is associated with an inflammatory response.7
Melatonin, which is a hormone released into the blood by the pineal gland, maintains circadian rhythms, improves sleep quality, and regulates mood.8 Melatonin concentrations vary in the plasma between day and night, and generally decrease with age in healthy human subjects.9 Additionally, an increase in expansion of body size plays a role in the decline in melatonin during childhood and adolescence.10 A previous study11 observed changes in melatonin metabolism in patients undergoing surgery. Wu et al.12 found that postoperative cognitive dysfunction was associated with fluctuations in endogenous melatonin levels. Lee et al.13 further suggested that melatonin ameliorates cognitive memory through the anti-inflammatory response in rats. Another study reported that propofol anesthesia significantly altered melatonin levels in blood plasma in rats.14 However, Scholtens et al.15 reported that preoperative cerebrospinal fluid melatonin levels did not differ between patients with and those without POD.
Therefore, we performed a prospective, observational study to investigate the association between serum melatonin levels and POD.
Materials and method
Study design
The study was approved by the ethics committee of The First Hospital of Jiaxing and all included patients signed informed consent forms. Older patients (≥65 years old) who had undergone major abdominal surgery with general anesthesia were included. The exclusion criteria were as follows: a history of neuropsychiatric diseases, such as depression and schizophrenia; a Mini-Mental State Examination (MMSE) score < 24; serious cardiovascular and cerebrovascular diseases; taking sedatives and hypnotics or narcotic analgesics; estimated duration of anesthesia < 2 hours and inability to understand Chinese; refusal or unsuitable for evaluation of delirium; and serum samples were not available.
Fentanyl, propofol, cisatracurium, and sevoflurane were used for the induction and maintenance of anesthesia in all patients. Intraoperative BI values were maintained in the range of 40 to 60. All operations were completed by 4:00 pm and lights in the ward were switched off at 10:00 pm.
We calculated the sample size using PASS 11 (NCSS 11 Statistical Software (2016); NCSS, LLC, Kaysville, UT, USA. ncss.com/software/ncss), with an overall incidence of delirium after gastrointestinal surgery in older patients of 35%,16 a power of 0.80, and an α level of 0.05. Based on our previous pilot study observations, the incidence of POD was set at 4% and 25% at different melatonin levels, with a total of 86 patients required. However, to account for possible loss of follow-up, we included 120 patients.
Assessment of delirium
Delirium was evaluated by a trained nurse on the basis of the Confusion Assessment Method.17 In this method, the diagnosis of delirium requires the presence of the following features: (1) an acute onset of cognitive impairment showing a fluctuating course; and (2) at least two of the following items of (a) perceptual disturbances, (b) a disorganized thought process, (c) disorientation and memory impairment, (d) an altered level of consciousness, and (e) hyper- or hypoactive psychomotor activity. Diagnostic interviews were conducted in the patients twice a day, from the second to the fifth day after the operation.
Measurements
Venous blood samples were collected at 9:00 pm, 1 day before (preoperative), and after (postoperative) the operation. The samples were placed in a serum separator tube and allowed to clot for 2 hours at room temperature, before centrifugation for 10 minutes. The serum samples were then transferred into Eppendorf tubes and stored in a −80°C freezer. Melatonin concentrations were determined using an enzyme-linked immunosorbent assay (Melatonin kit; Shanghai Yuduo Biotechnology Co. Ltd., Shanghai, China). The procedure included a standard, addition of samples, incubation, dilution, washing, enzyme addition, color development, addition of a stop buffer, and measurement. The standard curve was plotted with the concentration of the standard substance on the x-axis and the optical density (OD) on the y-axis. The corresponding concentration was obtained from the standard curve on the basis of the OD value of the sample and was multiplied by the dilution factor. The detection limit was between 1 and 80 ng/L.
Data collection
The collected demographic data included age, sex, American Society of Anesthesiologists scale, living situation, preoperative MMSE score, serum albumin levels, and the type of surgery. The duration of anesthesia, the requirement of postoperative analgesia, blood loss, and transfusion data were also recorded.
Statistical analyses
The Kolmogorov–Smirnov test and Shapiro–Wilk test were performed to examine the normality of continuous data. Normally distributed data are presented as mean ± standard deviation, and they were compared with the t-test. Abnormally distributed data are expressed as the median and interquartile range, and were compared with the Wilcoxon rank-sum test. Binary data are expressed as percentages and were compared using the chi-square and Fisher’s exact tests. Potential confounders with a significant effect on POD (P < 0.05), as confirmed by univariate analysis, were investigated with multivariate logistic regression analysis. The results of multivariate logistic regression are reported as odds ratio (OR) values and 95% confidence intervals (CIs). Statistical significance was set at P < 0.05.
Results
Patients’ characteristics
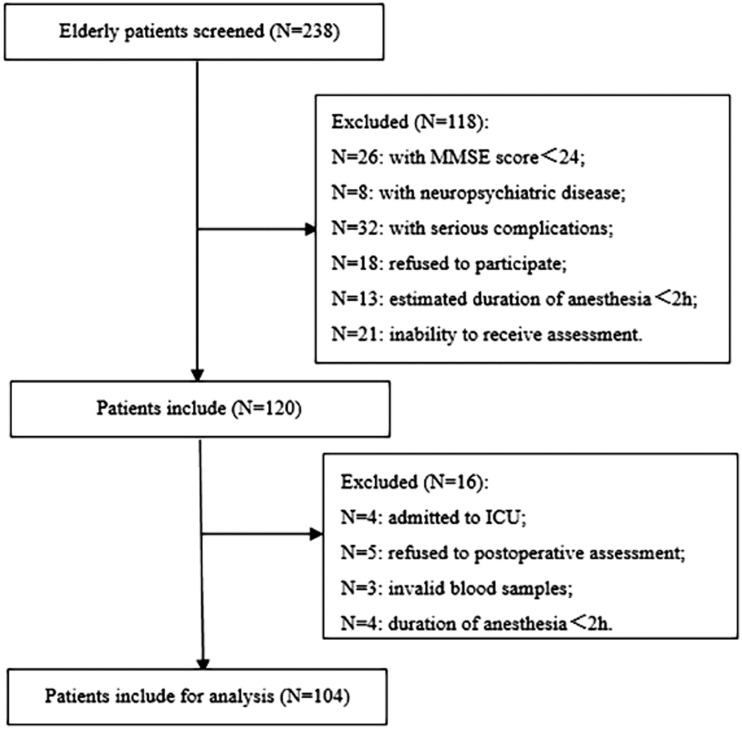
This study was conducted from October 2018 to September 2019. We screened 238 older patients who underwent abdominal surgery and selected 120 patients. However, 16 patients were further excluded from the study for the following reasons. Four patients were admitted to the intensive care unit (ICU) because of unstable hemodynamics, five refused diagnostic interviews, three blood samples from different patients did not have melatonin concentration measurements, and four patients had < 2 hours of anesthesia. A total of 104 patients were finally analyzed. A flow chart of selection of the patients is shown in Figure 1 and the baseline patients’ characteristics are listed in Table 1. The POD group contained 29 patients who were diagnosed with POD, while 75 patients did not have POD (non-POD group).
Figure 1.
Flow chart of selection of the patients. ICU: intensive care unit; MMSE: Mini-Mental State Examination.
Table 1.
Baseline characteristics of the patients.
| Variable | POD | Non-POD | P value |
|---|---|---|---|
| Age (years) | 79.00 ± 2.51 | 72.05 ± 2.94 | <0.01 |
| Sex (female/male) | 9/20 | 28/47 | 0.55 |
| Living alone (Y/N) | 17/12 | 38/37 | 0.47 |
| Type of surgery(gastrectomy/enterectomy) | 6/23 | 16/59 | 0.83 |
| ASA scale (II/III) | 17/12 | 59/16 | 0.04 |
| Serum albumin levels (g/L) | 44.10 ± 3.56 | 45.38 ± 2.34 | 0.02 |
| Preoperative MMSE score | 26 (25, 27) | 27 (26, 27) | 0.62 |
| Duration of anesthesia (minutes) | 165.97 ± 10.36 | 147.89 ± 9.06 | <0.01 |
| Blood loss (mL) | 336.59 ± 22.74 | 328.36 ± 23.18 | 0.11 |
| Transfusion (Y/N) | 6/23 | 8/67 | 0.18 |
| Requirement of postoperative analgesia (Y/N) | 10/19 | 21/54 | 0.52 |
| Serum melatonin levels | |||
| Preoperative (ng/L) | 68.35 ± 7.94 | 66.72 ± 8.21 | 0.36 |
| Postoperative (ng/L) | 43.61 ± 3.60 | 52.34 ± 5.73 | <0.01 |
| Change in melatonin | 26.50 ± 8.35 | 14.38 ± 10.67 | <0.01 |
Abbreviations: ASA, American Society of Anesthesiologists; MMSE, Mini-Mental State Examination; POD, postoperative delirium; Y: yes; N: no.
Melatonin levels
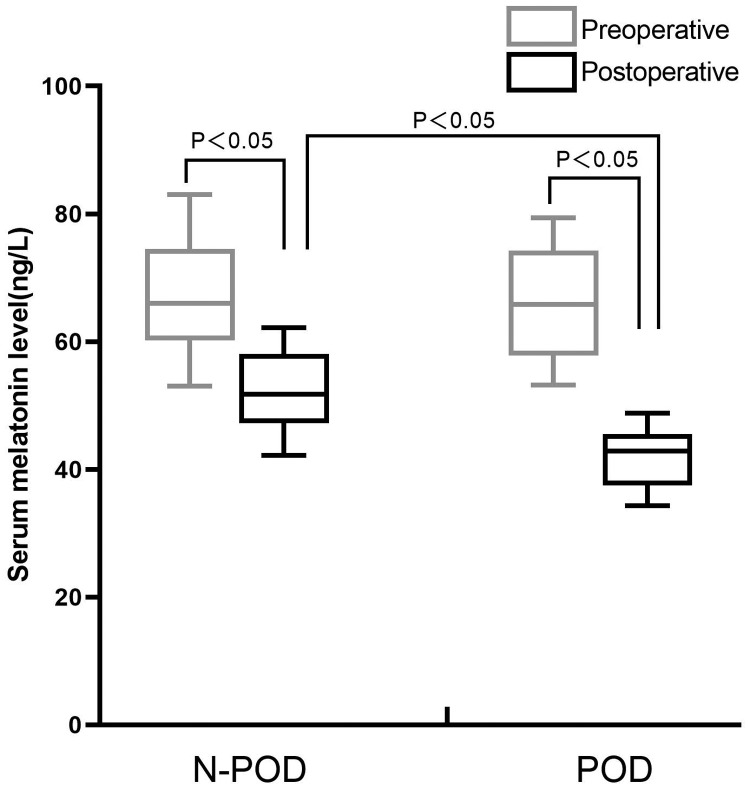
Postoperative serum melatonin levels were significantly lower than preoperative levels in both groups (both P < 0.05). Preoperative serum melatonin levels were not significantly different between the groups. However, postoperative serum melatonin levels were significantly lower in the POD group than in the non-POD group (P < 0.05) (Figure 2).
Figure 2.
Comparison of serum melatonin levels between the POD and non-POD groups. POD: postoperative delirium.
Risk factors of POD
The potential risk factors of POD were initially analyzed by using univariate analysis for later entry of significant variables into multivariate logistic regression analysis. Multivariate logistic regression analysis showed that patients in the POD group had a significantly longer duration of anesthesia, advanced age, and lower postoperative melatonin levels (all P < 0.05) (Table 2).
Table 2.
Multivariate analysis of risk factors for postoperative delirium.
| Factor | B | S.E. | Wald | P value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Age | 1.211 | 0.457 | 7.0366 | <0.01 | 3.358 | (1.372, 8.218) |
| ASA scale (II/III) | −0.960 | 1.698 | 0.320 | 0.57 | 0.383 | (0.014, 10.664) |
| Duration of anesthesia | 0.256 | 0.121 | 4.470 | 0.026 | 1.292 | (1.019, 1.637) |
| Postoperative serum melatonin levels | −0.703 | 0.357 | 3.873 | 0.038 | 0.495 | (0.246, 0.997) |
| Serum albumin levels | −0.282 | 0.525 | 0.289 | 0.591 | 0.754 | (0.270, 2.110) |
Abbreviations: S.E.: standard error; OR: odds ratio; CI: confidence interval; ASA, American Society of Anesthesiologists.
Discussion
In the current study, we observed a decline in serum melatonin levels postoperatively. This observation is consistent with the previous results,18 and could be attributed to the following reasons: (1) a reduction in melatonin synthesis due to N-acetyltransferase inhibition, caused by increased cortisol levels during surgery;19,20 (2) a reduction in melatonin levels through γ-aminobutyric acid antagonists by general anesthetics such as propofol;21 and (3) disappearance of oxidized melatonin by reactive oxygen free radicals.22
Melatonin is secreted in the pineal gland and reaches maximal levels at approximately 2:00 am in response to changes in light.23,24 Melatonin is an important neuroendocrine hormone that maintains the circadian rhythm and regulates the sleep arousal cycle. Furthermore, neuroprotective and anti-inflammatory effects of melatonin have been reported.13,25 Several studies have focused on the correlation between delirium and melatonin.12,15 Wu et al.12 suggested that urinary 6‐sulfatoxymelatonin levels could serve as a method for diagnosing POD. However, because light is an inevitable factor that could affect results, we turned off the lights at 10:00 pm and collected blood samples at 9:00 pm to reduce bias.
Our study showed an inverse correlation between serum melatonin levels and the incidence of POD. Miyazaki et al.26 reported a possible correlation between ICU psychosis and melatonin rhythm in patients after undergoing thoracic esophagectomy, but only patients in the ICU were included. Another study that was conducted on 40 critically ill patients reported that regulating light and noise in the ICU improved recovery after major surgery by increasing melatonin levels.27 However, the size of the sample in this study was small. Therefore, we investigated the correlation between melatonin levels and the incidence of POD in the current study. However, we excluded the patients with a score of <24 on the MMSE, which may have decreased the number of patients with POD. Adogwa et al.28 suggested that postoperative delirium is associated with decreased preoperative cognitive reserve.
The incidence of POD was relatively high in the present study and could be attributed to the longer duration of anesthesia (longer than 2 hours). Zhang et al.29 reported that continuous anesthesia and surgery induced delirium-like behavior in susceptible mice. Wu and his team evaluated the postoperative cognitive situation in patients who underwent surgery that lasted longer than 2 hours.12 To reduce clinical bias, we recruited patients who had longer than 2 hours of anesthesia.
In our study, risk factors of POD were a longer duration of anesthesia and advanced age, which are consistent with previous studies.4,30 Decreased prior cognitive function with aging might increase the incidence of POD.3 However, we found that in patients who required postoperative analgesia, preoperative serum albumin levels and transfusion were not significantly associated with the risk of POD. We attributed this finding to the small sample of patients who suffered from POD and performing laparoscopic surgery, which causes less trauma. Additionally, improvement of preoperative serum albumin levels could have been a factor related to this lack of risk associated with POD.
This study has the following limitations. (1) Although we turned off the lights at the same time, the environment and noise in the hospital may have affected production of melatonin. (2) Blood samples were only collected twice and individual variance may have resulted in bias. (3) We did not assess the severity of delirium. (4) Only patients who had longer than 2 hours of anesthesia were included. (5) Although we excluded patients with MMSE scores <24, we did not assess other aspects of preoperative cognitive status for the included patients.
Conclusion
Our study shows that melatonin levels are significantly reduced after surgery. Significantly decreased postoperative melatonin levels could be used to predict the incidence of POD in older patients who undergo major abdominal surgery with general anesthesia. However, more extensive studies are required to confirm these conclusions
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by grants from Jiaxing Key Discipline of Medicine–Anesthesiology (2019-zc-06) and Jiaxing Science and Technology Bureau (2018AD32080).
ORCID iD
Qi-hong Shen https://orcid.org/0000-0003-3365-779X
References
- 1.Abelli M, Pini S, Martinelli R, et al. [Delirium: a reappraisal of clinical characteristics and treatment perspectives after the transition from the DSM-IV to the DSM-5]. Riv Psichiatr 2019; 54: 218–223. DOI: 10.1708/3249.32186. [DOI] [PubMed] [Google Scholar]
- 2.Igwe EO, Traynor V, Rodgers S, et al. Knowledge, opinions and clinical practice regarding postoperative delirium in older patients: a survey of nurses and anaesthetists. J Clin Anesth 2019; 57: 108–109. [DOI] [PubMed] [Google Scholar]
- 3.Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly risk factors and outcomes. Ann Surg 2009; 249: 173–178. DOI: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 4.Zhu C, Wang B, Yin J, et al. Risk factors for postoperative delirium after spinal surgery: a systematic review and meta-analysis. Aging Clin Exp Res Epub ahead of print 30 August 2019. DOI: 10.1007/s40520-019-01319-y. [DOI] [PubMed] [Google Scholar]
- 5.Mueller A, Spies CD, Eckardt R, et al. Anticholinergic burden of long-term medication is an independent risk factor for the development of postoperative delirium: a clinical trial. J Clin Anesth 2020; 61: 109632. DOI: 10.1016/j.jclinane.2019.109632. [DOI] [PubMed] [Google Scholar]
- 6.Luo C, Zou W. Cerebral monitoring of anaesthesia on reducing cognitive dysfunction and postoperative delirium: a systematic review. J Int Med Res 2018; 46: 4100–4110. DOI: 10.1177/0300060518786406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plaschke K, Fichtenkamm P, Schramm C, et al. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med 2010; 36: 2081–2089. DOI: 10.1007/s00134-010-2004-4. [DOI] [PubMed] [Google Scholar]
- 8.Brzezinski A. Melatonin in humans. N Engl J Med 1997; 336: 186–195. [DOI] [PubMed] [Google Scholar]
- 9.Iguichi H, Kato KI, Ibayashi H. Age-dependent reduction in serum melatonin concentrations in healthy human subjects. J Clin Endocrinol Metab 1982; 55: 27–29. [DOI] [PubMed] [Google Scholar]
- 10.Waldhauser F, Weiszenbacher G, Tatzer E, et al. Alterations in nocturnal serum melatonin levels in humans with growth and aging. J Clin Endocrinol Metab 1988; 66: 648–652. [DOI] [PubMed] [Google Scholar]
- 11.Scholtens RM, van Munster BC, van Faassen M, et al. Plasma melatonin levels in hip fracture patients with and without delirium: a confirmation study. Mech Ageing Dev 2017; 167: 1–4. DOI: 10.1016/j.mad.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Wang J, Wu A, et al. Do fluctuations in endogenous melatonin levels predict the occurrence of postoperative cognitive dysfunction (POCD)? Int J Neurosci 2014; 124: 787–791. [DOI] [PubMed] [Google Scholar]
- 13.Lee B, Shim I, Lee H, et al. Melatonin ameliorates cognitive memory by regulation of cAMP-response element-binding protein expression and the anti-inflammatory response in a rat model of post-traumatic stress disorder. BMC Neurosci 2018; 19: 38. DOI: 10.1186/s12868-018-0439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dispersyn G, Pain L, Touitou Y. Propofol anesthesia significantly alters plasma blood levels of melatonin in rats. Anesthesiology 2010; 112: 333–337. DOI: 10.1097/ALN.0b013e3181c920e2. [DOI] [PubMed] [Google Scholar]
- 15.Scholtens RM, de Rooij SE, Vellekoop AE, et al. Preoperative CSF melatonin concentrations and the occurrence of delirium in older hip fracture patients: a preliminary study. PLoS One 2016; 11: e0167621. DOI: 10.1371/journal.pone.0167621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyagawa Y, Yokoyama Y, Fukuzawa S, et al. Risk factors for postoperative delirium in abdominal surgery: a proposal of a postoperative delirium risk score in abdominal surgery. Dig Surg 2017; 34: 95–102. DOI: 10.1159/000449044. [DOI] [PubMed] [Google Scholar]
- 17.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990; 113: 941–948. [DOI] [PubMed] [Google Scholar]
- 18.Cronin AJ, Keifer JC, Davies MF, et al. Melatonin secretion after surgery. Lancet 2000; 356: 1244–1245. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran N, Smyth N, Thorn L, et al. Relationship between post-awakening salivary cortisol and melatonin secretion in healthy participants. Stress 2016; 19: 260–263. [DOI] [PubMed] [Google Scholar]
- 20.Simons SSH, Beijers R, Cillessen AHN, et al. Development of the cortisol circadian rhythm in the light of stress early in life. Psychoneuroendocrinology 2015; 62: 292–300. [DOI] [PubMed] [Google Scholar]
- 21.Dispersyn G, Pain L, Touitou Y. Propofol anesthesia significantly alters plasma blood levels of melatonin in rats. Anesthesiology 2010; 112: 333–337. [DOI] [PubMed] [Google Scholar]
- 22.Mowafi HA, Ismail SA. Melatonin improves tourniquet tolerance and enhances postoperative analgesia in patients receiving intravenous regional anesthesia. Anesth Analg 2008; 107: 1422–1426. [DOI] [PubMed] [Google Scholar]
- 23.Lewy AJ, Wehr TA, Goodwin FK, et al. Light suppresses melatonin secretion in humans. Science 1980; 210: 1267–1269. [DOI] [PubMed] [Google Scholar]
- 24.Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003; 26: 342–392. [DOI] [PubMed] [Google Scholar]
- 25.Yon JH, Carter LB, Reiter RJ, et al. Melatonin reduces the severity of anesthesia-induced apoptotic neurodegeneration in the developing rat brain. Neurobiol Dis 2006; 21: 522–530. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki T, Kuwano H, Kato H, et al. Correlation between serum melatonin circadian rhythm and intensive care-unit psychosis after thoracic esophagectomy. Surgery 2003; 133: 662–668. [DOI] [PubMed] [Google Scholar]
- 27.Yoshitaka S, Egi M, Morimatsu H, et al. Perioperative plasma melatonin concentration in postoperative critically ill patients: its association with delirium. J Crit Care 2013; 28: 236–242. [DOI] [PubMed] [Google Scholar]
- 28.Adogwa O, Elsamadicy AA, Vuong VD, et al. Association between baseline cognitive impairment and postoperative delirium in elderly patients undergoing surgery for adult spinal deformity. J Neurosurg Spine 2018; 28: 103–108. DOI: 10.3171/2017.5.Spine161244. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Gao J, Guo G, et al. Anesthesia and surgery induce delirium-like behavior in susceptible mice: the role of oxidative stress. Am J Transl Res 2018; 10: 2435–2444. [PMC free article] [PubMed] [Google Scholar]
- 30.Galyfos GC, Geropapas GE, Sianou A, et al. Risk factors for postoperative delirium in patients undergoing vascular surgery. Journal Vasc Surg 2017; 66: 937–946. [DOI] [PubMed] [Google Scholar]