Abstract
Brain arteriovenous malformations have a high risk of intracranial hemorrhage, which is a substantial cause of morbidity and mortality in patients with brain arteriovenous malformations. Although a variety of genetic factors leading to hereditary brain arteriovenous malformations have been extensively investigated, their pathogenesis is still not well elucidated, especially in sporadic brain arteriovenous malformations. The authors have reviewed the updated data of not only the genetic aspects of sporadic brain arteriovenous malformations, but also the architecture of microvasculature, the roles of the angiogenic factors, and the signaling pathways. This knowledge may allow us to infer the pathogenesis of sporadic brain arteriovenous malformations and develop pre-emptive treatments for them.
Keywords: Arteriovenous malformation, non-hereditary, pathology, signal pathway
Introduction
Brain arteriovenous malformations (bAVMs) are major causes of intracerebral hemorrhage. Treatments for bAVMs are mainly directed to reduce the risk of rupture, which could be fatal. Two mechanisms for bAVM formation have been postulated1: (1) abnormal sprouting angiogenesis leading to an anomalous direct arterial-venous connection and (2) the progressive dilation of existing capillary beds resulting in high-flow shunting from arterial to venous circulations. Both mechanisms have been described and appear specific to the rodent model,2,3 but the pathogenesis of bAVM has still not been elucidated. bAVMs can occur as part of hereditary syndromes, such as hereditary hemorrhagic telangiectasia (HHT) and capillary malformation (CM)-arteriovenous malformation (AVM), where they result from germline mutations in genes that have known or plausible roles in angiogenesis and vascular remodeling, such as ENG, ALK1, SMAD4, and RASA1, among others.4,5 Similarly, familial inheritance of sporadic bAVMs has been ascribed to mutations in ALK1.6 Less is known about the cause of sporadic AVMs, which account for the majority of the disease burden in the general population.
Studies of AVM tissue suggest a dynamic and biologically active angiogenic and inflammatory lesion, rather than a static congenital vascular malformation.7,8 Molecular biology and gene expression studies have been performed to investigate the nature of AVMs. Sporadic vascular malformations, including sporadic AVMs and cavernous malformations,9,10 are much more common and seem to be associated with somatic mutations. In this communication, the formation of bAVMs, the architecture of microvasculature, the roles of the angiogenic factors, and the signaling pathways are reviewed. The emerging research geared toward a postulated genetic source for lesions once thought to be “sporadic” or without underlying genetic mutation is also summarized.
The rupture of bAVM
The underlying mechanisms for bAVM rupture are not fully understood. Hemodynamic factor (high flow shunt, restricted venous drainage, etc.) and vessel wall weakness are two main factors that may cause bAVM rupture. From the biological point of view, it is likely postulated that multiple mechanisms including inflammation, remodeling, and brain endothelial cell (EC) abnormalities contribute to bAVM’s tendency to exhibit hemorrhage.11 Several vascular wall cells, i.e. smooth muscle cells,12 pericytes,13 or both (mural cells),14 may cause vessel wall fragility. Inflammatory cells (macrophages and neutrophils) have been detected in surgical specimens of human bAVM, even in those without a history of hemorrhage or previous embolization or radiosurgery.15 Macrophages and neutrophils tend to invade AVM tissue even in the absence of radiographically-evident hemorrhage. Both relative neutrophilia and increases in macrophage migration inhibitory factor appear to contribute to the instability of bAVM nidus, contributing to apoptosis and possibly rupture. In the clinical scene, vessel wall magnetic resonance imaging may contribute to identify the rupture point in a ruptured bAVM.16
In addition, silent intralesional hemorrhage has been found in 30% of unruptured bAVMs.17 Nearly one-third of patients without a clinical history of AVM hemorrhage had hemosiderin in surgical specimens, indicating silent bAVM hemorrhage.18 Hemosiderin positivity was strongly associated with macrophages, suggesting that bAVMs with silent hemorrhage are more biologically active and inflamed lesions. These findings are consistent with the significantly higher risk of subsequent hemorrhage in patients with silent hemorrhage than in unruptured bAVM patients without it.18
Vasculogenesis and angiogenesis
During embryonic development, formation of blood vessel networks relies on two processes: vasculogenesis (de novo vessel formation during embryogenesis) and angiogenesis (the expansion of a pre-existing vascular network through sprouting or splitting of vessels).19,20 Subsequent growth of the vertebrate vasculature occurs entirely by angiogenesis, the first phase of which involves vascular EC proliferation and migration; the second phase of angiogenesis is vascular stabilization, during which ECs form capillary tubes, strengthen their intercellular junctions, and recruit smooth muscles cells (SMCs) to their walls.19–21
Brain AVMs form at the interface between arterial and venous endothelium where capillary endothelium normally lies.22 The angiogenic process, most severely disrupted by the vascular malformations, is that of vascular stabilization, the process whereby vascular ECs form capillary tubes, strengthen their intercellular junctions, and recruit SMCs to the vessel wall.23,24 The AVM nidus expresses markers specific to arteries and veins, as well as capillaries. The AVM nidus is thought to consist of aberrant vessels that are not finally differentiated and inadequately matured,25 and it histopathologically lacks a true capillary bed.22
Microvasculature
Cerebral blood vessels are anatomically comprised of ECs, vascular SMCs, and pericytes. To date, most bAVM research has focused on the endothelium. On histologic evaluation, significant endothelial heterogeneity has been described.1 Disruption of the blood–brain barrier (BBB) is well documented,13 and microhemorrhages are frequently observed in unruptured bAVMs and may predict future rupture.18 Whether the function of vascular ECs and SMCs affects the phenotypic presentation of bAVMs is still unknown.
Endothelial cells
Brain ECs form the monolayer cell lining of the vascular lumen, serving as the vital interface between the blood and brain parenchyma known as the BBB. At a molecular level, ECs express higher levels of pro-angiogenic factors,26,27 and, as a result, frequently assume a pro-angiogenic phenotype in bAVMs. ECs of bAVMs proliferate and migrate more rapidly and form aberrant vascular tubules in vitro.28,29 The experimental models reproduce some features observed in human bAVMs (e.g. dilated vessels, an arteriovenous shunt, a high-flow lesion, and formation of a nidus).30,31 In the mouse model, the combination of the EC-specific deletion of ALK1 and administration of vascular endothelial growth factor (VEGF) in the brain causes a lesion similar to human AVM with dilated vessels and proliferation of ECs.32 Adenovirus-mediated EC-selective ALK1 deletion and overexpression of VEGF induce lesions resembling human bAVM as well,3 suggesting involvement of changes in EC function and angiogenesis in the pathogenesis.
The endothelium in a bAVM also assumes a pro-inflammatory phenotype. Upregulation of endothelial adhesion molecules and cytokines and BBB breakdown facilitate the infiltration of circulating blood-derived inflammatory cells observed in bAVMs.15,33 Whole-exome sequencing of bAVMs showed that some vascular ECs contained KRAS mutations,10 and further investigation showed that cultured ECs that had the same mutation were phenotypically larger and elongated, demonstrated faster migration, and had more cytoskeletal actin projections.10
Smooth muscle cells
Vascular SMCs are the predominant cellular constituent of the vessel wall in arteries and veins. SMCs derived from bAVMs formed tubes in culture that were longer than those formed by normal brain vascular SMCs. The migration and proliferation of SMCs in bAVMs exceeded those of normal brain vascular SMCs.34 The reductions in vascular SMCs in bAVMs have been described.35 Wnt signaling was activated in vascular ECs and SMCs in low-flow bAVMs.36
Pericytes
Pericytes are embedded within a vascular basement membrane that is shared with the adjacent endothelium. The close spatial proximity during vascular remodeling between sprouting ECs and neighboring pericytes suggests crosstalk and, potentially, a reciprocal signaling relationship between these two cell types during vascular remodeling.37
Pericytes are important in promoting vascular stability and maturation throughout the human body.38,39 A reduction in pericytes has been described in both human bAVMs and rodent models,13,14 and it is greatest in ruptured human AVMs.13 In unruptured AVMs, the magnitude of pericyte loss correlates with the severity of BBB disruption and microhemorrhage.13 Pericytes have important roles in angiogenesis and maintaining vessel stability through crosstalk with angiopoietin (ANGPT) signaling. Angiopoietin-2 (Ang-2) is overexpressed in bAVMs compared to controls.27,40 Misregulated VEGF-A activity impairs pericyte coverage and distribution by disrupting pericyte migration.41 The vascular abnormalities associated with bAVMs result, in part, from downstream defects in pericyte behaviors and their underlying signaling mechanisms.
Angiogenic factors
EC behavior during vascular development is regulated by prominent growth factor families such as VEGF, fibroblast growth factor (FGF), ANGPT, and transforming growth factor beta (TGF-β)/bone morphogenetic protein (BMP) families. One important pathway for EC-vascular SMC and pericyte crosstalk is the platelet-derived growth factor (PDGF)-B/PDGFRβ pathway. In addition to growth factors, vascular development and homeostasis are regulated by many other signaling inputs, including ligand-receptor signaling pathways such as Wnt and Notch.
Abnormal expressions of PDGFB and PDGFRβ have been described in bAVMs in rodent models and humans.13,35,42 Recent work showed that both human and mouse bAVM vessels have less mural cell (vascular SMCs and pericytes) coverage than normal brain vessels,13,14 suggesting abnormal vascular remodeling in bAVMs. Immunohistochemistry of AVM vessels has shown the abundant production of potent angiogenic factors, such as VEGF and other proliferative factors (e.g. basic FGF and TGF-α).43,44 Significant overexpression of VEGF receptors and ANGPT receptors (e.g. Tie-1 and Tie-2) in ECs has been reported in surgically resected AVMs compared with controls.40
VEGF
VEGF is a potent EC mitogen that is thought to play a key role in angiogenesis,45 and abnormal expression of VEGF has been repeatedly observed, making it increasingly more evident that a disruption in angiogenic signaling systems is involved in the formation and progression of bAVMs.46,47 Increased VEGF expressions in the endothelium and other cells of the surgical specimens of bAVM implied that VEGF was involved in EC proliferation and angiogenesis in bAVMs.26,27 Another study also demonstrated the abundant expression of VEGF and Ang-2 protein in human bAVM lesions compared with normal cerebral cortex.48 Furthermore, the nidus size is larger in lesions positive for VEGF-A or Flt-1 (VEFR-A receptors) staining than in those without positive signals, suggesting the pathogenic role of VEGF-mediated angiogenesis in the enlargement of the lesion.26 VEGF also plays an important role in maintaining the normal permeability of vessel walls.49 Extrapolating from animal models, VEGF may contribute to the hemorrhagic tendency of bAVMs.50,51
Fatty acid binding protein 4
Fatty acid binding protein 4 (FABP4) is an intracellular lipid chaperone that is involved in cell proliferation and differentiation. FABP4 is potently induced by proangiogenic mediators, such as VEGF. FABP4 is not expressed in normal brain vasculature. However, FABP4 expression in EC and perivascular cells has been detected in 65% and 100% of AVM surgical specimens, respectively.52,53
TGF-β
TGF-β is a multifunctional cytokine that has multiple effects on brain vascular development implicated in vascular malformations, including both bAVMs and cavernous malformations.39,54 In humans, it has been suggested that single nucleotide polymorphisms in ALK1 or ENG may be associated with heightened risk of sporadic AVMs.55,56 More recently, whole exome sequencing has identified a novel SMAD9 mutation associated with a recurrent, sporadic AVM. Oligonucleotide-mediated knockdown in developing zebrafish confirmed formation of brain arterial-venous shunts, suggesting a causal role.57 However, the role of TGF-β signaling in the formation of non-HHT, sporadic bAVMs remains to be defined.
Bone morphogenetic proteins
The BMPs are members of the TGF-β superfamily. BMP signaling plays a critical role in modulating the fates of the tip and stalk cells during sprouting angiogenesis. BMP and Notch signaling pathways are essential for vascular development and homeostasis, and disruption of these pathways is known to cause vascular diseases. Recent studies showed that lack of BMP inhibition induces the expression of Notch components in ECs, which results in bAVMs.58,59 In human brain tissue, immunofluorescent staining demonstrated a vascular predominance of SMAD9 at the protein level. Vascular SMAD9 was markedly reduced in peri-nidal blood vessels of bAVMs, which was accompanied by decreased phosphorylated SMAD4, a downstream effector protein of the BMP signaling pathway. In a zebrafish model, the morpholino splice site and translation-blocking knockdown of SMAD9 resulted in abnormal brain artery-to-vein connections with morphologic similarities to human AVMs.60
Notch
The Notch pathway is a critical mediator of the differentiation of arteries and veins.61,62 An AVM arises due to impaired arterial or venous differentiation during early angiogenesis consequent to imbalanced Ephrin proteins, particularly EPHB2 and EPHB4.
Notch components are considered to be critical mediators of EC fate decisions and vascular lumen formation,63,64 and both loss-of-function and gain-of-function Notch mutations result in arteriovenous shunting.65,66 Notch receptors and ligands are differentially expressed in arteries and veins, with Notch1 and Notch4 expressed on arterial ECs, and Notch2 and Notch3 expressed on venous ones.67 In young mice, constitutive activation of the Notch4 intracellular domain has led to the production of brain arteriovenous shunts.2,68,69 Thomas et al. reported augmented expressions of EphrinB2, Hey2, and DLL4 in the nidus structures of bAVMs when compared to normal brain arteries,25 and they suggested that deregulated arterial specification signaling might have a significant role in the pathogenesis of AVM.
Recent work has suggested a role for Notch signaling in the formation of non-syndromic, sporadic bAVMs.70 Elevated expressions of Notch1 and Notch intracellular domain and increased activity of the ligands Jagged-1 and DLL-4 have been described in human bAVM specimens.68,71 Other studies have shown that Notch1, but not Notch4, was overexpressed in ruptured AVMs compared to unruptured ones.72 Polymorphisms of Notch4 gene have also been associated with human AVM formation and hemorrhage.73,74
Wnt signaling
Gene ontology analysis identified that Wnt signaling, an important signaling pathway associated with embryonic angiogenesis, was involved in the development of bAVMs.75 In vascular development, Wnt and Notch interact with each other to determine proper EC differentiation, vascular remodeling, and arteriovenous specification. Wnt signaling increases Sox17 transcription, subsequently activating Notch and promoting arterial identity.76 The Sox17-associated pathway is expressed in the bAVM nidus.75 High expression of the Sox17-associated pathway in thick-walled veins indicates the process of arterialization in response to the hemodynamic stress. The increased activation of the Sox17-associated pathway in medium-sized and small arteries suggests that ECs in bAVMs are primarily abnormal.75
Gene mutations
The identification of gene mutations and genetic risk factors associated with bAVMs has enabled understanding of the genetics of this disease. The genetic hypothesis of the formation of AVMs is a “two-hit” mechanism, in which an inherited mutation in one copy of a cerebrovascular malformation gene is followed by a somatic mutation in a second counterpart.19,77 The second “hit” could be environmental, in the form of a localized physiological or pathological perturbation.19 Therefore, there is an interaction between hemodynamic factors and genetic factors in vasculogenesis.
KRAS
A recent study showed that the majority of sporadic bAVMs also harbored somatic activating KRAS mutations driving downstream mitogen-activated protein kinase (MAPK)–extracellular signal-regulated kinase (ERK) signaling.10 Activating KRAS mutations were noted in 62.5% of 72 bAVMs, but in none of 21 paired blood samples.10 The presence of activating KRAS mutations in more than half of bAVM tissue samples may indicate the pathogenic role of these KRAS mutations.78 In sporadic bAVMs, KRAS mutations were also detected in 9 of 15 specimens (60%), and seven of them were G12V or G12D mutations.79 Priemer et al. demonstrated the first reported instance of a KRAS p.G12C mutation in a bAVM.80 The mean age of patients with KRAS-mutant bAVMs was lower than that of patients in the non-mutant group, and the mean size was larger. Histologic characteristics were equally distributed between KRAS-mutant and non-mutant groups.80 They postulated that these mechanisms may result in potentially distinguishable clinical and/or histologic features between KRAS-mutant and non-mutant bAVMs.
KRAS mutations were detected in ECs from human bAVMs in vitro, and it was noted that mutant KRAS expression initiated increased ERK activity that was counteracted by inhibition of MAPK–ERK signaling.10 Interestingly, KRAS is mostly implicated in tumorigenesis and cancer, where mutations promote unregulated activation of growth-promoting signal transduction pathways resulting in cell transformation and genomic instability.81
There is an opinion that cells with the mutated KRAS seem to be a therapeutic target in the treatment of bAVM.82,83 On the other hand, Priemer et al. insisted that it may indicate that the histology of bAVMs is a reflection of MAPK–ERK activation in general, regardless of the initiating event. From another point of view, KRAS G12D mutation may be the result of a repair of ECs damaged by excessive hemodynamic force from arterial blood flow, which is usually diminished by intervening capillaries. They suggest that the presence of KRAS mutations within AVMs may be of little clinical or pathologic importance.78–80
BRAF
Hong et al. reported the first evidence of activating BRAF mutations in bAVMs and spinal AVMs (sAVMs). The total prevalence of KRAS/BRAF mutations was 87.1% (27 of 31 patients) in their cohort.84 The prevalence of KRAS/BRAF mutations was 81.0% (17 of 21 patients) in bAVMs and 100% (10 of 10 patients) in sAVMs. KRAS p.G12D and p.G12V were mutation hotspots both in sAVMs and bAVMs, with a prevalence of 30.0% and 30.0% in sAVMs, and 52.4% and 19.0% in bAVMs, respectively, whereas BRAF p.V600E was rare and found in only one bAVM patient and one sAVM patient.84 They found that mutation variant frequencies correlated negatively with nidus volumes and largest diameters, but not with age.84
Signaling pathways
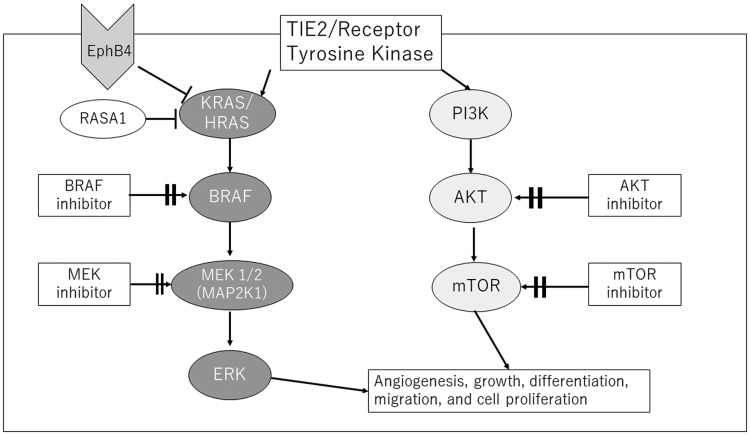
Increased KRAS activity can potentially affect multiple downstream signal pathways (Figure 1). Most vascular malformations are associated with mutations commonly found in cancer, mainly in phosphoinositide 3-kinase (PI3K)–AKT–mammalian target of rapamycin (mTOR) in low-flow vascular malformations including venous and lymphatic malformations85,86 and the RAS–MAPK–ERK pathway in high-flow lesions including bAVMs.9,10,87 bAVMs without detectable KRAS mutations also had high levels of phosphorylated ERK1/2, suggesting that RAS–MAPK–ERK pathway activation is a hallmark of all bAVMs.10 The PI3K signaling pathway is a critical regulator of the angiogenic process by controlling proliferation, migration, and survival of ECs.
Figure 1.
Signal transduction pathways in endothelial cells and the main genetic mutations associated with vascular malformations. Key signaling pathway PI3K/AKT/mTOR and RAS/RAF/MEK/ERK control cellular growth, apoptosis, and differentiation through complex transcriptional regulation. Potential treatments are shown in a square.
mTOR: mammalian target of rapamycin.
Germline autosomal dominant RASA1 mutations have been identified in 50% of CM-AVM1 patients, including those with Parkes Weber syndrome. RASA1 encodes p120-RasGAP protein that inhibits activity of RAS protein. Loss of function mutations of RASA1 may therefore lead to activation of RAS and increased downstream signaling via the MEK–ERK1/2 and PI3K–AKT–mTOR pathways that can be potentially targeted.88
Additional recent studies demonstrated autosomal dominant EPHB4 mutations, named CM-AVM2.89 This gene encodes a trans-membrane receptor expressed primarily in venous ECs during vascular development interacting with its ligand, EphrinB2, on arterial ECs. EPHB4 also activates p120-RasGAP, and therefore exerts similar downstream effects on the MEK/ERK pathway as RASA1. The phenotypic similarity between CM-AVM1 and CM-AVM2 suggests that RASA1 and EPHB4 play an overlapping role in vascular development during embryogenesis.90,91
Mechanistically, endothelial BMP signaling is likely linked to the hemodynamic response, because blood flow potentiates BMP9 signaling by inducing complex formation between ENG and ALK1. AVMs in BMP9, ENG, ALK1, and SMAD4 mutants arise largely due to over-activation of PI3K–AKT signaling downstream of blood flow and VEGF-A signaling.92,93 Reducing VEGFR2 activity or PI3K–AKT largely normalized the AVM phenotype caused by disrupting BMP9/ENG/ALK1/SMAD4 function.92–94
Somatic mutations have been noted in bAVMs, not only in the RAS–MAPK pathway, but in the PI3K pathway, as well as in other vascular malformations. The RAS–MAPK signaling pathway is the most promising therapeutic target for bAVMs. The initiating events for AVMs without RAS mutations are uncertain. The PI3K–AKT–mTOR pathway has a possibility of being a therapeutic target for bAVMs, although the somatic mutations in the PI3K pathway in high-flow AVMs have not previously emerged. Further study will demonstrate the increasing importance of genetic diagnosis for both germ-line and somatic mutations for future molecular target therapies.95
Future therapeutic targets
There are multiple treatment options for bAVMs, including surgical resection, radiosurgery, embolization, or medical treatment, but these modalities are not without risk.
An ideal therapy would be non-invasive, immediate in action, and would be specific to the abnormal cells of the AVM without affecting adjacent blood vessels or brain. Considerable efforts have been made to use existing therapies to target molecular pathways disrupted in bAVMs, including TGF-β, Notch, and VEGF. For example, losartan, an angiotensin II receptor antagonist used for treatment of hypertension, decreased vascular dysplasia and arteriovenous-shunting in a zebrafish model of bAVMs induced through knockdown of the TGF-β receptor ALK1.57 In HHT, treatment with thalidomide was shown to restore endothelial PDGFB expression, leading to recruitment of mural cells and vessel stabilization.96 Thalidomide or lenalidomide treatment reduced the number of dysplastic vessels and hemorrhage, and increased mural cell (vascular SMCs and pericytes) coverage in bAVM lesion.35
Inhibitors of Notch signaling may represent another prospective therapy for future development. With extracranial vascular malformations, Notch inhibitors reduce the rate of endothelial migration and formation of vascular networks.97 Direct targeting of pro-angiogenic pathways such as VEGF has also attracted attention for prospective therapeutic development in bAVMs.
VEGF neutralization prevented and normalized AVM in an animal model for HHT2, an autosomal-dominant disorder characterized by telangiectasia and AVMs in multiple organs.98 Others have begun to explore a direct approach using bevacizumab, a humanized VEGF monoclonal antibody. Bevacizumab has an established safety profile and has been trialed as anti-angiogenic therapy in a number of neoplastic conditions, including glioblastoma.99 In ALK1-deficient rodents with bAVMs, treatment with bevacizumab induced vascular apoptosis, reducing the number of proliferating vascular cells and dysplastic vessels.45
Malformations due to mutations affecting the RAS/BRAF/MEK/ERK pathway (e.g. CM, CM-AVM, bAVM, sAVM) could perhaps be targeted by a BRAF inhibitor (e.g. vemurafenib) and/or MEK inhibitors (e.g. trametinib, cobimetinib) that are available.84,90,100,101 An AVM overlying the left scapular region in a child was treated by a genotype-guided approach. Exome sequencing from a specimen of the AVM and saliva showed in-frame deletion of MAP2K1; therefore, treatment with a MEK inhibitor, trametinib, was given, with a significant reduction in overall volume after six months.102 Whether these agents may be repurposed to accelerate translation of a similar approach in human patients with bAVMs remains to be elucidated. Initial experiments with the MAPK–ERK pathway promoted vascular barrier properties and quiescence in patient-derived KRAS-mutant ECs in vitro.10 Common MEK inhibitors are currently used for the treatment of melanoma.103,104 A mutation of MEK1 (downstream effector of BRAF) has been identified in more than 50% of cases of extracranial AVMs, though it has also been described in some cases of bAVM.9,84,105
Vascular anomalies with mutations affecting the PI3K–AKT–mTOR pathway (e.g. venous malformation, venous malformation cutaneo-mucosal, multifocal venous malformation, and lymphatic malformation) are known to respond to mTOR inhibitors (sirolimus, everolimus, and temsirolimus). In vivo animal models showed that sirolimus inhibits angiogenesis via downregulating the PI3K/AKT signaling pathway and the expression of VEGF.106 All six head and neck AVM patients (four male and two female patients) responded favorably to the combination of sirolimus therapy followed by endovascular embolization, and four patients showed a near-complete response.107 mTOR signaling has been shown to mediate angiogenesis via increased expression of VEGF.108
Only recently have the contributions of other cell-types, such as pericytes, vascular SMCs, and inflammatory cells, begun to be appreciated in bAVMs.13 How molecular cross-talk among these cell types is disrupted remains poorly understood in bAVMs, and systematic characterization of other cell types, including astrocytes and resident microglia, has yet to be performed.1 A more comprehensive understanding of the dysfunction of the neurovascular unit in its entirety will likely yield additional targets for therapeutic development.
Conclusions
The pathogenesis of non-hereditary bAVMs is not clearly understood, but the identification of gene mutations and genetic risk factors associated with bAVMs has enabled understanding of the genetics of this disease and provided new insights. Knowledge from several research aspects, such as gene mutations, signal pathways, and molecular cross-talk of microvasculature, may deepen our understanding of the pathogenesis and provide novel therapeutic approaches to bAVMs in the near future.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Takahiro Ota https://orcid.org/0000-0002-5108-6719 Masaki Komiyama https://orcid.org/0000-0003-0998-6315
References
- 1.Winkler EA, Lu AY, Raygor KP, et al. Defective vascular signaling & prospective therapeutic targets in brain arteriovenous malformations. Neurochem Int 2019; 126: 126–138. [DOI] [PubMed] [Google Scholar]
- 2.Murphy PA, Kim TN, Huang L, et al. Constitutively active Notch4 receptor elicits brain arteriovenous malformations through enlargement of capillary-like vessels. Proc Natl Acad Sci 2014; 111: 18007–18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker EJ, Su H, Shen F, et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol 2011; 69: 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walcott BP, Smith ER, Scott RM, et al. Pial arteriovenous fistulae in pediatric patients: associated syndromes and treatment outcome. J Neurointerv Surg 2013; 5: 10–14. [DOI] [PubMed] [Google Scholar]
- 5.Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation – arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet 2003; 73: 1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yilmaz B, Toktas ZO, Akakin A, et al. Familial occurrence of brain arteriovenous malformation: a novel ACVRL1 mutation detected by whole exome sequencing. J Neurosurg 2017; 126: 1879–1883. [DOI] [PubMed] [Google Scholar]
- 7.Rothbart D, Awad IA, Lee J, et al. Expression of angiogenic factors and structural proteins in central nervous system vascular malformations. Neurosurgery 1996; 38: 915–925. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto T, Wen G, Lawton MT, et al. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke 2003; 34: 925–930. [DOI] [PubMed] [Google Scholar]
- 9.Couto JA, Huang AY, Konczyk DJ, et al. Somatic MAP2K1 mutations are associated with extracranial arteriovenous malformation. Am J Hum Genet 2017; 100: 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikolaev SI, Vetiska S, Bonilla X, et al. Somatic activating KRAS mutations in arteriovenous malformations of the brain. N Engl J Med 2018; 378: 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rangel-Castilla L, Russin JJ, Martinez-del-Campo E, et al. Molecular and cellular biology of cerebral arteriovenous malformations: a review of current concepts and future trends in treatment. Neurosurg Focus 2014; 37: E1. [DOI] [PubMed] [Google Scholar]
- 12.Frösen J, Joutel A. Smooth muscle cells of intracranial vessels: from development to disease. Cardiovas Res 2018; 114: 501–512. [DOI] [PubMed] [Google Scholar]
- 13.Winkler EA, Birk H, Burkhardt J-K, et al. Reductions in brain pericytes are associated with arteriovenous malformation vascular instability. J Neurosurg 2018; 129: 1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, Guo Y, Walker EJ, et al. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler Thromb Vasc Biol 2013; 33: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Zhu W, Bollen AW, et al. Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery 2008; 62: 1340–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhogal P, Lansley J, Wong K, et al. Vessel wall enhancement of a ruptured intra-nidal aneurysm in a brain arteriovenous malformation. Interv Neuroradiol 2019; 25: 310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y, Saunders T, Su H, et al. Silent intralesional microhemorrhage as a risk factor for brain arteriovenous malformation rupture. Stroke 2012; 43: 1240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abla AA, Nelson J, Kim H, et al. Silent arteriovenous malformation hemorrhage and the recognition of ‘unruptured’ arteriovenous malformation patients who benefit from surgical intervention. Neurosurgery 2015; 76: 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leblanc GG, Golanov E, Awad IA, et al. Biology of vascular malformations of the brain. Stroke 2009; 40: 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vorbrodt AW, Dobrogowska DH, Tarnawski M. Immunogold study of interendothelial junction-associated and glucose transporter proteins during postnatal maturation of the mouse blood-brain barrier. J Neurocytol 2001; 30: 705–716. [DOI] [PubMed] [Google Scholar]
- 21.Etchevers HC, Vincent C, Le Douarin NM, et al. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 2001; 128: 1059–1068. [DOI] [PubMed] [Google Scholar]
- 22.McCormick WF. The pathology of vascular (“arteriovenous”) malformations. J Neurosurg 1966; 24: 807–816. [DOI] [PubMed] [Google Scholar]
- 23.Glading A, Han J, Stockton RA, et al. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell-cell junctions. J Cell Biol 2007; 179: 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clatterbuck RE, Eberhart CG, Crain BJ, et al. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J Neurol Neurosurg Psychiatry 2001; 71: 188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas JM, Surendran S, Abraham M, et al. Gene expression analysis of nidus of cerebral arteriovenous malformations reveals vascular structures with deficient differentiation and maturation. PLoS One 2018; 13: e0198617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koizumi T, Shiraishi T, Hagihara N, et al. Expression of vascular endothelial growth factors and their receptors in and around intracranial arteriovenous malformations. Neurosurgery 2002; 50: 117–124. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto T, Wu Y, Lawton MT, et al. Coexpression of angiogenic factors in brain arteriovenous malformations. Neurosurgery 2005; 56: 1058–1065. [PubMed] [Google Scholar]
- 28.Ferreira R, Amar A, Chen TC, et al. MicroRNA-18a improves human cerebral arteriovenous malformation endothelial cell function. Stroke 2014; 45: 293–297. [DOI] [PubMed] [Google Scholar]
- 29.Jabbour MN, Elder JB, Samuelson CG, et al. Aberrant angiogenic characteristics of human brain arteriovenous malformation endothelial cells. Neurosurgery 2009; 64: 139–148. [DOI] [PubMed] [Google Scholar]
- 30.Komiyama M. Pathogenesis of brain arteriovenous malformations. Neurol Med Chir (Tokyo) 2016; 56: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen CM, Huang L, Murphy PA, et al. Mouse models of cerebral arteriovenous malformation. Stroke 2016; 47: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W, Sun Z, Han Z, et al. De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke 2014; 45: 900–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su H, Tihan T, Young W, et al. Distinctive distribution of lymphocytes in unruptured and previously untreated brain arteriovenous malformation. Neuroimmunol Neuroinflamm 2014; 1: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J, Song J, Qu M, et al. MicroRNA-137 and microRNA-195* inhibit vasculogenesis in brain arteriovenous malformations. Ann Neurol 2017; 82: 371–384. [DOI] [PubMed] [Google Scholar]
- 35.Zhu W, Chen W, Zou D, et al. Thalidomide reduces hemorrhage of brain arteriovenous malformations in a mouse model. Stroke 2018; 49: 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huo R, Fu W, Li H, et al. RNA sequencing reveals the activation of Wnt signaling in low flow rate brain arteriovenous malformations. J Am Heart Assoc 2019; 8: e012746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne LB, Zhao H, James CC, et al. The pericyte microenvironment during vascular development. Microcirculation 2019; 26: e12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kisler K, Nelson AR, Rege SV, et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci 2017; 20: 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 2016; 19: 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto T, Lam T, Boudreau NJ, et al. Abnormal balance in the angiopoietin-tie2 system in human brain arteriovenous malformations. Circ Res 2001; 89: 111–113. [DOI] [PubMed] [Google Scholar]
- 41.Darden J, Payne LB, Zhao H, et al. Excess vascular endothelial growth factor-A disrupts pericyte recruitment during blood vessel formation. Angiogenesis 2019; 22: 167–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yildirim O, Bicer A, Ozkan A, et al. Expression of platelet-derived growth factor ligand and receptor in cerebral arteriovenous and cavernous malformations. J Clin Neurosci 2010; 17: 1557–1562. [DOI] [PubMed] [Google Scholar]
- 43.Young WL, Yang GY. Are there genetic influences on sporadic brain arteriovenous malformations? Stroke 2004; 35: 2740–2745. [DOI] [PubMed] [Google Scholar]
- 44.Sandalcioglu IE, Asgari S, Wende D, et al. Proliferation activity is significantly elevated in partially embolized cerebral arteriovenous malformations. Cerebrovasc Dis 2010; 30: 396–401. [DOI] [PubMed] [Google Scholar]
- 45.Walker EJ, Su H, Shen F, et al. Bevacizumab attenuates VEGF-induced angiogenesis and vascular malformations in the adult mouse brain. Stroke 2012; 43: 1925–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashimoto T, Lawton MT, Wen G, et al. Gene microarray analysis of human brain arteriovenous malformations. Neurosurgery 2004; 54: 410–425. [DOI] [PubMed] [Google Scholar]
- 47.Kim GH, Hahn DK, Kellner CP, et al. Plasma levels of vascular endothelial growth factor after treatment for cerebral arteriovenous malformations. Stroke 2008; 39: 2274–2279. [DOI] [PubMed] [Google Scholar]
- 48.Wu CY, Wang YH, Meng FG, et al. Development of stereotactic neurosurgery in China. Neurosurgery 2005; 56: 851–860. [DOI] [PubMed] [Google Scholar]
- 49.Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol 2012; 24: 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee CZ, Xue Z, Zhu Y, et al. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke 2007; 38: 2563–2568. [DOI] [PubMed] [Google Scholar]
- 51.Cheng P, Ma L, Shaligram S, et al. Effect of elevation of vascular endothelial growth factor level on exacerbation of hemorrhage in mouse brain arteriovenous malformation. J Neurosurg 2019; 1: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dalton A, Dobson G, Prasad M, et al. De novo intracerebral arteriovenous malformations and a review of the theories of their formation. Br J Neurosurg 2018; 32: 305–311. [DOI] [PubMed] [Google Scholar]
- 53.Cataltepe S, Arikan MC, Liang X, et al. Fatty acid binding protein 4 expression in cerebral vascular malformations: implications for vascular remodelling. Neuropathol Appl Neurobiol 2015; 41: 646–656. [DOI] [PubMed] [Google Scholar]
- 54.Cunha SI, Magnusson PU, Dejana E, et al. Deregulated TGF-β/BMP signaling in vascular malformations. Circ Res 2017; 121: 981–999. [DOI] [PubMed] [Google Scholar]
- 55.Pawlikowska L, Tran MN, Achrol AS, et al. Polymorphisms in transforming growth factor-β-related genes ALK1 and ENG are associated with sporadic brain arteriovenous malformations. Stroke 2005; 36: 2278–2280. [DOI] [PubMed] [Google Scholar]
- 56.Simon M, Franke D, Ludwig M, et al. Association of a polymorphism of the ACVRL1 gene with sporadic arteriovenous malformations of the central nervous system. J Neurosurg 2006; 104: 945–949. [DOI] [PubMed] [Google Scholar]
- 57.Walcott BP. BMP signaling modulation attenuates cerebral arteriovenous malformation formation in a vertebrate model. J Cereb Blood Flow Metab 2014; 34: 1688–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Janes MR, Zhang J, Li L-S, et al. Targeting KRAS mutant cancers with a covalent G12C-specific inhibitor. Cell 2018; 172: 578–589.e17. [DOI] [PubMed] [Google Scholar]
- 59.Yao J, Guihard PJ, Blazquez-Medela AM, et al. Matrix Gla protein regulates differentiation of endothelial cells derived from mouse embryonic stem cells. Angiogenesis 2016; 19: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walcott BP, Winkler EA, Zhou S, et al. Identification of a rare BMP pathway mutation in a non-syndromic human brain arteriovenous malformation via exome sequencing. Hum Genome Var 2018; 5: 18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill-Felberg S, Wu HH, Toms SA, et al. Notch receptor expression in human brain arteriovenous malformations. J Cell Mol Med 2015; 19: 1986–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murphy PA, Kim TN, Lu G, et al. Notch4 normalization reduces blood vessel size in arteriovenous malformations. Sci Transl Med 2012; 4: 117ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benedito R, Trindade A, Hirashima M, et al. Loss of Notch signalling induced by Dll4 causes arterial calibre reduction by increasing endothelial cell response to angiogenic stimuli. BMC Dev Biol 2008; 8: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sainson RCA, Aoto J, Nakatsu MN, et al. Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. FASEB J 2005; 19: 1027–1029. [DOI] [PubMed] [Google Scholar]
- 65.Krebs LT, Shutter JR, Tanigaki K, et al. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev 2004; 18: 2469–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawson ND, Scheer N, Pham VN, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 2001; 128: 3675–3683. [DOI] [PubMed] [Google Scholar]
- 67.Villa N, Walker L, Lindsell CE, et al. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev 2001; 108: 161–164. [DOI] [PubMed] [Google Scholar]
- 68.Murphy PA, Lu G, Shiah S, et al. Endothelial Notch signaling is upregulated in human brain arteriovenous malformations and a mouse model of the disease. Lab Invest 2009; 89: 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carlson TR, Yan Y, Wu X, et al. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous. PNAS 2005; 102: 9884–9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mouchtouris N, Jabbour PM, Starke RM, et al. Biology of cerebral arteriovenous malformations with a focus on inflammation. J Cereb Blood Flow Metab 2015; 35: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.ZhuGe Q, Zhong M, Zheng W, et al. Notch-1 signalling is activated in brain arteriovenous malformations in humans. Brain 2009; 132: 3231–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li S, Wang R, Wang Y, et al. Receptors of the Notch signaling pathway are associated with hemorrhage of brain arteriovenous malformations. Mol Med Rep 2014; 9: 2233–2238. [DOI] [PubMed] [Google Scholar]
- 73.Murphy PA, Lam MTY, Wu X, et al. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc Natl Acad Sci 2008; 105: 10901–10906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Delev D, Pavlova A, Grote A, et al. NOTCH4 gene polymorphisms as potential risk factors for brain arteriovenous malformation development and hemorrhagic presentation. J Neurosurg 2016; 126: 1552–1559. [DOI] [PubMed] [Google Scholar]
- 75.Hermanto Y, Takagi Y, Ishii A, et al. Immunohistochemical analysis of Sox17 associated pathway in brain arteriovenous malformations. World Neurosurg 2016; 87: 573–583.e2. [DOI] [PubMed] [Google Scholar]
- 76.Corada M, Orsenigo F, Morini MF, et al. Sox17 is indispensable for acquisition and maintenance of arterial identity. Nat Commun 2013; 4: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Norris JS, Valiante TA, Wallace MC, et al. A simple relationship between radiological arteriovenous malformation hemodynamics and clinical presentation: a prospective, blinded analysis of 31 cases. J Neurosurg 2009; 90: 673–679. [DOI] [PubMed] [Google Scholar]
- 78.Oka M, Kushamae M, Aoki T, et al. KRAS G12D or G12V mutation in human brain arteriovenous malformations. World Neurosurg 2019; 126: e1365–e1373. [DOI] [PubMed] [Google Scholar]
- 79.Al-Olabi L, Polubothu S, Dowsett K, et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J Clin Invest 2018; 128: 1496–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Priemer DS, Vortmeyer AO, Zhang S, et al. Activating KRAS mutations in arteriovenous malformations of the brain: frequency and clinicopathologic correlation. Hum Pathol 2019; 89: 33–39. [DOI] [PubMed] [Google Scholar]
- 81.Simanshu DK, Nissley DV, McCormick F. RAS proteins and their regulators in human disease. Cell 2017; 170: 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Papke B, Der CJ. Drugging RAS: know the enemy. Science 2017; 355: 1158–1163. [DOI] [PubMed] [Google Scholar]
- 83.Saeed O, Lopez-Beltran A, Fisher KW, et al. RAS genes in colorectal carcinoma: pathogenesis, testing guidelines and treatment implications. J Clin Pathol 2019; 72: 135–139. [DOI] [PubMed] [Google Scholar]
- 84.Hong T, Yan Y, Li J, et al. High prevalence of KRAS/BRAF somatic mutations in brain and spinal cord arteriovenous malformations. Brain 2019; 142: 23–34. [DOI] [PubMed] [Google Scholar]
- 85.Karpathiou G, Chauleur C, Da Cruz V, et al. Vascular lesions of the female genital tract: clinicopathologic findings and application of the ISSVA classification. Pathophysiology 2017; 24: 161–167. [DOI] [PubMed] [Google Scholar]
- 86.Limaye N, Kangas J, Mendola A, et al. Somatic activating PIK3CA mutations cause venous malformation. Am J Hum Genet 2015; 97: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Couto JA, Vivero MP, Kozakewich HPW, et al. A somatic MAP3K3 mutation is associated with verrucous venous malformation. Am J Hum Genet 2015; 96: 480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zúñiga-Castillo M, Teng CL, Teng JMC. Genetics of vascular malformation and therapeutic implications. Curr Opin Pediatr 2019; 31: 498–508. [DOI] [PubMed] [Google Scholar]
- 89.Saliou G, Eyries M, Iacobucci M, et al. Clinical and genetic findings in children with central nervous system arteriovenous fistulas. Ann Neurol 2017; 82: 972–980. [DOI] [PubMed] [Google Scholar]
- 90.Queisser A, Boon LM, Vikkula M. Etiology and genetics of congenital vascular lesions. Otolaryngol Clin North Am 2018; 51: 41–53. [DOI] [PubMed] [Google Scholar]
- 91.Yu J, Streicher JL, Medne L, et al. EPHB4 mutation implicated in capillary malformation – arteriovenous malformation syndrome: a case report. Pediatr Dermatol 2017; 34: e227–e230. [DOI] [PubMed] [Google Scholar]
- 92.Ola R, Dubrac A, Han J, et al. PI3 kinase inhibition improves vascular malformations in mouse models of hereditary haemorrhagic telangiectasia. Nat Commun 2016; 7: 13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ola R, Künzel SH, Zhang F, et al. SMAD4 prevents flow induced arteriovenous malformations by inhibiting casein kinase 2. Circulation 2018; 138: 2379–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jin Y, Muhl L, Burmakin M, et al. Endoglin prevents vascular malformation by regulating flow-induced cell migration and specification through VEGFR2 signalling. Nat Cell Biol 2017; 19: 639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moteki Y, Akagawa H, Niimi Y, et al. Novel RASA1 mutations in Japanese pedigrees with capillary malformation-arteriovenous malformation. Brain Dev 2019; 41: 812–816. [DOI] [PubMed] [Google Scholar]
- 96.Lebrin F, Srun S, Raymond K, et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med 2010; 16: 420–428. [DOI] [PubMed] [Google Scholar]
- 97.Davis RB, Pahl K, Datto NC, et al. Notch signaling pathway is a potential therapeutic target for extracranial vascular malformations. Sci Rep 2018; 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han C, Choe S, Kim YH, et al. VEGF neutralization can prevent and normalize arteriovenous malformations in an animal model for hereditary hemorrhagic telangiectasia 2. Angiogenesis 2014; 17: 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014; 370: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Starke RM, McCarthy D, Komotar RJ, et al. Somatic KRAS mutation found in sporadic arteriovenous malformations. Clin Neurosurg 2018; 83: E14–E15. [DOI] [PubMed] [Google Scholar]
- 101.Kangas J, Nätynki M, Eklund L. Development of molecular therapies for venous malformations. Basic Clin Pharmacol Toxicol 2018; 123: 6–19. [DOI] [PubMed] [Google Scholar]
- 102.Lekwuttikarn R, Lim YH, Admani S, et al. Genotype-guided medical treatment of an arteriovenous malformation in a child. JAMA Dermatol 2019; 155: 256–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014; 371: 1877–1888. [DOI] [PubMed] [Google Scholar]
- 104.Rutkowski P, Lugowska I, Kosela-Paterczyk H, et al. Trametinib: a MEK inhibitor for management of metastatic melanoma. Onco Targets Ther 2015; 8: 2251–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Greene AK, Goss JA. Vascular anomalies: from a clinicohistologic to a genetic framework. Plast Reconstr Surg 2018; 141: 709e–717e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Phung TL, Ziv K, Dabydeen D, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell 2006; 10: 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chelliah MP, Do HM, Zinn Z, et al. Management of complex arteriovenous malformations using a novel combination therapeutic algorithm. JAMA Dermatol 2018; 154: 1316–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Adams DM, Trenor CC, III, Hammill AM, et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies 2016; 137: e20153257. [DOI] [PMC free article] [PubMed] [Google Scholar]