Abstract
Although there are well-known limitations of the human cognitive system in performing two tasks simultaneously (dual-tasking) or alternatingly (task-switching), the question for a common vs. distinct neural basis of these multitasking limitations is still open. We performed two Activation Likelihood Estimation meta-analyses of neuroimaging studies on dual-tasking or task-switching and tested for commonalities and differences in the brain regions associated with either domain. We found a common core network related to multitasking comprising bilateral intraparietal sulcus (IPS), left dorsal premotor cortex (dPMC), and right anterior insula. Meta-analytic contrasts revealed eight fronto-parietal clusters more consistently activated in dual-tasking (bilateral frontal operculum, dPMC, and anterior IPS, left inferior frontal sulcus and left inferior frontal gyrus) and, conversely, four clusters (left inferior frontal junction, posterior IPS, and precuneus as well as frontomedial cortex) more consistently activated in task-switching. Together with sub-analyses of preparation effects in task-switching, our results argue against purely passive structural processing limitations in multitasking. Based on these findings and drawing on current theorizing, we present a neuro-cognitive processing model of multitasking.
Keywords: Multitasking, Cognitive control, Executive function, ALE meta-analysis, fMRI
Introduction
In our daily lives, we often do several things at once, such as talking on the phone while walking or driving. People are usually unaware of difficulties in performing multiple tasks concurrently or in close succession. Yet, behavioral studies have demonstrated that humans exhibit a disproportional deceleration in their responses to external stimuli when faced with pairs of simple cognitive tasks, relative to performing these tasks separately (e.g., Kiesel et al. 2010; Monsell 2003; Pashler 1994, 2000). These performance decrements have not only been observed in situations that require doing two tasks simultaneously (i.e., dual-tasking) but also in situations that require the repeated shifting between two tasks (i.e., task-switching; Koch et al. 2018).
Studies that investigated the effects of aging on dual-tasking and task-switching performance found greater age-related performance deficits in dual-tasking as compared to task-switching (Kliegl et al. 1994; Mayr and Kliegl 1993; Mayr et al. 1996). A meta-analysis investigating the effect of aging on dual-task performance concluded that the multiplicative age effect in dual-tasking might be a result of “multiple and repeated switches between processing streams” in dual-tasking, “even if each of the switching steps by itself carries an additive deficit” (Verhaeghen et al. 2003, p. 453). Thus, it seems like similar cognitive and neural mechanisms may underlie the performance decrements in both multitasking contexts. Independently of each other, both multitasking paradigms (i.e., dual-tasking and task-switching) have become an extremely active research field in experimental psychology and cognitive neuroscience (e.g., Kiesel et al. 2010; Marois and Ivanoff 2005; Monsell 2003; Pashler 1994, 2000; Wager et al. 2005; Koch et al. 2018). With regard to the neural basis of task-switching, several neuroimaging meta-analyses have already been published. The most recent one (Kim et al. 2012) investigated switching-related brain activity that was domain general (associated with the switching process in general) vs. domain specific (associated with distinct kinds of switching). For domain-general switching, this meta-analysis revealed consistent brain activation in bilateral inferior frontal junction (IFJ) and posterior parietal cortex, consistent with earlier meta-analyses (Buchsbaum et al. 2005; Derrfuss et al. 2005; Wager et al. 2004).
In contrast, despite numerous neuroimaging experiments on dual-tasking, no meta-analysis has been done yet to synthesize these findings. Here, we set out to fill this gap. Furthermore, to our knowledge, only one neuroimaging study has directly compared the neural correlates of dual-tasking and task-switching and found brain activation common to both tasks in bilateral superior frontal gyrus and inferior parietal lobule (IPL), right middle frontal gyrus (MFG) and middle occipital gyrus, as well as left inferior frontal gyrus (IFG), pre-supplementary motor area (preSMA), cerebellum, and inferior temporal gyrus (Dreher and Grafman 2003). Such an analysis would be important to further elucidate potentially common and distinct neural mechanisms underlying multitasking performance decrements. Therefore, we tested for commonalities and differences of brain activity related to dual-tasking vs. task-switching on a meta-analytic scale. To provide a detailed context for our analyses, we start out by reviewing the current state of behavioral research and theorizing as well as summarizing recent findings on brain activity associated with dual-tasking and task-switching.
Dual-tasking: behavioral findings and cognitive models
A common observation in dual-task experiments is the slowing of response times (RTs) relative to single-task performance. This performance decrement in dual-tasking is known as the “dual-task interference effect” (Pashler 1994; Schubert 1999; Welford 1952). According to an influential theoretical account, the passive bottleneck theory, this interference effect occurs at a central decision stage in human information processing, which can only operate serially, while peripheral (perceptual and motor) stages can operate in parallel (Pashler 1994). In speeded choice-reaction tasks as typically used in laboratory dual-task experiments, the central decision stage is often equated with response selection, that is, the mapping of a response to a stimulus according to an arbitrary rule. Thus, following the bottleneck model, when faced with the need to select two responses (i.e., in Task 1 and Task 2) in parallel, a queuing effect is observed. That is, response selection in Task 2 is assumed to be stalled until response selection in Task 1 has been completed. Numerous behavioral experiments have shown this interference effect in dual-tasking, with delayed Task 2 responding when two tasks are presented in parallel or with a delay of 500 ms or less. As this delay between stimulus onsets in Tasks 1 and 2, the so-called stimulus onset asynchrony (SOA), increases, RT in Task 2 decreases until there is no temporal overlap between the processing of the two tasks anymore, so that Task 2 performance becomes independent of Task 1 (Pashler 1994; Sigman and Dehaene 2006). The time window of 500 ms or less, in which interference is observed, is also called the “psychological refractory period” (PRP; Welford 1952), and performance decrements within the PRP are known as the “PRP effect”. According to the classic bottleneck theory, RT in Task 1 should not be affected by varying SOAs, as Task 1 processing is thought to proceed independently of Task 2, even if Task 2 is presented in parallel or with minimal delay.
In contrast to these predictions, however, prolonged RTs in Task 1 have also been observed in dual-tasking. For example, in a situation when two tasks are presented in random order (Szameitat et al. 2006; Sigman and Dehaene 2008), responses in both Task 1 and Task 2 were found to be slowed down as compared to single-tasking (Hirsch et al. 2017; Kübler et al. 2017). Luria and Meiran (2003) interpreted these findings as additional time-consuming active setting of the bottleneck to process the first expected task first, referred to as “active bottleneck model”.
However, findings of prolonged Task 1 responses are also in line with models that assume interference effects due to neural capacity limitations (e.g., Logan and Gordon 2001; Navon and Miller 2002; Tombu and Jolicoeur 2003). These models are based on the premise of a limited central “resource”, which corresponds to the brain’s information processing capacity in situations that require top-down cognitive control. This controlled processing capacity is thought to be shareable across concurrent tasks in a graded manner. Therefore, the total amount of available capacity limits the amount of information that can be processed simultaneously. According to these capacity limitation models, dual-task situations exceed the finite resource capacity, which then constitutes a functional bottleneck, leading to interference effects. In PRP paradigms, this would be reflected in response slowing in both Task 1 and Task 2. However, although the capacity sharing model explains the response slowing in Task 1 of PRP dual-task situations, it fails to explain the disproportionally greater slowing in Task 2 compared to Task 1.
Summing up, the passive bottleneck model assumes that dual-task costs arise from the serial nature of the response selection process, while the capacity limitation model assumes that these costs arise from sharing a finite, limited resource. The passive bottleneck model can well explain the PRP effect in Task 2 but only the active bottleneck model can explain an analogous slowing in Task 1. Capacity limitation models can also explain slowing in both Task 1 and Task 2 but fail to explain the disproportionally greater slowing in Task 2 vs. Task 1. To further elucidate the underlying neural mechanisms, numerous neuroimaging and neurophysiological studies investigated brain activity associated with dual-tasking. The next section gives a brief overview of relevant neuroimaging and neurophysiological studies of dual-tasking.
Brain activity associated with dual-tasking
Previous studies using functional magnetic resonance imaging (fMRI) reported different neural correlates of dual-tasking such as increased activity in the lateral frontal, prefrontal, dorsal premotor, anterior cingulate, and posterior parietal cortex (e.g., Herath et al. 2001; Jiang 2004; Jiang et al. 2004; Marois et al. 2005; Szameitat et al. 2002). Most imaging studies used activity strength (peak amplitude) as the dependent variable, but did not test temporal characteristics of brain responses.
Dux et al. (2006), however, used time-resolved fMRI to investigate the temporal characteristics of brain activity changes during dual-tasking for several regions that had been associated with interference-related processing. The authors hypothesized that if the interference effect is due to serial processing as predicted by the passive bottleneck model, brain regions should show clear differences in peak latency but only a slight change (if any) in response amplitude. The left IFJ showed an activation pattern consistent with the serial postponement prediction of the passive bottleneck model: peak latency was significantly greater in dual-task than in single-task situations and occurred later for slow responses than for fast ones. The authors concluded that the IFJ forms a central structural bottleneck of information processing that limits the ability to multitask.
Other studies that simultaneously used electroencephalography (EEG) and fMRI, however, did not support Dux et al.’s (2006) findings. Instead, these studies found a strong association between the PRP-related postponement of the P3 component of the event-related potential (ERP) and increased hemodynamic responses in parietal cortex (Dell’Acqua et al. 2005; Hesselmann et al. 2011; Sigman and Dehaene 2008). Observations of greater response amplitudes associated with the PRP effect in brain areas such as the inferior frontal gyrus (IFG; Herath et al. 2001; Jiang et al. 2004; Marois et al. 2005) or dorsal premotor cortex (dPMC; Marois et al. 2005) suggest an increased (rather than simply delayed) recruitment of these brain regions during dual-tasking. There are two possible explanations for increased brain activation that is not associated with serial queuing processes in dual-tasking. First, in line with Kahneman’s (1973) flexible resource model, increased brain activation in dual- vs. single-tasking might reflect a central resource enhancement due to greater effort exertion in response to higher task demands during dual-tasking. Second, increased brain activation might reflect capacity sharing as an additional cognitive control process in dual-tasking due to the allocation and monitoring of split resources. This view is consistent with findings of a general executive control (or “multiple demand”) network (e.g., Cieslik et al. 2015; Cole and Schneider 2007; Duncan 2010; Langner et al. 2018). Hence, the aforementioned findings do not only suggest an involvement of several brain regions but also the possibility of different underlying mechanisms associated with dual-task-related response slowing.
Summing up, as is true for behavioral studies, the results of neuroimaging studies pertaining to dual-tasking seem to be rather inconsistent: Dux et al. (2006) favored frontal areas (IFJ) as location of a neural bottleneck; other studies, however, located the neural bottleneck in parietal areas (Dell’Acqua et al. 2005; Hesselmann et al. 2011; Sigman and Dehaene 2008). Yet others (Mochizuki et al. 2007; Stelzel et al. 2006, 2008) suggested finite neural resources as limiting factor in dual-tasking, favoring some kind of capacity sharing model. Therefore, our meta-analysis tested for any consistent brain activity across a wide range of neuroimaging studies related to dual-tasking. Based on the methodological considerations of Dux et al. (2006), we reasoned that activation increases in dual- vs. single-task conditions would be at odds with a purely passive structural bottleneck model. This is because this model predicts a delay of response selection processes and associated brain activity in Task 2, but, given the assumption of the bottleneck being passive, it does not predict additional processing and, therefore, no increase in brain activity. Activation increases would be expected, however, when assuming that dual-tasking evokes additional top-down modulatory processing to cope with higher control demands in order to keep more complex task sets activated. Thus, this rationale allowed us to bring the results of brain activation studies to bear on the question of whether the bottleneck in dual-tasking is passive structural (no activity increase expected in dual- vs. single-tasking) or rather more active-functional (activity increase expected in dual- vs. single-tasking).
Task-switching: behavioral findings and cognitive models
Task-switching experiments require participants to alternate repeatedly between two different tasks. That is, in contrast to dual-tasking, the two tasks are not presented simultaneously but alternatingly in close succession. There are two basic versions: (1) the “mixed-task vs. single-task blocks” paradigm, and (2) the task-cuing paradigm (Kiesel et al. 2010). In the former, participants need to switch between two tasks in some blocks of trials (mixed-task blocks) and perform only one of the tasks in others (single-task blocks). In the mixed-task blocks, the tasks predictably switch either every two trials (alternating-run design) or after some other fixed number of trials. In the second paradigm type, task switches are unpredictable and a task cue precedes or accompanies the stimulus to signal the task to be performed. Only preceding task cues enable preparation for a task switch, similar to expectable switches in the alternating-run paradigm (Jost et al. 2013). Compared to single-task blocks, performance in mixed-task blocks decreases with respect to speed and accuracy, which is referred to as “global switch costs” or “mixing costs”. These global costs have been attributed to higher executive control demands in mixed-task blocks, in which two task sets have to be maintained and shielded from each other, as compared to single-task blocks, in which only one task set has to be maintained without interference from a competing second one (Rogers and Monsell 1995). On top of these global costs, performance further decreases in switch trials relative to repetition trials (“local switch costs”; for review, see Kiesel et al. 2010; Monsell 2003). Like the PRP effect in dual-tasking, these local switch costs are assumed to result from interference between the two tasks and become smaller with increasing inter-task intervals (Karayanidis et al. 2003; Rogers and Monsell 1995; Meiran et al. 2000; Nicholson et al. 2005). However, even with long inter-task intervals (in alternating-run paradigms) or long intervals between task cue and stimulus (in task-cuing paradigms), residual switch costs remain (Rogers and Monsell 1995). It thus seems that interference in task-switching is not completely reducible with preparation. As most experiments included in our meta-analysis focused on local task switches, the following paragraphs will focus on this process, too.
Different cognitive models have been developed to explain local switch costs. Some of them interpret switch costs as reflecting an additional cognitive control process (Meiran 2000; Rogers and Monsell 1995; Rubinstein et al. 2001), while others suggest that switch costs arise from time-consuming bottom-up processes (Allport et al. 1994; Koch and Allport 2006; Logan and Bundesen 2003; Wylie and Allport 2000). Models assuming an active control process in switch trials often think of it as intentional task-set reconfiguration. Following Meiran (2000), this reconfiguration process is assumed to bias the stimulus set (the mental representation of the appropriate stimuli) toward the currently relevant task. In task-switching situations that allow preparation, this reconfiguration process is thought to be proactive and to occur after task-cue onset and before stimulus identification [i.e., during the cue–stimulus interval (CSI)], or in alternating-run designs after finishing the previous trial [i.e., during the response–stimulus interval (RSI)]. In task-switching situations that do not allow preparation (task-cuing paradigms with a CTI of 0 ms), this reconfiguration process is assumed to be reactive and to occur in parallel to stimulus identification. Other accounts suggest that local switch costs result from carry-over effects of the previous task, which cause a conflict between previous and current task-setting processes (due to limited cognitive resources), or from a persisting inhibition of the previously irrelevant task in a switch-back trial (Allport et al. 1994; Schuch and Koch 2003; Wylie and Allport 2000). Evidence for the view that switch costs depend on a passive decay of activation of the preceding task is, however, mixed. As an alternative to Allport et al.’s (1994) passive-decay hypothesis, Koch et al. (2010) suggested that inhibitory processes of the previous irrelevant task set might lead to this observed persistent activation.
Taken together, models explaining switch costs due to additional cognitive control processes assume that a process of task-set reconfiguration prolongs RT (e.g., Meiran 2000), while others associate switch costs with bottom-up guided carry-over effects (Allport et al. 1994; Logan and Bundesen 2003; Wylie and Allport 2000) or inhibitory processes related to the previous task (Koch et al. 2010). Considering that dual-tasking also involves task-switching processes, all models might also account for the switching-related performance costs in dual-tasking (Hirsch et al. 2018). To shed more light on possible underlying neural mechanisms of switch costs, the next section briefly surveys relevant findings regarding the neural correlates of task-switching.
Brain activity associated with task-switching
According to the reconfiguration model, switch trials should activate areas associated with attention and executive functions such as frontal and parietal regions (Miller and Cohen 2001). Indeed, switching-specific fronto-parietal activation is commonly found in bilateral medial and lateral PFC, supramarginal gyrus and superior parietal lobule, fusiform gyrus, occipital gyri, as well as subcortical structures (caudate nucleus and thalamus) (Kim et al. 2012; Wager et al. 2004). Also, the reconfiguration model predicts that switch vs. repeat contrasts should yield an increased activation of brain areas that are not activated in repeat trials. Indeed, some studies found exclusive brain activation associated with switching, mainly in parietal lobe (Barber and Carter 2005; Chiu and Yantis 2009; Kimberg et al. 2000).
Similarly, ERP studies analyzing components time-locked to the preparatory interval (CSI) revealed a larger posterior positivity on switch (vs. repeat) trials, which might reflect stronger brain activity due to proactive top-down control processes (e.g., Goffaux et al. 2006; Kieffaber and Hetrick 2005; Poulsen et al. 2005). Another study combined fMRI and ERP measures and found CSI-related early ERP components associated with activation in dorsolateral prefrontal cortex (dlPFC) and another later ERP component after stimulus onset associated with activation in posterior parietal cortex (Jamadar et al. 2010a). The authors interpreted these findings as support for the two-stage reconfiguration model, with the early ERP component reflecting proactive preparatory top-down processes and the later component reflecting reconfiguration processes after stimulus onset. Thus, beside the search of the neural correlates of switching, there are specific open questions about preparatory control in task-switching concerning proactive (top-down-guided cognitive control) vs. reactive control mechanisms and their neural correlates (for a review, see Ruge et al. 2013). For example, while some studies found an increase in brain activation in prepared vs. unprepared switch trials in prefrontal and parietal cortices (Badre and Wagner 2006; Barber and Carter 2005; Braver et al. 2003; Chiu and Yantis 2009; Ruge et al. 2010; Rushworth et al. 2001, 2002; Wylie et al. 2006), others did not support this finding (Brass and von Cramon 2002, 2004; Bunge et al. 2002; Cavina-Pratesi et al. 2006; Gruber et al. 2006; Luks et al. 2002; Ruge et al. 2005, 2009).
Coming back to the question of switching-related brain activity, according to the reconfiguration theory, an on-off function after task repetition trials would be expected. Instead, De Baene and Brass (2011) found a gradual reduction of brain activation in task-associated frontal and parietal areas, which rather argues for a passive decay of activation of the preceding task (Allport et al. 1994; Logan and Bundesen 2003; Wylie and Allport 2000). Further, supporting passive bottom-up, between-task competition models, it has been shown that activation in fronto-parietal areas associated with the previous task persists even after the onset of the next, switched-to task (for details, see Wylie et al. 2006; Yeung et al. 2006).
In summary, the neuroscientific evidence for or against the different theoretical accounts is mixed and equivocal: supporting the reconfiguration model, switching-specific fronto-parietal activation was found (Barber and Carter 2005; Chiu and Yantis 2009; Kim et al. 2012; Kimberg et al. 2000; Wager et al. 2004). ERP studies provided evidence for proactive top-down control processes in more frontal regions (e.g., Goffaux et al. 2006; Kieffaber and Hetrick 2005; Poulsen et al. 2005) as well as reactive reconfiguration processes associated with posterior parietal activation (Jamadar et al. 2010a). Conversely, a gradual reduction instead of a clear-cut absence of task-associated frontal and parietal activation (De Baene and Brass 2011) as well as persisting fronto-parietal activation after switch trials (Wylie et al. 2006; Yeung et al. 2006) argues for the passive bottom-up model.
Present study
Keeping in mind that task-switching processes might also play a role in dual-tasking, it is not surprising that similar mechanisms of interference have been discussed. Regarding both multitasking domains, there is a debate of whether performance decrements result from bottom-up processes (serial task queuing, task-set inertia) or, rather, from the necessity to apply top-down cognitive control to cope with the demands imposed by the multitasking situation (e.g., capacity sharing, reconfiguration processes). Regarding neurophysiological findings, there tends to be more evidence for additional cognitive control processes. As to dual-tasking, increased response amplitudes in IFG and dPMC (Herath et al. 2001; Jiang et al. 2004; Marois et al. 2005) argue for an additional top-down-guided recruitment of brain regions that have also been involved in a general executive control network (e.g., Cieslik et al. 2015; Cole and Schneider 2007; Duncan 2010). As to task-switching, findings of exclusive brain activation associated with switching (Barber and Carter 2005; Chiu and Yantis 2009; Kimberg et al. 2000) as well as findings of ERP studies of proactive and reactive reconfiguration processes in fronto-parietal regions (e.g., Goffaux et al. 2006; Jamadar et al. 2010a; Kieffaber and Hetrick 2005; Poulsen et al. 2005) provide evidence for an additional recruitment of brain areas associated with executive control processes.
Because of these commonalities, we hypothesized that both domains share several neural correlates in fronto-parietal regions due to similar subprocesses. This also implies that we expected partially distinct neural activation patterns for both domains, reflecting several distinct subprocesses presumably due to timing differences of both tasks. Furthermore, for task-switching, we additionally examined whether prepared vs. unprepared switching shows consistent activation differences. We hypothesized that proactive preparatory control processes should be reflected by additional brain activation in studies that investigated brain activation related to task-cue onset. In task-switching studies that investigated stimulus-related brain activation, however, we hypothesized less brain activation associated with proactive control processes, because we assumed proactive control to be finished at stimulus onset. Hence, we performed the following analyses:
Two separate ALE meta-analyses for dual-tasking and task-switching experiments to investigate consistent neural correlates of either paradigm.
A conjunction and a contrast analysis between the two above meta-analyses to investigate neural commonalities and differences, respectively.
A contrast analysis between prepared and unprepared task-switching to investigate effects of proactive preparation on brain activation in task-switching.
A meta-analytic correlation analysis of preparatory interval length and corresponding brain activation to investigate the neural correlates of reactive control processes in task-switching.
Finally, based on our findings and previous theorizing, we developed a neuro-cognitive processing model of multitasking.
Materials and methods
Data used for the meta-analysis
We performed a literature search in PubMed (http://www.pubmed.org) using the search strings: „dual task*“ OR „task-switch*“ in combination with “fMRI” OR “PET”. The references in the retrieved articles as well as in relevant reviews were also assessed to identify additional neuroimaging studies on dual-tasking or task-switching. Only studies that reported results of whole-brain group analyses as coordinates in a standard reference space [Talairach or Montreal Neurological Institute (MNI)] were included in the analysis, while single-subject reports and results of region-of-interest analyses were excluded. Likewise, experiments investigating between- or within-group effects pertaining to disease states or any sort of intervention were excluded. Finally, only positive activations were analyzed, as deactivations were only very inconsistently reported in the retrieved literature. Differences in coordinate spaces (MNI vs. Talairach space) between experiments were accounted for by transforming coordinates reported in Talairach space into MNI coordinates (Lancaster et al. 2007).
The current meta-analysis only included experiments that contrasted brain activity in a dual-task condition with that in single-task conditions. Additionally, we included PRP studies that contrasted brain activity related to parallel dual-tasking (short SOA between Task 1 and Task 2) with that related to serial dual-tasking (long SOA). Although short- vs. long-SOA contrasts in dual-tasking do not exactly reflect the same as dual- vs. single-tasking contrasts (cf. Koch et al. 2018), we assume that both capture partially overlapping processes central to this study, namely those involved in solving the interference between two tasks presented at once. In fact, all included experiments induced dual-task performance costs, and the neural mechanisms behind these costs were one of the foci of our study.
As for task-switching, we included studies that assessed switching attention between perceptual features of a stimulus or between response selection rules as well as S–R mapping reversal paradigms and paradigms that required shifting between task rules or cognitive sets. We included 50 experiments that contrasted brain activity during switch trials with that during repeat trials, thus testing for switching-related processes that are typically reflected in local switch costs. Furthermore, we included four experiments that contrasted brain activity during switch trials with brain activation at rest, and consequently involve more than just switching-related effects. Another six experiments were included that contrasted brain activity during switch trials with that during a sensorimotor control task, thus testing for processes related to both local and global switch costs. We included all 60 task-switching experiments in our main analysis but also performed a supplementary analysis including only the 50 switch vs. repeat contrasts. The results of this additional analysis are shown and discussed in the Supplementary Material. In all but three experiments (cf. Appendix 1), significant switch costs were observed. Regarding task specificity, for both dual-tasking and task-switching paradigms, only those experiments were included that reported choice-reaction tasks, as it is assumed that response selection is the key process that causes response slowing in Task 2 or in a switch trial, respectively (Kiesel et al. 2010; Pashler 1994, 2000). Our final sample comprised 18 dual-tasking studies (in total: 26 experiments, 378 participants, see Appendix 1) and 46 task-switching studies (in total: 60 experiments, 1362 participants, see Appendix 2).
Based on these samples, the following analyses were conducted: (1) main effect of dual-tasking; (2) main effect of task-switching; (3) activity shared between dual-tasking and task-switching (conjunction analysis); and (4) activity differences between dual-tasking and task-switching (contrast analyses). Besides, we conducted the following supplementary analyses: (5) activity differences between “prepared task-switching” and “unprepared task-switching” (contrast analyses), and (6) to further analyze preparatory effects in task-switching, a correlation analysis between brain activation likelihood and preparatory interval length.
Activation likelihood estimation
The data were analyzed using the current ALE algorithm for coordinate-based meta-analysis of neuroimaging experiments (Eickhoff et al. 2009, 2012; Turkeltaub et al. 2002, 2012) using in-house software implemented in Matlab (The MathWorks Inc., Natick, MA, USA). The algorithm aims to identify areas showing a significant convergence of reported spatial association, as compared to random associations. The core idea behind ALE is to treat the reported foci not as single points but rather as centres for 3-D Gaussian probability distributions capturing the spatial uncertainty associated with each reported focus. The width of these uncertainty functions was previously determined based on empirical data on the between-subject and between-template variance, which represent the main components of this uncertainty (Eickhoff et al. 2009). The ALE algorithm weights the between-subject variance by the number of examined subjects per study, accommodating the notion that larger sample sizes should provide more reliable approximations of the ‘true’ activation effect and should, therefore, be modeled by ‘smaller’ Gaussian distributions.
The probabilities of all foci reported in a given experiment were then combined for each voxel, resulting in a modeled activation (MA) map (Turkeltaub et al. 2012). Taking the union across these MA maps yielded voxel-wise ALE scores describing the convergence of results at each particular location of the brain. To distinguish ‘true’ convergence across studies from random convergence (i.e., noise), ALE scores were compared to a null-distribution reflecting a random spatial association between experiments. Hereby, a random-effects inference is invoked, focusing on inference on the above-chance convergence across studies, not clustering of foci within a particular study. Computationally, this nullhypothesis is derived by analytically solving the probability distribution that would ensue when repeatedly sampling a voxel at random from each of the MA maps and taking the union of these values in the same manner as done for the (spatially contingent) voxels in the true analysis (Eickhoff et al. 2012). The p value of ‘true’ ALE was then given by the proportion of equal or higher values obtained under the null-distribution. The resulting non-parametric p values for each meta-analysis were then thresholded at cluster-level p < 0.05 (cluster-forming threshold at voxel level: p < 0.001) and transformed into z scores for display. In this inference, the extent threshold necessary to control the cluster-level family-wise error (FWE) rate was derived from a Monte Carlo simulation of the excursion set above the cluster-forming threshold based on the analysis of randomly distributed foci under otherwise identical settings. Simulating 5000 of such random analyses allowed deriving a null-distribution of the above-threshold cluster sizes (more precisely, the maximum size of any cluster in the excursion set within each iteration). This distribution was then used to identify the cluster size, which was only exceeded in 5% of all random realizations, as the critical threshold for cluster-level FWE correction. Importantly, this critical extent threshold is strongly dependent on the number of experiments in the particular meta-analysis (as well as the characteristics of their foci). It was, therefore, calculated specifically for each of the presented meta-analyses.
Meta-analytic conjunctions, contrasts, and correlation
Conjunction analyses aimed at identifying those voxels where a significant effect was present in two separate analyses. To compute the conjunction between two ALE analyses, we used the conservative minimum statistic (Nichols et al. 2005), which is equivalent to identifying the intersection between the two cluster-level FWE-corrected results (Langner et al. 2018). That is, only regions significant at a corrected significance level in both individual analyses were considered. To exclude smaller regions of presumably incidental overlap between the thresholded ALE maps of the individual analyses, an additional cluster-extent threshold of k > 50 voxels was applied.
Differences between conditions were tested by first performing separate ALE analyses for each condition and computing the voxel-wise difference between the ensuing ALE maps (Eickhoff et al. 2012). All experiments contributing to either analysis were then pooled and randomly divided into two groups of the same size as the two original sets of experiments reflecting the contrasted ALE analyses. ALE scores for these two randomly assembled groups were calculated and the difference between these ALE-scores was recorded for each voxel in the brain. Repeating this process 10,000 times yielded an empirical null-distribution of ALE-score differences under the assumption of exchangeability. The ‘true’ difference in ALE scores was then tested against this null-distribution yielding a posterior probability that the difference was not due to random noise in an exchangeable set of labels, based on the proportion of lower differences in the random exchange. The resulting probability values were thresholded at p > 0.95 (> 95% chance of a true difference) and inclusively masked by the respective main effects, i.e., the significant effects of the ALE analysis for the particular condition. In addition, a cluster-extent threshold of k > 50 voxels was applied. Significant differences resulting from these meta-analytic contrasts indicate stronger convergence of activation (i.e., more consistent support) at a given location in the brain across the experiments included in a particular meta-analysis, as compared to the other analysis. Such differences in estimated activation likelihood can be interpreted as greater confidence in a given ALE cluster in one direction of the contrast. We note that contrast and conjunction effects are not mutually exclusive but rather may overlap if each of two sets of experiments (e.g., dual-tasking and task-switching) converges significantly in a given region but one of them more strongly than the other.
Finally, by means of rank-correlation analysis we tested whether and how the likelihood of task-switching-related activation in a given voxel (as obtained from every experiment’s MA map) was correlated with preparatory interval length across task-switching experiments (cf. Langner and Eickhoff 2013).
Anatomical labeling
All resulting areas were anatomically labeled by reference to probabilistic cytoarchitectonic maps of the human brain included in the SPM Anatomy Toolbox version 1.7 (Eickhoff et al. 2005, 2007). Using maximum probability maps, peaks of meta-analytic convergence were assigned to the most probable histologically defined brain area at their respective locations. Details on these cytoarchitectonic maps may be found in publications reporting on Broca’s region (Amunts et al. 1999), premotor cortex (Geyer 2004), and parietal cortex (Caspers et al. 2008; Choi et al. 2006; Scheperjans et al. 2008a, b). Regions that had not yet been cytoarchitectonically mapped at the time of analysis were labeled macroanatomically.
Results
Meta-analyses for individual multitasking domains
Dual-tasking
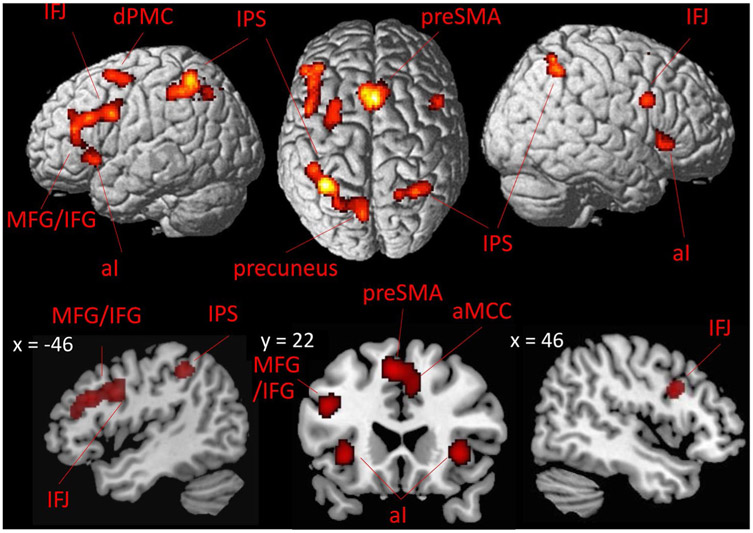
To assess the main effect of dual-tasking on brain activity, we conducted a meta-analysis across all 26 dual-tasking experiments (Appendix 2). This analysis revealed significant convergence of activation in six bilateral clusters including dPMC, IPS, and fO extending into right al. Furthermore, we found consistent brain activation in left IFS extending into MFG, as well as in left inferior frontal gyrus (IFG) extending into the anterior aspect of left temporal gyrus (STG; Fig. 1; Table S1).
Fig. 1.
Convergence of brain activation across all dual-tasking experiments included
Task-switching
To assess the main effect of task-switching on brain activity, we conducted a meta-analysis across all 60 task-switching experiments (Appendix 1). This analysis revealed significantly converging activation in seven bilateral clusters: al, preSMA extending into right aMCC, IFJ extending in the left hemisphere anteriorly into MFG and IFG, as well as IPS and adjacent IPL and SPL extending into left precuneus. Furthermore, we found consistent brain activation in left dPMC (Fig. 2; Table S2).
Fig. 2.
Convergence of brain activation across all task-switching experiments included
Conjunction analysis
To isolate multitasking-related brain activity independent of the specific paradigm, we conducted a conjunction analysis across the two individual meta-analyses reported above. This conjunction revealed shared convergence of brain activation in bilateral middle IPS (mIPS) and adjacent SPL, in the rostral part of the left dPMC, and in the right aI (Fig. 3; Table 1).
Fig. 3.
Shared convergence of brain activation in dual-tasking and task-switching experiments (conjunction analysis)
Table 1.
Shared convergence of brain activation of dual-tasking and task-switching experiments
| Cluster | Voxel | Macroanatomical location |
Cytoarchitectonic assignmenta |
MNI coordinates | |||
|---|---|---|---|---|---|---|---|
| x | y | z | z score | ||||
| 1 | 214 | Left mIPS | 7A, 7PC | − 32 | − 52 | 58 | 4.59 |
| − 38 | − 48 | 54 | 4.51 | ||||
| 2 | 148 | Right aI | - | 34 | 24 | 0 | 5.14 |
| 3 | 110 | Right mIPS | hIP3, 7A, 7PC | 30 | − 54 | 50 | 4.26 |
| 4 | 70 | Left dPMC | - | − 28 | 0 | 54 | 4.23 |
| - | − 24 | 8 | 58 | 3.18 | |||
mIPS middle inferior parietal sulcus, aI anterior insula, dPMC dorsal premotor cortex
References for histological assignments: 7A 7PC: Scheperjans et al. (2008b); hIP3: Scheperjans et al. (2008b)
In addition to the strict conjunction analysis, we performed a separate meta-analysis of the multitasking main effect across all dual-task and task-switching experiments. This analysis yielded significant convergence of activation in an extensive fronto-parietal network (Fig. S1, Table S3 in the Supplementary Material).
Contrast analyses
Dual-tasking vs. task-switching
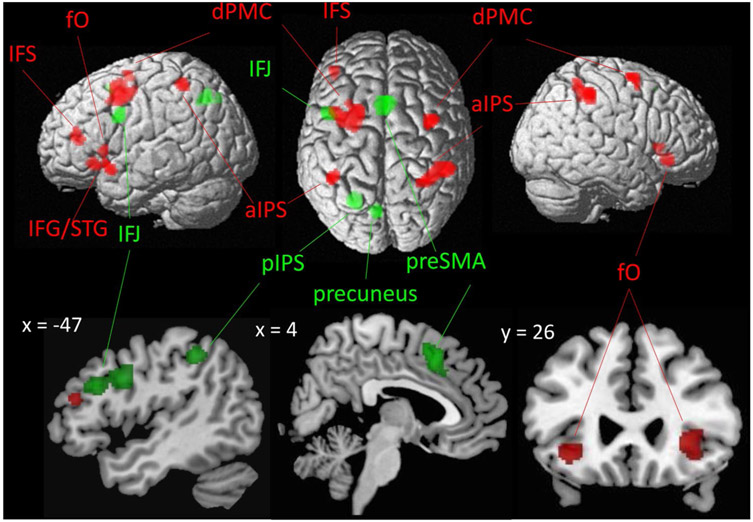
To evaluate which brain areas were more consistently associated with dual-tasking or task-switching, respectively, two ALE contrast analyses were performed on the main effects of dual-tasking and task-switching. In comparison with task-switching, dual-tasking experiments showed significantly stronger convergence of activation in six bilateral clusters: fO, dPMC, and anterior IPS (right hemisphere: 7PC, Area 2, PFt, 5L; left hemisphere: lPC, hlP2, Area 2). In addition, stronger convergence was found in left IFS and left IFG extending into the anterior aspect of left temporal gyrus (Fig. 4 as shown in red; Table 2).
Fig. 4.
Differences in convergence of brain activation between dual-tasking (red) and task-switching (green)
Table 2.
Differences between dual-tasking and task-switching experiments
| Cluster | Voxel | Macroanatomical location | Cytoarchitectonic assignmenta | MNI coordinates | |||
|---|---|---|---|---|---|---|---|
| x | y | z | z score | ||||
| Task-switching > dual-tasking | |||||||
| 1 | 243 | preSMA | - | 0 | 14 | 50 | 2.94 |
| 2 | 98 | Left pIPS | hIP3, hIP1 | − 26 | − 66 | 42 | 2.74 |
| - | − 26 | − 60 | 40 | 2.20 | |||
| 3 | 85 | Left IFJ | - | − 46 | 2 | 28 | 2.46 |
| - | − 50 | 4 | 30 | 2.34 | |||
| - | − 42 | 4 | 26 | 2.18 | |||
| 4 | 56 | Left precuneus | 7A | − 7 | − 72 | 42 | 2.27 |
| Dual-tasking > task-switching | |||||||
| 1 | 473 | Left dPMC | Area 6 | − 22 | 6 | 52 | 4.89 |
| - | − 34 | 4 | 44 | 3.94 | |||
| - | − 18 | 8 | 54 | 3.78 | |||
| - | − 24 | − 2 | 66 | 2.06 | |||
| - | − 24 | − 6 | 60 | 1.98 | |||
| 2 | 391 | Right aIPS | 7PC, Area 2 | 36 | − 46 | 56 | 3.45 |
| - | 48 | − 34 | 48 | 3.28 | |||
| - | 26 | − 48 | 62 | 2.26 | |||
| 3 | 96 | Right fO | - | 30 | 28 | − 6 | 3.09 |
| - | 38 | 30 | − 12 | 2.79 | |||
| - | 28 | 20 | 0 | 1.86 | |||
| 4 | 92 | Right dPMC | - | 38 | − 4 | 64 | 3.19 |
| - | 40 | 0 | 64 | 3.16 | |||
| - | 34 | 2 | 68 | 3.08 | |||
| - | 36 | 2 | 56 | 2.21 | |||
| - | 32 | 2 | 54 | 2.07 | |||
| 5 | 90 | Left fO | - | − 36 | 24 | − 12 | 2.88 |
| - | − 30 | 26 | − 10 | 2.51 | |||
| 6 | 84 | Left IFG/STG | Area 45, Area 44 | − 48 | 12 | − 12 | 2.79 |
| - | − 52 | 16 | − 10 | 2.68 | |||
| - | − 50 | 18 | 2 | 2 | |||
| 7 | 69 | Left aIPS | 7PC, hIP2, Area 2 | − 44 | − 46 | 56 | 2.91 |
| 8 | 60 | Left IFS | − 42 | 42 | 12 | 2.48 | |
preSMA pre-supplementary motor area, aIPS anterior part of the inferior parietal sulcus, pIPS posterior part of the inferior parietal sulcus, IFJ inferior frontal junction, dPMC dorsal premotor cortex, fO frontal operculum, IFG inferior frontal gyrus, STG superior temporal gyrus, IFS inferior frontal sulcus
References for histological assignments: hIP1: Choi et al. (2006); hIP3: Scheperjans et al. (2008b); 7A,7PC: Scheperjans et al. (2008b); Area 2: Grefkes et al. (2001); Area 6: Geyer (2004)
Task-switching vs. dual-tasking
As compared to dual-tasking, activations in task-switching experiments were more consistently found in four clusters: preSMA, left posterior IPS (hlP3, hlP1), left precuneus, as well as left IFJ (Fig. 4 as shown in green; Table 2).
Preparation effects in task-switching
To examine the effect of preparation, we divided the sample of task-switching experiments according to CTI length (i.e., in cued switching paradigms: the time interval between task-cue and target-stimulus onsets; in alternating-run paradigms: the intertrial interval (ITI) between previous and current target-stimulus onset): based on the classification of Ruge et al. (2013), experiments with CTIs/ITIs of 500/850 ms or more (n = 26) were considered to reflect “prepared task-switching”, as this interval would allow for intentional, proactive preparation of the upcoming switch. Conversely, experiments with CTIs below 500 ms (including simultaneous cue–target presentation) were considered to reflect “unprepared task-switching”, as such intervals would be too short for proper switch preparation and would, instead, encourage or enforce reactive control to implement switching at the moment of target occurrence.
ALE contrast analysis (see the Supplementary Material for individual main effects of prepared and unprepared task-switching, respectively) revealed that unprepared (vs. prepared) task-switching showed more consistent activation in three different clusters: preSMA and adjacent right aMCC, left IFS, and left aI/fO (Fig. 5 as shown in green; Table S4). In contrast, prepared (vs. unprepared) task-switching was more consistently associated with activation in right IFG (Fig. 5 as shown in red; Table S4).
Fig. 5.
Differences in convergence of brain activation between prepared (red) and unprepared (green) task-switching
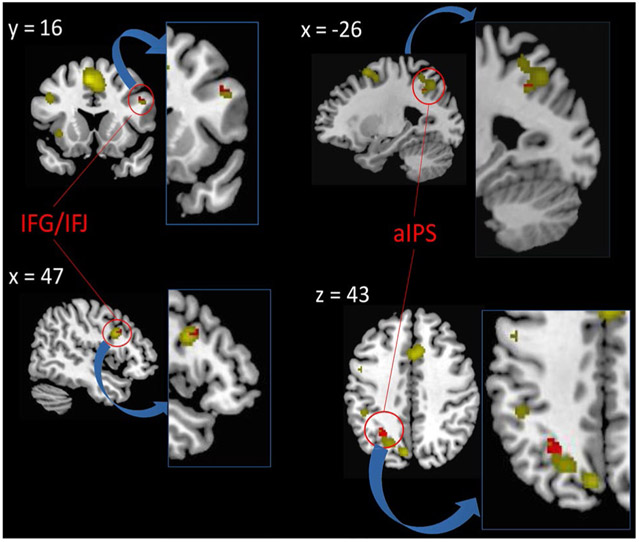
In addition, we conducted a rank-correlation analysis to investigate the association between the likelihood of task-switching-related brain activity and preparatory interval length across experiments. This correlation was inclusively masked by the main effect of task-switching. The analysis revealed a significantly negative correlation in the posterior part of the right IFS adjacent to the IFJ (MNI coordinates of the peak voxel: 52, 12, 30) and in left anterior IPS (− 24, − 58, 42) (Fig. 6). Hence, the shorter the preparatory interval, the higher was the activation likelihood in these regions. There was no significant positive correlation.
Fig. 6.
Brain regions that show a negative correlation between task-switching-related brain activation and length of the preparatory interval before a switch
Discussion
Our meta-analyses addressed the question whether information processing limitations related to dual-tasking or task-switching are reflected in converging neural mechanisms. In particular, we used the ALE algorithm for comparing reported brain activations associated with interference effects in dual-tasking and switch costs in task-switching. The only regions that showed consistent involvement in both dual-tasking and task-switching were the bilateral mIPS, left dPMC, and right aI, as revealed by a conjunction analysis. Contrast analyses showed that eight clusters were more consistently activated in dual-tasking (bilateral fO, dPMC, and anterior IPS, left IFS and left IFG), while four clusters in right preSMA/aMCC, left IFJ, left pIPS, and left precuneus were more consistently activated in task-switching. We conclude that the bilateral mIPS, left dPMC, and right aI play a pivotal role in dealing with multitasking demands in general, while other frontal and parietal regions are associated more specifically with subprocesses differentially engaged in dual-tasking or task-switching, respectively.
Investigating preparation effects in task-switching revealed the preSMA and adjacent right aMCC, left IFS, and left aI/fO to be more strongly associated with unprepared task-switching, while the right IFG was more strongly associated with prepared task-switching. We conclude that switching-related reactive control processes preferentially involve preSMA/aMCC, left IFS, and left aI/fO, while residual switching-related processes after preparation preferentially involve right anterior IFG. Furthermore, we observed a negative correlation between preparatory interval length and the likelihood of task-switching-related brain activity in right posterior IFG/IFJ and left IPS.
Commonalities and differences in brain activity related to dual-tasking and task-switching
The conjunction analysis across dual-tasking and task-switching experiments revealed convergent brain activation in bilateral mIPS and adjacent SPL, left dPMC, and right al. Only one neuroimaging study has previously compared neural correlates of dual-tasking and task-switching directly (Dreher and Grafman 2003). The authors found brain activation common to both tasks in bilateral superior frontal gyrus and IPL, right MFG and middle occipital gyrus, as well as left IFG, preSMA, cerebellum, and inferior temporal gyrus. This apparent non-overlap with our results might be due to idiosyncrasies and methodological limitations of Dreher and Grafman’s study. Apart from the moderate sample size, the common activations reported in that study resulted from contrasting the tasks against rest (rather than an active control task), and thus may not only reflect multitasking-specific activity but also more basic stimulus- and response-related processing. Moreover, Dreher and Grafman analyzed their data using a fixed-effects model, which restricts the generalizability of their inference. In contrast, the majority of experiments included in our meta-analysis reported comparisons against active control tasks and used random-effects models for statistical inference. We suggest that the multitasking-related activity in bilateral mIPS, left dPMC, and right aI during both task-switching and dual-tasking represents common subprocesses in controlling the efficient performance of two tasks, either in parallel or in close succession. As will be discussed below in more detail, we think these subprocesses comprise task-set activation and alertness regulation as well as attentional shifting and action reprogramming.
Anterior insula and frontal operculum
A large-scale meta-analysis (Kurth et al. 2010) showed that the aI is associated with a broad range of cognitive tasks, as would be expected from a highly integrative region. The aI has previously been found to be part of a core system that subserves the implementation and maintenance of task sets (Dosenbach et al. 2006, 2007). Since both dual-tasking and task-switching require the implementation and maintenance of multiple task (sub)sets, the right aI might be recruited more strongly to meet those increased demands, relative to single-tasking or task repetitions. However, managing multiple (vs. single) tasks also entails an increased difficulty, which is likely to be encountered by higher effort investment. Here, the aI might also play a role: For instance, Eckert et al. (2009) demonstrated that the right aI is not only functionally connected with frontal regions implicated in executive functioning but also that its activity correlated positively with activity in brain regions specifically engaged by tasks with varying perceptual and behavioral demands. Activity in the aI has also been related to the basic but effortful task of maintaining attention and response readiness to simple, easy-to-detect stimuli over time (i.e., intrinsic alertness; Langner et al. 2012). Based on this and other evidence, Langner and Eickhoff (2013) suggested that aI activity may signal the need to exert effort to maintain the relevant (i.e., goal-directed) task set sufficiently activated and succeed in performing the task at hand. As dual-tasking and task-switching alike put more demands on top-down control than do single-tasking or task repetitions, this demand needs to be translated into a motivational signal calling for increased effort expenditure. In fact, this increase in aI activity might be a neural correlate of the effort-based increase in general processing capacity in response to higher task demands as proposed by Kahneman (1973). In keeping with these notions, we interpret the aI’s role in multitasking as subserving the managing of multiple task sets and signaling the need for increased effort investment required to achieve their correct implementation by solving any mutual between-set interference.
Intriguingly, dual-tasking, as compared to task-switching, revealed more consistent activation in bilateral fO. This brain region, rostrally adjacent to the al, has been found to regulate the attentional selection of information held in working memory (Higo et al. 2011). Dual-tasking poses stronger demands for selection from working memory than does single-tasking or task-switching, as the S–R mappings for two tasks (and their order) need to be held active in parallel, forming a complex “compound task set”, from which each of the two perception–action cycles (i.e., Task 1 and Task 2) needs to be selected and passed onto “lower” levels for correct execution. The parallel presence of the Task 2 imperative stimulus or the wrong task priming from modality-incompatible S–R mappings might produce additional between-task crosstalk, enhancing the need for controlled attentional selection from working memory. We conjecture that these selection demands in dual-tasking are met by enhanced bilateral fO recruitment. If these selection processes failed, we would predict backward crosstalk from Task 2 onto Task 1, as has been shown for elderly individuals (Hein and Schubert 2004), for whom the neurofunctional network integrity of the fO/aI region has been found to be compromised (Langner et al. 2015).
Intraparietal sulcus and precuneus
We found different clusters of brain activation in the parietal lobe. The middle part of the IPS (mIPS) was consistently activated in both paradigms, whereas the anterior part (aIPS) was more consistently activated in dual-tasking (vs. task-switching), and the posterior part (pIPS) and adjacent precuneus were more consistently activated in task-switching (vs. dual-tasking). The mIPS is considered a part of the dorsomedial reach pathway and to project via the SPL to the dPMC (Grafton 2010). Several studies associated brain areas around the IPS and SPL with response selection processes (e.g., Bunge et al. 2002; Göbel et al. 2004; Sigman and Dehaene 2008), or more precisely with S–R mapping (Cavina-Pratesi et al. 2006; Schumacher et al. 2003; Cieslik et al. 2010). The mIPS also covers the human homologue of the monkey’s lateral intraparietal cortex, which was shown to be associated with shifts of visual attention and saccadic control in monkeys (Andersen et al. 1992; Blatt et al. 1990). In dual-tasking, mIPS activation may reflect S–R mapping processes, which are more demanding than during single-tasking, since two different mappings need to be performed instead of just one. In task-switching, stronger mIPS activation in switch (vs. repeat) trials may reflect the controlled mapping of a given stimulus (or stimulus feature) to the response that is adequate according to the currently active (i.e., just updated) task set, including the associated shifting of (mainly visual) attention to the now relevant stimulus or stimulus dimension.
The aIPS has been found to project primarily to ventral premotor areas and to be associated with sensorimotor processing in hand movements (Binkofski et al. 1998; Matelli et al. 1986; Murata et al. 2000; Rizzolatti et al. 1998). It has been suggested that the aIPS is related to action planning and reorienting of motor attention (Rushworth et al. 2003). In line with our results, Cieslik et al. (2010) found the aIPS to be related to top-down reorienting of attention when performing spatially incongruent responses. Considering the processing of two different motor tasks in dual-tasking, our results of more consistent aIPS activation in dual-tasking (compared to task-switching) agree well with these findings: supporting an association of the aIPS with top-down guided reorienting of motor attention and action planning (Rushworth et al. 2003). In accordance with the view of the aIPS’s role in reorienting motor attention and in action planning, our correlation analysis revealed that the shorter the preparatory interval in task-switching, the stronger the aIPS was activated. This is in line with our above interpretation, suggesting that the less time there is to get prepared for the upcoming (new) task, the more reactive control for reorienting motor attention and action planning is needed.
Blangero et al. (2009) found a posterior–anterior gradient of visuo-motor action processing in the parietal lobes. That is, posterior parietal areas were predominantly involved in bilateral spatial processing, while anterior areas were more involved in attention to contralateral limb movements. More recently, the pIPS has been found to facilitate target discrimination after a conflict trial by directing attention to task-relevant stimulus features (Soutschek et al. 2013). Using a combined fMRI–transcranial magnetic stimulation approach, Capotosto et al. (2013) supported a causal role of the pIPS in target discrimination. In line with our results, we therefore propose that dual-task-specific aIPS activation reflects enhanced attentional demands for planning and controlling the near-parallel motor output during the two tasks. In contrast, switching-specific pIPS activation might subserve increased demands for directing visual selective attention to the stimulus features that are relevant for the current task (Bisley and Goldberg 2003; Blatt et al. 1990; Chambers et al. 2004; Green and McDonald 2008; Rushworth et al. 2001; Rushworth and Taylor 2006).
Several studies have shown that the precuneus is transiently activated by shifts of spatial attention (Shulman et al. 2009; Tosoni et al. 2012; Vandenberghe and Gillebert 2009). Importantly, it has been demonstrated that the attention signal is not only modulated by spatial characteristics of the shift but also by non-spatial stimulus features, objects, sensory modalities or cognitive demands (Chiu and Yantis 2009; Langner et al. 2012; Shomstein and Yantis 2004; Yantis et al. 2002). It, therefore, appears that the precuneus plays a general, domain-independent role in shifting attention. Significantly more consistent activation in the precuneus during task-switching (vs. dual-tasking) might, therefore, reflect switching attention from the previous to the current task. In contrast, more consistent activation in pIPS might reflect attentional involvement to facilitate target discrimination in the upcoming task (cf. above). Such a process would be disadvantageous in dual-tasking, given that stimulus features of both tasks have to be processed nearly simultaneously, such that focusing attention to stimulus features of only one task would result in increased processing costs for the second task. Regarding the leftward asymmetry of brain activation in pIPS and precuneus in task-switching, several studies found contralateral activation in parietal lobe during motor execution as well as a leftward asymmetry in righthanders (Begliomini et al. 2008; Blangero et al. 2008; Stark and Zohary 2008). To sum up, the results of our analyses and previous research suggest an association between mIPS activity and S–R mapping, between aIPS activity and both action planning and motor attention (especially in dual-tasking), between pIPS activity and feature-specific attention, as well as between precuneus activity and attentional shifting (especially in task-switching).
Dorsal premotor cortex
The mIPS and SPL have been shown to project to dPMC, which further projects to the primary motor area (Grafton 2010). We found bilateral dPMC more consistently activated in dual-tasking, as compared to task-switching, while the left dPMC showed significant convergence across both paradigms. The dPMC clusters obtained in our meta-analysis overlap with the presumed location of the human frontal eye field (FEF; Paus 1996; zu Eulenburg et al. 2012). The FEF is supposed to facilitate visual target detection (Grosbras and Paus 2003) by biasing perception through attentional top-down signals (Corbetta and Shulman 2002; Langner et al. 2011). At the same time, FEF activity is thought to be biased itself by signals arising from content held in working memory (Ptak 2011). Moreover, the left dPMC was found to play a key role in rapid action reprogramming involving the selective suppression of inappropriate action codes (Hardwick et al. 2013; Hartwigsen and Siebner 2015; Petrides 1997) as well as response activation in humans (Pastor-Bernier et al. 2012) and in monkeys (Hoshi and Tanji 2000; Nakayama et al. 2008), usually together with the left IPL (Hartwigsen and Siebner 2015; Rizzolatti et al. 1998; Rizzolatti and Luppino 2001). As our conjunction analysis across dual-task and task-switching experiments revealed increased activation in left dPMC, we conjecture that the left dPMC may subserve increased demands for action (re)programming in multitasking. We consider this plausible because in both paradigms pre-activated action codes (related to Task 1 in dual-tasking or the previous task in task-switching, respectively) need to be suppressed for correctly performing Task 2 or the alternative task, respectively.
Our contrast analysis revealed stronger convergence in right dPMC for dual-tasking, relative to task-switching. A recent connectivity-based parcellation study (Genon et al. 2017) subdivided the dPMC into five anatomically and functionally different independent clusters: rostral, caudal, central, ventral, and dorsal cluster of the right dPMC. Our results overlap with the central cluster and adjacent caudal and dorsal clusters. Genon et al. showed that the central cluster has strong connections to the IPS and SPL and is engaged in motor and cognitive functions like action execution and working memory. Further, the central cluster was found to be coupled with all other clusters, suggesting a core role in linking the functionally more specialized clusters within the right dPMC. The caudal cluster was found to be functionally connected to right fronto-parietal operculum and to be engaged in action execution, motor learning, and interoception, suggesting an association with the organization of movement or action formulation (Schubotz and von Cramon 2003). The dorsal cluster of the right dPMC was found to be connected to bilateral prefrontal regions, insula, right putamen, and right MCC; functionally, it was associated with motor and cognitive networks, particularly with hand/finger movements (see also Sadato et al. 1997). Considering that the right dPMC is more consistently activated in dual-tasking, as compared to task-switching, we, therefore, reason that activation in right dPMC is associated with intentional action formulation and execution, especially under conditions of interference from a competing parallel or immediately preceding movement.
Inferior frontal gyrus and inferior frontal sulcus
The left IFG was found to be more consistently activated in dual-tasking, as compared to task-switching. Koechlin and Jubault (2006) proposed that the IFG subserves sequential behavior by “selecting/inhibiting simple action chunks through top-down interactions that initiate and terminate successive selections of simple chunk components occurring in the premotor regions (i.e., single motor acts or sensorimotor associations)” (p. 964). Nelson et al. (2009) associated the IFG with interference resolution during retrieval from WM, and Swick et al. (2008) showed that patients with lesions in left IFG (vs. in orbitofrontal cortex or healthy controls) showed a selective deficit in inhibiting motor responses. It therefore appears that the IFG plays a key role for sequencing movements by interference resolution. A recent connectivity-based parcellation study (Clos et al. 2013) subdivided the left IFG into five anatomically and functionally different independent clusters. Our result overlaps with Clos et al.’s cluster 4, which was found to be functionally connected with bilateral insula, thalamus, and basal ganglia. Functionally, cluster 4 showed a strong association with action and action imitation as well as with sequencing of motor tasks (see also Stevens et al. 2007). In conclusion, we assume that brain activation in left IFG reflects top-down processes of sequencing movements in dual-tasking, effectively controlling task order (Luria and Meiran 2003; Meyer and Kieras 1997a, b; Sigman and Dehaene 2006).
Similar to the IFG, we also found the left IFS to be more consistently activated in dual-tasking than in task-switching. The IFS has been found to be involved in task-order control processes during dual-tasking (Stelzel et al. 2008; Szameitat et al. 2006). Additionally, it has been related to resolving interference during retrieval from working memory (Nelson et al. 2009). We therefore conjecture that activity in left IFS during dual-tasking, in concert with left IFG, is related to task-order control processes in association with maintaining the withheld task set of the second task. However, further research is needed to demonstrate a causal involvement of these brain areas.
Pre-supplementary motor area and anterior midcingulate cortex
The preSMA was more consistently activated in task-switching than in dual-tasking. The preSMA has been associated with response inhibition and selection of the appropriate response among alternatives (Barber et al. 2013; Mostofsky and Simmonds 2008; Nachev et al. 2008). Moreover, electrophysiological recordings in non-human primates revealed that the preSMA plays a specific role in switching from automatic to controlled response selection (Isoda and Hikosaka 2007), which is characteristic for shifting to a given task after having (repeatedly) performed the alternative task. In addition, the main effect of task-switching yielded consistent activation in adjacent aMCC. The aMCC has been thought to mediate the interaction between action intentions and motivational state (Paus 2001), or to “energize” the currently relevant task set (Stuss et al. 2005; Langner and Eickhoff 2013). For instance, the aMCC was shown to signal motivational significance when the correct choice among actions was linked to high rewards (Kouneiher et al. 2009), and in the context of self-chosen actions it was found to translate intentions into specific motor output (i.e., intentional motor control; Hoffstaedter et al. 2014). Furthermore, the aMCC has been linked to performance monitoring and conflict detection (Botvinick et al. 2004; Ullsperger et al. 2014). Based on the above-mentioned research, we propose that in switch trials the preSMA and aMCC guide action selection via signaling action values in accordance with the currently appropriate (i.e., updated) task set to achieve and optimize goal-congruent performance.
Inferior frontal junction
Our contrast analysis yielded stronger across-study convergence of activations in left IFJ for task-switching, relative to dual-tasking, while we observed a negative correlation between preparatory interval length and the likelihood of task-switching-related brain activity in right IFG/IFJ. Brain activation in IFJ has been related to task preparation processes after a task cue and to the updating of task rule representations to adjust behavior in line with instructions (Brass and von Cramon 2002, 2004). The left IFJ in particular has been reported to be involved in implementing new S–R rules (Hartstra et al. 2011, 2012), which agrees with an earlier study that revealed selective deficits in patients with left (vs. medial or right) frontal lesions (including IFJ) in acquiring new S–R mappings in a choice-reaction task (Alexander et al. 2005). The right IFJ, in turn, has been shown to be involved in detecting infrequent but action-relevant signals (Verbruggen et al. 2010; Chikazoe et al. 2009), in line with a neuroimaging meta-analysis on vigilant attention, which found the IFJ to be consistently activated in tasks with longer attention maintenance (Langner and Eickhoff 2013). Another meta-analysis found the right IFJ to be conjointly activated across Stroop tasks, spatial interference tasks, stop-signal tasks, and go/no-go tasks (Cieslik et al. 2015), suggesting an involvement of the right IFJ in more than just simple detection. Hence, we propose that the IFJ in task-switching is associated with retrieving and representing the current (i.e., non-dominant but appropriate) S–R mapping rules.
Preparation effects in task-switching
Contrasting prepared (CTI/RSI length > 500 ms) with unprepared task-switching (CTI/RSI length < 500 ms) revealed more consistent activation in right IFG for prepared switching, while unprepared switching showed more consistent activation in preSMA/aMCC, left IFS, and left aI/fO. Further, we observed a negative correlation between time for preparation and the likelihood of activity in the posterior part of the right IFS (adjacent to the IFJ) and in the left anterior IPS. That is, the shorter the time available for preparing to switch, the more likely those regions were activated across experiments.
The right midlateral prefrontal cortex, of which the observed IFG cluster forms a part, has been found strongly functionally connected to the IPS and associated with cognitive action control and working memory (Cieslik et al. 2013; Rottschy et al. 2012). We argue that during prepared task switches, when the upcoming task is clear and reconfiguration processes have been done, strong and specific control signals that guide attentional selection can be sent. That is, in the absence of task uncertainty, attention can be intensely directed to the stimulus features and response options that are relevant to the task at hand, which might be reflected in this region’s increased activity.
Unprepared task-switching, in turn, requires reactive setlevel control at the moment of stimulus occurrence since the appropriate task set could not be configured beforehand. These processing demands are reflected by increased activity in preSMA/aMCC, aI, and IFS, all of which are regions known to be involved in task-set activation (cf. “Commonalities and differences in brain activity related to dual-tasking and task-switching”). Finally, our correlation analysis revealed an increasing likelihood of recruiting the right posterior IFS and left aIPS when more reactive control is needed in switch trials. The right IFS/IFJ has been previously implicated in the control of S–R mappings by subserving the (re)activation of non-dominant but adequate mappings (Anderson et al. 2016; Cieslik et al. 2015). The alPS, in turn, has been related to the top-down reorienting of motor attention and action planning (Cieslik et al. 2015; Rushworth et al. 2003). We conclude that the more reactive control is needed at task onset due to time restrictions on preparation, the more the right IFS and left aIPS are recruited, possibly for boosting the adequate S-R mapping and reorienting motor attention, respectively.
Theoretical implications and a neuro-cognitive processing model of multitasking
Our meta-analysis of dual-tasking experiments provided some indirect evidence bearing on the source of behavioral dual-task costs, which might be due to a passive structural bottleneck or to additional processing requirements, such as the active allocation of limited-capacity resources for sharing available resources efficiently or top-down control modulations for resolving between-task interference. We reasoned that activation increases in dual- vs. single-task conditions would be at odds with a purely passive structural bottleneck model, as this model predicts a delay of response selection processes and associated brain activity in Task 2, but it does not predict increased activity associated with this passive delay. Our finding of consistent brain activity increases during dual-tasking is, therefore, more in line with functional perspectives on dual-task performance decrements like the capacity-sharing model. In this framework, dual-tasking-related brain activations would reflect active, functional adaptations to cope with increased processing demand, for instance via mobilizing additional processing resources through effort to resolve between-task crosstalk and/or via actively controlling resource allocation to the two tasks (“prioritizing”). This is not to say, however, that there is no structural bottleneck (or even several bottlenecks), for which neuroscientific evidence has been provided as well (cf. Hesselmann et al. 2011; Sigman and Dehaene 2008).
Directly comparing dual-tasking and task-switching results reveals more neural differences than commonalities. This implies that only a few cognitive subprocesses are shared between both multitasking paradigms, likely forming core processes in dealing with multiple tasks. Beyond this common core, however, our results support the view that there are fundamental differences in the processes underlying either type of multitasking. Future research needs to clarify to what extent and under what conditions multitasking costs result from paradigm-specific mechanisms vs. common, multitasking-general mechanisms, as for instance suggested by age-related deficits in both dual-tasking and task-switching (Verhaeghen et al. 2003; Wasylyshyn et al. 2011).
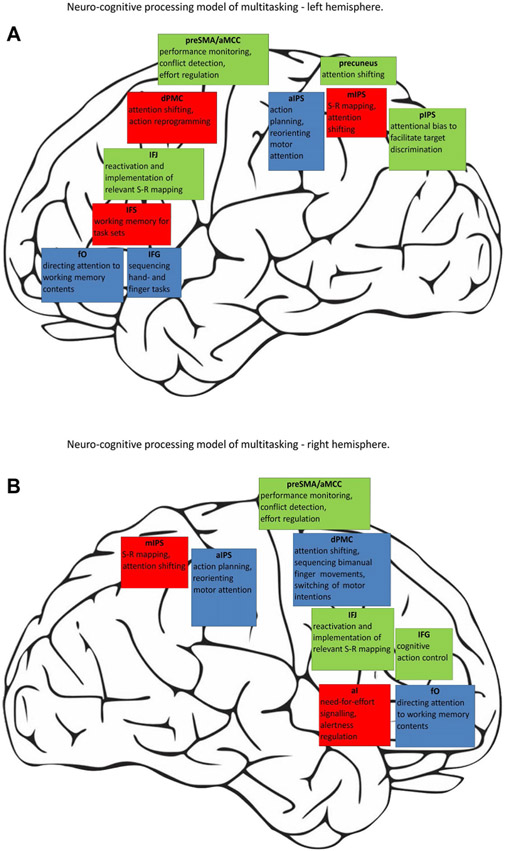
To provide some guidance for further research efforts, we conclude our meta-analytic review and comparison by proposing a neuro-cognitive processing model of multitasking (see Fig. 7). In this model, we summarize previous and our current findings regarding cognitive subcomponents of both multitasking paradigms and their putative neuroanatomical localization. We are aware of the model’s hypothetical nature and hope it will inspire and possibly guide more targeted research on brain–behavior relationships in multitasking. Open questions include the connectional architecture of the functional networks that mediate our ability to successfully juggle several tasks as well as training-induced improvements and age- or disease-related impairments of this ability.
Fig. 7.
Neuro-cognitive processing model of multitasking (a, left hemisphere; b, right hemisphere). Processes relevant for both multitasking domains are outlined in red, dual-task-specific processes in blue, and switching-specific processes in green
Conclusion
Our meta-analyses of brain activity associated with dual-tasking or task-switching, respectively, yielded two partly overlapping networks of fronto-parietal regions consistently associated with either multitasking paradigm. The shared core network comprised the intraparietal sulcus bilaterally, the left dorsal premotor cortex, and the right anterior insula. Drawing on previous research, this suggests that shifting attention and motor intentions as well as effort regulation for implementing the correct task rules may form the common thread throughout both multitasking settings. Apart from these commonalities, however, our data imply substantial processing differences between both multitasking paradigms. Finally, given that the increase in brain activity during dual-tasking, relative to single-tasking, reflects additional or more intense processing, we conclude that the usual performance costs incurred by doing two things at once are not only due to structural limitations of the cognitive processing architecture but also to demands for additional, effortful processing related to managing multiple task sets and solving between-task crosstalk.
Supplementary Material
Acknowledgements
We thank all contacted authors who contributed results of relevant contrasts not explicitly reported in the original publications, and we apologize to all authors whose eligible papers we might have missed.
Funding This study was supported by the Deutsche Forschungsgemeinschaft (LA 3071/3-1 to R.L. and S.B.E.; EI 816/4-1 to S.B.E.), the National Institute of Mental Health (R01-MH074457 to S.B.E.), the Helmholtz Portfolio Theme “Supercomputing and Modeling for the Human Brain” (S.B.E.), and the European Union Seventh Framework Programme (FP7/2007-2013) under Grant agreement no. 604102 (S.B.E.).
Appendix
Appendix 1:
Overview of all task-switching experiments included in the analysis
| Publication | No. of subjects | Contrast | Stimulus modality |
Effector modality |
Task order | Preparedness | CTI/ITI (ms) |
|---|---|---|---|---|---|---|---|
| Badre and Wagner (2006) | 10 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 250 |
| Barber and Carter (2005) | 13 | Switch > repeat | Vis vis | Man man | Random | Prepared | 7500 |
| Braver et al. (2003) | 13 | Switch > repeat | Vis vis | Man man | Random | Prepared | 2500 |
| Chiu and Yantis (2009) | 16 | Switch > repeat | Vis vis | Man man | Random | Prepared | 6250 |
| Cole and Schneider (2007) | 9 | Switch > repeat | Vis vis | Man man | Fixed | Unprepared | 1000 |
| Crone et al. (2006)b | 19 | Switch > repeat | Vis vis | Man man | Random | Prepared | 1500 |
| Crone et al. (2006)c | 19 | Switch > repeat | Vis vis | Man man | Random | Prepared | 1500 |
| De Baene and Brass (2011) | 19 | Switch > repeat | Vis vis | Man man | Random | Mixed | 2482 |
| Dibbets et al. (2010) | 14 | Switch > repeat | Vis vis | Man man | Random | Prepared | 1000 |
| DiGirolamo et al. (2001) | 8 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Dove et al. (2000) | 16 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Gazes et al. (2012) | 47 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Gu et al. (2008)a | 21 | Switch > repeat | Vis vis | Man man | Random | Prepared | 1170 |
| Halari et al. (2009) | 21 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Hedden and Gabrieli (2010)d | 17 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Hedden and Gabrieli (2010)e | 17 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Hyafil et al. (2009) | 24 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Jamadar et al. (2010a) | 18 | Switch > repeat | Vis vis | Man man | Random | Prepared | 700 |
| Jamadar et al. (2010b) | 12 | Switch > repeat | Vis vis | Man man | Random | Prepared | 700 |
| Kim et al. (2012) | 16 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Kimberg et al. (2000)f | 9 | Switch > repeat | Vis vis | Man man | Fixed | Prepared | 8000 |
| Kimberg et al. (2000)g | 9 | Switch > repeat | Vis vis | Man man | Fixed | Prepared | 8000 |
| Liston et al. (2006)h | 19 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Liston et al. (2006)i | 19 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Liston et al. (2006)j | 19 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Luks et al. (2002) | 11 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 3750 |
| Luks et al. (2002) | 11 | Switch > repeat | Vis vis | Man man | Random | Prepared | 3750 |
| Madden et al. (2010) | 40 | Switch > repeat | Vis vis | Man man | Random | Prepared | 1500 |
| Nee et al. (2011) | 27 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 4000 |
| Philipp et al. (2013) | 23 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 200 |
| Piguet et al. (2013) | 18 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 150 |
| Rodehacke et al. (2014) | 213 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Ravizza and Carter (2008) | 14 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Ruge et al. (2005) | 18 | Switch > repeat | Vis vis | Man man | Random | Mixed | 550 |
| Ruge et al. (2005) | 18 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 100 |
| Savine and Braver (2010) | 16 | Switch > repeat | Vis vis | Man man | Random | Prepared | 3050 |
| Shi et al. (2010) | 14 | Switch > repeat | Vis vis | Man man | Random | Prepared | 1200 |
| Smith et al. (2004) | 20 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 200 |
| Sohn et al. (2000) | 12 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Sohn et al. (2000) | 12 | Switch > repeat | Vis vis | Man man | Fixed | Prepared | 6000 |
| Wager et al. (2005) | 43 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Wilkinson et al. (2001) | 12 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 0 |
| Woodcock et al. (2010) | 8 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 100 |
| Wylie et al. (2006)a,k | 14 | Switch > repeat | Vis vis | Man man | Random | Prepared | 3000 |
| Wylie et al. (2006)a,l | 14 | Switch > repeat | Vis vis | Man man | Random | Prepared | 3000 |
| Witt and Stevens (2012) | 134 | Switch > repeat | Vis vis | Man man | Random | Prepared | 1200 |
| Witt and Stevens (2013) | 83 | Switch > repeat | Vis vis | Man man | Random | Prepared | 1200 |
| Yeung et al. (2006) | 15 | Switch > repeat | Vis vis | Man man | Random | Unprepared | 450 |
| Yoshida et al. (2010)a,m | 16 | Switch > repeat | Vis vis | Man man | Random | Prepared | 6000 |
| Yoshida et al. (2010)a,n | 16 | Switch > repeat | Vis vis | Man man | Random | Prepared | 6000 |
CTI cue–trial interval, ITI intertrial interval, vis visual, man manual
No significant switch costs were observed
univalent switches-repetitions
bivalent switch repetitions
incongruent shifting > neutral non-shifting
neural shifting > incongruent non-shifting
S–R at T0
S–R at T1
shift > repeat
high-response conflict switches > low-response conflict switches
high-stimulus conflict switches > low-stimulus conflict switches
color switch targets > color repeat targets
switch targets > speed repeat targets
rule switch > exploitation
meta-rule switch > exploitation
Appendix 2:
Overview of all dual-tasking experiments included in the analysis
| Publication | No. of subjects | Contrast | Stimulus modality |
Effector modality |
S–R modality compatibility |
Task order |
|---|---|---|---|---|---|---|
| Deprez et al. (2013) | 33 | Dual task > single tasks | Vis aud | Man man | Incompatible | Fixed |
| Dreher and Grafman (2003) | 8 | Dual task > baseline | Vis vis | Man man | Compatible | Random |
| Erickson et al. (2005) | 33 | Dual task > single tasks | Vis vis | Man man | Compatible | Random |
| Hartley et al. (2011)a | 12 | Short SOA > long SOA | Vis vis | Man man | Compatible | Fixed |
| Hartley et al. (2011)b | 12 | Short SOA > long SOA | Vis vis | Man man | Compatible | Fixed |
| Herath et al. (2001) | 10 | Dual task > single tasks | Vis som | Man man | Incompatible | Random |
| Herath et al. (2001) | 10 | Short SOA > long SOA | Vis som | Man man | Incompatible | Random |
| Hesselmann et al. (2011) | 12 | Dual task > single tasks | Vis vis | Man man | Compatible | Fixed |
| Houtkamp and Braun (2010) | 12 | Dual task > single tasks | Vis vis | Man man | Compatible | Random |
| Jiang (2004) | 10 | Short SOA > long SOA | Vis vis | Man man | Compatible | Fixed |
| Jiang et al. (2004) | 26 | Short SOA > long SOA | Vis vis | Man man | Compatible | Fixed |
| Koechlin et al. (1999) | 6 | Dual task > single tasks | Vis vis | Man man | Compatible | Random |
| Mochizuki et al. (2007) | 15 | Dual task > single tasks | Vis aud | Man man | Incompatible | Fixed |
| Mochizuki et al. (2007) | 15 | Dual task > single tasks | Vis aud | Man voc | Compatible | Fixed |
| Schubert and Szameitat (2003) | 11 | Dual task > single tasks | Vis aud | Man man | Incompatible | Fixed |
| Sigman and Dehaene (2008) | 21 | Dual task > single tasks | Vis aud | Man man | Incompatible | Fixed |
| Stelzel et al. (2006) | 10 | Dual task > single tasks | Vis aud | Man voc | Incompatible | Fixed |
| Stelzel et al. (2008)c | 13 | Dual task > baseline | Vis aud | Man man | Incompatible | Fixed |
| Stelzel et al. (2008)d | 13 | Dual task > baseline | Vis aud | Man man | Incompatible | Fixed |
| Stelzel et al. (2008)e | 13 | Dual task > baseline | Vis aud | Man man | Incompatible | Random |
| Stelzel et al. (2008)f | 13 | Dual task > baseline | Vis aud | Man man | Incompatible | Random |
| Szameitat et al. (2002) | 11 | Dual task > single tasks | Vis aud | Man man | Incompatible | Fixed |
| Szameitat et al. (2002) | 11 | Dual task > single tasks | Vis aud | Man man | Incompatible | Random |
| Tombu et al. (2011) | 12 | Dual task > single tasks | Vis aud | Man voc | Compatible | Random |
| Vetter et al. (2011)g | 18 | Dual task > single tasks | Vis vis | Man man | Compatible | Fixed |
| Vetter et al. (2011)h | 18 | Dual task > single tasks | Vis vis | Man man | Compatible | Fixed |
vis visual, man manual, aud auditory
Younger participants
older participants
Set-4
Set-8
Set-4
Set-8
high load > single task
low load > single task
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval For this type of study formal consent is not required.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00429-019-01870-4) contains supplementary material, which is available to authorized users.
References
- Alexander MP, Stuss DT, Shallice T, Picton TW, Gillingham S (2005) Impaired concentration due to frontal lobe damage from two distinct lesion sites. Neurology 65:572–579 [DOI] [PubMed] [Google Scholar]
- Allport DA, Styles EA, Hsieh S (1994) Shifting intentional set: Exploring the dynamic control of tasks In: Umilta C, Moscovitc M (eds) Attention and performance XV. MIT Press, Cambridge, pp 421–452 [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K (1999) Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 412:319–341 [DOI] [PubMed] [Google Scholar]
- Andersen RA, Brotchie PR, Mazzoni P (1992) Evidence for the lateral intraparietal area as the parietal eye field. Curr Opin Neurobiol 2(6):840–846 [DOI] [PubMed] [Google Scholar]
- Anderson MC, Bunce JG, Barbas H (2016) Prefrontal-hippocampal pathways underlying inhibitory control over memory. Neurobiol Learn Mem 134:145–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD (2006) Computational and neurobiological mechanisms underlying cognitive flexibility. Proc Natl Acad Sci USA 103:7186–7191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AD, Carter CS (2005) Cognitive control involved in overcomeing prepotent response tendencies and switching between tasks. Cereb Cortex 15:899–912 [DOI] [PubMed] [Google Scholar]
- Barber AD, Caffo BS, Pekar JJ, Mostofsky SH (2013) Effects of working memory demand on neural mechanisms of motor response selection and control. J Cogn Neurosci 25:1235–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begliomini C, Nelini C, Caria A, Grodd W, Castiello U (2008) Cortical activations in humans grasp-related areas depend on hand used and handedness. PLoS One 3:e3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Dohle C, Posse S, Stephan KM, Hefter H, Seitz RJ, Freund HJ (1998) Human anterior intraparietal area subserves prehension: a combined lesion and functional MRI activation study. Neurology 50:1253–1259 [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME (2003) Neuronal activity in the lateral intraparietal area and spatial attention. Science 299:81–86 [DOI] [PubMed] [Google Scholar]
- Blangero A, Gaveau V, Luauté J, Rode G, Salemme R, Guinard M, Boisson D, Rossetti Y, Pisella L (2008) A hand and a field effect in on-line motor control in unilateral optic ataxia. Cortex 44:560–568 [DOI] [PubMed] [Google Scholar]
- Blangero A, Menz MM, McNamara A, Binkofski F (2009) Parietal modules for reaching. Neuropsychologia 47:1500–1507 [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR (1990) Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol 299:421–445 [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS (2004) Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8:539–546 [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY (2002) The role of the frontal cortex in task preparation. Cereb Cortex 12:908–914 [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY (2004) Decomposing components of task preparation with functional magnetic resonance imaging. J Cogn Neurosci 16:609–620 [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI (2003) Neural mechanisms of transient and sustained cognitive control during task switching. Neuron 39:713–726 [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF (2005) Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp 25:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JDE (2002) Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage 17:1562–1571 [DOI] [PubMed] [Google Scholar]
- Capotosto P, Tosoni A, Spadone S, Sestieri C, Perrucci MG, Romani GL, Della Penna S, Corbetta M (2013) Anatomical segregation of visual selection mechanisms in human parietal cortex. J Neurosci 33:6225–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K (2008) The human inferior parietal lobule in stereotaxic space. Brain Struct Funct 212:481–495 [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Valyear KF, Culham JC, Köhler S, Obhi SS, Marzi CA, Goodale MA (2006) Dissociating arbitrary stimulus–response mapping from movement planning during preparatory period: evidence from event-related functional magnetic resonance imaging. J Neurosci 26:2704–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Payne JM, Stokes MG, Mattingley JB (2004) Fast and slow parietal pathways mediate spatial attention. Nat Neurosci 7:217–218 [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, Konishi S (2009) Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb Cortex 19:146–152 [DOI] [PubMed] [Google Scholar]
- Chiu YC, Yantis S (2009) A domain-independent source of cognitive control for task sets: shifting spatial attention and switching categorization rules. J Neurosci 29:3930–3938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, Amunts K (2006) Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J Comp Neurol 495:53–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Kurth F, Eickhoff SB (2010) Dissociating bottom-up and top-down processes in a manual stimulus–response compatibility task. J Neurophysiol 104:1472–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB (2013) Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex 23:2677–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Mueller VI, Eickhoff CR, Langner R, Eickhoff SB (2015) Three key regions for supervisory attentional control: evidence from neuroimaging meta-analyses. Neurosci Biobehav Rev 48:22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos M, Amunts K, Laird AR, Fox PT, Eickhoff SB (2013) Tackling the multifunctional nature of Broca’s region meta-analytically: co-activation-based parcellation of area 44. Neuroimage 83:174–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W (2007) The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage 37:343–360 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215 [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA (2006) Neural evidence for dissociable components of task-switching. Cereb Cortex 16:475–486 [DOI] [PubMed] [Google Scholar]
- De Baene W, Brass M (2011) Cue-switch effects do not rely on the same neural systems as task-switch effects. Cogn Affect Behav Neurosci 11:600–607 [DOI] [PubMed] [Google Scholar]
- Dell’Acqua R, Jolicoeur P, Vespignani F, Toffanin P (2005) Central processing overlap modulates P3 latency. Exp Brain Res 165:54–682 [DOI] [PubMed] [Google Scholar]
- Deprez S, Vandenbulcke M, Peeters R, Emsell L, Amant F, Sunaert S (2013) The functional neuroanatomy of multitasking: combining dual tasking with a short term memory task. Neuropsychologia 51:2251–2260 [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY (2005) Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Hum Brain Mapp 25:22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbets P, Evers EA, Hurks PP, Bakker K, Jolles J (2010) Differential brain activation patterns in adult attention-deficit hyperactivity disorder (ADHD) associated with task switching. Neuropsychology 24:413–423 [DOI] [PubMed] [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, Wszalek TM, Cohen NJ, Banich MT, Webb A, Belopolsky AV, McAuley E (2001) General and task-specific frontal lobe recruitment in older adults during executive processes: a fMRI investigation of task-switching. NeuroReport 12:2065–2071 [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE (2006) A core system for the implementation of task sets. Neuron 50:799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE (2007) Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104:11073–11078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY (2000) Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res 9:103–109 [DOI] [PubMed] [Google Scholar]
- Dreher JC, Grafman J (2003) Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cereb Cortex 13:329–339 [DOI] [PubMed] [Google Scholar]
- Duncan J (2010) The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci 14:172–179 [DOI] [PubMed] [Google Scholar]
- Dux PE, Ivanoff J, Asplund CL, Marois R (2006) Isolation of a central bottleneck of information processing with time-resolved FMRI. Neuron 56:1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR (2009) At the heart of the ventral attention system: the right anterior insula. Hum Brain Mapp 30:2530–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbas MH, Evans AC, Zilles K, Amunts K (2007) Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36:511–521 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009) Coordinate- based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT (2012) Activation likelihood estimation meta-analysis revisited. Neuroimage 59:2349–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kramer AF (2005) Neural correlates of dual-task performance after minimizing task-preparation. Neuroimage 28:967–979 [DOI] [PubMed] [Google Scholar]
- Gazes Y, Rakitin BC, Habeck C, Steffener J, Stern Y (2012) Age differences of multivariate network expressions during task-switching and their associations with behavior. Neuropsychologia 50:3509–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genon S, Li H, Fan L, Müller VI, Cieslik EC, Hoffstaedter F, Reid AT, Langner R, Grefkes C, Fox PT, Moebus S, Caspers S, Amunts K, Jiang T, Eickhoff SB (2017) The right dorsal premotor mosaic: organization, functions, and connectivity. Cereb Cortex 27:2095–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S (2004) The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cell Biol 174:1–89 [DOI] [PubMed] [Google Scholar]
- Göbel SM, Johansen-Berg H, Behrens T, Rushworth MF (2004) Response-selection-related parietal activation during number comparison. J Cogn Neurosci 16:1536–1551 [DOI] [PubMed] [Google Scholar]
- Goffaux P, Phillips NA, Sinai M, Pushkar D (2006) Behavioural and electrophysiological measures of task switching during single and mixed-task conditions. Biol Psychol 72:278–290 [DOI] [PubMed] [Google Scholar]
- Grafton ST (2010) The cognitive neuroscience of prehension: recent developments. Exp Brain Res 204:475–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JJ, McDonald JJ (2008) Electrical neuroimaging reveals timing of attentional control activity in human brain. PLoS Biol 6:730–738 [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K (2001) Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. NeuroImage 14:617–631 [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Paus T (2003) Transcranial magnetic stimulation of the human frontal eye field facilitates visual awareness. Eur J Neurosci 18:3121–3126 [DOI] [PubMed] [Google Scholar]
- Gruber O, Karch S, Schlueter EK, Falkai P, Goschke T (2006) Neural mechanisms of advance preparation in task switching. Neuroimage 31:887–895 [DOI] [PubMed] [Google Scholar]
- Gu BM, Park JY, Kang DH, Lee SJ, Yoo SY, Jo HJ, Choi CH, Lee JM, Kwon JS (2008) Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain 131:155–164 [DOI] [PubMed] [Google Scholar]
- Halari R, Simic M, Pariante CM, Papadopoulos A, Cleare A, Brammer M, Fombonne E, Rubia K (2009) Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naïve adolescents with depression compared to controls. J Child Psychol Psychiatry 50:307–316 [DOI] [PubMed] [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB (2013) A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage 67:283–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley AA, Jonides J, Sylvester CY (2011) Dual-task processing in younger and older adults: similarities and differences revealed by fMRI. Brain Cogn 75:281–291 [DOI] [PubMed] [Google Scholar]
- Hartstra E, Kühn S, Verguts T, Brass M (2011) The implementation of verbal instructions: an fMRI study. Hum Brain Mapp 32:1811–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartstra E, Waszak F, Brass M (2012) The implementation of verbal instructions: dissociating motor preparation from the formation of stimulus-response associations. Neuroimage 63:1143–1153 [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, Siebner HR (2015) Joint contribution of left dorsal premotor cortex and supramarginal gyrus to rapid action reprogramming. Brain Stimul 8:945–952 [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD (2010) Shared and selective neural correlates of inhibition, facilitation, and shifting processes during executive control. Neuroimage 51:421–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G, Schubert T (2004) Aging and input processing in dual-task situations. Psychol Aging 19:416–432 [DOI] [PubMed] [Google Scholar]
- Herath P, Klingberg T, Young J, Amunts K, Roland P (2001) Neural correlates of dual task interference can be dissociated from those of divided attention: an fMRI study. Cereb Cortex 11:796–805 [DOI] [PubMed] [Google Scholar]
- Hesselmann G, Flandin G, Dehaene S (2011) Probing the cortical network underlying the psychological refractory period: a combined EEG-fMRI study. Neuroimage 56:1608–1621 [DOI] [PubMed] [Google Scholar]
- Higo T, Mars RB, Boorman ED, Buch ER, Rushworth MF (2011) Distributed and causal influence of frontal operculum in task control. Proc Natl Acad Sci USA 108:4230–4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch P, Nolden S, Koch I (2017) Higher-order cognitive control in dual tasks: evidence from task-pair switching. J Exp Psychol Hum Percept Perform 43(3):569–580 [DOI] [PubMed] [Google Scholar]
- Hirsch P, Nolden S, Declerck M, Koch I (2018) Common cognitive control processes underlying performance in task-switching and dual-task contexts. Adv Cogn Psychol 14:62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, Fox PT, Eickhoff SB (2014) The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp 35:2741–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tanji J (2000) Integration of target and body-part information in the premotor cortex when planning action. Nature 408:466–470 [DOI] [PubMed] [Google Scholar]
- Houtkamp R, Braun J (2010) Cortical response to task-relevant stimuli presented outside the primary focus of attention. J Cogn Neurosci 22:1980–1992 [DOI] [PubMed] [Google Scholar]
- Hyafil A, Summerfield C, Koechlin E (2009) Two mechanisms for task switching in the prefrontal cortex. J Neurosci 29:5135–5142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O (2007) Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci 10:240–248 [DOI] [PubMed] [Google Scholar]
- Jamadar S, Hughes M, Fulham WR, Michie PT, Karayanidis F (2010a) The spatial and temporal dynamics of anticipatory preparation and response inhibition in task-switching. Neuroimage 51:432–449 [DOI] [PubMed] [Google Scholar]
- Jamadar S, Michie P, Karayanidis F (2010b) Compensatory mechanisms underlie intact task-switching performance in schizophrenia. Neuropsychologia 48:1305–1323 [DOI] [PubMed] [Google Scholar]
- Jiang Y (2004) Resolving dual-task interference: an fMRI study. Neuroimage 22:748–754 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Saxe R, Kanwisher N (2004) Functional magnetic resonance imaging provides new constraints on theories of the psychological refractory period. Psychol Sci 15:390–396 [DOI] [PubMed] [Google Scholar]
- Jost K, De Baene W, Koch I, Brass M (2013) A review of the role of cue processing in task switching. J Psychol 221:5–14 [Google Scholar]
- Kahneman D (1973) Attention and effort. Prentice Hall, New York [Google Scholar]
- Karayanidis F, Coltheart M, Michie PT, Murphy K (2003) Electrophysiological correlates of anticipatory and poststimulus components of task switching. Psychophysiology 40:329–348 [DOI] [PubMed] [Google Scholar]
- Kieffaber PD, Hetrick WP (2005) Event-related potential correlates of task switching and switch costs. Psychophysiology 42:56–71 [DOI] [PubMed] [Google Scholar]
- Kiesel A, Steinhauser M, Wendt M, Falkenstein M, Jost K, Philipp AM, Koch I (2010) Control and interference in task switching-a review. Psychol Bull 136:849–874 [DOI] [PubMed] [Google Scholar]
- Kim C, Cilles SE, Johnson NF, Gold BT (2012) Domain general and domain preferential brain regions associated with different types of task switching: a meta-analysis. Hum Brain Mapp 33:130–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, D’Esposito M (2000) Modulation of task-related neural activity in task-switching: an fMRI study. Brain Res Cogn Brain Res 10:189–196 [DOI] [PubMed] [Google Scholar]
- Kliegl R, Mayr U, Krampe RT (1994) Time–accuracy functions for determining process and person differences: an application to cognitive aging. Cogn Psychol 26:134–164 [DOI] [PubMed] [Google Scholar]
- Koch I, Allport A (2006) Cue-based preparation and stimulus-based priming of tasks in task switching. Mem Cogn 34:433–444 [DOI] [PubMed] [Google Scholar]
- Koch I, Gade M, Schuch S, Philipp AM (2010) The role of inhibition in task switching: a review. Psychon Bull Rev 17:1–14 [DOI] [PubMed] [Google Scholar]
- Koch I, Poljac E, Müller H, Kiesel A (2018) Cognitive structure, flexibility, and plasticity in human multitasking: an integrative review of dual-task and task-switching research. Psychol Bull 144:557–583 [DOI] [PubMed] [Google Scholar]
- Koechlin E, Jubault T (2006) Broca’s area and the hierarchical organization of human behavior. Neuron 50:963–974 [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J (1999) The role of the anterior prefrontal cortex in human cognition. Nature 399:148–151 [DOI] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E (2009) Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci 12:939–945 [DOI] [PubMed] [Google Scholar]
- Kubler S, Reimer CB, Strobach T, Schubert T (2017) The impact of free-order and sequential-order instructions on task-order regulation in dual tasks. Psychol Res 82(1):40–53 [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB (2010) A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct 5–6:519–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007) Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 28:1194–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB (2013) Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol Bull 139:870–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Kellermann T, Boers F, Sturm W, Willmes K, Eickhoff SB (2011) Modality-specific perceptual expectations selectively modulate baseline activity in auditory, somatosensory, and visual cortices. Cereb Cortex 21:2850–2862 [DOI] [PubMed] [Google Scholar]
- Langner R, Kellermann T, Eickhoff SB, Boers F, Chatterjee A, Willmes K, Sturm W (2012) Staying responsive to the world: modality-specific and -nonspecific contributions to speeded auditory, tactile, and visual stimulus detection. Hum Brain Mapp 33:398–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Cieslik EC, Behrwind SD, Roski C, Caspers S, Amunts K, Eickhoff SB (2015) Aging and response conflict solution: behavioural and functional connectivity changes. Brain Struct Funct 220:1739–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Leiberg S, Hoffstaedter F, Eickhoff SB (2018) Towards a human self-regulation system: common and distinct neural signatures of emotional and behavioural control. Neurosci Biobehav Rev 90:400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ (2006) Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron 50:643–653 [DOI] [PubMed] [Google Scholar]
- Logan GD, Bundesen C (2003) Clever homunculus: is there an endogenous act of control in the explicit task-cuing procedure? J Exp Psychol Hum Percept Perform 29:575–599 [DOI] [PubMed] [Google Scholar]
- Logan GD, Gordon RD (2001) Executive control of visual attention in dual-task situations. Psychol Rev 108:393–434 [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Feiwell RJ, Miller WL (2002) Evidence for anterior cingulate cortex involvement in monitoring preparatory attentional set. Neuroimage 17:792–802 [PubMed] [Google Scholar]
- Luria R, Meiran N (2003) Online order control in the psychological refractory period paradigm. J Exp Psychol Hum Percept Perform 29:556–574 [DOI] [PubMed] [Google Scholar]
- Madden DJ, Costello MC, Dennis NA, Davis SW, Shepler AM, Spaniol J, Bucur B, Cabeza R (2010) Adult age differences in functional connectivity during executive control. Neuroimage 52:643–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois R, Ivanoff J (2005) Capacity limits of information processing in the brain. Trends Cogn Sci 9:296–305 [DOI] [PubMed] [Google Scholar]
- Marois R, Larson JM, Chun MM, Shima D (2005) Response-specific sources of dual-task interference in human premotor cortex. Psychol Res 11:1–12 [DOI] [PubMed] [Google Scholar]
- Matelli M, Camarda R, Glickstein M, Rizzolatti G (1986) Afferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol 251:281–298 [DOI] [PubMed] [Google Scholar]
- Mayr U, Kliegl R (1993) Sequential and coordinative complexity: age-based processing limitations in figural transformations. J Exp Psychol Learn Mem Cogn 19:1297–1320 [DOI] [PubMed] [Google Scholar]
- Mayr U, Kliegl R, Krampe R (1996) Sequential and coordinative processing dynamics in figural transformation across the life span. Cognition 59:61–90 [DOI] [PubMed] [Google Scholar]
- Meiran N (2000) Modeling cognitive control in task-switching. Psychol Res 63:234–249 [DOI] [PubMed] [Google Scholar]
- Meiran N, Chorev Z, Sapir A (2000) Component processes in task switching. Cogn Psychol 41:211–253 [DOI] [PubMed] [Google Scholar]
- Meyer DE, Kieras DE (1997a) A computational theory of executive cognitive processes and multiple-task performance: I. Basic mechanisms. Psychol Rev 104:3–65 [DOI] [PubMed] [Google Scholar]
- Meyer DE, Kieras DE (1997b) A computational theory of executive cognitive processes and multiple-task performance: 2. Accounts of the psychological refractory-period phenomena. Psychol Rev 104:749–791 [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202 [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Tashiro M, Gyoba J, Suzuki M, Okamura N, Itoh M, Yanai K (2007) Brain activity associated with dual-task management differs depending on the combinations of response modalities. Brain Res 1172:82–92 [DOI] [PubMed] [Google Scholar]
- Monsell S (2003) Task switching. Trends Cogn Sci 7:134–140 [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ (2008) Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci 20:751–761 [DOI] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H (2000) Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol 83:2580–2601 [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M (2008) Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9:856–869 [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Yamagata T, Tanji J, Hoshi E (2008) Transformation of a virtual action plan into a motor plan in the premotor cortex. J Neurosci 8:10287–10297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon D, Miller J (2002) Queuing or sharing? A critical evaluation of the single-bottleneck notion. Cogn Psychol 44:193–251 [DOI] [PubMed] [Google Scholar]
- Nee DE, Kastner S, Brown JW (2011) Functional heterogeneity of conflict, error, task-switching, and unexpectedness effects within medial prefrontal cortex. Neuroimage 54:528–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JK, Reuter-Lorenz PA, Persson J, Sylvester CY, Jonides J (2009) Mapping interference resolution across task domains: a shared control process in left inferior frontal gyrus. Brain Res 1256:92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB (2005) Valid inference with the minimum statistic. Neuroimage 25:653–660 [DOI] [PubMed] [Google Scholar]
- Nicholson R, Karayanidis F, Poboka D, Heathcote A, Michie PT (2005) Electrophysiological correlates of anticipatory task-switching processes. Psychophysiology 42:540–554 [DOI] [PubMed] [Google Scholar]
- Pashler H (1994) Dual-task interference in simple tasks: data and theory. Psychol Bull 116:220–244 [DOI] [PubMed] [Google Scholar]
- Pashler H (2000) Task switching and multitask performance In: Monsell S, Driver J (eds) Attention and performance XVIII: control of mental processes. MIT Press, Cambridge, pp 277–307 [Google Scholar]
- Pastor-Bernier A, Tremblay E, Cisek P (2012) Dorsal premotor cortex is involved in switching motor plans. Front Neuroeng 5:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T (1996) Location and function of the human frontal eyefield: a selective review. Neuropsychologia 34:475–483 [DOI] [PubMed] [Google Scholar]
- Paus T (2001) Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2:417–424 [DOI] [PubMed] [Google Scholar]
- Petrides M (1997) Visuo-motor conditional associative learning after frontal and temporal lesions in the human brain. Neuropsychologia 7:989–997 [DOI] [PubMed] [Google Scholar]
- Philipp AM, Weidner R, Koch I, Fink G (2013) Differential roles of inferior frontal and inferior parietal cortex in task switching: evidence from stimulus-categorization switching and responsemodality switching. Hum Brain Mapp 34:1919–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet C, Sterpenich V, Desseilles M, Cojan Y, Bertschy G, Vuilleumier P (2013) Neural substrates of cognitive switching and inhibition in a face processing task. Neuroimage 82:489–499 [DOI] [PubMed] [Google Scholar]
- Poulsen C, Luu P, Davey C, Tucker DM (2005) Dynamics of task sets: evidence from dense-array event-related potentials. Brain Res Cogn Brain Res 24:133–154 [DOI] [PubMed] [Google Scholar]
- Ptak R (2011) The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. Neuroscientist 18:502–515 [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Carter CS (2008) Shifting set about task switching: behavioral and neural evidence for distinct forms of cognitive flexibility. Neuropsychologia 46:2924–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G (2001) The cortical motor system. Neuron 31:889–901 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M (1998) The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol 106:283–296 [DOI] [PubMed] [Google Scholar]
- Rodehacke S, Mennigen E, Müller KU, Ripke S, Jacob MJ, Hübner T, Schmidt DH, Goschke T, Smolka MN (2014) Interindividual differences in mid-adolescents in error monitoring and post-error adjustment. PLoS One 9:e88957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Monsell S (1995) The costs of a predictable switch between simple cognitive tasks. J Exp Psychol Gen 124:207–231 [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB (2012) Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage 60:830–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein J, Meyer DE, Evans JE (2001) Executive control of cognitive processes in task switching. J Exp Psychol Hum Percept Perform 27:763–797 [DOI] [PubMed] [Google Scholar]
- Ruge H, Brass M, Koch I, Rubin O, Meiran N, von Cramon DY (2005) Advance preparation and stimulus-induced interference in cued task switching: further insights from BOLD fMRI. Neuropsychologia 43:340–355 [DOI] [PubMed] [Google Scholar]
- Ruge H, Braver T, Meiran N (2009) Attention, intention, and strategy in preparatory control. Neuropsychologia 47:1670–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruge H, Müller SC, Braver TS (2010) Anticipating the consequences of action: an fMRI study of intention-based task preparation. Psychophysiology 47:1019–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruge H, Jamadar S, Zimmermann U, Karayanidis F (2013) The many faces of preparatory control in task switching: reviewing a decade of fMRI research. Hum Brain Mapp 34:12–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Taylor PC (2006) TMS in the parietal cortex: updating representations for attention and action. Neuropsychologia 44:2700–2716 [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Paus T, Sipila PK (2001) Attention systems and the organization of the human parietal cortex. J Neurosci 15:5262–5271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK (2002) Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol 87:2577–2592 [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen-Berg H, Göbel SM, Devlin JT (2003) The left parietal and premotor cortices: motor attention and selection. Neuroimage 20(Suppl 1):89–100 [DOI] [PubMed] [Google Scholar]
- Sadato N, Yonekura Y, Waki A, Yamada H, Ishii Y (1997) Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. J Neurosci 17:9667–9674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savine AC, Braver TS (2010) Motivated cognitive control: reward incentives modulate preparatory neural activity during task-switching. J Neurosci 30:10294–10305 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Scheperjans F, Eickhoff SB, Homke L, Mohlberg H, Hermann K, Amunts K, Zilles K (2008a) Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex 18:2141–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K (2008b) Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex 18:846–867 [DOI] [PubMed] [Google Scholar]
- Schubert T (1999) Processing differences between simple and choice reactions affect bottleneck localization in overlapping tasks. J Exp Psychol Hum Percept Perform 25:408–425 [Google Scholar]
- Schubert T, Szameitat AJ (2003) Functional neuroanatomy of interfereence in overlapping dual tasks: an fMRI study. Brain Res Cogn Brain Res 17:733–746 [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY (2003) Functional–anatomical concepts of human premotor cortex: evidence from fMRI and PET studies. Neuroimage 20:120–131 [DOI] [PubMed] [Google Scholar]
- Schuch S, Koch I (2003) The role of response selection for inhibition of task sets in task shifting. J Exp Psychol Hum Percept Perform 29:92–105 [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Elston PA, D’Esposito M (2003) Neural evidence for representation-specific response selection. J Cogn Neurosci 15:1111–1121 [DOI] [PubMed] [Google Scholar]
- Shi Y, Zhou X, Müller HJ, Schubert T (2010) The neural implementation of task rule activation in the task-cuing paradigm: an event-related fMRI study. Neuroimage 51:1253–1264 [DOI] [PubMed] [Google Scholar]
- Shomstein S, Yantis S (2004) Control of attention shifts between vision and audition in human cortex. J Neurosci 24:10702–10706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Franke D, Pope DL, Snyder AZ, McAvoy MP, Corbetta M (2009) Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia cortical networks. J Neurosci 29:4392–4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman M, Dehaene S (2006) Dynamics of the central bottleneck: dual-task and task uncertainty. PLoS Biol 4:e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman M, Dehaene S (2008) Brain mechanisms of serial and parallel processing during dual-task performance. J Neurosci 28:7585–7598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Rubia K (2004) Neural correlates of switching set as measured in fast, event-related functional magnetic resonance imaging. Hum Brain Mapp 21:247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS (2000) The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA 97:13448–13453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutschek A, Taylor PC, Müller HJ, Schubert T (2013) Dissociable networks control conflict during perception and response selection: a transcranial magnetic stimulation Study. J Neurosci 33:5647–5654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark A, Zohary E (2008) Parietal mapping of visuomotor transformations during human tool grasping. Cereb Cortex 18:2358–2368 [DOI] [PubMed] [Google Scholar]
- Stelzel C, Schumacher EH, Schubert T, D’Esposito M (2006) The neural effect of stimulus–response modality compatibility on dual-task performance: an fMRI study. Psychol Res 70:514–525 [DOI] [PubMed] [Google Scholar]
- Stelzel C, Kraft A, Brandt SA, Schubert T (2008) Dissociable neural effects of task order control and task set maintenance during dual-task performance. J Cogn Neurosci 20:613–628 [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson G, Calhoun VD (2007) Functional neural circuits for mental timekeeping. Hum Brain Mapp 28:394–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, Macdonald R, Borowiec A, Katz DI (2005) Multiple frontal systems controlling response speed. Neuropsychologia 43:396–417 [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU (2008) Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci 9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szameitat AJ, Schubert T, Müller K, Von Cramon DY (2002) Localization of executive functions in dual-task performance with fMRI. J Cogn Neurosci 14:1184–1199 [DOI] [PubMed] [Google Scholar]
- Szameitat AJ, Lepsien J, von Cramon DY, Sterr A, Schubert T (2006) Task-order coordination in dual-task performance and the lateral prefrontal cortex: an event-related fMRI study. Psychol Res 70:541–552 [DOI] [PubMed] [Google Scholar]
- Tombu M, Jolicoeur P (2003) A central capacity sharing model of dual-task performance. J Exp Psychol Hum Percept Perform 29:3–18 [DOI] [PubMed] [Google Scholar]
- Tombu MN, Asplund CL, Dux PE, Godwin D, Martin JW, Marois R (2011) A Unified attentional bottleneck in the human brain. Proc Natl Acad Sci USA 108:13426–13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosoni A, Shulman GL, Pope AL, McAvoy MP, Corbetta M (2012) Distinct representations for shifts of spatial attention and changes of reward contingencies in the human brain. Cortex 49:1733–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002) Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16:765–780 [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P (2012) Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp 33:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Danielmeier C, Jocham G (2014) Neurophysiology of performance monitoring and adaptive behavior. Physiol Rev 94:35–79 [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Gillebert CR (2009) Parcellation of parietal cortex: convergence between lesion-symptom mapping and mapping of the intact functioning brain. Behav Brain Res 199:171–182 [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Stevens MA, Chambers CD (2010) Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc Natl Acad Sci USA 107:13966–13971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Steitz DW, Sliwinski MJ, Cerella J (2003) Aging and dual-task performance: a meta-analysis. Psychol Aging 18:443–460 [DOI] [PubMed] [Google Scholar]
- Vetter P, Butterworth B, Bahrami B (2011) A candidate for the attentional bottleneck: set-size specific modulation of the right TPJ during attentive enumeration. J Cogn Neurosci 23:728–736 [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S (2004) Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage 22:1679–1693 [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Smith EE, Nichols TE (2005) Toward a taxonomy of attention shifting: individual differences in fMRI during multiple shift types. Cogn Affect Behav Neurosci 5:127–143 [DOI] [PubMed] [Google Scholar]
- Wasylyshyn C, Verhaeghen P, Sliwinski MJ (2011) Aging and task switching: a meta-analysis. Psychol Aging 26:15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford A (1952) The ‘psychological refractory period’ and the timing of high-speed performance: a review and a theory. Br J Psychol 43:2–9 [Google Scholar]
- Wilkinson DT, Halligan PW, Marshall JC, Büchel C, Dolan RJ (2001) Switching between the forest and the trees: brain systems involved in local/global changed-level judgments. Neuroimage 13:56–67 [DOI] [PubMed] [Google Scholar]
- Witt ST, Stevens MC (2012) Overcoming residual interference in mental set switching: neural correlates and developmental trajectory. Neuroimage 62:2055–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ST, Stevens MC (2013) fMRI task parameters influence hemodynamic activity in regions implicated in mental set switching. Neuroimage 65:139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock KA, Humphreys GW, Oliver C, Hansen PC (2010) Neural correlates of task switching in paternal 15q11-q13 deletion Prader–Willi syndrome. Brain Res 1363:128–142 [DOI] [PubMed] [Google Scholar]
- Wylie G, Allport A (2000) Task switching and the measurement of “switch costs”. Psychol Res 63:212–233 [DOI] [PubMed] [Google Scholar]
- Wylie GR, Javitt DC, Foxe JJ (2006) Jumping the gun: is effective preparation contingent upon anticipatory activation in task-relevant neural circuitry? Cereb Cortex 16:394–404 [DOI] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM (2002) Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci 5:995–1002 [DOI] [PubMed] [Google Scholar]
- Yeung N, Nystrom LE, Aronson JA, Cohen JD (2006) Between-task competition and cognitive control in task switching. J Neurosci 26:1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida W, Funakoshi H, Ishii S (2010) Hierarchical rule switching in prefrontal cortex. Neuroimage 50:314–322 [DOI] [PubMed] [Google Scholar]
- zu Eulenburg P, Caspers S, Roski C, Eickhoff SB (2012) Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage 60:162–169 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.