Abstract
Background
Physalis L. is a genus of herbaceous plants of the family Solanaceae, which has important medicinal, edible, and ornamental values. The morphological characteristics of Physalis species are similar, and it is difficult to rapidly and accurately distinguish them based only on morphological characteristics. At present, the species classification and phylogeny of Physalis are still controversial. In this study, the complete chloroplast (cp) genomes of four Physalis species (Physalis angulata, P. alkekengi var. franchetii, P. minima and P. pubescens) were sequenced, and the first comprehensive cp genome analysis of Physalis was performed, which included the previously published cp genome sequence of Physalis peruviana.
Results
The Physalis cp genomes exhibited typical quadripartite and circular structures, and were relatively conserved in their structure and gene synteny. However, the Physalis cp genomes showed obvious variations at four regional boundaries, especially those of the inverted repeat and the large single-copy regions. The cp genomes’ lengths ranged from 156,578 bp to 157,007 bp. A total of 114 different genes, 80 protein-coding genes, 30 tRNA genes, and 4 rRNA genes, were observed in four new sequenced Physalis cp genomes. Differences in repeat sequences and simple sequence repeats were detected among the Physalis cp genomes. Phylogenetic relationships among 36 species of 11 genera of Solanaceae based on their cp genomes placed Physalis in the middle and upper part of the phylogenetic tree, with a monophyletic evolution having a 100% bootstrap value.
Conclusion
Our results enrich the data on the cp genomes of the genus Physalis. The availability of these cp genomes will provide abundant information for further species identification, increase the taxonomic and phylogenetic resolution of Physalis, and assist in the investigation and utilization of Physalis plants.
Keywords: Physalis, Chloroplast genome, Molecular markers, Species identification, Phylogenetic relationship
Background
The genus Physalis L., consisting of 75–120 species, is a well-known genera of the family Solanaceae because of its significant economic value, owing to the medicinal, edible and ornamental uses of its members [1–3]. It is mainly distributed in the tropical and temperate Americas, with only few species are found in Eurasia and Southeast Asia [1, 4–6]. China has approximately five species and two varieties of Physalis plants, which were used as medicinal herbs for more than 2000 years by the Chinese people. Many Physalis species have a variety of pharmacological activities, leading to anti-inflammatory, anti-oxidant, and anti-cancer benefits, and are used to treat many illnesses, including malaria, rheumatism, hepatitis, asthma, cancer, and liver disorders [2, 7–11]. The Pharmacopoeia of the People’s Republic of China included Physalis alkekengi var. franchetii as a standard Physalis medical plant in 2015 [7]. Moreover, many Physalis species, such as P. pubescens, P. peruviana, P. alkekengi var. franchetii, and P. philadelphica, are cultivated in many regions of the world for their edible fruit or as ornamental plants [4, 12].
The chloroplast (cp) is an important organelle in plant cells and plays an important role in many plant cell functions, such as photosynthesis, carbon fixation, and stress response [13, 14]. In most plants, the cp genome’s structure is very conservative, being circular with a length of 120–170 kb, including four typical areas: two inverted repeats (IRs), large single-copy (LSC) and small single-copy (SSC) regions [15]. In a cp genome, the gene content and gene composition are highly conserved, generally containing 120–130 genes [16]. In addition, the evolutionary rate of a cp genome is usually slow compared with nuclear DNA sequence [17]. However, some significant structural genomic changes, including gene losses, large inversions, and contraction or expansion of IR regions, have been observed during the evolution of the cp genomes of some angiosperms [16–18]. For example, the infA, rpl22, rpl33, rps16, ycf1, ycf2, ycf4 and accD genes have been lost in some plant species [16, 19–21]. Furthermore, the IR regions of some species, such as Pisum sativum [22], Glycine max [23], Crytomeria japonica [24], Taxus chinensis var. mairei [25], and Vigna radiata [26] showed complete or partial losses. These cp genomic differences may be the results of differential indels and substitutions rates during the evolution of plant species [27]. Owing to the conserved structure, moderate evolutionary rates, and uniparental inheritance of cp genomes, the sequences are often used as genetic markers for DNA barcoding, and phylogenetic and evolutionary studies [17, 28–30].
In recent years, because of their various significant commercial values, the taxonomy of Physalis has become a concern, and its characterization is regarded as one of the most challengingly in Solanaceae [1, 3, 31, 32]. Traditionally, the genus Physalis was divided into species groups by morphological and/or geographical characters, such as habit, hair type, and number of calyx angles [5, 31]. Lately, with the raise of molecular taxonomy, the ribosomal internal transcribed spacer (ITS) 1 and ITS2, chloroplast ndhF, trnL-F and psbA-trnH sequences, and Waxy genes, have been used in species identification and phylogenetic analyses of Physalis, as well as to determine their relationship to other genera in the Solanaceae family [1, 3, 32, 33]. In addition, some DNA marker systems, including simple sequence repeat (SSR), inter-simple sequence repeats, and sequence-characterized amplified region markers, have been used in the genetic study of Physalis plants [4, 12, 34, 35]. However, owing to the limited information on these traditional genetic markers, there are still some controversies regarding the species identification and taxonomy of Physalis [3, 28]. The application and development of the cp genome in plant phylogenetic studies provide a new research idea for the better study of phylogenetic classification of Physalis. Advances in next-generation sequencing techniques have facilitated rapid progress in the field of cp genomics [36, 37]. By September 2019, more than 3000 complete cp genome sequences, including P. peruviana (GenBank accession number: NC_026570) as sole representative of Physalis genus without further analysis or study, were released into the National Center for Biotechnology Information (NCBI) organelle genome database (https://www.ncbi.nlm.nih.gov/genome/organelle/).
Here, we sequenced the cp genomes of four Physalis species (P. angulata, P. alkekengi var. franchetii, P. minima and P. pubescens), and performed an in deep analysis of the genomes, representing the first comprehensive analysis of cp genomes of Physalis, including the previously released P. peruviana cp genome. Our study’s aims were: (1) to present the complete cp genome sequences of four Physalis species; (2) to characterize and compare the global structural patterns of available Physalis cp genomes; (3) to examine variations in the SSRs and repeat sequences among the five Physalis cp genomes; and (4) to improve our understanding of the evolutionary and systematics positions of the genus Physalis within Solanaceae based on their cp genome sequences.
Results
Overall genome sequencing and assembly
Total genomic DNA was extracted from ~ 0.1 g of a six individuals pool of healthy, clean and fresh leaves per each Physalis species (Additional File 1: Table S1), and used to generate the corresponding Illumina MiSeq libraries by long-range PCR (see Methods section). After Illumina sequencing (paired-end, 250x), reads were QC filtered, mapped against P. peruviana cp reference genome (NC_026570) and assembled to obtain the four complete cp genomes. Clean bases mapped to the P. peruviana cp reference genome, with mean coverages ranging from 480x to 1756x (Additional File 1: Table S2).
Physalis cp genome features
The full length of Physalis cp genomes ranged from 156,578 bp (P. alkekengi var. franchetii) to 157,007 bp (P. pubescens) (Table 1). The gene maps of the newly sequenced Physalis cp genomes were provided in Fig. 1 (P. angulata) and in Additional File 2: Fig. S1–S3 (P. alkekengi var. franchetii, P. minima and P. pubescens). Like most angiosperms, the Physalis cp genomes also exhibited the typical quadripartite structure, distributed in one LSC region (86,845 bp–88,309 bp), one SSC region (18,363 bp–18,503 bp), and a pair of IR regions (A and B; 24,953 bp–25,685 bp). The overall GC content of each cp genome was comparable, ranging from 37.52 to 37.65%. Whereas the GC content was distributed differentially between each region, showing greater GC content at IR regions than in the LSC or SSC (Table 1). Compared with P. peruviana, the new cp genomes contained 2 more genes each (total genes 132 vs 130), some of them found in duplicate generally located at the IR regions (see Table 1). When duplicated genes in the IR regions were counted only once, each of the new four cp genomes (P. angulata, P. alkekengi var. franchetii, P. minima, and P. pubescens) contained the same 114 genes, distributed as 80 protein-coding genes, 4 rRNA genes, and 30 tRNA genes. While the P. peruviana cp genome contained only 113 genes, missing a protein-coding gene. These 114/113 genes encode for self-replication-related functions, photosynthesis-related, and other proteins, and as well as unknown proteins (Table 2). Of these 114/113 genes, 17 are intron-containing genes, 15 that contain one intron (rpl2, rpl16, rpoC1, rps12, rps16, trnA-UGC, trnG-GCC, trnI-GAU, trnK-UUU, trnL-UAA, trnV-UAC, atpF, ndhA, ndhB, and petB) and two that contain two introns (clpP and ycf3).
Table 1.
Summaries of complete chloroplast genomes of five Physalis species
| P. angulata | P. alkekengi var. franchetii | P. minima | P. pubescens | P. peruviana | |
|---|---|---|---|---|---|
| Genome size (bp) | 156,905 | 156,578 | 156,692 | 157,007 | 156,706 |
| Large single copy (LSC, bp) | 87,108 | 88,309 | 86,845 | 87,137 | 86,995 |
| Small single copy (SSC, bp) | 18,469 | 18,363 | 18,503 | 18,500 | 18,393 |
| Inverted repeat (IR, bp) | 25,664 | 24,953 | 25,672 | 25,685 | 25,659 |
| GC content (%) | |||||
| Total genome | 37.52 | 37.65 | 37.54 | 37.53 | 37.54 |
| LSC | 35.58 | 35.76 | 35.60 | 35.59 | 35.57 |
| SSC | 31.32 | 31.72 | 31.40 | 31.35 | 31.36 |
| IR | 43.05 | 43.20 | 43.03 | 43.06 | 43.08 |
| Gene (total /different) | 132/114 | 132/114 | 132/114 | 132/114 | 130/113 |
| genes duplicated in IR | 18 | 18 | 18 | 18 | 17 |
| protein-coding genes (total/in IR) | 87/7 | 87/7 | 87/7 | 87/7 | 85/6 |
| rRNA (total/different) | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 |
| tRNA (total/different) | 37/30 | 37/30 | 37/30 | 37/30 | 37/30 |
| GenBank accession | MH045574 | MH045575 | MH045577 | MH045576 | NC_026570 |
| References | This study | This study | This study | This study | Genbank |
Fig. 1.
Gene map of the P. angulata chloroplast genome. Genes shown outside the outer circle are transcribed clockwise, and those inside are transcribed counterclockwise. Genes belonging to different functional groups are color coded. The darker gray in the inner circle indicates the GC content, and the lighter gray indicates the AT content. The inner circle also indicates that the chloroplast genome contains two copies of the inverted repeat (IRA and IRB), a large single-copy region (LSC) and a small single-copy region (SSC). The map was constructed using OrganellarGenomeDRAW
Table 2.
Genes in the Physalis chloroplast genomes
| Category for genes | Group of genes | Name of genes |
|---|---|---|
| Self-replication | Large subunit of ribosome | ①*rpl2(×2), rpl14, *rpl16, rpl20, rpl22, rpl23(× 2), rpl32, rpl33, rpl36 |
| DNA dependent RNA polymerase | rpoA, rpoB, *rpoC1, rpoC2 | |
| Small subunit of ribosome | rps2, rps3, rps4, rps7(×2), rps8, rps11, *rps12(× 2), rps14, rps15, *rps16, rps18, rps19 | |
| rRNA Genes | rrn4.5S(×2), rrn5S(× 2), rrn16S(× 2), rrn23S(× 2) | |
| tRNA Genes | *trnA-UGC(×2), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnfM-CAU, *trnG-GCC, trnG-UCC, trnH-GUG, *trnI-GAU(× 2), trnI-CAU(× 2), *trnK-UUU, trnL-CAA(× 2), *trnL-UAA, trnL-UAG, trnM-CAU, trnN-GUU(× 2), trnP-UGG, trnQ-UUG, trnR-ACG(× 2), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC(× 2), *trnV-UAC, trnW-CCA, trnY-GUA | |
| Photosynthesis | Subunits of ATP synthase | atpA, atpB, atpE, *atpF, atpH, atpI |
| Subunits of NADH-dehydrogenase | *ndhA, *ndhB(×2), ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| Subunits of cytochrome b/f complex | petA, *petB, petD, petG, petL, petN | |
| Subunits of photosystem I | psaA, psaB, psaC, psaI, psaJ, ycf4 | |
| Subunits of photosystem II | psbA, ②psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, ③psbZ | |
| Subunit of rubisco | rbcL | |
| Other genes | LhbA | ④lhbA |
| Subunit of Acetyl-CoA-carboxylase | accD | |
| c-type cytochrom synthesis gene | ccsA | |
| Envelop membrane protein | cemA | |
| Protease | **clpP | |
| Translational initiation factor | infA | |
| Maturase | matK | |
| Unknown function | Conserved open reading frames | ycf1, ycf2(×2), **ycf3, ycf15(×2) |
Note: (×2): Two gene copies in IRs; *: gene containing a single intron; **: gene containing two introns; ①:One copy of rpl2 gene is missing in the chloroplast genome of P. peruviana; ②:psbB gene is missing in the chloroplast genome of P. peruviana; ③:psbZ gene exists only in chloroplast of P. minima; ④: lhbA gene is missing in the chloroplast genome of P. minima
Codon usage in Physalis cp genomes
After alignment of the five Physalis cp genomes in MEGA, a total of 20 amino acids were found encoded with differential usage depending on the trnL codons. Methionine and tryptophan only presented one trnL each. Whereas phenylalanine, tyrosine, histidine, glutamine, asparagine, lysine, aspartic acid, glutamic acid, and cysteine were encoded by two synonymous codons (Additional File 1: Table S3 and Additional File 2: Fig. S4).
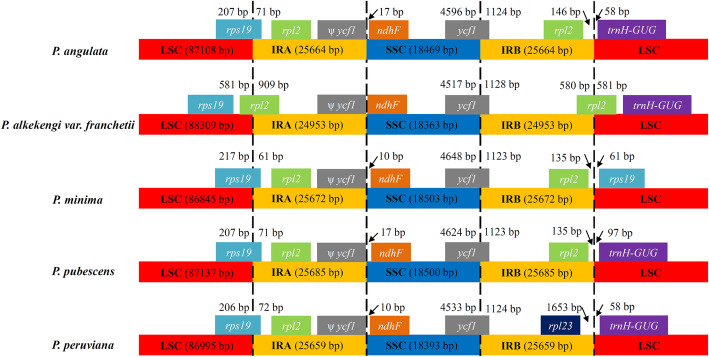
IR expansion and contraction
The IR regions (A and B) of the five Physalis cp genomes are the most conserved regions, being 24,953 to 25,685 bp in length. However, there are potential expansions and contractions of IR borders, which are considered to be evolutionary events and the main cause of cp genome length changes. The LSC/IR and SSC/IR borders of the Physalis cp genomes were compared (Fig. 2). The rps19, rpl2, rpl23 and trnH-GUG genes were mainly distributed near the LSC/IR border, while ycf1 and ndhF genes were distributed near the SSC/IR border. The gene ycf1 crossed the SSC/IRB region, and the pseudogene fragmentψycf1 was located at the IR-A region, near the SSC/IR-A border. Compared with the SSC/IR border, the LSC/IR border displayed a large variation. In P. alkekengi var. franchetii, the rps19 gene was located completely in the LSC region. However, the rps19 genes of P. angulata, P. minima, P. pubescens, and P. peruviana extended into the IRA region by 71, 61, 71, and 72 bp, respectively. There were two copies of the rpl2 genes in P. angulata, P. minima, and P. pubescens, and they were located in the IR-A and IRB regions, near the LSC/IR borders. In P. alkekengi var. franchetii, the two copies of the rpl2 gene span the LSC/IRA and LSC/IRB borders, respectively. One copy of the rpl2 gene was missing at the LSC/IRB border in P. peruviana; instead, there was a rpl23 gene at 1653 bp in the IRB region of the LSC/IRB border.
Fig. 2.
Comparisons of the borders of LSC, SSC, and IR regions among five Physalis chloroplast genomes
Genomes sequence divergence among Physalis species
The complete cp genomes of the five Physalis species were compared and plotted using mVISTA software by aligning the four cp genomes with the reference P. angulata, to elucidate the levels of sequence divergence (Fig. 3). LSC and SSC regions had higher sequence divergences than the IR regions. The sequence divergence in the coding region was limited, and most of the sequence divergence was concentrated in the non-coding region. At the genome level, the genetic distances among the five Physalis species ranged from 0.0007 to 0.0048, and the average genetic distance was just 0.0029 (Additional file 1: Table S4).
Fig. 3.
Comparative plots based on sequence identity of chloroplast genome of Physalis species, using P. angulata as the reference genome (upper plot). Plots were constructed with mVISTA software. Chloroplast coding regions are indicated in blue and non-coding regions in red, notice the reduction in sequence identity by reduction of the blue/red shadowing (white spaces)
Repeat sequences and SSR analysis
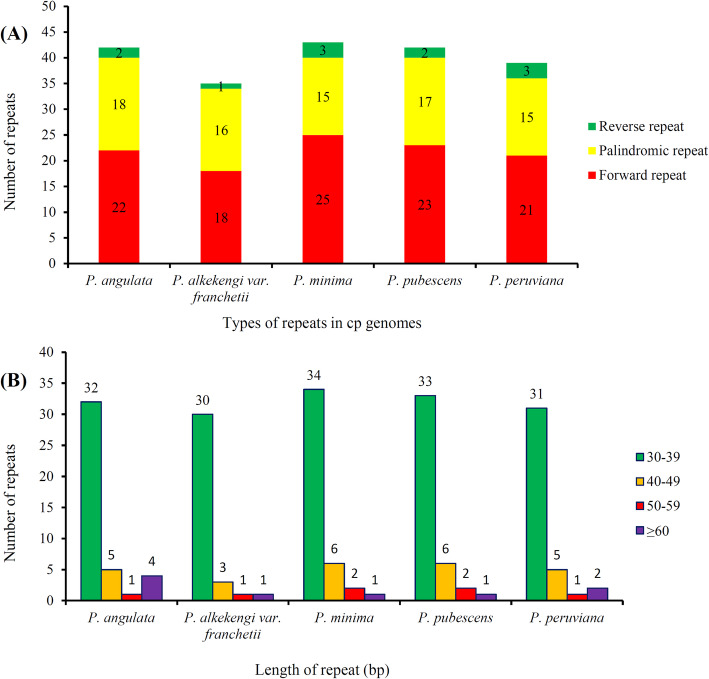
REPuter was used to analyze the repeat sequences in each cp genome. A total of 201 repeat sequences were identified, including 109 forward repeats, 81 palindromic repeats, and 11 reverse repeats of at least 30 bp per repeat unit with a sequence identity ≥90% (Fig. 4). The distribution of repeats per genome, and length of repeat and number of such repeated sequences per species are shown in Fig. 4 a and b, respectively.
Fig. 4.
Repeated sequences in five Physalis chloroplast genomes. a Total of three repeat types in five Physalis chloroplast genomes; b Numbers of repeat sequences by length
The SSRs, which usually consist of a series repeat units of 1–6 bp in length (labelled as mono- to hexa- mer in Fig. 5a), were distributed throughout the genome. In total 286 SSRs, with lengths of at least 10 bp, were detected, with a distribution ranging from 51 to 61 SSRs per genome (Fig. 5a). The majority of these SSRs were mononucleotides (poly-A or poly-T mainly), with 30–40 members in each cp genome (Fig. 5b–f). Only dinucleotides AT or TA were found in all species, and the sole hexanucleotide (TTTTTA) was detected only in P. peruviana (Fig. 5f). Trinucleotides (AAG, ACT, TAA, TTA, and/or TTC), tetranucleotides (AAAC, AATA, CTAT, CTTA, TTTA, and/or TTTG) and pentanucleotide SSRs (AATTG and/or AAATA), were found with a specific distribution, that may be used for future population studies (Fig. 5b to f).
Fig. 5.
SSR loci analysis of five Physalis chloroplast genomes. a Numbers of different SSRs types detected in the five genomes; b–e: Frequency rates of identified SSR motifs in different repeat class types
Phylogenetic analysis
To examine the phylogenetic positions of the five Physalis species and their relationships within Solanaceae, ML and NJ phylogenetically analyses were performed using 38 complete cp genomes from 36 species belonging to 11 genera of Solanaceae. Phylogenetic reconstruction by ML and NJ (Fig. 6 and Additional File 2: Fig. S5) divided all species into six groups (I to VI), with slightly differences based bootstrap support (BS) values for each tree topology. Group I was the most complex, with 12 species, and was further divided into two subgroups. One subgroup containing the five species Physalis species studied in this work (I-2, BS = 100%), with P. alkekengi var. franchetii as basal species. And the second sub-group (I-1, BS = 100%) with species from Iochroma, Dunalia, Saracha, and Vassobia genera. Group II contained four Capsicum species (C. annuum, C. annuum var. glabriusculum, C. lycianthoides and C. frutescens) in both the ML and NJ phylogenetic trees with 100% bootstrap values. Group III included 13 Solanum species (BS = 100% for both ML and NJ trees). Datura stramonium clustered into Group IV in both ML and NJ phylogenetic trees with 100% bootstrap values. Group V included Atropa belladonna and Hyoscyamus niger. The four Nicotiana species, N. sylvestris, N. tomentosiformis, N. undulate, and N. tabacum, were distant from any other Solanaceae species and were assigned into group VI (BS = 100% for both ML and NJ trees).
Fig. 6.
Maximum-likelihood (ML) tree based on the complete chloroplast genome sequences for 36 species of Solanaceae. Numbers above branches indicate bootstrap support, and circled by the red dotted lines are the five Physalis species
Discussion
Cp genome structure and sequence differences
In this study, four Physalis cp genomes were obtained using Illumina MiSeq and were compared with the published cp genome of P. peruviana. Illumina MiSeq is a next-generation sequencer that integrates amplification, sequencing, and data analysis on a single instrument, and was released by Illumina in 2011 [38]. Illumina MiSeq is quite closely matched in terms of utility and ease of workflow, and has good applications for the chloroplast genome sequencing [17, 39]. The comparative analysis of the five Physalis cp genomes showed highly conserved genes and structures. Like those of most angiosperms, the cp genomes of the five Physalis have a quadripartite structure that is typically composed of one LSC, one SSC, and two IR regions [15, 40]. The sizes of the cp genomes of P. angulata, P. alkekengi var. franchetii, P. minima, P. pubescens, and P. peruviana ranged from 156,578 bp to 157,007 bp, which suggested that the cp genome length in Physalis is highly conserved. The cp genomes of angiosperms evolve at relatively fast rates, and inversions and gene losses occur during the process of evolution [16]. In terms of gene composition, most of the coding genes, tRNAs and rRNAs of the five Physalis species are the same, but there are also slight differences. For example, the cp genome of P. minima has an additional psbZ gene, while the lhbA gene is missing, suggesting that gene deletion and insertion have occurred during the evolution of P. minima. In addition, the psbB gene and one copy of the rps12 gene are missing in the cp genome of P. peruviana. In fact, in other plant cp genomes, there have been many reports of the loss of lhbA, infA, rpl22 and rps16, as well as intron and copy deletions of rpl2, clpP and rps12 [20, 41–43].
The cp genomes of land angiosperms are highly conserved, but the expansion and contraction of the IR and SC boundaries are believed to be the main reasons for changes in cp genome size [40, 44]. For example, inversions and/or gene loss events were identified in the cp genome of Astragalus membranaceus [16], and that of Taxus chinensis var. mairei was found to lack a copy of the IR region [25]. Tetracentron cp genomes showed expansion/contraction events in the IR region [45], and those of Veroniceae contained rps19 gene duplications in the IR region [46]. After comparing the cp genomes among the five Physalis species, we found that the boundary region between the SSC and two IR regions was relatively conserved, and the distribution and specific locations of gene types in this region were highly consistent. Compared with the other four Physalis species, the IR region of P. alkekengi var. franchetii showed shrinkage, and its length was the smallest (24,953 bp), mainly because the rpl2 gene located at the LSC/IR boundary expanded the LSC region by 581 bp. In P. peruviana, there was no rpl2 gene at the LSC/IRB boundary, unlike in the other four Physalis species. Additionally, only the rps19 gene of P. minima was found at the LSC/IRA and LSC/IRB boundaries of the five plants, indicating that it was replicated. This phenomenon also found in the cp genome of Veroniceae nakaiana [46]. Therefore, changes in the LSC/IRB boundary appear to be the main contributors to the expansion/contraction of IR regions in Physalis.
Codon usage is a key factor in expressing genetic information correctly [47]. All five Physalis species shared the same codon usage, including 61 amino acid codons (containing one initiation codon, AUG) and three termination codons (UAA, UAG, and UGA). There were differences in the number and types of codons encoding 20 amino acids, and there was preferential codon usage. Most of the preferred amino acid-encoding codons had A or U as the third nucleotide. This phenomenon has been found in many angiosperms, such as Aconitum barbatum var. Puberulum [47], Nicotiana otophora [48] and Oryza minuta [49]. The codon usage frequency was different in other cp genomes, which might be related to the hydrophilicity, synonymy substitution rate, and/or expression level of the codon [50]. Codon preference is closely correlated with the evolutionary pattern of the species. Therefore, the study of codon use is of great value to further understand the historical evolution of the genus Physalis.
In most higher plants, there is less variation in the IR regions than in the SC regions, and this is mainly caused by repeated corrections caused by gene transformations between the two IR regions [40]. The mVISTA results showed that the cp genomes of Physalis had a low degree of sequence divergence, and the conservation of the IR regions was higher than that of the SC region. In addition, the conservation of coding region was higher than that of non-coding region, which was consistent with most cp genomes of higher angiosperms [40].
Repeat sequences and SSR sites
Repeat sequences are useful in phylogenetic studies and play crucial roles in genome recombination [48]. Furthermore, comparative studies of different cp genomes have shown that repeated sequences are important factors causing gene insertion, deletion, and replacement [51, 52]. A repeat analysis of the five Physalis cp genomes detected 201 repeat sequences, most of which are 30–39 bp in length. Among the five Physalis species, P. minima has the largest number of repeated sequences. Genome recombination and sequence variation are mainly caused by slipped-strand mismatches and inappropriate recombinations of repeated sequences [48, 51]. These repeats are the basis of genetic markers for population and phylogenetic studies, being widely used because of their high polymorphism rates among other characteristics [53–56]. In this study, 286 SSR loci were detected, most of them of A/T type as previously reported [56, 57].
Phylogenetic analysis
Owing to the large number of species, similar morphology, and wide distribution areas, Physalis plants are considered to be a relatively complex taxonomic group at both the morphological and molecular levels. Whitson and Manos (2005) used the ITS sequence and Waxy gene to conduct phylogenetic studies on the genus Physalis and its relatives [1]. Many morphological characteristics of Physalis appear to be homoplasious, and several previously defined intrageneric taxa of Physalis are not monophyletic [1]. Olmstead et al. (2008) presented a phylogenic study of Solanaceae, which included the five Physalis species P. heterophylla, P. peruviana, P. philadelphica, P. alkekengi, and P. carpenter, based on the cp DNA regions ndhF and trnL-F [32]. The study indicated that the genus Physalis is closely related to the genera Margaranthus, Chamaesaracha, Quincula, and Oryctes, and that P. alkekengi and P. carpenteri are not monogamous in evolution compared the other three Physalis species. In our previous studies in 2016 and 2018 [3, 33], the ITS2 sequence and cp psbA-trnH region, respectively, were used for the molecular identification and phylogenetic analysis of Physalis species. The conclusions were similar to those obtained by Whitson and Manos (2005) [1] and Olmstead et al. (2008) [32]. A systematic classification of Physalis species should be further explored. These studies have laid an important foundation for the classification and identification of Physalis species. However, the lengths of nuclear/cp gene sequence segments are relatively short, in which limits phylogenetic studies and results in phylogenetic trees that have low support values. Based on whole cp genome sequences, the present study conducted a phylogenetic analysis of 36 species in 11 genera (including the genus Physalis) of Solanaceae. ML and NJ analyses results showed that the tested Physalis species formed a single line in the phylogenetic evolution of Solanaceae (support rate of 100%) and are closely related to other genera, including Iochroma, Dunalia, Saracha, and Vassobia. P. alkekengi var. franchetii was distantly related to the other four Physalis species (support rate of 100%); therefore, we speculated that P. alkekengi var. franchetii differentiated earlier than the other four Physalis species during genetic evolution. To some extent, this result also supports the opinion that P. alkekengi var. franchetii should be classified into a small genus [1, 3]. Of course, only partial cp genomic sequences of Physalis and Solanaceae plants are available at present; therefore, the systematic classification of Physalis species cannot be completed. We plan to obtain more cp genomes of Physalis species using high-throughput sequencing in the future, which will allow us to more accurately analyze the phylogenetic relationships among Physalis species.
Although many studies have shown that the use of cp genomes has advantages in phylogenetic studies, there are still many problems [28]. For example, different species have different evolutionary rates, and for some groups with rapid evolutionary rates, using the whole cp genome information alone cannot completely determine their phylogenetic evolution [58]. In addition, cp DNA is parthenogenetic, and its genomic information can only reflect the evolutionary process of the maternal or paternal line, but it cannot be used to completely interpret the whole systematic evolution of the species itself [59]. Therefore, to better reveal the phylogenetic evolution of Physalis species, in addition to studies of the cp genomes, future studies should be combined with data analyses of nuclear and mitochondrial genomes.
Conclusions
In this study, the cp genomes of four Physalis species, P. angulata, P. alkekengi var. franchetii, P. pubescens, and P. minima were first obtained through high-throughput sequencing. The comparative genomic analysis performed, which included the published cp genome of P. peruviana, allowed us to determine the circular nature with the typical quadripartite structure of the Physalis cp genome. The whole Physalis cp genomes were relatively conserved, with differences at the boundaries IR/SC and LSC/IR. Nearly 290 SSR loci have been identified, which can be used as molecular markers in a future Physalis intraspecific diversity study. Whole cp genome allowed to reconstruct the phylogenetic trees of Solanaceae, identifying six group of species, and finding Physalis as an independent clade within Solanaceae group I. Our results enrich the data on the cp genomes of the genus Physalis and lay an important foundation for the accurate molecular identification and phylogenetic reconstruction of Physalis species.
Methods
Plant materials, DNA extraction and sequencing
Four species widely distributed in China, P. angulata, P. alkekengi var. franchetii, P. minima and P. pubescens, were field-collected (Details of sampling information of the four Physalis species collected in the study were shown in Additional File 1: Table S1). The formal identification of the plant material was undertaken by Dr. Huizhong Wang (Hangzhou Normal University). Voucher specimens of all the collected species were deposited at the Zhejiang Provincial Key Laboratory for Genetic Improvement and Quality Control of Medicinal Plants, Hangzhou Normal University (Additional File 1: Table S1). Permission was not necessary for collecting these species, which have not been included in the list of national key protected plants. Clean, healthy, fresh green leaves from the collected Physalis plants were sampled (6 specimens per each species). Leaves were surface washed, dried and stored at − 80 °C till DNA extraction.
Total genomic DNA was extracted from ~ 0.1 g of preserved leaves (mix of equal amounts of 6 individuals) according to a modified CTAB method [60]. The modification was mainly in the CTAB extraction buffer which contained 4% CTAB instead of 2%, ~ 0.2% DL-dithiothreitol (DTT) and 1% polyvinyl poly-pyrrolidone (PVP), the rest of the protocol was as described [60]. Complete cp genome of each species was obtained by Long-range PCR on total genomic DNA, as in previous works [17, 61]. Briefly, the PCR was carried out in 25 μL containing 1 × PrimeSTAR GXL buffer [10 mM Tris-HCl (pH 8.2), 1 mM MgCl2, 20 mM NaCl, 0.02 mM EDTA, 0.02 mM DDT, 0.02% Tween 20, 0.02% NP-40, and 10% glycerol], 1.6 mM dNTPs, 0.5 μM of each primer pair (as described in Yang et al. [61]) (Additional File 1: Table S5), 1 U PrimeSTAR GXL DNA polymerase (TaKaRa BIO INC.; Dalian, China), and 50 ng genomic DNA template. The PCR was performed using a GeneAmp PCR System 9700 DNA Thermal Cycler (PerkinElmer, Norwalk, CT, USA) with the following PCR program: 94 °C for 1 min, followed by 30 cycles 68 °C for 15 min, and a final extension at 72 °C for 10 min. Nine PCR reactions were performed for each Physalis species. The PCR products from the above reactions were then mixed in roughly equal proportions for Illumina MiSeq sequencing. These mixtures were fragmented and used for short insert (500 bp) library construction, following the manufacturer’s protocol (Illumina) [17]. DNA libraries of different species were run on an Illumina Miseq machine with paired-end, 250 bp reads at the Germplasm Bank of Wild Species in Southwest China, Kunming Institution of Botany, Chinese Academy of Sciences.
Genome assembly, annotation and comparative analysis
De novo and reference-guided strategies were used to assemble cp genomes. First, Illumina short reads were assembled into contigs using NGS QC Toolkit v2.3.3 (www.nipgr.res.in/ngsqctoolkit.html). Second, the high quality pair-ended reads were assembled using CLC Genomics Workbench version 8 (CLC Bio, Aarhus, Denmark) and SOAPdenovo (http://soap.genomics.org.cn/soapdenovo.html) with a k-mer length of 63. Third, highly similar genome sequences were identified using BLAST (http://blast.ncbi.nlm.nih.gov/) with default parameters. Output scaffolds/contigs larger than 1000 bps were mapped to the reference cp genome of P. peruviana (NC_026570). Finally, we determined the order of aligned scaffolds/contigs according to the reference genome and resolved any gaps that were present by mapping the raw reads to the assembly.
The Dual Organellar GenoMe Annotator (DOGMA) (http://dogma.ccbb.utexas.edu/) tool [62] was used to annotate the four complete Physalis cp genomes. Start and stop codons of protein-coding genes and intron positions were manually corrected based on the reference genome (NC_026570). DOGMA and tRNA scan-SE version 1.21 [63] were used to obtain and identify tRNA genes. The circular gene maps were constructed using the OrganellarGenomeDRAW tool followed by manual modification [64]. The cp genomes after annotation were submitted to the GenBank database (GenBank accession numbers: MH045574, MH045575, MH045576 and MH045577). Cp genome comparisons among the five Physalis species were performed using the mVISTA program (http://genome.lbl.gov/vista/mvista/about.shtml). MEGA 6 software was used to analyze GC content, codon usage and phylogenetic analyses as described below [65].
Repeat sequences and SSR analysis
The Perl script MISA (http://pgrc.ipk-gatersleben.de/misa/) [66] was used to detect potential microsatellites (SSRs) in the Physalis cp genomes. The parameters were set as follows: 10 repeat units for mononucleotide SSRs, 5 repeat units for dinucleotide SSRs, 4 repeat units for trinucleotide SSRs, and 3 repeat units for tetra-, penta- and hexanucleotide repeats. REPuter was used to identify forward (direct), reverse, and palindromic repeats, within the cp genome, with a minimum repeat size of 30 bp and 90% sequence identity (Hamming distance of 3) [67].
Phylogenetic analysis
To elucidate the phylogenetic positions of Physalis species within the Solanaceae family, multiple alignments were performed using the complete cp genome sequences of 36 Solanaceae species representing 11 genera (Additional File 1: Table S6), including Scutellaria baicalensis (NC_027262) and S. insignis (NC_028533), as outgroups. The MAFFT7.017 and ClustalX alignment software were used to compare and analyze the complete cp genome sequences of all the species, manual adjustments were made where necessary [68]. Maximum-likelihood (ML) and neighbor-joining (NJ) analyses were performed using MEGA 6 [65], using the general time reversible model with substitution-rate among sites of gamma distribution with invariant sites (GTR + G + I), with complete gap elimination and 1000 bootstrap repeats to ascertain branch support, as implemented in MEGA. Nucleotide and phylogeny inference models were selected after model testing in MEGA.
Supplementary information
Additional file 1: Table S1. Information on the four Physalis species used in the study. Table S2. Quality control of the Illumina sequencing of chloroplast genome of Physalis species. Table S3. Relative synonymous codon usage (RSCU) in five Physalis chloroplast genomes. Table S4. Evolutionary divergence among Physalis species based on complete chloroplast genome sequences. Table S5. Universal primers for amplifying complete chloroplast genomes. Table S6. The 36 studied species belonging to 11 genera of Solanaceae, and the corresponding chloroplast whole genome GenBank accession number.
Additional file 2: Figure S1. Gene map of the P. alkekengi var. franchetii chloroplast genome. Genes shown outside the outer circle are transcribed clockwise, and those inside are transcribed counterclockwise. Genes belonging to different functional groups are color coded. The darker gray in the inner circle indicates the GC content, and the lighter gray indicates the AT content. The inner circle also indicates that the chloroplast genome contains two copies of the inverted repeat (IRA and IRB), a large single-copy region (LSC) and a small single-copy region (SSC). The map was constructed using OrganellarGenomeDRAW. Figure S2. Gene map of the P. minima chloroplast genome. Genes shown outside the outer circle are transcribed clockwise, and those inside are transcribed counterclockwise. Genes belonging to different functional groups are color coded. The darker gray in the inner circle indicates the GC content, and the lighter gray indicates the AT content. The inner circle also indicates that the chloroplast genome contains two copies of the inverted repeat (IRA and IRB), a large single-copy region (LSC) and a small single-copy region (SSC). The map was constructed using OrganellarGenomeDRAW. Figure S3. Gene map of the P. pubescens chloroplast genome. Genes shown outside the outer circle are transcribed clockwise, and those inside are transcribed counterclockwise. Genes belonging to different functional groups are color coded. The darker gray in the inner circle indicates the GC content, and the lighter gray indicates the AT content. The inner circle also indicates that the chloroplast genome contains two copies of the inverted repeat (IRA and IRB), a large single-copy region (LSC) and a small single-copy region (SSC). The map was constructed using OrganellarGenomeDRAW. Figure S4. Amino acid frequencies in the chloroplast genomes of five Physalis species. Figure S5. Neighbor-joining (NJ) tree based on the complete chloroplast genome sequences of 36 species of Solanaceae. Numbers above branches indicate bootstrap support, and circled by the red dotted lines are the five Physalis species.
Acknowledgments
We would like to thank the College of Life and Environmental Science, Hangzhou Normal University for supporting this work. We are also grateful to the Germplasm Bank of Wild Species (KUN) for technical support. And last but not least, we thank the personnel of International Science Editing company for their services in editing this manuscript.
Abbreviations
- Cp
Chloroplast
- ITS
Internal transcribed spacer
- SSR
Simple sequence repeat
- IRs
Inverted repeats
- LSC
Large single-copy
- SSC
Small single-copy
- ML
Maximum-likelihood
- NJ
Neighbor-joining
- BS
Branch support
Authors’ contributions
SF and HW conceived of the study, designed experiments, sequenced chloroplast genomes, drafted the manuscript, and given final approval of the version to be published; SF, KZ and KJ carried out the molecular studies; SF, KZ, KJ, YC, CC, YM, LW, XZ and QY analyzed the data; HW secured funding and helped to draft the manuscript. All authors read and approved the final manuscript.
Funding
Our work was funded by the National Natural Science Foundation of China (31970346); the Zhejiang Provincial Natural Science Foundation of China (LY20H280012, LY19C160001); the Hangzhou Scientific and Technological Program of China (20191203B02); Key project at central government level: The ability establishment of sustainable use for valuable Chinese medicine resources (2060302); Zhejiang Provincial Key Research & Development Project Grants (2018C02030); the college students’ science and technology innovation project of Zhejiang (2019R426027). None of these funding bodies have any relationship with the publication of this manuscript.
Availability of data and materials
The complete chloroplast genomes of P. angulata, P. alkekengi var. franchetii, P. minima and P. pubescens were submitted to the NCBI database (https://www.ncbi.nlm.nih.gov/) with GenBank accession numbers MH045574 (P. angulata), MH045575 (P. alkekengi var. franchetii), MH045577 (P. minima) and MH045576 (P. pubescens). All other data and material generated in this manuscript are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
The collected Physalis species are widely distributed in China. Experimental researches with Physalis species comply with Hangzhou Normal University guidelines (https://hsdsbc.hznu.edu.cn/c/2014-09-15/897442.shtml), and do not include the genetic transformation of the same, preserving the genetic background of the species used. It does not require ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12870-020-02429-w.
References
- 1.Whitson M, Manos PS. Untangling Physalis (Solanaceae) from the Physaloids: a two-gene phylogeny of the Physalinae. Syst Bot. 2005;30(1):216–230. [Google Scholar]
- 2.Zhang WN, Tong WY. Chemical constituents and biological activities of plants from the genus Physalis. Chem Biodivers. 2016;13(1):48–65. doi: 10.1002/cbdv.201400435. [DOI] [PubMed] [Google Scholar]
- 3.Feng SG, Jiang MY, Shi YJ, Jiao KL, Shen CJ, Lu JJ, Ying QC, Wang HZ. Application of the ribosomal DNA ITS2 region of Physalis (Solanaceae): DNA barcoding and phylogenetic study. Front Plant Sci. 2016;7:1047. doi: 10.3389/fpls.2016.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei JL, Hu XR, Yang JJ, Yang WC. Identification of single-copy orthologous genes between Physalis and Solanum lycopersicum and analysis of genetic diversity in Physalis using molecular markers. PLoS One. 2012;7(11):e50164. doi: 10.1371/journal.pone.0050164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez M. Revision of Physalis section Epeteiorhiza (Solanaceae) Ann Ins Biol Bot. 1998;69:71–117. [Google Scholar]
- 6.Chinese academy of sciences . Flora of China. China: Science press; 1978. p. 50. [Google Scholar]
- 7.National pharmacopoeia committee . Pharmmacopoeia of the People's Republic of China. Beijing: Chemical Industry Press; 2015. pp. 360–361. [Google Scholar]
- 8.Ji L, Yuan YL, Ma ZJ, Chen Z, Gan LS, Ma XQ, Huang DS. Induction of quinone reductase (QR) by withanolides isolated from Physalis pubescens L. (Solanaceae) Steroids. 2013;78(9):860–865. doi: 10.1016/j.steroids.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Ding H, Hu ZJ, Yu LY, Ma ZJ, Ma XQ, Chen Z, Wang D, Zhao XF. Induction of quinone reductase (QR) by withanolides isolated from Physalis angulata L. var. villosa Bonati (Solanaceae) Steroids. 2014;86:32–38. doi: 10.1016/j.steroids.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Xu XM, Guan YZ, Shan SM, Luo JG, Kong LY. Withaphysalin-type withanolides from Physalis minima. Phytochem Lett. 2016;15:1–6. [Google Scholar]
- 11.Zhan XR, Liao XY, Luo XJ, Zhu YJ, Feng SG, Yu CN, Lu JJ, Shen CJ, Wang HZ. Comparative metabolomic and proteomic analyses reveal the regulation mechanism underlying MeJA-induced bioactive compound accumulation in cutleaf groundcherry (Physalis angulata L.) hairy roots. J Agric Food Chem. 2018;66(25):6336–6347. doi: 10.1021/acs.jafc.8b02502. [DOI] [PubMed] [Google Scholar]
- 12.Zamora-Tavares P, Vargas-Ponce O, Sanchez-Martinez J, Cabrera-Toledo D. Diversity and genetic structure of the husk tomato (Physalis philadelphica lam.) in Western Mexico. Genet Resour Crop Ev. 2015;62(1):141–153. [Google Scholar]
- 13.Redwan RM, Saidin A, Kumar SV. Complete chloroplast genome sequence of MD-2 pineapple and its comparative analysis among nine other plants from the subclass Commelinidae. BMC Plant Biol. 2015;15:196. doi: 10.1186/s12870-015-0587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin Avila E, Gisby MF, Day A. Seamless editing of the chloroplast genome in plants. BMC Plant Biol. 2016;16(1):168. doi: 10.1186/s12870-016-0857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diekmann K, Hodkinson TR, Wolfe KH, van den Bekerom R, Dix PJ, Barth S. Complete chloroplast genome sequence of a major allogamous forage species, perennial ryegrass (Lolium perenne L.) DNA Res. 2009;16(3):165–176. doi: 10.1093/dnares/dsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei WJ, Ni DP, Wang YJ, Shao JJ, Wang XC, Yang D, Wang JS, Chen HM, Liu C. Intraspecific and heteroplasmic variations, gene losses and inversions in the chloroplast genome of Astragalus membranaceus. Sci Rep. 2016;6:21669. doi: 10.1038/srep21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Y, Ma PF, Li HT, Yang JB, Wang H, Li DZ. Plastid phylogenomic analyses resolve Tofieldiaceae as the root of the early diverging monocot order Alismatales. Genome Biol Evol. 2016;8(3):932–945. doi: 10.1093/gbe/evv260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y, Cullis C. A novel inversion in the chloroplast genome of marama (Tylosema esculentum) J Exp Bot. 2017;68(8):2065–2072. doi: 10.1093/jxb/erw500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doyle JJ, Doyle JL, Palmer JD. Multiple independent losses of two genes and one intron from legume chloroplast genome. Syst Bot. 1995;20(3):272–294. [Google Scholar]
- 20.Millen RS, Olmstead RG, Adams KL, Palmer JD, Lao NT, Heggie L, Kavanagh TA, Hibberd JM, Gray JC, Morden CW, et al. Many parallel losses of infA from chloroplast DNA during angiosperm evolution with multiple independent transfers to the nucleus. Plant Cell. 2001;13(3):645–658. doi: 10.1105/tpc.13.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guisinger MM, Chumley TW, Kuehl JV, Boore JL, Jansen RK. Implications of the plastid genome sequence of Typha (Typhaceae, Poales) for understanding genome evolution in Poaceae. J Mol Evol. 2010;70(2):149–166. doi: 10.1007/s00239-009-9317-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro DR, Tewari KK. Nucleotide sequences of transfer RNA genes in the Pisum sativum chloroplast DNA. Plant Mol Biol. 1986;6(1):1–12. doi: 10.1007/BF00021301. [DOI] [PubMed] [Google Scholar]
- 23.Saski C, Lee SB, Daniell H, Wood TC, Tomkins J, Kim HG, Jansen RK. Complete chloroplast genome sequence of Gycine max and comparative analyses with other legume genomes. Plant Mol Biol. 2005;59(2):309–322. doi: 10.1007/s11103-005-8882-0. [DOI] [PubMed] [Google Scholar]
- 24.Hirao T, Watanabe A, Kurita M, Kondo T, Takata K. Complete nucleotide sequence of the Cryptomeria japonica D. Don chloroplast genome and comparative chloroplast genomics: diversified genomic structure of coniferous species. BMC Plant Biol. 2008;8:70. doi: 10.1186/1471-2229-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang YZ, Ma J, Yang BX, Li RY, Zhu W, Sun LL, Tian JK, Zhang L. The complete chloroplast genome sequence of Taxus chinensis var. mairei (Taxaceae): loss of an inverted repeat region and comparative analysis with related species. Gene. 2014;540(2):201–209. doi: 10.1016/j.gene.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 26.Tangphatsornruang S, Sangsrakru D, Chanprasert J, Uthaipaisanwong P, Yoocha T, Jomchai N, Tragoonrung S. The chloroplast genome sequence of mungbean (Vigna radiata) determined by high-throughput pyrosequencing: structural organization and phylogenetic relationships. DNA Res. 2010;17(1):11–22. doi: 10.1093/dnares/dsp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer JD, Thompson WF. Rearrangements in the chloroplast genomes of mung bean and pea. Proc Natl Acad Sci U S A. 1981;78(9):5533–5537. doi: 10.1073/pnas.78.9.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XW, Yang Y, Henry RJ, Rossetto M, Wang YT, Chen SL. Plant DNA barcoding: from gene to genome. Biol Rev Camb Philos Soc. 2015;90(1):157–166. doi: 10.1111/brv.12104. [DOI] [PubMed] [Google Scholar]
- 29.Dong WP, Xu C, Wu P, Cheng T, Yu J, Zhou SL, Hong DY. Resolving the systematic positions of enigmatic taxa: manipulating the chloroplast genome data of Saxifragales. Mol Phylogenet Evol. 2018;126:321–330. doi: 10.1016/j.ympev.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z, Zhao TT, Ma QH, Liang LS, Wang GX. Comparative genomics and phylogenetic analysis revealed the chloroplast genome variation and interspecific relationships of Corylus (Betulaceae) species. Front Plant Sci. 2018;9:927. doi: 10.3389/fpls.2018.00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Axelius B. The phylogenetic relationships of the physaloid genera (Solanaceae) based on morphological data. Amer J Bot. 1996;83:118–124. [Google Scholar]
- 32.Olmstead RG, Bohs L, Migid HA, Santiago-Valentin E, Garcia VF, Collier SM. A molecular phylogeny of the Solanaceae. Taxon. 2008;57(4):1159–1181. [Google Scholar]
- 33.Feng SG, Jiao KL, Zhu YJ, Wang HF, Jiang MY, Wang HZ. Molecular identification of species of Physalis (Solanaceae) using a candidate DNA barcode: the chloroplast psbA-trnH intergenic region. Genome. 2018;61(1):15–20. doi: 10.1139/gen-2017-0115. [DOI] [PubMed] [Google Scholar]
- 34.Vargas-Ponce O, Perez-Alvarez LF, Zamora-Tavares P, Rodriguez A. Assessing genetic diversity in Mexican husk tomato species. Plant Mol Biol Rep. 2011;29(3):733–738. [Google Scholar]
- 35.Feng SG, Zhu YJ, Yu CL, Jiao KL, Jiang M, JJ YL, Shen CJ, Ying QC, Wang HZ. Development of species-specific SCAR markers, based on a SCoT analysis, to authenticate Physalis (Solanaceae) species. Front Genet. 2018;9:192. doi: 10.3389/fgene.2018.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore MJ, Dhingra A, Soltis PS, Shaw R, Farmerie WG, Folta KM, Soltis DE. Rapid and accurate pyrosequencing of angiosperm plastid genomes. BMC Plant Biol. 2006;6:17. doi: 10.1186/1471-2229-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Yang CP, Zhao XY, Chen S, Qu GZ. Complete chloroplast genome sequence of Betula platyphylla: gene organization, RNA editing, and comparative and phylogenetic analyses. BMC Genomics. 2018;19(1):950. doi: 10.1186/s12864-018-5346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quail MA, Smith M, Coupland P, Otto TD, Harris SR, Connor TR, Bertoni A, Swerdlow HP, Gu Y. A tale of three next generation sequencing platforms: comparison of ion torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genomics. 2012;13:341. doi: 10.1186/1471-2164-13-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang YX, Iaffaldano BJ, Zhuang XF, Cardina J, Cornish K. Chloroplast genome resources and molecular markers differentiate rubber dandelion species from weedy relatives. BMC Plant Biol. 2017;17(1):34. doi: 10.1186/s12870-016-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang YJ, Du LW, Liu A, Chen JJ, Wu L, Hu WM, Zhang W, Kim K, Lee SC, Yang TJ, et al. The complete chloroplast genome sequences of five Epimedium species: lights into phylogenetic and taxonomic analyses. Front Plant Sci. 2016;7:306. doi: 10.3389/fpls.2016.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo J, Hou BW, Niu ZT, Liu W, Xue QY, Ding XY. Comparative chloroplast genomes of photosynthetic orchids: insights into evolution of the Orchidaceae and development of molecular markers for phylogenetic applications. PLoS One. 2014;9(6):e99016. doi: 10.1371/journal.pone.0099016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansen RK, Wojciechowski MF, Sanniyasi E, Lee S-B, Daniell H. Complete plastid genome sequence of the chickpea (Cicer arietinum) and the phylogenetic distribution of rps12 and clpP intron losses among legumes (Leguminosae) Mol Phylogenet Evol. 2008;48(3):1204–1217. doi: 10.1016/j.ympev.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuo LH, Shang AQ, Zhang S, Yu XY, Ren YC, Yang MS, Wang JM. The first complete chloroplast genome sequences of Ulmus species by de novo sequencing: genome comparative and taxonomic position analysis. PLoS One. 2017;12(2):e0171264. doi: 10.1371/journal.pone.0171264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim KJ, Lee HL. Complete chloroplast genome sequences from Korean ginseng (Panax schinseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. DNA Res. 2004;11(4):247–261. doi: 10.1093/dnares/11.4.247. [DOI] [PubMed] [Google Scholar]
- 45.Sun YX, Moore MJ, Meng AP, Soltis PS, Soltis DE, Li JQ, Wang HC. Complete plastid genome sequencing of Trochodendraceae reveals a significant expansion of the inverted repeat and suggests a Paleogene divergence between the two extant species. PLoS One. 2013;8(4):e60429. doi: 10.1371/journal.pone.0060429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi KS, Chung MG, Park S. The complete chloroplast genome sequences of three Veroniceae species (Plantaginaceae): comparative analysis and highly divergent regions. Front Plant Sci. 2016;7:355. doi: 10.3389/fpls.2016.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen XC, Li QS, Li Y, Qian J, Han JP. Chloroplast genome of Aconitum barbatum var. puberulum (Ranunculaceae) derived from CCS reads using the PacBio RS platform. Front Plant Sci. 2015;6:42. doi: 10.3389/fpls.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asaf S, Khan AL, Khan AR, Waqas M, Kang SM, Khan MA, Lee SM, Lee IJ. Complete chloroplast genome of Nicotiana otophora and its comparison with related species. Front Plant Sci. 2016;7:843. doi: 10.3389/fpls.2016.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asaf S, Waqas M, Khan AL, Khan MA, Kang SM, Imran QM, Shahzad R, Bilal S, Yun BW, Lee IJ. The complete chloroplast genome of wild rice (Oryza minuta) and its comparison to related species. Front Plant Sci. 2017;8:304. doi: 10.3389/fpls.2017.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang H, Shi C, Liu Y, Mao SY, Gao LZ. Thirteen Camellia chloroplast genome sequences determined by high-throughput sequencing: genome structure and phylogenetic relationships. BMC Evol Biol. 2014;14:151. doi: 10.1186/1471-2148-14-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yi X, Gao L, Wang B, Su YJ, Wang T. The complete chloroplast genome sequence of Cephalotaxus oliveri (Cephalotaxaceae): evolutionary comparison of cephalotaxus chloroplast DNAs and insights into the loss of inverted repeat copies in gymnosperms. Genome Biol Evol. 2013;5(4):688–698. doi: 10.1093/gbe/evt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao X, Tan YH, Liu YY, Song Y, Yang JB, Corlett RT. Chloroplast genome structure in Ilex (Aquifoliaceae) Sci Rep. 2016;6:28559. doi: 10.1038/srep28559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Li L, Yan TL, Liu Q. Complete chloroplast genome sequences of Praxelis (Eupatorium catarium Veldkamp), an important invasive species. Gene. 2014;549(1):58–69. doi: 10.1016/j.gene.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 54.Nie XJ, Lv SZ, Zhang YX, Du XH, Wang L, Biradar SS, Tan XF, Wan FH, Weining S. Complete chloroplast genome sequence of a major invasive species, Crofton weed (Ageratina adenophora) PLoS One. 2012;7(5):e36869. doi: 10.1371/journal.pone.0036869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pauwels M, Vekemans X, Gode C, Frerot H, Castric V, Saumitou-Laprade P. Nuclear and chloroplast DNA phylogeography reveals vicariance among European populations of the model species for the study of metal tolerance, Arabidopsis halleri (Brassicaceae) New Phytol. 2012;193(4):916–928. doi: 10.1111/j.1469-8137.2011.04003.x. [DOI] [PubMed] [Google Scholar]
- 56.Liu LX, Wang YW, He PZ, Li P, Lee J, Soltis DE, Fu CX. Chloroplast genome analyses and genomic resource development for epilithic sister genera Oresitrophe and Mukdenia (Saxifragaceae), using genome skimming data. BMC Genomics. 2018;19(1):235. doi: 10.1186/s12864-018-4633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen JH, Hao ZD, Xu HB, Yang LM, Liu GX, Sheng Y, Zheng C, Zheng WW, Cheng TL, Shi JS. The complete chloroplast genome sequence of the relict woody plant Metasequoia glyptostroboides Hu et Cheng. Front Plant Sci. 2015;6:447. doi: 10.3389/fpls.2015.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore MJ, Soltis PS, Bell CD, Burleigh JG, Soltis DE. Phylogenetic analysis of 83 plastid genes further resolves the early diversification of eudicots. P Natl Acad Sci USA. 2010;107(10):4623–4628. doi: 10.1073/pnas.0907801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang YJ, Li DZ. Advances in phylogenomics based on complete chloroplast genomes. Plant Diversity and Resources. 2011;33(4):365–375. [Google Scholar]
- 60.Doyle JJ. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- 61.Yang JB, Li DZ, Li HT. Highly effective sequencing whole chloroplast genomes of angiosperms by nine novel universal primer pairs. Mol Ecol Resour. 2014;14(5):1024–1031. doi: 10.1111/1755-0998.12251. [DOI] [PubMed] [Google Scholar]
- 62.Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20(17):3252–3255. doi: 10.1093/bioinformatics/bth352. [DOI] [PubMed] [Google Scholar]
- 63.Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005;33:W686–W689. doi: 10.1093/nar/gki366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lohse M, Drechsel O, Kahlau S, Bock R. OrganellarGenomeDRAW--a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013;41:W575–W581. doi: 10.1093/nar/gkt289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beier S, Thiel T, Munch T, Scholz U, Mascher M. MISA-web: a web server for microsatellite prediction. Bioinformatics. 2017;33(16):2583–2585. doi: 10.1093/bioinformatics/btx198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kurtz S, Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001;29(22):4633–4642. doi: 10.1093/nar/29.22.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Information on the four Physalis species used in the study. Table S2. Quality control of the Illumina sequencing of chloroplast genome of Physalis species. Table S3. Relative synonymous codon usage (RSCU) in five Physalis chloroplast genomes. Table S4. Evolutionary divergence among Physalis species based on complete chloroplast genome sequences. Table S5. Universal primers for amplifying complete chloroplast genomes. Table S6. The 36 studied species belonging to 11 genera of Solanaceae, and the corresponding chloroplast whole genome GenBank accession number.
Additional file 2: Figure S1. Gene map of the P. alkekengi var. franchetii chloroplast genome. Genes shown outside the outer circle are transcribed clockwise, and those inside are transcribed counterclockwise. Genes belonging to different functional groups are color coded. The darker gray in the inner circle indicates the GC content, and the lighter gray indicates the AT content. The inner circle also indicates that the chloroplast genome contains two copies of the inverted repeat (IRA and IRB), a large single-copy region (LSC) and a small single-copy region (SSC). The map was constructed using OrganellarGenomeDRAW. Figure S2. Gene map of the P. minima chloroplast genome. Genes shown outside the outer circle are transcribed clockwise, and those inside are transcribed counterclockwise. Genes belonging to different functional groups are color coded. The darker gray in the inner circle indicates the GC content, and the lighter gray indicates the AT content. The inner circle also indicates that the chloroplast genome contains two copies of the inverted repeat (IRA and IRB), a large single-copy region (LSC) and a small single-copy region (SSC). The map was constructed using OrganellarGenomeDRAW. Figure S3. Gene map of the P. pubescens chloroplast genome. Genes shown outside the outer circle are transcribed clockwise, and those inside are transcribed counterclockwise. Genes belonging to different functional groups are color coded. The darker gray in the inner circle indicates the GC content, and the lighter gray indicates the AT content. The inner circle also indicates that the chloroplast genome contains two copies of the inverted repeat (IRA and IRB), a large single-copy region (LSC) and a small single-copy region (SSC). The map was constructed using OrganellarGenomeDRAW. Figure S4. Amino acid frequencies in the chloroplast genomes of five Physalis species. Figure S5. Neighbor-joining (NJ) tree based on the complete chloroplast genome sequences of 36 species of Solanaceae. Numbers above branches indicate bootstrap support, and circled by the red dotted lines are the five Physalis species.
Data Availability Statement
The complete chloroplast genomes of P. angulata, P. alkekengi var. franchetii, P. minima and P. pubescens were submitted to the NCBI database (https://www.ncbi.nlm.nih.gov/) with GenBank accession numbers MH045574 (P. angulata), MH045575 (P. alkekengi var. franchetii), MH045577 (P. minima) and MH045576 (P. pubescens). All other data and material generated in this manuscript are available from the corresponding author upon reasonable request.