Abstract
Quercetin (Que) and its derivatives are naturally occurring phytochemicals with promising bioactive effects. The antidiabetic, anti-inflammatory, antioxidant, antimicrobial, anti-Alzheimer’s, antiarthritic, cardiovascular, and wound-healing effects of Que have been extensively investigated, as well as its anticancer activity against different cancer cell lines has been recently reported. Que and its derivatives are found predominantly in the Western diet, and people might benefit from their protective effect just by taking them via diets or as a food supplement. Bioavailability-related drug-delivery systems of Que have also been markedly exploited, and Que nanoparticles appear as a promising platform to enhance their bioavailability. The present review aims to provide a brief overview of the therapeutic effects, new insights, and upcoming perspectives of Que.
1. Introduction
Quercetin (Que, Figure 1) is an antioxidant flavonol belonging to the flavonoid group and generally present as Que glycoside.1−3 The Que aglycone is able to conjugate with glucose, xylose, or rutinose attaching to one of the Que’s hydroxyl groups with the consequent creation of various Que glycoside forms.4 Quercetin-3-O-glycoside mostly serves as a pigment in flowers, vegetables, and fruits.5 Nutritional Que is present mainly as glycosides rather than as aglycones.6 It shows a relatively higher bioavailability than other phytochemicals,7 with its main sources being grapes, berries, cherries, apples, citrus fruits, onions, buckwheat, kale, tomatoes, red wine, and black tea.3,8,9 However, the Que concentration can vary from one plant to another or even in different parts of the same plant.10,11
Figure 1.
Chemical structure of Quercetin.
Nutritional flavonoids, as is the case of Que, are even more powerful antioxidants than vitamins C and E,12 with those which contain in formula a 3-OH and 3′,4′-catechol revealing to be 10 times stronger toward peroxynitrite than ebselen.13 In different food products, onion-derived Que (containing Que glucoside) revealed a higher bioavailability than apple-derived Que (containing Que rhamnoside and Que galactoside).2
Que and its derivatives exert key biological effects on cell cycle progression and cellular signal transduction pathway regulation.14 Moreover, the metabolic plasticity of Que is a determinant in the plant adaptive reaction. This also has a great impact on the early-stage adaptation of plants to their local ecosystems.15 At the same time, Que aglycones are effective regulators of auxin transport, related to plant growth and development.16
The anti-inflammatory and antioxidant effects of Que are essential for its activity as oxidative, kinase, and cell cycle inhibitors, as well as for neuronal survival. Que apoptosis-inducing effects are the key for its anticancer potential.17 Que, like many other compounds with a flavone ring, also exerts a marked effect on the immunity and inflammation.18 Thus this review aims to review the therapeutic effects of Que using modern research techniques.
2. Results and Discussion
2.1. Preclinical Pharmacological Activities of Quercetin
2.1.1. Effects on Diabetes Complications
Many studies had shown that Que is a promising drug target for treating diabetes (Table 1). A number of mechanisms have been proposed for the antihyperglycemic action of Que, among which insulin sensitivity enhancement, glycogen synthesis promotion, and insulin resistance improvement are common. Que promotes the insulin sensitization effect by stimulating pancreatic β-cell proliferation, improving glucose metabolism and insulin secretion.19 Que has also been reported as an α-glucosidase and α-amylase inhibitor.20 In addition, Que improved plasma insulin levels and decreased blood glucose in streptozotocin (STZ)-induced diabetes models through maintaining the mass and function of β-cells and thus increasing the serum insulin effect. On the other hand, in alloxan-diabetic animal models, Que reduced islet cell failure, enhanced the insulin secretion of β-cells, and further prevented diabetes through decreasing oxidative stress.21
Table 1. Reported Pharmacological Activities for Quercetin.
| doses | In vitro/in vivo/ex vivo | route of administration | model | effect | refs |
|---|---|---|---|---|---|
| Antidiabetic Effect | |||||
| 20 mmol/L | ex vivo | INS-1 Pancreatic β-cells | potentiated insulin secretion, β-cells protected against oxidative damages | (22) | |
| 120 mg/kg/day for 8 weeks | in vivo | Oral | diabetic rats | decreased plasma triglycerides and weight of diabetic rats; decreased plasma cholesterol levels, fasting plasma insulin, and postprandial glucose and significantly increased insulin sensitivity index | (22) |
| 30 mg/kg b.w. for 14 days | in vivo | Intraperitoneal | STZ-induced diabetic SD rats | decreased serum blood glucose levels, enhanced insulin levels, and improved dyslipidemia, decreased oxidative stress injury | (23) |
| 10 and 15 mg/kg b.w. for 2 weeks | in vivo | Intraperitoneal | STZ-induced diabetic SD rats | regenerated pancreatic islets, increased insulin release, and reduced blood glucose and urine sugar levels. | (24) |
| 10 and 15 mg/kg b.w. for 8 weeks | in vivo | Intraperitoneal | alloxan-induced diabetic mice | decreased serum glucose levels and oxidative stress and inhibited apoptosis | (25) |
| 50 and 80 mg/kg b.w. for 45 days | in vivo | Oral | STZ-induced diabetic Wistar rats | regulated hyperglycemia, decreased the level of glycoprotein, scavenged ROS; modulated hepatic metabolism and antioxidant enzymes | (26) |
| 100 mg/kg b.w. for 49 days | in vivo | Oral | STZ-induced diabetic SD rats | attenuated fasting and postprandial hyperglycemia, reduced blood glycated hemoglobin | (20) |
| 25, 50 and 75 mg/kg for 28 days | in vivo | Oral | STZ-induced diabetic Wistar rats | decreased blood glucose and urine sugar. Enhanced plasma insulin and hemoglobin levels | (27) |
| 100 and 200 mg/kg b.w. for 6 weeks | in vivo | Oral | STZ-induced diabetic Wistar rats | controlled the blood sugar, decreased insulin resistance, and protected pancreatic cells | (28) |
| 50 mg/kg/day 4 weeks | in vivo | Intraperitoneal | after 2 weeks of HFD-fed, albino rats i.p. with a low dose of STZ 35 mg/kg | lowered blood glucose, increased serum insulin levels, preserved β-cell mass and function by increasing antioxidant activity | (29) |
| diet containing Que at 0.04% (wt/wt) and 0.08% for 6 weeks | in vivo | Oral | Type 2 diabetes C57BL/KsJ-db/db mice models | decreased plasma total cholesterol and increased HDL cholesterol, decreased lipid peroxides, attenuated mitochondrial dysfunction | (30) |
| 50 and 100 μM | ex vivo | cultured C2C12 skeletal muscles | activated glucose uptake through an insulin-independent mechanism involving AMPK | (31) | |
| ex vivo | cytological experiments on skeletal muscles | decreased blood sugar level, increased GLUT4 expression, strengthened glucose uptake on skeletal muscle cells surface by stimulating AMPK | (32) | ||
| 0.08% of diet combined with acarbose (0.03% of diet) | in vivo | Oral | db/db Mice | decreased plasma glucose level and improved insulin resistance, reduced cyclic adenosine phosphoric acid (cAMP) accumulation and the influx of free fatty acids, activated protein kinase A (PKA), preserving cyclic nucleotide-dependent phosphodiesterase 3B (PDE3B), and accumulating two acylglycerol (DAG) | (33) |
| Effect on Diabetic Complications, (A) Effect on Diabetic Liver Disorders | |||||
| 50 mg/kg b.w., per day for 30 days | in vivo | Oral | STZ-induced diabetic rats | increased CYP2E1 activity, reduced oxidative stress in liver, and decreased markers of liver damage | (34) |
| 50 mg/kg daily for 7 days | in vivo | Oral | Alloxan-induced diabetes (75 mg/kg) in mice | decreased the number of vacuolated cells and the degree of vacuolization | (35) |
| 15 mg/kg | in vivo | Intraperitoneal | STZ-induced diabetic rats | exerted significant preventive effect on liver cell damages | (36) |
| (B) Effect on Diabetic Reproductive Disorder | |||||
| 25 or 50 mg/kg for 5 weeks | in vivo | Oral | STZ-induced diabetic SD rats | enhanced sexual activity and mount frequency (MF) and intromission frequency (IF), also increased sperm count as well as motility | (37) |
| 50 mg/kg for 5 weeks | in vivo | Oral | STZ-induced diabetic rats | reduced testicular damage | (38) |
| 15 mg/kg for 8 weeks | in vivo | Oral | STZ-induced apoptosis of testicular cells in rats | attenuated diabetes-related testicular dysfunction and histopathologic changes | (39) |
| (C) Effect on Diabetic Neurodegenerative Disorders | |||||
| 5–20 mg/kg twice daily for 30 days | in vivo | Oral | STZ-induced diabetic rats | prevented changes in blood glucose, body weight, and performance in Morris water test and elevated the performance in plus-maze tasks | (40) |
| 40 mg/kg, twice daily for 31–35 days | in vivo | Oral | STZ-induced diabetic mice in Morris water maze task | decreased escape latency and increased the time spent by mice in the target quadrant during the Morris water maze task | (41) |
| 2.5, 5, and 10 mg/kg for 20 days | Intragastric | STZ-diabetic-induced brain injuries | decreased cerebral blood flow (CBF) and blood glucose level, prevented memory impairment, increased antioxidant enzymes activity, and attenuated brain energy metabolism and cholinergic dysfunction | (42) | |
| (D) Effect on Diabetic Retinopathy | |||||
| 150 mg/kg was administered per day for 20 weeks | in vivo | Gastric perfusion | diabetic retinopathy in adult male STZ-induced SD rats | alleviated retinopathy via downregulation of monocyte chemoattractant protein-1 (MCP-1), matrix metalloproteinase-9 (MMP-9), and vascular endothelial growth factor (VEGF) expression levels | (43) |
| Cardiovascular Effect | |||||
| 0.05 to 5 mg/kg | in vivo | Intravenous | conventional and spontaneously hypertensive rats | lowered blood pressure in both short- and long-term basis | (44) |
| 88.7 μmol/kg p.o, 45 min; 14.7 μmol/kg i.v., 5 min | in vivo | Oral Intravenous | experimental anesthetized rats | potentiated the hypotensive impact of bradykinin (10 nmol/kg i.v.) | (45, 46) |
| 1.5 g Que/kg for 7 days | in vivo | Oral | rat model | attenuated carotid hypertrophy and arterial blood pressure, reduced aorta medial wall thickening and normalized cardiac translocation | (47, 48) |
| 5 mg/kg and 10 mg/kg for 5 several weeks | in vivo | Oral | nitro-l-arginine methyl ester hydrochloride (l-NAME)-induced high blood pressure in Wistar Kyoto mice | reduced mean arterial pressure (12%) and heart rate (in STR) after 13 weeks | (49) |
| 10 mg/kg b.w., for 6 weeks | in vivo | Oral | STZ-induced suffering from diabetes male Wistar Kyoto mice | regenerated vascular function and reduced blood vessels sugar level and oxidative pressure | (50) |
| 20 mg/kg/day for 4 weeks | in vivo | Oral | young male normotensive control (C) and automatically hypertensive rats (SHR) over the period of their 5–8th week of age | damaged Na, K-ATPase action when all ATP and Na levels examined | (51) |
| 10 mg/kg/day for 7 days | in vivo | Oral | pretreatment to Wistar mice before induction of myocardial infarction by subcutaneous hypodermic injection of isoproterenol (100 mg/kg) at a period of 24 h for two times | reduced ST-segment level, decreased fat peroxidation in plasma and heart, and decreased free fatty acids levels in serum, serum phospholipids, total cholesterol, and triglycerides. | (52) |
| Autophagy | |||||
| 20 μM | in vitro | human umbilical vein endothelial cells (HUVECs) | promoted cell survival | (53) | |
| 100 mg/kg (in vivo) and 10–60 μmol (in vitro) | in vitro and in vivo | Intragastric | pulmonary arterial smooth muscle cells (PASMCs) | induced apoptotic and autophagic responses | (54) |
| 100 mg/kg | in vivo | Gavage | C57BL/6J mice on ethanol-containing Lieber De Carli liquids diets | reduced ethanol-induced liver injury and suppressed autophagic flux | (55) |
| Anti-Alzheimer’s Effect | |||||
| 50 mg/kg b.w., 2 times a week for 4 weeks | in vivo | Oral | homozygotic transgenic mouse line B6.129S7-Sod2tm1Leb/J Hydrogen peroxide- and Aβ-induced neurotoxicity | decreased ROS levels, improved the typical morphology of mitochondria, prevented mitochondrial dysfunction | (56) |
| 50 mg/kg b.w., every day for 10 weeks | in vivo | Oral | male C57BL/6J mice AMP-activated protein kinase activity on tau hyperphosphorylation | enhanced AMP-activated protein kinase activity, reduced tau hyperphosphorylation, and improved cognitive deficit | (57) |
| 25 mg/kg b.w., every 2 days for 2 months | in vivo | Oral | male SAMP8 mice model of AD | improved cognition deficit, enhanced memory impairments, reduced astrogliosis | (58) |
| 30 mg/kg b.w. every day for 8 days | in vivo | Intraperitoneal | scopolamine-induced cognitive dysfunction and neurodegeneration in male albino Wistar rats | abridged transfer latency, reduced avoidance response, decreased in 3,4-methylene dioxy amphetamine, acetylcholinesterase levels, increased brain catalase and glutathione levels | (59) |
| 10 mg/kg b.w. every day for 12 weeks | in vivo | Oral | aluminum-induced neurodegeneration in male albino Wistar rats | decreased ROS production, amplified mitochondrial superoxide dismutase activity | (60) |
| 25 mg/kg b.w. every day for 3 months | in vivo | Intraperitoneal | triple transgenic mouse model of AD | reduced Alzheimer’s pathology, protected cognitive deficit, improved emotional function | (61) |
| 500 mg/kg b.w. every day for 10 days | in vivo | Oral | five familial transgenic mouse model of AD | increased brain apolipoprotein E, reduced insoluble Aβ levels | (62) |
| 1% in mouse chow for from 3 to 13 months | in vivo | Oral | double transgenic female mice mouse model of AD | decreased neuroinflammation, reduced neurodegeneration | (63) |
| 10 and 50 mg/kg b.w. at 30 min, 12 h, and 24 h after subarachnoid hemorrhage | in vivo | Intraperitoneal | adult male SD rats, rat model of subarachnoid hemorrhage | improved brain damage, provided neuroprotection | (64) |
| 5 and 10 μM | ex vivo | cultured neurons Aβ42-induced oxidative cell toxicity | decreased oxidative stress, reduced neurotoxicity | (65) | |
| ex vivo | cell culture (PC12) H2O2-induced neurodegeneration | reduced oxidative stress, abated neurotoxicity | (66) | ||
| 0.5% in AIN93G diet for 5 weeks | in vivo | Oral | Aβ precursor protein 23 mice murine model of AD | reduced memory dysfunction, decreased oxidative stress | (67) |
| Wound Healing Effect | |||||
| 0.1, 1.0, and 10.0% concentration (w/v) | in vivo | Topical | experimentally wounded male rats | reduced wound area and increased wound contraction | (68) |
| Antiarthritic Effect | |||||
| 10–100 mg/kg | in vivo mice | Intraperitoneal | TiO2-induced arthritis model | inhibited knee joint mechanical hyperalgesia, edema and leukocyte recruitment | (69) |
| 30 mg/kg | in vivo mice (C57BL/6 mice) | Oral | collagen-induced arthritis (CIA) | reduced arthritic inflammation and protected cartilage and bone from destruction. | (70) |
| Antimicrobial Effect | |||||
| 100 mg/kg | in vivo mice | Subcutaneous | Streptococcus suis (virulent SS2 ZY05719 strain)-infected mice | reduced Streptococcus suis virulence | (71) |
| 5–30 mg/kg (in vivo) and 10–250 μM (in vitro) | in vitro and in vivo | Intraperitoneal | human mononuclear and polymorphonuclear leukocytes, and rat whole blood. | protected from gentamicin-induced oxidative stress | (72) |
| Effect on Liver Diseases | |||||
| 100 mg/kg | in vivo mice | Oral | chronic-plus-single-binge-ethanol feeding C57BL/6J mice | protected liver from ethanol-induced liver fat accumulation and liver damage | (73) |
| 5–20 mg/kg | in vivo rats | Subcutaneous | rotenone-induced hepatocellular dysfunction | attenuated rotenone-induced liver-metabolic imbalances | (74) |
| Antioxidant Effect | |||||
| 30 mg/kg b.w. | in vivo | Orally | streptozotocin-induced diabetic rat | decreased lipid peroxidation in both serum and liver tissue and controlled oxidative stress | (75) |
| 5.7 μM (for DPPH assay) and 0.78 μM (Fe3+-EDTA/H2O2 system) | in vitro | erythrocytes | exerted free-radical scavenging and ROS scavenging activity | (76) | |
| 87.5, 90.4, and 78.6 μg/mL (for DPPH, FRAP, and OH radical scavenging assays, respectively) | in vitro | attenuated oxidative stress through decreasing DPPH and OH radicals and reduction of ferric iron. | (77) | ||
| 10 mg/kg b.w. | in vivo | Intraperitoneal | LPS-mediated oxidative stress in spleen and bone marrow of Funambulus pennanti. | increased SOD, catalase, and GPx activities and decreased lipid peroxidation of bone marrow and spleen tissues | (78) |
| different serial concentrations from 3.33 mg/mL stock solution | in vitro | attenuated oxidative stress | (79) | ||
| 25 and 50 mg/kg b.w. day for 6 days | in vivo | Orally | phenylhydrazine-mediated oxidative stress male SD rats | controlled oxidative injury through modulating gene expression, and suppressed ROS generation, improved arterial blood pressure as well as resistance of peripheral vascular; increased response to bradykinin, acetylcholine, as well as phenylephrine in a dose-dependent manner; controlled blood glutathione; decreased plasma MDA levels, nitric oxide and superoxide anions. | (80) |
Que stimulate glucose uptake on isolated cells through stimulating the GLUT4 expression and endogenous GLUT4 translocation by upregulating the estrogen receptor-α, subsequently enhancing phosphorylation of both phosphatidylinositol-3-kinase/Akt (PI3K/Akt) and AMP-activated protein kinase/Akt (AMPK/Akt) signal pathways, increasing the glucose uptake in skeletal muscle cells.31,32 Que enhances the glucose utilization by acting on glucose transport and insulin-receptor signaling, which plays a similar role to rosiglitazone such as glycogen phosphorylase (GP) and peroxisome proliferator-activated receptor γ (PPARγ) agonist.81,82 The molecular interactions of the ligands, Que, gallic acid, and metformin were compared on various diabetes mellitus-related protein targets, such as GP and PPARγ. Que possesses good binding affinity to both targets.27
Que could restore the interference caused by hyperglycemia by modulating endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS).83,84 In addition, Que may activate silencing factor 1 (silent information regulator 1, SIRTl) to increase insulin sensitivity.
2.1.1.1. Effect on Diabetic Liver Disorders
The increase of CYP2E1 in liver in the case of diabetes has been reported85 thus and the enzyme inhibition mainly prevents the liver oxidative damage. In an in vivo study on rats, it was found that STZ-induced diabetes caused hyperglycemia, body weight loss, damaged hepatocyte ultrastructure, together with increased protein levels and CYP2E1 activity in liver. Thus, Que administration (50 mg/kg b.w., per day, during 30 days) led to a marked decrease in CYP2E1 activity, together with pro-oxidant–antioxidant balance promotion.86 Other studies are shown in Table 1.
2.1.1.2. Effect on Diabetic Nephropathy
Que may improve the kidney shape altered by hyperglycemia through protein kinase C (PKC) activity inhibition, transforming growth factor β 1 (TGF-β1) expression downregulation, extracellular matrix formation reduction, and renal hypertrophy delay.87 Que can also prevent renal pathological changes, delay diabetic nephropathy progression, and improve the glycolipid metabolism in type 2 diabetic rats. Moreover, Que could prevent diabetes-induced oxidative stress in renal tissues.88 Que attenuated the tissue dyslipidemic due to lipid deposits in diabetic rats with kidney disease via activating AMPK phosphorylation and suppressing sterol regulatory element binding protein (SREBP)-1c in kidney, thereby preventing renal progression.89
2.1.1.3. Effect on Diabetic Reproductive and Neurodegenerative Disorders
Many animal studies proved the improving effect of Que on diabetes-induced reproductive dysfunction processes, such as sexual dysfunction, testicular dysfunction, or infertility (Table 1). Moreover, studies that proved the Que protective effect on diabetes-induced neurodegenerative diseases are shown in Table 1.
2.1.1.4. Effect on Diabetic Retinopathy
The involved mechanisms in diabetes-induced diabetic retinopathy mainly include oxidative stress, inflammation, neurodegeneration, and damaged retinal vasculature.90 An in vitro experiment on human retinal endothelial cells proved that Que inhibited high-glucose-induced cell proliferation by reducing the vascular endothelial growth factor (VEGF) expression.91 Furthermore, Que exerted protection against STZ diabetes-induced retinal neurodegeneration by reducing oxidative damage and NF-κB-mediated inflammation,92 ameliorating neurotrophic factors levels and inhibiting the neurons apoptosis.93
2.1.2. Effect on Cardiovascular Diseases
Many studies were traced on the effects of Que on cardiovascular diseases (Table 1). The inhibitory effect of Que on the angiotensin-converting enzyme activity by converting bradykinin and angiotensin I was studied by Häckl et al.45 Bradykinin has a more significant effect on the blood pressure in rats on pretreatment with captopril or Que. Que also reduced the hypertensive response to angiotensin I.94 Moreover, the long-term use of Que diminished the risk of elevation of systolic blood pressure (SBP) levels in spontaneously hypertensive rats with a limited or full protection of most of the consequences caused by Nω-Nitro-l-arginine methyl ester hydrochloride (l-NAME).49
Moreover, the effects Que on vasoconstrictor and vasodilator reactions in the porcine-separated heart were studied by Suri et al.95 who reported that Que enhances both the cyclic guanosine monophosphate (cGMP) content of the artery and cGMP-dependent relaxations to glyceryl trinitrate (GTN) and sodium nitroprusside (SNP) as well as restricted receptor-mediated contractions of the porcine. In addition, Que at nutritionally essential levels reduced the responsiveness of typical L type Ca2+ channels to the pharmacological stimulation managed by (S)-(−)-methyl-1,4-dihydro-2,6-dimethyl-3-nitro-4-(2-trifluoromethylphenyl) pyridine-5-carboxylate (Bay K 8644), leading to cardioprotection. Dietary Que and its derivative epicatechin can improve the endothelial function through modulation of the blood nitric oxide concentration; they can also inhibit nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) that activates eNOS and hence enhance vascular activity.96
Another research has confirmed Que safety effect against myocardial ischemia-reperfusion injury (MIRI) in rabbits.97 Que restricted MIRI-induced NADPH oxidase 2 (NOX2), eNOS, and iNOS mRNA and protein expression. The actions of extracellular signal-regulated kinase (ERK1/2), p38 MAP kinase (mitogen-activated protein kinase), Akt, and glycogen synthase kinase-3 β (GSK3β) were considerably enhanced with pressure overload and attenuated by Que therapy. Moreover, Que may also improve the cardioprotective impact of amlodipine against damage activated in the heart by ischemia/reperfusion.98
Que also reduced the transcriptional action of NF-κB in human hepatocytes.99 In human C-reactive protein (CRP) transgenic mice, Que quenched interleukin-1β (IL-1β)-induced CRP appearance, as effected by sodium salicylate. In mice, Que significantly attenuated coronary artery illness by 40% (sodium salicylate by 86%).99 Yan et al.100 hypothesized that the Que heart hypertrophy prevention may be modulated by preventing activator protein 1 (c-fos, c-jun proteins) and initiating PPAR-γ signaling pathways.
Furthermore, in rat aorta, Que (0.1–100 μM) relaxed the contraction induced by pretreatment with 5 mM norepinephrine in a concentration-dependent manner. Finally, it was concluded that Que induces Ca2+ elevation, leading to NO production and Ca2+-activated K+ (KCa) channel activation in endothelium cell.101 Wang et al.102 reported that Que may act as a cardioprotector by initiating the PI3K/Akt signaling process and modulating the expression of Bcl-2 and Bax proteins through a study on male Sprague–Dawley rats.
In a placebo-controlled double-blind study, administration of 730 mg of Que per day for 28 days caused significant reductions in systolic (−7 ± 2 mm Hg), diastolic (−5 ± 2 mm Hg), and mean arterial pressures (−5 ± 2 mm Hg) in level 1 hypertensive patients.103 Overweight or obese subjects ranged between 25 and 65 years were exposed to a double-blind study of 6 weeks to examine the effects of Que (150 mg Que daily).104 Que plasma concentrations in fasting conditions increased from 71 to 269 nmol/L during Que treatment. Moreover, Que reduced SBP and HDL cholesterol levels, while total cholesterol levels, triacylglycerol (TAG), and the LDL:HDL cholesterol and TAG:HDL cholesterol rates were unaltered.
Que showed anti-inflammatory properties in patients with coronary artery disease (CAD), through a decrease in the transcriptional activity of the NF-κB. To prove this effect, Chekalina et al.105 designed a study to determine the effect of Que on the indicators of chronic systemic inflammation in CAD patients. The study involved 85 CAD patients, stable angina pectoris, functional class II, and heart failure.105 Thirty patients received Que at a daily dose of 120 mg for 2 months, while the remaining 55 patients considered as the control group received β-blockers, statins, and aspirin. An increase in the levels of IL-1β and tumor necrosis factor-α (TNF-α) and a moderate increase in IL-10 levels were detected in the serum of patients with CAD. Under the influence of Que, levels of IL-1β and TNF-α were reduced and IL-10 levels tended to decrease. Also, Que decreased the expression of the inhibitor of kappa βα (Ikβα) gene relative to the control group.
In a 10-week double-blind randomized clinical test on 72 females having type 2 diabetes, the subjects were assigned to Que (500 mg capsule daily) and placebo groups. The biochemical parameters were compared between the baseline and the end of the study. Consequently, Que consumption reduced systolic hypertension considerably relative to the placebo group, and the HDL levels were significantly reduced in both categories along with changes in total cholesterol levels, LDL, and triglycerides. Moreover, Que decreased the serum concentration of TNF-α and IL-6.106
In another clinical study, 23 healthy men were fed 4.3 g of onion extract (containing 51 mg of Que) once a day for 30 days.107 The onion extract consumption did not considerably affect fasting flow-mediated vasodilation (FMD) but enhanced the postprandial FMD considerably.
2.1.3. Autophagy
Autophagy is a degradation process through which macromolecules, misfolded proteins, and organelles are degraded by lysosomes and recycled,108 which constitutes an indispensable process for cellular homeostasis.109 In this context, targeting autophagy by natural products is considered a potential therapeutic strategy for the prevention and treatment of several disorders.
Que has been reported to promote autophagic responses and thus protects human umbilical vein endothelial cells (HUVECs) against high-glucose-induced damage.53 This study showed that Que can significantly promote cell survival via the induction of glutathione (GSH) and Beclin-1 and microtubule-associated protein light chain-3- II (LC3-II)/LC3-I ratio as well as reduction of oxidative stress marker levels. This promising activity can be beneficial in ECs insufficiency, and this will provide a high therapeutic potential for diabetic angiopathy.53
Similarly, Que has significant capacity to induce apoptotic and autophagic responses in pulmonary arterial smooth muscle cells (PASMCs).54 This activity was connected to the ability of Que to induce the expression and transcriptional activity of the autophagy regulator, forkhead box protein O1 (FOXO1). This indicates that Que is a promising candidate for treating hypoxia-associated pulmonary arterial hypertension via promoting hypoxia-induced autophagy. This is specifically applied in combination with autophagy inhibitors.54
Intriguingly, Que has significant abilities to ameliorate lysosome damage-mediated autophagy dysfunction in alcohol liver disease (ALD).55 mTOR-TFEB-mediated activity was associated with the induction of lysosomal-associated membrane protein 1 (LAMP-1), LAMP2, and Ras-related protein (Rab7) expression as well as reduction of LC3-II and p62 levels.55
2.1.4. Effect on Alzheimer’s Disease
Que is used for the development of anti-Alzheimer’s disease (AD) formulations due to its neuroprotective effect against oxidative stress and excitotoxicity via regulating apoptosis mechanisms.65 Diverse mechanisms have been suggested for the Que neuroprotective actions including amyloid-β (Aβ) aggregation inhibition, intracellular neurofibrillary tangles (NFTs) formation inhibition, amyloid precursor protein (APP) inhibition, cleaving enzyme (BACE1) inhibition, acetylcholinesterase (AChE) inhibition, and others attenuating the oxidative stress in AD (Table 1).
Que and its bioactive molecules are helpful to ameliorate neurogenesis and neuron longevity modulating signaling pathway such as P13 kinase, AKT/PKB tyrosine kinase, and protein kinase C in AD.61,110
Many in vitro studies demonstrated that the hydroxylfunctional groups of the B-ring of Que play an important role in Aβ aggregation inhibition and disrupt the mature fibrils by forming hydrogen bonds with the β-sheet structure of Aβ.110 Moreover, other studies have shown that Que increased in vitro neuronal culture survival.17 Additionally, Que at 100 μM displayed a considerable inhibition of β-site APP cleaving enzyme 1 (BACE1) in vitro in a cell-free system by 11.85%.111
The other characteristic pathological implication of AD is the appearance of NFTs, with the core protein being tau, and its accumulation into tangles involves phosphorylation.112 Que efficiently inhibited tau pathology via different mechanisms such as reducing hyperphosphorylation of tau protein with retrogressive action on the hyperphosphorylation process,111 inhibiting GSK3β activity and consequently inhibiting hyperphosphorylation of tau protein or possessing anti-HSP70 activity, and thus reducing tau pathology via attenuating the hyperphosphorylated tau level.110
The decline in cholinergic neurotransmission is a reliable and early symptom of AD, and AChE is a noteworthy therapeutic target for symptomatic improvement in AD.113 Que in vitro competitively inhibits AChE and diminished AChE activity in the hippocampal region.110 In one study, Que (50 mg/kg b.w.) stopped the increase of AChE activity due to diabetes in the cerebral cortex and hippocampus. In relation to this, through restoring the function of acetylcholine (ACh), Que mitigated the cholinergic signaling and facilitated regaining lost memory in diabetic rats.114 In another study, a single dose of Que avoided the memory impairment caused by scopolamine.115 Moreover, a molecular docking study predicted that Que is superior to conventional AchE inhibitor drugs.116
2.1.5. Effect on Tyrosinase Activity
Tyrosinase is the key enzyme in the pathway of melanogenesis, which involves three pigmentary specific enzymes in melanosomes117 and is widely present in mammalians, plants, bacteria, and fungi.118,119 Tyrosinase catalyzes the hydroxylation of monophenols to O-diphenols (monophenolase activity), then to O-quinone (diphenolase activity), and finally to melanin. Excessive melanin accumulation leads to several pigmentation disorders, such as melasma age spots, freckles, and melanoma.120 Inhibiting tyrosinase overexpression may decrease the generation of melanin. Que could inhibit both monophenolase and diphenolase activities of tyrosinase, and it inhibits diphenolase reversibly and competitively (IC50 of 3.08 ± 0.74 × 10–5 mol/L).121
2.1.6. Effect on Arthritis
Arthritis is associated with decreased motion anility, and its symptoms generally include swelling, pain, stiffness, and redness.122 There are over 100 types of arthritis, and the most common are osteo, gout, and rheumatoid arthritis. Genetic, hormonal, and environmental factors have been related to arthritis. Treatment of arthritis includes drug steroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and/or surgery.123,124 A prolonged use of steroids may lead to osteoporosis and fractures, while a prolonged use of NSAIDs can lead to gastric ulcers and renal damage. Chronic joint inflammation can lead to destruction of joints, thus requiring partial or total replacement of the destroyed tissues surfaces via arthroplasty.124
Que was reported to reduce pain and inflammation associated with arthritis.69 This dietary flavonoid inhibited, in mice, knee joint mechanical hyperalgesia, edema, and leukocyte recruitment in a dose-dependent manner without adverse effects on other organs such as liver, kidney, or stomach.69 Que mechanisms included inhibition of oxidative stress, cytokines and COX-2 production, and proteoglycan degradation as well as activation of nuclear factor-erythroid 2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) signaling pathway.69
Additionally, Que has been reported as a potential drug for the treatment of rheumatoid arthritis.70 The administration of Que was effective alone than methotrexate or in combination with methotrexate to reduce joint inflammation, in mice, and to provide the highest protection.70 The mechanisms included reduction of TNF-α, IL-1β, IL-17, and monocyte chemoattractant protein-1 (MCP-1) levels.
Therefore, Que represents a promising natural compound to treat pain and inflammation associated with arthritis.
2.1.7. Effect on Microbial Infections
Streptococcus suis is a globally distributed zoonotic Gram-positive bacterial pathogen.125,126S. suis-associated human infection, mostly from the pig industry, may lead to meningitis, septicemia, endocarditis, and deafness.127 Suilysin, a cytotoxic toxin secreted by S. suis, plays a potential role in infection. Thus, the effective interference of suilysin is a line of defense in the treatment of S. suis infection.
Que has been shown, in mice, to inhibit suilysin activity as well as decreasing S. suis-induced cytotoxicity. Its mechanism included the effective reduction IL-1β, IL-6, and TNF-α levels.55 Additionally, Que was effective to inhibit reactive oxygen species (ROS) production and lipid peroxidation in gentamicin-induced oxidative stress.72 Intriguingly, this study, which was performed in vitro on human leukocytes and in vivo on whole rat blood, showed that Que has more inhibitory capacity than ascorbic acid, the reference inhibitor.72 Thus, Que may be considered as a potential candidate for the treatment of S. suis infection by targeting suilysin and its associated inflammation.
2.1.8. Effect on Liver Diseases
Liver plays an indispensable role in energy metabolism, protein synthesis, production of biochemicals necessary for digestion, and detoxification of various metabolites and drugs.128 According to recent statistics, liver diseases cause approximately 2 million deaths/year worldwide, accounting for approximately 3.5% of worldwide deaths.129 Additionally, alcohol consumption was reported, according to the global burden of disease (2018), to account for approximately 10% of global deaths among populations aged 15–49 years in 2016.73 Que was shown to ameliorate ethanol-induced liver steatosis in chronic-plus-binge-ethanol feeding mice.73 This therapeutic potential was associated with the improvement of lipophagy. Additionally, Que attenuates liver damage, increased serum triglyceride, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels.73 Moreover, some xenobiotics can have severe adverse effects leading to liver toxicity. Among xenobiotics, rotenone, a natural pesticide, has been reported to cause liver toxicity.130 Que displayed significant ameliorative activity in rotenone-induced hepatocellular dysfunction.74
2.1.9. Anticancer Effects
Many reports were detected on the anticancer activity of Que, and they are summarized in Table 2.
Table 2. Reported Anticancer Activity of Quercetin.
| extract/compound | doses | In vitro/in vivo | route of administration | model | effect | refs |
|---|---|---|---|---|---|---|
| Que | 5 μM | in vitro | H1975 and A549 human lung cancer cell lines | Que significantly inhibited nickel-mediated invasion in H1975 and A549 human lung cancer cell lines; inhibited inflammatory mediators secretion; inhibited mRNA as well as protein expression of TLR4 and Myd88; reduced IKKβ as well as IκB phosphorylation; reduced NF-κB nuclear level; reduced MMP-9 expression; TLR4/NF-κB signaling pathway deactivation. | (131) | |
| Que | 50 and 75 μM | in vitro | PA-1 human ovarian cancer cell line | Que significantly reduced cell viability through a dose-dependent manner; enhanced apoptosis in invasive ovarian cancer cell lines (further assured by AO/EtBr dual stain, DAPI stain as well as DNA fragments); reduced Bcl-2 and Bcl-xL; increased Bad, Bid, Bax, caspase 3, caspase 9, as well as cytochrome c; enhanced mitochondrial-induced apoptotic pathways, so suppresses the invasive ovarian cancer cell growth. | (132) | |
| Que | 50 μM | in vitro | HSC-6 and SCC-9 human oral cancer cells | Que inhibited viability of cells, their migration and invasion (using MTT assay and western blot assay); decreased MMP-9 and MMP-2 abundance; downregulated miR-16 and upregulated HOXA10 (using qRT-PCR), where the relation linking miR-16 and HOXA10 has been tested using luciferase activity assay, RNA immunoprecipitation (RIP) as well as western blot analysis. | (133) | |
| Que | 10 mg/mL | in vitro | NPC039 nasopharyngeal cancer cell line. | Que decreased cell viability up to 36% compared to control after only 24 h (using MTT assay); suppressed VEGF expression and NF-κB activity (using RT-PCR and ELISA). | (134) | |
| Que | 40 μM | in vitro | LPS-mediated inflamed WI-38 lung fibroblast cells | Que may control LPS-mediated inflammation in lung fibroblasts through increasing cell viability and decreasing its apoptosis (using CCK-8 assay and Annexin V-FITC/PI Stain, respectively); reduced miR-221 expression level (using qRT-PCR), IL-6 as well as TNF-α (using ELISA) in the cells; suppressed NF-κB as well as JNK signaling pathways (using western blot). | (135) | |
| Que | 50 μM | in vitro | D44+/CD24– cancer stem cells and MCF-7 breast cancerous cell line. | Que may have a potential role in treating breast cancer through suppressing breast cancerous stem cells (CD44+/CD24−) via suppressing PI3K/Akt/mTOR- pathway; suppression of cell viability, formation of clones, and generation of mammospheres; arrest of G1 phase; suppression of Cyclin D1 as well as Bcell lymphoma2 overexpression; enhancement of Bcl-2-like protein4 expression; downregulation of α estrogen receptor. | (136) | |
| Que | 30 μM | in vitro | MCF-7 and MDA-MB-231 human breast cancer cell lines | Que may have a notable effect in controlling breast cancer through suppressing cancer cells mobility (trans-well invasion as well as wound healing assays) by suppressing glucose uptake as well as lactic acid production and reducing PKM2, GLUT1, and LDHA levels, so limiting the tumor cell migration; downregulation of MMP-2, MMP-9, and VEGF expression (western blot); induction of autophagy through deactivating Akt-mTOR pathway. | (137) | |
| Que and its metabolites | IC50 value: Que 14.0 mg/mL and its metabolites, methylQue 11.0 mg/mL and Que glucuronide 4.0 mg/mL. | in vitro | HL-60 Leukemic cells | Que and its metabolites (after microbial transformation), methylQue and Que glucuronide, proved their efficacy as cytotoxic agents (using MTT as well as TB assays). | (138) | |
| Que | 50 mg/kg b.w./day, for 9 days. | in vivo | Intraperitoneal | cisplatin-induced toxicity in kidney and tumor tissues Male Fischer F344 rats | Que succeeded in controlling the nephrotoxicity caused by cisplatin without affecting its antitumor action through decreasing the oxidative stress, inflammation and apoptotic effects. | (139) |
| Que | 40 μM | in vitro | CD44+/CD133+ and CD44+ prostate cancerous stem cells | Que may control prostate cancer in stem cells through inhibiting their proliferation through downregulation of MK expression, which led to reduction in cell migration and formation of spheroids; co-treatment of MK siRNA with Que decreased cell survival, increased apoptosis, and arrested G1 phase more efficiently than the single therapy; the co-therapy deactivated PI3K, AKT, and ERK1/2 pathways and decreased p38, ABCG2, and NF-κB expression. | (140) | |
| Que | 20, 40, 60, 80, or 100 μM | in vitro | U2OS and Saos-2 bone cancer cell lines | Que can inhibit cancerous cell proliferation and invasion through suppression of PTHR1 by reducing its expression; decreased cell viability and its adhesion and migration; attenuated MMP-2 and MMP-9. | (141) | |
| Que | 20 and 40 μM | in vitro | A431-III cell lines. | Que may have a potential effect in inhibiting metastasis of cancerous cells. Que decreased protein level as well as RPS19 activity through blockade of Akt/mTOR/c-Myc pathway. | (142) | |
| Que | 2, 4, and 8 μM | in vitro | Human A375 melanoma cells | Que combined with curcumin suppressed the cancerous cell proliferation through decreasing cell viability (MTT assay), decreasing the number of colonies (using colony assay), and altering Wnt/β-catenin signaling pathway (using western blot) | (143) | |
| Que | 20 μM | in vitro | Human MCF-7 breast cancer cell line | Que, combined with doxorubicin, enhanced the antiproliferative effect of breast cancerous cells through decreasing the cell viability (using SRB assay) | (144) | |
| Que | 20, 40, 60, and 80 μM | in vitro | Human SW480 cells and clone 26 colon cancer cell lines | Que decreased the cell viability of the cancerous cells (using MTT assay); decreased cyclin D1 expression (using RT-PCR and western blot); downregulation of Wnt/β-catenin pathway. | (145) | |
| Que | 20 μM | in vitro | 4T1 murine mammary cancer cells | Que presented a dose-dependent suppression of cell progression and prompted apoptosis in cancerous cell; inhibited luciferase activity; inhibited Wnt/β-catenin pathway through decreasing β-catenin protein stabilization. | (146) | |
| Que | 10 μM | in vitro | Human SK-Br3, MDA-MB-453, and MDA-MB-231 breast cancer cell lines | using a lower dose of Que also succeeded in arresting G1 phase of cancerous cell cycle through trapping E2F1; inhibited pRb phosphorylation of pRb; DNA damage; Chk2 activation; downregulated cyclin B1 as well as CDK1; inhibited the employment of NF-Y to cyclin B. | (147) | |
| Que | 25, 50, and 75 mg/kg b.w. | in vivo | Intraperitoneal | Murine Dalton’s lymphoma cells transplanted- AKR strain mice | Que had a potential role in the downregulation of PKC activity due to their antioxidant activity; improved apoptotic potential, as detected by measures of caspase 3, caspase 9, PARP, PKCa, as well as nuclear condensation; decreased cell survival; stimulated death receptor-induced apoptosis through TNFR1 in the cancerous cells. | (148) |
2.1.10. Antiparasitic Activity of Quercetin
Despite the pharmacological properties of Que, interest has been focused on its antiparasitic potential. In this sense, the capacity of this flavonol to affect the parasite growth and disease progression has been demonstrated in different preclinical models, as well the potential of Que-related compounds (Table 3). In this sense, the main reports have been dedicated to evaluate the in vitro potentialities on the main protozoa parasites that cause the major mortality and morbidity, including Leishmania, Trypanosoma, and Plasmodium. Nevertheless, other reports related to activity against Toxoplasma gondii, Giardia lamblia, and Entamoeba histolytica have also been evidenced (Table 3).
Table 3. Quercetin and Related Compounds with Reported Effects on Parasites of Medical Importance Using Different In Vitro or In Vivo Models.
| parasite target | experimental models/mechanism of action | refs |
|---|---|---|
| Quercetin | ||
| Encephalitozoon intestinalis | in vitro/antiparasitic activity | (149) |
| Giardia lamblia and Entamoeba histolytica | in vitro/high antigiardia activity and slight antiamebic activity | (150) |
| Leishmania amazonensis | in vivo/antileishmanial effect on due to the ROS generation and disrupted parasite mitochondrial function. | (151) |
| Leishmania braziliensis | in vitro/inhibition of promastigote forms due to the increase of the reactive oxygen species production, phosphatidylserine exposure, and loss of plasma membrane integrity. Reduction of parasites number in infected macrophages associated with the reduction of TNF-α and increase of IL-10 synthesis. | (152) |
| Leishmania donovani | in vitro/high activity but slight selectivity for parasite DNA topoisomerase I | (153) |
| Leishmania mexicana | in vitro/inhibition of parasite cathepsin L | (154) |
| Leishmania panamensis | in vitro/selective inhibition of nuclear DNA topoisomerase II enzymes from promastigotes | (155) |
| Plasmodium falciparum | in vitro/antiplasmodial potential | (156) |
| Plasmodium falciparum and Plasmodium berghei | in vivo/high reduction of parasitaemia in animal model | (157) |
| Toxoplasma gondii | in vitro/inhibits the synthesis of heat shock proteins (HSP90, HSP70, and HSP27). | (158) |
| Trypanosoma brucei | in vitro/inhibition of RNA triphosphatase Cet1 | (159) |
| Trypanosoma brucei | inhibition of hexokinases 1 | (160) |
| Trypanosoma brucei | in vitro/caused a loss of mitochondrial membrane potential and marked DNA degradation. | (161) |
| Trypanosoma brucei gambiense | in vitro/induced the death of parasite by apoptosis | (162) |
| 4-Hydroxycoumarin Derivatives (Isosters of Quercetin) | ||
| Trypanosoma cruzi | in vitro/moderate trypanocidal activity | (163) |
| Isoquercitin | ||
| Entamoeba histolytica | in vitro/inhibition of parasite viability and growth | (164) |
| Quercetin Analogues | ||
| Plasmodium falciparum | in vitro/antiplasmodial potential | (156) |
In another context, the antiparasitic activity of several natural-derived products has been attributed to the presence of Que or derived compounds (Table 4), which constitutes one of the most common metabolites in plants. Currently, an attractive alternative to synthetic drugs is the search for active molecules from natural sources, such as the medicinal plants that have been used in the treatment of parasites since ancient times. As could be observed, several plant-derived products with antiparasitic activity have been correlated with the presence of Que or some of the related compounds. In general, different families and plant species have been studied on parasites of medical relevance, including protozoa and helminths. Also, in this case, we note that some studies have been conducted on animal models, which provide interesting and more comprehensive information.
Table 4. Quercetin and Related Compounds from Plant-Derived Products with Reported Effects on Parasites of Medical Importance.
| family | natural source | isolated compound | parasite target | comments | refs |
|---|---|---|---|---|---|
| Amaranthaceae | Atriplex lindleyi Moq. | Que | Plasmodium falciparum | in vitro antimalarial activity. | (165) |
| Asteraceae | Bidens pilosa L. | Que-related glucopyranoside | Plasmodium spp. | reduction of parasitaemia in animal model. | (166) |
| Pluchea carolinensis (Jacq.) G. Don | Que | Leishmania amazonensis | activity against promastigotes and amastigotes. No effect on animal model of leishmaniasis. | (167) | |
| Crassulaceae | Kalanchoe pinnata (Lam) Pers. | Que aglycone-type structure | Leishmania amazonensis | activity against amastigotes and potent oral efficacy on cutaneous leishmaniasis. | (168) |
| Cucurbitaceae | Momordica charantia L. | Que-enriched subfraction | Fasciola hepatica | affect the embryonic development of eggs and strongly inhibited the miracidium hatching. | (169) |
| Fabaceae | Dimorphandra gardneriana Tul. | Que | Leishmania infantum | active on promastigotes and amastigotes. | (170) |
| Quercus infectoria Olivier | Que | Leishmania major | extract showed in vitro and in vivo antileishmanial activity, which was correlated with Que amount. | (171) | |
| Leguminosae | Mezoneuron benthamianum Baill. | Que | Plasmodium falciparum | in vitro antimalarial activity. | (172) |
| Malvaceae | Guazuma ulmifolia Lam. | ethanol extract | Leishmania braziliensis, Leishmania infantum and Trypanosoma cruzi. | significant antikinetoplastid in vitro inhibition. | (173) |
| Melastomataceae | Miconia langsdorffii Cogn. | Que derivatives | Schistosoma mansoni | cause a significantly reduction in motor activity of adult worms. | (174) |
| Meliaceae | Azadirachta indica A. Juss. | Que -3-rhamnoside, Que-3-rutinoside | Plasmodium falciparum | inhibition of substantial oxidant stress during malarial infection. | (175) |
| Myrtaceae | Psidium acutangulum Mart. ex DC | glycosylated Que derivatives | Plasmodium falciparum | in vitro antimalarial activity. | (176) |
| Psidium brownianum Mart. ex DC. and Psidium guajava L. | Que and derivatives | Trypanosoma cruzi, Leishmania brasiliensis and L. infantum | potent in vitro antikinetoplastidae activity. | (177) | |
| Poaceae | Cymbopogon citratus (DC.) Stapf | Que and derivatives | Giardia lamblia | in vitro and in vivo effect, correlated with phenolic compounds abundance. | (178) |
| Polygonaceae | Fagopyrum esculentum Moench | Que | Leishmania donovani | in vitro antileishmanial activity. | (179) |
| Rutaceae | Diosma pilosa I.J Williams | Que | Plasmodium falciparum | in vitro antimalarial activity. | (180) |
As could be appreciated in previous overview (Tables 3 and 4), remarkable efforts have contributed to elucidate the Que mechanism of action. In this sense, the antiparasite effect has been correlated with the disruption of mitochondrial function and the inhibition of different important enzymes and molecules including heat shock proteins (HSP), acetylcholinesterase enzyme, DNA topoisomerases, and kinases. Probably, the different targeted molecules promote the death in parasite by apoptosis, which has been evidenced in particular with the increase of ROS and DNA degradation. In addition, indirect biological effects of Que could have positive antiparasite effects, due to the induction of microbicidal response such as the production of nitric oxide and some cytokines.
In general, the development of drugs to treat parasitic diseases has been scarce, due to the fact that these diseases are more often presented in developing countries. However, as can be appreciated, Que and derivatives could constitute a source to explore new drugs with a wide antiparasitic spectrum. Several studies have been reported to prove the in vitro activity; although few reports described potentialities of this flavonol in animal models. However, some formulation has been designed and evaluated on experimental in vivo model, which is described in next work.
2.2. Quercetin in Preclinical and Clinical Trials
2.2.1. Preclinical Anticancer Effects
Cancer is an important problem in both developed and developing countries. Plants are important and promising sources for the treatment of cancers as well as many other diseases due to the secondary metabolites that they contain. Therefore, scientists are trying to discover new plant species and new active substances against different types of cancers, as well.181
Polyphenols, flavones, and flavonoids have great anticancer potential in different types of cancers.182 Que is an important flavonoid that might be considered as an important agent in various cancer cells, and since it is predominantly found in Western diet, we might benefit from its protective effect just by taking it via our diets of as a food supplement. Moreover, Que is considered to be a chemopreventive that acts on signal transduction pathways as modulators to prevent, inhibit, or reverse carcinogenesis. By this activity, it shows many activities such as apoptosis, cell migration, differentiation and proliferation, oxidative balance, and inflammation.183 It also shows antioxidative activity and inhibits enzymes that activate carcinogens.184
2.2.1.1. Pancreatic Cancer Preclinical Effects
Zhou et al.185 studied the efficacy of Que against pancreatic cancer, and it was observed that apoptosis resistance was reverted and proliferation, angiogenesis, cancer stem-cell marker expression were reduced with Que treatment, which is a dietary polyphenol. In another study by Serri et al.,186 the combination of gemcitabine with Que exerted a synergistic effect especially in the migration of pancreatic cancer cells. Though this is an in vitro study, it is worth mentioning that since pancreatic adenocarcinoma is one of the most fatal types of cancer, it is resistant to drugs, demonstrates aggressive behavior, leads to unpredictable genetic mutations, and is usually diagnosed at a late stage and usually after metastasis.
2.2.1.2. Osteosarcoma Preclinical Effects
Osteosarcoma is a bone disease; it is known to be a malignant neoplasm mostly in teenagers and also in adults, and it is a worldwide health problem. Que is reported to be beneficial against this disease by inhibiting the proliferation and apoptosis of human osteosarcoma cells U2OS/MTX300. It also reduces the invasion, adhesion, proliferation, and migration of osteosarcoma cells with the inhibition of parathyroid hormone receptor 1.141
2.2.1.3. Ovarian Cancer Preclinical Effects
Ovarian cancer is a fatal form of reproductive cancers due to its late diagnosis and silent and obscure symptoms,182 and it is the most leading cause of mortality among other gynecological cancer types.187 Common cancer therapies also tend to encounter resistance by these cells. On the other hand, Que was shown to be cytotoxic to cancer cells but does not exert harmful effect on health ones. It is considered to show effect due to its anti-inflammatory, pro-oxidative, apoptotic, cell cycle arrest induction, and antiproliferation effects and is known to be an ideal agent especially in combination with other anticancer drugs.188
In the treatment of ovarian cancer, usually platinum drugs (i.e., cisplatin, oxaliplatin) are used. However, these drugs have side effects and lead to drug resistance; therefore, other solutions were sought. In a study in which two human epithelial ovarian cancer cell lines (A2780 and its cisplatin resistant form A2780cisR) were used, it was concluded that the administration of Que 2 h before the usage of platinum drugs might sensitize cancer cells to the action of platinum drugs, and this would be beneficial in dealing with the development of drug resistance.
2.2.1.4. Breast Cancer Preclinical Effects
The beneficial property of Que was established in 1995 in a study by Singhal et al.,189 in which it was recommended in the treatment of relapsed breast carcinoma cases that cannot be operated. In highly invasive breast cancer cells, Que was administered along with doxorubicin and it was found to potentiate the antitumor effects of the synthetic drug. By combining these two substances together, Que was also shown to reduce the side effects of the synthetic drug in nontumoral cells; therefore, it is considered to be a promising agent in the development of chemotherapeutic combinations in the treatment of breast cancer.190
2.2.1.5. Cervical Cancer Preclinical Effects
Que was found to be beneficial against cervical cancer in addition to ovarian cancer and breast cancer. It exerts this effect via p63 induction and NF-κB inhibition and thus inhibits cancer progression. Due to these properties, it can be concluded that the compound might be a candidate for anticancer drug design.191
2.2.1.6. Leukemia Preclinical Effects
Acute myeloid leukemia (AML) is an important type of hemolytic malignancy that arises from the disorder of hematopoietic stem cells,192 and it is the most common acute type of leukemia in adults.193 In this disease, leukemia cells undergo mutations,193 and since conventional treatments also have cytotoxicity and side effects, many plant-derived agents are being used in the treatment of this disease and Que was also tested against this type of malignancy and shown to possess antileukemia ability since it contributes as an apoptosis inductor194 and also it can sensitize acute myeloid KG-1 cells against a TNF-related apoptosis-inducing ligand, which is a biological cytokine.193
In chronic lymphocytic leukemia, Que was found to inhibit kinases such as PIM1, which is expressed in the lymphocytes of patients having chronic lymphocytic leukemia. It is recommended to be evaluated further in the stabilization of increasing lymphocyte counts.195
2.2.1.7. Colon Cancer Preclinical Effects
Colorectal cancer is an important health issue in developed countries. ErbB2 and ErbB3 are receptor tyrosine kinases associated with the growth of human colon cancer, and it was found that their expressions are high in HT-29 cells. In a study by Kim et al.,194 Que decreased the levels of these two enzymes in a dose-dependent manner and thus inhibits cell growth of colon cancer cells and induces apoptosis in these cells.
In another study by Ranelletti et al.,196 Que showed blocking action in the G0/G1 phase in HT-29 cancer cell lines, along with WiDr, COLO21, and LS-174T human colon cancer cell lines. This cell proliferation inhibition was dose-dependent and reversible. The growth of primary colorectal tumors was also demonstrated to be inhibited with Que according to another study by Ranelletti et al.197 again.
2.2.1.8. Gastrointestinal Cancer Preclinical Effects
Que, which is found in foods in its free form in addition to the β-glycoside form like rutin and quercitrin, was reported to induce G1 arrest in human gastric cancer cells in a study conducted in 1990.198 In AGS human gastric cancer cells, Que induced morphological changes and reduced total viability via apoptotic cell death; decreased antiapoptotic proteins of Mcl-1, Bcl-2, and Bcl-x; and increased the proapoptotic protein of Bad, Bax, and Bid.199 Que protected intestinal porcine enterocyte cells against H2O2-induced apoptosis via the inhibition of the mitochondrial apoptosis pathway.200
2.2.1.9. Oral Cancer Preclinical Effects
Oral cancer has a high mortality rate throughout the world. It is among the most widespread tumors in the world with poor prognosis. Que has a beneficial effect via the regulation of miR-16 and HOXA10 in oral cancer cells, and with this regulation, it inhibits cell viability, migration, and invasion. These properties make it a promising agent in oncotherapy.133
2.2.1.10. Other Cancer-Related Preclinical Effects
In addition to possessing anticancer effect in various cancer types alone, Que exerts anticancer effect with other substances as combined. For example, it is reported to increase apoptosis in human lung cancer H1299 cells exposed to trichostatin A, i.e., it increases the antitumor activity of trichostatin A, which is a histone deacetylase inhibitor.201 When combined with kaempferol, they show synergism and lead to a decrease in cell proliferation in human gut cell lines HuTu-80 and Caco-2 and also in PMC42 human breast cell line.202
It was also reported to activate apoptosis and different types of cancers, for example, it was found to inhibit cell proliferation in the nonsmall cell lung cancer strongly and also increased apoptotic cell population in cervical cancer by decreasing NF-κB expression.203 Some researchers suggested that Que has a hormetic nature, that is, it has a biphasic dose–response relationship (for example, in low doses, it shows antioxidant effect; however, in high doses, it has pro-oxidant property). This hormetic feature of the compound makes it an excellent candidate in cancer prevention studies.204 As a result, it has been concluded that Que is an important and efficient antioxidant and can be considered as a potent anticancer agent.205
2.2.2. Antiobesity and Antidiabetes Preclinical Effects
Obesity and diabetes are major health problems in developing and developed countries. These are multifactorial, complex, and chronic diseases and risk factors in the establishment of various ailments like hypertension, coronary heart disease, cancer, osteoarthritis, respiratory problems, etc.206 They can also lead to metabolic syndrome that includes insulin resistance, hyperglycemia, dyslipidemia, and hypertension. Since chronic inflammation was considered as one of the factors contributing to obesity-related metabolic diseases, Que’s anti-inflammatory effect might be beneficial against the development of these two important health problems.207
It is also beneficial in the development of diabetic nonalcoholic fatty liver disease (NAFLD) via the regulation of genes related to lipid metabolism and secretion of inflammatory mediators. Que was shown to contribute to the prevention of hepatic lipid accumulation and liver injury due to chronic-plus-binge-ethanol feeding.73 As a conclusion, Que is demonstrated to have antidiabetic effects due to various positive activities, for example, it downregulates gluconeogenesis enzymes and reduces the making of glucose in hepatocytes.208
2.2.3. Antiaging Preclinical and Clinical Effects
Antioxidants influence the process of aging. Que alleviates human mesenchymal stem-cell senescence due to its property to promote self-renewal and differentiation; it also restores the heterochromatin architecture of human mesenchymal stem cells. Due to these beneficial properties, Que is considered as a geroprotective agent against premature aging.210
In a study performed on human volunteers, topical application of Que and its derivative Que-caprylate has positive effects in regard to elasticity, moisturization, and depth of wrinkles.209 This study was performed for cosmetic purposes; however, there are other aspects of aging in relation to Que, or polyphenols in general. For example, progeroid syndromes are rare genetic disorders characterized with clinical properties of premature aging (Wermer syndrome and Hutchinson–Gilford progeria syndrome are among these).
2.2.4. Hypertensive Clinical Effect
Hypertension is systolic blood pressure above 140 mm Hg and diastolic blood pressure over 90 mm Hg consistently, which is an important problem. Que may reduce blood pressure via nonendothelial mechanisms and is a promising agent in the treatment of hypertension.211
In a study by Gormaz et al.,212 Que lowered blood pressure in hypertensive patients when administered for 28 days at a dose of 1 g/day. This effect might be due to the mediation of improved endothelial function via both increased nitric oxide release and decreased endothelin-1 production. In a randomized, double-blind, placebo-controlled, crossover study performed with human patients with stage 1 hypertension, administration of high-dose Que was shown to lead to a decrease in systolic, diastolic, and mean arterial pressure, and these results show that it can be used for the treatment of early-stage hypertension.213
2.2.5. Cardiovascular Preclinical and Clinical Effects
Cardiovascular disease is an important health issue especially in industrialized countries and are considered to be among the main reasons for morbidity and mortality.99 Que is considered to be a promising agent due to its great potential in the prevention and treatment of chronic diseases such as cardiovascular problems.79 For example, both Que and its metabolites can be beneficial in the early stages of atherosclerosis due to their involvement in monocyte recruitment. And atherosclerosis is known to be the leading cause of cardiovascular disease mortality.214
Que supplementation might reduce cardiovascular problems and prevent obesity-associated mortality though it was found to have no positive effect on body weight.215 Nevertheless, it possesses important heart-related advantages such as inhibition of LDL oxidation, vasodilatory effects not dependent on endothelium, protective effect under oxidative stress on nitric oxide, and endothelial function.216 It might also exert positive effects on the cardiovascular system by reducing inflammation,217 and it is considered to possess antiatherogenic effect.212
2.2.6. Antioxidant Preclinical Effects
Oxidant compounds are known to lead to many important diseases in human body; therefore, antioxidant compounds have gained importance in the prevention of oxidation-associated diseases/disorders.
Que is an excellent antioxidant and probably the most potent scavenger of reactive oxygen species and reactive nitrogen species due to its two antioxidant pharmacophores for free-radical scavenging.218 One of the oxidant activity-associated disorders is age-related macular degeneration (AMD) that leads to vision loss in the elderly.219 Retinal pigment epithelial cells (RPEs) are quite susceptible to damage by reactive oxygen intermediates. Foods rich in antioxidant compounds might reduce the risk of the development of AMD, and since Que has cytoprotective effects, it is considered as a good candidate for the prevention of early pathologic changes in age-related macular degeneration. Not only in AMD but Que might also be beneficial in the inhibition of oxidative stress-mediated neuronal damage since oxidative stress is considered as one of the major reasons for the development of neurodegenerative disorders and vascular pathologies in the brain due to its radical-scavenging and metal-chelating activities.110 It might also be an effective protector against neurodegenerative diseases caused by oxidative stress and apoptosis.220
2.2.7. Anti-inflammatory Preclinical Effect
Que is known to possess anti-inflammatory effect. It is effective in various inflammation models, and the analgesic activity of the compound is also considered to be linked to its anti-inflammatory property.221 It also has beneficial effects as a topical product prepared for rheumatoid arthritis. Arthritic disorders are very common throughout the world, and this topical product containing Que in a nanoemulsion-based gel would be beneficial in the prevention of joint dysfunction and disability in adults.222
Respiratory diseases are widespread in the pediatric population, especially pneumonia. Lung fibroblasts undergoing inflammatory damage is seen as the occurrence and development of disease; therefore, Que might be effective against pneumonia since it can alleviate inflammatory damage by ameliorating lipopolysaccharide-originated inflammatory damage of WI-38 lung fibroblasts.135
2.2.8. Other Uses/Activities
Since Que is a multifaceted compound, it is indicated in other disorders and/or conditions as well. Some of them are summarized as follows:
It can be used as a protective factor against some drugs in the treatment of schizophrenia. This disease is a severe, chronic psychiatric disorder and is thought to be associated with oxidative stress. Drug use in the treatment of this disease, for example, haloperidol, is associated with a risk of developing important side effects (i.e., movement disorder like drug-induced parkinsonism, akathisia, and dystonia). Que reduces lipid peroxidation of plasma exposed to haloperidol or amisulpride; therefore, it can be beneficial in the protection against antipsychotic drugs.223
Que might be used as a sunscreen, and it was demonstrated in a study that it was as effective as the compound homosalate, a common ingredient found in sunscreen products.224
Isoquercitrin was demonstrated to have antiviral effects against African historical and Asian epidemic strains of ZIKV in human hepatoma, epithelial, and neroblastoma cell lines. This study was performed as an in vitro study; however, it is an important disease and therefore it might be worth mentioning it here. This derivative prevents the internalization of virus particles into the host cell and therefore prevents their entrance into the cell. Therefore, it can be a promising antiviral compound in the prevention of Zika virus infection.225
Que might protect against dimethoate-induced oxidative stress. This compound is an organophosphate insecticide that acts by interfering with the functioning of cholinesterase (an enzyme required for the proper functioning of the nervous system) and therefore may lead to human poisonings. Que decreases lipid peroxidation and protein oxidation, increases superoxide dismutase and catalase activities in human lymphocytes, and hence protects against dimethoate-induced oxidative stress.226
Whitening cosmetics are very popular throughout the world due to their antimelanogenesis activities. Que has been tested for this effect in human trials. Though Que was found to be ineffective in hypopigmentation of human skin, its tested glycosides were found to be somewhat effective in concentration-dependent behavior.227
Que was tested in 12 healthy men for 7 days on bike riding performance via the measurement of VO2 max and time to fatigue. Both these parameters were found to increase; therefore, it could be concluded that this flavonoid might increase the endurance capacity of the skeletal muscle.228
Eccentric exercises are involved in training programs of athletes, and they involve lengthening of the skeletal muscle while producing force. However, this produces a mechanical stress and induces breaking of contractile components and then the occurrence of an inflammatory phase that leads to secondary muscle damage. In a double-blind, placebo-controlled, randomized study with crossover design carried out on 12 moderately active men, Que promoted membrane stability by preserving excitation–contraction coupling in myocytes. Therefore, it can be said that Que might improve general physical fitness and even might improve performance.
2.3. Quercetin Bioavailability and Related Issues
Despite the immense benefits of Que, it is limited by poor aqueous solubility, poor permeability, instability in physiological medium (stomach and intestine), short biological half-life, and extensive first past metabolism in the liver before reaching the systemic circulation resulting in poor oral bioavailability,229−231 which limits the application of Que pharmaceutically. Nevertheless, recent progress in the field of drug delivery has enabled the consequential development of alternative drug-delivery systems to counteract the poor bioavailability of Que and achieve better therapeutic results tailored to enhance its biological activity. Ideal delivery systems must protect Que in the upper gastrointestinal tract to avoid its instability while releasing it at colon with prolonged sustained release with enhanced bioavailability.231
2.3.1. Quercetin Nanoformulations: A Way to Overcome Bioavailability-Related Issues
Nanoparticles offer a promising platform to enhance Que bioavailability owing to their large surface area, ability to be customized for targeted delivery of drugs, improved bioavailability, protection against enzymes, and physiological conditions, and they provide a controlled release of medication from a single dose.232
Natural polymers have been extensively studied for Que delivery, such as chitosan, which is a natural polysaccharide that has been used for the preparation of Que nanoparticles. Que-loaded chitosan nanoparticles with enhanced encapsulation efficiency and sustained release with small size (<200 nm) were prepared by Baksi et al.,233 which showed a significantly reduced IC50 value compared to free Que in cytotoxicity assay as well as a significant reduction of tumor volume in comparison to disease control groups in mice. Que-loaded chitosan nanoparticles were also investigated by encapsulating Que into pH-sensitive self-assembled amphiphilic chitosan nanoparticles; blank nanoparticles were nonantiproliferative, while Que maintains its metabolism inhibition against MCF-7 cells after encapsulation.234 Zein was also used for Que loading, which provided high and sustained levels of the drug in plasma and higher relative bioavailability with enhanced anti-inflammatory action.235
Synthetic polymers were also used for the formulation of Que delivery systems. Que nanoparticles using synthetic poly(lactide-co-glycolide) (PLGA) nanoparticles were formulated in several studies. One study utilized Que-loaded PLGA nanoparticles using pluronic as a stabilizer, and conjugation with transferrin (Tf-QrPLGA) showed significant antiproliferative and cytotoxic effects on metastatic cell lines as well as apoptosis and cycle cell arrest.236 Lou et al.237 prepared gold Que loaded into PLGA nanoparticles that induced cell autophagy and apoptosis in human neuroglioma cells as well as suppressed tumor growth through activation LC3/ERK/Caspase 3 and suppression AKT/mTOR signaling. The antidiabetic action of Que was also reported using PLGA nanoparticles, involving the emulsion–diffusion–evaporation method, which showed enhanced antioxidant properties as well as increased oral bioavailability (523%) of Que with sustained plasma concentration for 6 days in vivo, suggesting a reduced dosing frequency of the nanoformulations.238
Polymeric micelles have also been utilized in the synthesis of quercetin nanoparticles. Chen et al.57 developed a lecithin-based nanomicelle (LMPM) delivery system, which improved the solubility of Que as well as achieved sustained release and higher bioavailability as well as enhanced cytotoxicity against MCF-7 breast cancer cells with no toxic effects. Tan et al.239 utilized nanomicells for preparing Que from diblock copolymer of poly(ethylene glycol) (PEG)-derivatized phosphatidylethanolamine (PE) as oral anticancer dosage forms, which showed enhanced bioavailability and stability in simulated gastric fluid as well as enhanced antitumor activity using the A549 cancer cell line and murine xenograft model with no apparent toxicity.240
Metallic nanoparticles were also used in Que nanoparticle formulation. One study used phenylboronic acid (PBA)-conjugated zinc oxide nanoparticles (PBA-ZnO) loaded with Que.241 The presence of PBA moieties over the nanoparticle surface facilitates targeted delivery of Que to cancer cells. Moreover, Que-loaded PBA-ZnO nanoparticles showed pH-responsive drug-release behavior and induced apoptotic cell death in human breast cancer cells (MCF-7) via enhanced oxidative stress and mitochondrial damage. Braga et al.242 incorporated Que and silver nanoparticles (AgNPs) into poly(vinyl chloride) (PVC) matrix, which demonstrated significant antimicrobial activity against food pathogens (Escherichia coli, Salmonella Typhimurium, and Listeria monocytogenes) and considerable effect in inhibiting bacterial growth, indicating a possibility of the prevention of microbial dissemination in foods with a significant antioxidant effect. Another study prepared Que/CdSe/ZnS nanoparticles (QCZ NPs), which showed antibacterial activity against drug-resistant E. coli and Bacillus subtilis as well as anticancer activities by affecting cell proliferation and migration in BGC-823 cells.243 Que-loaded silica nanoparticles synthesized using the oil-in-water microemulsion method act as antioxidants and anti-inflammatory agents. The prepared nanoparticles showed superoxide radical scavenging effects as well as higher cell viability and lower amounts of proinflammatory cytokines produced by macrophages.244 Another study focused on loading of Que on silica nanoparticles and investigated its effect on MCF-7 breast cancer cell lines, which showed cell cycle arrest, apoptosis, and reduced cell vitality and growth rate, thereby offering a promising approach in breast cancer prevention.245
Lipid-based nanoparticles were also utilized in Que encapsulation. Solid lipid nanoparticles were formulated to encapsulate Que with sustained release till 48 h, showing enhanced cytotoxic effect and a lower IC50 than free Que in MCF-7 breast cancer cells.246 Hatahet et al.247 tested three approaches to improve Que delivery to skin—liposomes, lipid nanocapsules (LNC), and smart Crystals—and concluded that Que smart Crystals enabled the superficial deposition of Que on top of the skin. On the other hand, LNC seems to allow Que delivery to viable epidermis that holds the promise for skin inflammatory disorders such as psoriasis, while liposomes showed the least favorable characteristics in terms of encapsulation efficiency and particle size. Vijayakumar et al.248 prepared solid lipid nanoparticle (SLN) dispersion containing Que using tripalmitin and lecithin as lipid core, and then the surface was coated with chitosan, which showed enhanced in vitro release and cellular uptake of Que using Caco-2 cells. Que-loaded cationic nanostructured lipid carriers consisting of glycerol monostearate, medium chain triglycerides, and soy lecithin were found to exhibit a slower release in vitro during the digestion compared to those of Que suspended in 0.5% (w/v) sodium carboxymethylcellulose aqueous solution.249
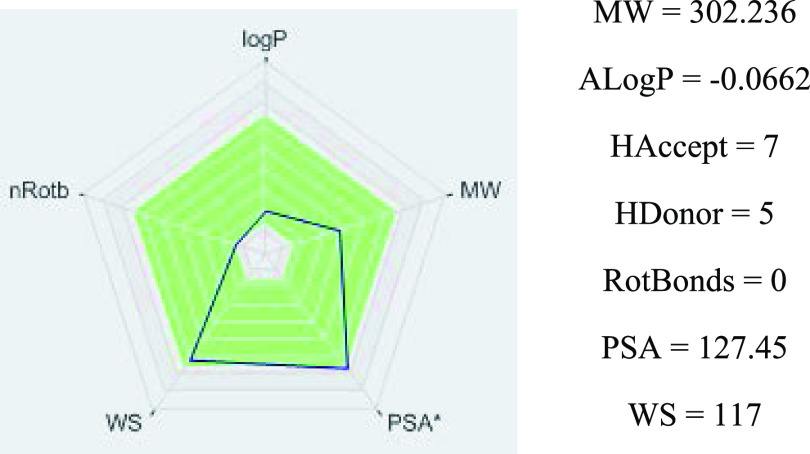
The poor bioavailability of flavonoids limits their biological effectiveness, which has been influenced by several factors.188,228 One of the more critical points is that in an aqueous solution, its solubility varies from 0.00215 g/L at 25 °C to 0.665 g/L at 140 °C. However, it shows high solubility at 25 °C in organic solvents such as ethanol (2 g/L) and dimethylsulfoxide (30 g/L).250 Then, as a consequence, the poor solubility and instability of this flavonol in aqueous intestinal fluids and the quick metabolism reduce its efficacy and oral bioavailability;218,251−253 which is almost 1% in humans.254 Nevertheless, Que is a druglike compliant with Lipinski’s rule of five without any violation (Figure 2), which predicts that a compound with ≤5 hydrogen-bond donors, ≤10 hydrogen-bond acceptors, molecular weight ≤500, and log P ≤ 5 more probably presents a high bioavailability.255
Figure 2.
Lipinski’s rule-of-five quercetin-like properties.
Different studies have demonstrated that Que circulated as types of conjugates in the blood. Thereafter, quercetin-3-O-β-d-glucuronide (Q3GA), a major metabolite of Que, and quercetin-3′-O-sulfate are distributed throughout the body where they may exert beneficial functions in target tissues.228,256 Hydrophilic Q3GA has been found to be deconjugated into hydrophobic Que aglycone at injured sites which, in turn, may improve the pathological conditions.228,257 Other Que-related compounds such as the Que monoglucosides present higher bioavailability,258 and isoquercetin (quercetin-3-O-β-d-glucopyranoside) exhibited a better pharmacokinetic profile in particular.259 However, it should be noted that nonconjugated aglycones, including methylquercetins, are scarcely detected in the human plasma.260
Alongside with this characteristic, of a BCS class II drug, a moderately short half-life after intravenous injection of Que was also observed.261,262 These drawbacks, which can limit the clinical administration of Que, demand urgent solutions to maintain free Que levels in blood and other tissues for a prolonged period.263 In this sense, nanoformulations have been strongly recommended as an alternative to Que therapy.
2.3.2. Nanodelivery Systems for Quercetin
Several biocompatible, biodegradable, affordable, and versatile nanoparticles are currently available for formulating bioactive compounds for health promotion and prevention. Nanoparticles can increase the solubility and stability of Que, enhancing its absorption, cellular uptake, and protection from premature degradation in the body, resulting in decreased toxicity.264
Numerous studies highlight that nanoencapsulation may elevate the bioactivities of Que, improving its sustained release.265−267 During an in vivo critical evaluation of Que as an antileishmanial agent through the use of different vesicular delivery systems, the nanoencapsulated Que was found to be the most potent in reducing the parasite burden in the spleen, with a better reduction of hepatotoxicity and renal toxicity compared to free drug or drug in other vesicular forms.264 Also, the gastro protection mediated by nanoencapsulated Que was better than by free Que, by preventing mitochondrial damage and regulation of key enzymes.267 The oral administration of Que-loaded poly(lactide-co-glycolide) nanocapsules containing triphenylphosphonium increased brain uptake and remarkable mitochondrial localization after cerebral ischemia reperfusion in young and aged rats.268 Besides, the encapsulation of Que in lipid-core nanocapsules of poly(ε-caprolactone) safely increased its oral efficacy in experimental cutaneous leishmaniasis.269
The potentiality of nano-Que as a promising adjuvant vehicle for chemotherapy has been demonstrated.270 In this sense, size, charge, and retention reached by a nanoform of Que in combination with anticancer drugs like adriamycin and mitoxantrone may solve multidrug resistance.271 Co-delivery of doxorubicin with phytosomes increased the efficacy of the drug in tumor microenvironment.272 Also, co-delivery of doxorubicin in a nanosized system based on gold nanocages increased the intracellular accumulation and could maximize the antitumor effect on malignant cells.273 Lately, co-loading of Que and vincristine through lipid polymeric nanocarriers exhibited the best in vitro and in vivo antitumor efficacy, showing a synergistic effect to overcome chemoresistant lymphoma.274
Chitosan has been used to design promising delivery systems capable of offering excellent protection and controlled released of Que. In this sense, micelles of a chitosan/polycaprolactam nanocomplex containing folic acid and Que efficiently inhibited breast cancer cells.275 Recently, the encapsulation of anticancer drugs into the nanomicelles of the Que–chitosan conjugate demonstrated that it could display a sustained-release profile in gastrointestinal simulation fluid and promote cellular uptake.276
Other polymers have allowed the preparation of biocompatible hybrid nanomaterials. To this effect, Que loading into nanosized polymeric micelles of methoxy poly(ethylene glycol)–cholesterol conjugate might be a potential formulation for the controlled and targeted drug delivery to tumor in a pH-sensitive manner.277 Likewise, conjugating Que into the backbone of poly(β-amino esters) guaranteed a uniform release and retention of its antioxidant activity.278 The synthesis of hybrid nanocomposite structures based on silica and PEG entrapping Que showed positive outcomes on the biocompatibility toward tested cell lines.279 Recently, a nanoplatform of poly-d-l-lactide-co-glycolide decorated with chitosan and PEG was suitable for the delivery of Que increasing its anticancer potency.280
Other therapeutic nanosystems containing Que have been reported as successful applications of nanotechnology to solve the problems of hydrolysis and short half-life. In this sense, new Que conjugated to gold nanoparticles was successfully evaluated for the first time against leishmanial macrophage infections, indicating its future application in the chemotherapy of wild- and resistant-type visceral leishmaniasis.281 Of note, solubility and bioavailability of Que were improved by a nanoliposomal presentation, which enhanced the cellular uptake and effectively displayed antioxidant protection.282
Moreover, the synthesis of a synergistic nanocomposite by conjugating Que and acetylcholine to the surface of Se nanoparticles enhanced the antibacterial activity against Methicillin-resistant Staphylococcus aureus.(283) Que-loaded nanolipidic carriers/solid lipid nanoparticles offered better drug loading and controlled release to brain with appreciable stability, with improvement of Que in vitro antioxidant performance.284 Que inclusion into silk fibroin nanoparticles conserved the antioxidant activity in comparison to the equivalent amount of free drug, being successfully transported and delivered gastrointestinally.285 Que-loaded phytosome nanoparticles were prepared using the thin-film hydration method, whose enhanced therapeutic efficacy over free Que was associated with better absorption.286
It stands out that the formulation of Que-loaded nanomicelles (Soluplus) improved the bioavailability of the drug and enhanced its antidiabetic effect.287 Lately, nano-Que significantly reduced the toxicity of the food mutagen 2-amino-3-methylimidazo[4,5-f]quinolone, which was higher than bulk Que.288
The previous observations raise the possibility of further studies using Que, in combination with existing clinically approved drugs to evaluate the synergism or using nanotechnology-related strategies.
3. Conclusions
There is an extraordinary attention to naturally occurring bioactive molecules, and Que is a great example of phenomenal therapeutic applications. The multitude of preclinical studies have allowed more in-depth studies, including the development of increasingly specific and targeted clinical studies. Apart from the several pharmacokinetic limitations of Que, the distinct techniques developed and currently applied have opened the possibility to overcome most of the previously identified gaps, thus allowing Que use under multiple preparations, for multiple purposes and even for different applications. Thus, the clinical applications and effectiveness of Que are remarkable. Nonetheless, as most of modern problems come from a wide variety of triggering agents, it would be of extreme importance to pay attention to the use of Que for preventive purposes, as well as in combination with multiple drugs to determine their abilities to potentiate or synergistically interact with these chemical agents, consequently reducing their side effects and related toxicity, at the same time increasing their overall efficacy and safety (as is the case of antitumor drugs).
Acknowledgments
This work was supported by CONICYT PIA/APOYO CCTE AFB170007.
Author Contributions
All authors contributed equally to the manuscript. J.S.-R. involved in the conceptualization; validation of resources, data curation, and writing were performed by all authors; J.S.-R., S.M.E., M.M., W.C.C., and N.M reviewed and edited the manuscript. All of the authors read and approved the final manuscript.
This research received no external funding.
The authors declare no competing financial interest.
References
- Spencer J. P.; Vauzour D.; Rendeiro C. Flavonoids and cognition: the molecular mechanisms underlying their behavioral effects. Arch. Biochem. Biophys. 2009, 492, 1–9. 10.1016/j.abb.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Kaşıkcı M. B.; Bağdatlıoğlu N. Bioavailability of quercetin. Curr. Res. Nutr. Food Sci. 2016, 4, 146–151. 10.12944/CRNFSJ.4.Special-Issue-October.20. [DOI] [Google Scholar]
- David A. V. A.; Arulmoli R.; Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytar O.; Kosyan A.; Taran N.; Smetanska I. Anthocyanin’s as marker for selection of buckwheat plants with high rutin content. Gesunde Pflanzen 2014, 66, 165. 10.1007/s10343-014-0331-z. [DOI] [Google Scholar]
- Miles S. L.; McFarland M.; Niles R. M. Molecular and physiological actions of quercetin: need for clinical trials to assess its benefits in human diseases. Nutr. Rev. 2014, 72, 720–734. 10.1111/nure.12152. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Bruno R. S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. 10.1016/j.jnutbio.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Russo M.; Spangnulo C.; Tedesco I.; Bilotto S.; Russo G. L. The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. 10.1016/j.bcp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Sultana B.; Anwar F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–888. 10.1016/j.foodchem.2007.11.053. [DOI] [PubMed] [Google Scholar]
- Pérez-Jiménez J.; Neveu V.; Vos F.; Scalbert A. Identification of the 100 richest dietary sources of polyphenols: An application of the phenol-explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. 10.1038/ejcn.2010.221. [DOI] [PubMed] [Google Scholar]
- Dinelli G.; Bonetti A.; Minelli M.; Marotti I.; Catizone P.; Mazzanti A. Content of flavonols in Italian bean (Phaseolus vulgaris L.) ecotypes. Food Chem. 2006, 99, 105–114. 10.1016/j.foodchem.2005.07.028. [DOI] [Google Scholar]
- Sytar O.; Bruckova K.; Hunkova E.; Zivcak M.; Konate K.; Brestic M. The application of muliplex flourimetric sensor for analysis flavonoids content in the medical herbs family Asteraceae, Lamiaceae, Rosaceae. Biol. Res. 2015, 48, 5. 10.1186/0717-6287-48-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokól-Lętowska A.; Osmianski J.; Wojdylo A. Antioxidant activity of phenolic compounds of hawthorn, pine and skullcap. Food Chem. 2007, 103, 853–859. 10.1016/j.foodchem.2006.09.036. [DOI] [Google Scholar]
- Haenen G. R.; Paquay J. B.; Korthouwer R. E.; Bast A. Peroxynitrite scavenging by flavonoids. Biochem. Biophys. Res. Commun. 1997, 236, 591–593. 10.1006/bbrc.1997.7016. [DOI] [PubMed] [Google Scholar]
- Dell’Albani P.; Di Marco B.; Grasso S.; Rocco C.; Foti M. C. Quercetin derivatives as potent inducers of selective cytotoxicity in glioma cells. Eur. J. Pharm. Sci. 2017, 101, 56–65. 10.1016/j.ejps.2017.01.036. [DOI] [PubMed] [Google Scholar]
- Mouradov A.; Spangenberg G. Flavonoids: a metabolic network mediating plants adaptation to their real estate. Front. Plant Sci. 2014, 5, 620. 10.3389/fpls.2014.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M.; Rubery P. H. Naturally occurring auxin transport regulators. Science 1988, 241, 346–349. 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Dajas F. Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 2012, 143, 383–396. 10.1016/j.jep.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Chirumbolo S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflammation Allergy: Drug Targets 2010, 9, 263–285. 10.2174/187152810793358741. [DOI] [PubMed] [Google Scholar]
- Adewole S. O.; Caxton-Martins E. A.; Ojewole J. A. Protective effect of quercetin on the morphology of pancreatic beta-cells of streptozotocin-treated diabetic rats. Afr. J. Tradit., Complementary Altern. Med. 2006, 4, 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H.; Kang M. J.; Choi H. N.; Jeong S. M.; Lee Y. M.; Kim J. I. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr. Res. Pract. 2011, 5, 107–111. 10.4162/nrp.2011.5.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G. J.; Li Y.; Cao Q. H.; Wu H. X.; Tang X. Y.; Gao X. H.; Yu J. Q.; Chen Z.; Yang Y. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019, 109, 1085–1099. 10.1016/j.biopha.2018.10.130. [DOI] [PubMed] [Google Scholar]
- Youl E.; Bardy G.; Magous R.; Cros G.; Sejalon F.; Virsolvy A.; Richard S.; Quignard J. F.; Gross R.; Petit P.; Bataille D.; Oiry C. Quercetin potentiates insulin secretion and protects INS-1 pancreatic beta-cells against oxidative damage via the ERK1/2 pathway. Br. J. Pharmacol. 2010, 161, 799–814. 10.1111/j.1476-5381.2010.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D. K.; Kang H. S. Anti-Diabetic Effect of Cotreatment with Quercetin and Resveratrol in Streptozotocin-Induced Diabetic Rats. Biomol. Ther. 2018, 26, 130–138. 10.4062/biomolther.2017.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessal M.; Hemmati M.; Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2003, 135, 357–364. 10.1016/S1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- Alam M. M.; Meerza D.; Naseem I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014, 109, 8–14. 10.1016/j.lfs.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Mahesh T.; Menon V. P. Quercetin allievates oxidative stress in streptozotocin-induced diabetic rats. Phytother. Res. 2004, 18, 123–127. 10.1002/ptr.1374. [DOI] [PubMed] [Google Scholar]
- Srinivasan P.; Vijayakumar S.; Kothandaraman S.; Palani M. Anti-diabetic activity of quercetin extracted from Phyllanthus emblica L. fruit: In silico and in vivo approaches. J. Pharm. Anal. 2018, 8, 109–118. 10.1016/j.jpha.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G.; Kumar S.; Sharma M.; Upadhyay N.; Kumar S.; Ahmed Z.; Mahindroo N. Anti-Diabetic, Anti-oxidant and anti-adipogenic potential of quercetin rich ethyl acetate fraction of Prunus persica. Pharmacogn. J. 2018, 10, 463–469. 10.5530/pj.2018.3.76. [DOI] [Google Scholar]
- Gaballah H. H.; Zakaria S. S.; Mwafy S. E.; Tahoon N. M.; Ebeid A. M. Mechanistic insights into the effects of quercetin and/or GLP-1 analogue liraglutide on high-fat diet/streptozotocin-induced type 2 diabetes in rats. Biomed. Pharmacother. 2017, 92, 331–339. 10.1016/j.biopha.2017.05.086. [DOI] [PubMed] [Google Scholar]
- Jeong S. M.; Kang M. J.; Choi H. N.; Kim J. H.; Kim J. I. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Pract. 2012, 6, 201–207. 10.4162/nrp.2012.6.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton K. E.; Rekman J. F.; Gunnink L. K.; Busscher B. M.; Scott J. L.; Tidball A. M.; Stehouwer N. R.; Johnecheck G. N.; Looyenga B. D.; Louters L. L. Quercetin inhibits glucose transport by binding to an exofacial site on GLUT1. Biochimie 2018, 151, 107–114. 10.1016/j.biochi.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X.; Ding Y.; Zhang Z.; Cai X.; Bao L.; Li Y. Quercetin but not quercitrin ameliorates tumor necrosis factor-alpha-induced insulin resistance in C2C12 skeletal muscle cells. Biol. Pharm. Bull. 2013, 36, 788–795. 10.1248/bpb.b12-00947. [DOI] [PubMed] [Google Scholar]
- Henagan T. M.; Cefalu W. T.; Ribnicky D. M.; Noland R. C.; Dunville K.; Campbell W. W.; Stewart L. K.; Forney L. A.; Gettys T. W.; Chang J. S.; Morrison C. D. In vivo effects of dietary quercetin and quercetin-rich red onion extract on skeletal muscle mitochondria, metabolism, and insulin sensitivity. Genes Nutr. 2015, 10, 451. 10.1007/s12263-014-0451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias A. S.; Porawski M.; Alonso M.; Marroni N.; Collado P. S.; Gonzalez-Gallego J. Quercetin decreases oxidative stress, NF-kappaB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. The. J. Nutr. 2005, 135, 2299–2304. 10.1093/jn/135.10.2299. [DOI] [PubMed] [Google Scholar]
- Sirovina D.; Orsolic N.; Koncic M. Z.; Kovacevic G.; Benkovic V.; Gregorovic G. Quercetin vs chrysin: effect on liver histopathology in diabetic mice. Hum. Exp. Toxicol. 2013, 32, 1058–1066. 10.1177/0960327112472993. [DOI] [PubMed] [Google Scholar]
- Khaki A.; Nouri M.; Fathiazad F.; Ahmadi-Ashtiani H.; Rastgar H.; Rezazadeh S. Protective Effects of Quercetin on Spermatogenesis in Streptozotocin-induced Diabetic Rat. J. Med. Plants 2009, 1, 57–64. [Google Scholar]
- Khaki A. A. Evaluation Effects of Quercetin on Liver Apoptosis in Streptozotocin-induced Diabetic Rat. J. Med. Plants 2009, 1, 70–78. [Google Scholar]
- Jahan S.; Iftikhar N.; Ullah H.; Rukh G.; Hussain I. Alleviative effect of quercetin on rat testis against arsenic: a histological and biochemical study. Syst. Biol. Reprod. Med. 2015, 61, 89–95. 10.3109/19396368.2014.998350. [DOI] [PubMed] [Google Scholar]
- Kanter M.; Aktas C.; Erboga M. Protective effects of quercetin against apoptosis and oxidative stress in streptozotocin-induced diabetic rat testis. Food Chem. Toxicol. 2012, 50, 719–725. 10.1016/j.fct.2011.11.051. [DOI] [PubMed] [Google Scholar]
- Nuckols T. K.; Keeler E.; Anderson L. J.; Green J.; Morton S. C.; Doyle B. J.; Shetty K.; Arifkhanova A.; Booth M.; Shanman R.; Shekelle P. Economic Evaluation of Quality Improvement Interventions Designed to Improve Glycemic Control in Diabetes: A Systematic Review and Weighted Regression Analysis. Diabetes care 2018, 41, 985–993. 10.2337/dc17-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom J. M.; Hernandez C.; Weber S. R.; Zhao Y.; Dunklebarger M.; Tiberti N.; Laremore T.; Simo-Servat O.; Garcia-Ramirez M.; Barber A. J.; Gardner T. W.; Simo R. Proteomic Analysis of Early Diabetic Retinopathy Reveals Mediators of Neurodegenerative Brain Diseases. Invest Ophthalmol. Visual Sci. 2018, 59, 2264–2274. 10.1167/iovs.17-23678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. H.; Xin X.; Wang Y.; Wu J. Z.; Jin Z. D.; Ma L. N.; Nie C. J.; Xiao X.; Hu Y.; Jin M. W. Pentamethylquercetin protects against diabetes-related cognitive deficits in diabetic Goto-Kakizaki rats. J. Alzheimer’s Dis. 2013, 34, 755–767. 10.3233/JAD-122017. [DOI] [PubMed] [Google Scholar]
- Chen B.; He T.; Xing Y.; Cao T. Effects of quercetin on the expression of MCP-1, MMP-9 and VEGF in rats with diabetic retinopathy. Exp. Ther. Med. 2017, 14, 6022–6026. 10.3892/etm.2017.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu E. P.; Cossu M.; Rassu G.; Giunchedi P.; Cerri G.; Pourova J.; Najmanova I.; Migkos T.; Pilarova V.; Novakova L.; Mladenka P.; Gavini E. Aqueous injection of quercetin: An approach for confirmation of its direct in vivo cardiovascular effects. Int. J. Pharm. 2018, 541, 224–233. 10.1016/j.ijpharm.2018.02.036. [DOI] [PubMed] [Google Scholar]
- Häckl L. P. N.; Cuttle G.; Dovichi S. S.; Lima-Landman M. T.; Nicolau M. Inhibition of angiotesin-converting enzyme by quercetin alters the vascular response to brandykinin and angiotensin I. Pharmacology 2002, 65, 182–186. 10.1159/000064341. [DOI] [PubMed] [Google Scholar]
- Linz W.; Wiemer G.; Gohlke P.; Unger T.; Scholkens B. A. Contribution of kinins to the cardiovascular actions of angiotensin-converting enzyme inhibitors. Pharmacol. Rev. 1995, 47, 25–49. [PubMed] [Google Scholar]
- Jalili T.; Takeishi Y.; Song G.; Ball N. A.; Howles G.; Walsh R. A. PKC translocation without changes in Galphaq and PLC-beta protein abundance in cardiac hypertrophy and failure. J. Am. Phys. 1999, 277, H2298–H2304. 10.1152/ajpheart.1999.277.6.H2298. [DOI] [PubMed] [Google Scholar]
- Collins J. F.; Pawloski-Dahm C.; Davis M. G.; Ball N.; Dorn G. W. 2nd; Walsh R. A. The role of the cytoskeleton in left ventricular pressure overload hypertrophy and failure. J. Mol. Cell. Cardiol. 1996, 28, 1435–1443. 10.1006/jmcc.1996.0134. [DOI] [PubMed] [Google Scholar]
- Sánchez M.; Galisteo M.; Vera R.; Villar I. C.; Zarzuelo A.; Tamargo J.; Perez-Vizcaino F.; Duarte J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 75–84. 10.1097/01.hjh.0000198029.22472.d9. [DOI] [PubMed] [Google Scholar]
- Machha A.; Achike F. I.; Mustafa A. M.; Mustafa M. R. Quercetin, a flavonoid antioxidant, modulates endothelium-derived nitric oxide bioavailability in diabetic rat aortas. Nitric Oxide 2007, 16, 442–447. 10.1016/j.niox.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Mezesova L.; Bartekova M.; Javorkova V.; Vlkovicova J.; Breier A.; Vrbjar N. Effect of quercetin on kinetic properties of renal Na,K-ATPase in normotensive and hypertensive rats. J. Physiol. Pharmacol. 2010, 61, 593–598. [PubMed] [Google Scholar]
- Prince P. S.; Sathya B. Pretreatment with quercetin ameliorates lipids, lipoproteins and marker enzymes of lipid metabolism in isoproterenol treated cardiotoxic male Wistar rats. Eur. J. Pharmacol. 2010, 635, 142–148. 10.1016/j.ejphar.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Rezabakhsh A.; Rahbarghazi R.; Malekinejad H.; Fathi F.; Montaseri A.; Garjani A. Quercetin alleviates high glucose-induced damage on human umbilical vein endothelial cells by promoting autophagy. Phytomedicine 2019, 56, 183–193. 10.1016/j.phymed.2018.11.008. [DOI] [PubMed] [Google Scholar]
- He Y.; Cao X.; Guo P.; Li X.; Shang H.; Liu J.; Xie M.; Xu Y.; Liu X. Quercetin induces autophagy via FOXO1-dependent pathways and autophagy suppression enhances quercetin-induced apoptosis in PASMCs in hypoxia. Free Radical Biol. Med. 2017, 103, 165–176. 10.1016/j.freeradbiomed.2016.12.016. [DOI] [PubMed] [Google Scholar]
- Li Y.; Chen M.; Wang J.; Guo X.; Xiao L.; Liu P.; Liu L.; Tang Y.; Yao P. Quercetin ameliorates autophagy in alcohol liver disease associated with lysosome through mTOR-TFEB pathway. J. Funct. Foods 2019, 52, 177–185. 10.1016/j.jff.2018.10.033. [DOI] [Google Scholar]
- Godoy J. A.; Lindsay C. B.; Quintanilla R. A.; Carvajal F. J.; Cerpa W.; Inestrosa N. C. Quercetin Exerts Differential Neuroprotective Effects Against H2O2 and Abeta Aggregates in Hippocampal Neurons: the Role of Mitochondria. Mol. Neurobiol. 2017, 54, 7116–7128. 10.1007/s12035-016-0203-x. [DOI] [PubMed] [Google Scholar]
- Chen L. C.; Chen Y. C.; Su C. Y.; Hong C. S.; Ho H. O.; Sheu M. T. Development and characterization of self-assembling lecithin-based mixed polymeric micelles containing quercetin in cancer treatment and an in vivo pharmacokinetic study. Int. J. Nanomed. 2016, 11, 1557–1566. 10.2147/IJN.S103681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno L.; Puerta E.; Suarez-Santiago J. E.; Santos-Magalhaes N. S.; Ramirez M. J.; Irache J. M. Effect of the oral administration of nanoencapsulated quercetin on a mouse model of Alzheimer’s disease. Int. J. Pharm. 2017, 517, 50–57. 10.1016/j.ijpharm.2016.11.061. [DOI] [PubMed] [Google Scholar]
- Palle S.; Neerati P. Quercetin nanoparticles attenuates scopolamine induced spatial memory deficits and pathological damages in rats. Bull. Fac. Pharm. 2017, 55, 101–106. 10.1016/j.bfopcu.2016.10.004. [DOI] [Google Scholar]
- Sharma D. R.; Wani W. Y.; Sunkaria A.; Kandimalla R. J.; Sharma R. K.; Verma D.; Bal A.; Gill K. D. Quercetin attenuates neuronal death against aluminum-induced neurodegeneration in the rat hippocampus. Neuroscience 2016, 324, 163–176. 10.1016/j.neuroscience.2016.02.055. [DOI] [PubMed] [Google Scholar]
- Sabogal-Guáqueta A. M.; Munoz-Manco J. I.; Ramirez-Pineda J. R.; Lamprea-Rodriguez M.; Osorio E.; Cardona-Gomez G. P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. 10.1016/j.neuropharm.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Hu J.; Zhong L.; Wang N.; Yang L.; Liu C. C.; Li H.; Wang X.; Zhou Y.; Zhang Y.; Xu H.; Bu G.; Zhuang J. Quercetin stabilizes apolipoprotein E and reduces brain Abeta levels in amyloid model mice. Neuropharmacology 2016, 108, 179–192. 10.1016/j.neuropharm.2016.04.032. [DOI] [PubMed] [Google Scholar]
- JUNG S. H.; Murphy E. A.; McClellan J. L.; Carmichael M. D.; Davis J. M. The dietary flavonoid quercetin decreases neuroinflammation in a mouse model of Alzheimer’s disease. FASEB J. 2010, 24, 604–617. [Google Scholar]
- Dong Y. S.; Wang J. L.; Feng D. Y.; Qin H. Z.; Wen H.; Yin Z. M.; Gao G. D.; Li C. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int. J. Med. Sci. 2014, 11, 282–290. 10.7150/ijms.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M. A.; Abdul H. M.; Joshi G.; Opii W. O.; Butterfield D. A. Protective effect of quercetin in primary neurons against Abeta(1-42): relevance to Alzheimer’s disease. J. Nutr. Biochem. 2009, 20, 269–275. 10.1016/j.jnutbio.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo H. J.; Lee C. Y. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J. Agric. Food Chem. 2004, 52, 7514–7517. 10.1021/jf049243r. [DOI] [PubMed] [Google Scholar]
- Braidy N.; Behzad S.; Habtemariam S.; Ahmed T.; Daglia M.; Nabavi S. M.; Sobarzo-Sanchez E.; Nabavi S. F. Neuroprotective Effects of Citrus Fruit-Derived Flavonoids, Nobiletin and Tangeretin in Alzheimer’s and Parkinson’s Disease. CNS Neurol. Disord.: Drug Targets 2017, 16, 387–397. 10.2174/1871527316666170328113309. [DOI] [PubMed] [Google Scholar]
- Kant V.; Jangir B. L.; Nigam A.; Kumar V.; Sharma S. Dose regulated cutaneous wound healing potential of quercetin in male rats. Wound Med. 2017, 19, 82–87. 10.1016/j.wndm.2017.10.004. [DOI] [Google Scholar]
- Borghi S. M.; Mizokami S. S.; Pinho-Ribeiro F. A.; Fattori V.; Crespigio J.; Clemente-Napimoga J. T.; Napimoga M. H.; Pitol D. L.; Issa J. P. M.; Fukada S. Y.; Casagrande R.; Verri W. A. Jr. The flavonoid quercetin inhibits titanium dioxide (TiO2)-induced chronic arthritis in mice. J. Nutr. Biochem. 2018, 53, 81–95. 10.1016/j.jnutbio.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Haleagrahara N.; Miranda-Hernandez S.; Alim M. A.; Hayes L.; Bird G.; Ketheesan N. Therapeutic effect of quercetin in collagen-induced arthritis. Biomed. Pharmacother. 2017, 90, 38–46. 10.1016/j.biopha.2017.03.026. [DOI] [PubMed] [Google Scholar]
- Li G.; Shen X.; Wei Y.; Si X.; Deng X.; Wang J. Quercetin reduces Streptococcus suis virulence by inhibiting suilysin activity and inflammation. Int. Immunopharmacol. 2019, 69, 71–78. 10.1016/j.intimp.2019.01.017. [DOI] [PubMed] [Google Scholar]
- Bustos P. S.; Deza-Ponzio R.; Paez P. L.; Albesa I.; Cabrera J. L.; Virgolini M. B.; Ortega M. G. Protective effect of quercetin in gentamicin-induced oxidative stress in vitro and in vivo in blood cells. Effect on gentamicin antimicrobial activity. Environ. Toxicol. Pharmacol. 2016, 48, 253–264. 10.1016/j.etap.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Zeng H.; Guo X.; Zhou F.; Xiao L.; Liu J.; Jiang C.; Xing M.; Yao P. Quercetin alleviates ethanol-induced liver steatosis associated with improvement of lipophagy. Food Chem. Toxicol. 2019, 125, 21–28. 10.1016/j.fct.2018.12.028. [DOI] [PubMed] [Google Scholar]
- Akinmoladun A. C.; Oladejo C. O.; Josiah S. S.; Famusiwa C. D.; Ojo O. B.; Olaleye M. T. Catechin, quercetin and taxifolin improve redox and biochemical imbalances in rotenone-induced hepatocellular dysfunction: Relevance for therapy in pesticide-induced liver toxicity?. Pathophysiology 2018, 25, 365–371. 10.1016/j.pathophys.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Adeoye A. O.; Ojowu J.; Daniel O. O.; Olorunsogo O. O. Inhibition of liver mitochondrial membrane permeability transition pore opening by quercetin and vitamin E in streptozotocin-induced diabetic rats. Biochem. Biophys. Res. Commun. 2018, 504, 460–469. 10.1016/j.bbrc.2018.08.114. [DOI] [PubMed] [Google Scholar]
- Lee K. S.; Park S. N. Cytoprotective effects and mechanisms of quercetin, quercitrin and avicularin isolated from Lespedeza cuneata G. Don against ROS-induced cellular damage. J. Ind. Eng. Chem. 2019, 71, 160–166. 10.1016/j.jiec.2018.11.018. [DOI] [Google Scholar]
- Nile S. H.; Nile A. S.; Keum Y. S.; Sharma K. Utilization of quercetin and quercetin glycosides from onion (Allium cepa L.) solid waste as an antioxidant, urease and xanthine oxidase inhibitors. Food Chem. 2017, 235, 119–126. 10.1016/j.foodchem.2017.05.043. [DOI] [PubMed] [Google Scholar]
- Rastogi S.; Haldar C. Comparative effect of melatonin and quercetin in counteracting LPS induced oxidative stress in bone marrow mononuclear cells and spleen of Funambulus pennanti. Food Chem. Toxicol. 2018, 120, 243–252. 10.1016/j.fct.2018.06.062. [DOI] [PubMed] [Google Scholar]
- Lesjak M.; Beara I.; Simin N.; Pintać D.; Majkić T.; Bekvalac K.; Orčić D.; Mimica-Dukić N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. 10.1016/j.jff.2017.10.047. [DOI] [Google Scholar]
- Luangaram S.; Kukongviriyapan U.; Pakdeechote P.; Kukongviriyapan V.; Pannangpetch P. Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and oxidative stress in rats. Food Chem. Toxicol. 2007, 45, 448–455. 10.1016/j.fct.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Moreira L.; Araujo I.; Costa T.; Correia-Branco A.; Faria A.; Martel F.; Keating E. Quercetin and epigallocatechin gallate inhibit glucose uptake and metabolism by breast cancer cells by an estrogen receptor-independent mechanism. Exp. Cell Res. 2013, 319, 1784–1795. 10.1016/j.yexcr.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Dhanya R.; Arya A. D.; Nisha P.; Jayamurthy P. Quercetin, a Lead Compound against Type 2 Diabetes Ameliorates Glucose Uptake via AMPK Pathway in Skeletal Muscle Cell Line. Front. Pharmacol. 2017, 8, 336. 10.3389/fphar.2017.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.-N.; Jeong S.-M.; Huh G. H.; Kim J.-I. Quercetin ameliorates insulin sensitivity and liver steatosis partly by increasing adiponectin expression in ob/ob mice. Food Sci. Biotechnol. 2015, 24, 273–279. 10.1007/s10068-015-0036-9. [DOI] [Google Scholar]
- Babacanoglu C.; Yildirim N.; Sadi G.; Pektas M. B.; Akar F. Resveratrol prevents high-fructose corn syrup-induced vascular insulin resistance and dysfunction in rats. Food Chem. Toxicol. 2013, 60, 160–167. 10.1016/j.fct.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Dey A.; Kumar S. M. Cytochrome P450 2E1 and hyperglycemia-induced liver injury. Cell Biol. Toxicol. 2011, 27, 285–310. 10.1007/s10565-011-9188-4. [DOI] [PubMed] [Google Scholar]
- Maksymchuk O.; Shysh A.; Rosohatska I.; Chashchyn M. Quercetin prevents type 1 diabetic liver damage through inhibition of CYP2E1. Pharmacol. Rep. 2017, 69, 1386–1392. 10.1016/j.pharep.2017.05.020. [DOI] [PubMed] [Google Scholar]
- Xu X.; Chen P.; Zheng Q.; Wang Y.; Chen W. Effect of pioglitazone on diabetic nephropathy and expression of HIF-1alpha and VEGF in the renal tissues of type 2 diabetic rats. Diabetes Res. Clin. Pract. 2011, 93, 63–69. 10.1016/j.diabres.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Chen P.; Chen J.; Zheng Q.; Chen W.; Wang Y.; Xu X. Pioglitazone, extract of compound Danshen dripping pill, and quercetin ameliorate diabetic nephropathy in diabetic rats. J. Endocrinol. Invest. 2013, 36, 422–427. 10.3275/8763. [DOI] [PubMed] [Google Scholar]
- Roth G.; Kotzka J.; Kremer L.; Lehr S.; Lohaus C.; Meyer H. E.; Krone W.; Muller-Wieland D. MAP kinases Erk1/2 phosphorylate sterol regulatory element-binding protein (SREBP)-1a at serine 117 in vitro. The. J. Biol. Chem. 2000, 275, 33302–33307. 10.1074/jbc.M005425200. [DOI] [PubMed] [Google Scholar]
- Elbe H.; Vardi N.; Esrefoglu M.; Ates B.; Yologlu S.; Taskapan C. Amelioration of streptozotocin-induced diabetic nephropathy by melatonin, quercetin, and resveratrol in rats. Hum. Exp. Toxicol. 2015, 34, 100–113. 10.1177/0960327114531995. [DOI] [PubMed] [Google Scholar]
- Thomas A. A.; Feng B.; Chakrabarti S. ANRIL: A Regulator of VEGF in Diabetic Retinopathy. Invest Ophthalmol. Visual Sci. 2017, 58, 470–480. 10.1167/iovs.16-20569. [DOI] [PubMed] [Google Scholar]
- Kumar B.; Gupta S. K.; Srinivasan B. P.; Nag T. C.; Srivastava S.; Saxena R.; Jha K. A. Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc. Res. 2013, 87, 65–74. 10.1016/j.mvr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Ibarra J.; Bland M.; Gonzalez M.; Garcia C. Quercetin ameliorates hyperglycemia-induced inflammation and apoptosis in the retina and lateral geniculate nucleus in a rat model of type 2 diabetes mellitus (688.8). FASEB J. 2014, 28, 688-8. [Google Scholar]
- Greindling K. K.; Lassegue B.; Alexander R. W. Angiotensin receptors and their therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 281–306. 10.1146/annurev.pa.36.040196.001433. [DOI] [PubMed] [Google Scholar]
- Suri S.; Liu X. H.; Rayment S.; Hughes D. A.; Kroon P. A.; Needs P. W.; Taylor M. A.; Tribolo S.; Wilson V. G. Quercetin and its major metabolites selectively modulate cyclic GMP-dependent relaxations and associated tolerance in pig isolated coronary artery. Br. J. Pharmacol. 2010, 159, 566–575. 10.1111/j.1476-5381.2009.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz P.; Vita J. A. Testing endothelial vasomotor function: nitric oxide, a multipotent molecule. Circulation 2003, 108, 2049–2053. 10.1161/01.CIR.0000089507.19675.F9. [DOI] [PubMed] [Google Scholar]
- Wan L. L.; Xia J.; Ye D.; Liu J.; Chen J.; Wang G. Effects of quercetin on gene and protein expression of NOX and NOS after myocardial ischemia and reperfusion in rabbit. Cardiovasc. Ther. 2009, 27, 28–33. 10.1111/j.1755-5922.2009.00071.x. [DOI] [PubMed] [Google Scholar]
- Ahmed L. A.; Salem H. A.; Attia A. S.; El-Sayed M. E. Enhancement of amlodipine cardioprotection by quercetin in ischaemia/reperfusion injury in rats. J. Pharm. Pharmacol. 2009, 61, 1233–1241. 10.1211/jpp.61.09.0014. [DOI] [PubMed] [Google Scholar]
- Kleemann R.; Verschuren L.; Morrison M.; Zadelaar S.; van Erk M. J.; Wielinga P. Y.; Kooistra T. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 2011, 218, 44–52. 10.1016/j.atherosclerosis.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Yan L.; Zhang J. D.; Wang B.; Lv Y. J.; Jiang H.; Liu G. L.; Qiao Y.; Ren M.; Guo X. F. Quercetin inhibits left ventricular hypertrophy in spontaneously hypertensive rats and inhibits angiotensin II-induced H9C2 cells hypertrophy by enhancing PPAR-gamma expression and suppressing AP-1 activity. PLoS One 2013, 8, e72548 10.1371/journal.pone.0072548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H.; Nishida S. Cardio-electopharmacology and vasodilating mechanisms of quercetin. Med. Chem. 2014, 4, 523–530. 10.4172/2161-0444.1000189. [DOI] [Google Scholar]
- Wang Y.; Zhang Z. Z.; Wu Y.; Ke J. J.; He X. H.; Wang Y. L. Quercetin postconditioning attenuates myocardial ischemia/reperfusion injury in rats through the PI3K/Akt pathway. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas 2013, 46, 861–867. 10.1590/1414-431X20133036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knekt P.; Kumpulainen J.; Jarvinen R.; Rissanen H.; Heliovaara M.; Reunanen A.; Hakulinen T.; Aromaa A. Flavonoid intake and risk of chronic diseases. The American journal of clinical nutrition 2002, 76, 560–568. 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- Egert S.; Bosy-Westphal A.; Seiberl J.; Kurbitz C.; Settler U.; Plachta-Danielzik S.; Wagner A. E.; Frank J.; Schrezenmeir J.; Rimbach G.; Wolffram S.; Muller M. J. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. 10.1017/S0007114509359127. [DOI] [PubMed] [Google Scholar]
- Chekalina N.; Burmak Y.; Petrov Y.; Borisova Z.; Manusha Y.; Kazakov Y.; Kaidashev I. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian Heart J. 2018, 70, 593–597. 10.1016/j.ihj.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi M.; Ghiasvand R.; Feizi A.; Asgari G.; Darvish L. Does Quercetin Improve Cardiovascular Risk factors and Inflammatory Biomarkers in Women with Type 2 Diabetes: A Double-blind Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2013, 4, 777–785. [PMC free article] [PubMed] [Google Scholar]
- Nakayama H.; Tsuge N.; Sawada H.; Higashi Y. Chronic intake of onion extract containing quercetin improved postprandial endothelial dysfunction in healthy men. Journal of the American College of Nutrition 2013, 32, 160–164. 10.1080/07315724.2013.797858. [DOI] [PubMed] [Google Scholar]
- Xie M.; Morales C. R.; Lavandero S.; Hill J. A. Tuning flux: autophagy as a target of heart disease therapy. Curr. Opin. Cardiol. 2011, 26, 216–222. 10.1097/HCO.0b013e328345980a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y.; Ikeda Y.; Iwabayashi M.; Akasaki Y.; Ohishi M. The Impact of Autophagy on Cardiovascular Senescence and Diseases. Int Heart J 2017, 58, 666–673. 10.1536/ihj.17-246. [DOI] [PubMed] [Google Scholar]
- Suganthy N.; Devi K. P.; Nabavi S. F.; Braidy N.; Nabavi S. M. Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. Biomed. Pharmacother. 2016, 84, 892–908. 10.1016/j.biopha.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Jiménez-Aliaga K.; Bermejo-Bescos P.; Benedi J.; Martin-Aragon S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011, 89, 939–945. 10.1016/j.lfs.2011.09.023. [DOI] [PubMed] [Google Scholar]
- McGeer P. L.; McGeer E. G. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 2013, 126, 479–497. 10.1007/s00401-013-1177-7. [DOI] [PubMed] [Google Scholar]
- Anand R.; Gill K. D.; Mahdi A. A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76, 27–50. 10.1016/j.neuropharm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Maciel R. M.; Carvalho F. B.; Olabiyi A. A.; Schmatz R.; Gutierres J. M.; Stefanello N.; Zanini D.; Rosa M. M.; Andrade C. M.; Rubin M. A.; Schetinger M. R.; Morsch V. M.; Danesi C. C.; Lopes S. T. A. Neuroprotective effects of quercetin on memory and anxiogenic-like behavior in diabetic rats: Role of ectonucleotidases and acetylcholinesterase activities. Biomed. Pharmacother. 2016, 84, 559–568. 10.1016/j.biopha.2016.09.069. [DOI] [PubMed] [Google Scholar]
- Richetti S. K.; Blank M.; Capiotti K. M.; Piato A. L.; Bogo M. R.; Vianna M. R.; Bonan C. D. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav. Brain Res. 2011, 217, 10–15. 10.1016/j.bbr.2010.09.027. [DOI] [PubMed] [Google Scholar]
- Islam M. R.; Zaman A.; Jahan I.; Chakravorty R.; Chakraborty S. In silico QSAR analysis of quercetin reveals its potential as therapeutic drug for Alzheimer’s disease. J. Young Pharm. 2013, 5, 173–179. 10.1016/j.jyp.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai A.; Tanaka S.; Imai Y.; Yoshida H.; Kanaoka T.; Tanaka T.; Taniguchi M. New tyrosinase inhibitory decapeptide: Molecular insights into the role of tyrosine residues. J. Biosci. Bioeng. 2016, 121, 607–613. 10.1016/j.jbiosc.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Hridya H.; Amrita A.; Mohan S.; Gopalakrishnan M.; Dakshinamurthy T. K.; Doss G. P.; Siva R. Functionality study of santalin as tyrosinase inhibitor: A potential depigmentation agent. Int. J. Biol. Macromol. 2016, 86, 383–389. 10.1016/j.ijbiomac.2016.01.098. [DOI] [PubMed] [Google Scholar]
- Hu Y. H.; Zhuang J. X.; Yu F.; Cui Y.; Yu W. W.; Yan C. L.; Chen Q. X. Inhibitory effects of cefotaxime on the activity of mushroom tyrosinase. J. Biosci. Bioeng. 2016, 121, 385–389. 10.1016/j.jbiosc.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Oyama T.; Takahashi S.; Yoshimori A.; Yamamoto T.; Sato A.; Kamiya T.; Abe H.; Abe T.; Tanuma S. I. Discovery of a new type of scaffold for the creation of novel tyrosinase inhibitors. Bioorg. Med. Chem. 2016, 24, 4509–4515. 10.1016/j.bmc.2016.07.060. [DOI] [PubMed] [Google Scholar]
- Fan M.; Zhang G.; Hu X.; Xu X.; Gong D. Quercetin as a tyrosinase inhibitor: Inhibitory activity, conformational change and mechanism. Food Res. Int. 2017, 100, 226–233. 10.1016/j.foodres.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Athanasiou K. A.; Darling E. M.; Hu J. C.; DuRaine G. D.; Reddi A. H.. Articular Cartilage; CRC Press, 2017. [Google Scholar]
- Laev S. S.; Salakhutdinov N. F. Anti-arthritic agents: Progress and potential. Bioorg. Med. Chem. 2015, 23, 3059–3080. 10.1016/j.bmc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Cobelli N.; Scharf B.; Crisi G. M.; Hardin J.; Santambrogio L. Mediators of the inflammatory response to joint replacement devices. Nat. Rev. Rheumatol. 2011, 7, 600–608. 10.1038/nrrheum.2011.128. [DOI] [PubMed] [Google Scholar]
- Xu J. G.; Jing H. Q.; Ye C. Y. Highly virulent Streptococcus suis infection and problems involved in the disease prevention and control in China. Zhonghua Liu Xing Bing Xue Za Zhi 2005, 26, 629–632. [PubMed] [Google Scholar]
- Gao Z. Y.; Zhuang H. Human infection due to Streptococcus suis. Zhonghua Liu Xing Bing Xue Za Zhi 2005, 26, 645–648. [PubMed] [Google Scholar]
- Huang Y. T.; Teng L. J.; Ho S. W.; Hsueh P. R. Streptococcus suis infection. J. Microbiol. Immunol. Infect. 2005, 38, 306–313. [PubMed] [Google Scholar]
- Abdel-Misih S. R. Z.; Bloomston M. Liver Anatomy. Surg. Clin. North Am. 2010, 90, 643–653. 10.1016/j.suc.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrani S. K.; Devarbhavi H.; Eaton J.; Kamath P. S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Sanders L. H.; Timothy Greenamyre J. Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radical Biol. Med. 2013, 62, 111–120. 10.1016/j.freeradbiomed.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.-C.; Chan S.-T.; Chang C.-N.; Yu P.-S.; Chuang C.-H.; Yeh S.-L. Quercetin and chrysin inhibit nickel-induced invasion and migration by downregulation of TLR4/NF-κB signaling in A549 cells. Chem.-Biol. Interact. 2018, 292, 101–109. 10.1016/j.cbi.2018.07.010. [DOI] [PubMed] [Google Scholar]
- Teekaraman D.; Elayapillai S. P.; Viswanathan M. P.; Jagadeesan A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chem.-Biol. Interact. 2019, 300, 91–100. 10.1016/j.cbi.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Fang Z.; Zha Z.; Sun Q.; Wang H.; Sun M.; Qiao B. Quercetin inhibits cell viability, migration and invasion by regulating miR-16/HOXA10 axis in oral cancer. Eur. J. Pharmacol. 2019, 847, 11–18. 10.1016/j.ejphar.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Huang D.-Y.; Dai Z.-R.; Li W.-M.; Wang R.-G.; Yang S.-M. Inhibition of EGF expression and NF-κB activity by treatment with quercetin leads to suppression of angiogenesis in nasopharyngeal carcinoma. Saudi J. Biol. Sci. 2018, 25, 826–831. 10.1016/j.sjbs.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Qu Z.; Kong L.; Xu L.; Zhang M.; Liu J.; Yang Z. Quercetin ameliorates lipopolysaccharide-caused inflammatory damage via down-regulation of miR-221 in WI-38 cells. Exp. Mol. Pathol. 2019, 108, 1–8. 10.1016/j.yexmp.2019.03.002. [DOI] [PubMed] [Google Scholar]
- Li X.; Zhou N.; Wang J.; Liu Z.; Wang X.; Zhang Q.; Liu Q.; Gao L.; Wang R. Quercetin suppresses breast cancer stem cells (CD44+/CD24−) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018, 196, 56–62. 10.1016/j.lfs.2018.01.014. [DOI] [PubMed] [Google Scholar]
- Jia L.; Huang S.; Yin X.; Zan Y.; Guo Y.; Han L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 2018, 208, 123–130. 10.1016/j.lfs.2018.07.027. [DOI] [PubMed] [Google Scholar]
- Araújo K. C. F.; de MB Costa E. M.; Pazini F.; Valadares M. C.; de Oliveira V. Bioconversion of quercetin and rutin and the cytotoxicity activities of the transformed products. Food Chem. Toxicol. 2013, 51, 93–96. 10.1016/j.fct.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Sánchez-González P. D.; López-Hernández F. J.; Dueñas M.; Prieto M.; Sánchez-López E.; Thomale J.; Ruiz-Ortega M.; López-Novoa J. M.; Morales A. I. Differential effect of quercetin on cisplatin-induced toxicity in kidney and tumor tissues. Food Chem. Toxicol. 2017, 107, 226–236. 10.1016/j.fct.2017.06.047. [DOI] [PubMed] [Google Scholar]
- Erdogan S.; Turkekul K.; Dibirdik I.; Doganlar O.; Doganlar Z. B.; Bilir A.; Oktem G. Midkine downregulation increases the efficacy of quercetin on prostate cancer stem cell survival and migration through PI3K/AKT and MAPK/ERK pathway. Biomed. Pharmacother. 2018, 107, 793–805. 10.1016/j.biopha.2018.08.061. [DOI] [PubMed] [Google Scholar]
- Li S.; Pei Y.; Wang W.; Liu F.; Zheng K.; Zhang X. Quercetin suppresses the proliferation and metastasis of metastatic osteosarcoma cells by inhibiting parathyroid hormone receptor 1. Biomed. Pharmacother. 2019, 114, 108839 10.1016/j.biopha.2019.108839. [DOI] [PubMed] [Google Scholar]
- Chen K.-C.; Hsu W.-H.; Ho J.-Y.; Lin C.-W.; Chu C.-Y.; Kandaswami C. C.; Lee M.-T.; Cheng C.-H. Flavonoids Luteolin and Quercetin Inhibit RPS19 and contributes to metastasis of cancer cells through c-Myc reduction. J. Food Drug Anal. 2018, 26, 1180–1191. 10.1016/j.jfda.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava N. S.; Srivastava R. A. K. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine 2019, 52, 117–128. 10.1016/j.phymed.2018.09.224. [DOI] [PubMed] [Google Scholar]
- Elmadany N.; Khalil E.; Vaccari L.; Birarda G.; Yousef I.; Abu-Dahab R. Antiproliferative activity of the combination of doxorubicin/quercetin on MCF7 breast cancer cell line: A combined study using colorimetric assay and synchrotron infrared microspectroscopy. Infrared Phys. Technol. 2018, 95, 141–147. 10.1016/j.infrared.2018.10.014. [DOI] [Google Scholar]
- Shan B.-E.; Wang M.-X.; Li R.-q. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/β-catenin signaling pathway. Cancer invest. 2009, 27, 604–612. 10.1080/07357900802337191. [DOI] [PubMed] [Google Scholar]
- Kim H.; Seo E.-M.; Sharma A. R.; Ganbold B.; Park J.; Sharma G.; Kang Y.-H.; Song D.-K.; Lee S.-S.; Nam J.-S. Regulation of Wnt signaling activity for growth suppression induced by quercetin in 4T1 murine mammary cancer cells. Int. J. Oncol. 2013, 43, 1319–1325. 10.3892/ijo.2013.2036. [DOI] [PubMed] [Google Scholar]
- Jeong J. H.; An J. Y.; Kwon Y. T.; Rhee J. G.; Lee Y. J. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell. Biochem. 2009, 106, 73–82. 10.1002/jcb.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya A. K.; Vinayak M. Modulation of PKC signaling and induction of apoptosis through suppression of reactive oxygen species and tumor necrosis factor receptor 1 (TNFR1): key role of quercetin in cancer prevention. Tumor Biol. 2015, 36, 8913–8924. 10.1007/s13277-015-3634-5. [DOI] [PubMed] [Google Scholar]
- Mead J.; McNair N. Antiparasitic activity of flavonoids and isoflavones against Cryptosporidium parvum and Encephalitozoon intestinalis. FEMS Microbiol. Lett. 2006, 259, 153–157. 10.1111/j.1574-6968.2006.00263.x. [DOI] [PubMed] [Google Scholar]
- Calzada F.; Correa-Basurto J.; Barbosa E.; Mendez-Luna D.; Yepez-Mulia L. Antiprotozoal Constituents from Annona cherimola Miller, a Plant Used in Mexican Traditional Medicine for the Treatment of Diarrhea and Dysentery. Pharmacogn. Mag. 2017, 13, 148–152. 10.4103/0973-1296.203976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca-Silva F.; Inacio J. D.; Canto-Cavalheiro M. M.; Almeida-Amaral E. E. Reactive oxygen species production and mitochondrial dysfunction contribute to quercetin induced death in Leishmania amazonensis. PLoS One 2011, 6, e14666 10.1371/journal.pone.0014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataneo A. H. D.; Tomiotto-Pellissier F.; Miranda-Sapla M. M.; Assolini J. P.; Panis C.; Kian D.; Yamauchi L. M.; Colado Simao A. N.; Casagrande R.; Pinge-Filho P.; Costa I. N.; Verri W. A. Jr; Conchon-Costa I.; Pavanelli W. R. Quercetin promotes antipromastigote effect by increasing the ROS production and anti-amastigote by upregulating Nrf2/HO-1 expression, affecting iron availability. Biomed. Pharmacother. 2019, 113, 108745 10.1016/j.biopha.2019.108745. [DOI] [PubMed] [Google Scholar]
- Jean-Moreno V.; Rojas R.; Goyeneche D.; Coombs G. H.; Walker J. Leishmania donovani: differential activities of classical topoisomerase inhibitors and antileishmanials against parasite and host cells at the level of DNA topoisomerase I and in cytotoxicity assays. Exp. Parasitol. 2006, 112, 21–30. 10.1016/j.exppara.2005.08.014. [DOI] [PubMed] [Google Scholar]
- de Sousa L. R.; Wu H.; Nebo L.; Fernandes J. B.; da Silva M. F.; Kiefer W.; Schirmeister T.; Vieira P. C. Natural products as inhibitors of recombinant cathepsin L of Leishmania mexicana. Exp. Parasitol. 2015, 156, 42–48. 10.1016/j.exppara.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Cortázar T. M.; Coombs G. H.; Walker J. Leishmania panamensis: comparative inhibition of nuclear DNA topoisomerase II enzymes from promastigotes and human macrophages reveals anti-parasite selectivity of fluoroquinolones, flavonoids and pentamidine. Exp. Parasitol. 2007, 116, 475–482. 10.1016/j.exppara.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Ganesh D.; Fuehrer H. P.; Starzengruber P.; Swoboda P.; Khan W. A.; Reismann J. A.; Mueller M. S.; Chiba P.; Noedl H. Antiplasmodial activity of flavonol quercetin and its analogues in Plasmodium falciparum: evidence from clinical isolates in Bangladesh and standardized parasite clones. Parasitol. Res. 2012, 110, 2289–2295. 10.1007/s00436-011-2763-z. [DOI] [PubMed] [Google Scholar]
- Penna-Coutinho J.; Aguiar A. C.; Krettli A. U. Commercial drugs containing flavonoids are active in mice with malaria and in vitro against chloroquine-resistant Plasmodium falciparum. Mem. Inst. Oswaldo Cruz 2018, 113, e180279 10.1590/0074-02760180279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerboeuf D.; Riou M.; Guegnard F. Flavonoids and related compounds in parasitic disease control. Mini-Rev. Med. Chem. 2008, 8, 116–128. 10.2174/138955708783498168. [DOI] [PubMed] [Google Scholar]
- Smith P.; Ho C. K.; Takagi Y.; Djaballah H.; Shuman S. Nanomolar Inhibitors of Trypanosoma brucei RNA Triphosphatase. mBio 2016, 7, e00058 10.1128/mBio.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson H. C.; Lyda T. A.; Chambers J. W.; Morris M. T.; Christensen K. A.; Morris J. C. Quercetin, a fluorescent bioflavanoid, inhibits Trypanosoma brucei hexokinase 1. Exp. Parasitol. 2011, 127, 423–428. 10.1016/j.exppara.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthen C.; Jensen B. C.; Parsons M. Diverse effects on mitochondrial and nuclear functions elicited by drugs and genetic knockdowns in bloodstream stage Trypanosoma brucei. PLoS Neglected Trop. Dis. 2010, 4, e678 10.1371/journal.pntd.0000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamani-Matsuda M.; Rambert J.; Malvy D.; Lejoly-Boisseau H.; Daulouede S.; Thiolat D.; Coves S.; Courtois P.; Vincendeau P.; Mossalayi M. D. Quercetin induces apoptosis of Trypanosoma brucei gambiense and decreases the proinflammatory response of human macrophages. Antimicrob. Agents Chemother. 2004, 48, 924–929. 10.1128/AAC.48.3.924-929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Cruz F.; Serra S.; Delogu G.; Lapier M.; Maya J. D.; Olea-Azar C.; Santana L.; Uriarte E. Antitrypanosomal and antioxidant properties of 4-hydroxycoumarins derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5569–5573. 10.1016/j.bmcl.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Calzada F.; Alanis A. D. Additional antiprotozoal flavonol glycosides of the aerial parts of Helianthemum glomeratum. Phytother. Res. 2007, 21, 78–80. 10.1002/ptr.2031. [DOI] [PubMed] [Google Scholar]
- El Souda S. S.; Matloub A. A.; Nepveuc F.; Valentin A.; Roques C. Phenolic composition and prospective anti-infectious properties of Atriplex lindleyi. Asian Pac. J. Trop. Dis. 2015, 5, 786–791. 10.1016/S2222-1808(15)60931-8. [DOI] [Google Scholar]
- Oliveira F. Q.; Andrade-Neto V.; Krettli A. U.; Brandao M. G. New evidences of antimalarial activity of Bidens pilosa roots extract correlated with polyacetylene and flavonoids. J. Ethnopharmacol. 2004, 93, 39–42. 10.1016/j.jep.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Montrieux E.; Perera W. H.; Garcia M.; Maes L.; Cos P.; Monzote L. In vitro and in vivo activity of major constituents from Pluchea carolinensis against Leishmania amazonensis. Parasitol. Res. 2014, 113, 2925–2932. 10.1007/s00436-014-3954-1. [DOI] [PubMed] [Google Scholar]
- Muzitano M. F.; Falcao C. A.; Cruz E. A.; Bergonzi M. C.; Bilia A. R.; Vincieri F. F.; Rossi-Bergmann B.; Costa S. S. Oral metabolism and efficacy of Kalanchoe pinnata flavonoids in a murine model of cutaneous leishmaniasis. Planta Med. 2009, 75, 307–311. 10.1055/s-0028-1088382. [DOI] [PubMed] [Google Scholar]
- Pereira C. A. J.; Oliveira L. L. S.; Coaglio A. L.; Santos F. S. O.; Cezar R. S. M.; Mendes T.; Oliveira F. L. P.; Conzensa G.; Lima W. S. Anti-helminthic activity of Momordica charantia L. against Fasciola hepatica eggs after twelve days of incubation in vitro. Vet. Parasitol. 2016, 228, 160–166. 10.1016/j.vetpar.2016.08.025. [DOI] [PubMed] [Google Scholar]
- Vila-Nova N. S.; Morais S. M.; Falcão M. J. C.; Bevilaqua C. M. L.; Rondon F. C. M.; Wilson M. E.; Vieira I. G. P.; Andrade H. F. Leishmanicidal and cholinesterase inhibiting activities of phenolic compounds of Dimorphandra gardneriana and Platymiscium floribundum, native plants from Caatinga biome. Pesq. Vet. Bras. 2012, 32, 1164–1168. 10.1590/S0100-736X2012001100015. [DOI] [Google Scholar]
- Kheirandish F.; Delfan B.; Mahmoudvand H.; Moradi N.; Ezatpour B.; Ebrahimzadeh F.; Rashidipour M. Antileishmanial, antioxidant, and cytotoxic activities of Quercus infectoria Olivier extract. Biomed. Pharmacother. 2016, 82, 208–215. 10.1016/j.biopha.2016.04.040. [DOI] [PubMed] [Google Scholar]
- Jansen O.; Tchinda A. T.; Loua J.; Esters V.; Cieckiewicz E.; Ledoux A.; Toukam P. D.; Angenot L.; Tits M.; Balde A. M.; Frederich M. Antiplasmodial activity of Mezoneuron benthamianum leaves and identification of its active constituents. J. Ethnopharmacol. 2017, 203, 20–26. 10.1016/j.jep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- Calixto Júnior J. T.; de Morais S. M.; Gomez C. V.; Molas C. C.; Rolon M.; Boligon A. A.; Athayde M. L.; de Morais Oliveira C. D.; Tintino S. R.; Henrique Douglas M. C. Phenolic composition and antiparasitic activity of plants from the Brazilian Northeast “Cerrado”. Saudi J. Biol. Sci. 2016, 23, 434–440. 10.1016/j.sjbs.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha N. L.; Uchoa C. J.; Cintra L. S.; de Souza H. C.; Peixoto J. A.; Silva C. P.; Magalhaes L. G.; Gimenez V. M.; Groppo M.; Rodrigues V.; da Silva Filho A. A.; Andrade E. S. M. L.; Cunha W. R.; Pauletti P. M.; Januario A. H. In vitro schistosomicidal activity of some brazilian cerrado species and their isolated compounds. J. Evidence-Based Complementary Altern. Med. 2012, 2012, 173614 10.1155/2012/173614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwu M. M.; Obidoa O.; Anazodo M. Biochemical mechanism of the antimalarial activity of Azadirachta indica leaf extract. Pharmacol. Res. Commun. 1986, 18, 81–91. 10.1016/0031-6989(86)90161-X. [DOI] [PubMed] [Google Scholar]
- Houël E.; Nardella F.; Jullian V.; Valentin A.; Vonthron-Senecheau C.; Villa P.; Obrecht A.; Kaiser M.; Bourreau E.; Odonne G.; Fleury M.; Bourdy G.; Eparvier V.; Deharo E.; Stien D. Wayanin and guaijaverin, two active metabolites found in a Psidium acutangulum Mart. ex DC (syn. P. persoonii McVaugh) (Myrtaceae) antimalarial decoction from the Wayana Amerindians. J. Ethnopharmacol. 2016, 187, 241–248. 10.1016/j.jep.2016.04.053. [DOI] [PubMed] [Google Scholar]
- de Souza C. E. S.; da Silva A. R. P.; Gomez M. C. V.; Rolom M.; Coronel C.; da Costa J. G. M.; Sousa A. K.; Rolim L. A.; de Souza F. H. S.; Coutinho H. D. M. Anti-Trypanosoma, anti-Leishmania and cytotoxic activities of natural products from Psidium brownianum Mart. ex DC. and Psidium guajava var. Pomifera analysed by LC-MS. Acta Trop. 2017, 176, 380–384. 10.1016/j.actatropica.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Méabed E. M. H.; Abou-Sreea A. I. B.; Roby M. H. H. Chemical analysis and giardicidal effectiveness of the aqueous extract of Cymbopogon citratus Stapf. Parasitol. Res. 2018, 117, 1745–1755. 10.1007/s00436-018-5855-1. [DOI] [PubMed] [Google Scholar]
- Das B. B.; Sen N.; Roy A.; Dasgupta S. B.; Ganguly A.; Mohanta B. C.; Dinda B.; Majumder H. K. Differential induction of Leishmania donovani bi-subunit topoisomerase I-DNA cleavage complex by selected flavones and camptothecin: activity of flavones against camptothecin-resistant topoisomerase I. Nucleic Acids Res. 2006, 34, 1121–1132. 10.1093/nar/gkj502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid S. A.; Farouk A.; Geary T. G.; Jensen J. B. Potential antimalarial candidates from African plants: and in vitro approach using Plasmodium falciparum. J. Ethnopharmacol. 1986, 15, 201–209. 10.1016/0378-8741(86)90156-X. [DOI] [PubMed] [Google Scholar]
- Gibellini L.; Pinti M.; Nasi M.; Montagna J. P.; De Biasi S.; Roat E.; Bertoncelli L.; Cooper E. L.; Cossarizza A. Quercetin and cancer chemoprevention. J. Evidence-Based Complementary Altern. Med. 2011, 2011, 591356 10.1093/ecam/neq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafabakhsh R.; Asemi Z. Quercetin: a natural compound for ovarian cancer treatment. J. Ovarian Res. 2019, 12, 55 10.1186/s13048-019-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper M.; Güneş H. The anticancer and anti-inflammatory effects of Centaurea solstitialis extract on human cancer cell lines. Turk. J. Pharm. Sci. 2019, 16, 273–281. 10.4274/tjps.galenos.2018.27146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami A.; Ashida H.; Terao J. Multitargeted cancer prevention by quercetin. Cancer letters 2008, 269, 315–325. 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- Zhou W.; Kallifatidis G.; Baumann B.; Rausch V.; Mattern J.; Gladkich J.; Giese N.; Moldenhauer G.; Wirth T.; Buchler M. W.; Salnikov A. V.; Herr I. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int. J. Oncol. 2010, 37, 551–561. 10.3892/ijo_00000704. [DOI] [PubMed] [Google Scholar]
- Serri C.; Quagliariello V.; Iaffaioli R. V.; Fusco S.; Botti G.; Mayol L.; Biondi M. Combination therapy for the treatment of pancreatic cancer through hyaluronic acid-decorated nanoparticles loaded with quercetin and gemcitabine: A preliminary in vitro study. J. Cell. Physiol. 2019, 234, 4959–4969. 10.1002/jcp.27297. [DOI] [PubMed] [Google Scholar]
- Nessa M. U.; Beale P.; Chan C.; Yu J. Q.; Huq F. Synergism from combinations of cisplatin and oxaliplatin with quercetin and thymoquinone in human ovarian tumour models. Anticancer Res. 2011, 31, 3789–3797. [PubMed] [Google Scholar]
- Lesser S.; Wolffram S. Oral bioavailability of the flavonol quercetin - A review. Curr. Top. Nutraceutical Res. 2006, 4, 239–256. [Google Scholar]
- Singhal R. L.; Yeh Y. A.; Praja N.; Olah E.; Sledge G. W. Jr; Weber G. Quercetin down-regulates signal transduction in human breast carcinoma cells. Biochem. Biophys. Res. Commun. 1995, 208, 425–431. 10.1006/bbrc.1995.1355. [DOI] [PubMed] [Google Scholar]
- Staedler D.; Idrizi E.; Kenzaoui B. H.; Juillerat-Jeanneret L. Drug combinations with quercetin: doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother. Pharmacol. 2011, 68, 1161–1172. 10.1007/s00280-011-1596-x. [DOI] [PubMed] [Google Scholar]
- Vidya Priyadarsini R.; Senthil Murugan R.; Maitreyi S.; Ramalingam K.; Karunagaran D.; Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-kappaB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91. 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Calgarotto A. K.; Maso V.; Junior G. C. F.; Nowill A. E.; Filho P. L.; Vassallo J.; Saad S. T. O. Antitumor activities of Quercetin and Green Tea in xenografts of human leukemia HL60 cells. Sci. Rep. 2018, 8, 3459 10.1038/s41598-018-21516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimi A.; Entezari A.; Hagh M. F.; Hassanzadeh A.; Saraei R.; Solali S. Quercetin sensitizes human myeloid leukemia KG-1 cells against TRAIL-induced apoptosis. J. Cell. Physiol. 2019, 234, 13233–13241. 10.1002/jcp.27995. [DOI] [PubMed] [Google Scholar]
- Kim W. K.; Bang M. H.; Kim E. S.; Kang N. E.; Jung K. C.; Cho H. J.; Park J. H. Quercetin decreases the expression of ErbB2 and ErbB3 proteins in HT-29 human colon cancer cells. J. Nutr. Biochem. 2005, 16, 155–162. 10.1016/j.jnutbio.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Baron B. W.; Thirman M. J.; Giurcanu M. C.; Baron J. M. Quercetin Therapy for Selected Patients with PIM1 Kinase-Positive Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: A Pilot Study. Acta Haematol. 2018, 139, 132–139. 10.1159/000486361. [DOI] [PubMed] [Google Scholar]
- Ranelletti F. O.; Ricci R.; Larocca L. M.; Maggiano N.; Capelli A.; Scambia G.; Benedetti-Panici P.; Mancuso S.; Rumi C.; Piantelli M. Growth-inhibitory effect of quercetin and presence of type-II estrogen-binding sites in human colon-cancer cell lines and primary colorectal tumors. Int. J. Cancer 1992, 50, 486–492. 10.1002/ijc.2910500326. [DOI] [PubMed] [Google Scholar]
- Ranelletti F. O.; Maggiano N.; Serra F. G.; Ricci R.; Larocca L. M.; Lanza P.; Scambia G.; Fattorossi A.; Capelli A.; Piantelli M. Quercetin inhibits p21-RAS expression in human colon cancer cell lines and in primary colorectal tumors. Int. J. Cancer 2000, 85, 438–445. . [DOI] [PubMed] [Google Scholar]
- Yoshida M.; Sakai T.; Hosokawa N.; Marui N.; Matsumoto K.; Fujioka A.; Nishino H.; Aoike A. The effect of quercetin on cell cycle progression and growth of human gastric cancer cells. FEBS Lett. 1990, 260, 10–13. 10.1016/0014-5793(90)80053-L. [DOI] [PubMed] [Google Scholar]
- Shang H. S.; Lu H. F.; Lee C. H.; Chiang H. S.; Chu Y. L.; Chen A.; Lin Y. F.; Chung J. G. Quercetin induced cell apoptosis and altered gene expression in AGS human gastric cancer cells. Environ. Toxicol. 2018, 33, 1168–1181. 10.1002/tox.22623. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Yuan Q.; Xu G.; Chen H.; Lei H.; Su J. Effects of Quercetin on Proliferation and H2O2-Induced Apoptosis of Intestinal Porcine Enterocyte Cells. Molecules 2018, 23, 2012. 10.3390/molecules23082012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang C. H.; Chan S. T.; Chen C. H.; Yeh S. L. Quercetin enhances the antitumor activity of trichostatin A through up-regulation of p300 protein expression in p53 null cancer cells. Chem.-Biol. Interact. 2019, 306, 54–61. 10.1016/j.cbi.2019.04.006. [DOI] [PubMed] [Google Scholar]
- Ackland M. L.; van de Waarsenburg S.; Jones R. Synergistic antiproliferative action of the flavonols quercetin and kaempferol in cultured human cancer cell lines. In Vivo 2005, 19, 69–76. [PubMed] [Google Scholar]
- Yalçın A. S.; Yılmaz A. M.; Altundağ E. M.; Koçtürk S. Kurkumin, kuersetin ve çay kateşinlerinin anti-kanser etkileri. Marmara Pharm. J. 2016, 21, 19–29. 10.12991/marupj.259877. [DOI] [Google Scholar]
- Vargas A. J.; Burd R. Hormesis and synergy: pathways and mechanisms of quercetin in cancer prevention and management. Nutrition reviews 2010, 68, 418–428. 10.1111/j.1753-4887.2010.00301.x. [DOI] [PubMed] [Google Scholar]
- Nam J. S.; Sharma A. R.; Nguyen L. T.; Chakraborty C.; Sharma G.; Lee S. S. Application of Bioactive Quercetin in Oncotherapy: From Nutrition to Nanomedicine. Molecules 2016, 21, 108. 10.3390/molecules21010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre L.; Arias N.; Macarulla M. T.; Gracia A.; Portillo M. P. Beneficial effects of quercetin on obesity and diabetes. Open Nutraceuticals J. 2011, 4, 189–198. 10.2174/1876396001104010189. [DOI] [Google Scholar]
- Chen S.; Jiang H.; Wu X.; Fang J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediators Inflammation 2016, 2016, 9340637 10.1155/2016/9340637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostadmohammadi V.; Milajerdi A.; Ayati E.; Kolahdooz F.; Asemi Z. Effects of quercetin supplementation on glycemic control among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1330–1340. 10.1002/ptr.6334. [DOI] [PubMed] [Google Scholar]
- Chondrogianni N.; Kapeta S.; Chinou I.; Vassilatou K.; Papassideri I.; Gonos E. S. Anti-ageing and rejuvenating effects of quercetin. Exp. Gerontol. 2010, 45, 763–771. 10.1016/j.exger.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Geng L.; Liu Z.; Zhang W.; Li W.; Wu Z.; Wang W.; Ren R.; Su Y.; Wang P.; Sun L.; Ju Z.; Chan P.; Song M.; Qu J.; Liu G. H. Chemical screen identifies a geroprotective role of quercetin in premature aging. Protein Cell 2019, 10, 417–435. 10.1007/s13238-018-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson A. J.; Symons J. D.; Jalili T. Quercetin: A Treatment for Hypertension?-A Review of Efficacy and Mechanisms. Pharmaceuticals 2010, 3, 237–250. 10.3390/ph3010237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormaz J. G.; Quintremil S.; Rodrigo R. Cardiovascular Disease: A Target for the Pharmacological Effects of Quercetin. Curr. Top. Med. Chem. 2015, 15, 1735–1742. 10.2174/1568026615666150427124357. [DOI] [PubMed] [Google Scholar]
- Khurana S.; Venkataraman K.; Hollingsworth A.; Piche M.; Tai T. C. Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, 3779–3827. 10.3390/nu5103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribolo S.; Lodi F.; Connor C.; Suri S.; Wilson V. G.; Taylor M. A.; Needs P. W.; Kroon P. A.; Hughes D. A. Comparative effects of quercetin and its predominant human metabolites on adhesion molecule expression in activated human vascular endothelial cells. Atherosclerosis 2008, 197, 50–56. 10.1016/j.atherosclerosis.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Carrasco-Pozo C.; Cires M. J.; Gotteland M. Quercetin and Epigallocatechin Gallate in the Prevention and Treatment of Obesity: From Molecular to Clinical Studies. J. Med. food 2019, 22, 753–770. 10.1089/jmf.2018.0193. [DOI] [PubMed] [Google Scholar]
- Patel R. V.; Mistry B. M.; Shinde S. K.; Syed R.; Singh V.; Shin H. S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. 10.1016/j.ejmech.2018.06.053. [DOI] [PubMed] [Google Scholar]
- D’Andrea G. Quercetin: A flavonol with multifaceted therapeutic applications?. Fitoterapia 2015, 106, 256–271. 10.1016/j.fitote.2015.09.018. [DOI] [PubMed] [Google Scholar]
- Boots A. W.; Haenen G. R.; Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Kook D.; Wolf A. H.; Yu A. L.; Neubauer A. S.; Priglinger S. G.; Kampik A.; Welge-Lussen U. C. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol. Visual Sci. 2008, 49, 1712–1720. 10.1167/iovs.07-0477. [DOI] [PubMed] [Google Scholar]
- Suematsu N.; Hosoda M.; Fujimori K. Protective effects of quercetin against hydrogen peroxide-induced apoptosis in human neuronal SH-SY5Y cells. Neurosci. Lett. 2011, 504, 223–227. 10.1016/j.neulet.2011.09.028. [DOI] [PubMed] [Google Scholar]
- Carullo G.; Cappello A. R.; Frattaruolo L.; Badolato M.; Armentano B.; Aiello F. Quercetin and derivatives: useful tools in inflammation and pain management. Future Med. Chem. 2017, 9, 79–93. 10.4155/fmc-2016-0186. [DOI] [PubMed] [Google Scholar]
- Gokhale J. P.; Mahajan H. S.; Surana S. J. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: In vivo and in vitro studies. Biomed. Pharmacother. 2019, 112, 108622 10.1016/j.biopha.2019.108622. [DOI] [PubMed] [Google Scholar]
- Dietrich-Muszalska A.; Olas B. Inhibitory effects of polyphenol compounds on lipid peroxidation caused by antipsychotics (haloperidol and amisulpride) in human plasma in vitro. World J. Biol. Psychiatry 2010, 11, 276–281. 10.3109/15622970902718790. [DOI] [PubMed] [Google Scholar]
- Rabinovich L.; Kazlouskaya V. Herbal sun protection agents: Human studies. Clin. Dermatol. 2018, 36, 369–375. 10.1016/j.clindermatol.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Gaudry A.; Bos S.; Viranaicken W.; Roche M.; Krejbich-Trotot P.; Gadea G.; Despres P.; El-Kalamouni C. The Flavonoid Isoquercitrin Precludes Initiation of Zika Virus Infection in Human Cells. Int. J. Mol. Sci. 2018, 19, 1093. 10.3390/ijms19041093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargouri B.; Mansour R. B.; Abdallah F. B.; Elfekih A.; Lassoued S.; Khaled H. Protective effect of quercetin against oxidative stress caused by dimethoate in human peripheral blood lymphocytes. Lipids Health Dis. 2011, 10, 149. 10.1186/1476-511X-10-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M. H.; Shin H. J. Anti-melanogenesis effect of quercetin. Cosmetics 2016, 3, 18. 10.3390/cosmetics3020018. [DOI] [Google Scholar]
- Kawabata K.; Mukai R.; Ishisaka A. Quercetin and related polyphenols: new insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. 10.1039/C4FO01178C. [DOI] [PubMed] [Google Scholar]
- Graefe E. U.; Wittig J.; Mueller S.; Riethling A.; Uehleke B.; Drewelow B.; Pforte H.; Jacobasch G.; H H. D.; Veit M. Pharmacokinetics and Bioavailability of Quercetin Glycosides in Humans. J. Clin. Psychopharmacol. 2001, 41, 492–499. 10.1177/00912700122010366. [DOI] [PubMed] [Google Scholar]
- Almeida A. F.; Borge G. I. A.; Piskula M.; Tudose A.; Tudoreanu L.; Valentová K.; Williamson G.; Santos C. N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. 10.1111/1541-4337.12342. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P.; Prajapati A. K. Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers – a review. RSC Adv. 2015, 5, 97547–97562. 10.1039/C5RA18896B. [DOI] [Google Scholar]
- Rizvi S. A. A.; Saleh A. M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. 10.1016/j.jsps.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksi R.; Singh D. P.; Borse S. P.; Rana R.; Sharma V.; Nivsarkar M. In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed. Pharmacother. 2018, 106, 1513–1526. 10.1016/j.biopha.2018.07.106. [DOI] [PubMed] [Google Scholar]
- de Oliveira Pedro R.; Hoffmann S.; Pereira S.; Goycoolea F. M.; Schmitt C. C.; Neumann M. G. Self-assembled amphiphilic chitosan nanoparticles for quercetin delivery to breast cancer cells. Eur. J. Pharm. Biopharm. 2018, 131, 203–210. 10.1016/j.ejpb.2018.08.009. [DOI] [PubMed] [Google Scholar]
- Penalva R.; Gonzalez-Navarro C. J.; Gamazo C.; Esparza I.; Irache J. M. Zein nanoparticles for oral delivery of quercetin: Pharmacokinetic studies and preventive anti-inflammatory effects in a mouse model of endotoxemia. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 103–110. 10.1016/j.nano.2016.08.033. [DOI] [PubMed] [Google Scholar]
- Halder A.; Mukherjee P.; Ghosh S.; Mandal S.; Chatterji U.; Mukherjee A. Smart PLGA nanoparticles loaded with Quercetin: Cellular uptake and in-vitro anticancer study. Mater. Today: Proc. 2018, 5, 9698–9705. 10.1016/j.matpr.2017.10.156. [DOI] [Google Scholar]
- Lou M.; Zhang L. N.; Ji P. G.; Feng F. Q.; Liu J. H.; Yang C.; Li B. F.; Wang L. Quercetin nanoparticles induced autophagy and apoptosis through AKT/ERK/Caspase-3 signaling pathway in human neuroglioma cells: In vitro and in vivo. Biomed. Pharmacother. 2016, 84, 1–9. 10.1016/j.biopha.2016.08.055. [DOI] [PubMed] [Google Scholar]
- Chitkara D.; Nikalaje S. K.; Mittal A.; Chand M.; Kumar N. Development of quercetin nanoformulation and in vivo evaluation using streptozotocin induced diabetic rat model. Drug Delivery Transl. Res. 2012, 2, 112–123. 10.1007/s13346-012-0063-5. [DOI] [PubMed] [Google Scholar]
- Tan B. J.; Liu Y.; Chang K. L.; Lim B. K.; Chiu G. N. Perorally active nanomicellar formulation of quercetin in the treatment of lung cancer. Int. J. Nanomed. 2012, 7, 651–661. 10.2147/IJN.S26538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.; Wang X. Spectrometric study on the interaction of sodium cholate aggregates with quercetin. Colloids Surf., A 2015, 481, 31–37. 10.1016/j.colsurfa.2015.04.023. [DOI] [Google Scholar]
- Sadhukhan P.; Kundu M.; Chatterjee S.; Ghosh N.; Manna P.; Das J.; Sil P. C. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater. Sci. Eng., C 2019, 100, 129–140. 10.1016/j.msec.2019.02.096. [DOI] [PubMed] [Google Scholar]
- Braga L. R.; Pérez L. M.; Soazo M. dV.; Machado F. Evaluation of the antimicrobial, antioxidant and physicochemical properties of Poly(Vinyl chloride) films containing quercetin and silver nanoparticles. Lwt 2019, 101, 491–498. 10.1016/j.lwt.2018.11.082. [DOI] [Google Scholar]
- Yang X.; Zhang W.; Zhao Z.; Li N.; Mou Z.; Sun D.; Cai Y.; Wang W.; Lin Y. Quercetin loading CdSe/ZnS nanoparticles as efficient antibacterial and anticancer materials. J. Inorg. Biochem. 2017, 167, 36–48. 10.1016/j.jinorgbio.2016.11.023. [DOI] [PubMed] [Google Scholar]
- Lee G. H.; Lee S. J.; Jeong S. W.; Kim H. C.; Park G. Y.; Lee S. G.; Choi J. H. Antioxidative and antiinflammatory activities of quercetin-loaded silica nanoparticles. Colloids Surf., B 2016, 143, 511–517. 10.1016/j.colsurfb.2016.03.060. [DOI] [PubMed] [Google Scholar]
- Aghapour F.; Moghadamnia A. A.; Nicolini A.; Kani S. N. M.; Barari L.; Morakabati P.; Rezazadeh L.; Kazemi S. Quercetin conjugated with silica nanoparticles inhibits tumor growth in MCF-7 breast cancer cell lines. Biochem. Biophys. Res. Commun. 2018, 500, 860–865. 10.1016/j.bbrc.2018.04.174. [DOI] [PubMed] [Google Scholar]
- Niazvand F.; Orazizadeh M.; Khorsandi L.; Abbaspour M.; Mansouri E.; Khodadadi A. Effects of Quercetin-Loaded Nanoparticles on MCF-7 Human Breast Cancer Cells. Medicina 2019, 55, 114. 10.3390/medicina55040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatahet T.; Morille M.; Hommoss A.; Devoisselle J. M.; Muller R. H.; Begu S. Liposomes, lipid nanocapsules and smartCrystals(R): A comparative study for an effective quercetin delivery to the skin. Int. J. Pharm. 2018, 542, 176–185. 10.1016/j.ijpharm.2018.03.019. [DOI] [PubMed] [Google Scholar]
- Vijayakumar A.; Baskaran R.; Jang Y. S.; Oh S. H.; Yoo B. K. Quercetin-Loaded Solid Lipid Nanoparticle Dispersion with Improved Physicochemical Properties and Cellular Uptake. AAPS PharmSciTech 2017, 18, 875–883. 10.1208/s12249-016-0573-4. [DOI] [PubMed] [Google Scholar]
- Liu L.; Tang Y.; Gao C.; Li Y.; Chen S.; Xiong T.; Li J.; Du M.; Gong Z.; Chen H.; Liu L.; Yao P. Characterization and biodistribution in vivo of quercetin-loaded cationic nanostructured lipid carriers. Colloids Surf., B 2014, 115, 125–131. 10.1016/j.colsurfb.2013.11.029. [DOI] [PubMed] [Google Scholar]
- Srinivas K.; King J. W.; Howard L. R.; Monrad J. K. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. J. Food Eng. 2010, 100, 208–218. 10.1016/j.jfoodeng.2010.04.001. [DOI] [Google Scholar]
- Biasutto L.; Marotta E.; Garbisa S.; Zoratti M.; Paradisi C. Determination of quercetin and resveratrol in whole blood--implications for bioavailability studies. Molecules 2010, 15, 6570–6579. 10.3390/molecules15096570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A.; Kumar V.; Yadav S. K. Plant extract synthesized PLA nanoparticles for controlled and sustained release of quercetin: a green approach. PLoS One 2012, 7, e41230 10.1371/journal.pone.0041230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza M. P.; Vaz A. F. M.; Correia M. T. S.; Cerqueira M. A.; Vicente A. A.; Carneiro-da-Cunha M. G. Quercetin-loaded lecithin/chitosan nanoparticles for functional food applications. Food Bioprocess Technol. 2014, 7, 1149–1159. 10.1007/s11947-013-1160-2. [DOI] [Google Scholar]
- Gugler R.; Leschik M.; Dengler H. J. Disposition of quercetin in man after single oral and intravenous doses. Eur. J. Clin. Pharmacol. 1975, 9, 229–234. 10.1007/BF00614022. [DOI] [PubMed] [Google Scholar]
- Lipinski C. A. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discovery Today: Technol. 2004, 1, 337–341. 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Kawai Y. Understanding metabolic conversions and molecular actions of flavonoids in vivo:toward new strategies for effective utilization of natural polyphenols in human health. J. Med. Invest. 2018, 65, 162–165. 10.2152/jmi.65.162. [DOI] [PubMed] [Google Scholar]
- de Boer V. C.; Dihal A. A.; van der Woude H.; Arts I. C.; Wolffram S.; Alink G. M.; Rietjens I. M.; Keijer J.; Hollman P. C. Tissue distribution of quercetin in rats and pigs. J. Nutr. 2005, 135, 1718–1725. 10.1093/jn/135.7.1718. [DOI] [PubMed] [Google Scholar]
- Olthof M. R.; Hollman P. C.; Vree T. B.; Katan M. B. Bioavailabilities of quercetin-3-glucoside and quercetin-4′-glucoside do not differ in humans. J. Nutr. 2000, 130, 1200–1203. 10.1093/jn/130.5.1200. [DOI] [PubMed] [Google Scholar]
- Stopa J. D.; Neuberg D.; Puligandla M.; Furie B.; Flaumenhaft R.; Zwicker J. I. Protein disulfide isomerase inhibition blocks thrombin generation in humans by interfering with platelet factor V activation. JCI insight 2017, 2, e89373 10.1172/jci.insight.89373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day A. J.; Mellon F.; Barron D.; Sarrazin G.; Morgan M. R.; Williamson G. Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin. Free Radical Res. 2001, 35, 941–952. 10.1080/10715760100301441. [DOI] [PubMed] [Google Scholar]
- Hollman P. C. H.; Arts I. C. W. Flavonols, flavones and flavanols – nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1081–1093. . [DOI] [Google Scholar]
- Manach C.; Donovan J. L. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radical Res. 2004, 38, 771–785. 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]
- Li W.; Yi S.; Wang Z.; Chen S.; Xin S.; Xie J.; Zhao C. Self-nanoemulsifying drug delivery system of persimmon leaf extract: Optimization and bioavailability studies. Int. J. Pharm. 2011, 420, 161–171. 10.1016/j.ijpharm.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Sarkar S.; Mandal S.; Sinha J.; Mukhopadhyay S.; Das N.; Basu M. K. Quercetin: critical evaluation as an antileishmanial agent in vivo in hamsters using different vesicular delivery modes. J. Drug Targeting 2002, 10, 573–578. 10.1080/106118021000072681. [DOI] [PubMed] [Google Scholar]
- Li H.; Zhao X.; Ma Y.; Zhai G.; Li L.; Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Controlled Release 2009, 133, 238–244. 10.1016/j.jconrel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Ghosh D.; Ghosh S.; Sarkar S.; Ghosh A.; Das N.; Das Saha K.; Mandal A. K. Quercetin in vesicular delivery systems: evaluation in combating arsenic-induced acute liver toxicity associated gene expression in rat model. Chem.-Biol. Interact. 2010, 186, 61–71. 10.1016/j.cbi.2010.03.048. [DOI] [PubMed] [Google Scholar]
- Chakraborty S.; Stalin S.; Das N.; Choudhury S. T.; Ghosh S.; Swarnakar S. The use of nano-quercetin to arrest mitochondrial damage and MMP-9 upregulation during prevention of gastric inflammation induced by ethanol in rat. Biomaterials 2012, 33, 2991–3001. 10.1016/j.biomaterials.2011.12.037. [DOI] [PubMed] [Google Scholar]
- Ghosh S.; Sarkar S.; Choudhury S. T.; Ghosh T.; Das N. Triphenyl phosphonium coated nano-quercetin for oral delivery: Neuroprotective effects in attenuating age related global moderate cerebral ischemia reperfusion injury in rats. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2439–2450. 10.1016/j.nano.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Sousa-Batista A. J.; Poletto F. S.; Philipon C.; Guterres S. S.; Pohlmann A. R.; Rossi-Bergmann B. Lipid-core nanocapsules increase the oral efficacy of quercetin in cutaneous leishmaniasis. Parasitology 2017, 144, 1769–1774. 10.1017/S003118201700097X. [DOI] [PubMed] [Google Scholar]
- Tian F.; Dahmani F. Z.; Qiao J.; Ni J.; Xiong H.; Liu T.; Zhou J.; Yao J. A targeted nanoplatform co-delivering chemotherapeutic and antiangiogenic drugs as a tool to reverse multidrug resistance in breast cancer. Acta Biomater. 2018, 75, 398–412. 10.1016/j.actbio.2018.05.050. [DOI] [PubMed] [Google Scholar]
- Saha C.; Kaushik A.; Das A.; Pal S.; Majumder D. Anthracycline Drugs on Modified Surface of Quercetin-Loaded Polymer Nanoparticles: A Dual Drug Delivery Model for Cancer Treatment. PLoS One 2016, 11, e0155710 10.1371/journal.pone.0155710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minaei A.; Sabzichi M.; Ramezani F.; Hamishehkar H.; Samadi N. Co-delivery with nano-quercetin enhances doxorubicin-mediated cytotoxicity against MCF-7 cells. Mol. Biol. Rep. 2016, 43, 99–105. 10.1007/s11033-016-3942-x. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Xu S.; Wang Y.; Yu Y.; Li F.; Zhu H.; Shen Y.; Huang S.; Guo S. Near-infrared triggered co-delivery of doxorubicin and quercetin by using gold nanocages with tetradecanol to maximize anti-tumor effects on MCF-7/ADR cells. J. Colloid Interface Sci. 2018, 509, 47–57. 10.1016/j.jcis.2017.08.097. [DOI] [PubMed] [Google Scholar]
- Zhu B.; Yu L.; Yue Q. Co-delivery of vincristine and quercetin by nanocarriers for lymphoma combination chemotherapy. Biomed. Pharmacother. 2017, 91, 287–294. 10.1016/j.biopha.2017.02.112. [DOI] [PubMed] [Google Scholar]
- Rezvani M.; Mohammadnejad J.; Narmani A.; Bidaki K. Synthesis and in vitro study of modified chitosan-polycaprolactam nanocomplex as delivery system. Int. J. Biol. Macromol. 2018, 113, 1287–1293. 10.1016/j.ijbiomac.2018.02.141. [DOI] [PubMed] [Google Scholar]
- Mu Y.; Fu Y.; Li J.; Yu X.; Li Y.; Wang Y.; Wu X.; Zhang K.; Kong M.; Feng C.; Chen X. Multifunctional quercetin conjugated chitosan nano-micelles with P-gp inhibition and permeation enhancement of anticancer drug. Carbohydr. Polym. 2019, 203, 10–18. 10.1016/j.carbpol.2018.09.020. [DOI] [PubMed] [Google Scholar]
- Li J.; He Z.; Yu S.; Li S.; Ma Q.; Yu Y.; Zhang J.; Li R.; Zheng Y.; He G.; Song X. Micelles based on methoxy poly(ethylene glycol)-cholesterol conjugate for controlled and targeted drug delivery of a poorly water soluble drug. J. Biomed. Nanotechnol. 2012, 8, 809–817. 10.1166/jbn.2012.1433. [DOI] [PubMed] [Google Scholar]
- Gupta P.; Authimoolam S. P.; Hilt J. Z.; Dziubla T. D. Quercetin conjugated poly(beta-amino esters) nanogels for the treatment of cellular oxidative stress. Acta Biomater. 2015, 27, 194–204. 10.1016/j.actbio.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catauro M.; Bollino F.; Nocera P.; Piccolella S.; Pacifico S. Entrapping quercetin in silica/poly(ethylene glycol) hybrid materials: Chemical characterization and biocompatibility. Mater. Sci. Eng., C 2016, 68, 205–212. 10.1016/j.msec.2016.05.082. [DOI] [PubMed] [Google Scholar]
- Abd-Rabou A. A.; Ahmed H. H. CS-PEG decorated PLGA nano-prototype for delivery of bioactive compounds: A novel approach for induction of apoptosis in HepG2 cell line. Adv. Med. Sci. 2017, 62, 357–367. 10.1016/j.advms.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Das S.; Roy P.; Mondal S.; Bera T.; Mukherjee A. One pot synthesis of gold nanoparticles and application in chemotherapy of wild and resistant type visceral leishmaniasis. Colloids Surf., B 2013, 107, 27–34. 10.1016/j.colsurfb.2013.01.061. [DOI] [PubMed] [Google Scholar]
- Rezaei-Sadabady R.; Eidi A.; Zarghami N.; Barzegar A. Intracellular ROS protection efficiency and free radical-scavenging activity of quercetin and quercetin-encapsulated liposomes. Artif. Cells, Nanomed., Biotechnol. 2016, 44, 128–134. 10.3109/21691401.2014.926456. [DOI] [PubMed] [Google Scholar]
- Huang X.; Chen X.; Chen Q.; Yu Q.; Sun D.; Liu J. Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs. Acta Biomater. 2016, 30, 397–407. 10.1016/j.actbio.2015.10.041. [DOI] [PubMed] [Google Scholar]
- Kumar P.; Sharma G.; Kumar R.; Singh B.; Malik R.; Katare O. P.; Raza K. Promises of a biocompatible nanocarrier in improved brain delivery of quercetin: Biochemical, pharmacokinetic and biodistribution evidences. Int. J. Pharm. 2016, 515, 307–314. 10.1016/j.ijpharm.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Lozano-Pérez A. A.; Rivero H. C.; Perez Hernandez M. D. C.; Pagan A.; Montalban M. G.; Villora G.; Cenis J. L. Silk fibroin nanoparticles: Efficient vehicles for the natural antioxidant quercetin. Int. J. Pharm. 2017, 518, 11–19. 10.1016/j.ijpharm.2016.12.046. [DOI] [PubMed] [Google Scholar]
- Abd El-Fattah A. I.; Fathy M. M.; Ali Z. Y.; El-Garawany A. E. A.; Mohamed E. K. Enhanced therapeutic benefit of quercetin-loaded phytosome nanoparticles in ovariectomized rats. Chem.-Biol. Interact. 2017, 271, 30–38. 10.1016/j.cbi.2017.04.026. [DOI] [PubMed] [Google Scholar]
- Singh J.; Mittal P.; Vasant Bonde G.; Ajmal G.; Mishra B. Design, optimization, characterization and in-vivo evaluation of Quercetin enveloped Soluplus(R)/P407 micelles in diabetes treatment. Artif. Cells, Nanomed., Biotechnol. 2018, 46, S546–S555. 10.1080/21691401.2018.1501379. [DOI] [PubMed] [Google Scholar]
- Habas K.; Abdulmwli M.; Demir E.; Jacob B. K.; Najafzadeh M.; Anderson D. DNA damage protection by bulk and nano forms of quercetin in lymphocytes of patients with chronic obstructive pulmonary disease exposed to the food mutagen 2-amino-3-methylimidazo [4,5-f]quinolone (IQ). Environ. Res. 2018, 166, 10–15. 10.1016/j.envres.2018.05.012. [DOI] [PubMed] [Google Scholar]