Abstract
Primary central nervous system lymphoma (PCNSL) typically shows a strong uptake of 18F-fludeoxyglucose (FDG) imaged by positron emission tomography (PET). Uncommonly, PCNSL demonstrates a low uptake on FDG PET. We investigated the clinicopathological characteristics of the unusual cases of PCNSL with low FDG uptake.
We retrospectively enrolled 104 consecutive patients with newly diagnosed PCNSL who underwent baseline brain FDG PET. The degree of FDG uptake of PCNSL was visually scored by 4 grades (0, ≤contralateral white matter; 1, >contralateral white matter and <contralateral gray matter; 2, = contralateral gray matter; 3, >contralateral gray matter). Grades 0–2 were considered as PCNSL with low uptake. We investigated association of low uptake of PCNSL with the following clinicopathological factors: age, sex, steroid treatment, lactate dehydrogenase level, cerebrospinal fluid protein level, condition of PET scanning, immunohistochemical markers (cluster of differentiation 10 [CD10], B-cell lymphoma 6 [BCL-6], B-cell lymphoma 2 [BCL-2], multiple myeloma oncogene 1 [MUM1], Epstein-Barr virus [EBV] protein, and Ki67), location of lesions, tumor size, multiplicity of lesions, involvement of deep brain structures, and cystic or necrotic appearance of lesions.
Of the 104 patients with PCNSL, 14 patients (13.5%) showed PCNSL with low FDG uptake on PET. Among various clinicopathological factors, MUM1 negativity was the only factor associated with low FDG uptake PCNSL by univariate (P = .002) and multivariate analysis (P = .007).
This study suggests that the different clinicopathological characteristics between patients with high uptake and low uptake of PCNSL on FDG PET is closely associated with lack of MUM1, a protein known to be a crucial regulator of B-cell development and tumorigenesis.
Keywords: 18F-fludeoxyglucose positron emission tomography, characteristic, multiple myeloma oncogene 1, primary central nervous system lymphoma
1. Introduction
Primary central nervous system lymphoma (PCNSL) is a rare subtype of extranodal non-Hodgkin lymphoma (NHL) and is confined to the brain, leptomeninges, spinal cord, or eyes, without evidence of systemic disease.[1] Although the worldwide incidence of PCNSL has increased over the last 2 decades, little is known regarding its molecular biology, clinical features, or prognostic biomarkers.[2] Many studies used 18F-fludeoxyglucose (FDG) positron emission tomography (PET) to reveal biological features of tumors, such as proliferation, histologic type, differentiation, and hypoxia.[3,4,5,6] Since PCNSL has a high cellular density and high glycolytic activity, it shows a strong accumulation of FDG, and at a higher level than that seen in high-grade gliomas and normal brain tissue. FDG PET is, therefore, a promising diagnostic tool in the field of PCNSL.[7,8,9] Rosenfeld et al[10] demonstrated a mean tumor to normal tissue FDG uptake ratio of 1.6 for high-grade glioma and a significantly higher value of 2.0 for PCNSL, while Palmedo et al[11] found that the ratio in PCNSL was even higher, at 2.8.
Uncommonly, PCNSL demonstrates a low uptake on FDG PET. The detection and differential diagnosis of a PCNSL lesion can be difficult in such cases, as it can mimic other types of tumors or non-tumorous disease (e.g., multiple sclerosis, stroke, or infection).[12] Some studies concluded that such low FDG uptake by PCNSL is due to steroid use prior to the PET examination.[8,9,10] One study showed that FDG uptake differed between steroid-treated and untreated cases of PCNSL, but not significantly (P = .4), and the small number of patients studied (10 PCNSL patients, of whom only 4 took dexamethasone) did not allow any definitive conclusion to be drawn.[10]
To date, there have been no reports on the clinicopathological features of PCNSL with atypically low uptake on FDG PET, presumably due in part to the rarity of this disease. This study based on the hypothesis that PCNSL with low uptake could be related to previously unrecognized clinical or histopathological tumor factors. We therefore investigated the different clinicopathological characteristics between patients with high uptake and low uptake of PCNSL on FDG PET.
2. Methods
2.1. Patients
A total of 151 consecutive patients with pathologically confirmed CNSL by stereotactic biopsy or resection at the Asan Medical Center between 2002 and 2013 were retrospectively reviewed. Of these patients, 107 patients who underwent brain FDG PET or PET/computed tomography (CT) before chemotherapy or radiation therapy were included. Among these patients, 3 patients were excluded because systemic lymphoma was identified by chest, abdominal, and pelvic CT, bone marrow biopsy, and/or body PET on initial work up. The remaining 104 PCNSL patients (61 men, 43 women; mean age: 56.7 ± 14.3 years, range: 7–78 years) were analyzed in the present study.
The clinical data reviewed from the electronic medical recording system of the Asan Medical Center were as follows: age at diagnosis, sex, steroid treatment before PET, dose and duration of steroid administration if any, serum lactate dehydrogenase (LDH) level, and cerebrospinal fluid (CSF) protein level, immunohistochemical markers (cluster of differentiation 10 [CD10], B-cell lymphoma 6 [BCL-6], B-cell lymphoma 2 [BCL-2], multiple myeloma oncogene 1 [MUM1], Epstein-Barr virus [EBV] protein, and Ki67). The location of brain lesions (supratentorial or subtentorial), tumor size, multiplicity of lesions, involvement of deep brain structures (periventricular area, corpus callosum, basal ganglia, brain stem, and cerebellum), cystic or necrotic lesions were determined by MR images. To assess the condition of PET scanning, blood glucose level at the time of injection of FDG, FDG injection dose, scan start time after FDG injection were reviewed from PET reporting results in electronic medical recording system. The association of these clinicopathological factors of PCNSL with low uptake on FDG PET was investigated.
This study was conducted in accordance with the Helsinki Declaration. The Institutional Review Board of the Asan Medical Center approved this retrospective cohort study (Approval no. 2013–0835), and informed consent from patients was waived due to the retrospective nature of this study.
2.2. 18F-FDG PET imaging
Data were acquired using ECAT EXACT HR+ (Siemens Medical Systems, Knoxville, TN), PET/CT Biograph Sensation 16 (Siemens), PET/CT TruePoint 40 (Siemens), PET/CT Discovery STE (GE Healthcare, Milwaukee, WI), and PET/CT D690 scanning systems (GE). PET emission data were acquired for 10 to 15 minutes in the 3-dimensional mode. For attenuation correction, a transmission scan was performed using an external source of 68Ge in the ECAT EXACT HR + scanner or a brain CT was performed in the spiral mode at 120 kVp and 380 mAs (reference standard) in the other scanners. Attenuation-corrected emission images were reconstructed with 6 iterations and 21 subsets. All patients fasted for at least 6 hours before FDG tracer injections, and each had a serum glucose level below 150 mg/dL at the time of FDG injection. All patients rested in a quiet, dimly lit room prior to injection with FDG, and were then positioned in the tomography gantry with the imaging plane parallel to the canthomeatal line. FDG PET images were obtained approximately 60 minutes after intravenous injection of 447.7 ± 111 MBq of FDG.
2.3. Immunohistochemistry and pathologic evaluation
Immunohistochemical staining was performed on 4-μm-thick sections from the tissue microarray block using the BenchMark XT autostainer (Ventana Medical Systems, Tucson, AZ). Briefly, these sections were deparaffinized by xylene and ethanol. After epitope retrieval, samples were incubated with diluted primary antibodies for 1 hour at room temperature. The slides were washed again and incubated with multimer-labeled anti-mouse or rabbit IgG, after which staining was developed using the staining kit according to the manufacturer's protocol. Finally, samples were counterstained with hematoxylin. The staining results were reviewed by an experienced pathologist. All pathological assessments were performed for routine clinical purposes. Pathology results were obtained from the electronic medical records: tissue immunohistochemical marker expression (CD10, BCL-6, BCL-2, and MUM1), Epstein-Barr virus infection and high Ki67 index (≥70% of tumor cells). Using the results of immunohistochmistry, the cases were classified into germinal center B-cell like (GCB) or non-GCB type according to the algorithm of Hans et al.[13]
2.4. Image analysis
Visual analysis of FDG uptakes of PCNSL lesions on brain PET was performed by the consensus of 2 board-certified nuclear medicine physicians (HOK and SYC) who were blinded to the clinical information. The location of brain lesions was determined by visually comparing the PET images with the corresponding MR images (contrast-enhanced T1 weighted scans in all cases; time interval from PET to MR imaging: 5.1 ± 4.8 days, maximum of 34 days), and FDG uptake by the brain lesions was evaluated by comparison with normal brain tissue. The degree of FDG uptake by the tumor was classified using a 4 grade visual scoring system as follows: grade 0, less than or equal to contralateral white matter; grade 1, greater than contralateral white matter and less than contralateral gray matter; grade 2, equal to contralateral gray matter; grade 3, greater than contralateral gray matter (Fig. 1). Grade 3 was considered as representing the typical finding in PCNSL, while grades 0–2 were considered as low uptake of PCNSL on FDG PET.
Figure 1.
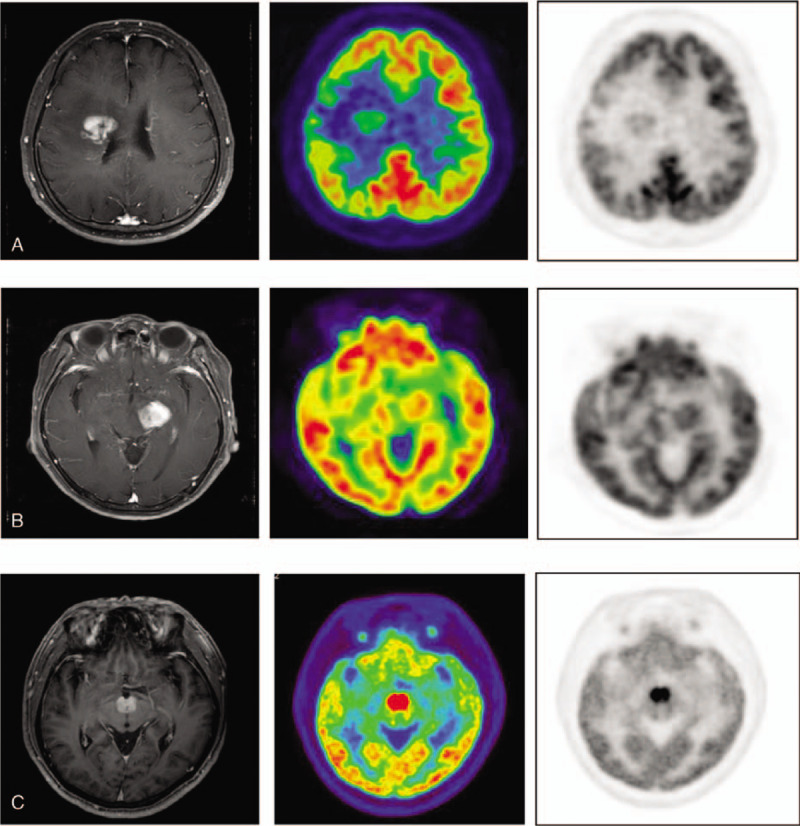
Representative images for visual grading scales of FDG uptake by PCNSL. In a grade 1 tumor (A) the tumor uptake is greater than that of the contralateral white matter and less than that of the contralateral gray matter, (B) grade 2 is equal in uptake to the contralateral gray matter, and (C) grade 3 uptake is greater than that of the contralateral gray matter. FDG = Fludeoxyglucose, PCNSL = primary central nervous system lymphoma.
2.5. Statistical analysis
Descriptive statistical analyses were performed on all patients. Continuous and categorical variables are presented as mean ± standard deviation (SD) and numbers (%). To evaluate the association of clinicopathological factors of PCNSL with low uptake, the PCNSL lesions were divided into 2 groups: PCNSL with low uptake and PCNSL with high uptake. The associations between continuous data such as age, tumor size, blood glucose level, scan start time after FDG injections, and PCNSL with low uptake were assessed using the independent 2-sample t test, or Mann–Whitney U test. Categorical data such as sex, steroid treatment before PET, elevation of serum LDH level, elevation of CSF protein level, location of brain lesions, multiplicity of lesions, involvement of deep brain structures, presence of cystic or necrotic lesions were compared using Pearson chi-square test or Fisher exact test. Logistic regression analysis was carried out for evaluating the relationship between PCNSL with low uptake and clinicopathological factors. On univariate analysis, variables for which the unadjusted P value was <.1 in logistic regression were entered for inclusion in the multivariate model. Multiple logistic regression analysis was performed using backward elimination to examine independent associated factors of PCNSL with low uptake. All statistical analyses were performed using IBM SPSS Version 21 for Windows (SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. Patient characteristics
The characteristics of the 104 patients with PCNSL are shown in Table 1. The mean age of the patients was 56.7 ± 14.3 years (range, 7–78 years). The mean time interval between brain biopsy and FDG PET was 4.7 ± 9.9 days. The subtypes of PCNSL in the study population were as follows: diffuse large B-cell lymphoma (DLBCL; n = 100), Burkitt lymphoma (n = 1), extranodal marginal zone B-cell lymphoma (n = 1), and peripheral T-cell lymphoma (n = 2).
Table 1.
Characteristics of the PCNSL patients.

Fifty-six (53.8%) patients were receiving corticosteroid treatment at the time of the PET scans. For these patients, the mean daily dose was 12.4 mg of dexamethasone (4–18 mg), the time interval between start of steroid administration and PET imaging was 4.2 ± 4.6 days (range, 1–29 days), and the mean treatment duration was 3.5 ± 3.5 days (range, 1–22 days).
Immunohistochemical analysis of PCNSL lesions revealed the following positivity rates: CD10, 26.0%; BCL-6, 73.7%; BCL-2, 70.0%; MUM1, 90.9%; and high Ki67, 80.8%. Applying the phenotypic algorithm of Hans et al,[13] these data indicated that 29.0% (20/69) of the cases were of the GCB subtype and 71.0% (49/69) the non-GCB subtype.
3.2. Characteristics of patients with PCNSL with low FDG uptake
None of the 104 PCNSL cases had FDG uptake of grade 0, 6 (5.8%) were grade 1, 8 (7.7%) were grade 2, and 90 (86.5%) were grade 3. Therefore, 14 cases (13.5%) showed PCNSL with low uptake and 90 cases (86.5%) showed typical presentation on FDG PET. In 13 of 14 cases showing low FDG uptake, pathological diagnosis was DLBCL, and 1 was extranodal marginal zone B-cell lymphoma.
Multiple clinicopathological factors of PCNSL are summarized in Table 2 and compared between patients with high and low FDG uptake by univariate analysis. In univariate analysis, the only factor significantly associated with PCNSL with low uptake was MUM1 expression (P = .002). MUM1 positivity was found in 5/9 (55.6%) of PCNSL with low uptake, while 55/57 (96.5%) with high uptake were positive. Among various clinicopathological factors analyzed by multivariate analysis, again only expression of MUM1 was associated with PCNSL with low uptake (odds ratio = 15.893; 95% confidence interval [CI] = 2.102–120.160; P = .007; Table 3).
Table 2.
Clinicopathological factors associated with PCNSL with low FDG uptake on FDG PET.

Table 3.
Univariate and multivariate logistic regression models of factors associated with PCNSL with low FDG uptake.

The proportion of patients who had received steroid treatment was higher among those with low FDG uptake PCNSL than those with typical high uptake PCNSL (71.4% vs 51.1%), but the difference was not statistically significant (P = .249). In a subgroup analysis of the 48 steroid-naïve cases, 4 cases showed low FDG uptake, and MUM1 expression was also the only factor associated with low uptake (P = .016). In the steroid-treated group, the duration of steroid treatment tended to be longer in patients with PCNSL with low uptake than in those with PCNSL with high uptake (5.7 ± 6.7 days vs 3.0 ± 2.2 days), but the difference was not statistically significant (P = .245).
4. Discussion
We reviewed the characteristics of patients with PCNSL with low FDG uptake seen at our institute over a 10-year period; the total of 104 constitutes a large cohort. Our data suggest that PCNSL with low uptake is closely related to negativity for MUM1 expression by the tumor. MUM1 is a member of the interferon regulatory family of transcription factors, and plays an essential role in cell proliferation, survival, and differentiation.[14,15] Expression of MUM1 by DLBCL is indicative of a non-GCB cell phenotype, with a less favorable prognosis than cases of GCB type.[16] MUM1 is a key regulator of diverse steps in dendritic, myeloid, and lymphoid cell differentiation and maturation,[17] and the level of MUM1 expression varies in a stage- and lineage-specific manner, acting as an important regulator of the later stages of B-cell differentiation that occur after the expression of CD10 and Bcl-6.[18] MUM1 is also crucial for several stages of myeloid and T-cell differentiation.[17] Shaffer et al[17] discovered an extensive network of MUM1 target genes and identified a direct interaction with MYC in activated B-cells and myeloma cells using gene expression profiling and genome-wide chromatin immunoprecipitation analyses. A recent study by Tabata et al[19] showed that MUM1 induces MYC expression, and, in turn, MYC transactivates MUM1, creating a positive autoregulatory feedback loop. Several reports show that MYC directly controls the upregulation of many glucose metabolism-related genes, such as GLUT1, LDHA, HK2, PFKM, and ENO1, thereby contributing directly to the Warburg effect.[20,21,22] In human breast cancer, over-expression of MYC is the molecular alteration most highly associated with high FDG uptake.[21] Moreover, MYC strongly influences the glutamine pathway by regulating the RNA levels of several pathway members, shown to have increased transcript levels in FDG-avid and FDG signature-positive tumors.[20,21,23] The findings of the present study suggest that the FDG avidity of MUM1-positive tumors is related to the extensive network of interactions that associate MUM1 and MYC.
The abbreviation MUM1 stands for multiple myeloma oncogene 1, so called because it is an oncogene that was originally identified in multiple myeloma. Its inhibition has a toxic effect on myeloma cell lines, regardless of the transforming oncogenic mechanism.[24] Data on MUM1 expression in PCNSL are limited; hence there is no clue thus far to a possible oncogenic involvement of MUM1 in this tumor. Coupland et al[25] found MUM1 immunoreactivity in 45/50 (90%) cases of PCNSL. In the present study, we also found MUM1 reactivity in most (60/66, 90.9%) cases of PCNSL. However, we found that MUM1 immunoreactivity was negative in 5/9 (55.6%) cases of PCNSL with low uptake. This finding provides new insight into cases of PCNSL with lower metabolic activity as in such cases lack of MUM1 expression could reflect reduced aggressiveness and a more favorable clinical outcome.
It was reported by Rosenfeld et al[10] that steroid therapy substantially reduced FDG uptake in PCNSL after its administration. However, the difference between high and low uptake cases of PCNSL among those who did and did not undergo steroid treatment did not reach statistical significance. By contrast, another study noted that dexamethasone affected the FDG metabolism of the cerebral cortex, but not that of the tumor.[26,27] Hustinx et al[27] reported no difference in standardized uptake values (SUVs) on PET whether the patients were taking dexamethasone or not. In the present study, our analysis of the steroid-naïve PCNSL patient subgroup showed that they also had tumors with low FDG uptake (4/56, 7.1%), and that only MUM1 expression was consistently associated with PCNSL with low uptake. Since steroids have a cytotoxic effect in lymphomas,[28] such steroid administrations might have influenced FDG uptake in PCNSL, but they do not appear to be a critical factor affecting FDG uptake in PCNSL.
Previous studies show that FDG uptake in PCNSL is associated with patient outcome.[29,30] One demonstrated, using univariate analyses, that a high SUV for FDG is associated with decreased progression-free survival (PFS) and overall survival (OS) durations,[29] while another, analyzing 42 patients with PCNSL, showed that the baseline evaluation of FDG uptake in PCNSL is an independent predictor of PFS and OS.[30] PCNSL has a poor prognosis relative to other types of extranodal NHL. Current treatment modalities for patients with newly diagnosed PCNSL include chemotherapy and/or radiotherapy, and autologous stem cell transplantation. These treatments improve patient survival, but there is a high incidence of severe complications. Among them, neurotoxicity is potentially devastating,[2,31,32] and patients often die as a result of a neurotoxicity complication, even in the absence of active PCNSL. To maintain acceptable cognitive function and improvement of the quality of life, a new, modified, approach to treatment is required.[7] PCNSL has a heterogeneous clinical behavior, and therefore tumor characteristics and prognostic biomarkers with which to stratify PCNSL and enable tailored treatment urgently need to be identified. The majority of cases of PCNSL show robust FDG uptake and high MUM1 immunoreactivity, features that may be associated with a poor prognosis. That MUM1 is being considered as a target for the therapy of some MUM1-positive cancers[14,17] is therefore promising for such cases of PCNSL. Risk stratification of PCNSL based on FDG PET uptake could further help to discriminate those patients at low risk, and allow their treatment to be tailored accordingly. Furthermore, we believe that the metabolic information gleaned from PET will improve our understanding of PCNSL and aid development of novel, targeted therapies, and prognostication.
This study has several limitations. First, it was only included pathologic confirmed PCNSL patients who underwent brain PET. Thus, the results may have been subject to selection bias. Second, it was retrospective in nature, and only 79.8% of the patients had immunohistochemical staining data available. In addition, the data collected over a long period of time between 2002 and 2013, the immunohistochemical staining methods might not be completely homogeneous and interobserver variations were also taken into consideration. A larger prospective series of patients will be necessary to validate the association that we found between the immunohistochemistry results and FDG uptake in PCNSL.
In conclusion, this study suggests that the atypical presentation of PCNSL with low FDG uptake is closely associated with a lack of MUM1, a protein known to be a crucial regulator of B-cell development and tumorigenesis. We believe that further investigation of the biology and risk stratification of PCNSL based on FDG PET findings is now warranted.
Author contributions
Conceptualization: Jae Seung Kim, Hye Ok Kim.
Formal analysis: Sun Young Chae, Minjung Seo, Suk Hyun Lee.
Investigation: Jin-Sook Ryu, Joo-ryung Huh, Jeong Hoon Kim.
Methodology: Seon-Ok Kim, Seung Jun Oh, Jungsu S. Oh.
Supervision: Jae Seung Kim.
Writing – original draft: Hye Ok Kim.
Footnotes
Abbreviations: BCL-2 = B-cell lymphoma 2, BCL-6 = B-cell lymphoma 6, CD10 = cluster of differentiation 10, CSF = cerebrospinal fluid, EBV = Epstein-Barr virus, FDG = Fludeoxyglucose, GCB = Germinal center B-cell subtype, LDH = lactate dehydrogenase, MUM1 = multiple myeloma oncogene 1, NHL = extranodal non-Hodgkin lymphoma, PCNSL = primary central nervous system lymphoma, PET = positron emission tomography.
How to cite this article: Kim HO, Kim JS, Kim SO, Chae SY, Oh SJ, Seo M, Lee SH, Oh JS, Ryu JS, Huh Jr, Kim JH. Clinicopathological characteristics of primary central nervous system lymphoma with low 18F-FDG uptake on brain PET. Medicine. 2020;99:20(e20140).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C2383).
The authors have no conflicts of interest to disclose.
References
- [1].Abla O, Weitzman S, Blay JY, et al. Primary CNS lymphoma in children and adolescents: a descriptive analysis from the International Primary CNS Lymphoma Collaborative Group (IPCG). Clin Cancer Res 2011;17:346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Batchelor T, Loeffler JS. Primary CNS lymphoma. J Clin Oncol 2006;24:1281–8. [DOI] [PubMed] [Google Scholar]
- [3].Choi H, Paeng JC, Kim DW, et al. Metabolic and metastatic characteristics of ALK-rearranged lung adenocarcinoma on FDG PET/CT. Lung Cancer 2013;79:242–7. [DOI] [PubMed] [Google Scholar]
- [4].Vesselle H, Schmidt RA, Pugsley JM, et al. Lung cancer proliferation correlates with [F-18]fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res 2000;6:3837–44. [PubMed] [Google Scholar]
- [5].Jadvar H, Alavi A, Gambhir SS. 18F-FDG uptake in lung, breast, and colon cancers: molecular biology correlates and disease characterization. J Nucl Med 2009;50:1820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee D, Lee J, Kim J, et al. 18F-FDG PET in patients with primary systemic anaplastic large cell lymphoma: differential features according to expression of anaplastic lymphoma kinase. Nucl Med Mol Imaging 2013;47:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Basso U, Brandes AA. Diagnostic advances and new trends for the treatment of primary central nervous system lymphoma. Eur J Cancer 2002;38:1298–312. [DOI] [PubMed] [Google Scholar]
- [8].Roelcke U, Leenders KL. Positron emission tomography in patients with primary CNS lymphomas. J Neurooncol 1999;43:231–6. [DOI] [PubMed] [Google Scholar]
- [9].Kosaka N, Tsuchida T, Uematsu H, et al. 18F-FDG PET of common enhancing malignant brain tumors. AJR Am J Roentgenol 2008;190:W365–9. [DOI] [PubMed] [Google Scholar]
- [10].Rosenfeld SS, Hoffman JM, Coleman RE, et al. Studies of primary central nervous system lymphoma with fluorine-18-fluorodeoxyglucose positron emission tomography. J Nucl Med 1992;33:532–6. [PubMed] [Google Scholar]
- [11].Palmedo H, Urbach H, Bender H, et al. FDG-PET in immunocompetent patients with primary central nervous system lymphoma: correlation with MRI and clinical follow-up. Eur J Nucl Med Mol Imaging 2006;33:164–8. [DOI] [PubMed] [Google Scholar]
- [12].Kawai N, Okubo S, Miyake K, et al. Use of PET in the diagnosis of primary CNS lymphoma in patients with atypical MR findings. Ann Nucl Med 2010;24:335–43. [DOI] [PubMed] [Google Scholar]
- [13].Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–82. [DOI] [PubMed] [Google Scholar]
- [14].Gualco G, Weiss LM, Bacchi CE. MUM1/IRF4: a review. Appl Immunohistochem Mol Morphol 2010;18:301–10. [DOI] [PubMed] [Google Scholar]
- [15].Naresh KN. MUM1 expression dichotomises follicular lymphoma into predominantly, MUM1-negative low-grade and MUM1-positive high-grade subtypes. Haematologica 2007;92:267–8. [DOI] [PubMed] [Google Scholar]
- [16].Gualco G, Weiss LM, Harrington WJ, Jr, et al. Nodal diffuse large B-cell lymphomas in children and adolescents: immunohistochemical expression patterns and c-MYC translocation in relation to clinical outcome. Am J Surg Pathol 2009;33:1815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shaffer AL, Emre NC, Romesser PB, et al. IRF4: immunity. Malignancy! Therapy? Clin Cancer Res 2009;15:2954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gaidano G, Carbone A. MUM1: a step ahead toward the understanding of lymphoma histogenesis. Leukemia 2000;14:563–6. [DOI] [PubMed] [Google Scholar]
- [19].Tabata R, Yasumizu R, Tabata C, et al. Double-hit lymphoma demonstrating t(6;14;18)(p25;q32;q21), suggesting two independent dual-hit translocations, MYC/BCL-2 and IRF4/BCL-2. J Clin Exp Hematop 2013;53:141–50. [DOI] [PubMed] [Google Scholar]
- [20].Miller DM, Thomas SD, Islam A, et al. c-Myc and cancer metabolism. Clin Cancer Res 2012;18:5546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Palaskas N, Larson SM, Schultz N, et al. 18F-fluorodeoxy-glucose positron emission tomography marks MYC-overexpressing human basal-like breast cancers. Cancer Res 2011;71:5164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 2009;15:6479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xu J, Chen Y, Olopade OI. MYC and breast cancer. Genes Cancer 2010;1:629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shaffer AL, Emre NC, Lamy L, et al. IRF4 addiction in multiple myeloma. Nature 2008;454:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Coupland SE, Loddenkemper C, Smith JR, et al. Expression of immunoglobulin transcription factors in primary intraocular lymphoma and primary central nervous system lymphoma. Invest Ophthalmol Vis Sci 2005;46:3957–64. [DOI] [PubMed] [Google Scholar]
- [26].Roelcke U, Blasberg RG, von Ammon K, et al. Dexamethasone treatment and plasma glucose levels: relevance for fluorine-18-fluorodeoxyglucose uptake measurements in gliomas. J Nucl Med 1998;39:879–84. [PubMed] [Google Scholar]
- [27].Hustinx R, Smith RJ, Benard F, et al. Can the standardized uptake value characterize primary brain tumors on FDG-PET? Eur J Nucl Med 1999;26:1501–9. [DOI] [PubMed] [Google Scholar]
- [28].Deckert M, Engert A, Bruck W, et al. Modern concepts in the biology, diagnosis, differential diagnosis and treatment of primary central nervous system lymphoma. Leukemia 2011;25:1797–807. [DOI] [PubMed] [Google Scholar]
- [29].Kawai N, Zhen HN, Miyake K, et al. Prognostic value of pretreatment 18F-FDG PET in patients with primary central nervous system lymphoma: SUV-based assessment. J Neurooncol 2010;100:225–32. [DOI] [PubMed] [Google Scholar]
- [30].Kasenda B, Haug V, Schorb E, et al. 18F-FDG PET is an independent outcome predictor in primary central nervous system lymphoma. J Nucl Med 2013;54:184–91. [DOI] [PubMed] [Google Scholar]
- [31].Hashemi-Sadraei N, Peereboom DM. Chemotherapy in newly diagnosed primary central nervous system lymphoma. Ther Adv Med Oncol 2010;2:273–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 2005;23:5034–43. [DOI] [PubMed] [Google Scholar]


