Abstract
To determine the short-term clinical outcomes of single-segment cervical spondylotic radiculopathy treated with posterior percutaneous endoscopic cervical discectomy (PPECD).
Data of a total of 24 patients who underwent PPECD and local anesthesia for single-level segmental cervical spondylotic radiculopathy between March 2016 and December 2017 were reviewed. The Japanese Orthopaedic Association, visual analog scale (VAS), and neck disability index scores at preoperative 1 day, postoperative 1 day, 1 week, 1 month, 3 months, 6 months, and 1 year were recorded. The modified MacNab criteria at the last follow-up were re-recorded for the evaluation of clinical effectiveness.
All operations were successfully completed under endoscopic guidance. No patient showed spinal cord, nerve root, vascular injuries, dural tears or other complications. The postoperative VAS scores of the arm and neck were significantly reduced compared with the preoperative VAS scores (P < .05), while postoperative the Japanese Orthopaedic Association scores were significantly increased (P < .05). The postoperative neck disability index scores were significantly reduced compared with preoperative scores (P < .05). The modified MacNab criteria at the last follow-up showed 16 excellent cases, 8 good cases, 0 fine cases, and 0 poor cases. Postoperative magnetic resonance imaging and cervical 3-dimensional computed tomography reconstruction showed that the intervertebral disc was adequately resected and the nerve root was not under compression.
PPECD is safe and effective for the treatment of single-segment cervical spondylotic radiculopathy.
Keywords: endoscopic cervical discectomy, percutaneous, radiculopathy
1. Introduction
Cervical spondylotic radiculopathy (CSR) is the most common type of cervical spondylosis, accounting for 60% to 70% of all types of cervical spondylosis.[1] The typical clinical manifestation is radicular pain and numbness, and the primary etiological factor is intervertebral disc herniation. CSR is classified as median, paramedian, or lateral[2] according to the intervertebral disc herniation location. The diagnostic criteria for single-level posterolateral CSR include[3] single-level herniation of the lateral cervical intervertebral discs from C2/3 to C7/T1 on magnetic resonance imaging (MRI) and computed tomography (CT) scans, and compression of the cervical nerve with numbness or pain radiating to the arm. Failed conservative treatment or symptom recurrence or worsening indicate surgical treatment. Anterior cervical discectomy and fusion (ACDF) remains the gold standard for treating CSR.[4,5,6] However, postoperative intervertebral disc height loss, reduced mobility, and adjacent segment degeneration are still a concern.[7,8,9,10]
In recent years, with the continuous development of minimally invasive spine techniques and minimally invasive concepts, cervical endoscopic spinal surgery has shown exponential growth.[11] This is particularly true for the posterior percutaneous endoscopic cervical discectomy (PPECD), which is associated with a smaller incision, less bleeding, faster recovery, and similar decompression results to open surgery. In addition, PPECD can maintain the anatomical and biomechanical structures without the loss of motion segments associated with ACDF, thus has been accepted by more spine surgeons.[3]
Here we analyze the early clinical efficacy and safety of PPECD in the treatment of single-level CSR in 24 patients.
2. Methods
2.1. Patients
From March 2016 to December 2017, 105 patients were treated for cervical spondylosis at the Department of Spine Surgery, Guizhou Orthopedics Hospital, of which 40 agreed to be treated with PPECD. Sixteen patients were excluded, including 6 patients with cervical spondylotic myelopathy or vertebral artery type cervical spondylopathy, 5 patients with multi-level cervical spondylosis, and 5 patients with coronary heart disease or chronic obstructive pulmonary disease. Finally, the clinical data of 24 patients with a confirmed diagnosis of single-level CSR were retrospectively collected. Patients were treated with PPECD with local anesthesia. Fourteen patients had C5/6 segment CSR and 10 patients had C6/7 segment CST. The diagnostic criteria for single-segment posterolateral CSR include single segment herniation of the lateral cervical intervertebral discs from C2/3 to C7/T1 on MRI and CT scans and compression of the cervical nerve with pain radiating to the arm. All patients had unilateral upper limb and finger pain and numbness, with or without neck and shoulder pain, and decreased muscle strength.
The indications for surgery are[12,13]:
-
(1)
Signs and symptoms of CSR consistent with the imaging results;
-
(2)
Failed conservative treatment or symptom recurrence and worsening; and
-
(3)
Single gap and unilateral soft and radicular compression symptoms.
The contraindications for surgery include[12,13]:
-
(1)
Median intervertebral disc herniation;
-
(2)
History of open posterior cervical surgery;
-
(3)
Cervical spondylotic myelopathy;
-
(4)
Skin infection behind the neck or a cervical spine infection; and
-
(5)
Flexion-extension X-ray of the cervical spine showing cervical instability.
All patients provided written informed consent and the study was approved by the ethics committee of Guizhou Orthopedics Hospital.
2.2. Surgical procedure
A single surgeon performed all the operations. The patient was positioned prone and local anesthesia was performed with the neck slightly flexed and the head slightly higher than the feet. A C-arm X-ray was used for lateral fluoroscopy and localization of the surgical segment. Anteroposterior fluoroscopy was used to determine the posterior side of the ipsilateral articular process, which was labeled by drawing lines on the skin. A mark was made 1.5 cm away from the midline of the spinous process for needle insertion. Twenty milliliters of 1% lidocaine were used for layer-by-layer infiltration anesthesia to the inferior margin of the upper lamina to the diseased segment. The cervical spine guide needle was then inserted into the lamina externa. Anteroposterior fluoroscopy was used to confirm that the guide needle was located at the inferior margin of the upper lamina of the diseased segment and 1.5 cm at the lateral side of the spinous process. The guide needle was used as a center and 6 to 7 cm of skin was incised to the deep fascia. A guide dilator and a working cannula were inserted along the guide needle before the dilator was removed. The Joimax endoscopic system was used for the surgery (Joimax GmbH, Germany; TESSYS instrument system, working cannula diameter of 6.9 mm, low-temperature radiofrequency ablation system). The tissue structures were differentiated with an aqueous medium and endoscope. A plasma knife was used to ablate the fibrous tissues outside the lamina to expose the inferior margin of the upper laminar and intravertebral triangle.
The lateral side of the lamina externa and the medial side of the articular process were removed using a high-speed drill. A laminar clamp was used to remove the upper and lower lamina interna to expose the ligamentum flavum within the surgical site. A nerve hook was used to lift up the lateral edge of the ligamentum flavum. A grasper was used to remove the ligamentum flavum and expose part of the dural sac and nerve root, which were compressed with high nerve root tension. The herniated intervertebral disc tissue was completely removed to relieve nerve root compression and allow for sufficient decompression. The excised disk samples were weighed and sent for pathological biopsy.
Radiofrequency electrocoagulation was used to achieve hemostasis and ablate the intervertebral disc nucleus pulposus tissue before flushing with saline. At this point, the nerve root and dural sac showed good pulsation, and the surface vascular circulation of the nerve root was restored showing good filling. Following that, a nerve hook/probe was applied to the inferior margin of the pedicle of the upper vertebral arch and the superior margin of the pedicle of the lower vertebral arch to monitor for good nerve root decompression and restoration of a normal pulse. Complete hemostasis was achieved and a drainage tube was inserted.
2.3. Postoperative management
After surgery, the patient rested for 12 to 24 hours before wearing a neck brace and leaving the bed for activities. For patients with severe traction and irritation of the nerve root during surgery, dexamethasone and mannitol were used for symptomatic treatment. Patients were discharged 1 to 3 days after surgery and instructed to wear the neck brace for 3 to 4 weeks. After surgery, patients underwent cervical extension and flexion exercises under the guidance of our hospital physiatrist.
2.4. Outcome assessment
The scoring criteria for the cervical spondylotic myelopathy (17 point system) proposed by the Japanese Orthopaedic Association (JOA)[14] were used to measure the neurological function: JOA scores of the upper limb movement and sensory function on Day 1 before surgery as well as Day 1, Month 1, Month 3, Month 6, and Year 1 after surgery were compared. The visual analog scale (VAS)[15] criteria were used to evaluate the improvement status at various timepoints (Day 1 before surgery and Day 1, Month 1, Month 3, Month 6, and Year 1 after surgery). The neck disability index (NDI)[16] was used to assess improvement in neurological function at various timepoints (Day 1 before surgery and Day 1, Month 1, Month 3, Month 6, and Year 1 after surgery). The modified MacNab criteria[17] were used to assess treatment efficacy at the last follow-up visit. Postoperative cervical spine MRI and 3-dimensional CT scans were performed to observe the intervertebral disc tissue removal and nerve root decompression status. The extent of the ipsilateral articular process removed during surgery was indirectly recorded.
2.5. Statistical analysis
The data were imported into SPSS 23.0 software for analysis and processing. Quantitative data are expressed as mean ± standard deviation. Repeated-measures analysis of variance was employed to compare neck VAS, arm VAS, JOA, and NDI scores between different timepoints. Subsequently, paired t-tests were used to compare timepoints after surgery and Day 1 before surgery. A P-value less than .05 was considered statistically significant.
3. Results
Our patients included 10 males and 14 females with a mean age of 40 years (range, 30–50 years). All surgeries were successfully completed. No spinal cord, nerve, or blood vessel damage and no dural tears or other complications occurred during surgery.
There were 17 cases in which the surgical segment was C5/6 and 7 cases in which the surgical segment was C6/7. The mean length of surgery was 85.8 ± 8.6 min and the mean intraoperative blood loss volume was 18.6 ± 1.4 mL. All patients underwent follow-up for a mean 15.6 ± 2.8 months (Table 1).
Table 1.
The baseline characteristics of patients.
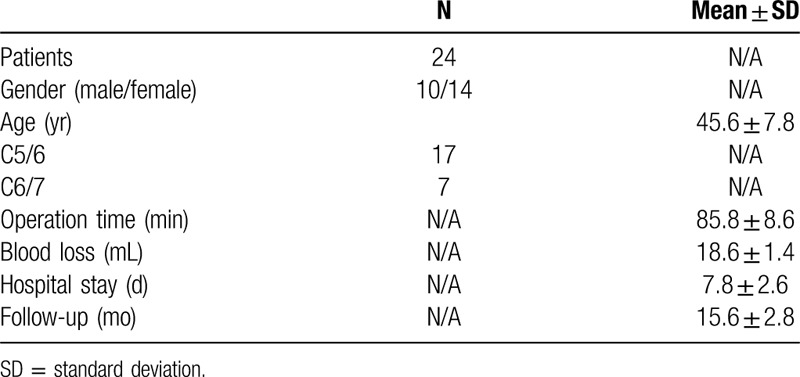
The neck VAS scores on Day 1 before surgery, Day 1, Month 1, Month 3, and Month 6 after surgery, and at the last follow-up visit were 8.86 ± 0.82, 3.38 ± 0.58, 2.84 ± 0.74, 2.12 ± 0.48, 1.94 ± 0.92, and 1.19 ± 0.62, respectively. The arm VAS scores on Day 1 before surgery, Day 1, Month 1, Month 3, and Month 6 after surgery, and at the last follow-up visit were 9.40 ± 0.92, 3.84 ± 0.42, 2.67 ± 0.56, 2.08 ± 0.48, 1.84 ± 0.63, and 1.04 ± 0.86, respectively. The JOA scores on 1 day before surgery, Day 1, Month 1, Month 3, and Month 6 after surgery, and at the last follow-up visit were 9.24 ± 0.68, 12.26 ± 0.46, 13.88 ± 0.52, 14.78 ± 0.64, 15.24 ± 0.48, and 16.24 ± 0.47, respectively. The NDI scores (%) on 1 day before surgery, Day 1, Month 1, Month 3, and Month 6 after surgery, and at the last follow-up visit were 60.5 ± 5.6, 33.8 ± 5.2, 31.7 ± 4.8, 30.8 ± 5.8, 28.9 ± 4.7, and 20.9 ± 3.2, respectively. The results showed that the neck and arm VAS scores were significantly decreased after surgery (P < .05). Postoperative JOA scores were significantly increased (P < .05). Postoperative NDI scores were significantly decreased compared with preoperative NDI scores (P < .05; Table 2 and Fig. 1). According to the modified MacNab criteria scores at the last follow-up visit, 16, 8, 0, and 0 patients had excellent, good, fair, and poor grades. Objective cervical spine MRI and three-dimensional CT results in all patients on Day 1 after surgery confirmed removal of the intervertebral disc tissue and alleviation of the nerve root compression. The 3-dimensional CT reconstruction showed that the extent of ipsilateral articular process removed did not exceed 1/2 of the entire articular process (Figs. 2 and 3).
Table 2.
Comparison of preoperative and postoperative VAS, JOA, and NDI scores (mean ± standard deviation; n = 24).
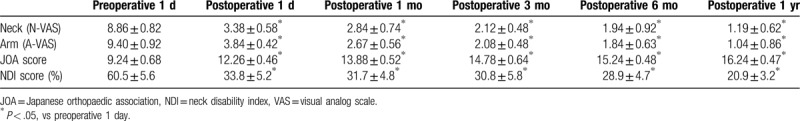
Figure 1.
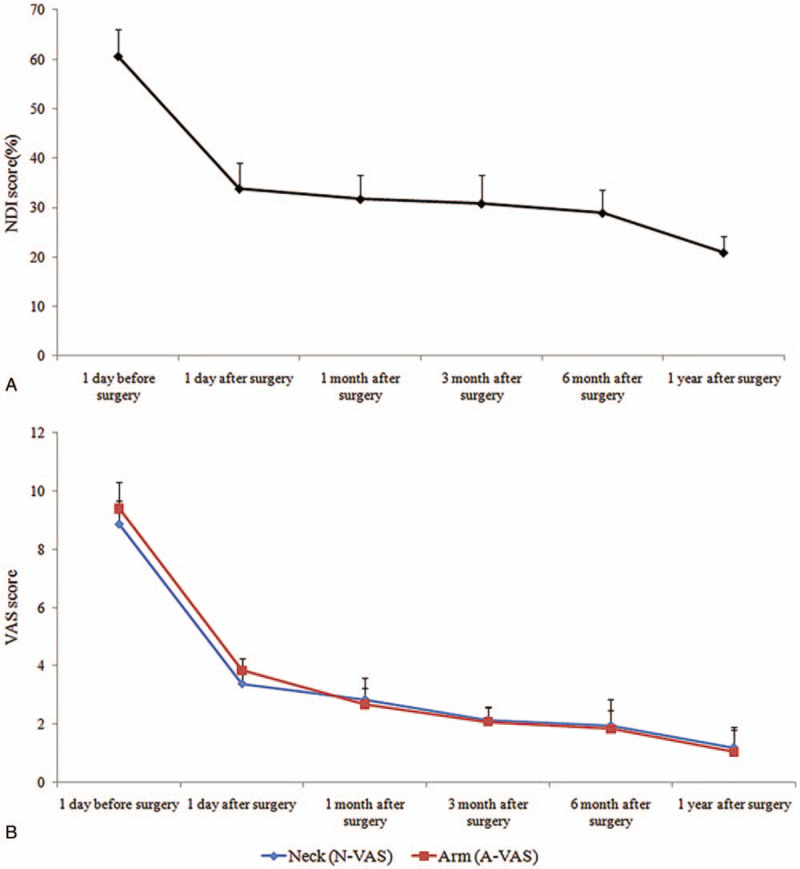
Neck and arm NDI and VAS scores. A, NDI score. B, vas score. NDI = neck disability index, VAS = visual analog scale.
Figure 2.
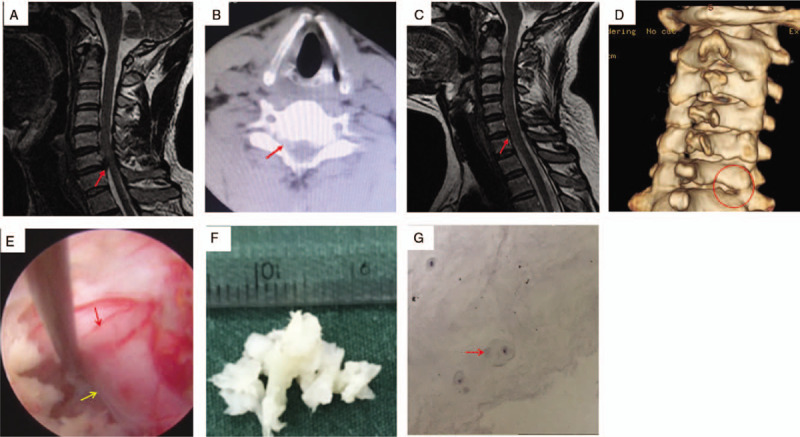
Case 1.A. Preoperative cervical spine MRI: red arrows show C6/7 intervertebral disc herniation and dural sac compression; B. Preoperative cervical spine CT: red arrows show C6/7 intervertebral disc (soft) herniation (right-sided); C. Year 1 postoperative cervical spine MRI suggests that the C6/7 intervertebral disc herniation has been removed and dural sac compression has been alleviated; D. Year 1 postoperative cervical spine 3D CT reconstruction (red circle) suggests good healing of the ground lamina and articular process; E. Intraoperative exposure of the nerve root (yellow arrows) and boundaries of the dural sac (red arrow); F. Intraoperative removal of intervertebral disc nucleus pulposus tissue; G. Postoperative pathology report shows intervertebral disc nucleus pulposus tissue (red arrows). CT = computed tomography, MRI = magnetic resonance imaging.
Figure 3.
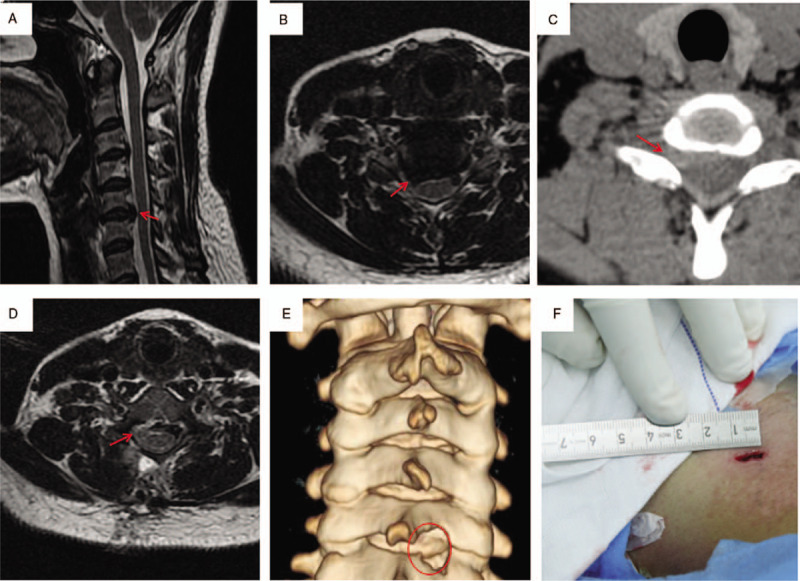
Case 2.A, B. Preoperative cervical spine MRI: red arrows show C5/6 intervertebral disc herniation and dural sac compression; C. preoperative cervical CT: red arrows shows C5/6 intervertebral disc (soft) herniation (right-sided); D. Year 1 postoperative cervical spine MRI suggests removal of the C5/6 intervertebral disc herniation; E. Year 1 postoperative cervical spine 3D CT reconstruction (red circle) suggests good healing of the drilled lamina and articular process; F. Postoperative incision was approximately 1 cm. CT = computed tomography, MRI = magnetic resonance imaging.
4. Discussion
The conventional methods for the treatment of CSR include classical ACDF and posterior approach open surgery. In the former method, the adjacent segment degeneration and the motion segments are a concern. In the latter method, the widespread muscle stripping may result in destruction of the muscle vascular supply and denervation, which increases the risk of postoperative neck axial pain and segmental instability. With the development of endoscopic minimally invasive vertebral techniques, these problems can be resolved in suitable patients.
Lee et al[18] reported the long-term follow-up results for 37 patients with single-level CSR who underwent percutaneous endoscopic cervical discectomy. These patients showed no intervertebral disc height loss and degeneration of the intervertebral disc involved. Percutaneous endoscopic cervical discectomy can be classified as the anterior approach and the posterior approach (PPECD). The factors affecting the surgical approaches[12] include:
-
(1)
Location of the intervertebral disc herniation;
-
(2)
Neural foraminal stenosis and height of the intervertebral disc space; and
-
(3)
Anesthesia.
Yang et al[19] believed that the anterior approach is better for median intervertebral disc herniation but may collapse the vertebral body and intervertebral foramen height and that the posterior approach is more suitable for foraminal stenosis and lateral intervertebral disc herniation. The posterior approach is associated with less damage to the blood vessels, sufficient decompression of the spinal canal, faster postoperative recovery, less complications, shorter hospitalization, and lower costs. Ruetten et al[13] reported on the 2-year follow-up results of 83 patients who underwent PPECD and found satisfactory clinical efficacy results. In the following year, the authors reported on a randomized controlled study comparing PPECD with conventional microsurgical anterior decompression and fusion. There were no significant differences in the incidence of complications and clinical efficacy between the 2 methods, but the former exhibited significant advantages in surgical trauma, intraoperative bleeding, and postoperative hospitalization period.[20] Some researchers found that the symptoms of 90% of patients with CSR due to neural foraminal stenosis were relieved after posterior cervical foraminotomy. At the same time, adjacent segmental degeneration caused by ADCF was prevented. Therefore, the posterior cervical foraminotomy is an effective substitute surgical procedure.[21,22] In our study, the 24 patients all had single-level unilateral nerve root compression symptoms. Therefore, we selected PPECD to treat these patients.
The crux to PPECD is sufficient decompression. In PPECD, the intervertebral foramen is dilated under endoscopic guidance to remove the nucleus pulposus that is compressing the nerve. Therefore, dilation of the intervertebral foramen is a key and challenge in PPECD. Kim et al[23] recommended using the V-point as the center for drilling the intervertebral foramen, ie, using the convergence of the inferior margin of the cephalic lamina and the superior margin of the caudal lamina on the medial junction of the facet joints as the center. In all patients in our study, drilling started at the median edge of the upper intervertebral foramen and the radius of the drill is usually 3 to 4 mm. During surgery, a probe hook was used to examine the medial edge of the vertebral arch to prevent instability caused by excessive facet joint removal. Chen et al[24] reported that at least 50% of the facet joint should be retained to maintain the biomechanical stability of the neck. Kim et al[23] performed a 25-month follow-up on 32 PPECD patients and measured the postoperative cervical curvature, segmental Cobb's angle, and intervertebral disc space height of the surgical segment. The results showed that PPECD will not result in worsening of the cervical curvature if >50% of the facet joint is retained. Postoperative three-dimensional CT scans showed that >50% of the facet joints were retained in the 24 patients in our study.
Due to the visual field limitations under the working cannula, nerve root injury during PPECD also requires attention. The anatomical relationship between the nerve root in the intervertebral foramen and the intervertebral disc can be divided into 4 types:[25]
-
(1)
Infra-axillary, in which the intervertebral disc is located at the tail of the nerve root;
-
(2)
Supra-shoulder, in which the intervertebral disc is adjacent to the base of the nerve root;
-
(3)
Anterior, in which the intervertebral disc is located anteriorly to the nerve root;
-
(4)
Free, in which the intervertebral disc is not connected to the nerve root.
In our study, there were 17 patients with the infra-axillary, 5 patients with the supra-shoulder, and 2 patients with the anterior relationships. Based on our experience, the supra-shoulder relationship is the safest for PPECD. This is because the ligamentum flavum is cut after the articular process is drilled and the adipose tissues at the dorsal side of the spinal nerve are carefully separated. A nerve root elevator can be used to examine the intervertebral disc rupture. The infra-axillary form requires the excision of more ligamentum flavum, which makes it prone to bleeding and affects the endoscopic visual field. This increases the risk of nerve injury. Therefore, preoperative cervical spine MRI is useful to determine the location of the nerve root and the intervertebral disc, as well as the position of the nucleus pulposus during surgery, reducing the risk of nerve injury.
In our study, 24 patients with single-level CSR with consistent signs, symptoms, and radiological findings underwent PPECD under local anesthesia. After surgery, the neck, shoulder, and upper-limb pain and numbness all showed significant alleviation. Statistical analysis found significant improvements when postoperative JOA, VAS, and NDI scores were compared with preoperative scores as well as in modified MacNab scores at the last follow-up visit. Postoperative cervical spine MRI showed significant removal of intervertebral disc tissue and sufficient nerve root decompression. Postoperative three-dimensional CT scans showed that more than 50% of the facet joint was retained.
The following should be noted in PPECD:
-
(1)
After entry to the spinal canal, the ligamentum flavum and the dural sac should be carefully separated to ensure that there is no dural sac tear, the adjacent blood vessels, nerves, and tissues are protected, and nerve root decompression is possible; and
-
(2)
Dilation of the lateral side of the facet joint and excessive resection of the lateral side of the intervertebral foramen should be avoided.
This may damage the venous plexus surrounding the vertebral arteries and cause dark black venous bleeding. At this time, destructive manipulation of the lateral side should be avoided to effectively prevent damage to the vertebral arteries. Complications after PPECD are uncommon with reported incidences ranging 2% to 9%. The most common complications include cerebrospinal fluid leakage, spinal cord injury, bleeding, air embolism, and wound complications.[20,26] Our retrospective study had a small sample size, which may have resulted in selection bias. In addition, the follow-up period was short and the long-term outcomes remain unknown.
In conclusion, PPECD is safe and effective for the treatment of the single-segment CSR. Further investigation with a larger sample size and longer follow-up time is needed.
Acknowledgments
Not applicable.
Author contributions
Conceptualization: Beiping Ouyang, Shudan Yao, Tingsheng Lu, Chunshan Luo.
Data curation: Beiping Ouyang, Shudan Yao, Tingsheng Lu, Chunshan Luo.
Formal analysis: Qiling Chen, Chunshan Luo.
Writing – original draft: Beiping Ouyang, Shudan Yao, Tingsheng Lu.
Writing – review & editing: Beiping Ouyang, Shudan Yao, Tingsheng Lu, Qiling Chen, Chunshan Luo.
Footnotes
Abbreviations: ACDF = anterior cervical discectomy and fusion, CSR = cervical spondylotic radiculopathy, CT = computed tomography, JOA = the Japanese Orthopaedic Association, MRI = magnetic resonance imaging, NDI = neck disability index, PPECD = posterior percutaneous endoscopic cervical discectomy, VAS = visual analog scale.
How to cite this article: Yao S, Ouyang B, Lu T, Chen Q, Luo C. Treatment of cervical spondylotic radiculopathy with posterior percutaneous endoscopic cervical discectomy: Short-term outcomes of 24 cases. Medicine. 2020;99:20(e20216).
BO, SY, and TL are co-first authors and contributed equally to this work.
Funding was not applicable.
The authors have no conflicts of interest to disclose.
References
- [1].Yadav YR, Parihar V, Ratre S, et al. Endoscopic decompression of cervical spondylotic myelopathy using posterior approach. Neurol India 2014;62:640–5. [DOI] [PubMed] [Google Scholar]
- [2].Kokubun S, Tanaka Y. Types of cervical disc herniation and relation to myelopathy and radiculopathy. J Back Musculoskelet Rehabil 1995;5:145–54. [DOI] [PubMed] [Google Scholar]
- [3].Quillo-Olvera J, Lin GX, Kim JS. Percutaneous endoscopic cervical discectomy: a technical review. Ann Transl Med 2018;6:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fraser JF, Hartl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine 2007;6:298–303. [DOI] [PubMed] [Google Scholar]
- [5].Baba H, Furusawa N, Tanaka Y, et al. Anterior decompression and fusion for cervical myeloradiculopathy secondary to ossification of the posterior ligament. Int Orthop 1994;18:204–9. [DOI] [PubMed] [Google Scholar]
- [6].Chen Y, Chen D, Wang X, et al. Anterior corpectomy and fusion for severe ossification of posterior longitudinal ligament in the cervical spine. Int Orthop 2009;33:477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tureyen K. Disc height loss after anterior cervical microdiscectomy with titanium intervertebral cage fusion. Acta Neurochir (Wien) 2003;145:565–9. [DOI] [PubMed] [Google Scholar]
- [8].Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine 2007;32:2310–7. [DOI] [PubMed] [Google Scholar]
- [9].Suk KS, Kim KT, Lee SH, et al. Prevertebral soft tissue swelling after anterior cervical discectomy and fusion with plate fixation. Int Orthop 2006;30:290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kraemer P, Fehlings MG, Hashimoto R, et al. A systematic review of definitions and classification systems of adjacent segment pathology. Spine 2012;37: 22 Suppl: S31–9. [DOI] [PubMed] [Google Scholar]
- [11].Middleton SD, Wagner R, Gibson JNA. Multi-level spine endoscopy: a review of available evidence and case report. EFORT open reviews 2017;2:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ahn Y. Percutaneous endoscopic cervical discectomy using working channel endoscopes. Expert Rev Med Devices 2016;13:601–10. [DOI] [PubMed] [Google Scholar]
- [13].Ruetten S, Komp M, Merk H, et al. A new full-endoscopic technique for cervical posterior foraminotomy in the treatment of lateral disc herniations using 6.9-mm endoscopes: prospective 2-year results of 87 patients. Minim Invasive Neurosurg 2007;50:219–26. [DOI] [PubMed] [Google Scholar]
- [14].Yonenobu K, Abumi K, Nagata K, et al. Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine 2001;26:1890–4. [DOI] [PubMed] [Google Scholar]
- [15].Zanoli G, Stromqvist B, Jonsson B. Visual analog scales for interpretation of back and leg pain intensity in patients operated for degenerative lumbar spine disorders. Spine 2001;26:2375–80. [DOI] [PubMed] [Google Scholar]
- [16].Swanenburg J, Humphreys K, Langenfeld A, et al. Validity and reliability of a German version of the Neck Disability Index (NDI-G). Manual therapy 2014;19:52–8. [DOI] [PubMed] [Google Scholar]
- [17].Wen H, Wang X, Liao W, et al. Effective range of percutaneous posterior full-endoscopic paramedian cervical disc herniation discectomy and indications for patient selection. Biomed Res Int 2017;2017:3610385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee JH, Lee SH. Clinical and radiographic changes after percutaneous endoscopic cervical discectomy: a long-term follow-up. Photomed Laser Surg 2014;32:663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang JS, Chu L, Chen L, et al. Anterior or posterior approach of full-endoscopic cervical discectomy for cervical intervertebral disc herniation? A comparative cohort study. Spine 2014;39:1743–50. [DOI] [PubMed] [Google Scholar]
- [20].Ruetten S, Komp M, Merk H, et al. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: a prospective, randomized, controlled study. Spine 2008;33:940–8. [DOI] [PubMed] [Google Scholar]
- [21].Tumialan LM, Ponton RP, Gluf WM. Management of unilateral cervical radiculopathy in the military: the cost effectiveness of posterior cervical foraminotomy compared with anterior cervical discectomy and fusion. Neurosurg Focus 2010;28:E17. [DOI] [PubMed] [Google Scholar]
- [22].Fehlings MG, Gray RJ. Posterior cervical foraminotomy for the treatment of cervical radiculopathy. J Neurosurg 2009;10:343–4. [DOI] [PubMed] [Google Scholar]
- [23].Kim CH, Shin KH, Chung CK, et al. Changes in cervical sagittal alignment after single-level posterior percutaneous endoscopic cervical diskectomy. Global Spine J 2015;5:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen BH, Natarajan RN, An HS, et al. Comparison of biomechanical response to surgical procedures used for cervical radiculopathy: posterior keyhole foraminotomy versus anterior foraminotomy and discectomy versus anterior discectomy with fusion. J Spinal Disord 2001;14:17–20. [DOI] [PubMed] [Google Scholar]
- [25].Tanaka N, Fujimoto Y, An HS, et al. The anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine 2000;25:286–91. [DOI] [PubMed] [Google Scholar]
- [26].O’Toole JE, Sheikh H, Eichholz KM, et al. Endoscopic posterior cervical foraminotomy and discectomy. Neurosurg Clin N Am 2006;17:411–22. [DOI] [PubMed] [Google Scholar]


