Abstract
Background:
Antifibrinolytic agents have been successfully used to reduce blood transfusion demand in patients undergoing elective knee arthroplasty. The purpose of this study was to investigate different antifibrinolytic agents for patients undergoing total-knee arthroplasty (TKA).
Methods:
We searched the randomized controlled trials assessing the effect of antifibrinolytic agents on TKA in MEDLINE, PubMed, Embase, and the Cochrane Library. Participants are divided into antifibrinolytic agent group and control group under TKA. Double extraction technology is used and the quality of its methodology is evaluated before analysis. Outcomes analyzed included blood loss, number of blood transfusions, rates of blood transfusion, and deep vein thrombosis (DVT).
Results:
A total of 28 randomized controlled trials involving 1899 patients were included in this study. Compared with the control group, the antifibrinolytic agents group exhibited significantly reduced the amounts of total blood loss (weighted mean difference [WMD] with 95% confidence interval [CI]: −272.19, −338.25 to −206.4), postoperative blood loss (WMD with 95% CI: −102.83, −157.64 to −46.02), average units of blood transfusion (risk ratio with 95% CI: 0.7, 0.12 to 0.24), and average blood transfusion volumes (WMD with 95% CI: −1.34, −1.47 to −1,21). Antifibrinolytic agents significantly reduced the rate of blood transfusions and did not increase the occurrence risk of intraoperative blood loss and DVT. Several limitations should also be acknowledged such as the heterogeneity among the studies.
Conclusion:
The application of antifibrinolytic agents can significantly reduce blood loss and blood transfusion requirements. Additionally, these agents did not increase the risk of DVT in patients undergoing TKAs.
Keywords: antifibrinolytic agents, blood loss, meta-analysis, total-knee arthroplasty, tranexamic acid
1. Introduction
Total-knee arthroplasty (TKA) is widely known as one of the most effective treatments for severe osteoarthritis of the knee.[1] However, this surgery is prone to significant intraoperative and postoperative blood loss along with long operation times and large wound surfaces. Therefore, allogenic blood transfusions are often used in clinical practice.[2] However, this method increases the risk of immunologic and nonimmunologic adverse effects, such as a higher rate of postoperative infection, intravascular hemolysis, transfusion-induced coagulopathy, renal impairment or failure, and even death.[3] Many strategies, including regional anesthesia, autologous blood donations, autologous drain transfusions, and acute normovolemic hemodilution, have been used to reduce blood transfusion rates.[4] However, the strategies’ applications are limited by their clinical and financial efficacies and the fact that antifibrinolytic agents are needed to decrease blood loss.
Antifibrinolytic agents, including tranexamic acid (TXA), epsilon aminocaproic acid (EACA), and aprotinin, are widely used to reduce bleeding and for transfusions in cardiac, orthopedic, and hepatic surgeries.[5,6] TXA and EACA are synthetic amino acid derivatives that reversibly bind to plasminogen, thereby inhibiting fibrin binding and plasmin activation.[7] Aprotinin, which is a naturally occurring, single-chain, 58-amino acid polypeptide, is a proteinase inhibitor that binds and inhibits plasmin.[8] Numerous studies have investigated their efficacies in reducing blood loss and transfusion requirements in patients undergoing orthopedic surgery.[9]
Recent studies have suggested that antifibrinolytic agents are effective in reducing blood loss and blood transfusions in patients undergoing total-hip arthroplasty and spine surgery.[10] Although these antifibrinolytic agents can reduce the perioperative bleeding of TKA, few prospective orthopedic studies directly compare these 3 agents. Therefore, we conducted this meta-analysis to investigate the effectiveness of antifibrinolytic agents (TXA, EACA, and aprotinin) and compare their benefits of intraoperative intravenous in reducing blood loss and blood transfusions for patients undergoing TKA.
2. Methods
2.1. Study registration and ethical approval
This review design was based on the methodology of the Cochrane Library in regards to conducting the meta-analysis. All of the data were reported according to the Quality of Reporting for Meta-analyses, which is provided by the Handbook for Systematic Reviews of Interventions (version 5.0).[11] This meta-analysis was conducted in accordance with the guidance of the preferred reporting items and meta-analysis statements for systematic evaluation of interventions and the Cochrane manual. All analyses are based on previously published literatures and do not belong to studies with experimental human or animal subjects, so ethical approval and patient consent are not required.
2.2. Search strategy
We identified the relevant studies that evaluated the use of antifibrinolytic agents in patients undergoing TKA. We searched PubMed, Embase, and the Cochrane Library (the search was last updated on August 2019) with a search algorithm based on a combination of the following seven factors. The search terms were listed as the following: “antifibrinolytics,” “cyklokapron,” “aprotinin,” “tranexamic acid,” “epsilon aminocaproic acid,” “total knee arthroplasty,” and “randomized controlled trials.” The search was restricted to “humans” and the “English” language.
2.3. Selection criteria
Studies were included if they met the following criteria: participants who underwent TKA; randomly assigned patients to the treatment group who received antifibrinolytic agents and control patients who received a placebo or no treatment; neither the antifibrinolytic agent-treated group nor the control group used anticoagulant drugs before the operation; both groups reported one of the following outcomes: blood loss, the number of patients who received allogeneic transfusions, the number of blood transfusion units per patient, and the number of patients with deep vein thrombosis (DVT). And exclusion criteria for this study as follows: they were non-English language studies; they were nonrandomized controlled trials; randomized controlled trials that did not contain any of the above outcomes were also excluded. The initial electronic database retrieval that identified the potential studies for inclusion and that were based on title and abstract information were performed by two independent authors (QM, GH).
2.4. Data extraction
Data extractions for the included studies were conducted by 2 independent authors (QM, GH). Any disagreement between the 2 authors was resolved by consensus or consultation with the senior author (TJ). For each report, the relevant information was extracted, including the name of first author, journal, country of origin, year of publication, studied population, study design (prospective or retrospective), patient enrollment procedure, sample size, study design, subject age, the type of surgery, the dose and timing of the antifibrinolytic agents, and outcome data. In addition, adverse outcomes were recorded. For studies without adverse outcomes, authors were contacted for confirmation or for more information regarding adverse events if necessary. Each eligible study was evaluated by 2 of the authors independently, and discrepancies were resolved by discussion with a 3rd author.
2.5. Risk of bias
I2 statistics were used to evaluate the between study heterogeneity analysis in this meta-analysis. The heterogeneity among studies was tested by I2 statistic. As a guide, I2 values <50% indicated moderated and >50% indicated high heterogeneity.
2.6. Statistical analysis
All analyses were conducted using RevMan version 5.0 (The Nordic Cochrane Centre, The Cochrane Collaboration) with P < 0.05 being considered statistically significant. The weighted mean difference (WMD) and 95% confidence interval (CI) via the use of the Mantel–Haenszel method were used for analysis of the continuous data, the risk ratio (RR) and 95% CI were applied for analysis of the dichotomous data. The data from each study were pooled by a fixed- or random-effects model based on the degree of heterogeneity.[12] Heterogeneity across the included studies by using the value of I2 and the result of the Chi-squared test. Substantial heterogeneity was considered present when P < .05 and an I2 value of >50% were considered to be suggestive of statistical heterogeneity. A subgroup analysis was also performed to explore the heterogeneity.
3. Results
3.1. Study selection and characteristics
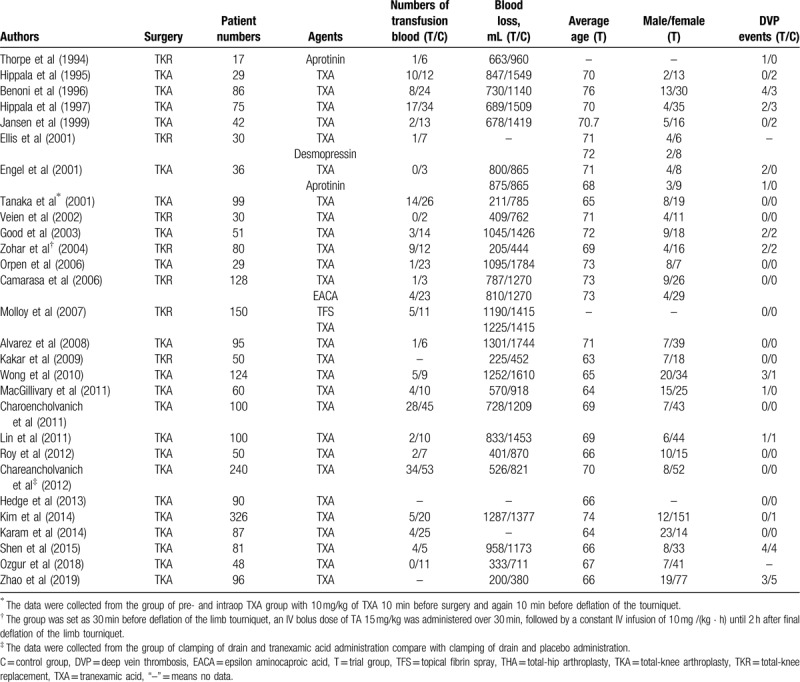
A total of 479 abstracts and titles were reviewed. Of these abstracts and titles, 28 randomized controlled trials met the eligibility criteria and were included in this study[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] (Fig. 1). Seventeen hundred and five patients were enrolled in the randomized controlled trials. The characteristics of the included studies are presented in Table 1. In total, 28 studies involved TXA,[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,34,40,41] 2 studies involved aprotinin, and 1 study involved EACA.[23] A total of 18 studies involved bleeding analyses,[13,14,15,16,18,20,21,23,24,25,26,27,31,34] and 26 studies were included in the DVT analysis.[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,34] The characteristics of the included studies are presented in Table 1.
Figure 1.
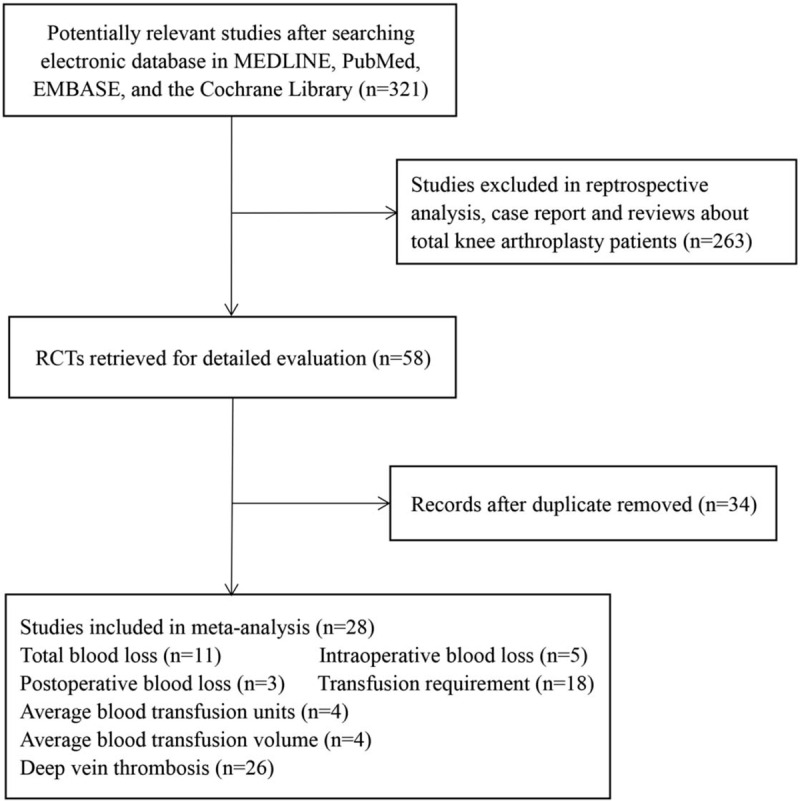
Flow diagram of the literature search and screening process in this meta-analysis. n = number of papers, RCTs = randomized controlled trials.
Table 1.
Characteristics of the included clinical trials and participants.
3.2. Risk of bias
I2 statistics were used to evaluate the between study heterogeneity analysis in this meta-analysis. The heterogeneity among studies was tested by Q statistic and quantified by I2 statistic. As a guide, I2 values <50% indicated moderated and >50% indicated high heterogeneity.
3.3. Total blood loss
Total blood loss was examined in 9 trials involving a total of 282 patients. The meta-analysis results showed that the use of antifibrinolytic agents significantly reduced total blood loss (WMD with 95% CI: −272.19, −338.25 to −206.4, I2 = 0%, random effect model) (Figs. 2 and 3).
Figure 2.
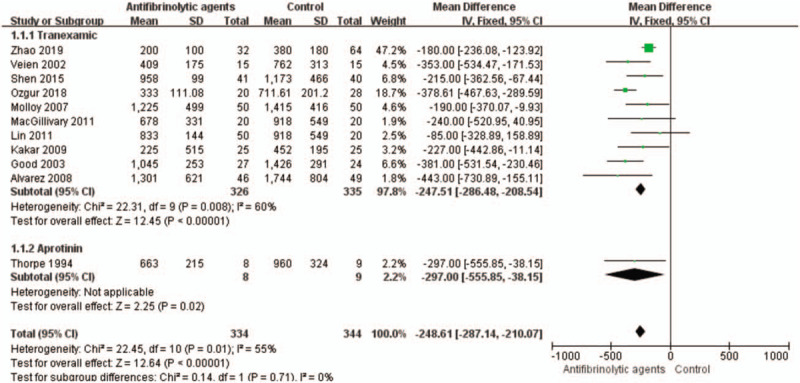
Forest plot of the total blood loss. CI = confidence interval, SD = standard deviation.
Figure 3.
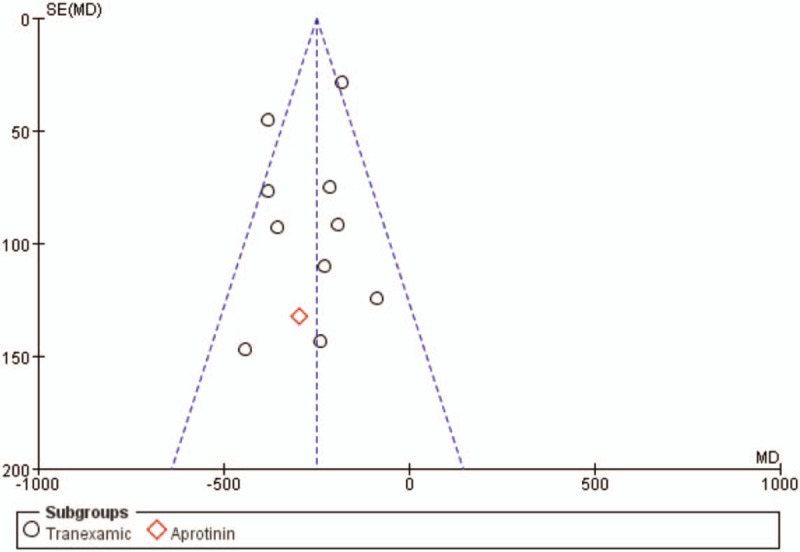
Funnel plot of the total blood loss. MD = mean difference, SE = standard error.
3.4. Intraoperative blood loss
Five studies with a total of 244 patients were eligible for this meta-analysis. These randomized trials included 124 patients who received antifibrinolytic agents and 120 patients who received control treatments. The use of antifibrinolytic agents did not significantly reduced intraoperative blood loss (WMD with 95% CI: 2.67, −34.72 to 40.07, I2 = 0, fixed-effects model) (Figs. 4 and 5).
Figure 4.
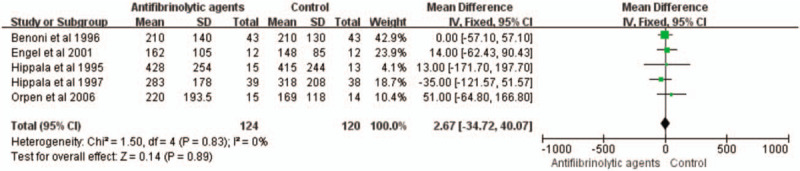
Forest plot of the intraoperative blood loss. CI = confidence interval, SD = standard deviation.
Figure 5.
Funnel plot of the intraoperative blood loss. MD = mean difference, SE = standard error.
3.5. Postoperative blood loss
Three studies with a total of 170 patients were eligible for this meta-analysis. The use of antifibrinolytic agents reduced postoperative blood loss (WMD with 95% CI: −102.83, −157.64 to −46.02, I2 = 38, fixed-effects model) (Figs. 6 and 7).
Figure 6.
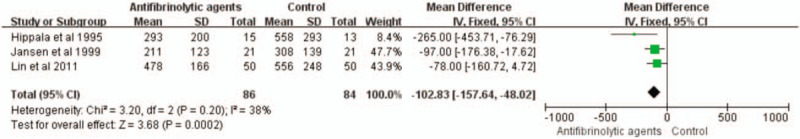
Forest plot of the postoperative blood loss. CI = confidence interval, SD = standard deviation.
Figure 7.
Funnel plot of the postoperative blood loss. MD = mean difference, SE = standard error.
3.6. Rate of blood transfusion
Data on transfusion rates were examined in 18 trials involving a total of 1227 patients. Antifibrinolytic agents significantly reduced the rate of blood transfusion (RR with 95% CI: 0.7, 0.12 to 0.24, I2 = 0, fixed-effects model) (Figs. 8 and 9).
Figure 8.
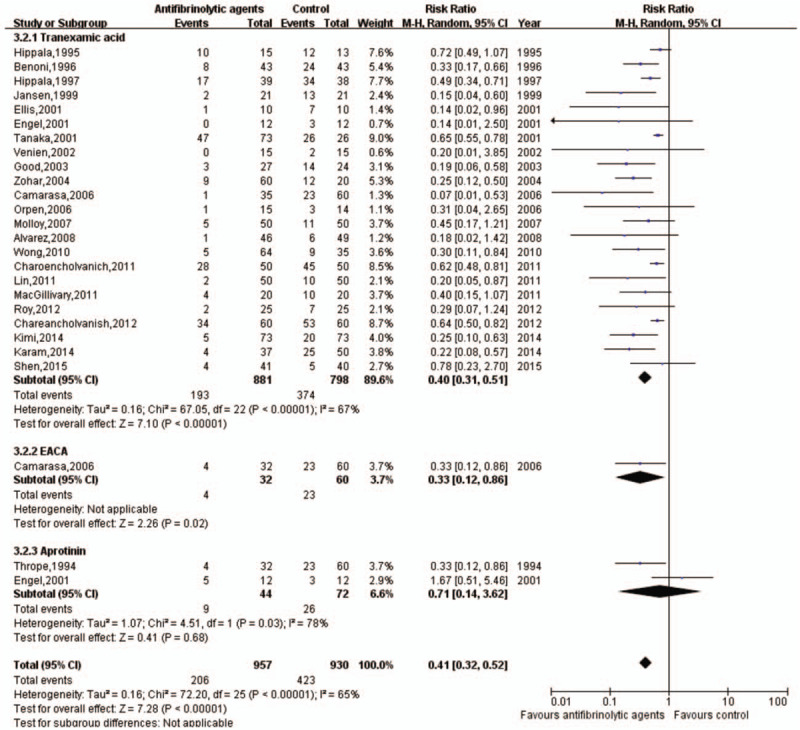
Forest plot of the rate of blood transfusion. CI = confidence interval, EACA = epsilon aminocaproic acid.
Figure 9.
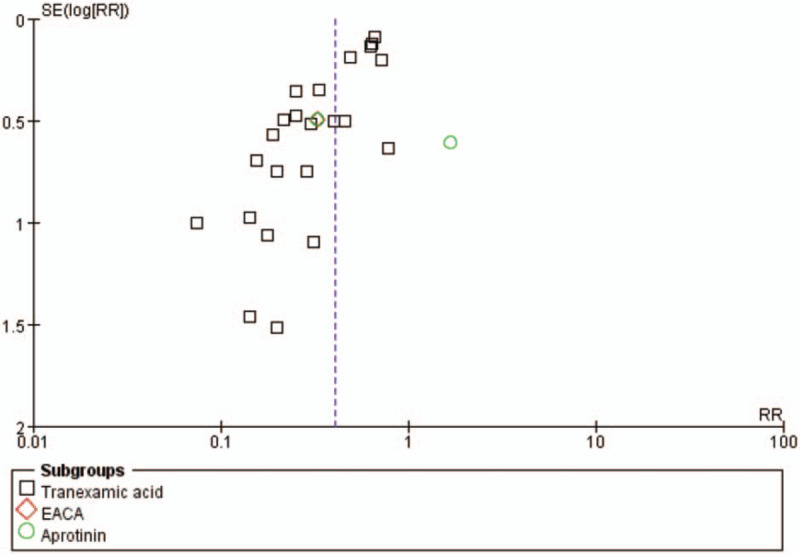
Funnel plot of the rate of blood transfusion. CI = confidence interval, EACA = epsilon aminocaproic acid.
3.7. Average blood transfusion units
This outcome measure was available in 4 trials, which involved a total of 278 patients. The use of antifibrinolytic agents significantly reduced the average units of blood transfusions compared with the control group (WMD with 95% CI: −1.34, −1.47 to −1,21, I2 = 0, fixed-effects model) (Figs. 10 and 11).
Figure 10.
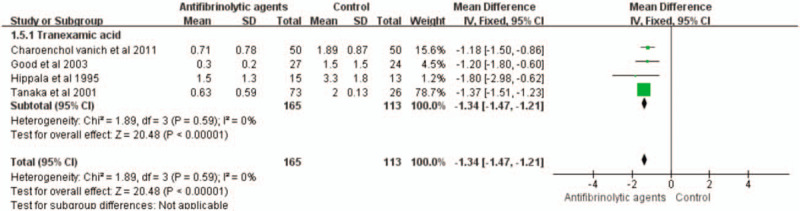
Forest plot of the average units of blood transfusion. CI = confidence interval, SD = standard deviation.
Figure 11.
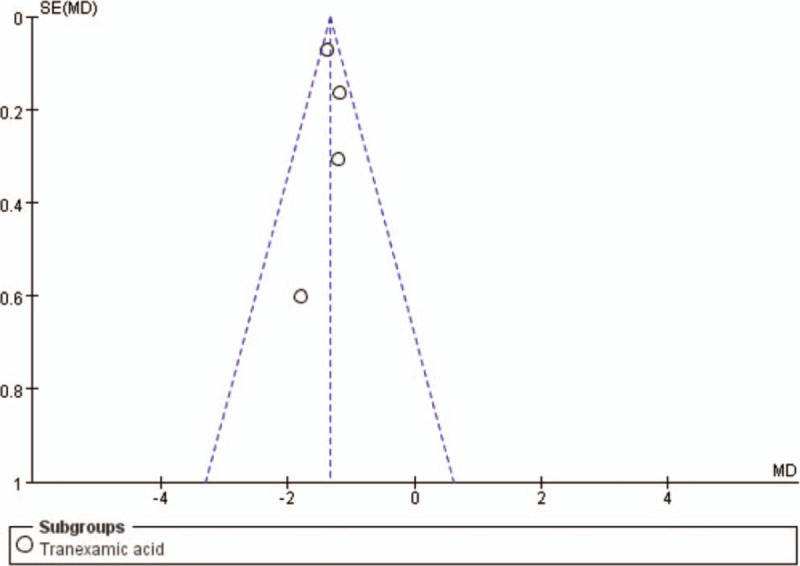
Funnel plot of the average units of blood transfusion. MD = mean difference, SE = standard error.
3.8. Average blood transfusion volume
Two studies with a total of 150 patients reported mean transfusion volumes. The use of antifibrinolytic agents significantly reduced the average blood transfusion volume compared with the control group (WMD with 95% CI: −244.04, −284.89 to −203.19, I2 = 0, fixed-effects model) (Figs. 12 and 13).
Figure 12.
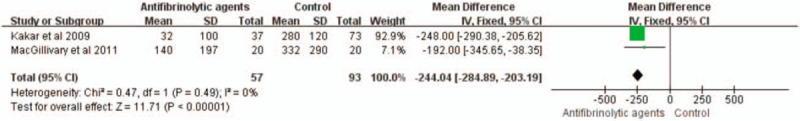
Forest plot of the average volumes of blood transfusion. CI = confidence interval, SD = standard deviation.
Figure 13.
Funnel plot of the average volumes of blood transfusion. MD = mean difference, SE = standard error.
3.9. Rate of DVTs
Twenty-six trials with 1818 patients reported DVT complications. Patients receiving antifibrinolytic agents (n = 919) had 23 episodes of DVT, while 899 patients who did not receive antifibrinolytic agents had a total of 21 episodes of DVT. The rate of DVT was not associated with the use of antifibrinolytic agents when the group was compared with the control group (RR: 1.02; 95% CI: 0.58–1.81, I2 = 0, fixed-effects model) (Figs. 14 and 15).
Figure 14.
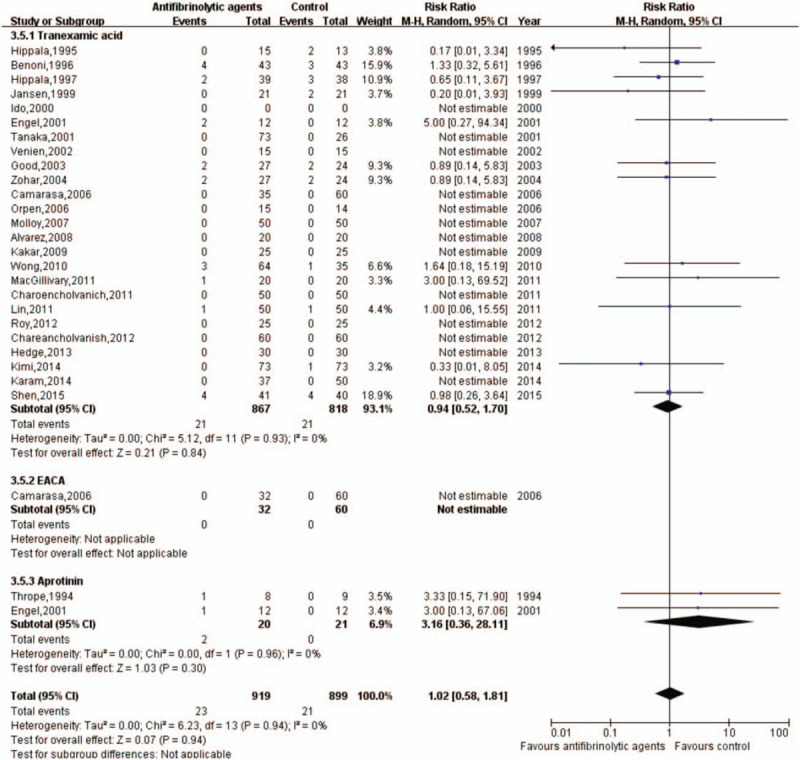
Forest plot of the effect of antifibrinolytic agents on deep vein thrombosis. CI = confidence interval, EACA = epsilon aminocaproic acid.
Figure 15.
Funnel plot of the effect of antifibrinolytic agents on deep vein thrombosis. EACA = epsilon aminocaproic acid, RR = risk ratio, SE = standard error.
4. Discussion
The significant blood loss and risk for blood transfusions are important features that need to be considered in TKA.[42] This requirement is a result of several factors that are inherent to the surgery itself, such as extensive bone decortication, fluid therapy-induced dilutional coagulopathy, and the presence of microthrombi in the transfusion blood.[43] Previous studies have shown that approximately 38% patients undergoing TKA require blood transfusions for a total average blood loss of 1500 mL during the perioperative period.[44] Many patients with tissue extravasation after TKA present with lower limb swelling, pain, and effects in functional exercise.[45] Such techniques as autologous blood transfusions, intraoperative hemodilution, hypotensive anesthesia, and modern modified uses of drainage increase additional logistical problems and may be immunomodulatory.[46]
After that, the use of antifibrinolytic agents can avoid many of these complications and are widely available and inexpensive.[6] Though published literature on antifibrinolytic agents has dramatically expanded over the past several years, the literature lacks a comprehensive review on the efficacy of antifibrinolytic agents in primary TKA. We performed a meta-analysis of different antifibrinolytic agents in the setting of TKA to investigate the variables associated with administration of antifibrinolytic agents as part of the supporting evidence for the combined clinical practice guidelines. In the early, Kagoma et al studied the effects of antifibrinolytic agents in reducing blood transfusions after total-knee and total-hip arthroplasties, and the authors reviewed the evidence for using TXA, aprotinin, and EACA in total blood loss and transfusion rates in orthopedic surgery.[47]
Our results showed that the use of antifibrinolytic agents (as well as subgroup analyses of TXA, EACA, and aprotinin) for patients undergoing TKA is effective in reducing blood loss and blood transfusions, as well as in reducing the units and volumes of transfusion, and did not appear to increase incidences of DVT. Statistically significant differences were observed in blood loss, transfusion requirements, and the number of transfusions between the antifibrinolytic agent-treated and control groups. Furthermore, the use of antifibrinolytic agents also reduced the probability of receiving a blood transfusion by 41%. Another, Tan et al performed a review of antifibrinolytic agents and found them to be effective and safe in total-hip arthroplasty.[48] The evidence of the use of antifibrinolytic agents in reducing the need for blood transfusions is strong. However, according to the action mechanism of the antifibrinolytic agents, the increased risk of DVT is another concern in major orthopedic surgery.[49]
The TXA has been shown to be effective and safe in major orthopedic surgery.[50] Aprotinin has been used in cardiac surgery and noncardiac procedures, including liver transplantations and major vascular reconstructions, and it is effective in reducing blood loss and blood transfusions.[51,52] Several studies have previously shown that aprotinin is effective in decreasing surgical blood loss in various orthopedic surgeries,[53] then the European Medicine Agency recommended the lifting of the restrictions on the use of aprotinin.[54]
Compare with other studies, although a lot of research has been done so far, there are few prospective randomized controlled trials compare different antifibrinolytic agents in patients undergoing total-knee replacement. Recently, Boese et al reported the results of 194 TKA patients who were given TXA or EACA.[55] Their study used tourniquets, but no drainage after surgery, and reported that TXA resulted in lower estimated blood loss. There was no need for blood transfusion in both groups, so the authors concluded that both TXA and EACA had clinical efficacy. Camarasa et al conducted a randomized controlled trial of 127 TKA patients, who were divided into TXA, EACA, and placebo groups.[23] They found that total blood loss, changes in hemoglobin levels and transfusion rates were significantly lower in the 2 antifibrinolytic groups than in the placebo group. Our study with TKA patients is consistent with these studies. Our data support the view that postoperative loss (measured by postoperative drainage and blood volume loss) may higher in the EACA group compare with TXA and aprotinin, but does not lead to more blood transfusion.
At the same time, several limitations should also be acknowledged. First, we systematically searched a range of databases for published and unpublished trials. However, we cannot exclude the possibility that some studies were missed. If many unpublished trials showed little or no effect of the antifibrinolytic agents on the reduction of blood loss and transfusion requirements, the treatment effect of antifibrinolytic agents could be overestimated in this meta-analysis. Second, the funnel plot showed the presence of a publication bias in this meta-analysis. Publication bias is a well-known problem affecting the accuracy of the results of a meta-analysis because positive results tend to be accepted by journals, whereas negative results are often subjected to rejection and a lack of publication. Third, there was significant heterogeneity among the studies when considering intraoperative blood loss, postoperative blood loss, and blood units or volumes transfused per patient. Furthermore, the estimation of blood loss was variable because blood loss resulting from hematomas or tissue extravasations were rarely measured, which could cause inaccurate results. It is likely that these inconsistencies across all of the studies contributed to the high I2 values that were observed in the statistical analyses. Thus, more accurate results could be obtained if the total red blood cell loss could be calculated by using the reservoir's blood hematocrit levels and if a unified standard blood transfusion protocol was adopted. The other variations that may have accounted for such heterogeneity include differences in the surgical techniques, the sample sizes, and the variations in the patient characteristics. Thus, more high-quality, randomized controlled trial studies with large sample sizes and complete data are required for further meta-analyses to compare these antifibrinolytic agents.
Given the results of 1899 patients in this study, it can be concluded that these antifibrinolytic agents are equally safe and effective in preventing excessive bleeding during TKA surgery. Because of its low cost, EACA may be seen as a suitable alternative front-line agent to reduce the financial burden on healthcare providers. From the comparative advantage of economic and technical means, we can compare different antifibrinolytic agents in the future, so as to get more appropriate way for patients under TKA.
5. Conclusion
Our research provides evidence that the use of antifibrinolytic agents may significantly reduce blood loss and blood transfusion requirements. Additionally, this analysis did not increase the risk of DVT in patients undergoing TKAs. Therefore, these findings support the routine use of antifibrinolytic agents in TKA. However, given the heterogeneity of the pooled estimates and the low number of studies, larger studies are needed to examine blood loss, transfusions, and thromboembolic complications in the use of antifibrinolytic agents during TKA. Considering the economic and technical problems, more research results need to be analyzed so as to provide better antifibrinolytic agents for patients under TKA.
Author contributions
Data curation: Qi-ming Ma.
Formal analysis: Guo-song Han.
Investigation: Guo-song Han, Bo-wen Li.
Methodology: Qi-ming Ma.
Project administration: Ting Jiang.
Resources: Qi-ming Ma, Bo-wen Li.
Software: Guo-song Han.
Supervision: Ting Jiang.
Validation: Guo-song Han.
Visualization: Xiao-jing Li.
Writing – original draft: Bo-wen Li, Xiao-jing Li.
Writing – review & editing: Ting Jiang.
Footnotes
Abbreviations: CI = confidence interval, DVT = deep vein thrombosis, EACA = epsilon aminocaproic acid, RR = risk ratio, TKA = total-knee arthroplasty, TXA = tranexamic acid, WMD = weighted mean difference.
How to cite this article: Ma Qm, Han Gs, Li Bw, Li Xj, Jiang T. Effectiveness and safety of the use of antifibrinolytic agents in total-knee arthroplasty: a meta-analysis. Medicine. 2020;99:20(e20214).
QM and G-sH are the first authors of this study.
The data sets generated during and/or analyzed during the present study are publicly available.
The authors declared no conflict of interest. And no funding was provided for this study. Research did not involve human participants and animals.
References
- [1].Price AJ, Alvand A, Troelsen A, et al. Knee replacement. Lancet 2018;392:1672–82. [DOI] [PubMed] [Google Scholar]
- [2].Huang Z, Huang C, Xie J, et al. Analysis of a large data set to identify predictors of blood transfusion in primary total hip and knee arthroplasty. Transfusion 2018;58:1855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Theusinger OM, Spahn DR. Perioperative blood conservation strategies for major spine surgery. Best Pract Res Clin Anaesthesiol 2016;30:41–52. [DOI] [PubMed] [Google Scholar]
- [4].Bible JE, Mirza M, Knaub MA. Blood-loss management in spine surgery. J Am Acad Orthop Surg 2018;26:35–44. [DOI] [PubMed] [Google Scholar]
- [5].Sprigg N, Flaherty K, Appleton JP, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet 2018;391:2107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Walsh M. Antifibrinolytic agents in acquired bleeding disorders. Clin Adv Hematol Oncol 2017;15:357–61. [PubMed] [Google Scholar]
- [7].Lum ZC, Manoukian MAC, Pacheco CS, et al. Intravenous tranexamic acid versus topical aminocaproic acid: which method has the least blood loss and transfusion rates? J Am Acad Orthop Surg Glob Res Rev 2018;2:e072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gao P, Wu S, Chang X, et al. Aprotinin encapsulated gold nanoclusters: a fluorescent bioprobe with dynamically nuclear targeting and selective detection of trypsin and heavy metal. Bioconjug Chem 2018;29:4140–8. [DOI] [PubMed] [Google Scholar]
- [9].Soroceanu A, Oren JH, Smith JS, et al. Effect of antifibrinolytic therapy on complications, thromboembolic events, blood product utilization, and fusion in adult spinal deformity surgery. Spine (Phila Pa 1976) 2016;41:E879–86. [DOI] [PubMed] [Google Scholar]
- [10].White CC4th, Eichinger JK, Friedman RJ. Minimizing blood loss and transfusions in total knee arthroplasty. J Knee Surg 2018;31:594–9. [DOI] [PubMed] [Google Scholar]
- [11].Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354:1896–900. [DOI] [PubMed] [Google Scholar]
- [12].Melsen WG, Bootsma MC, Rovers MM, et al. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect 2014;20:123–9. [DOI] [PubMed] [Google Scholar]
- [13].Hiippala S, Strid L, Wennerstrand M, et al. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth 1995;74:534–7. [DOI] [PubMed] [Google Scholar]
- [14].Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br 1996;78:434–40. [PubMed] [Google Scholar]
- [15].Hiippala ST, Strid LJ, Wennerstrand MI, et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg 1997;84:839–44. [DOI] [PubMed] [Google Scholar]
- [16].Jansen AJ, Andreica S, Claeys M, et al. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth 1999;83:596–601. [DOI] [PubMed] [Google Scholar]
- [17].Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg 2000;120:518–20. [DOI] [PubMed] [Google Scholar]
- [18].Engel JM, Hohaus T, Ruwoldt R, et al. Regional hemostatic status and blood requirements after total knee arthroplasty with and without tranexamic acid or aprotinin. Anesth Analg 2001;92:775–80. [DOI] [PubMed] [Google Scholar]
- [19].Tanaka N, Sakahashi H, Sato E, et al. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br 2001;83:702–5. [DOI] [PubMed] [Google Scholar]
- [20].Veien M, Sorensen JV, Madsen F, et al. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand 2002;46:1206–11. [DOI] [PubMed] [Google Scholar]
- [21].Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth 2003;90:596–9. [DOI] [PubMed] [Google Scholar]
- [22].Zohar E, Ellis M, Ifrach N, et al. The postoperative blood-sparing efficacy of oral versus intravenous tranexamic acid after total knee replacement. Anesth Analg 2004;99:1679–83. [DOI] [PubMed] [Google Scholar]
- [23].Camarasa MA, Olle G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth 2006;96:576–82. [DOI] [PubMed] [Google Scholar]
- [24].Orpen NM, Little C, Walker G, et al. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee 2006;13:106–10. [DOI] [PubMed] [Google Scholar]
- [25].Molloy DO, Archbold HA, Ogonda L, et al. Comparison of topical fibrin spray and tranexamic acid on blood loss after total knee replacement: a prospective, randomised controlled trial. J Bone Joint Surg Br 2007;89:306–9. [DOI] [PubMed] [Google Scholar]
- [26].Alvarez JC, Santiveri FX, Ramos I, et al. Tranexamic acid reduces blood transfusion in total knee arthroplasty even when a blood conservation program is applied. Transfusion 2008;48:519–25. [DOI] [PubMed] [Google Scholar]
- [27].Kakar PN, Gupta N, Govil P, et al. Efficacy and safety of tranexamic acid in control of bleeding following TKR: a randomized clinical trial. Indian J Anaesth 2009;53:667–71. [PMC free article] [PubMed] [Google Scholar]
- [28].Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am 2010;92:2503–13. [DOI] [PubMed] [Google Scholar]
- [29].MacGillivray RG, Tarabichi SB, Hawari MF, et al. Tranexamic acid to reduce blood loss after bilateral total knee arthroplasty: a prospective, randomized double blind study. J Arthroplasty 2011;26:24–8. [DOI] [PubMed] [Google Scholar]
- [30].Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA: a prospective randomized controlled trial. Clin Orthop Relat Res 2011;469:2874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lin PC, Hsu CH, Chen WS, et al. Does tranexamic acid save blood in minimally invasive total knee arthroplasty? Clin Orthop Relat Res 2011;469:1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Roy SP, Tanki UF, Dutta A, et al. Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2012;20:2494–501. [DOI] [PubMed] [Google Scholar]
- [33].Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, et al. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord 2012;13:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hegde C, Wasnik S, Kulkarni S, et al. Simultaneous bilateral computer assisted total knee arthroplasty: the effect of intravenous or intraarticular tranexamic acid. J Arthroplasty 2013;28:1888–91. [DOI] [PubMed] [Google Scholar]
- [35].Kim TK, Chang CB, Kang YG, et al. Clinical value of tranexamic acid in unilateral and simultaneous bilateral TKAs under a contemporary blood-saving protocol: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 2014;22:1870–8. [DOI] [PubMed] [Google Scholar]
- [36].Karam JA, Bloomfield MR, DiIorio TM, et al. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty 2014;29:501–3. [DOI] [PubMed] [Google Scholar]
- [37].Shen PF, Hou WL, Chen JB, et al. Effectiveness and safety of tranexamic acid for total knee arthroplasty: a prospective randomized controlled trial. Med Sci Monit 2015;21:576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Thorpe CM, Murphy WG, Logan M. Use of aprotinin in knee replacement surgery. Br J Anaesth 1994;73:408–10. [DOI] [PubMed] [Google Scholar]
- [39].Ellis MH, Fredman B, Zohar E, et al. The effect of tourniquet application, tranexamic acid, and desmopressin on the procoagulant and fibrinolytic systems during total knee replacement. J Clin Anesth 2001;13:509–13. [DOI] [PubMed] [Google Scholar]
- [40].Senturk O. Can low-dose tranexamic acid decrease blood loss and transfusion requirements in total knee arthroplasty? Cureus 2018;10:e2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhao M, Geng X, Wang C, et al. The value of tranexamic acid for patients with preoperative anemia in primary total knee arthroplasty. Eur J Med Res 2019;24:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cundy WJ, Theodoulou A, Ling CM, et al. Blood loss in total knee arthroplasty. J Knee Surg 2017;30:452–9. [DOI] [PubMed] [Google Scholar]
- [43].Mufarrih SH, Qureshi NQ, Ali A, et al. Total knee Arthroplasty: risk factors for allogeneic blood transfusions in the South Asian population. BMC Musculoskelet Disord 2017;18:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Boutsiadis A, Reynolds RJ, Saffarini M, et al. Factors that influence blood loss and need for transfusion following total knee arthroplasty. Ann Transl Med 2017;5:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Spicka J, Lostak J, Gallo J, et al. Influence of enhanced recovery regime on early outcomes of total knee arthroplasty [in Czech]. Acta Chir Orthop Traumatol Cech 2017;84:361–7. [PubMed] [Google Scholar]
- [46].Yao Y, Kang P, Xue C, et al. A prospective randomized controlled study of total knee arthroplasty via mini-subvastus and conventional approach [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2018;32:162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kagoma YK, Crowther MA, Douketis J, et al. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res 2009;123:687–96. [DOI] [PubMed] [Google Scholar]
- [48].Tan J, Chen H, Liu Q, et al. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res 2013;184:880–7. [DOI] [PubMed] [Google Scholar]
- [49].Saleh J, El-Othmani MM, Saleh KJ. Deep vein thrombosis and pulmonary embolism considerations in orthopedic surgery. Orthop Clin North Am 2017;48:127–35. [DOI] [PubMed] [Google Scholar]
- [50].Rohrich RJ, Cho MJ. The role of tranexamic acid in plastic surgery: review and technical considerations. Plast Reconstr Surg 2018;141:507–15. [DOI] [PubMed] [Google Scholar]
- [51].Godier A, Hunt BJ. Aprotinin as an alternative to tranexamic acid in cardiac surgery - is this where we started from? Anaesth Crit Care Pain Med 2017;36:79–81. [DOI] [PubMed] [Google Scholar]
- [52].Mangus RS, Kinsella SB, Fridell JA, et al. Aminocaproic Acid (amicar) as an alternative to aprotinin (trasylol) in liver transplantation. Transplant Proc 2014;46:1393–9. [DOI] [PubMed] [Google Scholar]
- [53].Ipema HJ, Tanzi MG. Use of topical tranexamic acid or aminocaproic acid to prevent bleeding after major surgical procedures. Ann Pharmacother 2012;46:97–107. [DOI] [PubMed] [Google Scholar]
- [54].Howell N, Senanayake E, Freemantle N, et al. Putting the record straight on aprotinin as safe and effective: results from a mixed treatment meta-analysis of trials of aprotinin. J Thorac Cardiovasc Surg 2013;145:234–40. [DOI] [PubMed] [Google Scholar]
- [55].Boese CK, Centeno L, Walters RW. Blood conservation using tranexamic acid is not superior to epsilon-aminocaproic acid after total knee arthroplasty. J Bone Joint Surg Am 2017;99:1621–8. [DOI] [PubMed] [Google Scholar]


