Taenia solium neurocysticercosis (NCC) is endemic in most of the world and contributes significantly to the burden of epilepsy and other neurological morbidity. Also present in developed countries because of immigration and travel, NCC is one of few diseases targeted for eradication. This paper reviews all aspects of its life cycle (taeniasis, porcine cysticercosis, human cysticercosis), with a focus on recent advances in its diagnosis, management, and control. Diagnosis of taeniasis is limited by poor availability of immunological or molecular assays.
KEYWORDS: Peru, Taenia solium, cysticercosis, epilepsy, neurocysticercosis
SUMMARY
Taenia solium neurocysticercosis (NCC) is endemic in most of the world and contributes significantly to the burden of epilepsy and other neurological morbidity. Also present in developed countries because of immigration and travel, NCC is one of few diseases targeted for eradication. This paper reviews all aspects of its life cycle (taeniasis, porcine cysticercosis, human cysticercosis), with a focus on recent advances in its diagnosis, management, and control. Diagnosis of taeniasis is limited by poor availability of immunological or molecular assays. Diagnosis of NCC rests on neuroimaging findings, supported by serological assays. The treatment of NCC should be approached in the context of the particular type of infection (intra- or extraparenchymal; number, location, and stage of lesions) and has evolved toward combined symptomatic and antiparasitic management, with particular attention to modulating inflammation. Research on NCC and particularly the use of recently available genome data and animal models of infection should help to elucidate mechanisms of brain inflammation, damage, and epileptogenesis.
INTRODUCTION
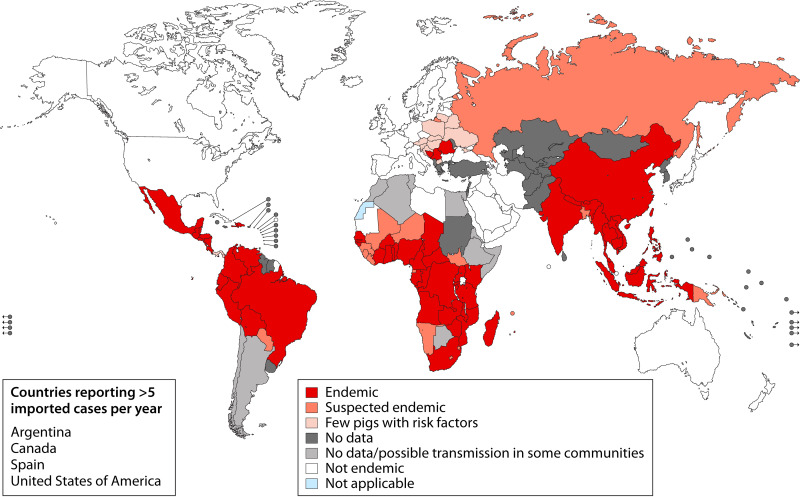
The relation between human infection by the cystic larvae (cysticerci) of the pork tapeworm Taenia solium and neurological disease has been known since the 16th century, when Rumler in 1558 and Panarolus in 1652 described vesicles in the dura mater and corpus callosum of epileptic individuals (1). It was only 2 centuries later that Kuchenmeister demonstrated in Germany that ingestion of cysticerci resulted in intestinal taeniasis (demonstrated in the necropsy of an executed prisoner 72 h after feeding him cysts), closing the parasite life cycle (2). Taenia solium was widely endemic in most of Europe until the early 1900s and remains endemic in wide areas of the world, including most of Latin America, sub-Saharan Africa, Southeast Asia, the Indian subcontinent, and parts of China (3–11) (Fig. 1). In these regions, infection of the human brain by cysticerci (neurocysticercosis [NCC]) accounts for approximately one-third of the cases of epilepsy (3, 5, 6, 8, 9, 11–15). Traveling and immigration make NCC a health burden even in regions of nonendemicity such as the United States and Europe/United Kingdom (10, 16–24).
FIG 1.
Geographic distribution of Taenia solium taeniasis/cysticercosis (WHO, 2015).
BIOLOGY OF THE PARASITE
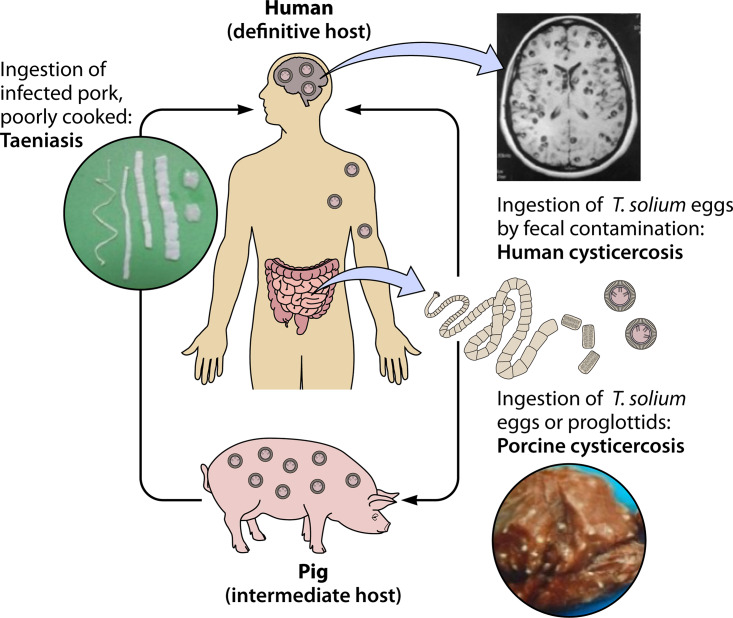
Like most helminths, Taenia solium has a complex life cycle that involves a usual intermediate host (pig) that harbors the parasitic larvae in its tissues and a sole definitive host (human) that hosts the adult tapeworm in its intestines. In the usual cycle, the adult tapeworm expels eggs or proglottids with the feces of the human definitive host, each egg containing an infective hexacanth embryo or oncosphere protected by a thick keratin embryophore (25, 26). In areas with deficient sanitary conditions, free-roaming pigs have access to human feces and feed on them, ingesting the tapeworm eggs (27). The embryos are liberated from the eggshells and, activated by the action of gastric and intestinal juices, free themselves from the surrounding embryophoric membrane by using their three pairs of oncospheral hooks (28), attach to the intestinal epithelium, and actively cross the intestinal mucosa in a process facilitated by the secretion of parasite proteases (29, 30). After crossing the intestinal mucosa, the embryos reach the circulatory system of the pig. Infective embryos are then distributed by the bloodstream, become established, and develop into cystic, fluid-filled larvae or cysticerci, each containing an invaginated scolex with a double crown of hooks and four muscular suckers (Fig. 2). T. solium taeniasis occurs when humans ingest poorly cooked pork containing cysticerci. The scolex in the cyst evaginates following exposure to bile and intestinal juices, attaches to the intestinal mucosa by the action of its suckers and its double crown of hooks, and begins producing proglottids at its neck region, forming a strobila to develop into an adult tapeworm (25, 26).
FIG 2.
Life cycle of Taenia solium. (Adapted from reference 276.)
HUMAN TAENIASIS
The human carrier of an intestinal T. solium tapeworm is the sole source of infection for pigs and for other humans in its surroundings (31, 32). Despite its importance in establishing transmission and maintaining the endemicity of the disease, we know surprisingly little about human taeniasis. Yoshino published in the 1930s a seminal series of articles on the early stages of porcine cysticercosis, and to this purpose he infected himself with Taenia solium cysts (33, 34). From this published report and other series and anecdotal reports (35, 36), including another case of self-infection of a well-known British parasitologist, P. S. Craig (this time with the cysts of the harmless beef tapeworm Taenia saginata), we can conclude that in the human host, the tapeworm matures and begins expelling gravid proglottids approximately 3 to 4 months after infection with cysticerci. The adult T. solium tapeworm lives in the proximal small intestine and is reported to measure between 2 and 7 m (26), in our experience usually below 4 m. Attempts to establish the usual life span of the tapeworm are hampered by minimal or nil knowledge of the proportion of tapeworms who die before reaching patency (most sources of information refer to stool-positive cases). Even so, experts assume that the adult tapeworm lives approximately 3 years on average (37–39).
The genome of T. solium was initially published in 2013 (40), and now there is also available one genome from China (41), published in 2014, and two from Peru from 2015 (42). The size of the T. solium genome seems to be around 112 to 130 Mb, with 18 chromosomes and a GC content of 43%. Genome data have already been used to compare species evolution (40), as well as to identify and characterize host-parasite pathways (43, 44), microsatellite markers (42), and antigenic proteins (45, 46). The three human-infecting taenias, T. asiatica, T. saginata, and T. solium, share many common genomic features but differ from each other in evolution and diversification of certain specialized gene families. Comparison of homologous genes among these human tapeworms revealed that 90.3% of T. asiatica genes had homologues in T. saginata and T. solium (41), suggesting that these parasites share many proteins involved in host-parasite interactions, as well as molecular targets for diagnostics and treatment. Partial cysticercal transcriptomes have been produced using next-generation sequencing for expressed sequence tags or transcriptome sequencing (RNA-seq) (47–49). A few proteomic studies looking to characterize biological stages through protein profiles (50), explore host-parasite interactions (51), identify antigenic proteins (52, 53), and compare protein profiles according to the infected organ (brain versus muscle) have already been published (54, 55).
Besides T. solium, two other large tapeworms in the genus Taenia (Taenia saginata and Taenia asiatica) can infect humans as their definitive host. T. saginata, the beef tapeworm, is a much longer tapeworm and is endemic in wide regions of the world, including Europe and parts of the United States, and its cycle involves humans as tapeworm carriers and cysticercotic cattle. Proglottids of T. saginata are motile and can appear in the underwear of tapeworm carriers. T. asiatica, on the other hand, is geographically restricted to Korea, Japan, China, and Southeast Asia. It is closer to T. saginata, but its usual intermediate host is the pig, with the characteristic that cysts are found in the viscera and peritoneum of the pig (56, 57) rather than in the muscle tissues, as seen with porcine T. solium cysticercosis. Neither T. saginata nor T. asiatica causes human cysticercosis.
Diagnosis of Taeniasis
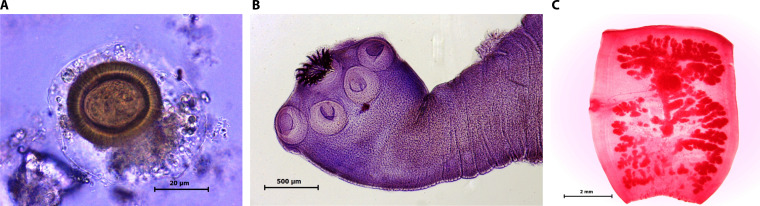
Finding the tapeworm scolex with its characteristic double crown of hooks is diagnostic, although rarely obtainable. Similarly, finding T. solium proglottids in stools allows species diagnosis by observation of the number of main uterine branches, or more recently by DNA tests (58, 59). However, proglottid excretion is intermittent and not reliable as a diagnostic approach. Historically, taeniasis has been diagnosed by microscopic examination of human stools. Direct examination of stool to detect T. solium eggs is poorly sensitive (26), although it is unclear whether T. solium does not release many eggs or whether they are released only intermittently. Concentration methods, particularly those using sedimentation, increase the diagnostic yield; however, the overall sensitivity of serial stool exams using concentration methods is still suboptimal. Antibodies stage specific to the adult tapeworm can be detected in serum (60, 61), and T. solium DNA can be detected in the stools of tapeworm carriers (62, 63), although these tests are not commercially available. The introduction of stool antigen (coproantigen) detection by ELISA greatly enhanced diagnostic sensitivity (64, 65) and provided a sensitive method to confirm treatment efficacy (66), although this assay is also poorly available outside academic research laboratories. In regions of endemicity, the prevalence of Taenia solium taeniasis by microscopy usually ranges around 1% (27, 67–69). The use of coproantigen detection increases the diagnostic yield by at least 60% (65). Caution should be exercised, however, when assessing putative population prevalence of taeniasis based solely on positive coproantigen ELISA results without parasitological confirmation (Fig. 3).
FIG 3.
Taenia solium. (A) Egg; (B) scolex; (C) gravid proglottid. (All images courtesy of Juan Jimenez, Lima, Peru.)
Treatment of Taeniasis
Two drugs are effective for the treatment of human taeniasis, niclosamide and praziquantel (PZQ). Niclosamide (2 g orally in a single dose for adults) is safe and well tolerated, with only mild and transient side effects. Its efficacy in treatment series has been reported to be above 80% (70). In a large-scale community-based deworming program, however, its efficacy was only 67% (71). Praziquantel seems to be effective at either 5 or 10 mg/kg (also a single oral dose) (72). If a person has taeniasis and simultaneously has latent, asymptomatic neurocysticercosis, praziquantel treatment for taeniasis may affect previously silent brain cysts and trigger seizures or other neurological symptoms (73–75). Judging by the numbers of doses of praziquantel used for schistosomiasis in regions of cysticercosis endemicity in Africa, this event seems to be extremely uncommon, but the impact of newly developed seizures or neurological symptoms in one or a few individuals could still be devastating for a community-based control program. On the other hand, only a few studies with mass chemotherapy for schistosomiasis were designed with sufficient follow-up to detect adverse central nervous system (CNS) events developing days after treatment. Niclosamide is not absorbed from the intestinal tract and thus it does not affect brain cysts. The reported efficacy of benzimidazoles such as mebendazole and albendazole (ABZ) to treat taeniasis in single-dose regimes is low (76, 77), requiring multiple doses and several days of treatment (78–81).
PORCINE CYSTICERCOSIS
In the porcine host (with a usual pig life span of 8 to 9 months), it is common to find muscular and subcutaneous cysts, as well as cysts in the nervous system. The few available studies of porcine cysticercosis using systematic thin-cut necropsy specimens demonstrate that most infected pigs have only a few (<10) cysts in the entire carcass (82). As expected, pigs with brain cysts are a subset of all infected pigs. Also, pigs with only degenerated cysts in the carcass (or with a majority of degenerating cysts) can be found, demonstrating that in some cases the infection can resolve by natural evolution. A minority of pigs host enormous numbers of cysts, in the range of thousands, and in these cases, the cysts are all viable (Fig. 4).
FIG 4.
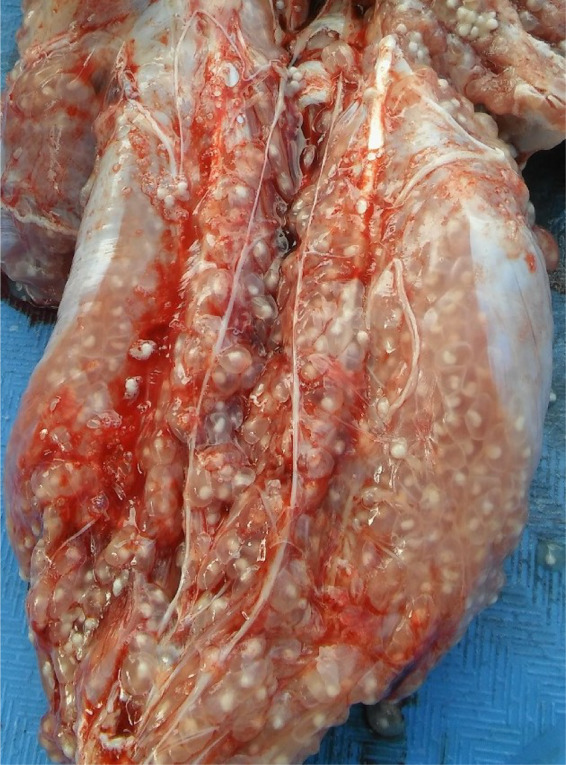
Massive cysticercosis infection in a pig. (Courtesy of the Cysticercosis Elimination Program in Tumbes, Peru.)
Diagnosis of Porcine Cysticercosis
Traditional public health manuals advocate slaughterhouse pig inspection (83). Slaughterhouse inspection is limited to a few cuts to not damage the market value of the carcass and may only rarely find infections with low numbers of cysts (84). Similarly, villagers in areas of cysticercosis endemicity are familiar with examination of the tongue of the pig visually and by palpation (85). This method can identify most heavily infected pigs, although similarly to slaughterhouse inspection, its sensitivity drops markedly for mild infections (86). Seizures may occur in heavily infected pigs (87), but they are quite rare. Other methods have been proposed to diagnose and characterize porcine cysticercosis infection, including serology (antibody or antigen detection) (88, 89), DNA-based assays (90), or the gold standard of detailed dissection of the entire pig carcass (91). None of these has yet proven practical to be routinely used, outside specific research studies.
Treatment of Porcine Cysticercosis
Initial trials of drug treatment for porcine cysticercosis used flubendazole without much success. Praziquantel was the first drug to show promise, and it was initially used at 50 mg/kg/day for 15 days (92) and later in a single-day regime (93). Overall, the efficacy of praziquantel in porcine cysticercosis is partial and not consistent (91). An initial study using a 1-month regime of albendazole at 15 mg/kg, published in 1995, indicated significant efficacy, but the regime was not widely adopted due to its long duration, which made it impractical for use in the field (281). Three days of albendazole at 30 mg/kg/day destroys all cysts (94). The introduction of oxfendazole (OXF) provided a more efficacious agent, killing all cysts in the carcasses of pigs receiving a single dose of 30 mg/kg (95–97). Further reports confirmed this high efficacy for muscle cysts but also demonstrated that a proportion of brain cysts survive a single dose of oxfendazole (91, 98). Niclosamide, nitazoxanide, or triclabendazole does not show significant cysticidal efficacy (91).
HUMAN CYSTICERCOSIS AND NEUROCYSTICERCOSIS
In areas where T. solium is endemic, NCC is a common diagnosis in individuals with seizures and other neurological symptoms. Cases of human and porcine cysticercosis cluster around human tapeworm carriers, the source of infection (27, 31). In most regions of endemicity, the spectrum of symptomatic disease is varied and involves cases of single and multiple parenchymal NCC lesions as well as extraparenchymal NCC. Calcified lesions, usually single, often in patients with no recognized symptoms, are also frequently found (99–102). In the Indian subcontinent, however, the spectrum of disease seems to involve mostly young individuals with a single intraparenchymal cyst (103). The reason for this difference in clinical expression is unknown, although it could be related to less contact with tapeworm carriers, as similar patterns of disease are seen in people infected in regions where the disease is not endemic and in travelers (104).
Localization of Cysts in Human Tissues
In the human host (with a long life span), most infections are detected in the nervous system. This is partly due to the evident nature of seizures, intracranial hypertension, or other neurological symptoms in comparison to that of viable cysts in the muscles or other organs, where lesions may go unnoticed. It was initially believed that the establishing cysts localized preferentially in the brain, but evidence from the porcine host (105), old necropsy studies and radiological reports (106, 107), and radiological evidence of residual calcifications in other tissues (108) suggest that embryos are distributed to all tissues and are commonly destroyed by the immune response of the host, surviving preferentially in the brain and eye with the help of the blood-brain barrier (BBB) and the hemato-ocular barrier (109–111). In addition, viable cysticerci have been shown to use multiple active mechanisms of immune evasion, including the secretion of molecules able to block the complement system, affect the cellular response, increase regulatory T cells, degrade attacking immunoglobulins (including immunoglobulin-cleaving proteases, protease inhibitors and antioxidants, immunosuppressor factors, and other molecules like paramyosin, sulfated proteoglycans, prostaglandin E2, taeniaestatin, and neuropeptides such as substance P and somatostatin) (112–114), or even cover itself with host immunoglobulins (110, 111, 115, 116). Eventually, intraparenchymal cysts degenerate and resolve, either by natural involution (117) or following treatment with antiparasitic agents (118), in a sequence well described decades ago and revisited in 2002 by Escobar and Weidenheim (119). Dead parasites resolve completely (in most cases) or leave a calcified scar (120).
Localization of Cysts in the Nervous System
Clinical manifestations of NCC differ according to the parasite location in the human CNS, inside or outside the brain parenchyma (intra- or extraparenchymal NCC). The presence of cysts or cyst clusters outside the brain parenchyma is a major driver of morbidity and mortality. Unlike parenchymal cysts that establish as small cysts, manifest with headache or seizures, and rarely grow beyond 2 cm in diameter, cysts in the ventricles or particularly those in the subarachnoid space tend to grow and spread into the surrounding spaces, causing clinical manifestations related to mass effects, hydrocephalus, chronic arachnoiditis, and vasculitis, with a much poorer prognosis.
TYPES OF NEUROCYSTICERCOSIS
The combination of lesion location and evolutionary stage of lesions results in a wide array of clinical presentations of NCC. A simplified, nonexclusive categorization is shown in Table 1.
TABLE 1.
Types of NCCa
| Location | Stage | Perilesional inflammation/edema |
|---|---|---|
| Parenchymal (single or multiple) | Viable | Variable |
| Degenerating | Usually present and marked | |
| Calcified | May be present (associated with symptoms) | |
| Extraparenchymal, intraventricular | Viable or in degeneration, rarely calcified | No |
| Extraparenchymal, subarachnoid | Viable or in degeneration, rarely calcified | Arachnoiditis or pachymeningitis, occasionally without a defined parasitic lesion |
Parenchymal NCC
As mentioned above, patients with parenchymal NCC usually present with seizures and chronic headache (3, 121–128), although patients with large cysts may present with focal deficits (129). Cognitive/memory deficits and psychiatric symptoms such as depression are frequently found (130–132), but these are usually not the reason why patients seek care. Overall, NCC, especially parenchymal brain cysticercosis, seems to account for one-third of all epilepsy cases in regions of endemicity, and as such, it represents the most important cause of acquired epilepsy worldwide.
Cysts go from a viable, quiescent state to complete resolution or calcification, passing through an involution process that involves focal inflammation, followed by cyst degeneration and then calcification. Cyst degeneration involves perilesional inflammation and is frequently associated with the onset or exacerbation of neurological symptoms (118). The boundaries between a viable cyst with inflammation and a degenerated parenchymal cyst are poorly defined. Cysts with perilesional contrast enhancement and edema are considered by some authors indistinctly as either cysts with inflammation or degenerating cysts, while other authors refer to changes in the density of cyst contents (133). Considering that in pigs, cysts may evaginate even after 2 weeks of antiparasitic treatment despite signs of inflammation and changes in the appearance of the cystic fluid (96), we have used in the past the absence of liquid content signal (hypodense on computed tomography [CT], hyperintense on T2 magnetic resonance imaging [MRI]) as the marker of parasite degeneration (134). In this view, cysts showing liquid contents are still considered cysts with inflammation and the absence of discernible liquid contents categorize the lesion as a degenerating cyst.
A subset of patients present with a single small parenchymal lesion, viable or in degeneration (9, 135). This type of NCC is particularly common on the Indian subcontinent, where it has been exhaustively described, affecting young individuals (8, 9, 135–140). It carries a much more benign prognosis, frequently with complete cure, low proportions of residual calcification, and low frequency of seizure relapses.
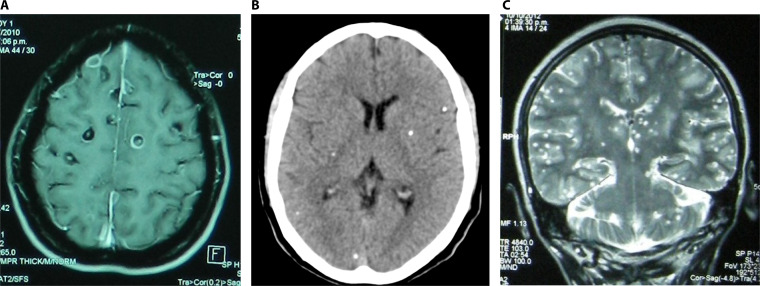
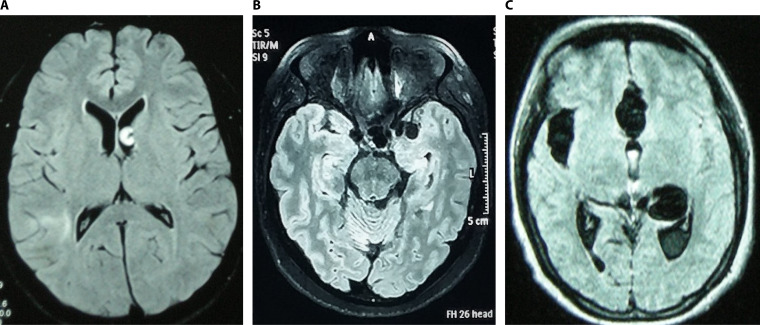
In rare cases, the patients with parenchymal NCC may show hundreds of small degenerating cysts, a particular and severe presentation named “cysticercotic encephalitis,” where diffuse brain inflammation places the patient at risk of death (141) (Fig. 5).
FIG 5.
Parenchymal neurocysticercosis. (A) Viable and degenerating cysts; (B) calcified lesions; (C) cysticercosis encephalitis.
Perilesional alterations, inflammation, and seizures.
Seizures in patients with NCC may occur in relation to cysts in any stage, most frequently with degenerating cysts but also with viable and calcified cysts (142, 143). Patients frequently seek neurological attention after months or years with seizures. In these patients, seizures are usually of the same type and are localization related to a parasitic lesion (142).
Although viable cysts show minimal signs of inflammation, gliosis, pericystic neuronal damage, and vascular alterations have been demonstrated (144–146). In symptomatic individuals, it is common to find perilesional edema and contrast enhancement around at least one of their cysts (118). While pericystic inflammation has been interpreted as marking the onset of parasite degeneration (119), some of these lesions may survive and continue to be apparently viable for months (120) or years (147, 148). Treatment with antiparasitic agents triggers a similar focal pericystic inflammatory response in temporal association with an exacerbation of seizures and other symptoms (149, 150).
Resolution of the parasitic lesions usually results in a decrease in symptoms. Individuals with calcified NCC cysts do not usually show perilesional inflammation, although perilesional edema is seen after a seizure in one-third to one-half of cases, a finding that may lead to the erroneous diagnosis of a degenerating cysticercus (151, 152). Most authors view pericalcification edema as an episodic immune response to parasite antigens remaining in the calcified matrix; however, the role of seizure activity as a cause of BBB disruption contributing to perilesional edema is yet unclear (153). Local inflammation around a cyst or a calcification has also been associated with an increased risk of later seizure relapses (154). Scarring also plays a role: perilesional gliosis around calcified cysts can be demonstrated by MRI, and it is also associated with an increased frequency of seizure relapses (155–157).
All of the above lead to the consideration that inflammation contributes to focal damage as well as to early and late seizure manifestations, and as such, seizures in NCC patients result from a combination of both focal damage and inflammation. Interestingly, chronic calcific NCC seems to be associated with hippocampal sclerosis, also suggesting distant damage (158–164).
Extraparenchymal NCC
Most cases of extraparenchymal NCC locate in the cerebral ventricles or in the subarachnoid spaces. Intraventricular cysts (particularly cysts in the third or fourth ventricle) can cause hydrocephalus or may cause symptoms due to direct compression of the brainstem (as in fourth ventricle cysts). More rarely, large cysts in the horns of the lateral ventricles can also cause mass effects. In the lateral ventricles, it is important to discern whether the cysts are attached to the wall or freely floating. Migration of cysts from one ventricle to another has been described with some frequency and seems to be unique to NCC (165–167). The degree of inflammation may be important for surgical (neuroendoscopical) resection, as cysts with surrounding inflammation may be adherent, difficult to remove, and prone to bleed.
The more frequent locations of subarachnoid NCC cysts are the Sylvian fissures, basal cisterns, and interhemispheric spaces, frequently affecting multiple sites. In these regions, and particularly in the Sylvian fissures, lesions tend to form large aggregates (“giant” cysts or cyst clusters). Lesions in the basal subarachnoid spaces or in the interhemispheric areas also tend to spread into the surrounding spaces. Conversely, small, well-defined cysts in the convexity of the cerebral hemispheres may behave similarly to parenchymal NCC (125) (Fig. 6).
FIG 6.
Extraparenchymal neurocysticercosis. (A) Intraventricular cyst; (B) basal subarachnoid cysticercosis; (C) Sylvian fissure and interhemispheric cysticercosis.
Subarachnoid NCC is, however, a very complex disease. Patients with basal subarachnoid NCC are on average 10 to 15 years older than patients with parenchymal NCC, and the few cases of long-term immigrants seen with subarachnoid NCC in the United States also had remained in the country for many years without further exposure, suggesting a very long incubation period (168–170). A second important characteristic is the proliferative nature of the subarachnoid NCC cyst membrane. Unlike the typical cyst membrane, subarachnoid cysts have a hypercellular epithelium with areas of exuberant membrane growth (that may involve internal areas of necrosis or fibrosis). This amount of parasite tissue is associated with high levels of circulating parasite antigens, strong antibody responses, and, in general, an inflammatory response reflected in abnormal cerebrospinal fluid (CSF) (hypercellular, with eosinophils, high protein, and occasionally low glucose) (17, 171). Mass effects and the chronic inflammatory response with resultant fibrosis frequently lead to hydrocephalus and the common presentation of intracranial hypertension. Inflammation may also result in vasculitis and associated ischemic events (172). The classic literature named subarachnoid cyst clusters “racemose” cysticercosis, in allusion to its resemblance to a bunch of grapes. The lack of a scolex structure in the pieces was noted, and multiple hypotheses tried to explain its disappearance. The most accepted hypothesis is that the uncontrolled growth of the membrane “incorporates” the scolex structures in its membranous expansion (173).
DIAGNOSTIC TOOLS
The diagnosis of NCC rests on neuroimaging tools and is supported by immunodiagnostic tests. Molecular tests are slowly being introduced, but so far these have not yet reached the required levels of sensitivity (174–177). Routine hematological tests are of poor use, and even eosinophilia has been found not to be frequent in newly diagnosed NCC patients (178).
Neuroimaging
The introduction of CT was one of the major advances in the knowledge of human NCC, providing clinicians with the capacity to visualize lesions in the brain parenchyma (up to that point, imaging was limited to the detection of calcifications [X-rays], distortions in the ventricular/cisternal anatomy [pneumoencephalography], or distortions in the vascular anatomy [arteriography]). The advent of CT changed the landscape of NCC by unveiling many cases with mild disease, much more benign than the severe cases seen before, which were limited to those that could be detected by old, less-sensitive techniques. The introduction of MRI a few years later further improved imaging definition and added the capacity to present images in different planes. In general, MRI is more sensitive than CT in detecting parenchymal and extraparenchymal disease, although its sensitivity to detect calcified lesions, particularly small ones, is quite limited (133, 179–181). Lesions in the ventricles and the cisterns are better visualized using volumetric balanced steady-state gradient echo sequences (FIESTA, BFFE, or CISS, depending on the company) (181–183). Current U.S. guidelines for the diagnosis of NCC suggest that whenever possible, patients should be assessed by both techniques (184).
Neuroimaging is more helpful than serology in the sense that it provides data on the number, size, location, and stage of lesions, as well as perilesional inflammation (133, 179, 180, 185). Therapeutic decisions beyond symptomatic therapy cannot be made in the absence of this information.
Immunodiagnosis
While neuroimages may be highly compatible with NCC (in fact, multiple cystic images with a scolex are pathognomonic of the disease, although care has to be taken to avoid confusion with other structures that may mimic a scolex) (180, 184, 186, 187), in many cases the diagnosis is not conclusive. In these cases, specific serology plays a major role in confirming the diagnosis. Antibody detection is most frequently used because of its higher sensitivity, while antigen detection provides additional information on the presence of living parasites (188).
Antibody detection.
The assay of choice for antibody detection is the enzyme-linked immunoelectrotransfer blot (EITB) assay using lentil lectin purified parasite glycoprotein antigens (LLGP) (189). This test has a sensitivity above 98% in patients with more than one live brain cyst, and its specificity is close to 100% (190, 191). EITB sensitivity drops in patients with a single viable brain cyst. Antibody detection ELISAs available in the market use less-purified antigens, resulting in lower sensitivity and, more importantly, frequent cross-reactions with related cestodes such as the ubiquitous Hymenolepis nana or Echinococcus sp. (hydatid disease) (192, 193).
The seven LLGP antigens used in the LLGP-EITB assay belong to three families, with low-molecular-weight antigens associated with viable disease and appearing after weeks or months of infection (190). Heavier-molecular-weight antigens appear first and are the latest to disappear after the patient is cured and all the parasites have died. Patients may be antibody positive for months or years after successful therapy (190). Drawbacks of the LLGP-EITB assay include its limited availability and complex processing, as well as the need for parasite material to produce the antigen mix. Efforts are under way to produce a simpler version based on recombinant or synthetic antigens (194).
Antigen detection.
Detecting circulating parasite antigen is a difficult task because unlike antibodies, antigen is limited in amount and not multiplied by the immune system (resulting in decreased sensitivity) and also because helminths share many diagnostic epitopes (resulting in frequent cross-reactions) (188). The production of monoclonal antibodies against Taenia saginata allowed the development of antigen-capture ELISAs with good specificity, although their sensitivity is still lower than that of the LLGP-EITB (195, 196). On the positive side, detecting parasite antigen confirms the presence of living parasites and as such it informs therapeutic decisions (197). Antigen levels also serve to monitor the efficacy of antiparasitic treatment (198, 199).
Molecular Tests
Taenia solium DNA has been detected by PCR or deep genomic sequencing using cerebrospinal fluid (CSF) of patients with subarachnoid NCC (175–177, 200, 201), although there are no reports of its use in parenchymal NCC cases, even less in patients with a single brain lesion where most diagnostic problems arise. Cell-free T. solium DNA has been demonstrated in the urine and serum of patients with NCC (174, 202), and recent data suggest that monocyte gene expression and serum mass spectrometry profiles could be used to identify NCC cases (203, 204). To date, however, molecular biology assays are not directly applied for routine case assessment.
Diagnostic Criteria
Diagnostic criteria for cysticercosis were developed more than 20 years ago by Del Brutto et al. (205), to homogenize the diagnostic approach and to reduce errors that occur when epidemiological data, clinical manifestations, and complementary tests are used by themselves to diagnose the disease. A second set of criteria, confined to the diagnosis of NCC, was reported in 2001 (206). This set used four categories of diagnostic criteria (absolute, major, minor, and epidemiologic), stratified according to their diagnostic strength, and two degrees of diagnostic certainty (definitive and probable). These criteria have been widely used for the diagnosis of NCC in both hospital and field settings and have proven useful in areas of endemicity and nonendemicity. The more recent version (187) emphasizes the importance of neuroimaging as the basis for a diagnosis of NCC, and as such, it is organized into absolute, neuroimaging, and clinical/exposure criteria. Likewise, proper interpretation of these criteria allows two degrees of diagnostic certainty, definitive and probable (Table 2). While this revised set permits a diagnosis of probable NCC in individuals presenting with suggestive clinical manifestations (mainly seizures) and evidence of exposure to cysticercosis, a definitive diagnosis of NCC cannot be established without the evidence provided by neuroimaging.
TABLE 2.
Revised Del Brutto’s diagnostic criteria and degrees of diagnostic certainty for neurocysticercosisa
| Category | Criterion or definitionb |
|---|---|
| Diagnostic criteria | |
| Absolute criteria | Histological demonstration of the parasite from biopsy specimen of a brain or spinal cord lesion |
| Visualization of subretinal cysticercus | |
| Conclusive demonstration of a scolex within a cystic lesion on neuroimaging studies | |
| Neuroimaging criteria | |
| Major | Cystic lesions without a discernible scolex |
| Enhancing lesionsc | |
| Multilobulated cystic lesions in the subarachnoid space | |
| Typical parenchymal brain calcificationsc | |
| Confirmative | Resolution of cystic lesions after cysticidal drug therapy |
| Spontaneous resolution of single small enhancing lesionsd | |
| Migration of ventricular cysts documented on sequential neuroimaging studiesc | |
| Minor | Obstructive hydrocephalus (symmetric or asymmetric) or abnormal enhancement of basal leptomeninges |
| Clinical/exposure criteria | |
| Major | Detection of specific anticysticercal antibodies or cysticercal antigens by well-standardized immunodiagnostic testsc |
| Cysticercosis outside the central nervous systemc | |
| Evidence of a household contact with T. solium infection | |
| Minor | Clinical manifestations suggestive of neurocysticercosisc |
| Individuals coming from or living in an area where cysticercosis is endemicc | |
| Degrees of diagnostic certainty | |
| Definitive diagnosis | One absolute criterion |
| Two major neuroimaging criteria plus any clinical/exposure criteria | |
| One major and one confirmative neuroimaging criteria plus any clinical/exposure criteria | |
| One major neuroimaging criteria plus two clinical/exposure criteria (including at least one major clinical/exposure criterion), together with the exclusion of other pathologies producing similar neuroimaging findings | |
| Probable diagnosis | One major neuroimaging criteria plus any two clinical/exposure criteria |
| One minor neuroimaging criteria plus at least one major clinical/exposure criteria |
Adapted from reference 187 with permission of Elsevier.
Definitions: cystic lesions, rounded, well-defined lesions with liquid contents of signal similar to that of CSF on CT or MRI; enhancing lesions, single or multiple, ring- or nodule-enhancing lesions of 10 to 20 mm in diameter, with or without surrounding edema, but not displacing midline structures; typical parenchymal brain calcifications, single or multiple solid lesions, most usually of <10 mm in diameter; migration of ventricular cyst, demonstration of a different location of ventricular cystic lesions on sequential CTs or MRIs; well-standardized immunodiagnostic tests, to date, antibody detection by enzyme-linked immunoelectrotransfer blot assay using lentil lectin purified T. solium antigens and detection of cysticercal antigens by monoclonal antibody-based ELISA; cysticercosis outside the central nervous system, demonstration of cysticerci from biopsy of subcutaneous nodules, X-ray films, or CT showing cigar-shape calcifications in soft tissues, or visualization of the parasite in the anterior chamber of the eye; suggestive clinical manifestations, mainly seizures (often starting in individuals aged 20 to 49 years; the diagnosis of seizures in this context is not excluded if patients are outside the typical age range), but other manifestations include chronic headaches, focal neurologic deficits, intracranial hypertension, and cognitive decline; area of cysticercosis endemicity, a place where active transmission is documented.
Operational definition.
The use of corticosteroids makes this criterion invalid.
MANAGEMENT OF NEUROCYSTICERCOSIS
Symptomatic NCC requires medical therapy with symptomatic medication and/or antiparasitic drugs and, less frequently, surgical interventions.
Medical Treatment
Symptomatic treatment.
Patients with symptomatic cysticercosis seek medical attention because of neurological symptoms. Symptomatic medication, including analgesics, antiepileptic drugs, mannitol, and steroids, are in general indicated as they would be administered for seizures, headache, or intracranial hypertension from any other etiology. Symptomatic management is important and should be well established before considering the onset of antiparasitic drug therapy (207).
Antiparasitic treatment.
Destroying live or degenerating cysticerci by using antiparasitic drugs is indicated in most cases (125). An interesting peculiarity of the use of antiparasitic drugs in NCC is that no immediate improvement is expected in the initial days or weeks. Conversely, its use triggers local perilesional inflammation that may cause or worsen neurological symptoms, and thus steroids or other agents are simultaneously administered to modulate this undesirable effect (208). In the long term, however, using antiparasitic drugs to destroy viable parenchymal NCC lesions results in fewer relapses of seizures with generalization (120, 209), and individuals who cure all their viable lesions also demonstrate fewer overall seizures (134). In addition, use of antiparasitic drugs to resolve subarachnoid NCC lesions reduces the significant mortality associated with this aggressive form of the disease (210).
The use of antiparasitic drugs in NCC has not been exempt from controversy (211). Praziquantel (PZQ) was first introduced in Mexico in 1979 (212). Clinicians in Latin America, treating mainly multicystic parenchymal disease and subarachnoid NCC, rapidly embraced the availability of antiparasitic therapy (213–215). In contrast, practitioners in the United States and India, treating mainly patients with single enhancing parenchymal NCC cysts, noted a relatively benign course with symptomatic therapy (216–218). The initial series of praziquantel and later albendazole reported significant neurological side effects occurring in the initial days of treatment, including death in some cases, rightly attributed to an exacerbated inflammatory reaction against the degenerating parasites (150). Simultaneous use of steroids helped to control treatment-associated symptoms (149). Some publications suggested that praziquantel and albendazole do not really result in cyst destruction and may even result in long-term sequelae (219) and also do not improve the prognosis of the underlying seizure disorder (220). Subsequent randomized controlled trials demonstrated beneficial effects of antiparasitic treatment to resolve viable cysts (with the associated reduction of mass effects), reduce the likelihood of disease progression, and improve the evolution of seizures due to parenchymal NCC, and it is accepted as the option of choice in most cases (120, 134, 184, 209, 221, 222). A proportion of patients, however, will continue having seizures despite the resolution of all of their brain cysts.
The regimen of choice for single parenchymal NCC is albendazole at 15 mg/kg/day for 7 to 15 days. Albendazole (at the same dose) combined with praziquantel at 50 mg/kg/day for 10 days demonstrated superior antiparasitic efficacy in cases with multiple viable cysts (134, 223). Longer regimens of albendazole are required in subarachnoid NCC. Some authors report continued use for several months until complete lesion resolution is seen in neuroimaging (169) or suggest the use of higher doses (224, 225). Yet there is no controlled comparative data on the safety and efficacy of combined albendazole and praziquantel in this type of disease. Higher plasma levels of albendazole sulfoxide (the active metabolite of albendazole) seem associated with higher parasiticidal efficacy (226). It should be kept in mind that antiparasitic therapy is contraindicated in the setting of uncontrolled elevated intracranial pressure, as seen with diffuse cerebral edema. Careful steroid management is important to modulate inflammation-related side effects and for the control of vasculitis and avoidance of vascular complications that can occur as the steroids are tapering (118, 227). More recently, methotrexate and etanercept have been used with this purpose (228–230).
Surgical Approaches
Surgery may be required in patients with NCC. The most frequent procedure is the insertion of a ventricle-peritoneal shunt to control hydrocephalus. Also, in the past 20 years, neuroendoscopy became the approach of choice for the management of intraventricular NCC. This procedure seems safe and effective, although caution is required when cysts are adhered to the ventricular wall, because of the risk of intraventricular bleeding (231). Open surgery is occasionally used to excise large cysts or cyst masses, most commonly in the Sylvian fissures or in the fourth ventricle (232).
CONTROL AND ELIMINATION
Development eliminates cysticercosis by canceling the conditions required to maintain the cycle (poor sanitation and noncommercial, domestic pig raising) (233–235). In poor localities, however, major changes in living conditions are unlikely to occur in the short term and active interventions are required to control or eliminate Taenia solium. Taeniasis/cysticercosis was recognized as potentially eradicable long ago (236), on the basis of availability of diagnostics and treatments, a life cycle that involves a domestic animal, and the lack of an invertebrate vector. Early efforts using mass human deworming with praziquantel in Ecuador (237) were followed by mass chemotherapy experiences in other Latin American countries (68, 238–241) and later in Asia and Africa (242–244). Addition of chemotherapy and vaccines to eliminate the pig reservoir increased the feasibility of interrupting transmission (37). Multiple control initiatives can be found in the literature. Early studies combining health education and human mass chemotherapy with praziquantel in Mexico gave inconclusive results (241, 245, 246). A study on pig confinement plus chemotherapy in Tanzania failed to control transmission (247), and assessment of mass praziquantel treatment for schistosomiasis showed a partial impact on the prevalence of taeniasis and porcine cysticercosis after three rounds of treatment but not in a village that received two rounds only (248). A study using daily doses of albendazole for 3 days in repeated campaigns, plus porcine vaccination in a small village in Lao People’s Democratic Republic, found a marked decrease in the prevalence of human taeniasis (78, 80), and a trial of porcine vaccine plus oxfendazole treatment protected pigs from cysticercosis in a population of approximately 200 pigs in Nepal (244). Health education interventions have resulted in very partial decreases in transmission indicators (249–251). In Peru, a large integrated program combining human and porcine mass chemotherapy, pig vaccination, and coproantigen detection-based case confirmation over 1 year has proven effective in achieving focal elimination of transmission in a wide area of the northern coast of the country (71). This program was able to interrupt T. solium transmission in 105 of 107 villages in a rural region comprising more than 80,000 individuals.
CURRENT RESEARCH IN TAENIA SOLIUM
There are many active fronts in Taenia solium research involving a very wide spectrum, from basic science (144, 202, 203) to mathematical modeling of transmission (252–255). Some of the most promising study fields include the following.
In Vitro and Animal Models
The lack of suitable in vitro and animal models has always been a drawback in cysticercosis research. The recent description of in vitro oncospheral development until early cyst stages (256, 257) now allows the systematic assessment of metabolic processes and antigen expression in these early stages of the parasite. Regarding animal models, infection had been reported in mice (258, 259), and more recently, reproducible brain infection in rats was obtained by intracranial oncosphere injection (144, 145, 256, 260). Oral infection of pigs is impractical because of variable rates of infection (261). Intracranial infection of piglets is feasible (262). Oncospheral injection in the pig carotid artery results in high rates of brain infection with small numbers of cysts, a model that resembles human NCC more closely than purchasing heavily infected pigs from villages where the disease is endemic, since the latter group has too many brain cysts, in the range of tens or hundreds (263).
Epileptogenesis
Despite claims that NCC might cause only acute, inflammation-related seizures and not epilepsy (264), there is much evidence that brain cysticerci result in the development of epileptogenic circuits (142). Moreover, recent studies demonstrate a consistent association between calcified NCC lesions and hippocampal sclerosis (158–161, 163), suggesting the possibility of distant formation of epileptogenic foci (158–160, 163, 265–268). Information to date suggests that development of a residual calcification (269) and lesion degeneration in the absence of antiparasitic treatment (270) are both associated with a poorer prognosis in terms of seizure relapses. Studies in animal models and in human cases may identify early markers of epileptogenesis and eventually serve to test interventions to prevent it. Studies in brain cysticercosis lesions in rodents have shown pro- and anti-inflammatory cytokine activity, disruption of the blood-brain barrier, angiogenesis, collagen deposition, and glial scarring (144, 271–273), and some authors suggest that substance P or other molecules produced early in the host granulomatous reaction to the dying parasite is capable of inducing seizure activity (274, 275). Local inflammatory damage and perilesional scarring may contribute to the presence and persistence of symptoms, particularly seizures. Along this line, since residual calcifications have consistently been associated with increased rates of seizure relapse, therapies that decrease the likelihood of residual calcification by modulating the inflammatory response or by directly influencing the deposit of calcium may result in improved clinical evolution.
FUTURE PERSPECTIVES
A better understanding of the mechanisms involved in parasite degeneration and clearance as well as the mechanisms of damage to the surrounding neural tissue should lead to improved clinical outcomes and perhaps even avoidance of epileptogenesis. Similarly, deciphering the dynamics of antigen and antibody reactions or assessing parasite DNA, as well as determining appropriate therapeutic serum levels of the most common antiparasitic drugs, could lead to improved monitoring of disease evolution and fine-tuning of therapy. Clearly, advanced tools such as genetic manipulation and omics studies should also lead to significant improvements in our understanding of the biology of T. solium and the pathogenesis of cysticercosis. On a higher level, however, the perspective for elimination and potential eradication of Taenia solium taeniasis/cysticercosis should be a major target for public health practitioners and decision makers.
Biographies

Hector H. Garcia, M.D., Ph.D., is the director of the Center for Global Health and professor at the Department of Microbiology at the Universidad Peruana Cayetano Heredia, as well as head of the Cysticercosis Unit at the Instituto Nacional de Ciencias Neurologicas in Lima, Peru. He earned his Ph.D. in 2002 from The Johns Hopkins University Bloomberg School of Public Health and his M.D. in 1989 from The Universidad Peruana Cayetano Heredia in Lima, Peru. Dr. Garcia is a coordinating member of the Cysticercosis Working Group in Peru, a multi-institutional network which is one of the foremost research groups worldwide in the study of neurocysticercosis, working in varied aspects of cysticercosis research that offers hands-on training in global health research to local scientists and health professionals.

Armando E. Gonzalez, D.V.M., Ph.D., a past Dean of the School of Veterinary Medicine at San Marcos University in Lima, Peru, and also a member of the Coordinating Board of the Cysticercosis Working Group in Peru, is an accomplished veterinary epidemiologist. He graduated from San Marcos University and obtained his M.S.C. degree in microbiology at the Universidad Peruana Cayetano Heredia and his Ph.D. in Veterinary Economics at the University of Reading in the United Kingdom. With over 200 publications in indexed journals on cysticercosis and other veterinary parasitoses, Prof. Gonzalez is one of the most productive scientists in Peru and also a mentor for many young veterinary researchers.

Robert H. Gilman, M.D., D.T.M.H., is a senior researcher based at the Department of International Health of the Johns Hopkins School of Public Health in Baltimore, MD. The long trajectory of Prof. Gilman has resulted in over 800 publications and important findings that include not only the work of the Cysticercosis Working Group in Peru but also seminal work in other infectious diseases, such as the discovery of Cyclospora cayetanensis and the development of the MODs test for tuberculosis. With more than 30 years of living in Peru, Prof. Gilman mentored not only Drs. Garcia and Gonzalez but also a large group of Peruvian researchers that have now returned to Peru and established their own research groups, building the critical mass for the future of biomedical research in the country.
REFERENCES
- 1.Grove DI. 1990. A history of human helminthology. CABI, Wallingford, England. [Google Scholar]
- 2.Kean BH, Mott KE, Russell AJ. 1978. Tropical medicine and parasitology. Classic investigations, vol 2 Cornell University Press, Ithaca, NY. [Google Scholar]
- 3.Brizzi K, Pelden S, Tshokey T, Nirola DK, Diamond MB, Klein JP, Tshering L, Deki S, Nidup D, Bruno V, Dorny P, Garcia HH, Mateen FJ, Bhutan Epilepsy Project. 2016. Neurocysticercosis in Bhutan: a cross-sectional study in people with epilepsy. Trans R Soc Trop Med Hyg 110:517–526. doi: 10.1093/trstmh/trw066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debacq G, Moyano LM, Garcia HH, Boumediene F, Marin B, Ngoungou EB, Preux PM. 2017. Systematic review and meta-analysis estimating association of cysticercosis and neurocysticercosis with epilepsy. PLoS Negl Trop Dis 11:e0005153. doi: 10.1371/journal.pntd.0005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melki J, Koffi E, Boka M, Toure A, Soumahoro MK, Jambou R. 2018. Taenia solium cysticercosis in West Africa: status update. Parasite 25:49. doi: 10.1051/parasite/2018048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mwape KE, Blocher J, Wiefek J, Schmidt K, Dorny P, Praet N, Chiluba C, Schmidt H, Phiri IK, Winkler AS, Gabriel S. 2015. Prevalence of neurocysticercosis in people with epilepsy in the Eastern province of Zambia. PLoS Negl Trop Dis 9:e0003972. doi: 10.1371/journal.pntd.0003972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owolabi LF, Adamu B, Jibo AM, Owolabi SD, Imam AI, Alhaji ID. 2020. Neurocysticercosis in people with epilepsy in Sub-Saharan Africa: a systematic review and meta-analysis of the prevalence and strength of association. Seizure 76:1–11. doi: 10.1016/j.seizure.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Rajshekhar V. 2018. Evolution of concepts in the management of cysticercosis of the brain: then (1970) and now (2018). Neurol India 66:919–927. doi: 10.4103/0028-3886.236969. [DOI] [PubMed] [Google Scholar]
- 9.Singhi P, Malhi P, Suthar R, Deo B, Khandelwal NK. 2018. Long-term cognitive outcome of children with parenchymal neurocysticercosis: a prospective observation study. J Child Neurol 33:468–473. doi: 10.1177/0883073818766985. [DOI] [PubMed] [Google Scholar]
- 10.Trevisan C, Sotiraki S, Laranjo-Gonzalez M, Dermauw V, Wang Z, Karssin A, Cvetkovikj A, Winkler AS, Abraham A, Bobic B, Lassen B, Cretu CM, Vasile C, Arvanitis D, Deksne G, Boro I, Kucsera I, Karamon J, Stefanovska J, Koudela B, Pavlova MJ, Varady M, Pavlak M, Sarkunas M, Kaminski M, Djurkovic-Djakovic O, Jokelainen P, Jan DS, Schmidt V, Dakic Z, Gabriel S, Dorny P, Omeragic J, Alagic D, Devleesschauwer B. 2018. Epidemiology of taeniosis/cysticercosis in Europe, a systematic review: eastern Europe. Parasit Vectors 11:569. doi: 10.1186/s13071-018-3153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu W, Jia F, Wang W, Huang Y, Huang Y. 2013. Antiparasitic treatment of cerebral cysticercosis: lessons and experiences from China. Parasitol Res 112:2879–2890. doi: 10.1007/s00436-013-3459-3. [DOI] [PubMed] [Google Scholar]
- 12.Bruno V, Klein JP, Nidup D, Nirola DK, Tshering L, Deki S, Clark SJ, Linn KA, Shinohara RT, Dorji C, Pokhrel DR, Dema U, Mateen FJ, Bhutan Epilepsy Project. 2017. Yield of brain MRI in clinically diagnosed epilepsy in the Kingdom of Bhutan: a prospective study. Ann Glob Health 83:415–422. doi: 10.1016/j.aogh.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, Dickey M, Reynolds S, Stoner JA. 2010. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis 4:e870. doi: 10.1371/journal.pntd.0000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segamwenge IL, Kioko NP, Mukulu C, Jacob O, Humphrey W, Augustinus J. 2016. Neurocysticercosis among patients with first time seizure in Northern Namibia. Pan Afr Med J 24:127. doi: 10.11604/pamj.2016.24.127.8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh BB, Khatkar MS, Gill JP, Dhand NK. 2017. Estimation of the health and economic burden of neurocysticercosis in India. Acta Trop 165:161–169. doi: 10.1016/j.actatropica.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Angheben A, Buonfrate D, Zammarchi L, Strohmeyer M, Gobbi F, Degani M, Anselmi M, Marchese V, Bartoloni A, Bisoffi Z. 2018. Seroprevalence of Taenia solium antibodies in a cohort of Bolivian immigrants in Italy. Acta Trop 185:107–109. doi: 10.1016/j.actatropica.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Coyle CM. 2019. Neurocysticerosis: an individualized approach. Infect Dis Clin North Am 33:153–168. doi: 10.1016/j.idc.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Coyle CM, Mahanty S, Zunt JR, Wallin MT, Cantey PT, White AC Jr, O'Neal SE, Serpa JA, Southem PM, Wilkins P, McCarthy AE, Higgs ES, Nash TE. 2012. Neurocysticercosis: neglected but not forgotten. PLoS Negl Trop Dis 6:e1500. doi: 10.1371/journal.pntd.0001500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flecker RH, O'Neal SE, Townes JM. 2016. Evaluating healthcare claims for neurocysticercosis by using all-payer all-claims data, Oregon, 2010–2013. Emerg Infect Dis 22:2168–2170. doi: 10.3201/eid2212.160370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Federal Register. 2015. Designating additions to the current list of tropical diseases in the Federal Food, Drug, and Cosmetic Act. Final order. Fed Regist 80:50559–50564. [PubMed] [Google Scholar]
- 21.Laranjo-Gonzalez M, Devleesschauwer B, Trevisan C, Allepuz A, Sotiraki S, Abraham A, Afonso MB, Blocher J, Cardoso L, Correia da Costa JM, Dorny P, Gabriel S, Gomes J, Gomez-Morales MA, Jokelainen P, Kaminski M, Krt B, Magnussen P, Robertson LJ, Schmidt V, Schmutzhard E, Smit GSA, Soba B, Stensvold CR, Staric J, Troell K, Rataj AV, Vieira-Pinto M, Vilhena M, Wardrop NA, Winkler AS, Dermauw V. 2017. Epidemiology of taeniosis/cysticercosis in Europe, a systematic review: Western Europe. Parasit Vectors 10:349. doi: 10.1186/s13071-017-2280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Keefe KA, Eberhard ML, Shafir SC, Wilkins P, Ash LR, Sorvillo FJ. 2015. Cysticercosis-related hospitalizations in the United States, 1998–2011. Am J Trop Med Hyg 92:354–359. doi: 10.4269/ajtmh.14-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Neal SE, Flecker RH. 2015. Hospitalization frequency and charges for neurocysticercosis, United States, 2003–2012. Emerg Infect Dis 21:969–976. doi: 10.3201/eid2106.141324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zammarchi L, Bonati M, Strohmeyer M, Albonico M, Requena-Mendez A, Bisoffi Z, Nicoletti A, Garcia HH, Bartoloni A, COHEMI Project Study Group. 2017. Screening, diagnosis and management of human cysticercosis and Taenia solium taeniasis: technical recommendations by the COHEMI project study group. Trop Med Int Health 22:881–894. doi: 10.1111/tmi.12887. [DOI] [PubMed] [Google Scholar]
- 25.Flisser A. 2013. State of the art of Taenia solium as compared to Taenia asiatica. Korean J Parasitol 51:43–49. doi: 10.3347/kjp.2013.51.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flisser A. 1994. Taeniasis and cysticercosis due to Taenia solium. Prog Clin Parasitol 4:77–116. [PubMed] [Google Scholar]
- 27.Sarti E, Schantz PM, Plancarte A, Wilson M, Gutierrez IO, Lopez AS, Roberts J, Flisser A. 1992. Prevalence and risk factors for Taenia solium taeniasis and cysticercosis in humans and pigs in a village in Morelos, Mexico. Am J Trop Med Hyg 46:677–685. doi: 10.4269/ajtmh.1992.46.677. [DOI] [PubMed] [Google Scholar]
- 28.Mendlovic F, Garza-Rodríguez A, Carrillo-Farga J, González-Domínguez F, Maravilla P, Flisser A. 2014. From stillness to motion: 80 years after the first description of Taenia solium oncosphere hatching. Parasit Vectors 7:12. doi: 10.1186/1756-3305-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White AC Jr, Baig S, Robinson P. 1996. Taenia saginata oncosphere excretory/secretory peptidases. J Parasitol 82:7–10. doi: 10.2307/3284107. [DOI] [PubMed] [Google Scholar]
- 30.Zimic M, Pajuelo M, Gilman RH, Gutierrez AH, Rueda LD, Flores M, Chile N, Verastegui M, Gonzalez A, Garcia HH, Sheen P, Cysticercosis Working Group in Peru. 2012. The highly antigenic 53/25 kDa Taenia solium protein fraction with cathepsin-L like activity is present in the oncosphere/cysticercus and induces non-protective IgG antibodies in pigs. Vet Immunol Immunopathol 145:171–178. doi: 10.1016/j.vetimm.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lescano AG, Pray IW, Gonzalez AE, Gilman RH, Tsang VCW, Gamboa R, Guezala MC, Aybar V, Rodriguez S, Moulton LH, Leontsini E, Gonzalvez G, O’Neal SE, Garcia HH, Cysticercosis Working Group in Peru. 2019. Clustering of necropsy-confirmed porcine cysticercosis surrounding Taenia solium tapeworm carriers in Peru. Am J Trop Med Hyg 100:314–322. doi: 10.4269/ajtmh.18-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pray IW, Ayvar V, Gamboa R, Muro C, Moyano LM, Benavides V, Flecker RH, Garcia HH, O'Neal SE. 2017. Spatial relationship between Taenia solium tapeworm carriers and necropsy cyst burden in pigs. PLoS Negl Trop Dis 11:e0005536. doi: 10.1371/journal.pntd.0005536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshino K. 1933. Studies on the post-embryonal development of Taenia solium: II. On the youngest form of Cysticercus cellulosae and on the migratory course of the oncosphera of Taenia solium within the intermediate host. J Med Assoc Formosa 32:155–158. [Google Scholar]
- 34.Yoshino K. 1933. Studies on the post-embryonal development of Taenia solium: III. On the development of Cysticercus cellulosae within the definitive intermediate host. J Med Assoc Formosa 32:166–169. [Google Scholar]
- 35.Tembo A, Craig PS. 2015. Taenia saginata taeniosis: copro-antigen time-course in a voluntary self-infection. J Helminthol 89:612–619. doi: 10.1017/S0022149X14000455. [DOI] [PubMed] [Google Scholar]
- 36.Tsuboi M, Hayakawa K, Yamasaki H, Katanami Y, Yamamoto K, Kutsuna S, Takeshita N, Kanagawa S, Ohmagari N, Kato Y. 2018. Clinical characteristics and epidemiology of intestinal tapeworm infections over the last decade in Tokyo, Japan: a retrospective review. PLoS Negl Trop Dis 12:e0006297. doi: 10.1371/journal.pntd.0006297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez AE. 1997. Evaluation of a control program for Taenia solium targetting human and porcine health. University of Reading, Reading, United Kingdom. [Google Scholar]
- 38.Hoberg EP. 2002. Taenia tapeworms: their biology, evolution and socioeconomic significance. Microbes Infect 4:859–866. doi: 10.1016/S1286-4579(02)01606-4. [DOI] [PubMed] [Google Scholar]
- 39.Lescano AG, Garcia HH, Gilman RH, Guezala MC, Tsang VC, Gavidia CM, Rodriguez S, Moulton LH, Green JA, Gonzalez AE. 2007. Swine cysticercosis hotspots surrounding Taenia solium tapeworm carriers. Am J Trop Med Hyg 76:376–383. doi: 10.4269/ajtmh.2007.76.376. [DOI] [PubMed] [Google Scholar]
- 40.Tsai IJ, Zarowiecki M, Holroyd N, Garciarrubio A, Sánchez-Flores A, Brooks KL, Tracey A, Bobes RJ, Fragoso G, Sciutto E, Aslett M, Beasley H, Bennett HM, Cai X, Camicia F, Clark R, Cucher M, De Silva N, Day TA, Deplazes P, Estrada K, Fernández C, Holland PWH, Hou J, Hu S, Huckvale T, Hung SS, Kamenetzky L, Keane JA, Kiss F, Koziol U, Lambert O, Liu K, Luo X, Luo Y, Macchiaroli N, Nichol S, Paps J, Parkinson J, Pouchkina-Stantcheva N, Riddiford N, Rosenzvit M, Salinas G, Wasmuth JD, Zamanian M, Zheng Y, Taenia solium Genome Consortium, Cai J, Soberón X, Olson PD, Laclette JP, Brehm K, Berriman M. 2013. The genomes of four tapeworm species reveal adaptations to parasitism. Nature 496:57–63. doi: 10.1038/nature12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Wang S, Luo Y, Xiao L, Luo X, Gao S, Dou Y, Zhang H, Guo A, Meng Q, Hou J, Zhang B, Zhang S, Yang M, Meng X, Mei H, Li H, He Z, Zhu X, Tan X, Zhu X-Q, Yu J, Cai J, Zhu G, Hu S, Cai X. 2016. Comparative genomics reveals adaptive evolution of Asian tapeworm in switching to a new intermediate host. Nat Commun 7:12845. doi: 10.1038/ncomms12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pajuelo MJ, Eguiluz M, Dahlstrom E, Requena D, Guzman F, Ramirez M, Sheen P, Frace M, Sammons S, Cama V, Anzick S, Bruno D, Mahanty S, Wilkins P, Nash T, Gonzalez A, Garcia HH, Gilman RH, Porcella S, Zimic M, Cysticercosis Working Group in Peru. 2015. Identification and characterization of microsatellite markers derived from the whole genome analysis of Taenia solium. PLoS Negl Trop Dis 9:e0004316. doi: 10.1371/journal.pntd.0004316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adalid-Peralta L, Rosas G, Arce-Sillas A, Bobes RJ, Cardenas G, Hernandez M, Trejo C, Meneses G, Hernandez B, Estrada K, Fleury A, Laclette JP, Larralde C, Sciutto E, Fragoso G. 2017. Effect of transforming growth factor-beta upon Taenia solium and Taenia crassiceps cysticerci. Sci Rep 7:12345. doi: 10.1038/s41598-017-12202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aguilar-Diaz H, Nava-Castro KE, Escobedo G, Dominguez-Ramirez L, Garcia-Varela M, Del Rio-Araiza VH, Palacios-Arreola MI, Morales-Montor J. 2018. A novel progesterone receptor membrane component (PGRMC) in the human and swine parasite Taenia solium: implications to the host-parasite relationship. Parasit Vectors 11:161. doi: 10.1186/s13071-018-2703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leon-Janampa N, Liendo R, Gilman RH, Padilla C, Garcia HH, Gonzales A, Sheen P, Pajuelo MJ, Zimic M, Cysticercosis Working Group in Peru. 2019. Characterization of a novel cathepsin L-like protease from Taenia solium metacestodes for the immunodiagnosis of porcine cysticercosis. Vet Parasitol 267:9–16. doi: 10.1016/j.vetpar.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponce R, Leon-Janampa N, Gilman RH, Liendo R, Roncal E, Luis S, Quinones-Garcia S, Silverstein Z, Garcia HH, Gonzales A, Sheen P, Zimic M, Pajuelo MJ, Cysticercosis Working Group in Peru. 2018. A novel enolase from Taenia solium metacestodes and its evaluation as an immunodiagnostic antigen for porcine cysticercosis. Exp Parasitol 191:44–54. doi: 10.1016/j.exppara.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Almeida CR, Stoco PH, Wagner G, Sincero TC, Rotava G, Bayer-Santos E, Rodrigues JB, Sperandio MM, Maia AA, Ojopi EP, Zaha A, Ferreira HB, Tyler KM, Davila AM, Grisard EC, Dias-Neto E. 2009. Transcriptome analysis of Taenia solium cysticerci using Open Reading Frame ESTs (ORESTES). Parasit Vectors 2:35. doi: 10.1186/1756-3305-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lundstrom J, Salazar-Anton F, Sherwood E, Andersson B, Lindh J. 2010. Analyses of an expressed sequence tag library from Taenia solium, Cysticerca. PLoS Negl Trop Dis 4:e919. doi: 10.1371/journal.pntd.0000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang D, Fu Y, Wu X, Xie Y, Nie H, Chen L, Nong X, Gu X, Wang S, Peng X, Yan N, Zhang R, Zheng W, Yang G. 2012. Annotation of the transcriptome from Taenia pisiformis and its comparative analysis with three Taeniidae species. PLoS One 7:e32283. doi: 10.1371/journal.pone.0032283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santivanez SJ, Hernandez-Gonzalez A, Chile N, Oleaga A, Arana Y, Palma S, Verastegui M, Gonzalez AE, Gilman R, Garcia HH, Siles-Lucas MM, Cysticercosis Working Group in Peru. 2010. Proteomic study of activated Taenia solium oncospheres. Mol Biochem Parasitol 171:32–39. doi: 10.1016/j.molbiopara.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navarrete-Perea J, Moguel B, Mendoza-Hernandez G, Fragoso G, Sciutto E, Bobes RJ, Laclette JP. 2014. Identification and quantification of host proteins in the vesicular fluid of porcine Taenia solium cysticerci. Exp Parasitol 143:11–17. doi: 10.1016/j.exppara.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 52.da Costa GCV, Peralta RHS, Kalume DE, Alves A, Peralta JM. 2018. A gel-free proteomic analysis of Taenia solium and Taenia crassiceps cysticerci vesicular extracts. Parasitol Res 117:3781–3790. doi: 10.1007/s00436-018-6080-7. [DOI] [PubMed] [Google Scholar]
- 53.Gomez S, Adalid-Peralta L, Palafox-Fonseca H, Cantu-Robles VA, Soberón X, Sciutto E, Fragoso G, Bobes RJ, Laclette JP, Yauner L. d P, Ochoa-Leyva A. 2015. Genome analysis of excretory/secretory proteins in Taenia solium reveals their abundance of antigenic regions (AAR). Sci Rep 5:9683. doi: 10.1038/srep09683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Navarrete-Perea J, Isasa M, Paulo JA, Corral-Corral R, Flores-Bautista J, Hernández-Téllez B, Bobes RJ, Fragoso G, Sciutto E, Soberón X, Gygi SP, Laclette JP. 2017. Quantitative multiplexed proteomics of Taenia solium cysts obtained from the skeletal muscle and central nervous system of pigs. PLoS Negl Trop Dis 11:e0005962. doi: 10.1371/journal.pntd.0005962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Navarrete-Perea J, Moguel B, Bobes RJ, Villalobos N, Carrero JC, Sciutto E, Soberón X, Laclette JP. 2017. Protein profiles of Taenia solium cysts obtained from skeletal muscles and the central nervous system of pigs: Search for tissue-specific proteins. Exp Parasitol 172:23–29. doi: 10.1016/j.exppara.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Ale A, Victor B, Praet N, Gabriel S, Speybroeck N, Dorny P, Devleesschauwer B. 2014. Epidemiology and genetic diversity of Taenia asiatica: a systematic review. Parasit Vectors 7:45. doi: 10.1186/1756-3305-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taniyama D, Inoue I, Kawano M, Arakawa C, Adachi T, Morishima Y, Yamasaki H, Sugiyama H. 2019. Possible reintroduction of Taenia asiatica in the Kanto region of Japan. Jpn J Infect Dis 72:62–63. doi: 10.7883/yoken.JJID.2018.160. [DOI] [PubMed] [Google Scholar]
- 58.Mayta H, Talley A, Gilman RH, Jimenez J, Verastegui M, Ruiz M, Garcia HH, Gonzalez AE. 2000. Differentiating Taenia solium and Taenia saginata infections by simple hematoxylin-eosin staining and PCR-restriction enzyme analysis. J Clin Microbiol 38:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamasaki H, Nakao M, Sako Y, Nakaya K, Sato MO, Mamuti W, Okamoto M, Ito A. 2002. DNA differential diagnosis of human taeniid cestodes by base excision sequence scanning thymine-base reader analysis with mitochondrial genes. J Clin Microbiol 40:3818–3821. doi: 10.1128/jcm.40.10.3818-3821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verastegui M, Gilman RH, Garcia HH, Gonzalez AE, Arana Y, Jeri C, Tuero I, Gavidia CM, Levine M., Tsang VC, Cysticercosis Working Group in Peru. 2003. Prevalence of antibodies to unique Taenia solium oncosphere antigens in taeniasis and human and porcine cysticercosis. Am J Trop Med Hyg 69:438–444. doi: 10.4269/ajtmh.2003.69.438. [DOI] [PubMed] [Google Scholar]
- 61.Wilkins PP, Allan JC, Verastegui M, Acosta M, Eason AG, Garcia HH, Gonzalez AE, Gilman RH, Tsang VC. 1999. Development of a serologic assay to detect Taenia solium taeniasis. Am J Trop Med Hyg 60:199–204. doi: 10.4269/ajtmh.1999.60.199. [DOI] [PubMed] [Google Scholar]
- 62.Mayta H, Gilman RH, Prendergast E, Castillo JP, Tinoco YO, Garcia HH, Gonzalez AE, Sterling CR, Cysticercosis Working Group in Peru. 2008. Nested PCR for specific diagnosis of Taenia solium taeniasis. J Clin Microbiol 46:286–289. doi: 10.1128/JCM.01172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamasaki H, Allan JC, Sato MO, Nakao M, Sako Y, Nakaya K, Qiu D, Mamuti W, Craig PS, Ito A. 2004. DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR. J Clin Microbiol 42:548–553. doi: 10.1128/jcm.42.2.548-553.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allan JC, Avila G, Garcia Noval J, Flisser A, Craig PS. 1990. Immunodiagnosis of taeniasis by coproantigen detection. Parasitology 101:473–477. doi: 10.1017/s0031182000060686. [DOI] [PubMed] [Google Scholar]
- 65.Allan JC, Velasquez-Tohom M, Torres-Alvarez R, Yurrita P, Garcia-Noval J. 1996. Field trial of the coproantigen-based diagnosis of Taenia solium taeniasis by enzyme-linked immunosorbent assay. Am J Trop Med Hyg 54:352–356. doi: 10.4269/ajtmh.1996.54.352. [DOI] [PubMed] [Google Scholar]
- 66.Bustos JA, Rodriguez S, Jimenez JA, Moyano LM, Castillo Y, Ayvar V, Allan JC, Craig PS, Gonzalez AE, Gilman RH, Tsang VC, Garcia HH. 2012. Detection of Taenia solium taeniasis coproantigen is an early indicator of treatment failure for taeniasis. Clin Vaccine Immunol 19:570–573. doi: 10.1128/CVI.05428-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allan JC, Mencos F, Garcia-Noval J, Sarti E, Flisser A, Wang Y, Liu D, Craig PS. 1993. Dipstick dot ELISA for the detection of Taenia coproantigens in humans. Parasitology 107:79–85. doi: 10.1017/s0031182000079439. [DOI] [PubMed] [Google Scholar]
- 68.Garcia HH, Gonzalez AE, Gilman RH, Moulton LH, Verastegui M, Rodriguez S, Gavidia C, Tsang VC. 2006. Combined human and porcine mass chemotherapy for the control of T. solium. Am J Trop Med Hyg 74:850–855. doi: 10.4269/ajtmh.2006.74.850. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez-Canul R, Fraser A, Allan JC, Dominguez-Alpizar JL, Argaez-Rodriguez F, Craig PS. 1999. Epidemiological study of Taenia solium taeniasis/cysticercosis in a rural village in Yucatan state, Mexico. Ann Trop Med Parasitol 93:57–67. doi: 10.1080/00034989958807. [DOI] [PubMed] [Google Scholar]
- 70.Pawlowski ZS. 2006. Role of chemotherapy of taeniasis in prevention of neurocysticercosis. Parasitol Int 55(Suppl):S105–S109. doi: 10.1016/j.parint.2005.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia HH, Gonzalez AE, Tsang VC, O'Neal SE, Llanos-Zavalaga F, Gonzalvez G, Romero J, Rodriguez S, Moyano LM, Ayvar V, Diaz A, Hightower A, Craig PS, Lightowlers MW, Gauci CG, Leontsini E, Gilman RH, Cysticercosis Working Group in Peru. 2016. Elimination of Taenia solium transmission in northern Peru. N Engl J Med 374:2335–2344. doi: 10.1056/NEJMoa1515520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pearson RD, Guerrant RL. 1983. Praziquantel: a major advance in anthelminthic therapy. Ann Intern Med 99:195–198. doi: 10.7326/0003-4819-99-2-195. [DOI] [PubMed] [Google Scholar]
- 73.Flisser A, Madrazo I, Plancarte A, Schantz P, Allan J, Craig P, Sarti E. 1993. Neurological symptoms in occult neurocysticercosis after single taeniacidal dose of praziquantel. Lancet 342:748. doi: 10.1016/0140-6736(93)91743-6. [DOI] [PubMed] [Google Scholar]
- 74.Johnson RB. 1986. Potential hazard of mass praziquantel use. Am J Med 80:A88. doi: 10.1016/0002-9343(86)90953-8. [DOI] [PubMed] [Google Scholar]
- 75.Torres JR. 1989. Use of praziquantel in populations at risk of neurocysticercosis. Rev Inst Med Trop Sao Paulo 31:290. doi: 10.1590/s0036-46651989000400014. [DOI] [PubMed] [Google Scholar]
- 76.Chavarria AP, Villarejos VM, Zeledon R. 1977. Mebendazole in the treatment of taeniasis solium and taeniasis saginata. Am J Trop Med Hyg 26:118–120. doi: 10.4269/ajtmh.1977.26.118. [DOI] [PubMed] [Google Scholar]
- 77.Steinmann P, Utzinger J, Du ZW, Jiang JY, Chen JX, Hattendorf J, Zhou H, Zhou XN. 2011. Efficacy of single-dose and triple-dose albendazole and mebendazole against soil-transmitted helminths and Taenia spp.: a randomized controlled trial. PLoS One 6:e25003. doi: 10.1371/journal.pone.0025003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ash A, Okello A, Khamlome B, Inthavong P, Allen J, Thompson RCA. 2017. Controlling Taenia solium and soil transmitted helminths in a northern Lao PDR village: impact of a triple dose albendazole regime. Acta Trop 174:171–178. doi: 10.1016/j.actatropica.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 79.de Kaminsky RG. 1991. Albendazole treatment in human taeniasis. Trans R Soc Trop Med Hyg 85:648–650. doi: 10.1016/0035-9203(91)90378-C. [DOI] [PubMed] [Google Scholar]
- 80.Okello AL, Thomas L, Inthavong P, Ash A, Khamlome B, Keokamphet C, Newberry K, Gauci CG, Gabriel S, Dorny P, Thompson RA, Lightowlers MW, Allen J. 2016. Assessing the impact of a joint human-porcine intervention package for Taenia solium control: results of a pilot study from northern Lao PDR. Acta Trop 159:185–191. doi: 10.1016/j.actatropica.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 81.Steinmann P, Zhou XN, Du ZW, Jiang JY, Xiao SH, Wu ZX, Zhou H, Utzinger J. 2008. Tribendimidine and albendazole for treating soil-transmitted helminths, Strongyloides stercoralis and Taenia spp.: open-label randomized trial. PLoS Negl Trop Dis 2:e322. doi: 10.1371/journal.pntd.0000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sciutto E, Martínez JJ, Villalobos NM, Hernández M, José MV, Beltrán C, Rodarte F, Flores I, Bobadilla JR, Fragoso G, Parkhouse ME, Harrison LJS, de Aluja AS. 1998. Limitations of current diagnostic procedures for the diagnosis of Taenia solium cysticercosis in rural pigs. Vet Parasitol 79:299–313. doi: 10.1016/S0304-4017(98)00180-0. [DOI] [PubMed] [Google Scholar]
- 83.Gemmell M, Matyas Z, Pawlowski Z, Soulsby EJL, Larralde C, Nelson GS, Rosicky B. 1983. Guidelines for surveillance, prevention and control of taeniasis/cysticercosis. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 84.Sithole MI, Bekker JL, Tsotetsi-Khambule AM, Mukaratirwa S. 2019. Ineffectiveness of meat inspection in the detection of Taenia solium cysticerci in pigs slaughtered at two abattoirs in the Eastern Cape Province of South Africa. Vet Parasitol Reg Stud Rep 17:100299. doi: 10.1016/j.vprsr.2019.100299. [DOI] [PubMed] [Google Scholar]
- 85.Gonzalez AE, Cama V, Gilman RH, Tsang VC, Pilcher JB, Chavera A, Castro M, Montenegro T, Verastegui M, Miranda E, et al. . 1990. Prevalence and comparison of serologic assays, necropsy, and tongue examination for the diagnosis of porcine cysticercosis in Peru. Am J Trop Med Hyg 43:194–199. doi: 10.4269/ajtmh.1990.43.194. [DOI] [PubMed] [Google Scholar]
- 86.Chembensofu M, Mwape KE, Van Damme I, Hobbs E, Phiri IK, Masuku M, Zulu G, Colston A, Willingham AL, Devleesschauwer B, Van Hul A, Chota A, Speybroeck N, Berkvens D, Dorny P, Gabriël S. 2017. Re-visiting the detection of porcine cysticercosis based on full carcass dissections of naturally Taenia solium infected pigs. Parasit Vectors 10:572. doi: 10.1186/s13071-017-2520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trevisan C, Mkupasi EM, Ngowi HA, Forkman B, Johansen MV. 2016. Severe seizures in pigs naturally infected with Taenia solium in Tanzania. Vet Parasitol 220:67–71. doi: 10.1016/j.vetpar.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Assana E, Awah-Ndukum J, Djonmaila JD, Djiatche HD, Awe C, Manchang TK, Zoli AP. 2019. A comparison of Taenia solium and Taenia hydatigena infection in pigs using serological diagnosis and post-mortem inspection methods in Benoue division, North Cameroon. Vet Parasitol Reg Stud Rep 17:100306. doi: 10.1016/j.vprsr.2019.100306. [DOI] [PubMed] [Google Scholar]
- 89.Bustos JA, Ninaquispe BE, Rodriguez S, Castillo Y, Yang SY, Gilman RH, Dorny P, Gabriel S, Garcia HH, Gonzalez AE, Cysticercosis Working Group in Peru. 2019. Performance of a sandwich antigen-detection ELISA for the diagnosis of porcine Taenia solium cysticercosis. Am J Trop Med Hyg 100:604–608. doi: 10.4269/ajtmh.18-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Satyaprakash K, Khan WA, Chaudhari SP, Shinde SV, Kurkure NV, Kolte SW. 2018. Pathological and molecular identification of porcine cysticercosis in Maharashtra, India. Acta Parasitol 63:784–790. doi: 10.1515/ap-2018-0094. [DOI] [PubMed] [Google Scholar]
- 91.Gonzalez AE, Bustos JA, Jimenez JA, Rodriguez ML, Ramirez MG, Gilman RH, Garcia HH, Cysticercosis Working Group in Peru. 2012. Efficacy of diverse antiparasitic treatments for cysticercosis in the pig model. Am J Trop Med Hyg 87:292–296. doi: 10.4269/ajtmh.2012.11-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flisser A, Gonzalez D, Shkurovich M, Madrazo I, Correa D, Rodriguez-Carbajal J, Cohen S, Rodriguez-del-Rosal E, Collado M, Fernandez B. 1990. Praziquantel treatment of porcine brain and muscle Taenia solium cysticercosis. 1. Radiological, physiological and histopathological studies. Parasitol Res 76:263–269. doi: 10.1007/BF00930823. [DOI] [PubMed] [Google Scholar]
- 93.Torres A, Plancarte A, Villalobos AN, de Aluja AS, Navarro R, Flisser A. 1992. Praziquantel treatment of porcine brain and muscle Taenia solium cysticercosis. 3. Effect of 1-day treatment. Parasitol Res 78:161–164. doi: 10.1007/bf00931659. [DOI] [PubMed] [Google Scholar]
- 94.Gonzalez AE, Garcia HH, Gilman RH, Lopez MT, Gavidia C, McDonald J, Pilcher JB, Tsang VC. 1995. Treatment of porcine cysticercosis with albendazole. Am J Trop Med Hyg 53:571–574. doi: 10.4269/ajtmh.1995.53.571. [DOI] [PubMed] [Google Scholar]
- 95.Gonzales AE, Garcia HH, Gilman RH, Gavidia CM, Tsang VC, Bernal T, Falcon N, Romero M, Lopez-Urbina MT. 1996. Effective, single-dose treatment or porcine cysticercosis with oxfendazole. Am J Trop Med Hyg 54:391–394. doi: 10.4269/ajtmh.1996.54.391. [DOI] [PubMed] [Google Scholar]
- 96.Gonzalez AE, Falcon N, Gavidia C, Garcia HH, Tsang VC, Bernal T, Romero M, Gilman RH. 1998. Time-response curve of oxfendazole in the treatment of swine cysticercosis. Am J Trop Med Hyg 59:832–836. doi: 10.4269/ajtmh.1998.59.832. [DOI] [PubMed] [Google Scholar]
- 97.Gonzalez AE, Falcon N, Gavidia C, Garcia HH, Tsang VC, Bernal T, Romero M, Gilman RH. 1997. Treatment of porcine cysticercosis with oxfendazole: a dose-response trial. Vet Rec 141:420–422. doi: 10.1136/vr.141.16.420. [DOI] [PubMed] [Google Scholar]
- 98.Sikasunge CS, Johansen MV, Willingham AL 3rd, Leifsson PS, Phiri IK. 2008. Taenia solium porcine cysticercosis: viability of cysticerci and persistency of antibodies and cysticercal antigens after treatment with oxfendazole. Vet Parasitol 158:57–66. doi: 10.1016/j.vetpar.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 99.Del Brutto OH, Arroyo G, Del Brutto VJ, Zambrano M, García HH. 2017. On the relationship between calcified neurocysticercosis and epilepsy in an endemic village: a large-scale, computed tomography-based population study in rural Ecuador. Epilepsia 58:1955–1961. doi: 10.1111/epi.13892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fleury A, Gomez T, Alvarez I, Meza D, Huerta M, Chavarria A, Carrillo Mezo RA, Lloyd C, Dessein A, Preux PM, Dumas M, Larralde C, Sciutto E, Fragoso G. 2003. High prevalence of calcified silent neurocysticercosis in a rural village of Mexico. Neuroepidemiology 22:139–145. doi: 10.1159/000068748. [DOI] [PubMed] [Google Scholar]
- 101.Moyano LM, O'Neal SE, Ayvar V, Gonzalvez G, Gamboa R, Vilchez P, Rodriguez S, Reistetter J, Tsang VC, Gilman RH, Gonzalez AE, Garcia HH, Cysticercosis Working Group in Peru. 2016. High prevalence of asymptomatic neurocysticercosis in an endemic rural community in Peru. PLoS Negl Trop Dis 10:e0005130. doi: 10.1371/journal.pntd.0005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prasad KN, Verma A, Srivastava S, Gupta RK, Pandey CM, Paliwal VK. 2011. An epidemiological study of asymptomatic neurocysticercosis in a pig farming community in northern India. Trans R Soc Trop Med Hyg 105:531–536. doi: 10.1016/j.trstmh.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 103.Chandy MJ, Rajshekhar V, Prakash S, Ghosh S, Joseph T, Abraham J, Chandi SM. 1989. Cysticercosis causing single, small CT lesions in Indian patients with seizures. Lancet 333:390–391. doi: 10.1016/S0140-6736(89)91771-6. [DOI] [PubMed] [Google Scholar]
- 104.Del Brutto OH, Nash TE, Garcia HH. 2012. Cysticerci-related single parenchymal brain enhancing lesions in non-endemic countries. J Neurol Sci 319:32–36. doi: 10.1016/j.jns.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gonzalez AE, Gauci CG, Barber D, Gilman RH, Tsang VC, Garcia HH, Verastegui M, Lightowlers MW. 2005. Vaccination of pigs to control human neurocysticercosis. Am J Trop Med Hyg 72:837–839. doi: 10.4269/ajtmh.2005.72.837. [DOI] [PubMed] [Google Scholar]
- 106.Brailsford JF. 1941. Cysticercus cellulosae, its radiographic detection in the musculature and the central nervous system. Br J Radiol 14:79–93. doi: 10.1259/0007-1285-14-159-79. [DOI] [Google Scholar]
- 107.Morrison WK. 1934. Cysticercosis in twin brothers aged 13 years. Br Med J 1:13–14. doi: 10.1136/bmj.1.3809.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bustos JA, Garcia HH, Dorregaray R, Naranjo M, Pretell EJ, Gonzalez AE, Gilman RH, Cysticercosis Working Group in Peru. 2005. Detection of muscle calcifications by thigh CT scan in neurocysticercosis patients. Trans R Soc Trop Med Hyg 99:775–779. doi: 10.1016/j.trstmh.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 109.Fleury A, Cardenas G, Adalid-Peralta L, Fragoso G, Sciutto E. 2016. Immunopathology in Taenia solium neurocysticercosis. Parasite Immunol 38:147–157. doi: 10.1111/pim.12299. [DOI] [PubMed] [Google Scholar]
- 110.Garcia HH, Rodriguez S, Friedland JS, Cysticercosis Working Group in Peru. 2014. Immunology of Taenia solium taeniasis and human cysticercosis. Parasite Immunol 36:388–396. doi: 10.1111/pim.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gonzales I, Rivera JT, Garcia HH, Cysticercosis Working Group in Peru. 2016. Pathogenesis of Taenia solium taeniasis and cysticercosis. Parasite Immunol 38:136–146. doi: 10.1111/pim.12307. [DOI] [PubMed] [Google Scholar]
- 112.Khumbatta M, Firozgary B, Tweardy DJ, Weinstock J, Firozgary G, Bhatena Z, Bulsara T, Siller R, Robinson P. 2014. 2014. Somatostatin negatively regulates parasite burden and granulomatous responses in cysticercosis. Biomed Res Int 2014:247182. doi: 10.1155/2014/247182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leid RW, Grant RF, Suquet CM. 1987. Inhibition of equine neutrophil chemotaxis and chemokinesis by a Taenia taeniaeformis proteinase inhibitor, taeniaestatin. Parasite Immunol 9:195–204. doi: 10.1111/j.1365-3024.1987.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 114.Terrazas LI, Bojalil R, Rodriguez-Sosa M, Govezensky T, Larralde C. 1999. Taenia crassiceps cysticercosis: a role for prostaglandin E2 in susceptibility. Parasitol Res 85:1025–1031. doi: 10.1007/s004360050676. [DOI] [PubMed] [Google Scholar]
- 115.Arce-Sillas A, Álvarez-Luquín DD, Cárdenas G, Casanova-Hernández D, Fragoso G, Hernández M, Proaño Narváez JV, García-Vázquez F, Fleury A, Sciutto E, Adalid-Peralta L. 2016. Interleukin 10 and dendritic cells are the main suppression mediators of regulatory T cells in human neurocysticercosis. Clin Exp Immunol 183:271–279. doi: 10.1111/cei.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meeusen E, Barcham GJ, Gorrell MD, Rickard MD, Brandon MR. 1990. Cysticercosis: cellular immune responses during primary and secondary infection. Parasite Immunol 12:403–418. doi: 10.1111/j.1365-3024.1990.tb00977.x. [DOI] [PubMed] [Google Scholar]
- 117.Tokushige SI, Nagashima Y, Maekawa R, Shiio Y. 2018. Sequential magnetic resonance imaging changes in neurocysticercosis. ANZ J Surg 88:512–514. doi: 10.1111/ans.13453. [DOI] [PubMed] [Google Scholar]
- 118.Nash TE, Mahanty S, Garcia HH, Cysticercosis Group in Peru. 2011. Corticosteroid use in neurocysticercosis. Expert Rev Neurother 11:1175–1183. doi: 10.1586/ern.11.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Escobar A, Weidenheim G. 2002. The pathology of neurocysticercosis, p 289–305. In Singh G, Prabhakar S (ed), Taenia solium cysticercosis: from basic to clinical science. CABI Publishing, Wallingford, United Kingdom. [Google Scholar]
- 120.Garcia HH, Pretell EJ, Gilman RH, Martinez SM, Moulton LH, Del Brutto OH, Herrera G, Evans CA, Gonzalez AE, Cysticercosis Working Group in Peru. 2004. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med 350:249–258. doi: 10.1056/NEJMoa031294. [DOI] [PubMed] [Google Scholar]
- 121.Cho TA. 2018. Helminthic infections of the central nervous system. Continuum (Minneapolis, Minn) 24:1489–1511. doi: 10.1212/CON.0000000000000646. [DOI] [PubMed] [Google Scholar]
- 122.Elliott EJ, Landaker EJ. 2017. Worsening migraine due to neurocysticercosis. Cleve Clin J Med 84:196–198. doi: 10.3949/ccjm.84a.16003. [DOI] [PubMed] [Google Scholar]
- 123.Fogang YF, Camara M, Diop AG, Ndiaye MM. 2014. Cerebral neurocysticercosis mimicking or comorbid with episodic migraine?. BMC Neurol 14:138. doi: 10.1186/1471-2377-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garcia HH, Del Brutto OH, Cysticercosis Working Group in Peru. 2017. Antiparasitic treatment of neurocysticercosis—the effect of cyst destruction in seizure evolution. Epilepsy Behav 76:158–162. doi: 10.1016/j.yebeh.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Garcia HH, Nash TE, Del Brutto OH. 2014. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol 13:1202–1215. doi: 10.1016/S1474-4422(14)70094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Millogo A, Kongnyu Njamnshi A, Kabwa-PierreLuabeya M. 2019. Neurocysticercosis and epilepsy in sub-Saharan Africa. Brain Res Bull 145:30–38. doi: 10.1016/j.brainresbull.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 127.Nash TE, Mahanty S, Loeb JA, Theodore WH, Friedman A, Sander JW, Singh G, Cavalheiro E, Del Brutto OH, Takayanagui OM, Fleury A, Verastegui M, Preux PM, Montano S, Pretell EJ, White AC Jr, Gonzales AE, Gilman RH, Garcia HH. 2015. Neurocysticercosis: a natural human model of epileptogenesis. Epilepsia 56:177–183. doi: 10.1111/epi.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saito EK, Mehta B, Wang F, Nakamoto B, McMurtray AM. 2017. Headaches more common among epilepsy sufferers with neurocysticercosis than other structural brain lesions. Hawaii J Med Public Health 76:152–155. [PMC free article] [PubMed] [Google Scholar]
- 129.Ghasemi R, Rowe A, Shah R, Venkatesan P, England TJ. 2016. Neurocysticercosis presenting as a 'Stroke Mimic'. Acute Med 15:79–83. [PubMed] [Google Scholar]
- 130.Nau AL, Mwape KE, Wiefek J, Schmidt K, Abatih E, Dorny P, Praet N, Chiluba C, Schmidt H, Phiri IK, Winkler AS, Gabriel S, Blocher J. 2018. Cognitive impairment and quality of life of people with epilepsy and neurocysticercosis in Zambia. Epilepsy Behav 80:354–359. doi: 10.1016/j.yebeh.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 131.Prasad R, Shambhavi OP, Mishra SK, Upadhyay TB, Singh UK. 2014. Cognitive and behaviour dysfunction of children with neurocysticercosis: a cross-sectional study. J Trop Pediatr 60:358–362. doi: 10.1093/tropej/fmu029. [DOI] [PubMed] [Google Scholar]
- 132.Ramirez-Bermudez J, Higuera-Calleja J, Espinola-Nadurille M, Corona T. 2017. Neuropsychiatric disorders in patients with neurocysticercosis. Asia Pac Psychiatry 9:e12250. doi: 10.1111/appy.12250. [DOI] [PubMed] [Google Scholar]
- 133.Dumas JL, Visy JM, Belin C, Gaston A, Goldlust D, Dumas M. 1997. Parenchymal neurocysticercosis: follow up and staging by MRI. Neuroradiology 39:12–16. doi: 10.1007/s002340050358. [DOI] [PubMed] [Google Scholar]
- 134.Garcia HH, Gonzales I, Lescano AG, Bustos JA, Zimic M, Escalante D, Saavedra H, Gavidia M, Rodriguez L, Najar E, Umeres H, Pretell EJ, Cysticercosis Working Group in Peru. 2014. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double-blind, randomised controlled trial. Lancet Infect Dis 14:687–695. doi: 10.1016/S1473-3099(14)70779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Singhi P, Suthar R, Deo B, Malhi P, Khandelwal NK. 2017. Long-term clinical and radiologic outcome in 500 children with parenchymal neurocysticercosis. Pediatr Infect Dis J 36:549–555. doi: 10.1097/INF.0000000000001536. [DOI] [PubMed] [Google Scholar]
- 136.Ghosh RN, Vyas S, Singh P, Khandelwal N, Sankhyan N, Singhi P. 2019. Perfusion magnetic resonance imaging in differentiation of neurocysticercosis and tuberculoma. Neuroradiology 61:257–263. doi: 10.1007/s00234-018-2118-x. [DOI] [PubMed] [Google Scholar]
- 137.Gulati S, Jain P, Sachan D, Chakrabarty B, Kumar A, Pandey RM, Gupta AK. 2014. Seizure and radiological outcomes in children with solitary cysticercous granulomas with and without albendazole therapy: a retrospective case record analysis. Epilepsy Res 108:1212–1220. doi: 10.1016/j.eplepsyres.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 138.Kumar N, Garg RK, Malhotra HS, Gupta RK, Verma R, Sharma PK. 2016. Natural course of typical and atypical parenchymal solitary cysticercus granuloma of the brain: a 3-year prospective clinico-radiological study. Neuroradiol J 29:19–29. doi: 10.1177/1971400915620437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mahajan L, Malhotra HS, Garg RK, Kumar N, Sharma PK, Verma R, Rizvi I. 2016. Predictors of lesion calcification in patients with solitary cysticercus granuloma and new-onset seizures. Am J Trop Med Hyg 95:623–628. doi: 10.4269/ajtmh.16-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rajshekhar V. 1991. Etiology and management of single small CT lesions in patients with seizures: understanding a controversy. Acta Neurol Scand 84:465–470. doi: 10.1111/j.1600-0404.1991.tb04996.x. [DOI] [PubMed] [Google Scholar]
- 141.Rangel R, Torres B, Bruto OD, Sotelo J. 1987. Cysticercotic encephalitis: a severe form in young females. Am J Trop Med Hyg 36:387–392. doi: 10.4269/ajtmh.1987.36.387. [DOI] [PubMed] [Google Scholar]
- 142.Duque KR, Escalaya AL, Zapata W, Burneo JG, Bustos JA, Gonzales I, Saavedra H, Pretell EJ, Garcia HH, Cysticercosis Working Group in Peru. 2018. Clinical topography relationship in patients with parenchymal neurocysticercosis and seizures. Epilepsy Res 145:145–152. doi: 10.1016/j.eplepsyres.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Singh AK, Garg RK, Rizvi I, Malhotra HS, Kumar N, Gupta RK. 2017. Clinical and neuroimaging predictors of seizure recurrence in solitary calcified neurocysticercosis: a prospective observational study. Epilepsy Res 137:78–83. doi: 10.1016/j.eplepsyres.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 144.Carmen-Orozco RP, Dávila-Villacorta DG, Cauna Y, Bernal-Teran EG, Bitterfeld L, Sutherland GL, Chile N, Céliz RH, Ferrufino-Schmidt MC, Gavídia CM, Sterling CR, García HH, Gilman RH, Verástegui MR. 2019. Blood-brain barrier disruption and angiogenesis in a rat model for neurocysticercosis. J Neurosci Res 97:137–148. doi: 10.1002/jnr.24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mejia Maza A, Carmen-Orozco RP, Carter ES, Dávila-Villacorta DG, Castillo G, Morales JD, Mamani J, Gavídia CM, Alroy J, Sterling CR, Gonzalez AE, García HH, Woltjer RL, Verástegui MR, Gilman RH, Cysticercosis Working Group in Peru. 2019. Axonal swellings and spheroids: a new insight into the pathology of neurocysticercosis. Brain Pathol 29:425–436. doi: 10.1111/bpa.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rabiela-Cervantes MT, Rivas-Hernandez A, Rodriguez-Ibarra J, Castillo-Medina S, Cancino FM. 1982. Anatomopathological aspects of human brain cysticercosis, p 179–200. In Flisser A, Willms K, Laclette JP, Larralde C, Ridaura C, Beltran F (ed), Cysticercosis: present state of knowledge and perspectives. Academic Press, New York, NY. [Google Scholar]
- 147.Giordani MT, Tamarozzi F, Cattaneo F, Brunetti E. 2014. Three cases of imported neurocysticercosis in Northern Italy. J Travel Med 21:17–23. doi: 10.1111/jtm.12066. [DOI] [PubMed] [Google Scholar]
- 148.Wiegand F, Koeppen S, Haussermann P, Delcker A. 1999. Neurocysticercosis. Current review of the literature based on a long-term study of 2 clinically distinct German cases. Nervenarzt 70:298–305. doi: 10.1007/s001150050440. [DOI] [PubMed] [Google Scholar]
- 149.Del Brutto OH, Sotelo J. 1988. Neurocysticercosis: an update. Rev Infect Dis 10:1075–1087. doi: 10.1093/clinids/10.6.1075. [DOI] [PubMed] [Google Scholar]
- 150.Spina Franca A, de Rezende GL. 1982. Changes in cerebrospinal fluid induced by praziquantel. Salud Publica Mex 24:633–636. [PubMed] [Google Scholar]
- 151.Nash TE, Bartelt LA, Korpe PS, Lopes B, Houpt ER. 2014. Calcified neurocysticercus, perilesional edema, and histologic inflammation. Am J Trop Med Hyg 90:318–321. doi: 10.4269/ajtmh.13-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nash TE, Bustos JA, Garcia HH, Cysticercosis Working Group in Peru. 2017. Disease Centered around calcified Taenia solium granuloma. Trends Parasitol 33:65–73. doi: 10.1016/j.pt.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ruber T, David B, Luchters G, Nass RD, Friedman A, Surges R, Stocker T, Weber B, Deichmann R, Schlaug G, Hattingen E, Elger CE. 2018. Evidence for peri-ictal blood-brain barrier dysfunction in patients with epilepsy. Brain 141:2952–2965. doi: 10.1093/brain/awy242. [DOI] [PubMed] [Google Scholar]
- 154.Herrick JA, Maharathi B, Kim JS, Abundis GG, Garg A, Gonzales I, Saavedra H, Bustos JA, Garcia HH, Loeb JA, Cysticercosis Working Group in Peru. 2018. Inflammation is a key risk factor for persistent seizures in neurocysticercosis. Ann Clin Transl Neurol 5:630–639. doi: 10.1002/acn3.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.de Souza A, Nalini A, Kovoor JM, Yeshraj G, Siddalingaiah HS, Thennarasu K. 2011. Perilesional gliosis around solitary cerebral parenchymal cysticerci and long-term seizure outcome: a prospective study using serial magnetization transfer imaging. Epilepsia 52:1918–1927. doi: 10.1111/j.1528-1167.2011.03189.x. [DOI] [PubMed] [Google Scholar]
- 156.Pradhan S, Kathuria MK, Gupta RK. 2000. Perilesional gliosis and seizure outcome: a study based on magnetization transfer magnetic resonance imaging in patients with neurocysticercosis. Ann Neurol 48:181–187. doi:. [DOI] [PubMed] [Google Scholar]
- 157.Saini J, Gupta PK, Gupta P, Yadav R, Yadav N, Gupta RK. 2019. 3D-double-inversion recovery detects perilesional gliosis better than 3D-FLAIR and postcontrast T1 imaging in calcified neurocysticercosis. Neurol India 67:136–141. doi: 10.4103/0028-3886.253614. [DOI] [PubMed] [Google Scholar]
- 158.Bianchin MM, Velasco TR, Wichert-Ana L, Araujo D Jr, Alexandre V Jr, Scornavacca F, Escorsi-Rosset SR, dos Santos AC, Carlotti CG Jr, Takayanagui OM, Sakamoto AC. 2015. Neuroimaging observations linking neurocysticercosis and mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res 116:34–39. doi: 10.1016/j.eplepsyres.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 159.de Oliveira Taveira M, Morita ME, Yasuda CL, Coan AC, Secolin R, Luiz Cunha da Costa A, Cendes F. 2015. Neurocysticercotic calcifications and hippocampal sclerosis: a case-control study. PLoS One 10:e0131180. doi: 10.1371/journal.pone.0131180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lama J, Zambrano M, García HH, Del Brutto VJ, Campos X, Del Brutto OH, Salgado P. 2015. Calcified neurocysticercosis associates with hippocampal atrophy: a population-based study. Am J Trop Med Hyg 92:64–68. doi: 10.4269/ajtmh.14-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Leon A, Saito EK, Mehta B, McMurtray AM. 2015. Calcified parenchymal central nervous system cysticercosis and clinical outcomes in epilepsy. Epilepsy Behav 43:77–80. doi: 10.1016/j.yebeh.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 162.Meguins LC, Adry RA, Silva Junior SC, Pereira CU, Oliveira JG, Morais DF, Araujo Filho GM, Marques LH. 2015. Longer epilepsy duration and multiple lobe involvement predict worse seizure outcomes for patients with refractory temporal lobe epilepsy associated with neurocysticercosis. Arq Neuropsiquiatr 73:1014–1018. doi: 10.1590/0004-282X20150175. [DOI] [PubMed] [Google Scholar]
- 163.Oliveira MC, Martin MG, Tsunemi MH, Vieira G, Castro LH. 2014. Small calcified lesions suggestive of neurocysticercosis are associated with mesial temporal sclerosis. Arq Neuropsiquiatr 72:510–516. doi: 10.1590/0004-282x20140080. [DOI] [PubMed] [Google Scholar]
- 164.Singh G, Sander JW. 2018. Neurocysticercosis as a probable risk factor for hippocampal sclerosis. Arq Neuropsiquiatr 76:783–790. doi: 10.1590/0004-282X20180130. [DOI] [PubMed] [Google Scholar]
- 165.Ghosh S, Al-Khalili R, Liu JK, Slasky SE. 2014. Paradoxical migrating cyst: an unusual presentation of intraventricular neurocysticercosis with a coincidental pituitary adenoma. J Clin Neurosci 21:1066–1068. doi: 10.1016/j.jocn.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 166.Rana S, Prasad A, Brar R, Rathore DS, Dwivedi A. 2018. Caught in the act: migrating intraventricular neurocysticercosis causing intermittent unilateral hydrocephalus due to foramen of Monro obstruction. Acta Neurol Belg 118:509–511. doi: 10.1007/s13760-017-0831-6. [DOI] [PubMed] [Google Scholar]
- 167.Shah A, Vutha R, Sankhe S, Goel A. 2017. Transventricular migration of neurocysticercosis. World Neurosurg 105:1043.e11–1043.e13. doi: 10.1016/j.wneu.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 168.Del Brutto OH. 2012. Neurocysticercosis among international travelers to disease-endemic areas. J Travel Med 19:112–117. doi: 10.1111/j.1708-8305.2011.00592.x. [DOI] [PubMed] [Google Scholar]
- 169.Nash TE, O'Connell EM, Hammoud DA, Wetzler L, Ware JM, Mahanty S. 2020. Natural history of treated subarachnoid neurocysticercosis. Am J Trop Med Hyg 102:78–89. doi: 10.4269/ajtmh.19-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Serpa JA, Graviss EA, Kass JS, White AC Jr. 2011. Neurocysticercosis in Houston, Texas: an update. Medicine (Baltimore) 90:81–86. doi: 10.1097/MD.0b013e318206d13e. [DOI] [PubMed] [Google Scholar]
- 171.Mahale RR, Mehta A, Rangasetty S. 2015. Extraparenchymal (racemose) neurocysticercosis and its multitude manifestations: a comprehensive review. J Clin Neurol 11:203–211. doi: 10.3988/jcn.2015.11.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cantu C, Barinagarrementeria F. 1996. Cerebrovascular complications of neurocysticercosis. Clinical and neuroimaging spectrum. Arch Neurol 53:233–239. doi: 10.1001/archneur.1996.00550030039021. [DOI] [PubMed] [Google Scholar]
- 173.Rabiela-Cervantes MT, Rivas-Hernandez A, Castillo-Medina S, Gonzalez-Angulo A. 1985. Morphological evidence indicating that C. cellulosae and C. racemosus are larval stages of Taenia solium. Arch Invest Med (Mex) 16:81–92. [PubMed] [Google Scholar]
- 174.Goyal G, Phukan AC, Hussain M, Lal V, Modi M, Goyal MK, Sehgal R. 2020. Sorting out difficulties in immunological diagnosis of neurocysticercosis: development and assessment of real time loop mediated isothermal amplification of cysticercal DNA in blood. J Neurol Sci 408:116544. doi: 10.1016/j.jns.2019.116544. [DOI] [PubMed] [Google Scholar]
- 175.Michelet L, Fleury A, Sciutto E, Kendjo E, Fragoso G, Paris L, Bouteille B. 2011. Human neurocysticercosis: comparison of different diagnostic tests using cerebrospinal fluid. J Clin Microbiol 49:195–200. doi: 10.1128/JCM.01554-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.O'Connell EM, Harrison S, Dahlstrom E, Nash T, Nutman TB. 2019. A novel, highly sensitive quantitative polymerase chain reaction assay for the diagnosis of subarachnoid and ventricular neurocysticercosis and for assessing responses to treatment. Clin Infect Dis 70:1875–1881. doi: 10.1093/cid/ciz541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yera H, Dupont D, Houze S, Ben M'rad M, Pilleux F, Sulahian A, Gatey C, Andrieu FG, Dupouy-Camet J. 2011. Confirmation and follow-up of neurocysticercosis by real-time PCR in cerebrospinal fluid samples of patients living in France. J Clin Microbiol 49:4338–4340. doi: 10.1128/JCM.05839-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ware JM, Nash TE. 2016. The lack of association of eosinophilia and neurocysticercosis at clinical presentation: a retrospective analysis of cases seen at the National Institutes of Health, 1985–2015. Am J Trop Med Hyg 95:1432–1434. doi: 10.4269/ajtmh.16-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.García HH, Del Brutto OH. 2003. Imaging findings in neurocysticercosis. Acta Trop 87:71–78. doi: 10.1016/S0001-706X(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 180.Hernandez RD, Duran BB, Lujambio PS. 2014. Magnetic resonance imaging in neurocysticercosis. Top Magn Reson Imaging 23:191–198. doi: 10.1097/RMR.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 181.Lerner A, Shiroishi MS, Zee CS, Law M, Go JL. 2012. Imaging of neurocysticercosis. Neuroimaging Clin N Am 22:659–676. doi: 10.1016/j.nic.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 182.Mont'Alverne Filho FEF, Machado LDR, Lucato LT, Leite CC. 2011. The role of 3D volumetric MR sequences in diagnosing intraventricular neurocysticercosis: preliminar results. Arq Neuropsiquiatr 69:74–78. doi: 10.1590/s0004-282x2011000100015. [DOI] [PubMed] [Google Scholar]
- 183.Neyaz Z, Patwari SS, Paliwal VK. 2012. Role of FIESTA and SWAN sequences in diagnosis of intraventricular neurocysticercosis. Neurol India 60:646–647. doi: 10.4103/0028-3886.105205. [DOI] [PubMed] [Google Scholar]
- 184.White AC, Coyle CM, Rajshekhar V, Singh G, Hauser WA, Mohanty A, Garcia HH, Nash TE. 2018. Diagnosis and treatment of neurocysticercosis: 2017 Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis 66:1159–1116. doi: 10.1093/cid/ciy157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Venkat B, Aggarwal N, Makhaik S, Sood R. 2016. A comprehensive review of imaging findings in human cysticercosis. Jpn J Radiol 34:241–257. doi: 10.1007/s11604-016-0528-4. [DOI] [PubMed] [Google Scholar]
- 186.Costa R, Costa RB, Bacchi C, Sarinho F. 2014. Adenocarcinoma of the lung presenting with atypical cystic brain lesions. BMJ Case Rep 2014:bcr2013203506. doi: 10.1136/bcr-2013-203506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Del Brutto OH, Nash TE, White AC Jr, Rajshekhar V, Wilkins PP, Singh G, Vasquez CM, Salgado P, Gilman RH, Garcia HH. 2017. Revised diagnostic criteria for neurocysticercosis. J Neurol Sci 372:202–210. doi: 10.1016/j.jns.2016.11.045. [DOI] [PubMed] [Google Scholar]
- 188.Rodriguez S, Wilkins P, Dorny P. 2012. Immunological and molecular diagnosis of cysticercosis. Pathog Glob Health 106:286–298. doi: 10.1179/2047773212Y.0000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tsang VC, Brand JA, Boyer AE. 1989. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis 159:50–59. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 190.Arroyo G, Rodriguez S, Lescano AG, Alroy KA, Bustos JA, Santivanez S, Gonzales I, Saavedra H, Pretell EJ, Gonzalez AE, Gilman RH, Tsang VCW, Garcia HH, Cysticercosis Working Group in Peru. 2018. Antibody banding patterns of the enzyme-linked immunoelectrotransfer blot and brain imaging findings in patients with neurocysticercosis. Clin Infect Dis 66:282–288. doi: 10.1093/cid/cix774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Garcia HH, O'Neal SE, Noh J, Handali S, Gilman RH, Gonzalez AE, Tsang VCW, Rodriguez S, Martinez M, Gonzales I, Saavedra H, Verastegui M, Bustos JA, Zimic M, Mayta H, Castillo Y, Castro Y, Lopez MT, Gavidia CM, Moyano LM, Gamboa R, Muro C, Vilchez P, Nash TE, Mahanty S, Friedland J, Cysticercosis Working Group in Peru. 2018. Laboratory diagnosis of neurocysticercosis (Taenia solium). J Clin Microbiol 56:e00424-18. doi: 10.1128/JCM.00424-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Carod JF, Randrianarison M, Razafimahefa J, Ramahefarisoa RM, Rakotondrazaka M, Debruyne M, Dautigny M, Cazal P, Andriantseheno ML, Charles ER. 2012. Evaluation of the performance of 5 commercialized enzyme immunoassays for the detection of Taenia solium antibodies and for the diagnosis of neurocysticercosis. Diagn Microbiol Infect Dis 72:85–89. doi: 10.1016/j.diagmicrobio.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 193.Garcia HH, Castillo Y, Gonzales I, Bustos JA, Saavedra H, Jacob L, Del Brutto OH, Wilkins PP, Gonzalez AE, Gilman RH, Cysticercosis Working Group In Peru. 2018. Low sensitivity and frequent cross-reactions in commercially available antibody detection ELISA assays for Taenia solium cysticercosis. Trop Med Int Health 23:101–105. doi: 10.1111/tmi.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Noh J, Rodriguez S, Lee YM, Handali S, Gonzalez AE, Gilman RH, Tsang VC, Garcia HH, Wilkins PP. 2014. Recombinant protein- and synthetic peptide-based immunoblot test for diagnosis of neurocysticercosis. J Clin Microbiol 52:1429–1434. doi: 10.1128/JCM.03260-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Brandt JR, Geerts S, De Deken R, Kumar V, Ceulemans F, Brijs L, Falla N. 1992. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol 22:471–477. doi: 10.1016/0020-7519(92)90148-E. [DOI] [PubMed] [Google Scholar]
- 196.Harrison LJ, Joshua GW, Wright SH, Parkhouse RM. 1989. Specific detection of circulating surface/secreted glycoproteins of viable cysticerci in Taenia saginata cysticercosis. Parasite Immunol 11:351–370. doi: 10.1111/j.1365-3024.1989.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 197.Zea-Vera A, Cordova EG, Rodriguez S, Gonzales I, Pretell EJ, Castillo Y, Castro-Suarez S, Gabriël S, Tsang VCW, Dorny P, Garcia HH, Cysticercosis Working Group in Peru. 2013. Parasite antigen in serum predicts the presence of viable brain parasites in patients with apparently calcified cysticercosis only. Clin Infect Dis 57:e154–e159. doi: 10.1093/cid/cit422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bobes RJ, Hernandez M, Marquez C, Fragoso G, Garcia E, Parkhouse RM, Harrison LJ, Sciutto E, Fleury A. 2006. Subarachnoidal and intraventricular human neurocysticercosis: application of an antigen detection assay for the diagnosis and follow-up. Trop Med Int Health 11:943–950. doi: 10.1111/j.1365-3156.2006.01642.x. [DOI] [PubMed] [Google Scholar]
- 199.Garcia HH, Dorny P, Castillo Y, Pretell J, Rodriguez S, Mija L, Gonzalez AE, Gilman RH, Tsang VCW, Brandt J. 2010. Circulating antigen levels follow post-treatment evolution of subarachnoid neurocystercosis. J Neuroparasitol 1:1–3. doi: 10.4303/jnp/N100804. [DOI] [Google Scholar]
- 200.Liu P, Weng X, Zhou J, Xu X, He F, Du Y, Wu H, Gong Y, Peng G. 2018. Next generation sequencing based pathogen analysis in a patient with neurocysticercosis: a case report. BMC Infect Dis 18:113. doi: 10.1186/s12879-018-3015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wilson MR, Sample HA, Zorn KC, Arevalo S, Yu G, Neuhaus J, Federman S, Stryke D, Briggs B, Langelier C, Berger A, Douglas V, Josephson SA, Chow FC, Fulton BD, DeRisi JL, Gelfand JM, Naccache SN, Bender J, Dien Bard J, Murkey J, Carlson M, Vespa PM, Vijayan T, Allyn PR, Campeau S, Humphries RM, Klausner JD, Ganzon CD, Memar F, Ocampo NA, Zimmermann LL, Cohen SH, Polage CR, DeBiasi RL, Haller B, Dallas R, Maron G, Hayden R, Messacar K, Dominguez SR, Miller S, Chiu CY. 2019. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med 380:2327–2340. doi: 10.1056/NEJMoa1803396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Toribio L, Romano M, Scott AL, Gonzales I, Saavedra H, Garcia HH, Shiff C, Cysticercosis Working Group in Peru. 2019. Detection of Taenia solium DNA in the urine of neurocysticercosis patients. Am J Trop Med Hyg 100:327–329. doi: 10.4269/ajtmh.18-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hanas JS, Hocker JR, Ramajayam G, Prabhakaran V, Rajshekhar V, Oommen A, Manoj JJ, Anderson MP, Drevets DA, Carabin H. 2018. Distinguishing neurocysticercosis epilepsy from epilepsy of unknown etiology using a minimal serum mass profiling platform. Exp Parasitol 192:98–107. doi: 10.1016/j.exppara.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Prabhakaran V, Drevets DA, Ramajayam G, Manoj JJ, Anderson MP, Hanas JS, Rajshekhar V, Oommen A, Carabin H. 2017. Comparison of monocyte gene expression among patients with neurocysticercosis-associated epilepsy, idiopathic epilepsy and idiopathic headaches in India. PLoS Negl Trop Dis 11:e0005664. doi: 10.1371/journal.pntd.0005664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Del Brutto OH, Wadia NH, Dumas M, Cruz M, Tsang VCW, Schantz PM. 1996. Proposal of diagnostic criteria for human cysticercosis and neurocysticercosis. J Neurol Sci 142:1–6. doi: 10.1016/0022-510X(96)00130-X. [DOI] [PubMed] [Google Scholar]
- 206.Del Brutto OH, Rajshekhar V, White AC, Tsang VC, Nash TE, Takayanagui OM, Schantz PM, Evans CA, Flisser A, Correa D, Botero D, Allan JC, Sarti E, Gonzalez AE, Gilman RH, García HH. 2001. Proposed diagnostic criteria for neurocysticercosis. Neurology 57:177–183. doi: 10.1212/wnl.57.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bustos JA, Garcia HH, Del Brutto OH. 2016. Antiepileptic drug therapy and recommendations for withdrawal in patients with seizures and epilepsy due to neurocysticercosis. Expert Rev Neurother 16:1079–1085. doi: 10.1080/14737175.2016.1194757. [DOI] [PubMed] [Google Scholar]
- 208.Singh AK, Singh SK, Singh A, Gupta KK, Khatoon J, Prasad A, Rai RP, Gupta RK, Tripathi M, Husain N, Prasad KN. 2015. Immune response to Taenia solium cysticerci after anti-parasitic therapy. Int J Parasitol 45:749–759. doi: 10.1016/j.ijpara.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 209.Romo ML, Wyka K, Carpio A, Leslie D, Andrews H, Bagiella E, Hauser WA, Kelvin EA, Ecuadorian Neurocysticercosis Group. 2015. The effect of albendazole treatment on seizure outcomes in patients with symptomatic neurocysticercosis. Trans R Soc Trop Med Hyg 109:738–746. doi: 10.1093/trstmh/trv078. [DOI] [PubMed] [Google Scholar]
- 210.Proaño JV, Madrazo I, Avelar F, López-Félix B, Díaz G, Grijalva I. 2001. Medical treatment for neurocysticercosis characterized by giant subarachnoid cysts. N Engl J Med 345:879–885. doi: 10.1056/NEJMoa010212. [DOI] [PubMed] [Google Scholar]
- 211.Del Brutto OH. 2019. A personal account regarding the origin and evolution of controversies in the management of neurocysticercosis. Am J Trop Med Hyg 100:780–782. doi: 10.4269/ajtmh.18-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Robles C, Chavarría Chavarría M. 1979. Report of a clinical case of cerebral cysticercosis treated medically with a new drug: praziquantel. Salud Publica Mex 21:603–618. [PubMed] [Google Scholar]
- 213.Botero D, Castaño S. 1982. Treatment of cysticercosis with praziquantel in Colombia. Am J Trop Med Hyg 31:811–821. doi: 10.4269/ajtmh.1982.31.811. [DOI] [PubMed] [Google Scholar]
- 214.Sotelo J, del Brutto OH, Penagos P, Escobedo F, Torres B, Rodriguez-Carbajal J, Rubio-Donnadieu F. 1990. Comparison of therapeutic regimen of anticysticercal drugs for parenchymal brain cysticercosis. J Neurol 237:69–72. doi: 10.1007/bf00314663. [DOI] [PubMed] [Google Scholar]
- 215.Sotelo J, Escobedo F, Rodriguez-Carbajal J, Torres B, Rubio-Donnadieu F. 1984. Therapy of parenchymal brain cysticercosis with praziquantel. N Engl J Med 310:1001–1007. doi: 10.1056/NEJM198404193101601. [DOI] [PubMed] [Google Scholar]
- 216.Garg RK. 1997. Drug treatment of neurocysticercosis. Natl Med J India 10:173–177. doi: 10.4103/0970-258X.243422. [DOI] [PubMed] [Google Scholar]
- 217.Mitchell WG, Crawford TO. 1988. Intraparenchymal cerebral cysticercosis in children: diagnosis and treatment. Pediatrics 82:76–82. [PubMed] [Google Scholar]
- 218.Moodley M, Moosa A. 1989. Treatment of neurocysticercosis: is praziquantel the new hope?. Lancet 333:262–263. doi: 10.1016/S0140-6736(89)91268-3. [DOI] [PubMed] [Google Scholar]
- 219.Carpio A, Kelvin EA, Bagiella E, Leslie D, Leon P, Andrews H, Hauser WA, Equadorian Neurocysticercosis Group. 2008. Effects of albendazole treatment on neurocysticercosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry 79:1050–1055. doi: 10.1136/jnnp.2008.144899. [DOI] [PubMed] [Google Scholar]
- 220.Carpio A, Santillan F, Leon P, Flores C, Hauser WA. 1995. Is the course of neurocysticercosis modified by treatment with antihelminthic agents? Arch Intern Med 155:1982–1988. doi: 10.1001/archinte.1995.00430180088010. [DOI] [PubMed] [Google Scholar]
- 221.Otte WM, Singla M, Sander JW, Singh G. 2013. Drug therapy for solitary cysticercus granuloma: a systematic review and meta-analysis. Neurology 80:152–162. doi: 10.1212/WNL.0b013e31827b90a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhao BC, Jiang HY, Ma WY, Jin DD, Li HM, Lu H, Nakajima H, Huang TY, Sun KY, Chen SL, Chen KB. 2016. Albendazole and corticosteroids for the treatment of solitary cysticercus granuloma: a network meta-analysis. PLoS Negl Trop Dis 10:e0004418. doi: 10.1371/journal.pntd.0004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Garcia HH, Lescano AG, Gonzales I, Bustos JA, Pretell EJ, Horton J, Saavedra H, Gonzalez AE, Gilman RH, Cysticercosis Working Group in Peru. 2016. Cysticidal efficacy of combined treatment with praziquantel and albendazole for parenchymal brain cysticercosis. Clin Infect Dis 62:1375–1379. doi: 10.1093/cid/ciw134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Göngora-Rivera F, Soto-Hernández JL, González Esquivel D, Cook HJ, Márquez-Caraveo C, Hernández Dávila R, Santos-Zambrano J. 2006. Albendazole trial at 15 or 30 mg/kg/day for subarachnoid and intraventricular cysticercosis. Neurology 66:436–438. doi: 10.1212/01.wnl.0000195887.63124.dc. [DOI] [PubMed] [Google Scholar]
- 225.Osorio R, Carrillo-Mezo R, Romo ML, Toledo A, Matus C, González-Hernández I, Jung H, Fleury A. 2019. Factors associated with cysticidal treatment response in extraparenchymal neurocysticercosis. J Clin Pharmacol 59:548–556. doi: 10.1002/jcph.1346. [DOI] [PubMed] [Google Scholar]
- 226.Arroyo G, Bustos JA, Lescano AG, Gonzales I, Saavedra H, Rodriguez S, Pretell EJ, Bonato PS, Lanchote VL, Takayanagui OM, Horton J, Gonzalez AE, Gilman RH, Garcia HH, Cysticercosis Working Group in Peru. 2019. Albendazole sulfoxide plasma levels and efficacy of antiparasitic treatment in patients with parenchymal neurocysticercosis. Clin Infect Dis 69:1996–2002. doi: 10.1093/cid/ciz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Garcia HH, Gonzales I, Lescano AG, Bustos JA, Pretell EJ, Saavedra H, Nash TE, Cysticercosis Working Group in Peru. 2014. Enhanced steroid dosing reduces seizures during antiparasitic treatment for cysticercosis and early after. Epilepsia 55:1452–1459. doi: 10.1111/epi.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Anand P, Mukerji SS, Thon J, Gunaratne S, Cho TA, Venna N. 2019. Steroid-sparing agents for the treatment of inflammation in complicated neurocysticercosis. Neurol Neuroimmunol Neuroinflamm 6:e606. doi: 10.1212/NXI.0000000000000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mitre E, Talaat KR, Sperling MR, Nash TE. 2007. Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clin Infect Dis 44:549–553. doi: 10.1086/511040. [DOI] [PubMed] [Google Scholar]
- 230.Nash TE, Ware JM, Coyle CM, Mahanty S. 2019. Etanercept to control inflammation in the treatment of complicated neurocysticercosis. Am J Trop Med Hyg 100:609–616. doi: 10.4269/ajtmh.18-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Psarros TG, Krumerman J, Coimbra C. 2003. Endoscopic management of supratentorial ventricular neurocysticercosis: case series and review of the literature. Minim Invasive Neurosurg 46:331–334. doi: 10.1055/s-2003-812470. [DOI] [PubMed] [Google Scholar]
- 232.Rajshekhar V. 2010. Surgical management of neurocysticercosis. Int J Surg 8:100–104. doi: 10.1016/j.ijsu.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 233.Alarcon TA, Del Brutto OH. 2012. Neurocysticercosis: declining incidence among patients admitted to a large public hospital in Guayaquil, Ecuador. Pathog Glob Health 106:310–311. doi: 10.1179/2047773212Y.0000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Flisser A, Correa D. 2010. Neurocysticercosis may no longer be a public health problem in Mexico. PLoS Negl Trop Dis 4:e831. doi: 10.1371/journal.pntd.0000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Robertson LJ, Joshi H, Utaaker KS, Kumar A, Chaudhary S, Goyal K, Sehgal R. 2017. Changes in the seroprevalence of cysticercosis in suspected patients in Chandigarh, India between 1998 and 2014: analysis of 17 years of data. Epidemiol Infect 145:1159–1167. doi: 10.1017/S0950268816003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Schantz PM, Cruz M, Sarti E, Pawlowski Z. 1993. Potential eradicability of taeniasis and cysticercosis. Bull Pan Am Health Organ 27:397–403. [PubMed] [Google Scholar]
- 237.Cruz M, Davis A, Dixon H, Pawlowski ZS, Proano J. 1989. Operational studies on the control of Taenia solium taeniasis/cysticercosis in Ecuador. Bull World Health Organ 67:401–407. [PMC free article] [PubMed] [Google Scholar]
- 238.Allan JC, Velasquez-Tohom M, Fletes C, Torres-Alvarez R, Lopez-Virula G, Yurrita P, Soto de Alfaro H, Rivera A, Garcia-Noval J. 1997. Mass chemotherapy for intestinal Taenia solium infection: effect on prevalence in humans and pigs. Trans R Soc Trop Med Hyg 91:595–598. doi: 10.1016/S0035-9203(97)90042-0. [DOI] [PubMed] [Google Scholar]
- 239.Diaz Camacho SP, Candil Ruiz A, Suate Peraza V, Zazueta Ramos ML, Medina MF, Lozano R, Willms K. 1991. Epidemiologic study and control of Taenia solium infections with praziquantel in a rural village of Mexico. Am J Trop Med Hyg 45:522–531. doi: 10.4269/ajtmh.1991.45.522. [DOI] [PubMed] [Google Scholar]
- 240.Keilbach NM, de Aluja AS, Sarti-Gutierrez E. 1989. A programme to control taeniasis-cysticercosis (T. solium): experiences in a Mexican village. Acta Leidensia 57:181–189. [PubMed] [Google Scholar]
- 241.Sarti E, Schantz PM, Avila G, Ambrosio J, Medina-Santillán R, Flisser A. 2000. Mass treatment against human taeniasis for the control of cysticercosis: a population-based intervention study. Trans R Soc Trop Med Hyg 94:85–89. doi: 10.1016/S0035-9203(00)90451-6. [DOI] [PubMed] [Google Scholar]
- 242.Braae UC, Magnussen P, Ndawi B, Harrison W, Lekule F, Johansen MV. 2017. Effect of repeated mass drug administration with praziquantel and track and treat of taeniosis cases on the prevalence of taeniosis in Taenia solium endemic rural communities of Tanzania. Acta Trop 165:246–251. doi: 10.1016/j.actatropica.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 243.Ngowi HA, Winkler AS, Braae UC, Mdegela RH, Mkupasi EM, Kabululu ML, Lekule FP, Johansen MV. 2019. Taenia solium taeniosis and cysticercosis literature in Tanzania provides research evidence justification for control: a systematic scoping review. PLoS One 14:e0217420. doi: 10.1371/journal.pone.0217420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Poudel I, Sah K, Subedi S, Kumar Singh D, Kushwaha P, Colston A, Gauci CG, Donadeu M, Lightowlers MW. 2019. Implementation of a practical and effective pilot intervention against transmission of Taenia solium by pigs in the Banke district of Nepal. PLoS Negl Trop Dis 13:e0006838. doi: 10.1371/journal.pntd.0006838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sarti E. 1997. Taeniasis and cysticercosis due to Taenia solium. Salud Publica Mex 39:225–231. doi: 10.1590/S0036-36341997000300009. [DOI] [PubMed] [Google Scholar]
- 246.Sarti E, Flisser A, Schantz PM, Gleizer M, Loya M, Plancarte A, Avila G, Allan J, Craig P, Bronfman M, Wijeyaratne P. 1997. Development and evaluation of a health education intervention against Taenia solium in a rural community in Mexico. Am J Trop Med Hyg 56:127–132. doi: 10.4269/ajtmh.1997.56.127. [DOI] [PubMed] [Google Scholar]
- 247.Kabululu ML, Ngowi HA, Kimera SI, Lekule FP, Kimbi EC, Johansen MV. 2018. Effectiveness of an integrated intervention in the control of endo- and ectoparasites of pigs kept by smallholder farmers in Mbeya rural and Mbozi districts, Tanzania. Vet Parasitol Reg Stud Rep 13:64–73. doi: 10.1016/j.vprsr.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 248.Braae UC, Magnussen P, Harrison W, Ndawi B, Lekule F, Johansen MV. 2016. Effect of National Schistosomiasis Control Programme on Taenia solium taeniosis and porcine cysticercosis in rural communities of Tanzania. Parasite Epidemiol Control 1:245–251. doi: 10.1016/j.parepi.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Carabin H, Millogo A, Ngowi HA, Bauer C, Dermauw V, Kone AC, Sahlu I, Salvator AL, Preux PM, Some T, Tarnagda Z, Gabriel S, Cisse R, Ouedraogo JB, Cowan LD, Boncoeur-Martel MP, Dorny P, Ganaba R. 2018. Effectiveness of a community-based educational programme in reducing the cumulative incidence and prevalence of human Taenia solium cysticercosis in Burkina Faso in 2011–14 (EFECAB): a cluster-randomised controlled trial. Lancet Glob Health 6:e411–e425. doi: 10.1016/S2214-109X(18)30027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Garcia HH, Gonzalez AE, O'Neal SE, Gilman RH. 2018. Notes and recommendations for the establishment of control programs for taeniasis and cysticercosis due to Taenia solium in PeruNotes and recommendations for the establishment of control programs for Taeniasis/cysticercosis in Peru. Rev Peru Med Exp Salud Publica 35:132–138. doi: 10.17843/rpmesp.2018.351.3606. [DOI] [PubMed] [Google Scholar]
- 251.Ngowi HA, Carabin H, Kassuku AA, Mlozi MR, Mlangwa JE, Willingham AL III. 2008. A health-education intervention trial to reduce porcine cysticercosis in Mbulu District, Tanzania. Prev Vet Med 85:52–67. doi: 10.1016/j.prevetmed.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 252.Braae UC, Devleesschauwer B, Gabriel S, Dorny P, Speybroeck N, Magnussen P, Torgerson P, Johansen MV. 2016. CystiSim—an agent-based model for Taenia solium transmission and control. PLoS Negl Trop Dis 10:e0005184. doi: 10.1371/journal.pntd.0005184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gonzalez AE, Lopez-Urbina T, Tsang B, Gavidia C, Garcia HH, Silva ME, Ramos DD, Manzanedo R, Sanchez-Hidalgo L, Gilman RH, Tsang VC, Cysticercosis Working Group in Peru. 2006. Transmission dynamics of Taenia solium and potential for pig-to-pig transmission. Parasitol Int 55(Suppl):S131–S135. doi: 10.1016/j.parint.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 254.Sanchez-Torres NY, Bobadilla JR, Laclette JP, Jose MV. 2019. How to eliminate taeniasis/cysticercosis: porcine vaccination and human chemotherapy (Part 2). Theor Biol Med Model 16:4. doi: 10.1186/s12976-019-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhou H, Wang Q, Zhou J, Li T, Medina A, Felt SA, Rozelle S, Openshaw JJ. 2019. Structural equation modeling (SEM) of cysticercosis in school-aged children in Tibetan rural farming areas of western China: implications for intervention planning. Int J Environ Res Public Health 16:E780. doi: 10.3390/ijerph16050780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chile N, Clark T, Arana Y, Ortega YR, Palma S, Mejia A, Angulo N, Kosek JC, Kosek M, Gomez-Puerta LA, Garcia HH, Gavidia CM, Gilman RH, Verastegui M, Cysticercosis Working Group in Peru. 2016. In vitro study of Taenia solium postoncospheral form. PLoS Negl Trop Dis 10:e0004396. doi: 10.1371/journal.pntd.0004396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Palma S, Chile N, Carmen-Orozco RP, Trompeter G, Fishbeck K, Cooper V, Rapoport L, Bernal-Teran EG, Condori BJ, Gilman RH, Verastegui MR, Cysticercosis Working Group in Peru. 2019. In vitro model of postoncosphere development, and in vivo infection abilities of Taenia solium and Taenia saginata. PLoS Negl Trop Dis 13:e0007261. doi: 10.1371/journal.pntd.0007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ito A, Chung WC, Chen CC, Ito M, Endo S, Okamoto M, Fan PC. 1997. Human Taenia eggs develop into cysticerci in scid mice. Parasitology 114:85–88. doi: 10.1017/s0031182096008074. [DOI] [PubMed] [Google Scholar]
- 259.Wang IC, Ma YX, Guo JX, Chung WC, Lu SC, Ito A, Fan PC. 1999. Oncospheres of Taenia solium and T. saginata asiatica develop into metacestodes in normal and immunosuppressed mice. J Helminthol 73:183–186. doi: 10.1017/s0022149x99000281. [DOI] [PubMed] [Google Scholar]
- 260.Verastegui MR, Mejia A, Clark T, Gavidia CM, Mamani J, Ccopa F, Angulo N, Chile N, Carmen R, Medina R, Garcia HH, Rodriguez S, Ortega Y, Gilman RH. 2015. Novel rat model for neurocysticercosis using Taenia solium. Am J Pathol 185:2259–2268. doi: 10.1016/j.ajpath.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Santamaria E, Plancarte A, de Aluja AS. 2002. The experimental infection of pigs with different numbers of Taenia solium eggs: immune response and efficiency of establishment. J Parasitol 88:69–73. doi: 10.2307/3285392. [DOI] [PubMed] [Google Scholar]
- 262.Fleury A, Trejo A, Cisneros H, Garcia-Navarrete R, Villalobos N, Hernandez M, Villeda Hernandez J, Hernandez B, Rosas G, Bobes RJ, de Aluja AS, Sciutto E, Fragoso G. 2015. Taenia solium: development of an experimental model of porcine neurocysticercosis. PLoS Negl Trop Dis 9:e0003980. doi: 10.1371/journal.pntd.0003980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Alroy KA, Arroyo G, Gilman RH, Gonzales-Gustavson E, Gallegos L, Gavidia CM, Verastegui M, Rodriguez S, Lopez T, Gomez-Puerta LA, Alroy J, Garcia HH, Gonzalez AE, The Cysticercosis Working Group in Peru. 2018. Carotid Taenia solium oncosphere infection: a novel porcine neurocysticercosis model. Am J Trop Med Hyg 99:380–387. doi: 10.4269/ajtmh.17-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Carpio A, Hauser WA. 2009. Epilepsy in the developing world. Curr Neurol Neurosci Rep 9:319–326. doi: 10.1007/s11910-009-0048-z. [DOI] [PubMed] [Google Scholar]
- 265.Bianchin MM, Velasco TR, Wichert-Ana L, Alexandre V Jr, Araujo D Jr, dos Santos AC, Carlotti CG Jr, Takayanagui OM, Sakamoto AC. 2014. Characteristics of mesial temporal lobe epilepsy associated with hippocampal sclerosis plus neurocysticercosis. Epilepsy Res 108:1889–1895. doi: 10.1016/j.eplepsyres.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 266.Bianchin MM, Velasco TR, Wichert-Ana L, Dos Santos AC, Sakamoto AC. 2017. Understanding the association of neurocysticercosis and mesial temporal lobe epilepsy and its impact on the surgical treatment of patients with drug-resistant epilepsy. Epilepsy Behav 76:168–177. doi: 10.1016/j.yebeh.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 267.Del Brutto OH, Engel J, Eliashiv DS, García HH. 2016. Update on cysticercosis epileptogenesis: the role of the hippocampus. Curr Neurol Neurosci Rep 16:1. doi: 10.1007/s11910-015-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Del Brutto OH, Engel J, Eliashiv DS, Salamon N, García HH. 2014. Hippocampal sclerosis: the missing link of cysticercosis epileptogenesis? Epilepsia 55:2077–2078. doi: 10.1111/epi.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Del Brutto OH. 1994. Prognostic factors for seizure recurrence after withdrawal of antiepileptic drugs in patients with neurocysticercosis. Neurology 44:1706–1709. doi: 10.1212/wnl.44.9.1706. [DOI] [PubMed] [Google Scholar]
- 270.Jama-Antonio JMC, Yasuda CL, Cendes F. 2019. Neurocysticercosis and hippocampal atrophy: MRI findings and the evolution of viable or calcified cysts in patients with neurocysticercosis. Front Neurol 10:449. doi: 10.3389/fneur.2019.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Alvarez JI, Colegial CH, Castano CA, Trujillo J, Teale JM, Restrepo BI. 2002. The human nervous tissue in proximity to granulomatous lesions induced by Taenia solium metacestodes displays an active response. J Neuroimmunol 127:139–144. doi: 10.1016/S0165-5728(02)00101-7. [DOI] [PubMed] [Google Scholar]
- 272.Restrepo BI, Alvarez JI, Castano JA, Arias LF, Restrepo M, Trujillo J, Colegial CH, Teale JM. 2001. Brain granulomas in neurocysticercosis patients are associated with a Th1 and Th2 profile. Infect Immun 69:4554–4560. doi: 10.1128/IAI.69.7.4554-4560.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Restrepo BI, Llaguno P, Sandoval MA, Enciso JA, Teale JM. 1998. Analysis of immune lesions in neurocysticercosis patients: central nervous system response to helminth appears Th1-like instead of Th2. J Neuroimmunol 89:64–72. doi: 10.1016/S0165-5728(98)00112-X. [DOI] [PubMed] [Google Scholar]
- 274.Robinson P, Garza A, Weinstock J, Serpa JA, Goodman JC, Eckols KT, Firozgary B, Tweardy DJ. 2012. Substance P causes seizures in neurocysticercosis. PLoS Pathog 8:e1002489. doi: 10.1371/journal.ppat.1002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Stringer JL, Marks LM, White AC Jr, Robinson P. 2003. Epileptogenic activity of granulomas associated with murine cysticercosis. Exp Neurol 183:532–536. doi: 10.1016/S0014-4886(03)00179-1. [DOI] [PubMed] [Google Scholar]
- 276.Garcia HH, Martinez SM (ed). 1996. Taeniasis/cisticercosis por Taenia solium. Editorial Universo, Lima, Peru. [Google Scholar]
- 277.Alsina GA, Johnson JP, McBride DQ, Rhoten PR, Mehringer CM, Stokes JK. 2002. Spinal neurocysticercosis. Neurosurg Focus 12:e8. doi: 10.3171/foc.2002.12.6.9. [DOI] [PubMed] [Google Scholar]
- 278.Kumar V, Surve A, Kumar P, Sharma A, Azad S. 7 April 2019. Submacular cysticercosis. Eur J Ophthalmol doi: 10.1177/1120672119841542. [DOI] [PubMed] [Google Scholar]
- 279.Del Brutto OH, Del Brutto VJ. 2013. Intrasellar cysticercosis: a systematic review. Acta Neurol Belg 113:225–227. doi: 10.1007/s13760-013-0199-1. [DOI] [PubMed] [Google Scholar]
- 280.Im SH, Park SH, Oh DH, Kang BS, Kwon OK, Oh CW. 2005. Subdural cysticercosis mimicking a chronic subdural hematoma. Case illustration. J Neurosurg 102:389. doi: 10.3171/jns.2005.102.2.0389. [DOI] [PubMed] [Google Scholar]
- 281.Kaur M, Joshi K, Ganguly NK, Mahajan RC, Malla N. 1995. Evaluation of the efficacy of albendazole against the larvae of Taenia solium in experimentally infected pigs, and kinetics of the immune response. Int J Parasitol 25:1443–1450. doi: 10.1016/0020-7519(95)00057-7. [DOI] [PubMed] [Google Scholar]