In the 1980s, menstrual toxic shock syndrome (mTSS) became a household topic, particularly among mothers and their daughters. The research performed at the time, and for the first time, exposed the American public as well as the biomedical community, in a major way, to understanding disease progression and investigation. Those studies led to the identification of the cause, Staphylococcus aureus and the pyrogenic toxin superantigen TSS toxin 1 (TSST-1), and many of the risk factors, for example, tampon use.
KEYWORDS: diaphragms, menstrual cups, Staphylococcus aureus, tampons, toxic shock syndrome
SUMMARY
In the 1980s, menstrual toxic shock syndrome (mTSS) became a household topic, particularly among mothers and their daughters. The research performed at the time, and for the first time, exposed the American public as well as the biomedical community, in a major way, to understanding disease progression and investigation. Those studies led to the identification of the cause, Staphylococcus aureus and the pyrogenic toxin superantigen TSS toxin 1 (TSST-1), and many of the risk factors, for example, tampon use. Those studies in turn led to TSS warning labels on the outside and inside of tampon boxes and, as important, uniform standards worldwide of tampon absorbency labeling. This review addresses our understanding of the development and conclusions related to mTSS and risk factors. We leave the final message that even though mTSS is not commonly in the news today, cases continue to occur. Additionally, S. aureus strains cycle in human populations in roughly 10-year intervals, possibly dependent on immune status. TSST-1-producing S. aureus bacteria appear to be reemerging, suggesting that physician awareness of this emergence and mTSS history should be heightened.
INTRODUCTION
Toxic shock syndrome (TSS), as originally described, was a syndrome, which implies that we did not know its cause; at that time, it was solely a clinical entity (1–3). However, this is no longer the case since we now know the causes. The name persists in recognition of an important article in a 1978 issue of The Lancet, where the name was coined by a pediatrician, James Todd, who, together with his colleagues, described a serious infection affecting both young females and males (4). TSS comes in two major forms. Staphylococcal TSS is caused by Staphylococcus aureus and is the subject of this review (1, 2, 4). Streptococcus pyogenes (group A streptococcus) also causes TSS, which appears to be malignant scarlet fever (5, 6). It is now also recognized that other beta-hemolytic streptococci, particularly groups B, C, and G, may cause rare cases of streptococcal TSS (7–10). In retrospect, there are also cases of staphylococcal TSS that were originally described as staphylococcal scarlet fever (11–13). Today, we also know that many cases of adult Kawasaki syndrome are staphylococcal TSS. Kawasaki syndrome is a syndrome of unknown cause that primarily affects children ≤4 years of age (14–17). Early cases of staphylococcal TSS were called adult Kawasaki syndrome because of overlapping features (18). Often, physicians, if asked, will say the earliest cases of staphylococcal TSS that they saw, if they saw any, occurred after 1972, indicating an emergence at about that time. This timing is important because, as is shown later, the major toxin associated with menstrual TSS (mTSS) emerged coincidentally in 1972 (19–21). Additionally, the highest-absorbency tampons, most often associated with TSS, were marketed in 1976, 4 years after the emergence of the causative strain of S. aureus.
In examination of the historical record, we also now know that staphylococcal TSS, caused by the pyrogenic toxin superantigen (PTSAg) TSS toxin 1 (TSST-1), resulted in the death of 50% of children in what is referred to as the Bundaberg, Australia, disaster in 1928 (22). The cause was not recognized at that time, and indeed, the outbreak led to the identification of staphylococcal alpha-toxin (alpha-hemolysin and alpha-cytotoxin) as the likely cause. It turns out that the Bundaberg S. aureus strain produces little if any alpha-toxin but makes large amounts of TSST-1 (22). All affected children had defining clinical features of TSS, including fever, hypotension (and shock and death), and multiorgan changes that typify staphylococcal TSS. Unfortunately, TSST-1 was not identified until 1981, 53 years later (23, 24).
In 1987, the Minnesota Department of Health and colleagues reported a series of TSS cases of a new infection, termed postinfluenza TSS, in the Minneapolis-St. Paul, MN, area (25). All eight children described in that study succumbed to superinfection with TSST-1-producing S. aureus (TSST-1 S. aureus) following an influenza illness. The one surviving child had staphylococcal enterotoxin B (SEB)-associated S. aureus infection.
SEB is another PTSAg, related in activity to TSST-1 although not as potent (26–29). Unpublished clinical testing of pediatric influenza cases showed that 100% or nearly 100% of childhood deaths were due to TSST-1 S. aureus, as opposed to surviving children being superinfected with enterotoxin-producing S. aureus; however, enterotoxin-producing S. aureus could also cause deaths. In retrospect, postinfluenza TSS could have been the same disease that caused the plague of Athens (also known as Thucydides syndrome) in 430 to 427 BC (for example, see reference 30).
We know that there are multiple subsets of staphylococcal TSS. These include the subtypes listed in Table 1. We do not discuss further those subsets that are not menstruation associated.
TABLE 1.
Subsets of staphylococcal TSS
MENSTRUAL STAPHYLOCOCCAL TSS AND TSST-1
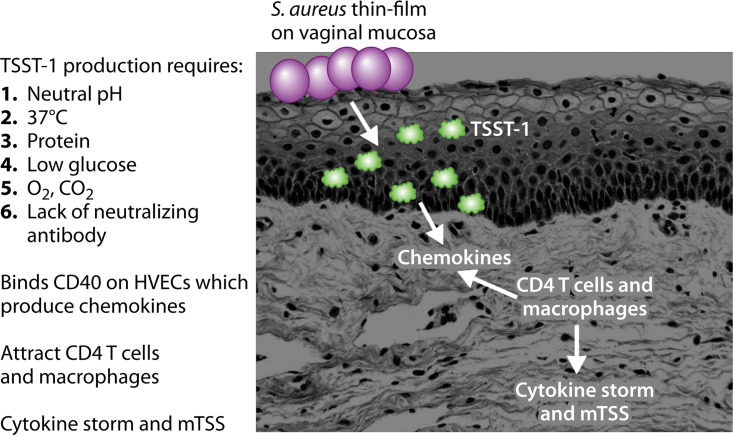
It is important to first define terms. Menstruation in this review refers to 3 days prior to menstruation, during menstruation, and up to 3 days after menstruation ceases; this designation has been adopted by the Centers for Disease Control and Prevention (CDC) in reference to mTSS cases (1). mTSS does not specify whether TSS was caused by S. aureus or beta-hemolytic streptococci, primarily group A streptococci, noting that both group A streptococci and S. aureus cause TSS in women of menstrual age primarily during their menstrual periods. This review focuses on menstrual staphylococcal TSS and not menstrual group A streptococcal TSS. Group A streptococci are aerotolerant anaerobes, whereas S. aureus is a facultative aerobe. A major reason proposed for the tampon association with staphylococcal TSS (and not streptococcal TSS) is that tampons introduce oxygen within the tampons as a function of absorbency into the normally anaerobic vagina (31, 32). The production of TSST-1 by S. aureus absolutely requires oxygen, whereas PTSAg production by group A streptococci is independent of oxygen (the possible role of oxygen in staphylococcal TSS is discussed in detail later in this review). No epidemiological study has implicated group A streptococci in menstrual, tampon-associated TSS.
mTSS does not highlight where the causative S. aureus isolate is found in the body. This review focuses on mTSS where the causative S. aureus isolate is present vaginally. However, mTSS cases occur with colonization of the skin and oral, respiratory, and gastrointestinal mucosae. Additionally, while most mTSS cases with vaginal S. aureus colonization are associated initially with tampon use, highly severe mTSS cases, including death, have occurred in women with vaginal colonization by TSS S. aureus who have never used tampons or are having recurrent TSS, where the women had been advised against and were not using tampons after their first episode (33–35). Thus, mTSS cases must be differentiated based on the location of S. aureus colonization and whether tampons were being used.
The major publicity associated with mTSS occurred on 7 June 1980 (a Saturday), with national spread the following Monday (36). It is now recognized that this form of TSS became the second greatest news event of 1980, second only to the Iran hostage crisis. It was quickly recognized that the majority of cases of this new syndrome were occurring in young women during their menstrual periods, including in young women, even of 12 years of age, and generally in women less than 25 years of age (1, 2). Although not recognized as mTSS at the time, these new types of cases were also present among some of the cases seen in the original 1978 description (4).
The connection between TSS and adult Kawasaki syndrome started in 1978. David Schlossberg, an adult infectious disease physician (recently deceased) from Harrisburg, PA, had seen a patient with a new severe scarlet fever-like disease, but he had not been able to culture group A streptococci, the usual causes of scarlet fever (37). Instead, he isolated vaginal S. aureus as the possible cause in association with menstruation. This led to our identification of three pyrogenic toxins (A, B, and C). We now know that pyrogenic exotoxin A is SEA, a PTSAg produced by the majority of TSST-1-positive (TSST-1+) S. aureus strains (38, 39). Pyrogenic exotoxin B (40) is the same as SEC, a PTSAg produced by 15% of TSST-1-positive S. aureus strains. Staphylococcal pyrogenic exotoxin C was first described in April 1981 in collaboration with researchers from the CDC (23). This toxin became known as TSST-1 in 1984 as the cause of 75% of all staphylococcal TSS cases, including 100% of mTSS cases (38, 41). In May of 1981, Merlin Bergdoll and colleagues described a staphylococcal enterotoxin, called SEF, also strongly associated with TSS (24). It became clear in 1984 that both pyrogenic exotoxin C and SEF were the same toxin, and thus, the name TSST-1 was given to this toxin at an international TSS symposium held in Madison, WI (41).
As originally described, SEF had emetic activity, a property which typifies staphylococcal enterotoxins. However, SEF was contaminated with small amounts of SEA, and this contaminant most likely led to the observed emetic activity. TSST-1 has no emetic activity (42). At that time, SEF was shown to have two cysteine amino acids, known to be present and required for emesis due to SEs (28, 29). TSST-1 has no cysteine residues, but SEA does (27, 43, 44). The biochemical properties and its high presence in TSST-1-positive S. aureus isolates make SEA the PTSAg most likely to account for the SEF emetic activity.
It is often asked why TSST-1 has the dash one, “-1.” This was added at the above-mentioned international symposium in case TSST-2 and others were identified. To date, and surprisingly, there have not been human TSST-1 variants described. TSST-ovine is a protein related to TSST-1 but is not toxic to humans and not found in human S. aureus isolates (45). TSST-ovine, from all sheep and goat mastitis strains of S. aureus, has seven amino acid differences from TSST-1, and these changes render TSST-ovine inactive against human cells (45, 46). TSST-ovine is active against sheep cells (45).
CLINICAL DESCRIPTION OF mTSS
The collaborative article by Todd and colleagues provided many of the clinical features of mTSS (4). These criteria were modified by the CDC for the purposes of studying epidemiologically the risk factors for mTSS (1). Unfortunately, the original CDC epidemiological clinical criteria were adopted by many physicians as the “gold-standard” definition of mTSS. These criteria are listed in Table 2; we have added one additional category of TSS as suggested by Parsonnet, namely, toxin-mediated staphylococcal disease (47).
TABLE 2.
Diagnostic criteria for staphylococcal TSSa
| Criterion or classification | Description |
|---|---|
| Diagnostic criteria | |
| Fever | Temp of ≥102.0°F |
| Rash | Sunburn like or scarlet fever like |
| Peeling of skin | Often but not necessarily upon recovery |
| Hypotension | Systolic blood pressure of ≤90 mm Hg or <5th percentile for children <16 yr of age |
| Multisystem involvement (at least 3 of the following) | |
| Gastrointestinal | Vomiting or diarrhea; usually the first symptoms seen |
| Muscular | Severe myalgia or creatine phosphokinase level ≥2× the upper limit of normal |
| Mucous membrane | Hyperemia of any mucosal surface |
| Renal | Blood urea nitrogen or creatinine levels ≥2× the upper limit of normal or urinary sediment with pyuria in the absence of urinary tract infection |
| Hepatic | Total bilirubin, alanine aminotransferase, or aspartate aminotransferase levels ≥2× the upper limit of normal |
| Hematological | Platelet counts of <100,000/mm3 |
| Central nervous system | Disorientation, combativeness, or other alterations in consciousness without focal neurological signs when fever/hypotension is absent |
| Laboratory diagnostic criteria | |
| Negative results, if obtained | Blood or cerebrospinal fluid cultures (blood culture may be positive for S. aureus) |
| Negative antibody tests for Rocky Mountain spotted fever, leptospirosis, or measles | |
| Case classification | |
| Confirmed TSS | Meets the laboratory criteria, and all 5 clinical criteria are present, including desquamation, unless the patient dies before skin peeling |
| Probable TSS | Meets the laboratory criteria, and 4 of 5 clinical criteria are present |
| Toxin-mediated staphylococcal disease | Case where more than 1 criterion is missing, S. aureus is isolated, and other causes are ruled outb |
mTSS is defined by high fever, hypotension, the presence of a sunburn-like rash, skin peeling often seen upon recovery, and a multiorgan component that includes many flu-like symptoms (1). Importantly, it is necessary to rule out other causes. Within a year of this CDC description, it was recognized that many patients with TSS do not meet all of the required clinical criteria (48). Indeed, cases occur that lack individually each of the mTSS criteria. This led to the establishment of a category called probable TSS in which one major clinical criterion was absent (48). This left open the issue, What if more than one criterion is missing? It is our sentiment that toxin-mediated staphylococcal disease (47) should be used to define those cases, making it even more critical to rule out other causes and find the presence of TSS S. aureus (27–29, 47).
TRAITS OF mTSS S. AUREUS
mTSS strains, as originally isolated, were referred to as combinations of bacteriophage types 29, 52, and 52a (19–21). The original bacteriophage typing of S. aureus is complex, with more than 180 bacteriophages required for complete determination of clonality. The CDC and others, as we use here, employ a pulsed-field electrophoresis method for the determination of S. aureus clones, referred to as clonal groups USA100 to USA1100. mTSS isolates are nearly all of the USA200 clonal group. Some research groups refer to the USA200 clonal group as clonal complex 30 (CC30) and sequence type 36 (ST36). As such, they have highly unique characteristics, including all members producing TSST-1. Additionally, most members of the clonal group (like the Bundaberg strain) lack or have greatly reduced production of staphylococcal alpha-toxin (49, 50). This greatly reduced production results from a mutation that leads to a truncated product (50). In studies using mass spectrometry and antibody-based methods, we can detect the production of a peptide up to the mutation and additionally a peptide encompassing the entire carboxyl part (49). This suggests that alpha-toxin is indeed produced at low levels by the isolates with the readthrough mutation (49). Of greatest importance, the reduced production of alpha-toxin leads to these strains by and large being confined to mucosal surfaces or damaged skin. Studies that suggest that USA200 strains are less virulent than skin isolates are unfounded (51). Comparing mucosal versus skin isolates is akin to comparing apples and oranges; all USA200 isolates cultured today produce the PTSAg TSST-1, the cause of 75% of all cases of staphylococcal TSS, 100% of mTSS cases, and 100% of fatal postinfluenza TSS cases (27–29).
The most important trait of USA200 mTSS S. aureus strains is the presence of the gene for TSST-1, referred to as tstH (43, 52). This gene is present on one of two pathogenicity islands, referred to as SaPI1 and SaPI2 (53). These pathogenicity islands, approximately 14 kb in size, were likely at one time components of bacteriophages that are now defective, nearly one-third the size of usual staphylococcal bacteriophages, and that have tstH near the end of the islands (53). The TSST-1 gene was likely acquired by bacteriophages from an unknown source and has become trapped in the chromosome (54); helper bacteriophages can sometime lead to excision and packaging, creating abnormal-appearing bacteriophages (55). TSST-1 is one of three PTSAgs that are produced in large amounts (micrograms per milliliter to milligrams per milliliter), the other two being SEs B and C (56). Nearly all TSST-1-positive strains produce 3 to 5 μg/ml in broth culture under conditions that favor production, and these same strains may produce 15,000 μg/ml when the organisms are cultured in biofilms (56). Occasional strains, such as MN8, may produce TSST-1 at up to 20 μg/ml in broth cultures (57). The basis for the greater production in biofilms and why MN8 produces more TSST-1 than other strains is not known. When the TSST-1 gene is present, TSST-1 protein is always produced (58).
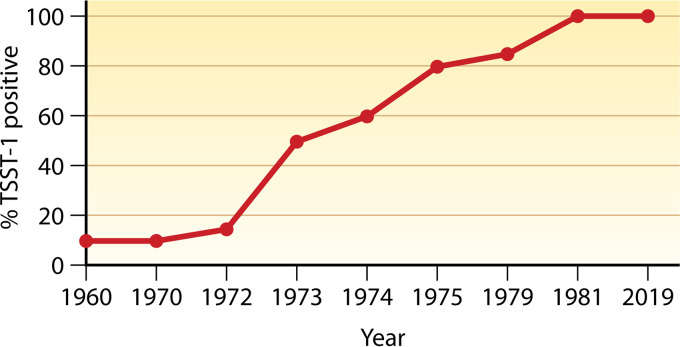
Analyses performed in a blind manner were used to determine if TSST-1 has always been present in USA200 strains (29/52/52A phage groups) from vaginal isolates. Through these studies (Fig. 1), USA200 strains producing TSST-1 were present in low numbers until they emerged in 1972, with essentially 100% producing TSST-1 by 1976 (19–21). The basis for this emergence is not known, but even today, 100% of USA200 strains are TSST-1 positive. There has been discussion about whether the use of high-absorbency tampons drove this emergence. This is not the case. The S. aureus emergence began in 1972, peaking at the same time as when the highest-absorbency tampons were first marketed, the tampons most associated with mTSS.
FIG 1.
Emergence of TSST-1-positive USA200 S. aureus based on phage typing, pulsed-field gel electrophoresis, and TSST-1 testing.
SaPIs also contain a gene of unknown function called ear, for enterotoxin-associated ampicillin resistance (53). This gene leads to the production of a small protein (EAR), which is massively secreted through the signal peptide mechanism. The exact biological function of EAR remains unknown. However, its gene is also adjacent to other PTSAg genes on pathogenicity islands in S. aureus, for example, SEB and SEC (54).
There is a PTSAg referred to as SE-like X (59). This PTSAg, like TSST-1, belongs to the group I PTSAgs, and interestingly, 100% of S. aureus isolates produce either TSST-1 or SE-like X, with very rare strains producing both toxins (29). The mechanism underlying this apparent exclusion remains unclear. As many as 15% of TSST-1-positive strains produce SEC, but only very rare strains produce both TSST-1 and SEB, a PTSAg highly related to SEC (29). The genetic basis for this exclusion is also unknown.
In early studies of mTSS S. aureus, it was observed that the presence of TSST-1 and the relative absence of alpha-toxin defined these strains (23, 50). However, there are several unique properties of these strains (Table 3). While the isolates do not differ from other S. aureus strains in known cell surface virulence factors, there are multiple other interesting properties. Nearly all mTSS isolates are methicillin sensitive (60). Those isolates that are methicillin resistant lack gold pigment and thus appear white (60). Nearly all USA200 strains lack plasmids, suggesting an unknown DNA restriction, and these strains are difficult to transform with exogenous DNA. USA200 organisms in the United States all exhibit penicillin and ampicillin resistance; these same organisms are resistant to both cadmium and arsenate. Typically, these traits are encoded by genes on plasmids, but this is clearly not the case for USA200 strains. Nearly all USA200 isolates contain the enterotoxin gene cluster of six PTSAgs, including SEG and SE-like I, M, N, O, and U (61). Of these, SEG has emetic activity and is thus an enterotoxin (62), and SE-like U has significant sequence similarity to SEB and -C. This family of six PTSAgs is considered important for local colonization with S. aureus, as they are produced in only very small amounts (nanograms per milliliter), and they are thus unable to cause TSS (61). Our previous studies have shown that 100% of S. aureus isolates produce at least one major PTSAg (for example, USA200 isolates produce TSST-1) and one cytotoxin (USA200 strains produce the wild-type cytotoxins beta-toxin, gamma-toxin, delta-toxin, phenol-soluble modulins, and epsilon-toxin) (60). It is interesting to note that USA200 strains are particularly unique compared to other USA clonal groups in their wild-type production of beta-toxin (63). Other clonal groups contain a bacteriophage that inserts into the beta-toxin structural gene, leading to exceptionally low-level production in vitro. However, in vivo, there is a loss of the bacteriophage in these other clonal groups, leading to greater beta-toxin production and suggesting that beta-toxin contributes to disease (63).
TABLE 3.
Properties of mTSS S. aureus
| Property | Result(s)a |
|---|---|
| TSST-1 production | 100% positive (strains are variously called USA200, CC30, and ST36) |
| Reduced alpha-toxin | 99% have reduced or no alpha-toxin |
| Antibiotic resistance | In the U.S., 100% resistance to penicillin and ampicillin; occasional MRSA isolates are seen, and these invariably lack pigment |
| Metal resistance | 100% cadmium and arsenate |
| Plasmids | Typically not present |
| Other toxins | 99% have the enterotoxin gene cluster of 6 PTSAg genes; 100% produce beta-toxin (lack the bacteriophage that inactivates this toxin); 100% are EAR gene positive (unknown function); 15% produce SEC but not SEB |
MRSA, methicillin-resistant S. aureus.
BIOCHEMICAL AND BIOLOGICAL PROPERTIES OF TSST-1
The biochemical and biological properties of TSST-1 are shown in Table 4. TSST-1 is a protein with a molecular weight of 22,000 (64). It has an isoelectric point of 7.0 to 7.2, migrating as two interconvertible bands during isoelectric focusing (64). The reason for this interconversion is unknown. The major secreted proteins of USA200 S. aureus strains are TSST-1 and EAR, thus making TSST-1 easy to purify (64). The simplest way to purify the PTSAg is 80% ethanol precipitation followed by thin-layer isoelectric focusing (65). From 5 liters of appropriate culture medium and under appropriate S. aureus growth conditions, the yield is usually 100 to 150 mg of highly purified TSST-1 (65). The protein thus purified is stable for years at temperatures of 4°C to −80°C. TSST-1 is stable against heating at 100°C for 1 h and highly resistant to many proteases and cyanogen bromide cleavage despite having 2 internal methionine amino acids (43). These data indicate that TSST-1 is tightly folded and must easily refold into the native structure after heat treatment. The protein is produced with a 40-amino-acid signal peptide that is cleaved away during the secretion process (43). The mature protein has 194 amino acids (43). Because TSST-1 secretion requires a signal peptide, the protein folds as it is secreted, reaching its final form only upon complete secretion. Intact TSST-1 is not present in S. aureus cells.
TABLE 4.
Biochemical and biological properties of TSST-1
| Biochemical or biological property | Result(s) |
|---|---|
| Biochemical properties | |
| Molecular mass | 22,000 Da |
| Isoelectric point | 7.2 |
| Variants | 2 interconvertible forms describeda |
| Stability against heat | 100°C for ≥1 h |
| Stability against proteases and CNBr | Trypsin; high-level resistance to CNBr |
| Biological properties | |
| Pyrogenicity | Potent |
| Superantigenicity | Binds to Vβ2-TCR, MHC-II, and CD28 |
| Epithelial cell chemokine production | Binds to CD40 |
| Endotoxin shock | Enhanced by up to 106-fold |
| Estimated lethal dose in humans | 0.1 μg |
See reference 64.
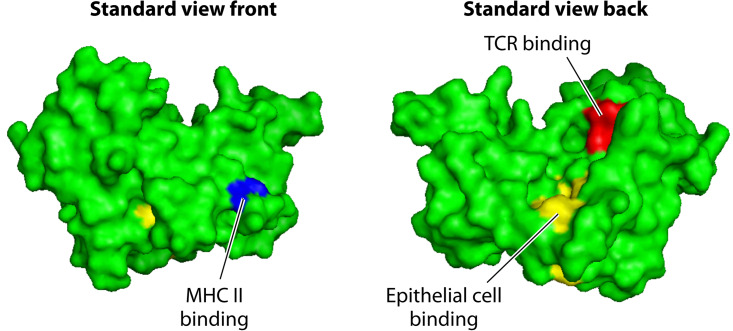
The three-dimensional structures of TSST-1 (Fig. 2) (66) and other PTSAgs have been determined (27–29). TSST-1 has the most basic structure, together with SE-like X. These are referred to as group I PTSAgs. As such, these two PTSAgs have receptor sites only for T lymphocyte receptors, major histocompatibility complex class II (MHC-II) molecules (27–29), and the immune-costimulatory molecules CD40 (26) and CD28 (67). For many years, the PTSAg family, including TSST-1, SEs, SE-like proteins, and streptococcal pyrogenic exotoxins (SPEs), were known as pyrogenic toxins since their abilities to cause fever were shared (68). This activity explains the high fever normally associated with TSS. Fever results from direct and cytokine-induced indirect (interleukin-1β [IL-1β]) stimulation of the hypothalamus, initially to induce prostaglandin E2 production, followed by alteration centrally of the ratio of serotonin and norepinephrine and the activation of α-adrenergic nerve receptors (69, 70). Additionally, all PTSAgs share the unusual activity of enhancing lethal shock due to lipopolysaccharide (LPS) by as much as a millionfold, by enhancing tumor necrosis factor (TNF) production (71–73). It has been known since the 1970s that pyrogenic toxins are major stimulators of T lymphocyte proliferation and that this property is important in human infections (23, 74). The term SAg was later coined to emphasize the unusual way in which pyrogenic toxins activate T lymphocytes (75). PTSAgs cross-bridge T lymphocyte receptors (T cell receptors [TCRs]) with MHC-II molecules on antigen-presenting cells (APCs) in a relatively nonspecific way, inducing the massive proliferation of T cells and activation of APCs, mainly macrophages (27–29). T cell and macrophage activation results in a “cytokine storm” that explains most symptoms of TSS (27–29). The massive production of TNF-α and TNF-β causes capillary leakage and results in hypotension, shock, death, and likely many of the multiorgan changes in mTSS (27–29). The rash associated with mTSS may or may not be present depending on the hypersensitivity state of the patient (76, 77). At least one prior exposure to TSST-1 appears to be required for the rash, which most closely resembles the scarlet fever rash associated with PTSAgs of group A streptococci (76, 78). These rashes appear to result from the amplification of cytokine (IL-2 and interferon gamma [IFN-γ]) delayed hypersensitivity to the PTSAgs, noting that delayed hypersensitivity results from T lymphocyte overactivation of macrophages (76–78). Upon autopsy, one of only a few TSS-specific findings is overactivation of macrophages such that the cells are phagocytizing other host cells (termed erythrophagocytosis) (33). The other two autopsy findings are fatty replacement of the liver, which typifies LPS-induced shock, and epithelial barrier sloughing, an effect that depends on cytokine production (the result of either recovery from edema or sloughing due to IL-2 and interferon gamma) (33).
FIG 2.
Three-dimensional structure of TSST-1 showing positions of T cell, MHC-II, and epithelial cell (CD40) receptor interactions.
Numerous studies have provided remarkable amounts of data regarding the three-dimensional structures and host cell receptor sites for most PTSAgs (27–29). We focus only on TSST-1 (Fig. 2). Readers are encouraged to consult other major reviews where more complete discussions of all PTSAg structural studies are presented (27–29). Studies that have provided the structures have shown that TSST-1 contains two major protein domains, an amino-terminal oligosaccharide/oligonucleotide binding (O/B) fold and a carboxy-terminal β-grasp domain, with domains connected by a central, diagonal α-helix. Based on small variations in this shared core structure, which defines TSST-1, TSST-ovine, and SE-like X, all members of the family can be categorized into 5 major groups (27). Group I PTSAgs are represented by TSST-1. Group I PTSAgs have unique amino acid sequences compared to other PTSAgs, and they contain only the α-chain MHC-II binding site, of low affinity, in their O/B folds. The TSST-1 interaction with the T cell receptor is known as Vβ-TCR (variable component of the β-chain of the T cell receptor); this binding site is positioned in a groove between the O/B fold and β-grasp domains, on the top back of the molecules as usually viewed (Fig. 2). In contrast, the non-group I PTSAgs bind Vβ-TCRs on the top front. The complete significance of this difference is unclear, but it could contribute to TSST-1’s greater toxicity than, for example, SEB and SEC (26). TSST-1 interacts only with human Vβ2-TCR (75). Human Vβ2 is present typically on 10% of T lymphocytes, but during acute TSS, these cells undergo proliferation such that they become up to 70% of all T lymphocytes in the patient; both CD4 and CD8 T cells are stimulated.
In addition to having up to two MHC-II binding sites, a site for Vβ-TCR binding, and a cystine loop needed for emesis by SEs, all PTSAgs also contain another host cell binding site, referred to as the dodecapeptide binding site (26, 67). This domain of PTSAgs is required for interaction with the immune-costimulatory molecule CD40 on epithelial cells (26). It has been known since the 1980s that TSST-1 binds to epithelial cells (79). However, the importance of this interaction was clarified only recently. Our studies indicate that the interaction of PTSAgs with epithelial cells initiates a cascade of events that result in disease by causing harmful inflammation at mucosal barriers (80, 81). The dodecapeptide region is relatively conserved among PTSAgs, with the greatest differences being observed between TSST-1 and other PTSAgs. This difference in the dodecapeptide binding region, and its direct binding to CD40, may explain the greater mucosal epithelium penetration of TSST-1 than of other PTSAgs and, thus, TSST-1’s unique association with mTSS (26). The dodecapeptide binding region is located near the base of the central, diagonal α-helix. TSS production depends to a large extent on TSST-1 stimulation of T lymphocytes and macrophages in the submucosa (26).
EPIDEMIOLOGY OF mTSS WITH RESPECT TO TAMPON ASSOCIATION
It is erroneous to think that only young, menstruating women using tampons develop TSS based on the early case reports and epidemiological studies that were published almost 40 years ago (1, 2, 4, 82). Other categories of staphylococcal TSS and streptococcal TSS occur, as discussed above. The most difficult conditions to study by epidemiological methods are those that are uncommon (83). TSS is relatively uncommon, affecting both males and females, and published cases indicate that TSS may also occur in infants and the elderly (1, 2, 4, 48, 82, 84, 85).
Clear case definitions are critical for cogent epidemiological investigations of outbreaks. The use of a similar or standard case definition allows for comparisons between investigations over time and location. The original TSS case definition included a constellation of signs and symptoms for the clear identification of cases (Table 2). These criteria were developed by a team that consisted of CDC representatives and other biomedical scientists (86). The diagnostic criteria for TSS did not (and still do not) include sex, menstruation status, the use or no use of tampons, the isolation of S. aureus from infection sites, or the absence of neutralizing antibody to TSST-1. The identification of patient gender, catamenial (menstruation control) device use, and menstrual history led to intense debate, that original diagnostic judgments by physicians or the subsequent diagnostic selections by investigators were biased when diagnosing TSS. In fact, Harvey and colleagues (87) designed a set of descriptive vignettes having diverse resemblances to TSS or Kawasaki syndrome. Physicians who participated in this research were more likely to diagnose TSS for menstruating tampon users or simply menstruating women than for men or nonmenstruating women. Strikingly, TSS was diagnosed in 85% of instances when the cases involved menstruating tampon users but in only 23% when the same scenario involved males.
Another potential bias using this strict case definition is that if a study enrolled only confirmed cases, probable or mild cases would not be counted. With increased awareness of the signs and symptoms, early management and/or treatment may be sought that does not lead to inpatient treatment at the hospital. For example, if a young woman with symptoms similar to those of influenza or mild TSS presents at the emergency room for treatment, she may be advised to drink fluids, rest, and treat the fever. The case may be assigned an International Statistical Classification of Disease revision 10 (ICD-10) code such as J06.9 (acute upper respiratory infection), instead of A48.3 (TSS). Thus, there would be underreporting of the disease when diagnosis-related groups (DRGs) are used (88).
Many of the published epidemiological studies on TSS involve retrospective data. These studies examined exposures to potential risk factors or, in some cases, protective ones, or the data were collected for reasons other than research. These were often chart reviews because the data sources (patient records) or surveillance of cases used passive methods. There are three types of retrospective studies, case reports, case series, and case-control studies, that generate odds ratios (ORs) to estimate risk.
Frequently found in public health research, matched case-control studies contain participants who serve as controls in the study, whereby an effort is made to ensure that the variables of interest (such as age, gender, sex, and other comorbidities) resemble those of the cases. Logistic regression is most often used to analyze these studies. This allows for adjustment for potential confounders by reducing variance as well as improving statistical efficiency (89). There were several active surveillance reports published on TSS (included in Table 5).
TABLE 5.
Incidence of TSS as reported by selected U.S. studies
| Method | Location of population | Sex | Age range (yr) | Yr(s) of study | No. of TSS cases/106 people | Reference(s) or source |
|---|---|---|---|---|---|---|
| DRG-ICD chart review | National | Both | All | 2003–2012 | 0.67 | 250 |
| Colorado | ♀ | 10–39 | 1993–2006 | 1.18 | 114 | |
| Minneapolis-St. Paul | ♀ | All | 2000–2003 | 0.69 | 113 | |
| 34 states | ♀ | All | 1999–2003 | 0.9 | 260 | |
| National | ♀ | Multiple | 1996–2001 | Not reported, but TSS peaked for girls aged 15–18 yr | 251 | |
| Northern California | ♀ | 10–39 | 1981–1996 | 0.8–3.4 | 252 | |
| 42 States | Both | All | 1981–1982 | 0.68–0.81 | 109 | |
| Northern California | ♀ | 15–34 | 1977–1987 | 1.5–2.4 | 253, 254 | |
| 2 Colorado counties | ♀ | 1–45 | 1986–1996 | 0–8.0 | 255 | |
| 2 Colorado counties | Both | <1–30 | 1970–1982 | 0.8–9.1 | 256 | |
| Chart review | Cincinnati | Both | 10–30 | 1970–1980 | 0–22.9 | 257 |
| Iowa | ♀ | 18–31 | 1976–1980 | 25 cases | 258 | |
| Active surveillance | 5 states and Los Angeles County | ♀ | 15–44 | 1986 | 1.05 | 110 |
| Minnesota | ♀ | 12–49 | 1980–1981 | 2.3–13.7 | 103 | |
| Utah | ♀ | 12–49 | 1976–1980 | 14.4 | 100 | |
| Utah | ♀ | 12–49 | 1980–1981 | 4.8–12.3 | 101 | |
| Wisconsin | ♀ | 12–49 | 1979–1980 | 6.2 | 2 | |
| Passive surveillance | Utah | Both | 12–49 | 1976–1983 | 1.7–12.3 | 259 |
| Iowa | 1979–1980 | 25 cases | 258 | |||
Case Reports and TSS
Case reports involve an evaluation of patients and may describe the transmission, natural clinical history, and response to treatment. Although case reports are based on only one or a few patients, they may yield important new epidemiological information regarding the disease. TSS was not new when named in 1978 (4). Case reports of a syndrome related to TSS had been published and studied for almost a century (12, 13, 90).
Case Series and TSS
A second type of descriptive epidemiological study is the case series. In this kind of study, data from a cluster of cases are reported. No comparison is made with controls. In 1978, Todd et al. (4) identified an acute, severe illness characterized by high fever, generalized skin rash, conjunctival hyperemia, diarrhea, hypotension, and renal failure in seven children (8 to 17 years of age). During convalescence, four girls and three boys had desquamation of the affected skin and peeling of the palms and soles of their feet. In more than 70% of the cases, S. aureus (phage group I) was isolated from the vagina, a localized abscess, or the nasopharynx; one patient succumbed. The following year, a case report of a woman (20 years of age) with Kawasaki-like syndrome, in whom S. aureus was isolated from a purulent vaginal discharge, appeared in the literature (37). The author of that study (recently deceased) reported later to P. M. Schlievert that the S. aureus strain (Harrisburg strain) produced TSST-1 and that the patient was a tampon user, but this was not reported in the manuscript. The association between TSS and tampon use was reported 2 years later in a case report (91) noting a possible association with herpes infection. We now know that TSST-1-positive S. aureus causes mTSS, but the immune dysregulation that occurs in mTSS may allow latent herpes infections to reactivate. In late 1980, a study of 11 cases in females from 13 to 43 years of age identified 5 vaginal cultures of S. aureus, and 3 of the isolates produced TSST-1; recurrences were reported (92).
The most reliable measure of incidence rates derives from hospital-based record reviews. Both diagnosed and undiagnosed cases of TSS are ascertained in an unbiased way by reviewing medical charts as well discharge diagnoses likely to be TSS, when included in the research data set.
Even with the challenges of the types of studies mentioned above, the published historical epidemiology information provided insight into mTSS and its etiology. The history of the studies and their results are presented in the following sections and provide information on the approaches to understand associated TSS (both menstrual and nonmenstrual) risk factors and implications from a regulatory perspective as well as to provide a reminder that studies can be flawed due to biases.
First Announcements and Subsequent Analytical Studies
In late 1979, Minnesota and Wisconsin epidemiologists recognized the occurrence of TSS in young women during menstruation, and they began an immediate, active search for additional cases and notified the CDC of their findings in January 1980 (reported at a National Academy of Medicine meeting in 1982). On 23 May 1980, the CDC reported that they had received information on 55 cases, 95% in women (93). Of the women whose menstrual history was known, 95% had onset of TSS during menstruation, and 13 had recurrence of symptoms with the subsequent menstrual period (93).
On 27 June of the same year, the CDC reported that the number of cases had increased to more than 100, with 96% of women aged 12 to 52 years with disease onset between December 1976 and June 1980 (94). There were 52 cases and controls (age and sex matched). There were several major observations from this study: (i) 16/17 (94%) cases had positive cultures for S. aureus; (ii) 50/52 cases (96%) had onset during menstruation, and all 52 cases had used tampons; (iii) more cases used tampons throughout their menstrual periods than did controls; (iv) no significant differences were noted for tampon brand or absorbency; (v) fewer cases than controls used contraceptives; and (vi) 14/44 (32%) had recurrent illness in the following menstrual periods.
In addition to their study, the CDC reported preliminary results from the Wisconsin case-control study of 31 TSS patients matched with 93 menstrual control subjects selected from a gynecologic clinic (94). The final study was published later that year (2), where 34/35 cases used tampons, versus 80/105 controls. Letters were sent to Wisconsin on 31 January 1980 summarizing the clinical features of TSS and mentioning the apparent association with menstruation (95). This communication may have provided a source of bias for case identification, as menstruation was not part of the case definition, and cases of non-mTSS may have been ignored. This study represented the first case-control study designed to investigate potential risk factors associated with TSS by asking the case patients various questions, including brands of tampons used, wear time, as well as contraceptive history. Several risk factors were noted: (i) tampon use was associated with cases of TSS, (ii) controls used contraceptives more frequently than cases, (iii) recurrences of mTSS cases were common, and (iv) a higher rate of mTSS was observed in women <30 years of age. It was reported that higher-absorbency tampons may be associated with alterations to the vaginal mucosa. It should be noted that cases were interviewed, while the controls completed a questionnaire. Tampon brands used by the women were identified via recall (potential for recall bias in early cases). The cases were questioned for tampon use in the menstrual period of TSS (which may have been as long ago as 1975), while the controls were questioned as to their present use of tampons; thus, there was another potential for recall bias by the cases included in the study. Wear time of tampons was not identified to be a risk factor.
The 19 September 1980 Morbidity and Mortality Weekly Report released preliminary data from an observational study in Minnesota reporting that 35% of 29 TSS cases and 18% of 50 matched controls used Rely tampons. No statistical comparisons were made (261). Before any of these studies began, Wisconsin made public reference to the possibility that TSS was associated with menstruation as well as tampon use. The 23 May 1980 Morbidity and Mortality Weekly Report (93) associating TSS with young women and menstruation also caused intense national media attention. Thus, another bias that may have impacted the results of investigations was the amount of publicity that occurred during the epidemiological surveys. In the National Academy of Medicine review of the information available on TSS (262), it was noted that publicity could lead to increased reporting and that the reported cases determine the composition of case-control studies. The report also noted that “the marked increase in TSS cases reported nationally with onset in August and September 1980 was undoubtedly related to the extensive media coverage of TSS and its association with tampons. The extent to which publicity may have biased TSS case reporting and caused a selective increase in TSS cases, who reported use of Rely before September 15, 1980, is difficult to assess.” The report went on to note that cases with Rely tampons may be overrepresented among the cases.
Epidemiological and Demographic Findings
Using a passive surveillance system, epidemiologists from the CDC reviewed information collected on 1,407 cases of TSS based on a nationwide surveillance system (96). Ninety-two percent (92%) were associated with menstruation, and almost all of those patients reported using tampons. The age distribution of mTSS cases ranged from 11 to 61 years. Sixty-five percent of the mTSS cases were in females younger than 25 years of age.
The highest numbers of reported cases were reported in Oregon, Utah, Minnesota, and Wisconsin, and of those, almost one-third of the total cases came from Wisconsin, Minnesota, and California, whereas 18 states reported five or fewer cases (96). The authors speculated that such differences could be due to differing levels of interest by state health departments and clinical investigators as well as regional variation of toxin-producing S. aureus and the availability and use of various tampon styles and brands. Temporal analysis of mTSS cases showed a small but steady increase starting in the spring of 1980; a rapid rise in summer, peaking in August and September (∼125 per month); and then a drop of cases almost in half during October 1980. Cases continued to be reported between November (n = ∼65) and December (n = ∼50), but by the early fall of September 1981, fewer than 5 cases were reported. The authors noted that the gradual increase in reported cases before the peak “probably reflected both a real increase in number of cases and improved recognition and reporting, and that changes in the use of different kinds of tampons may also have been factor[s], including a shift from using Rely.” The researchers stressed the importance for research to clarify the roles that the host, bacteria, and tampon may play in the development of mTSS and that the disease had not been eradicated (96). Almost all of the mTSS cases (97%) were diagnosed in white females.
A small number (n = 18) of cases, occurring between 1 December 1979 and 30 November 1980 in Oregon, which were not included in case-control studies conducted by the CDC, were sent questionnaires to complete (97). Two controls, consisting of one friend and one clinic control, were also matched to a case. Several differences from the cases were noted in the control groups. The average menstrual period for cases and friend controls exceeded that of the clinic controls, in addition to differences in marital status, family income, and the use of birth control. Rely tampons were used more often by cases during their index periods than by either of the controls. Two issues to consider with this study are the small number of cases and the long interval between the patient’s illness and the time when the questionnaire was administered (recall bias). In this study, the median number of tampon changes per day among tampon users was higher for the patients than for friend controls.
Due to the potential biases identified in the first CDC study, the CDC completed a second published study that focused on specific brands of tampons used by subjects (98, 99). Five brands of marketed tampons were part of the questionnaire, and it was noted that the “smaller number of non-Rely users among cases precluded the accurate estimates of risk among other tampon brands.” The population included 50 recent TSS patients who had not been included in the first study and 150 control subjects who were friends/acquaintances of the same sex, who lived in the same geographic area, and who were within 3 years of age of the controls; the CDC investigators telephoned each participant to complete information in the questionnaire. Cases had diagnosed disease during July or August 1980. The study was not performed in a blind manner, and the interviewers knew both the hypothesis to be tested and the case or control status of the interviewees. All cases used tampons during their illnesses, as did 125/150 (83%) controls. Seventy-one percent of the cases used Rely tampons exclusively, versus 26% of the controls. Cases and controls tended to use tampons with the same labeled absorbency 5 months after the completion of the original case-control interviews to assess changes. No new insights emerged on risk factors. The authors noted, however, that is was clear that “tampons alone do not cause TSS, as cases of TSS have been described in women using other menstrual devices and in nonmenstruating women and men.” The authors also noted that “regardless of brand name, high-absorbency tampons are associated with an increased risk of development of TSS.” The results were presented to the FDA; a small group of researchers, including Schlievert; and the Rely tampon manufacturer upon the completion of the study. The manufacturer voluntarily removed Rely tampons from the market on 22 September 1980 (86). They also provided refunds to consumers who had unused product and also launched a campaign to advise women against their use in the future. The CDC criteria for case identification were reiterated, and the association with S. aureus was mentioned.
Tampon manufactures were subsequently asked to place warnings on tampon boxes on 26 September 1980, and by 20 December 1982, regulations required consumer information to appear “prominently and legibly” in a package insert or on the box in ways that are understandable to the layperson.
Preliminary data from Utah (12 mTSS patients and 40 neighborhood controls) were presented in the 27 June 1980 issue of Morbidity and Mortality Weekly Report (94). All cases used tampons as menstrual protection, versus 80% of controls (not significantly different), and no other risk factors were identified at the time.
The Utah State Health Department also investigated TSS in its state (100, 101). Questionnaires were administered by a three-person team to 29 hospitalized TSS patients from Utah (onset of illness from January 1976 through August 1980) and approximately 4 neighborhood controls of menstrual age (n = 91). All cases were identified in women, and none were identified in men. The incidence of TSS was 14.4/100,000 in women aged 12 to 49 years. All questionnaires were administered in person by interviewers, and it was noted that the Utah State Health Department issued quarterly articles updating information on TSS to physicians, encouraging case reporting by them as well as self-reporting by patients. In addition, the disease received statewide reporting by the media. All cases reported tampon use during the month of illness, and 77% of controls used tampons. When tampon brand was evaluated as a risk factor, only patients and their matched controls, who used a single brand during menstrual illness, were included in the analysis. Sixty percent of cases versus 23% of controls used Rely-brand tampons. The relative risk for TSS was 6 times higher for Rely than for any other tampon if used as the only tampon during menses. Accuracy of recall, asked for by the study participants, may have presented a potential bias, as all women were interviewed after disease occurrence, and the oldest case was in January 1976. Controls were interviewed 1 week after the case interview.
The second published Utah study by Latham and colleagues (101) limited the study to hospitalized patients for whom medical records were available, and this led to the exclusion of many patients. It was noted that an active campaign encouraged case reporting and that the syndrome received statewide attention from the media. Fifteen TSS cases and 14 matched controls used a single brand (Rely) of tampon, and the relative risk was reported to be 6.1. Cases of TSS were reported with the Tampax (Tambrands, Palmer, MA), Kotex (Kimberly-Clark, Dallas, TX), and Playtex (Rochester, NY) brands as well, but no significant risk was identified. The study reported cases received by the Utah State Health Department from January 1976 through June 1981 but did not include or analyze data from ∼20 Utah cases that were reported after Rely was withdrawn from the market. The attack rate from January to September (1980) was 12.3/100,000 female subjects (12 to 49 years of age), and the rate of illness declined to 4.8/100,000 women during the following 9 months.
A decline in reported cases of TSS nationwide and in Utah after the removal of Rely tampons (22 September 1980) from commerce was not noted in all states. Minnesota continued active surveillance from early 1980 into 1982 and noted no change in the incidence after the removal of Rely tampons (102, 103). The results were not released until 1981, after the CDC completed the second nationwide study.
The often-referred-to gold-standard case-control study evaluated collective patients from Wisconsin, Minnesota, and Iowa (Tri-State TSS Study) using active surveillance methods (34, 35). The study was designed to evaluate risk factors associated with menstruation and therefore included only females. Incidence rates were not calculated. Cases (n = 80) with disease onset between 1 October 1979 and mid-September 1980 and twice as many (n = 160) age-matched neighborhood controls (±1 or 2 years) were enrolled in the study. Specifically, no cases involving carboxymethyl cellulose-polyester foam tampons were enrolled after the tampons were unavailable commercially. All interviews were done in person. Tampon absorbency (in vivo and Syngyna in vitro information provided by manufacturers) was evaluated as a risk factor. The study confirmed that tampon use during menses posed an increased risk; the added risk (odds ratio) among different brands varied from 5.9 to 27.2. In addition, Rely tampons had a higher relative risk than that predicted by absorbency alone. The added risk remained when absorbency was accounted for, and it appeared to be associated with brand’s regular-absorbency shelf-keeping unit (SKU) but not the superabsorbency SKU. Several other tampon brands had a higher risk than that experienced with no tampon use. The small number of tampon-brand styles in the highest- or lowest-absorbency categories, however, made it almost impossible to discern between the effects of tampon materials and absorbency on the risk of developing mTSS. It is important to note that no statistical significance was identified regarding tampon wear time and disease. There was, however, a trend toward significance at >13 h, but no statistically significant difference in wear time between TSS patients and controls was identified. Average wear times of a tampon by menstruating women in the United States are between 5.1 and 5.5 h (104) and between 7.6 and 7.8 h overnight (105) according to current reports. The wear time during the day is similar to that reported by the CDC. Cases in the TSS study also reported (i) a higher frequency of vaginitis within the preceding year and (ii) a higher occurrence of disease during menstruation preceding the index menstrual period.
Epidemiological studies have been conducted using results from the Tri-State TSS Study. The first one investigated the potential association between tampon absorbency and the risk of TSS. In order to investigate markers for other characteristics that create an environment that encourages the production of TSST-1 by S. aureus, results from the Tri-State TSS Study were used to estimate three tampon factors (oxygen content, absorbency, and chemical composition). Although few data were available, tampon oxygen content had the strongest risk of mTSS relative to absorbency or chemical composition. The authors concluded that “oxygen is either an important risk factor or a marker for another important risk factor not yet identified” (106, 107). The studies also investigated the possible effects of specific contraceptive methods and vaginitis on the incidence of TSS, controlling the confounding effects of recognized risk factors. A strong, positive association was found between TSS and tubal ligation. A negative correlation was noted with the use of oral contraceptives, and a positive association was noted if the woman reported recent vaginitis.
Researchers compared national TSS passive surveillance data on 285 tampon-associated mTSS cases (1983 to 1984) with data presented on age- and year-matched controls from national surveys of tampon usage (108). Women who used any tampon brand had an increased risk compared with non-tampon users (OR, 32.8; 95% confidence interval, 15.5 to 69.6). It is important to note that when tampon absorbency increased, the odds ratio for TSS also increased. Additionally, the odds ratios were also influenced by the chemical composition of the tampon. Polyacrylate-containing tampons had elevated odds ratios, but once tampon absorbency was controlled, they were lower than the odds ratios for cotton, rayon, and cotton-rayon-blend tampons. The authors noted that since there was a strong association of tampon absorbency with disease risk, the use of lower-absorbency tampons might lower the risk of TSS. It is important to note that control subjects were not matched geographically or racially, and the study excluded women with focal, nonvaginal sites of infection, even if the onset of illness occurred during menstruation.
A study by Miday and Wilson raised questions regarding the geographical distribution of TSS cases. Hospital record databases from the Commission on Professional and Hospital Activity Study (CPHA-PAS) were utilized to explore the incidence and geographic distribution (1 January 1981 to 30 September 1983) (109). Variations in TSS occurrence geographically were noted and were theorized to result from differences in surveillance activity. Minnesota, Wisconsin, and Utah were identified as states with intense surveillance efforts as well as higher reported incidence rates. The similar regional pattern found with the CPHA hospital-diagnosed cases indicated that these differences were real. Reported risk factors (female sex, menstruation, tampon use, and absorbency) had low variation from state to state and would not lead to a clear case distribution pattern. The highest incidence of TSS was noted for the Mountain region, at 4.5/100,000 (Montana, Idaho, Wyoming, Colorado, New Mexico, Arizona, Utah, and Nevada); the West North Central region, at 4.1/100,000 (Minnesota, Iowa, Missouri, North and South Dakota, Nebraska, and Kansas); and the Pacific region, at 3/100,000 (Washington, Oregon, California, Alaska, and Hawaii). Although cases were reported from all 50 states, marked regional differences in the incidence of the disease through the middle of June 1983 were noted in five states (Wisconsin, Minnesota, Colorado, Utah, and California). Reports from these states accounted for almost half of the reported cases, although they represented only a small percentage (16%) of the population at the time. Marked regional differences in the diagnosed cases may provide insight into the pathogenesis of this uncommon disease, and the authors indicated that there may be “one or more as yet unidentified risk factors that are regionally distributed” (109).
Active surveillance for TSS was established in 1986 in Washington, Tennessee, New Jersey, Missouri, Oklahoma, as well as Los Angeles County (110). One hundred sixteen definite and 63 probable cases were reported; 85% of the cases occurred in females, and the remainder (15%) occurred in males. Eighty-three cases, involving females (55%), were during menstruation, with a mean age of 23 years (range, 12 to 46 years) and an overall incidence of approximately 0.5/100,000. The cumulative incidence varied significantly by region, ranging from about 1.25/100,000 in Oklahoma to 0.20/100,000 in New Jersey. The incidence in the remaining locations ranged from 0.4/100,000 to 0.70/100,000. The incidence of mTSS was 1.05/100,000 in menstruating women (age range, 15 to 44 years). The peak in females occurred at the ages of 15 to 19 years (1.52/100,000). Although the authors recognized underreporting of cases in the passive surveillance system, they concluded that the proportion of mTSS cases identified through active surveillance was similar to that identified by the 1986 passive reporting system. Although active surveillance and education of hospital staff reduce the number of cases missed, they may not eliminate the diagnostic bias by health care providers who knew of the publicity linking TSS with females, menstruation, and tampons. The authors also reported a higher incidence of mTSS in whites than in nonwhites (1.21/100,000 versus 0.34/100,000).
One hundred twenty-five cases of TSS were reported to the Iowa Department of Public Health (1980 to 1986); 83% of the cases were mTSS (111). Of the 125 total TSS cases, almost one-half (49%) of the cases met all criteria for definite cases, and 30% were classified as probable TSS. Vaginal or cervical cultures were positive for S. aureus in 92% of the definite cases and 76% of the probable cases. In almost one-half (43%) of the definite cases, the woman used Playtex (Playtex, Inc., Dover, DE) tampons, in contrast to only 7% of probable and 19% of uncertain cases.
By 1989, both polyacrylate rayon and carboxymethyl cellulose-polyester foam had been removed from all tampons. Active surveillance for TSS was initiated in five states and Los Angeles County in 1986 through June 1987 (110). To evaluate the risk of mTSS with currently available tampon materials, another CDC case-control study enrolled 108 cases (71 definite and 31 probable cases), identified in the multistate surveillance study, and 372 controls (185 friends and 187 from the neighborhood). Ninety-eight percent had used tampons, and 81.5% had used a single brand (110). When in vitro and in vivo tampon absorbency, weight, oxygen content, and chemical composition were analyzed as risk factors, there was an increased risk with in vitro absorbency. The mTSS risk increased by 34% for each gram increase in tampon absorbency. The oxygen content correlated somewhat less well, and when corrections for absorbency were made, it disappeared as a risk factor. Initially, it was determined that cases were more likely to leave a single tampon in place for a longer mean maximum time. However, it was finally determined that longer wear time was not a risk factor for mTSS once absorbency and continuous use were accounted for in the analyses. Continuous tampon use for at least 1 day of the cycle was strongly correlated with the risk of mTSS after adjustment for absorbency. The influence of the chemical composition of any tampon could not be determined because of the size of the study. The results of this study supported the conclusions found in the Tri-State TSS Study. The investigators concluded that despite the many changes made in tampon construction and absorbency, the use of tampons in 1986 to 1987 was still associated with an increased risk of mTSS. The authors noted that one of the limitations of the study was that physicians were more likely to diagnose and/or report a case of mTSS if the patients used tampons during menstruation, and this factor could result in an overestimation of risk associated with tampon use versus no tampon use. Second, they noted that there were “insufficient cases to permit a meaningful assessment of the independent contributions of tampon absorbency, chemical composition, and other characteristics to the risk of developing TSS” (110).
The CDC (112) reviewed passive surveillance data and confirmed the downward trend in TSS that was previously noted in the 1986 active surveillance study. Both probable and confirmed cases were included. From 1979 to 1996, 5,296 cases (both mTSS and non-mTSS) were reported. Percentages of mTSS cases versus total TSS cases reported were as follows: 91% from 1979 to 1980, 71% from 1981 to 1986, and 59% from 1987 to 1996. Seventy-four percent of all TSS cases reported from 1979 to 1996 were menstrually related TSS. It should be remembered that a diagnostic bias toward menstrual cases may have existed in the early years of epidemiological research, as pointed out above, and that nonmenstrual cases did not come to the forefront of epidemiology research until 1982 (48). Less than half of the cases of mTSS reported the level of absorbency of the tampon used; of the 41% of cases who reported absorbency, 28% used regular and 71% used superabsorbent tampons. It was not specified if this was the tampon absorbency used throughout the menstrual period or if it was the absorbency used immediately prior to the diagnosis. The authors noted that one of the important changes in TSS epidemiology was the trend toward more nonmenstrual cases, specifically those associated with surgical procedures. In addition, the case-fatality rate of non-mTSS cases remained constant, although the case-fatality rate of mTSS declined. The authors attributed this decline in mTSS to several factors, such as standardized labeling required by the Food and Drug Administration (FDA), greater awareness of TSS among women, and education of the consumer via materials such as package inserts. The authors recommended further studies to clarify the risk factors associated with non-mTSS.
Using ICD-9 codes, an active surveillance study was completed during the years 2000 to 2006 in the Minneapolis-St. Paul, MN, region. This population-based surveillance for TSS utilized the CDC criteria for defining cases of TSS (113). The results of the study suggested a stability in the incidence of TSS (mTSS and non-mTSS) during the years 2000 to 2003, compared to the late 1980s. ICD-9 codes were used to identify potential cases of TSS diagnosed between 1 January 2000 and 31 December 2003 from 24 hospitals. The average annual incidence for all TSS cases was 0.52 cases/100,000 persons; that of mTSS was 0.69, and that of non-mTSS was 0.32. Younger females (aged 13 to 24 years) had the highest incidence, at 1.41/100,000 persons. There were no changes in incidence rates to indicate an increase in the number of TSS cases from the years 2000 to 2006.
mTSS continues to occur in the United States, even though significant public health efforts have occurred, such as the elimination of polyacrylate, carboxymethyl cellulose, and polyester foam from tampons; absorbency labeling standardization; and public safety announcements that have been put in place. Various approaches have been utilized to estimate the incidence, including discharge diagnosis, chart review, and passive or active surveillance. The best numbers place the current incidence at 0.5 to 1.0/100,000 population (113, 114). A summary of much of the published information appears in Table 5.
Knowing the vaginal colonization rate of TSST-1 S. aureus, the approximate percentage of women using tampons, the percentage of females 12 years of age or older lacking protective antibodies to TSST-1, and the approximately one-third of women who may not be able to transport TSST-1 across the vaginal mucosa, the maximum incidence of mTSS should be approximately 10/100,000 persons. This calculated incidence (P. M. Schlievert, unpublished data) agrees well with the highest reported incidences from early epidemiology studies.
TAMPONS AND TSST-1 PRODUCTION
Factors That Affect TSST-1 Production
The important factors that promote TSST-1 production are summarized in Table 6, providing critical data to help explain the tampon association with mTSS. TSST-1 is produced by USA200 strains at body temperature (37°C), with smaller amounts of TSST-1 produced at 37°C than at higher temperature (40°C) (31). Additionally, TSST-1 production required pHs of >6.5 but less than 9.0; vaginal pH during menstruation is pH 6.8 to 7.2 (31). It is worth noting that cases of mTSS may occur in the absence of menstrual blood, providing the basis for the CDC considering cases as mTSS that include 3 days prior to menstruation (115). Hormone changes just prior to menstruation reduce the ability of lactobacilli to control vaginal pH, allowing TSS S. aureus to grow to high numbers, even in the absence of menstrual blood. TSS S. aureus requires protein for TSST-1 production, with glucose functioning as a catabolite repressor of toxin production (31). S. aureus vaginally may grow from undetectable at times other than menstruation to an estimated 107 to 1011 bacteria vaginally during menstruation (116). It has been shown that S. aureus numbers vaginally peak on days 2 to 3 of menstruation, with the peak onset of mTSS occurring on days 3 to 4 of menstruation (116).
TABLE 6.
Environmental factors that affect TSST-1 production by USA200 S. aureus
| Factor | Effect(s) on TSST-1 production |
|---|---|
| Temp | 37°C upregulates; 40°C leads to greater upregulation |
| pH | pH 6.5 to 8.0 is optimal; pHs below 6.0 and at 9.0 are strongly inhibitory |
| Protein | Required |
| Glucose | Represses |
| Oxygen | ≥2% required |
| 5% carbon dioxide | Stimulates in the presence of oxygen |
Importantly, oxygen is required for TSST-1 production; S. aureus can grow anaerobically through fermentation, but TSST-1 production requires oxidative metabolism (31). The 1983 study by Schlievert and Blomster suggested that the role of tampons in mTSS was to introduce oxygen into a usually anaerobic environment. This would also explain why the risk for mTSS in general increases with tampon absorbency, namely, through the introduction of more oxygen in the tampon. In 1989, the role of air as an environmental factor required for TSST-1 production was confirmed (32).
In more recent studies, Yarwood et al. determined that conditions above 2% oxygen were needed for S. aureus to produce TSST-1; the presence of CO2 enhanced the oxygen effect (117). Yarwood and colleagues (118, 119) also demonstrated that there is a two-component system, referred to as SrrA/B, that directly or indirectly senses oxygen. Under anaerobic conditions, this two-component system represses tstH expression, whereas >2% oxygen derepresses the system, leading to TSST-1 production. The redox potential across the bacterial plasma membrane may be the actual signal sensed by SrrA/B (117–119). TSST-1 production is also partially regulated by other two-component systems, but those systems are regulated by SrrA/B and are not discussed in this review. They do not directly impact the tampon association with mTSS.
There remains at least one major problem with the oxygen theory. As many as 60% of women who developed a first episode of TSS will develop recurrences with continued tampon use (35). Yet a significant percentage of women using tampons associated with the first episode will develop recurrences even without the additional use of tampons. So far, there is no good explanation except to suggest that it is possible that those women developed TSS due to S. aureus colonization at mucosal sites other than the vagina. However, it is clear that a source of oxygen for S. aureus must be present for mTSS to occur.
History of Tampon Use
To discern the factors associated with device-associated mTSS, the history of U.S. regulations associated with tampon use; the impact of tampon use; physiological changes during menstruation, especially with younger users; menstrual fluid composition; the immune response to TSST-1; the vaginal microbiota; and vaginal epithelial characteristics are reviewed.
In the Western Hemisphere, 70% of women report using tampons during menstruation (120). In a descriptive research study, it has been reported that ∼80% of women (≤41 years of age) use tampons during menstruation (121). Women in the United States spend more than $2 billion on menstruation control products per year, and over an American woman’s reproductive lifetime, it has been estimated that she will use 16,800 tampons and pads before the onset of menopause (122).
The first tampons were made from a variety of materials found in nature. Ancient women in Egypt wound rolls of soft papyrus, Japanese women used paper, Greek women tied lint around pieces of wood, and Hawaiian and African women used native plants (123). In 1879, the British Medical Journal described “Dr. Aveling’s Vaginal Tampon-Tube” in its section on the latest innovations in medicine. It described a complex device complete with a “small unsilvered glass vaginal speculum, with a wooden rod,” used to insert 4 cotton wool pledgets connected with a stout string. A Colorado-based physician, Earle Haas, introduced the first commercial applicator tampon and patented it in 1933. It was to replace the bulky pads that his wife wore during menstruation. The tampon consisted of compressed cotton with a withdrawal string and could be inserted and removed without having to be touched. An applicator made of cardboard tubes would telescope and thereby insert the tampon into the vagina when engaged. Only the fingers of the hand would touch the applicator, not the tampon. The product was called Tampax, a combination of “tampon and vaginal packs.” Consumer Reports (1942 technical section, p. 157 to 158) noted that “tampons appealed to many women because of their compactness and small bulk…and they interfered less with normal activities than do pads.” Seven years later, a review of the product noted that women made “two primary demands of tampons: that they be comfortable and provide adequate protection” (263, 264). Tampons made history in 1983 and were included on the space voyage for astronaut Sally Ride (124).
Tampon Materials and Design
Modern tampons are mainly composed of cellulose absorbent material, either rayon or cotton, or a mixture of these fibers (125). A thin, smooth layer of nonwoven or perforated film is wrapped around the tampon to make insertion easier, and the addition of a cotton or other fiber cord is added to aid in removal. The overwrap also serves to reduce the loss of core fibers during wear. Tampons are each wrapped before being packed into cartons. The use of an applicator aids insertion, and once used, it can be disposed of in the tampon wrapper. Tampons that expand radially are most likely composed of a coiled fiber pad. These types of tampons either can be digitally inserted or need an applicator. Tampons made from a rectangular pad have the withdrawal cord sewn across the pad, and it is then compressed into a cylinder and utilizes an applicator to aid insertion into the vagina (see https://www.edana.org/docs/default-source/infographics/tampons-infographic-final.pdf?sfvrsn=4 for general information on how tampons are made via an infographic).
First Clinical Studies of Tampons
A study was designed (126) to determine if “self-tamponage” was an effective method “to collect catamenial discharge of women” and noted that the tampons constructed for the test (1.8 to 3.5 g) and used as the sole catamenial device for menses were not efficient in absorbing fluid. Dickenson surveyed the literature and identified 19 sources that collectively involved more than 6,500 women, 90% of whom approved of tampon use and were satisfied (127). This was particularly true of younger, educated women. Tampons absorbed 2 to 4 oz of fluid and minimized odor. Since that time, clinical safety-in-use studies have been conducted and published, indicating no adverse effects of tampons on key vaginal microbial species, vaginal tissue, or irritation (128).
Regulation of Tampon Safety in the United States
The enactment of the 1938 Food, Drug, and Cosmetic Act gave legal authority to the U.S. Food and Drug Administration to oversee the safety of drugs and food as well as protect consumers from unlawful cosmetics and medical devices. This law, as amended, is still in force today (129). Since 1976, the FDA has been required by law to classify and regulate the safety and performance of medical devices; tampons are class II medical devices (130).
On 22 June 1982, the final FDA regulation mandated to manufacturers that information sheets describing TSS and warning of the risk of tampon use be placed in all boxes of tampons (Federal Register [47 FR 26982]). Based on the Tri-State TSS Study data on absorbency, it was also required that tampon labeling advise women to use a tampon with the minimum absorbency needed to control menstrual flow. FDA testing demonstrated that terms such as junior, regular, and superabsorbent did not mean the same thing from one brand to another (131). The final rule standardized the four existing terms as follows, utilizing data from the in vitro Syngyna test (265–267): 6 g or less of fluid absorbed for junior, 6 to 9 g of fluid absorbed for regular, 9 to 12 g of fluid absorbed for super, and 12 to 15 g of fluid absorbed for super plus. Ultra-absorbency (15 to 18 g absorbed fluid) was subsequently added.
The FDA did not require the removal of polyacrylate fibers from tampons. A federal district court judge in Wichita, KS, used a remittitur of punitive damages in exchange for a product recall in O’Gilvie v. International Playtex (132). A remittitur is a ruling by a judge during a civil case and comes into play when the judge orders lowering the amount of damages granted by the jury to the plaintiff. In this case, the judge announced that he would remit nearly 90% of a punitive damage award if the defendant acknowledged the jury’s findings as fact and removed polyacrylate tampons from the market. The manufacturer (Playtex, Stamford, CT) announced the recall of the product and outlined a TSS public information campaign. Other companies using polyacrylate rayon fibers followed suit and removed them from tampons at the same time. Today, tampon absorbent core material in the United States is limited to cotton, viscose rayon, or a blend of these fibers.
A manufacturer who intends for a menstrual device to enter commerce in the United States should conform to the general controls of the federal Food, Drug, and Cosmetic Act, including premarket notification as described in 21 CFR 807 subpart E, and obtain a “substantial equivalence” determination from FDA prior to marketing the device. The manufacturer develops and submits premarket notifications. They follow the FDA guidance document (133):
FDA developed a guidance document to assist industry in preparing premarket notification submissions (510(k)) for menstrual tampons and pads that are subject to 510(k) requirements. This document covers the recommendations for the content of 510(k) submissions for these devices. Depending on any unique design, technology, indications, and performance characteristics of a given product, FDA may recommend additional information not described in this guidance.
Manufacturers are required to submit to the FDA a detailed risk assessment of the device components, the design, and test results for review and comment before a tampon is introduced into commerce.
Manufacturers of tampons also complete postmarket surveillance to ensure that their product is safe for consumer use. If they note a signal in the surveillance data, they may announce a voluntary product recall or withdrawal or a safety alert. The FDA posts the company’s announcement as a public service. As an example, on 11 December 2018, Kimberly-Clark announced a voluntary recall of Kotex Sleek tampons, regular absorbency, throughout the United States and Canada. Specific lots were listed as the company received consumer reports that they were “unraveling and/or coming apart upon removal” (134).
Menses Composition and Physical Characteristics
The process of menstruation occurs at the end of a woman’s reproductive cycle. This occurs after the corpus luteum shrinks, leading to decreases in progesterone. Menses is a complex biological fluid and consists of blood, desquamated endometrial tissue, vaginal epithelial cells, cervicovaginal secretions, and endogenous vaginal microbes (135). Fluid loss varies from 43 ml in women with low flow who use tampons to 213 ml in women with high flow who use only pads (136). The concentrations of many elements in menses are lower than those in venous blood. The blood content of menses depends on the extent of endometrial breakdown and dilution of blood- and tissue-derived constituents with cervicovaginal fluid. Vaginal fluid in menses contributes principally water, electrolytes, organic moieties, and up to 14 proteins with a molecular weight of up to 82,000. Menstrual blood is different from circulating blood, as evidenced by analyses of the two proteomes. Three hundred eighty-five unique proteins were identified in menses, compared to the 1,774 proteins in the proteome of circulating blood (137). Menstrual fluid pH is generally neutral, and the median pH is 7.2 (138). Menses are also depleted of certain clotting factors and have low platelet counts and activity (139). What appear to be menstrual blood clots actually represent blood-tissue agglomerates (135). The viscosity of menses is dependent on the shear rate and temperature with viscosity (decreased viscosity with increased temperatures and shear rates) (135). Menstrual blood loss in women has been reported to range from 36 ml to 79 ml (136), with up to 183 ml of fluid with a corresponding blood loss of 110 ml in women complaining of heavy menstrual bleeding. Menses contain matrix metalloproteinases (MMPs) from the sloughed endometrium lining the uterus. MMPs are activated in response to progesterone withdrawal during menstruation (140). In endometrial tissue, IL-1 type I receptor mRNA expression was evident throughout menstruation. IL-1 type I receptor mRNA expression levels are 4-fold higher in endometrial tissue that is sloughed during menses (141). PTSAgs, particularly TSST-1, are potent inducers of IL-1β from macrophages (142, 143) and contribute to the pathogenesis of TSS (144).
By investigating the local immune vaginal response, vaginal IgG levels are at the highest values after menstruation, and IgA levels are low throughout the cycle, close to the limit of detection of the assay (145). It was hypothesized that the contribution of immunoglobulins (IgA and IgG) through secretion and transudation into the vagina and its lumen is small. Total anti-TSST-1 antibody levels in vaginal lavage fluids from healthy women are negligible compared to serum values of healthy subjects (titer range of 2.5 to 23 in vaginal lavage fluid versus 1,476 to 1,540 in sera) (146). In contrast to the results for sera, total antibody titers in lavage fluid during menses are significantly higher than those during nonmenses, possibly due to the presence of menstrual blood. Interestingly, no anti-TSST-1 IgA could be identified during any phase of the menstrual cycle. The only isotypes detected in menstrual vaginal lavage fluids were IgG1 and IgG4 albeit at low values.
Menstrual fluid also contains oxygen. Mean values for both O2 and CO2 estimated for menses are 42 mm Hg PO2 and 43.5 mm Hg PCO2. These two values are in the range expected for venous blood (147). Using batch culture of TSST-1 S. aureus strain MN8 in the presence of CO2, TSST-1 production increased from low levels in the presence of 2% O2 to 2 μg/ml in the presence of 21% O2, demonstrating that both O2 and CO2 control TSST-1 production (117).
Previously, it was observed that staphylococcal exotoxins are not produced in vitro when S. aureus is grown in human blood and menses (115, 148). Culture conditions were ideal for exotoxin production, such as temperatures near 37°C, pH near neutrality, and protein-based medium, with a minimum above 2% O2 and 7% CO2 (31, 117). Human blood was fractionated into plasma and water-lysed red blood cells. Mixtures of the alpha- and beta-globins of hemoglobin inhibited exotoxin production but increased the production of staphylococcal protein A. Bacterial growth was not affected (148).
Puberty is an important developmental stage that has both physical and physiological changes that in females lead to breast development, hair in the pubic region and armpits, and the onset of menstruation, which usually occurs last. Information on normal values for peripheral blood T cells and other mononuclear cell subsets (involved in responses to TSST-1) in older children and adolescents is limited (149, 150). Researchers have found that the CD4+-to-CD8+ ratios were higher in males, but others found the ratios to be higher in females. Also, it has been suggested that the ratio was higher in blacks than in whites and that adolescent females aged 12 to 18 years had a higher proportion of CD4+ cells than males aged 17 to 18 years.
Characteristics of Adolescent Females
Initially, adolescent females experience irregular menstrual periods, and most are anovulatory due to immaturity of the hypothalamic-pituitary-ovarian axis. Ninety percent of menstrual cycles will occur within the range of 21 to 45 days. Variation happens, with short cycles of <20 days and long cycles of >45 days. By the third year after menarche, 60 to 80% of menstrual cycles are 21 to 34 days, as is typical of adults (151). Sex steroids change during pubertal development and continue to fluctuate in diurnal or monthly rhythms (152–154). Progesterone and/or estrogen may affect immune responses (149, 150).
Cervical ectopy is commonly found in adolescent females. The squamocolumnar junction is composed of tissue that lines the endocervix and extends onto the proximal portion of the cervix, immediately adjacent to the multilayer squamous epithelium. The transformation into squamous epithelium from columnar epithelium does not occur until puberty, leading to areas of immature tissue. Frequently, adolescents have large areas of cervical ectopy, which refers to the area of the ectocervix lined by the columnar epithelium. This thin tissue is friable due to high vascularization. Although the columnar epithelium of the cervix eventually transforms into squamous epithelium via metaplasia, this process is relatively silent until the onset of puberty. This results in relatively large areas of immature epithelia in adolescents (89).
Culture-independent methods have been applied to characterize the vaginal microbiota of adolescents. Vaginal swabs from 90 menarchial adolescents (aged 13 to 18 years) were characterized using terminal restriction fragment length polymorphism (T-RFLP) analyses of 16S rRNA genes (155). Four major clusters accounted for 97% of the cohort and could be divided into those dominated by Lactobacillus spp. and those dominated by a variety of lactic acid-producing, anaerobic bacteria, such as Atopobium vaginae and Streptococcus spp. The compositional and structural similarities of the vaginal microbiota of monarchial adolescents to that of adults suggested that the vaginal microbiota does not change significantly after the onset of menarche (155).
Tampon Characteristics
For more than a century, the compressed-fiber tampon has been cylindrical. The human vagina, however, is not. The vagina is the canal to the cervix, which separates the uterus from the vagina (127). The vaginal mucosa is made up of a nonkeratinized, stratified squamous epithelium that helps the vaginal walls stretch. The vaginal length varies from 7 to 15 cm (3 to 6 in.), with a range of widths of 5 to 6 cm (2 to 2.5 in.) (156). These figures were based on measurements from Caucasian women, and more variation was noted when data from African-American and Hispanic women were included (157). In the dorsal posture, the vaginal cavity holds 10 ml of fluid, and relaxed, it holds 20 to 45 ml of fluid. The vaginal surface is estimated to be 72 ± 21 cm2 (range, ∼34 to 164 cm2) (158).
The lateral spread of the passage is greatest at its upper end, where it reaches a breadth of 60 mm. The right lateral fornix is usually the deeper, the cervix is not in the midline, and the tampon often slips on one side of it if not behind it (127). An important factor is the sling of muscular and fascial layers that sweep downward and backward along the sides and rear of the passage, forming a sphincter group that retains the tampon within its upper two-thirds (127).
Risk factors associated with tampon use identified via epidemiological studies have been further evaluated in biological studies. One major factor thought to be necessary for TSST-1 production is an aerobic environment, and such an environment is present vaginally through the oxygen contained in menses as well as through the inherent oxygen contained within tampons (147). It is important to note that recent research refutes the initial theory that the vagina becomes oxygenated by the process of inserting a tampon by delivering a bolus of oxygen to the environment (159, 160). The previous work placed bulky Clark electrodes with the faces toward the tampon and more than likely measured the oxygen content of the tampon. Hill et al. (147) noted that the cervicovaginal partial pressure of O2 was in the hypoxic range of 4 to 14 mm Hg (2%) using Neotrend sensors (161) that could monitor the gaseous concentrations on the vaginal surface (147). The vaginal oxygen levels in both the midzone and cervical zones dropped with the insertion of tampons. Both studies noted that oxygen is contained in the void spaces of a tampon (147, 160). However, during tampon use, levels of O2 in the tampon decreased, and levels of CO2 increased over several hours. Tampon absorbency, menses loading, and wear time influenced the kinetics of changes observed. Colonization by S. aureus had no effect on gas profiles during menstruation.
Under low-oxygen conditions, vaginal lactobacilli produce primarily lactic acid, but in air (21% oxygen), many strains of lactobacilli preferentially produce acetic acid (162). A major factor considered necessary for TSST-1 production is the presence of an aerobic environment. We have shown that such an environment is present vaginally through the oxygen in menstrual blood as well as through the inherent oxygen in tampons (147). Statistical analysis of absolute vaginal gas levels in vivo showed no significant differences in the values measured by the sensors placed in the cervical midzone regions of the vagina throughout the course of these experiments (147).
Vaginal Mucosa
The healthy vaginal mucosa of reproductive-age women is comprised of a multilayered, stratified squamous epithelium that rests on a lamina propria. It is many cell layers thick, is lined by a stratified nonkeratinized epithelium, and is devoid of mucous-secreting cells. The lower part of the cervix (ectocervix) is composed of squamous epithelial cells, resembling the vaginal epithelium, while the cervical canal (connecting the vagina with the uterine body) is lined with columnar epithelial cells and contains underlying glandular structures (163).
External skin and the mucosal surfaces that line orifices of the body barriers form barriers to most bacterial pathogens and their exotoxins. Previously, it was demonstrated that TSST-1 upregulates over 500 genes and stimulates chemokine production from human vaginal epithelial cells through human CD40 (26, 164, 165). Multiple studies indicate that epithelial cells are important for the initiation of harmful inflammatory responses to disrupt the mucosal barrier and facilitate TSS onset (80, 81, 166).
To summarize the importance of the local environment in the development of mTSS (Fig. 3), (i) the submucosal vaginal environment is critical to the development of mTSS; (ii) intercellular ceramides contribute to a robust permeability barrier (163, 167, 168); (iii) some surfactants enhance the penetration of TSST-1; (iv) slight increases in temperature from 37°C or topical application of endotoxin does not the enhance penetration of TSST-1 (168); (v) TSST-1 binds to CD40, and the downstream consequence of this binding is epithelial cell production of chemokines that attract other components of the adaptive immune system (26); and (vi) the specificity of TSST-1 for menstrual TSS is in part dependent on higher-affinity interactions with CD40 (26).
FIG 3.
Model for the production of mTSS. S. aureus bacteria (purple spheres) are located as thin films on the vaginal mucosa. TSST-1 is produced under the conditions listed. TSST-1 binds to CD40 on human vaginal epithelial cells (HVECs). HVECs produce chemokines, which attract the immune system and lead to vaginal barrier disruption. CD4 T cells and macrophages are activated to produce interleukin-1, TNF-α, TNF-β, interferon gamma, and interleukin-2. These cytokines cause mTSS.
The surface epithelial layers of the stratified squamous mucosa form a permeability barrier that protects the deeper tissue. Once produced, TSST-1 must bind and penetrate the vaginal mucosa (79). It has been demonstrated that TSST-1 penetrated Caco-2 cell lines, modeling the intestinal, pseudostratified columnar epithelium (169). The histological architecture of the human vaginal mucosa is quite different, however, and includes a lipid permeability barrier composed of ceramides between the various layers of cells. Using a porcine vaginal tissue model with a ceramide composition like that of humans, we demonstrated that TSST-1 caused a non-dose-dependent rise in mucosal permeability and crossed the intact mucosa at a low rate, without disrupting tissue integrity. In incised vaginal mucosa, however, TSST-1 induced subepithelial tissue separation and atrophy that were similar to those in vaginal lesions that were reported in fatal cases of mTSS (167).
No irritation in the vaginal or cervical tissues after the use of tampons was found in 21 patients (170). Thornton came to the same conclusion after evaluating 110 subjects who used tampons for 1 to 2 years (171). A decade later, another study concluded that “there was no evidence that vaginal tampons are prejudicial to health” after tampon usage, noting no evidence of local inflammation, irritation, or alteration of pH or the glycogen content of epithelial cells (172).
Reports in the early 1980s (173, 174) indicated that prolonged use may contribute to microulcerations. Suggestions were made by the coauthors that this occurred when tampons, which are no longer sold, were worn at nonmenstruation times. Berkley et al. (108) determined that vaginal mucosal drying and layering were more common in all tampon users than in users of pads, most commonly in the rayon polyacrylate tampon group. Those tampons were withdrawn from the U.S. market in 1985. It is recommended that tampons be used only during menstruation and that the tampon absorbency should match the menstrual flow rate.
To compare the etiologies of various vaginal changes after intercourse, tampon use, and the use of contraceptive devices, 107 sexually active women (aged 18 to 35 years) were enrolled and evaluated by colposcopy (175). This study confirmed that relatively superficial vaginal conditions, which do not merit the pathological designation of “lesion,” are common in women under usual circumstances. Small pinpoint bruises (petechiae) were the most commonly noted changes. Sexual intercourse in the previous 24 h was most commonly associated with an increased frequency of epithelial changes. There was also a positive link between the number of minor vaginal changes and smoking (175). It is known that cigarette smoking has negative consequences on the squamous epithelium and makes it more susceptible to minor changes (176).
S. aureus and Tampons
Tampons are not the vaginal source of S. aureus. In a microbiological survey of almost 4,000 unused tampons in Australia, none were contaminated with S. aureus (177). Tampons were also inoculated with 1 × 106 S. aureus cells/ml with 0.1% peptone water plus 0.01% Tween 80, and the authors noted decreases of several logs by day 3, to almost undetectable cell numbers. In studies carried out in the United States, S. aureus was not recovered from unused tampons remaining in tampon boxes used by mTSS patients or tampons remining on shelves in stores (1, 178). Briancesco et al. (179) reported that the tampons evaluated in their laboratory were found to be of “good hygienic quality” and stressed the importance of good manufacturing practices during their production. It was hypothesized at this time also by the CDC that unused tampons may contain coagulase-negative staphylococci that produced TSST-1; the TSST-1 gene could be transferred to S. aureus. Our laboratories participated in a study of 22 coagulase-negative staphylococci that were isolated by the CDC from a large number of unused tampons; none produced TSST-1 (178).
S. aureus is a common and sometimes persistent colonizer of human epithelia as well as an important human pathogen. The percentage of healthy women who are colonized with S. aureus appears to be very consistent from one study to another. Healthy women can be colonized with S. aureus at various anatomical sites. Linneman et al. isolated S. aureus from the labia or vagina of 9.2% of women (n = 808), and approximately 13% of the strains were TSST-1 positive (180). The use of systemic antibiotics within 2 weeks of the vaginal culture decreased the rate of recovery. In the most recent highly cited studies, healthy, menstruating women (aged 13 to 40 years) were recruited from 5 separate geographical regions (Cincinnati, OH; East Brunswick, NJ; St. Petersburg, FL; Scottsdale, AZ; and Winnipeg, Manitoba, Canada) in North America (181, 182). S. aureus (TSST-1 positive and negative) colonization in three anatomical locations (nasal, anal, and vaginal) were documented in correlation with anti-TSST-1 IgG antibodies. Several groups of women were monitored to determine the answer to two questions: (i) Is carriage of S. aureus persistent within unique anatomical niches? and (ii) Could women without anti-TSST-1 IgG seroconvert once exposed to S. aureus? No significant differences were found in the rates of S. aureus colonization or toxigenic S. aureus colonization according to the geographic area, or across age groups, from which the subjects were recruited. No significant differences were found in the rates of S. aureus colonization (25 to 30%) across all body sites for the age groups evaluated. Twenty-eight percent of white subjects were colonized with TSST-1-positive S. aureus, which was more common than colonization with the same organism from African-American, Hispanic, or Asian subjects. This difference was not statistically significant. A significantly higher percentage of African-American subjects than European-American subjects were colonized vaginally with S. aureus (14% versus 8%).
The follow-up study (181) investigated the persistence of S. aureus and serum antibody (anti-TSST-1 IgG) among select groups of menstruating women, recalled from the previous study (182). Nasal carriage of S. aureus persisted and was the best predicator of subsequent colonization. Transient colonization was more common in anal and vaginal sites. Antibodies in women colonized with toxigenic S. aureus remained skewed toward higher titers, independent of whether the organism was persistent or transient. This suggested that colonization, at some point in time, was sufficient to elevate antibody titers, and those titers were persistent. The results also indicated that women can become seropositive without experiencing signs or symptoms of mTSS.
Bacteria can exist in biofilms, in which the bacterial community is encased in an extracellular polysaccharide-protein-DNA matrix and attached to a surface, either inert or living tissue (183, 184). There is cell-to-cell attachment and retention of biofilm macromolecules on the cell surface, as regulated by staphylococcal Agr, in fibrin-based aggregates (185). Veeh et al. (186) designed an elegant experiment to determine if bacteria (specifically S. aureus) could adhere to the vaginal mucosa and/or tampons during menstruation. Fluorescent in situ hybridization (FISH) probes were constructed and validated in order to examine vaginal epithelial cells and tampons from two groups of women who were determined to be either culture positive or culture negative for vaginal S. aureus. FISH detected S. aureus in all 44 specimens, and small S. aureus biofilm aggregates were observed in 37/44. Confirmation of the presence of S. aureus in specimens from all 18 women was performed by PCR of an S. aureus-specific nuclease gene. The results demonstrated that S. aureus small biofilm aggregates can form on tampons and menses components in vivo. Additionally, the research suggested that vaginal S. aureus carriage is more common than what is demonstrated by standard culturing techniques. It should be remembered that that vaginal mucosae turn over rapidly, and thus, the surface is not static to allow large biofilm communities to form. Also, the average tampon wear time (as discussed above) is less than 6 h, and given the doubling time of the organism, this would not allow large microbial communities to form on the inert fibers of the tampons.
Tampon Characteristics and TSST-1
A vaginal tampon can be a frequent yet incidental finding in a menstruating female who is imaged with multidetector computerized tomography (MDCT). The tampon conforms to the shape and orientation of the vaginal canal and the space occupied by the tampon. Due to the gas (oxygen) that is inherent with the tampon and located between the fibers, it has an appearance similar to that of air. The air-filled tampon acts as a negative contrast allowing visualization of the vagina and cervix (187).
When tampons are inserted into the vagina, they tend to rest in either the left or right fornices of the vagina and at least 2 cm from the cervical os. Through an analysis of radiographs of tampons placed in situ, it was demonstrated that none of the participants had a tampon directly blocking the cervix (188). The authors concluded that an “intravaginal tampon does not tend to produce a blockage of [menses] within the vaginal vault. The anatomic arrangement, as well as the muscular activity in the pelvic structures and the various body movements, tend to direct the vaginal tampon away from the cervical os, principally into one of the lateral fornices” (188). The data support the function of the device, which is to absorb fluid, wicking fluid into the central core of the tampon, not forming a plug.
One of the tampon innovations that increased absorbency was the introduction of rayon, a natural, refined fiber made from the cellulose of wood pulp. Unfortunately, there is misinformation available, suggesting that this natural fiber is less safe than “all-natural” cotton.
There are three major in vitro methods to assess device (tampons, menstrual cups, menstrual discs, diaphragms, and wound dressings) effects on S. aureus growth and TSST-1 production. These include the “shake flask” method, in which devices are placed into Erlenmeyer flasks with culture medium and S. aureus. The flasks are then shaken for designated periods of time, and S. aureus numbers and amounts of TSST-1 are determined (189). This test evaluates if the component parts of devices are antibacterial and/or inhibitory to TSST-1 production. Another method is to add tampons (and other devices, if desired) to culture medium plus S. aureus such that the tampons will absorb a known amount of medium; nonabsorbent devices should be added to the medium such that the culture medium is no more than 3 mm above the device. We have experimentally shown that sufficient oxygen can penetrate to 3 mm to provide this requirement (117). The cultures are then incubated, stationary, for the desired periods of time, and S. aureus counts and TSST-1 amounts are determined. This method, like the shake flask method, is designed to assess the effect of component parts of devices on S. aureus and TSST-1 production. The latter method is referred to as the “device sac” method (190). Tampons and other devices are added inside dialysis tubing containing S. aureus. The dialysis tubing is then submerged beneath soft agar and incubated for the desired periods of time. The effect on S. aureus growth and TSST-1 production inside the dialysis tubing is then assessed. This assay restricts exposure to oxygen to that within the devices. This assay makes use of the fact that the only exposure of S. aureus to medium is that brought into the dialysis bag from the soft agar, and TSST-1, as produced within the dialysis sac, remains within the sac due to molecular weight restriction. In a recent publication, Schlievert provides a clear description of all three of these methods (191). Figure 4 provides an example of a tampon sac.
FIG 4.

Example of the in vitro “tampon sac” method for assessing the effect of tampons (or other devices) on S. aureus and TSST-1 production. The tampon is inserted into dialysis tubing containing S. aureus without culture medium. The tubing is then submerged beneath soft agar. Nutrients required for S. aureus growth may be absorbed from the soft agar. Oxygen required for TSST-1 production is available only from within the tampon. Neither S. aureus nor TSST-1 can penetrate through the dialysis tubing. Upon completion of incubation for a designated time period, stationary at 37°C, the contents of the dialysis tubing are assayed for S. aureus counts and TSST-1 quantities.
Schlievert compared tampons made of 100% cotton and a mixture of cotton and rayon to determine the effects on the growth of S. aureus as well as TSST-1 production (192). Under stationary in vitro conditions, the same amount of or more TSST-1 was obtained with cotton tampons than with cotton-rayon tampons. It should be noted that test conditions included tampons that were either oversaturated, 50% saturated with culture medium, or assayed by a tampon sac method (190), designed to mimic a tampon in the vagina. The samples yielded the same amount of or more toxin in cotton tampons than in cotton-rayon tampons. Bacterial cell numbers generally paralleled toxin production. These data demonstrated that cotton tampons neither prevent TSST-1 production nor adsorb toxin onto the fibers to make toxin unavailable to cause TSS, in contrast to data from a previous study (192). A year later, Parsonnet et al. (193) demonstrated that there was no difference in TSST-1 when cotton versus cotton-rayon tampons were evaluated. The authors reported that after completing the studies in a double-blind manner and evaluating 16 commercially available tampons, “neither cotton nor rayon amplifies production of TSST-1 in vitro, and cotton tampons cannot be claimed to be inherently safer on the basis of such data.”
To summarize the cotton-versus-cotton-rayon controversy, two separate, independent studies failed to reproduce previous reports asserting that rayon was associated with a higher risk of TSS than cotton (194, 195). Epidemiological studies conducted in the 1980s confirmed that tampons made with cotton and/or rayon fibers had the lowest relative risk of TSS versus tampons made with other compositions.
The fluid uptake or absorbency of tampons is related to their composition as well as their weight, and the absorbency of tampons is one of the most important characteristics that have been associated epidemiologically with the development of mTSS (35, 108). Menstrual fluid uptake was demonstrated to be key for the internal oxygen concentration in a tampon to be consumed, along with carbon dioxide produced in vivo (147).
In another study evaluating tampon environmental conditions, researchers (196) investigated intravaginal temperature changes with simulated and actual menstrual tampon use. Tampons (with various absorbency compositions) were evaluated with a thermocouple sensor placed in the tampon. To simulate the human vagina, a condom was confined in a hollow glass tube, jacketed, placed in a 37°C water bath, and then dosed with human menses to fluid saturation; additionally, tests were run clinically during menstrual tampon use, up to 8 h in a stationary, supine position under controlled conditions. An increase in temperature in the tampon core was documented during menstrual fluid uptake both in vitro and clinically. Temperature profile characteristics varied from a transient spike with commercial cotton-rayon tampons of two different absorbencies to a small but sustained rise (≥6 h) with a carboxymethyl cellulose-containing prototype.
Early in vitro vaginal permeability work suggested that a linear increase in permeability occurred at temperatures of between 27°C and 37°C (197). When temperature gradations were evaluated (37°C, 39°C, and 41°C), permeability increased with temperature. Davis et al. investigated the impact of a small (from 37°C to 39°C) temperature increase on the permeability of TSST-1 through vaginal mucosa ex vivo and saw no enhanced penetration of the toxin (168).
TSST-1 can be produced vaginally with tampon use (115). Four healthy women who were vaginally colonized with S. aureus returned used tampons for evaluation, where TSST-1 and alpha-toxin concentrations were estimated. Two women with mTSS associated with tampons provided tampons for the same analyses. Tampons were sectioned into 0.5-cm pieces. It was noted that some sections contained menses and that others lacked menses but appeared to have absorbed clear vaginal fluid. The pH of tampon sections with or without menstrual blood was near 7. S. aureus bacteria were present in tampon sections at approximately equivalent counts (total counts were 1 × 108 to 2 × 109 bacteria/tampon). When sections containing little or no menstrual blood (low hemoglobin density) were evaluated, TSST-1 at concentrations from 2 to 80 μg/tampon were detected, and alpha-toxin concentrations of 28 to 30 μg/tampon were measured (115). Previous studies suggested that S. aureus exotoxins are not produced when the organism is cultured in human blood. In a follow-up study on the potential effects of alpha and beta chains of hemoglobin from blood, it was found that when human blood was fractionated into plasma and water-lysed red blood cells, mixtures of alpha- and beta-globins of hemoglobin inhibited S. aureus exotoxin production while increasing the production of protein A and not affecting bacterial growth. Pepsin but not trypsin digestion destroyed the ability of alpha- and beta-globins to inhibit exotoxin production. Methicillin susceptibility profiles did not affect the results (148).
Human Vaginal Microbiota and Tampons
The various microbiota associated with the human body are (i) important for the maintenance of health, (ii) influential on immune responses, and (iii) modulatory of responses to environmental impacts such as alterations in diet. Vaginal microbial communities provide an important line of defense by excluding pathogens associated with diseases. In recent years, much has been learned about the bacterial species compositions of these communities and how they differ between individuals of different ages, ethnicities, and stages in the ovarian cycle and during pregnancy (198, 199). Magrid and Gregor investigated the “bacterial flora” in the vagina and cervix after the use of tampons in 25 women over two menstrual periods. No clinically meaningful differences were noted (200). Brand subsequently determined that there was no appreciable change in vaginal bacterial flora (172). Several other studies have used culture-based techniques to analyze the vaginal microbiota for individual species in the vaginal niche during the menstrual cycle, with similar results (104, 116, 128, 201–204).
The Human Microbiome Project (HMP) was a National Institutes of Health research initiative to improve the understanding of microbial communities involved in human health and disease. Launched in 2007, the first phase (HMP1) focused on identifying and characterizing human microbiota associated with health (205). The “biggest initial revelation [from the study] was that the taxonomic composition of the microbiota in the human body was not a reliable predictor of host phenotype, such as disease susceptibility” (206). Several anatomical sites were evaluated in both men and women, and culture-independent analyses of microbial communities were matched with the metadata of the participants. The sites studied were the mouth, nose, skin, gastrointestinal tract, and reproductive tract of the human (vagina) (268). Keen interest in applying this knowledge to the human vagina during menstruation and various catamenial products is evidenced by the following articles.
The means by which vaginal microbiomes help prevent urogenital diseases in women and maintain health have been investigated using culture-independent techniques. There can be more than one kind of normal, healthy microbial composition of the vagina. The healthy vaginal microbiota has been described as being constituted mainly by Gram-positive bacilli of the genus Lactobacillus. A variety of species have been identified; the most common species are Lactobacillus crispatus, L. iners, L. gasseri, and L. jensenii. A fifth grouping of organisms was identified in women, demonstrating lower proportions of lactic acid bacteria and higher proportions of strictly anaerobic organisms, indicating that a potential key ecological function, the production of lactic acid, seems to be conserved in all communities (207).
Using culture-independent analyses, the vaginal bacterial communities of 396 asymptomatic, healthy North American women, representing four ethnic groups (white, black, Hispanic, and Asian), were sampled (207). The species composition was characterized by pyrosequencing of barcoded 16S rRNA genes. The proportions of each community group varied among the four ethnic groups, and these differences were statistically significant. It is important to note that the vaginal pHs of women in different ethnic groups also differed. The pHs were higher in Hispanic (pH 5.0 ± 0.6) and black (pH 4.7 ± 1) women than in Asian (pH 4.4 ± 0.6) and white (pH 4.2 ± 0.3) women at times other than menstruation.
MacPhee et al. investigated a potential risk factor for mTSS, an aberrant vaginal microbiota, and its impact on TSST-1 production (208). They postulated that aberrant microbiota characteristics of pathogenic bacteria could induce TSST-1 production. Those authors developed a reporter strain of TSST-1 S. aureus and grew it with vaginal swab contents collected from three groupings of women: women with a clinically healthy vaginal status, women with an intermediate status, and women with bacterial vaginosis. While clinical samples from healthy women and those with bacterial vaginosis suppressed toxin production, in vitro studies demonstrated that Streptococcus agalactiae and Enterococcus spp. significantly induced TSST-1 production, whereas some Lactobacillus spp. suppressed it.
Hickey et al. investigated the influence of menses on the vaginal microbiota and determined if tampons that differed in composition could influence communities (198). All women (n = 7) were dominated by Lactobacillus spp. at midcycle, and the compositions of those communities varied between menstrual cycles. Community dynamic patterns during menses varied considerably but were usually individualized. Vaginal microbiota composition changes during menses were common, but the magnitude of the changes varied among women. Regardless of these changes, most communities returned to compositions similar to those at previous midcycle sampling times following menstruation (207).
Researchers have evaluated if differences in the species composition of vaginal bacterial communities reflected a differential risk of colonization by TSST-1-producing S. aureus (209). Using terminal restriction fragment length polymorphism (T-RFLP) profiles and sequences of cloned 16S rRNA genes, the compositions of vaginal communities of women who were or were not colonized with tst-containing S. aureus were compared using this culture-independent technique. There were no significant differences in community composition or species rank abundance between women vaginally colonized with TSST-1-producing S. aureus and those who were not. “The results indicate that the numerically dominant members of vaginal communities do not preclude colonization and proliferation of mTSS S. aureus within indigenous microbial communities of the vagina” (209).
Recently, an elegant clinical study was designed to determine the potential impact of the vaginal microbial community composition on tampon colonization by S. aureus during menses; to achieve this goal, tampons from healthy women and mTSS cases were analyzed using culture-omics and 16S rRNA metabarcoding analysis (210). No differences in the richness, diversity, and ecological distance of the communities isolated from the tampon were observed between tampon vaginal fluids with and those without S. aureus and between healthy donors carrying S. aureus and mTSS patients. The tampon fluid samples that were positive for S. aureus did not cluster together. Clustering analysis did not distinguish a specific cluster grouping of a microbial community carrying TSST-1+ S. aureus or the microbiota of mTSS cases. These results are similar to the results reported by Pierson et al. (209). The authors concluded that the tampon microbiota exerts important effects through more complex interactions than simply inhibiting lactic acid production by lactic acid bacteria such as members of the genus Lactobacillus (210).
Using Bayesian networks (BNs), Noyes et al. reported that a woman’s age, ethnicity, or pregnancy was associated with the presence/absence of specific vaginal microbes (211). The resulting BN analysis identified multiple as-yet-undocumented associations between birth control usage, menstrual hygiene practices, and specific microbiome members. Many of these complex relationships were not specifically identified using common analytical methods. Based on the study results, it was unclear which microbiome members specifically were being affected and whether there were indirect or direct effects. The importance of having a large cohort to study the interactions of metadata and the microbiome was highlighted.
Immune Response to TSST-1
Infants under 6 months of age are protected from TSS because of the high level of maternal TSST-1 antibody (212). Using an enzyme-linked immunosorbent assay (ELISA), the titers of serum IgG antibody to TSST-1 in Japan were determined (212). The percentage of babies (<6 months old) with positive TSST-1 antibody titers was 78.6%. Only 21% of infants (6 to 12 months old) had positive titers. Titers began to increase again after the age of 3 years; titers increased again likely due to nasal colonization with TSST-1 S. aureus via active, natural immunization from the colonizing strain.
Parsonnet and colleagues reported the results of an extensive clinical study consisting of over 3,000 menstruating women (13 to 40 years of age) in order to explore trends in colonization and antibody prevalence among populations, reflective of the 1990 U.S. census (182). Subgroups by geographic region, race and/or ethnicity, and age were also compared. The results of the colonization study are addressed above in this paper. Using an ELISA, samples with titers of ≤1:4 (anti-TSST-1 IgG) were considered “negative” for antibody, and those with titers of ≥1:32 were considered “positive.” These groupings are based on prior clinical experience with hundreds of patients with either mTSS or TSST-1-induced nonmenstrual TSS. Of these patients, >90% had a titer of ≤1:4, and 100% had a titer of <1:32. Titers of 1:8 and 1:16 were classified as “intermediate” (i.e., likely to contain antibodies to TSST-1 but may not be associated with protection). Eighty-one percent of subjects (aged 13 to 18 years) developed positive antibody titers to TSST-1. Among these teenagers, 79% of subjects 13 to 15 years of age (n = 121) and 81% of subjects 16 to 18 years of age (n = 354) developed positive antibody titers to TSST-1. The teenage subjects (aged 13 to 18 years) had significantly lower levels of positive antibody than did older women. All teenage carriers of toxigenic S. aureus were already positive for antibodies to TSST-1. Overall, 85% of women aged 13 to 40 years had antibody titers ≥1:32, including 81% of those aged 13 to 18 years and 100% of TSST-1 carriers between the ages of 13 and 18 years. Only 8% of the study population could be considered antibody negative (titer of ≤1:4). The percentage of African-American subjects with positive antibody titers was significantly lower than that of European-American subjects. This is consistent with the findings of Merriman et al. in which African-American subjects were not colonized by TSST-1-positive S. aureus (199).
The presence of antibodies plays a critical role in PTSAg-mediated diseases due to their ability to bind to and neutralize these proteins. Some studies, however, have shown that the presence of an anti-PTSAg antibody is not always sufficient for neutralization (213, 214). Previous studies indicated that women with anti-TSST-1 titers (IgG) of ≥1:32 are more likely to be protected from mTSS (182, 215). Total anti-TSST-1 antibody titers (all immunoglobulin classes) in sera from these healthy subjects were observed to be high, regardless of the sampling time during the menstrual cycle. No difference in average total antibody titers was found between samples taken during menses and those taken during nonmenses. Indeed, the variation in antibody titers during menses and nonmenses for each subject was found to be less than the variation among the subjects. In summary, a clear difference exists between healthy women and mTSS patients with respect to the quantity and quality of anti-TSST-1 antibodies. This study not only cross-validated another study (216) showing that low anti-TSST-1 antibody titers are associated with a high risk of mTSS but also demonstrated a relationship between the antibody isotype and the ability to neutralize TSST-1.
It is acknowledged that women with high titers of neutralizing antibodies are normally protected from mTSS (181, 182, 213, 217) due to the ability of such antibodies to neutralize TSST-1. However, it is possible that additional host factors, beyond TSST-1-neutralizing antibodies, modulate disease severity. Host factors, which determine immunological responsiveness to TSST-1, have an impact on susceptibility to mTSS. Antibodies of the IgG class are important for the neutralization of the toxin. If TSST-1 is not neutralized, the PTSAg will bind to MHC class II molecules expressed by antigen-presenting cells and T cell receptor molecules on T cells (27–29). Latham et al. analyzed HLA genotypes among a small group of TSS patients but could not identify a correlation of specific HLA haplotypes with the immunosusceptibility of individuals (218). Other researchers analyzed multiple immune parameters after challenging transgenic mice expressing different HLA-DRB1 alleles (HLA-DRB1*15:01, HLA-DRB1*15:02, HLA-DRB1*03:01, and HLA-DRB1*04:01), and sharing the HLA-A1*01:01 chain, with purified TSST-1 (219). Among the HLA-DR alleles, mice expressing the HLA-DRB1*15:01 allele elicited a significantly higher serum cytokine/chemokine response, greater splenic T cell expansion, and the most severe organ pathology.
In toxicology, the dose-response relationship is an essential concept that correlates exposures with changes in health, body functions, and/or disease development. The relationship is based on data collected from cellular, animal, or human clinical studies. Determination of the relationship provides evidence that a chemical has induced an observed effect, and the threshold effect is where the lowest dose leads to an induced outcome. A cohort of mTSS patients was studied, and their T cells remained reactive to TSST-1 during the acute phase of the disease (220). It has been noted that exposure of mice to PTSAgs renders their T cells anergic to subsequent challenge (75); this does not occur with mTSS patients, as these women remain susceptible to multiple recurrences upon recovery from prior episodes (35). No information existed on the responsiveness of reproductive-age, healthy women. Kimber et al. reported immunological characteristics and responsiveness to TSST-1 by utilizing venous blood samples from healthy, reproductive-age women (221). This ex vivo study had as its objective the determination of whether it is possible to identify differences in responsiveness to TSST-1 and to establish an association of relative sensitivity with immunological analyses of monocyte responses and correlate these with HLA haplotypes of the women.
The immune response of peripheral blood mononuclear cells (PBMCs) to TSST-1 was calculated using 15% effective concentration (EC15) values for the high-, medium-, and low-sensitivity groups, which were 0.005 ± 0.05 ng/ml, 0.04 ± 0.01 ng/ml, and 0.32 ± 0.05 ng/ml, respectively, each being significantly different from the other two. PBMCs were challenged with phytohemagglutinin to ascertain their overall T cell responsiveness. The only group demonstrating statistically significant responsiveness was the high-responder group. Based on pilot studies, by using a range (0.01 to 10 ng/ml) of TSST-1 concentrations, 10 ng/ml maximized cytokine expression (particularly IL-2 and IFN-γ) across all donors. The most relevant and important observation made from this study was that there are substantial interindividual differences in the responsiveness of PBMCs to TSST-1 drawn from healthy, asymptomatic women, regardless of their S. aureus colonization. It was possible to assign all participants to one of the three groups (high, medium, and low sensitivity) based on differences in the PBMC proliferative responses, as defined by calculating their EC15 responses. There was an association between responsiveness to TSST-1 and certain haplotypes, with the frequencies of the DR7DQ2, DR14DQ5, DR4Q8, and DR8DQ4 haplotypes being higher among women with high sensitivity. This was confirmed by analysis of homozygous B cell lines.
In conclusion, from these antibody and HLA studies, it is proposed that consideration of neutralizing antibody titers associated with protection, along with the relative responsiveness of T lymphocytes to TSST-1 and the possible role of specific HLA-II haplotypes, may provide measures of enhanced susceptibility. Nevertheless, almost 80% of U.S. women have antibody titers that are protective, and colonization with vaginal TSST-1+ S. aureus occurs in less than 1% of the population and is transient in nature. Also, given the fact that the disease is uncommon, recognizable, and treatable, the best clinical management is to remind tampon users to follow the guidelines of matching the tampon absorbency to the menstrual flow rate, alternating tampons with pads, and not using a tampon for more than 8 h. If mTSS occurs, quick recognition of the symptoms and signs is paramount. Direct the patient to remove any tampons, and based on the clinical presentation of the patient, initiate empirical antibiotic therapy, fluid management/replacement, and possibly intravenous immunoglobulin (IVIG) and steroids.
One final note is worth mentioning here: the Schlievert laboratory is able to evaluate TSST-1 production by S. aureus strains, measure TSST-1 antibody in persons, and measure TSST-1 directly in fluid samples from patients (115, 222). It is worthwhile to assess the TSST-1 antibody status of young women upon their first gynecological examination; however, at present, there is no commercially available test.
Other Devices Associated with mTSS
Reports exist regarding other vaginal devices associated with TSS, such as diaphragms, intrauterine devices, pessaries, contraceptive sponges, and menstrual cups. (223–228). Starting in 1984, there were several reports identifying the use of contraceptive sponges with TSS (229, 230). Two research groups reported TSS associated with diaphragm use (223, 224). Mitchell et al. (225) reported a case of mTSS with the use of a menstrual cup, and subsequently, Nonfoux et al. (227) reported the results of testing four types of menstrual cups by the modified tampon sac in vitro method for understanding their potential impact on S. aureus growth and TSST-1 production. The latter group noted that “our results do not show that menstrual cups are safer than tampons.” The most recent publication related to other, nonabsorbent devices suggested that their association with mTSS may be coincidental, noting that women develop mTSS who have used only external menstrual pads or develop mTSS with S. aureus infection of other body sites (191).
A case of mTSS with the use of natural sea sponges was reported (269, 270). Smith et al. (231) reported the impact of various catamenial products used during menstruation on vaginal S. aureus colonization. Among the cultures done during that time, significantly higher rates of vaginal colonization with S. aureus, Escherichia coli, and other Enterobacteriaceae were noted among women (n = 25) who were using sea sponges to control menstrual flow. The authors noted that the association of sea sponges “with a high rate of S. aureus colonization suggests that they are not an alternative to tampons for women seeking to decrease the risk of toxic shock syndrome” (231). Public interest in the products led the University of Iowa Laboratory to examine 12 sea sponges in the latter part of 1980. The sponges contained sand, grit, bacteria, and various other substances. Subsequently, sponges collected by FDA district inspectors were analyzed by a Baltimore laboratory and again showed particles of grit, sand, bacteria, yeast, and mold, with one sample containing S. aureus. The Compliance Policy Guide Section 345.300 Menstrual Sponges (CPG) noted that sea sponges labeled as menstrual, hygienic, or sanitary sponges intended for menstrual use are regarded as a significant risk, and the sponsor must conduct a clinical safety-in-use study per 21 CFR 812. Jade & Pearl, Inc., received a warning letter from the FDA on 9 May 2014 (https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/jade-pearl-inc-05092014).
FLAWED mTSS THEORIES OVER THE DECADES
Early in the mTSS epidemic, there were many theories put forward, often in the media, concerning why tampons were associated with mTSS. Some of these are worth mentioning.
A theory was put forward that tampons cause mTSS. In fact, S. aureus causes all forms of staphylococcal TSS (23, 24). As shown in Table 1, there are multiple forms of TSS, many of which have no connection with menstruation or tampon use. The epidemiology data clearly show that some, but not all, tampons serve as risk factors for mTSS (1, 2, 35). Based on the personal knowledge of Schlievert, an unpublished study presented to the news media in the early 1980s reported that the administration of pieces of tampons into the uterus of experimental animals caused rapid hypotension and death. While this may be a true finding, it has nothing to do with staphylococcal mTSS. No S. aureus was present in the experimental animals. Additionally, a woman does not insert tampons into her uterus.
It was purported in the news media in 1982 that tampons form plugs after insertion into the vagina, creating anaerobic environments, facilitating TSST-1 production. There are two problems with this theory. Tampons do not rest under the cervix after insertion; in fact, tampons leak when oversaturated. Aerobic environments are required for TSST-1 production (31). Since 1981, when TSST-1 was identified, and up to the present, no one has found a condition to cause TSS S. aureus to produce TSST-1 under nonoxygenated conditions.
Tampons such as the Rely-brand tampon, associated with mTSS cases, contained carboxymethyl cellulose (cross-linked derivative in the case of Rely). Carboxymethyl cellulose can be degraded by cellulases to form glucose, which in turn was proposed to be used as a nutrient for energy to produce TSST-1 (232). There are multiple reasons to discount this theory. In addition to Rely, some tampons from the early 1980s, not associated epidemiologically with mTSS, also contained carboxymethyl cellulose. Glucose is a catabolite repressor of TSST-1 production (31). Finally, women do not normally have strong cellulase-producing bacteria vaginally to degrade carboxymethyl cellulose. Data concerning the early high association of Rely tampons, compared to other tampons of similar absorbency, must be viewed with caution. In the summer of 1980, when mTSS was first brought to the U.S. public’s attention, Rely tampon sales were changing dramatically, going from zero to a high market share in months. The increased market share of the product may have been a confounder in the study design that was not considered during analyses of epidemiological data. This makes it difficult to determine the elevated risk with mTSS compared to other tampons. Clearly, Rely tampons were associated with mTSS, but so were other high-absorbency tampons (35). Thus, absorbency was more important than composition.
A research group proposed that tampons with carboxymethyl cellulose form emulsions, which enhance TSST-1 production in the presence of aqueous fluids (194). It is the Schlievert laboratory’s experience that these emulsions simply reflect the amount of trapped oxygen. Indeed, prereduced emulsions, when cultured with S. aureus in an anaerobic chamber, give no TSST-1 production, indicating that emulsions yield TSST-1 due to trapped oxygen.
Studies suggest that the Rely tampon was associated with mTSS in part because of the presence of the surfactant pluronic L92 (PL92), an agent that increases TSST-1 production and/or vaginal mucosal penetration. While it is clear that very high concentrations (>60%) of pluronic L92 enhance TSST-1 production (233), it is unlikely that such high concentrations existed vaginally during the usage of Rely tampons. Conversely, many tampons of the highest absorbency in the early 1980s contained polyacrylate to increase absorbency. These tampons typically were inhibitory to the growth and TSST-1 production of S. aureus due to small amounts of bactericidal acrylic acid (189). Also, the ex vivo porcine vaginal tissue model demonstrated that relevant concentrations of PL92 applied topically to the tissue did not enhance the penetration of TSST-1 across the vaginal mucosa (180).
There was an attempt to produce a television show, involving Schlievert in the early 1980s, where it was suggested that tampon manufacturers added arsenic and other agents to tampons to increase menstrual flow. This would also select for mTSS S. aureus, most of which have genes, for example, to encode arsenate resistance. There is no evidence that such a practice has happened, and this is completely contrary to the health-first policies of tampon manufacturers.
A pervasive theory for the tampon association with mTSS was that tampons cause vaginal microulcerations, which increase TSST-1 penetration of the vaginal mucosa (173). There is no reason to invoke a causative role of microulcerations. Many tampons associated with TSS do not cause vaginal microulcerations. TSST-1, without additional factors, has the ability to penetrate the vaginal mucosal barrier while at the same time causing vaginal epithelial cells to produce chemokines, causing inflammation and further increasing mucosal penetration (26).
There is a theory that tampons composed of all cotton are safer than cotton-rayon and all-rayon tampons, as rayon is a synthetic fiber, which is harmful (195). This theory has been disproven multiple times, yet the theory exists even today. Cotton and rayon have the same basic structure, with cotton coming from cotton plants and rayon coming from wood pulp; rayon is not synthetic. Additionally, all studies except the one cited above (195), and in blind and nonblind formats, show that all tampons are similar in their effect on mTSS S. aureus and the production of TSST-1, regardless of composition (32, 192, 193). Because that sole study suggested that all-cotton tampons are safer than other tampons, women may choose to use higher-absorbency tampons than they should, ignoring the CDC recommendation to use the tampon of the lowest absorbency to control menstrual flow and thus putting themselves at an increased risk of mTSS.
It has been theorized that tampons contain dioxin and phthalates, which makes them unsafe. Concern has been expressed that rayon tampons contain dioxins as a result of chlorine bleaching, although these have not been identified as risk factors for mTSS. The chemical 2,3,7,8-tetrachlorodibenzo-p-dioxin is commonly meant when the generic term “dioxin” is used. Rayon tampons do not contain this chemical. The concentration of dioxin-like material theoretically available from tampons is at least 6 orders of magnitude lower than the estimated levels of daily food exposure to these contaminants (234, 235). Regarding phthalates, the vaginal practice of douching is most commonly associated with the presence of this group of chemical compounds. More recently, National Health and Nutrition Examination Survey (NHANES) data that were utilized to examine the potential association between the self-reported use of feminine hygiene products such as sanitary napkins, tampons, vaginal douches, feminine spray, feminine powder, and feminine wipes/towelettes and urinary concentrations of monoethyl phthalate (MEP) and mono-n-butyl phthalate (MnBP), metabolites of diethyl phthalate and dibutyl phthalate, indicated that douching was most likely associated with the presence of these chemical moieties. More African-American women than European-American or Mexican-American women reported the use of vaginal douches, feminine spray, feminine powder, and wipes/towelettes in the past month. White women were more likely than other racial/ethnic groups to report tampon use. Douching in the past month was associated with higher concentrations of MEP but not MnBP. None of the remaining feminine hygiene products were significantly associated with either MEP or MnBP (236).
FOR THE FUTURE
Since the description of mTSS, risk factors, and TSST-1 characteristics, an enormous amount of scientific literature has become available on the subject. Despite the wealth of knowledge, many remaining aspects merit consideration. Some of these are presented below.
Although we know that oxygen is required for mTSS (31), and studies have now suggested that oxygen introduction within tampons may explain the tampon association (147), we do not know why recurrences may occur in the absence of continued tampon use (35). Somehow, the vagina must become oxygenated, or alternatively, these cases occur coincidental with menstruation but are caused by TSST-1-producing S. aureus located at other body sites.
S. aureus strains appear to cycle in populations in roughly 10-year intervals. We may be entering a new cycle in which TSST-1-producing strains are emerging. We have discussed the emergence of bacteriophage types over time, and we have provided evidence for the emergence of TSST-1+ S. aureus beginning in about 1972 (19–21). More recently, we have seen the emergence and relative disappearance of USA300 and USA400 (237–240). TSST-1 production by USA200 S. aureus is under complex regulatory control (117–119, 241, 242). It is possible that strains may emerge in which the amounts of TSST-1 produced may change. For example, the mTSS strain MN8 produces approximately 10 times more TSST-1 than many other strains (57). The reason for this difference is unknown. The consequences of additional strains producing larger amounts of TSST-1 remain unclear. We need a better understanding of why new strains emerge and disappear and, at the same time, a better understanding of the regulation of PTSAg production.
When asked, a large percentage of the U.S. female population prefers the use of tampons compared to other forms of menstrual protection. In some countries, there is a significant increase in the use of menstrual cups for environmental reasons. There have been occasional associations with menstrual cups and diaphragms. Studies should continue in attempts to develop ways to reduce even more the risk of mTSS in women who use tampons and other forms of menstrual protection.
Studies should continue toward vaccine development against S. aureus infections, including mTSS. There are now only limited studies being done in the United States due to prior vaccine failures (243). There is no question that S. aureus is the most significant cause of serious infectious diseases in the United States, causing over 500,000 cases of surgical site infection, 70,000 cases of pneumonia, 40,000 cases of infective endocarditis, and 20,000 cases of sepsis, and is associated with atopic dermatitis, diabetes mellitus, and cystic fibrosis. We must do more to make a vaccine available; in this regard, a first-in-human trial of a toxoid vaccine has been performed in Austria (244).
Finally, it is of the upmost importance that the medical and scientific communities continue to recognize cases of mTSS. There is not the media attention associated with tampons and mTSS that was present across the 1980s. Cases may be missed or described as mystery illnesses. It must be remembered that PTSAgs such as TSST-1 are lethal at concentrations of as low as 0.1 μg/human intravenously (245). We do not yet know why many of the clinical findings in mTSS occur. As examples, mTSS patients often temporarily lose their hair. Why this occurs is unknown. Some mTSS patients temporarily, partially lose memories, sometimes for up to 1.5 years. Again, why this occurs is unknown.
ACKNOWLEDGMENTS
C.C.D. is a former employee of Procter & Gamble. She retired in 2019 and is now part of the Creighton University faculty. P.M.S. declares no conflict of interest.
Biographies

Patrick M. Schlievert received his bachelor’s degree (geology/general science) and Ph.D. (microbiology) from the University of Iowa. He was a postdoctoral associate at the University of Minnesota (UMN) from 1976 to 1979, studying streptococcal pyrogenic toxin superantigens as causes of scarlet fever and related illnesses. In 1979, Dr. Schlievert accepted a faculty position at UCLA in the Microbiology and Immunology Department, where he discovered toxic shock syndrome (TSS) toxin 1, the principal cause of TSS, and demonstrated the main reason why tampons are associated with TSS (oxygen introduction vaginally). Dr. Schlievert returned to UMN as a faculty member in the Microbiology Department, where he worked his way through the ranks, becoming a full professor in 1990. From 2011 to 2019, he was Chair of Microbiology and Immunology at the University of Iowa. Dr. Schlievert and his clinical colleagues have described many new superantigen-associated human illnesses, including streptococcal TSS in 1987 with Dr. Larry Cone, Eisenhower Medical Center, Palm Springs, CA. He has also performed collaborative studies to determine the structures of superantigens alone, complex structures with host cell receptors, and immunopathogenesis of disease. His major current interests continue in the description of new diseases, drug discovery, and vaccine development. Dr. Schlievert has published over 400 manuscripts. While at UMN, he received the university’s highest education honor for teaching microbiology and immunology for medical students. In 2016, Dr. Schlievert was named the ASM Graduate Educator of the Year. He was also a member of the Academic Health Center Academy of Excellence in Medical Research at UMN. Dr. Schlievert has cofounded two small biopharma companies.

Catherine C. Davis received her bachelor’s degree with distinction from the University of Nebraska—Lincoln, master of science degree in Pathology from Washington University in St. Louis, M.P.H. (Epidemiology) from the Colorado School of Public Health, and Certificate in Bioethics from Georgetown University. She received her Ph.D. in Medical Microbiology and Immunology from Creighton University, where she studied the role of alcohol in the pathogenesis of pneumococcal pneumonia in the laboratory of Dr. Laurel C. Preheim (VA Medical Center-Omaha). Her first faculty position was in the College of Dentistry, University of Iowa, where she served as Assistant Professor and subsequently joined the University of Colorado School of Dentistry, where she was Clinical Associate Professor in the Departments of Oral Pathology and Dental Hygiene. Students at both universities awarded her the Teacher of the Year Award numerous times, and she was appointed by the American Dental Association to serve on the National Test Board Construction Committees for Basic Sciences as well as Public Health and Infection Control. She joined The Procter & Gamble (P&G) Company in 1995, where she served as Principal Scientist and Project Leader for the Company’s global research program on toxic shock syndrome as well as the Vice Chair of the P&G Institutional Review Board. She was part of the NIH Human Microbiome Project Consortium that published research in Nature (2012) characterizing the human microbiome in a large cohort of healthy adults as well as the landmark study characterizing the vaginal microbiome in healthy women in Proceedings of the National Academy of Sciences of the United States of America (2011). She received the John Smale Award for Technical Excellence from P&G in 2003 and was elected to Fellowship status (2004) by the Infectious Diseases Society of America. She also served on several national committees in the American Society for Microbiology and was elected Chair of Division E. Dr. Davis retired from P&G in 2012 and currently serves as Clinical Professor of Medical Microbiology and Immunology in the School of Medicine, Creighton University, Omaha, NE. She has also taught in the M.P.H. programs of the University of Northern Colorado and Creighton University.
REFERENCES
- 1.Shands KN, Schmid GP, Dan BB, Blum D, Guidotti RJ, Hargrett NT, Anderson RL, Hill DL, Broome CV, Band JD, Fraser DW. 1980. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N Engl J Med 303:1436–1442. doi: 10.1056/NEJM198012183032502. [DOI] [PubMed] [Google Scholar]
- 2.Davis JP, Chesney PJ, Wand PJ, LaVenture M, Investigation and Laboratory Team . 1980. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med 303:1429–1435. doi: 10.1056/NEJM198012183032501. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb M, Long B, Koyfman A. 2018. The evaluation and management of toxic shock syndrome in the emergency department: a review of the literature. J Emerg Med 54:807–814. doi: 10.1016/j.jemermed.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 4.Todd J, Fishaut M, Kapral F, Welch T. 1978. Toxic-shock syndrome associated with phage-group-I staphylococci. Lancet ii:1116–1118. doi: 10.1016/s0140-6736(78)92274-2. [DOI] [PubMed] [Google Scholar]
- 5.Cone LA, Woodard DR, Schlievert PM, Tomory GS. 1987. Clinical and bacteriologic observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N Engl J Med 317:146–149. doi: 10.1056/NEJM198707163170305. [DOI] [PubMed] [Google Scholar]
- 6.Stevens DL, Tanner MH, Winship J, Swarts R, Ries KM, Schlievert PM, Kaplan E. 1989. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med 321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 7.Assimacopoulos AP, Stoehr JA, Schlievert PM. 1997. Mitogenic factors from group G streptococci associated with scarlet fever and streptococcal toxic shock syndrome. Adv Exp Med Biol 418:109–114. [DOI] [PubMed] [Google Scholar]
- 8.Hussain SM, Luedtke GS, Baker CJ, Schlievert PM, Leggiadro RJ. 1995. Invasive group B streptococcal disease in children beyond early infancy. Pediatr Infect Dis J 14:278–281. [DOI] [PubMed] [Google Scholar]
- 9.Schlievert PM, Gocke JE, Deringer JR. 1993. Group B streptococcal toxic shock-like syndrome: report of a case and purification of an associated pyrogenic toxin. Clin Infect Dis 17:26–31. doi: 10.1093/clinids/17.1.26. [DOI] [PubMed] [Google Scholar]
- 10.Wagner JG, Schlievert PM, Assimacopoulos AP, Stoehr JA, Carson PJ, Komadina K. 1996. Acute group G streptococcal myositis associated with streptococcal toxic shock syndrome: case report and review. Clin Infect Dis 23:1159–1161. doi: 10.1093/clinids/23.5.1159. [DOI] [PubMed] [Google Scholar]
- 11.Feldman CA. 1962. Staphylococcal scarlet fever. N Engl J Med 267:877–878. doi: 10.1056/NEJM196210252671709. [DOI] [PubMed] [Google Scholar]
- 12.Aranow H, Wood WB. 1942. Staphylococcal infection simulating scarlet fever. JAMA 119:1491–1495. doi: 10.1001/jama.1942.02830350023006. [DOI] [Google Scholar]
- 13.Stevens FA. 1927. The occurrence of Staphylococcus aureus with a scarlatiniform rash. JAMA 88:1957–1958. doi: 10.1001/jama.1927.02680510015006. [DOI] [Google Scholar]
- 14.Leung DY, Schlievert PM, Meissner HC. 1998. The immunopathogenesis and management of Kawasaki syndrome. Arthritis Rheum 41:1538–1547. doi:. [DOI] [PubMed] [Google Scholar]
- 15.Leung DY, Meissner C, Fulton D, Schlievert PM. 1995. The potential role of bacterial superantigens in the pathogenesis of Kawasaki syndrome. J Clin Immunol 15:11S–17S. doi: 10.1007/bf01540888. [DOI] [PubMed] [Google Scholar]
- 16.Leung DY, Meissner HC, Fulton DR, Murray DL, Kotzin BL, Schlievert PM. 1993. Toxic shock syndrome toxin-secreting Staphylococcus aureus in Kawasaki syndrome. Lancet 342:1385–1388. doi: 10.1016/0140-6736(93)92752-F. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. 1974. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics 54:271–276. [PubMed] [Google Scholar]
- 18.Hansen RC. 1983. Staphylococcal scalded skin syndrome, toxic shock syndrome, and Kawasaki disease. Pediatr Clin North Am 30:533–544. doi: 10.1016/s0031-3955(16)34401-7. [DOI] [PubMed] [Google Scholar]
- 19.Altemeier WA, Lewis SA, Schlievert PM, Bergdoll MS, Bjornson HS, Staneck JL, Crass BA. 1982. Staphylococcus aureus associated with toxic shock syndrome: phage typing and toxin capability testing. Ann Intern Med 96:978–982. doi: 10.7326/0003-4819-96-6-978. [DOI] [PubMed] [Google Scholar]
- 20.Altemeier WA, Lewis SA, Bjornson HS, Staneck JL, Schlievert PM. 1983. Staphylococcus in toxic shock syndrome and other surgical infections. Development of new bacteriophages. Arch Surg 118:281–284. doi: 10.1001/archsurg.1983.01390030013002. [DOI] [PubMed] [Google Scholar]
- 21.Altemeier WA, Lewis S, Schlievert PM, Bjornson HS. 1981. Studies of the staphylococcal causation of toxic shock syndrome. Surg Gynecol Obstet 153:481–485. [PubMed] [Google Scholar]
- 22.Mueller EA, Merriman JA, Schlievert PM. 2015. Toxic shock syndrome toxin-1, not alpha-toxin, mediated Bundaberg fatalities. Microbiology 161:2361–2368. doi: 10.1099/mic.0.000196. [DOI] [PubMed] [Google Scholar]
- 23.Schlievert PM, Shands KN, Dan BB, Schmid GP, Nishimura RD. 1981. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis 143:509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- 24.Bergdoll MS, Crass BA, Reiser RF, Robbins RN, Davis JP. 1981. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet i:1017–1021. doi: 10.1016/s0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald KL, Osterholm MT, Hedberg CW, Schrock CG, Peterson GF, Jentzen JM, Leonard SA, Schlievert PM. 1987. Toxic shock syndrome. A newly recognized complication of influenza and influenzalike illness. JAMA 257:1053–1058. doi: 10.1001/jama.1987.03390080043027. [DOI] [PubMed] [Google Scholar]
- 26.Schlievert PM, Cahill MP, Hostager BS, Brosnahan AJ, Klingelhutz AJ, Gourronc FA, Bishop GA, Leung DYM. 2019. Staphylococcal superantigens stimulate epithelial cells through CD40 to produce chemokines. mBio 10:e00214-19. doi: 10.1128/mBio.00214-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCormick JK, Yarwood JM, Schlievert PM. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol 55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 28.Dinges MM, Orwin PM, Schlievert PM. 2000. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaulding AR, Salgado-Pabón W, Kohler PL, Horswill AR, Leung DY, Schlievert PM. 2013. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev 26:422–447. doi: 10.1128/CMR.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langmuir AD, Worthen TD, Solomon J, Ray CG, Petersen E. 1985. The Thucydides syndrome. A new hypothesis for the cause of the plague of Athens. N Engl J Med 313:1027–1030. doi: 10.1056/NEJM198510173131618. [DOI] [PubMed] [Google Scholar]
- 31.Schlievert PM, Blomster DA. 1983. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J Infect Dis 147:236–242. doi: 10.1093/infdis/147.2.236. [DOI] [PubMed] [Google Scholar]
- 32.Fischetti VA, Chapman F, Kakani R, James J, Grun E, Zabriskie JB. 1989. Role of air in growth and production of toxic shock syndrome toxin 1 by Staphylococcus aureus in experimental cotton and rayon tampons. Rev Infect Dis 11:S176–S181. doi: 10.1093/clinids/11.Supplement_1.S176. [DOI] [PubMed] [Google Scholar]
- 33.Larkin SM, Williams DN, Osterholm MT, Tofte RW, Posalaky Z. 1982. Toxic shock syndrome: clinical, laboratory, and pathologic findings in nine fatal cases. Ann Intern Med 96:858–864. doi: 10.7326/0003-4819-96-6-858. [DOI] [PubMed] [Google Scholar]
- 34.Davis JP, Osterholm MT, Helms CM, Vergeront JM, Wintermeyer LA, Forfang JC, Judy LA, Rondeau J, Schell WL. 1982. Tri-state toxic-shock syndrome study. II. Clinical and laboratory findings. J Infect Dis 145:441–448. doi: 10.1093/infdis/145.4.441. [DOI] [PubMed] [Google Scholar]
- 35.Osterholm MT, Davis JP, Gibson RW, Mandel JS, Wintermeyer LA, Helms CM, Forfang JC, Rondeau J, Vergeront JM. 1982. Tri-state toxic-state syndrome study. I. Epidemiologic findings. J Infect Dis 145:431–440. doi: 10.1093/infdis/145.4.431. [DOI] [PubMed] [Google Scholar]
- 36.Nelson H. 7 June 1980. Toxic shock syndrome. Los Angeles Times, Los Angeles, CA. [Google Scholar]
- 37.Schlossberg D, Kandra J, Kreiser J. 1979. Possible Kawasaki disease in a 20-year-old woman. Arch Dermatol 115:1435–1436. doi: 10.1001/archderm.1979.04010120033014. [DOI] [PubMed] [Google Scholar]
- 38.Musser JM, Schlievert PM, Chow AW, Ewan P, Kreiswirth BN, Rosdahl VT, Naidu AS, Witte W, Selander RK. 1990. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proc Natl Acad Sci U S A 87:225–229. doi: 10.1073/pnas.87.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlievert PM, Schoettle DJ, Watson DW. 1979. Purification and physicochemical and biological characterization of a staphylococcal pyrogenic exotoxin. Infect Immun 23:609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlievert PM. 1980. Purification and characterization of staphylococcal pyrogenic exotoxin type B. Biochemistry 19:6204–6208. doi: 10.1021/bi00567a040. [DOI] [PubMed] [Google Scholar]
- 41.Bergdoll MS, Schlievert PM. 1984. Toxic-shock syndrome toxin. Lancet ii:691. doi: 10.1016/S0140-6736(84)91241-8. [DOI] [Google Scholar]
- 42.Schlievert PM, Jablonski LM, Roggiani M, Sadler I, Callantine S, Mitchell DT, Ohlendorf DH, Bohach GA. 2000. Pyrogenic toxin superantigen site specificity in toxic shock syndrome and food poisoning in animals. Infect Immun 68:3630–3634. doi: 10.1128/iai.68.6.3630-3634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blomster-Hautamaa DA, Kreiswirth BN, Kornblum JS, Novick RP, Schlievert PM. 1986. The nucleotide and partial amino acid sequence of toxic shock syndrome toxin-1. J Biol Chem 261:15783–15786. [PubMed] [Google Scholar]
- 44.Brehm RD, Tranter HS, Hambleton P, Melling J. 1990. Large-scale purification of staphylococcal enterotoxins A, B, and C2 by dye ligand affinity chromatography. Appl Environ Microbiol 56:1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee PK, Kreiswirth BN, Deringer JR, Projan SJ, Eisner W, Smith BL, Carlson E, Novick RP, Schlievert PM. 1992. Nucleotide sequences and biologic properties of toxic shock syndrome toxin 1 from ovine- and bovine-associated Staphylococcus aureus. J Infect Dis 165:1056–1063. doi: 10.1093/infdis/165.6.1056. [DOI] [PubMed] [Google Scholar]
- 46.Murray DL, Prasad GS, Earhart CA, Leonard BA, Kreiswirth BN, Novick RP, Ohlendorf DH, Schlievert PM. 1994. Immunobiologic and biochemical properties of mutants of toxic shock syndrome toxin-1. J Immunol 152:87–95. [PubMed] [Google Scholar]
- 47.Parsonnet J. 1998. Case definition of staphylococcal TSS: a proposed revision incorporating laboratory findings. Int Congr Symp Ser 229:15. [Google Scholar]
- 48.Reingold AL, Hargrett NT, Dan BB, Shands KN, Strickland BY, Broome CV. 1982. Nonmenstrual toxic shock syndrome: a review of 130 cases. Ann Intern Med 96:871–874. doi: 10.7326/0003-4819-96-6-871. [DOI] [PubMed] [Google Scholar]
- 49.Lin YC, Anderson MJ, Kohler PL, Strandberg KL, Olson ME, Horswill AR, Schlievert PM, Peterson ML. 2011. Proinflammatory exoprotein characterization of toxic shock syndrome Staphylococcus aureus. Biochemistry 50:7157–7167. doi: 10.1021/bi200435n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Reilly M, Kreiswirth B, Foster TJ. 1990. Cryptic alpha-toxin gene in toxic shock syndrome and septicaemia strains of Staphylococcus aureus. Mol Microbiol 4:1947–1955. doi: 10.1111/j.1365-2958.1990.tb02044.x. [DOI] [PubMed] [Google Scholar]
- 51.DeLeo FR, Kennedy AD, Chen L, Bubeck Wardenburg J, Kobayashi SD, Mathema B, Braughton KR, Whitney AR, Villaruz AE, Martens CA, Porcella SF, McGavin MJ, Otto M, Musser JM, Kreiswirth BN. 2011. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc Natl Acad Sci U S A 108:18091–18096. doi: 10.1073/pnas.1111084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kreiswirth BN, Löfdahl S, Betley MJ, O’Reilly M, Schlievert PM, Bergdoll MS, Novick RP. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 53.Lindsay JA, Ruzin A, Ross HF, Kurepina N, Novick RP. 1998. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol Microbiol 29:527–543. doi: 10.1046/j.1365-2958.1998.00947.x. [DOI] [PubMed] [Google Scholar]
- 54.Yarwood JM, McCormick JK, Paustian ML, Orwin PM, Kapur V, Schlievert PM. 2002. Characterization and expression analysis of Staphylococcus aureus pathogenicity island 3. Implications for the evolution of staphylococcal pathogenicity islands. J Biol Chem 277:13138–13147. doi: 10.1074/jbc.M111661200. [DOI] [PubMed] [Google Scholar]
- 55.Ruzin A, Lindsay J, Novick RP. 2001. Molecular genetics of SaPI1—a mobile pathogenicity island in Staphylococcus aureus. Mol Microbiol 41:365–377. doi: 10.1046/j.1365-2958.2001.02488.x. [DOI] [PubMed] [Google Scholar]
- 56.Schlievert PM, Peterson ML. 2012. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS One 7:e40350. doi: 10.1371/journal.pone.0040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlievert PM, Kelly JA. 1984. Clindamycin-induced suppression of toxic-shock syndrome-associated exotoxin production. J Infect Dis 149:471. doi: 10.1093/infdis/149.3.471. [DOI] [PubMed] [Google Scholar]
- 58.Bonventre PF, Weckbach L, Staneck J, Schlievert PM, Thompson M. 1983. Production of staphylococcal enterotoxin F and pyrogenic exotoxin C by Staphylococcus aureus isolates from toxic shock syndrome-associated sources. Infect Immun 40:1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson GJ, Seo KS, Cartwright RA, Connelley T, Chuang-Smith ON, Merriman JA, Guinane CM, Park JY, Bohach GA, Schlievert PM, Morrison WI, Fitzgerald JR. 2011. A novel core genome-encoded superantigen contributes to lethality of community-associated MRSA necrotizing pneumonia. PLoS Pathog 7:e1002271. doi: 10.1371/journal.ppat.1002271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schlievert PM, Strandberg KL, Lin YC, Peterson ML, Leung DY. 2010. Secreted virulence factor comparison between methicillin-resistant and methicillin-sensitive Staphylococcus aureus, and its relevance to atopic dermatitis. J Allergy Clin Immunol 125:39–49. doi: 10.1016/j.jaci.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stach CS, Vu BG, Merriman JA, Herrera A, Cahill MP, Schlievert PM, Salgado-Pabón W. 2016. Novel tissue level effects of the Staphylococcus aureus enterotoxin gene cluster are essential for infective endocarditis. PLoS One 11:e0154762. doi: 10.1371/journal.pone.0154762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munson SH, Tremaine MT, Betley MJ, Welch RA. 1998. Identification and characterization of staphylococcal enterotoxin types G and I from Staphylococcus aureus. Infect Immun 66:3337–3348. doi: 10.1128/IAI.66.7.3337-3348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salgado-Pabón W, Herrera A, Vu BG, Stach CS, Merriman JA, Spaulding AR, Schlievert PM. 2014. Staphylococcus aureus beta-toxin production is common in strains with the beta-toxin gene inactivated by bacteriophage. J Infect Dis 210:784–792. doi: 10.1093/infdis/jiu146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blomster-Hautamaa DA, Kreiswirth BN, Novick RP, Schlievert PM. 1986. Resolution of highly purified toxic-shock syndrome toxin 1 into two distinct proteins by isoelectric focusing. Biochemistry 25:54–59. doi: 10.1021/bi00349a009. [DOI] [PubMed] [Google Scholar]
- 65.Blomster-Hautamaa DA, Schlievert PM. 1988. Preparation of toxic shock syndrome toxin-1. Methods Enzymol 165:37–43. doi: 10.1016/s0076-6879(88)65009-9. [DOI] [PubMed] [Google Scholar]
- 66.Prasad GS, Earhart CA, Murray DL, Novick RP, Schlievert PM, Ohlendorf DH. 1993. Structure of toxic shock syndrome toxin 1. Biochemistry 32:13761–13766. doi: 10.1021/bi00213a001. [DOI] [PubMed] [Google Scholar]
- 67.Arad G, Levy R, Nasie I, Hillman D, Rotfogel Z, Barash U, Supper E, Shpilka T, Minis A, Kaempfer R. 2011. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol 9:e1001149. doi: 10.1371/journal.pbio.1001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bohach GA, Fast DJ, Nelson RD, Schlievert PM. 1990. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol 17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 69.Schlievert PM, Bettin KM, Watson DW. 1978. Effect of antipyretics on group A streptococcal pyrogenic exotoxin fever production and ability to enhance lethal endotoxin shock. Proc Soc Exp Biol Med 157:472–475. doi: 10.3181/00379727-157-40079. [DOI] [PubMed] [Google Scholar]
- 70.Schlievert PM, Watson DW. 1979. Biogenic amine involvement in pyrogenicity and enhancement of lethal endotoxin shock by group A streptococcal pyrogenic exotoxin. Proc Soc Exp Biol Med 162:269–274. doi: 10.3181/00379727-162-40663. [DOI] [PubMed] [Google Scholar]
- 71.Schlievert PM. 1982. Enhancement of host susceptibility to lethal endotoxin shock by staphylococcal pyrogenic exotoxin type C. Infect Immun 36:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dinges MM, Schlievert PM. 2001. Comparative analysis of lipopolysaccharide-induced tumor necrosis factor alpha activity in serum and lethality in mice and rabbits pretreated with the staphylococcal superantigen toxic shock syndrome toxin 1. Infect Immun 69:7169–7172. doi: 10.1128/IAI.69.11.7169-7172.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dinges MM, Schlievert PM. 2001. Role of T cells and gamma interferon during induction of hypersensitivity to lipopolysaccharide by toxic shock syndrome toxin 1 in mice. Infect Immun 69:1256–1264. doi: 10.1128/IAI.69.3.1256-1264.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barsumian EL, Schlievert PM, Watson DW. 1978. Nonspecific and specific immunological mitogenicity by group A streptococcal pyrogenic exotoxins. Infect Immun 22:681–688. doi: 10.1128/IAI.22.3.681-688.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marrack P, Kappler J. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 76.John CC, Niermann M, Sharon B, Peterson ML, Kranz DM, Schlievert PM. 2009. Staphylococcal toxic shock syndrome erythroderma is associated with superantigenicity and hypersensitivity. Clin Infect Dis 49:1893–1896. doi: 10.1086/648441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schlievert PM. 1983. Alteration of immune function by staphylococcal pyrogenic exotoxin type C: possible role in toxic-shock syndrome. J Infect Dis 147:391–398. doi: 10.1093/infdis/147.3.391. [DOI] [PubMed] [Google Scholar]
- 78.Schlievert PM, Bettin KM, Watson DW. 1979. Reinterpretation of the Dick test: role of group A streptococcal pyrogenic exotoxin. Infect Immun 26:467–472. doi: 10.1128/IAI.26.2.467-472.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kushnaryov VM, MacDonald HS, Reiser R, Bergdoll MS. 1984. Staphylococcal toxic shock toxin specifically binds to cultured human epithelial cells and is rapidly internalized. Infect Immun 45:566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brosnahan AJ, Schaefers MM, Amundson WH, Mantz MJ, Squier CA, Peterson ML, Schlievert PM. 2008. Novel toxic shock syndrome toxin-1 amino acids required for biological activity. Biochemistry 47:12995–13003. doi: 10.1021/bi801468w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brosnahan AJ, Schlievert PM. 2011. Gram-positive bacterial superantigen outside-in signaling causes toxic shock syndrome. FEBS J 278:4649–4667. doi: 10.1111/j.1742-4658.2011.08151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Todd JK. 1988. Toxic shock syndrome. Clin Microbiol Rev 1:432–446. doi: 10.1128/cmr.1.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stallones RA. 1982. A review of the epidemiologic studies of toxic shock syndrome. Ann Intern Med 96:917–920. doi: 10.7326/0003-4819-96-6-917. [DOI] [PubMed] [Google Scholar]
- 84.Chesney PJ, Jaucian RM, McDonald RA, Kapral FA, Bergdoll MS. 1983. Exfoliative dermatitis in an infant. Association with enterotoxin F-producing staphylococci. Am J Dis Child 137:899–901. doi: 10.1001/archpedi.1983.02140350073018. [DOI] [PubMed] [Google Scholar]
- 85.Reingold AL, Dan BB, Shands KN, Broome CV. 1982. Toxic-shock syndrome not associated with menstruation. A review of 54 cases. Lancet i:1–4. doi: 10.1016/S0140-6736(82)92552-1. [DOI] [PubMed] [Google Scholar]
- 86.Donawa ME, Schmid GR, Osterholm MT. 1984. Toxic shock syndrome: chronology of state and federal epidemiologic studies and regulatory decision-making. Public Health Rep 99:342–350. [PMC free article] [PubMed] [Google Scholar]
- 87.Harvey M, Horwitz RI, Feinstein AR. 1982. Toxic shock and tampons. Evaluation of the epidemiologic evidence. JAMA 248:840–846. [PubMed] [Google Scholar]
- 88.Doyle TJ, Glynn MK, Groseclose SL. 2002. Completeness of notifiable infectious disease reporting in the United States: an analytical literature review. Am J Epidemiol 155:866–874. doi: 10.1093/aje/155.9.866. [DOI] [PubMed] [Google Scholar]
- 89.Coppleson M, Pixley E, Reid B. 1978. Colposcopy: a scientific approach to the cervix and vagina in health and disease. Charles D. Thomas, Springfield, IL. [Google Scholar]
- 90.Dunnet WN, Schallibaum EM. 1960. Scarlet-fever-like illness due to staphylococcal infection. Lancet ii:1227–1229. doi: 10.1016/s0140-6736(60)92418-1. [DOI] [PubMed] [Google Scholar]
- 91.Schrock CG. 1980. Disease alert. JAMA 243:1231. [PubMed] [Google Scholar]
- 92.McKenna UG, Meadows JA III, Brewer NS, Wilson WR, Perrault J. 1980. Toxic shock syndrome, a newly recognized disease entity. Report of 11 cases. Mayo Clin Proc 55:663–672. [PubMed] [Google Scholar]
- 93.Centers for Disease Control. 1980. Toxic-shock syndrome—United States. MMWR Morb Mortal Wkly Rep 29:229–230. [Google Scholar]
- 94.Centers for Disease Control. 1980. Follow-up on toxic shock syndrome—United States. MMWR Morb Mortal Wkly Rep 29:297–299. [Google Scholar]
- 95.Schuchat A, Broome CV. 1991. Toxic shock syndrome and tampons. Epidemiol Rev 13:99–112. doi: 10.1093/oxfordjournals.epirev.a036080. [DOI] [PubMed] [Google Scholar]
- 96.Reingold AL, Hargrett NT, Shands KN, Dan BB, Schmid GP, Strickland BY, Broome CV. 1982. Toxic shock syndrome surveillance in the United States, 1980 to 1981. Ann Intern Med 96:875–880. doi: 10.7326/0003-4819-96-6-875. [DOI] [PubMed] [Google Scholar]
- 97.Helgerson SD, Foster LR. 1982. Toxic shock syndrome in Oregon: epidemiologic findings. Ann Intern Med 96:909–911. doi: 10.7326/0003-4819-96-6-909. [DOI] [PubMed] [Google Scholar]
- 98.Schlech WF III, Shands KN, Reingold AL, Dan BB, Schmid GP, Hargrett NT, Hightower A, Herwaldt LA, Neill MA, Band JD, Bennett JV. 1982. Risk factors for development of toxic shock syndrome. Association with a tampon brand. JAMA 248:835–839. [PubMed] [Google Scholar]
- 99.Shands KN, Schlech WF III, Hargrett NT, Dan BB, Schmid GP, Bennett JV. 1982. Toxic shock syndrome: case-control studies at the Centers for Disease Control. Ann Intern Med 96:895–898. doi: 10.7326/0003-4819-96-6-895. [DOI] [PubMed] [Google Scholar]
- 100.Kehrberg MW, Latham RH, Haslam BT, Hightower A, Tanner M, Jacobson JA, Barbour AG, Noble V, Smith CB. 1981. Risk factors for staphylococcal toxic-shock syndrome. Am J Epidemiol 114:873–879. doi: 10.1093/oxfordjournals.aje.a113257. [DOI] [PubMed] [Google Scholar]
- 101.Latham RH, Kehrberg MW, Jacobson JA, Smith CB. 1982. Toxic shock syndrome in Utah: a case-control and surveillance study. Ann Intern Med 96:906–908. doi: 10.7326/0003-4819-96-6-906. [DOI] [PubMed] [Google Scholar]
- 102.Osterholm MT, Forfang JC. 1982. Surveillance of toxic shock syndrome in Minnesota: comments on national surveillance. Ann Intern Med 96:887–890. doi: 10.7326/0003-4819-96-6-887. [DOI] [PubMed] [Google Scholar]
- 103.Osterholm MT, Forfang JC. 1982. Toxic-shock syndrome in Minnesota: results of an active-passive surveillance system. J Infect Dis 145:458–464. doi: 10.1093/infdis/145.4.458. [DOI] [PubMed] [Google Scholar]
- 104.Shehin SE, Jones MB, Hochwalt AE, Sarbaugh FC, Nunn S. 2003. Clinical safety-in-use study of a new tampon design. Infect Dis Obstet Gynecol 11:89–99. doi: 10.1080/10647440300025504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Woeller KE, Miller KW, Robertson-Smith AL, Bohman LC. 2015. Impact of advertising on tampon wear-time practices. Clin Med Insights Womens Health 8:29–38. doi: 10.4137/CMWH.S25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lanes SF, Rothman KJ. 1990. Tampon absorbency, composition and oxygen content and risk of toxic shock syndrome. J Clin Epidemiol 43:1379–1385. doi: 10.1016/0895-4356(90)90105-x. [DOI] [PubMed] [Google Scholar]
- 107.Lanes SF, Poole C, Dreyer NA, Lanza LL. 1986. Toxic shock syndrome, contraceptive methods, and vaginitis. Am J Obstet Gynecol 154:989–991. doi: 10.1016/0002-9378(86)90734-9. [DOI] [PubMed] [Google Scholar]
- 108.Berkley SF, Hightower AW, Broome CV, Reingold AL. 1987. The relationship of tampon characteristics to menstrual toxic shock syndrome. JAMA 258:917–920. [PubMed] [Google Scholar]
- 109.Miday RK, Wilson ER. 1988. Toxic shock syndrome: incidence and geographic distribution from a hospital medical records reporting system. Am J Public Health 78:578–580. doi: 10.2105/ajph.78.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reingold AL, Broome CV, Gaventa S, Hightower AW, Toxic Shock Syndrome Study Group . 1989. Risk factors for menstrual toxic shock syndrome: results of a multistate case-control study. Rev Infect Dis 11:S35–S41. doi: 10.1093/clinids/11.Supplement_1.S35. [DOI] [PubMed] [Google Scholar]
- 111.Helms CM, Wintermeyer LA. 1989. Toxic shock syndrome reporting in Iowa. Iowa Med 79:18–20. [PubMed] [Google Scholar]
- 112.Hajjeh RA, Reingold A, Weil A, Shutt K, Schuchat A, Perkins BA. 1999. Toxic shock syndrome in the United States: surveillance update, 1979-1996. Emerg Infect Dis 5:807–810. doi: 10.3201/eid0506.990611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.DeVries AS, Lesher L, Schlievert PM, Rogers T, Villaume LG, Danila R, Lynfield R. 2011. Staphylococcal toxic shock syndrome 2000-2006: epidemiology, clinical features, and molecular characteristics. PLoS One 6:e22997. doi: 10.1371/journal.pone.0022997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smit MA, Nyquist AC, Todd JK. 2013. Infectious shock and toxic shock syndrome diagnoses in hospitals, Colorado, USA. Emerg Infect Dis 19:1855–1858. doi: 10.3201/eid1011.121547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schlievert PM, Nemeth KA, Davis CC, Peterson ML, Jones BE. 2010. Staphylococcus aureus exotoxins are present in vivo in tampons. Clin Vaccine Immunol 17:722–727. doi: 10.1128/CVI.00483-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schlievert PM, Case LC, Strandberg KL, Tripp TJ, Lin YC, Peterson ML. 2007. Vaginal Staphylococcus aureus superantigen profile shift from 1980 and 1981 to 2003, 2004, and 2005. J Clin Microbiol 45:2704–2707. doi: 10.1128/JCM.02295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yarwood JM, Schlievert PM. 2000. Oxygen and carbon dioxide regulation of toxic shock syndrome toxin 1 production by Staphylococcus aureus MN8. J Clin Microbiol 38:1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pragman AA, Yarwood JM, Tripp TJ, Schlievert PM. 2004. Characterization of virulence factor regulation by SrrAB, a two-component system in Staphylococcus aureus. J Bacteriol 186:2430–2438. doi: 10.1128/jb.186.8.2430-2438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yarwood JM, McCormick JK, Schlievert PM. 2001. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J Bacteriol 183:1113–1123. doi: 10.1128/JB.183.4.1113-1123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nelson EA, Jordon M. 8 December 2000. Procter & Gamble seeks new markets for tampons: P&G faces cultural barriers, p A1, A9 The Wall Street Journal, New York, NY. [Google Scholar]
- 121.Czerwinski BS. 2000. Variation in feminine hygiene practices as a function of age. J Obstet Gynecol Neonatal Nurs 29:625–633. doi: 10.1111/j.1552-6909.2000.tb02076.x. [DOI] [PubMed] [Google Scholar]
- 122.Roos R. 4 August 2017. Homeless women in need of menstrual products. Period. NBC Connecticut, West Hartford, CT: https://www.nbcconnecticut.com/news/local/homeless-women-in-need-of-menstrual-products-period/19798/. [Google Scholar]
- 123.Friedman ND. 1981. Everything you must know about tampons. Berkley Books, New York, NY. [Google Scholar]
- 124.Friedman A. 19 June 2014. Astronaut Sally Ride and the burden of being the first. Am Prospect 54:66–69. [Google Scholar]
- 125.Ajmeri JR, Ajmeri CJ. 2011. Nonwoven material for medical applications, p 106–131. In Bartels VJ. (ed), Handbook of medical textiles. Woodhead Publishing, Philadelphia, PA. [Google Scholar]
- 126.Arnold L, Hagele M. 1938. Vaginal tamponage for catemenial sanitary protection. JAMA 110:790–792. doi: 10.1001/jama.1938.02790110016004. [DOI] [Google Scholar]
- 127.Dickenson RL. 1945. Tampons as menstrual guards. JAMA 128:490–494. [Google Scholar]
- 128.Hochwalt AE, Jones MB, Meyer SJ. 2010. Clinical safety assessment of an ultra absorbency menstrual tampon. J Womens Health (Larchmt) 19:273–278. doi: 10.1089/jwh.2009.1423. [DOI] [PubMed] [Google Scholar]
- 129.Food and Drug Administration. 2018. How did the federal Food, Drug, and Cosmetic Act come about? Food and Drug Administration, Rockville, MD: https://www.fda.gov/about-fda/fda-basics/how-did-federal-food-drug-and-cosmetic-act-come-about. [Google Scholar]
- 130.Rome E, Wolhander J. 1992. Can tampon safety be regulated?, p 261–273. In Dan AJ, Lewis LL (ed), Menstrual health in women’s lives. University of Illinois Press, Champaign, IL. [Google Scholar]
- 131.Nightingale SL. 1990. New requirements for tampon labeling. Am Fam Physician 41:999–1000. [PubMed] [Google Scholar]
- 132.O’Gilvie v International Playtex. 1985. 609 F. Supp. 817 (D. Kan. 1985) appeal docketed, no. 85-1861 (10th Cir. June 3, 1985) (argued, January 29, 1986).
- 133.FDA. 2005. Guidance document. Menstrual tampons and pads: information for premarket notification submissions (510(k)s)—guidance for industry and FDA staff. FDA, Rockville, MD: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/menstrual-tampons-and-pads-information-premarket-notification-submissions-510ks-guidance-industry. [Google Scholar]
- 134.Food and Drug Administration. 2018. Kimberly-Clark announces voluntary recall of U by Kotex Sleek tampons, regular absorbency, throughout U.S. and Canada. Food and Drug Administration, Rockville, MD: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/kimberly-clark-announces-voluntary-recall-u-kotexr-sleekr-tampons-regular-absorbencythroughout-us. [Google Scholar]
- 135.Farage MA, Hood WH, Hillard PJA. 2017. The menstrual cycle, the composition of menses on the skin, p 53–62. In Farage MA, Mailbach H (ed), The vulva, 2nd ed CRC Press, Boca Raton, FL. [Google Scholar]
- 136.Fraser IS, Warner P, Marantos PA. 2001. Estimating menstrual blood loss in women with normal and excessive menstrual fluid volume. Obstet Gynecol 98:806–814. doi: 10.1016/s0029-7844(01)01581-2. [DOI] [PubMed] [Google Scholar]
- 137.Yang H, Zhou B, Prinz M, Siegel D. 2012. Proteomic analysis of menstrual blood. Mol Cell Proteomics 11:1024–1035. doi: 10.1074/mcp.M112.018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cederholm-Williams SA, Rees MC, Turnbull AC. 1984. Consumption of fibrinolytic proteins in menstrual fluid from women with normal menstrual blood loss. J Clin Pathol 37:879–881. doi: 10.1136/jcp.37.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cederholm-Williams SA, Rees MC, Turnbull AC. 1984. Examination of certain coagulation factors in menstrual fluid from women with normal blood loss. Thromb Haemost 52:224–225. [PubMed] [Google Scholar]
- 140.Marbaix E, Kokorine I, Moulin P, Donnez J, Eeckhout Y, Courtoy PJ. 1996. Menstrual breakdown of human endometrium can be mimicked in vitro and is selectively and reversibly blocked by inhibitors of matrix metalloproteinases. Proc Natl Acad Sci U S A 93:9120–9125. doi: 10.1073/pnas.93.17.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Simon C, Piquette GN, Frances A, Westphal LM, Heinrichs WL, Polan ML. 1993. Interleukin-1 type I receptor messenger ribonucleic acid expression in human endometrium throughout the menstrual cycle. Fertil Steril 59:791–796. doi: 10.1016/s0015-0282(16)55861-0. [DOI] [PubMed] [Google Scholar]
- 142.Parsonnet J, Gillis ZA, Pier GB. 1986. Induction of interleukin-1 by strains of Staphylococcus aureus from patients with nonmenstrual toxic shock syndrome. J Infect Dis 154:55–63. doi: 10.1093/infdis/154.1.55. [DOI] [PubMed] [Google Scholar]
- 143.Parsonnet J, Hickman RK, Eardley DD, Pier GB. 1985. Induction of human interleukin-1 by toxic-shock-syndrome toxin-1. J Infect Dis 151:514–522. doi: 10.1093/infdis/151.3.514. [DOI] [PubMed] [Google Scholar]
- 144.Beezhold DH, Best GK, Bonventre PF, Thompson M. 1987. Synergistic induction of interleukin-1 by endotoxin and toxic shock syndrome toxin-1 using rat macrophages. Infect Immun 55:2865–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Usala SJ, Usala FO, Haciski R, Holt JA, Schumacher GF. 1989. IgG and IgA content of vaginal fluid during the menstrual cycle. J Reprod Med 34:292–294. [PubMed] [Google Scholar]
- 146.Kansal R, Davis C, Hansmann M, Seymour J, Parsonnet J, Modern P, Gilbert S, Kotb M. 2007. Structural and functional properties of antibodies to the superantigen TSST-1 and their relationship to menstrual toxic shock syndrome. J Clin Immunol 27:327–338. doi: 10.1007/s10875-007-9072-4. [DOI] [PubMed] [Google Scholar]
- 147.Hill DR, Brunner ME, Schmitz DC, Davis CC, Flood JA, Schlievert PM, Wang-Weigand SZ, Osborn TW. 2005. In vivo assessment of human vaginal oxygen and carbon dioxide levels during and post menses. J Appl Physiol 99:1582–1591. doi: 10.1152/japplphysiol.01422.2004. [DOI] [PubMed] [Google Scholar]
- 148.Schlievert PM, Case LC, Nemeth KA, Davis CC, Sun Y, Qin W, Wang F, Brosnahan AJ, Mleziva JA, Peterson ML, Jones BE. 2007. Alpha and beta chains of hemoglobin inhibit production of Staphylococcus aureus exotoxins. Biochemistry 46:14349–14358. doi: 10.1021/bi701202w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bartlett JA, Schleifer SJ, Demetrikopoulos MK, Delaney BR, Shiflett SC, Keller SE. 1998. Immune function in healthy adolescents. Clin Diagn Lab Immunol 5:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tollerud DJ, Ildstad ST, Brown LM, Clark JW, Blattner WA, Mann DL, Neuland CY, Pankiw-Trost L, Hoover RN. 1990. T-cell subsets in healthy teenagers: transition to the adult phenotype. Clin Immunol Immunopathol 56:88–96. doi: 10.1016/0090-1229(90)90172-m. [DOI] [PubMed] [Google Scholar]
- 151.Hickey M, Balen A. 2003. Menstrual disorders in adolescence: investigation and management. Hum Reprod Update 9:493–504. doi: 10.1093/humupd/dmg038. [DOI] [PubMed] [Google Scholar]
- 152.Evagelatou M, Farrant J. 1994. Effect of 17 beta-estradiol on immunoglobulin secretion by human tonsillar lymphocytes in vitro. J Steroid Biochem Mol Biol 48:171–177. doi: 10.1016/0960-0760(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 153.Athreya BH, Pletcher J, Zulian F, Weiner DB, Williams WV. 1993. Subset-specific effects of sex hormones and pituitary gonadotropins on human lymphocyte proliferation in vitro. Clin Immunol Immunopathol 66:201–211. doi: 10.1006/clin.1993.1026. [DOI] [PubMed] [Google Scholar]
- 154.Pacifici R, Vannice JL, Rifas L, Kimble RB. 1993. Monocytic secretion of interleukin-1 receptor antagonist in normal and osteoporotic women: effects of menopause and estrogen/progesterone therapy. J Clin Endocrinol Metab 77:1135–1141. doi: 10.1210/jcem.77.5.8077304. [DOI] [PubMed] [Google Scholar]
- 155.Yamamoto T, Zhou X, Williams CJ, Hochwalt A, Forney LJ. 2009. Bacterial populations in the vaginas of healthy adolescent women. J Pediatr Adolesc Gynecol 22:11–18. doi: 10.1016/j.jpag.2008.01.073. [DOI] [PubMed] [Google Scholar]
- 156.Pendergrass PB, Reeves CA, Belovicz MW, Molter DR, White JH. 1996. The shape and dimensions of the human vagina as seen in three dimensional vinyl polysiloxane casts. Gynecol Obstet Invest 42:178–182. doi: 10.1159/000291946. [DOI] [PubMed] [Google Scholar]
- 157.Pendergrass PB, Reeves CA, Belovicz MW, Molter DR, White JH. 2000. Comparison of vaginal shapes in Afro-American, Caucasian, and Hispanic women as seen with vinyl polysiloxane casting. Gynecol Obstet Invest 50:54–59. doi: 10.1159/000010281. [DOI] [PubMed] [Google Scholar]
- 158.Luo J, Betschart C, Ashton-Miller JA, DeLancey JOL. 2016. Quantitative analyses of variability in normal vaginal shape and dimension on MR images. Int Urogynecol J 27:1087–1095. doi: 10.1007/s00192-016-2949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wagner G, Ottesen B. 1982. Vaginal physiology during menstruation. Ann Intern Med 96:921–923. doi: 10.7326/0003-4819-96-6-921. [DOI] [PubMed] [Google Scholar]
- 160.Wagner G, Bohr L, Wagner P, Petersen LN. 1984. Tampon-induced changes in vaginal oxygen and carbon dioxide tensions. Am J Obstet Gynecol 148:147–150. doi: 10.1016/s0002-9378(84)80165-9. [DOI] [PubMed] [Google Scholar]
- 161.Morgan C, Newell SJ, Ducker DA, Hodgkinson J, White DK, Morley CJ, Church JM. 1999. Continuous neonatal blood gas monitoring using a multiparameter intra-arterial sensor. Arch Dis Child Fetal Neonatal Ed 80:F93–F98. doi: 10.1136/fn.80.2.f93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.O’Hanlon DE, Moench TR, Cone RA. 2013. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One 8:e80074. doi: 10.1371/journal.pone.0080074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Squier CA, Mantz MJ, Schlievert PM, Davis CC. 2008. Porcine vagina ex vivo as a model for studying permeability and pathogenesis in mucosa. J Pharm Sci 97:9–21. doi: 10.1002/jps.21077. [DOI] [PubMed] [Google Scholar]
- 164.Peterson ML, Ault K, Kremer MJ, Klingelhutz AJ, Davis CC, Squier CA, Schlievert PM. 2005. The innate immune system is activated by stimulation of vaginal epithelial cells with Staphylococcus aureus and toxic shock syndrome toxin 1. Infect Immun 73:2164–2174. doi: 10.1128/IAI.73.4.2164-2174.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Spaulding AR, Lin YC, Merriman JA, Brosnahan AJ, Peterson ML, Schlievert PM. 2012. Immunity to Staphylococcus aureus secreted proteins protects rabbits from serious illnesses. Vaccine 30:5099–5109. doi: 10.1016/j.vaccine.2012.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brosnahan AJ, Mantz MJ, Squier CA, Peterson ML, Schlievert PM. 2009. Cytolysins augment superantigen penetration of stratified mucosa. J Immunol 182:2364–2373. doi: 10.4049/jimmunol.0803283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Davis CC, Kremer MJ, Schlievert PM, Squier CA. 2003. Penetration of toxic shock syndrome toxin-1 across porcine vaginal mucosa ex vivo: permeability characteristics, toxin distribution, and tissue damage. Am J Obstet Gynecol 189:1785–1791. doi: 10.1016/s0002-9378(03)00873-1. [DOI] [PubMed] [Google Scholar]
- 168.Davis CC, Baccam M, Mantz MJ, Osborn TW, Hill DR, Squier CA. 2014. Use of porcine vaginal tissue ex-vivo to model environmental effects on vaginal mucosa to toxic shock syndrome toxin-1. Toxicol Appl Pharmacol 274:240–248. doi: 10.1016/j.taap.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 169.Shupp JW, Jett M, Pontzer CH. 2002. Identification of a transcytosis epitope on staphylococcal enterotoxins. Infect Immun 70:2178–2186. doi: 10.1128/iai.70.4.2178-2186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sackren HS. 1939. Vaginal tampons for menstrual absorption. Clin Med Surg 46:327–329. [Google Scholar]
- 171.Thornton MJ. 1943. The use of vaginal tampons for the absorption of menstrual discharge. Am J Obstet Gynecol 46:259–265. doi: 10.1016/S0002-9378(15)32918-5. [DOI] [Google Scholar]
- 172.Brand EN. 1952. Bacteriology of vaginal flora after use of internal tampons. Br Med J i:24–27. doi: 10.1136/bmj.1.4748.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Friedrich EG Jr, Siegesmund KA. 1980. Tampon-associated vaginal ulcerations. Obstet Gynecol 55:149–156. [PubMed] [Google Scholar]
- 174.Barrett KF, Bledsoe S, Greer BE, Droegemueller W. 1977. Tampon-induced vaginal or cervical ulceration. Am J Obstet Gynecol 127:332–333. doi: 10.1016/0002-9378(77)90483-5. [DOI] [PubMed] [Google Scholar]
- 175.Fraser IS, Lahteenmaki P, Elomaa K, Lacarra M, Mishell DR Jr, Alvarez F, Brache V, Weisberg E, Hickey M, Vallentine P, Nash HA. 1999. Variations in vaginal epithelial surface appearance determined by colposcopic inspection in healthy, sexually active women. Hum Reprod 14:1974–1978. doi: 10.1093/humrep/14.8.1974. [DOI] [PubMed] [Google Scholar]
- 176.Szarewski A, Jarvis MJ, Sasieni P, Anderson M, Edwards R, Steele SJ, Guillebaud J, Cuzick J. 1996. Effect of smoking cessation on cervical lesion size. Lancet 347:941–943. doi: 10.1016/S0140-6736(96)91417-8. [DOI] [PubMed] [Google Scholar]
- 177.Skopek A. 1982. Toxic shock syndrome. Incidence and survival of Staphylococcus aureus in unused tampons. Med J Aust 2:74–76. [PubMed] [Google Scholar]
- 178.Kreiswirth BN, Schlievert PM, Novick RP. 1987. Evaluation of coagulase-negative staphylococci for ability to produce toxic shock syndrome toxin 1. J Clin Microbiol 25:2028–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Briancesco R, Paduano S, Semproni M, Bonadonna L. 2018. A study on the microbial quality of sealed products for feminine hygiene. J Prev Med Hyg 59:E226–E229. doi: 10.15167/2421-4248/jpmh2018.59.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Linnemann CC Jr, Staneck JL, Hornstein S, Barden TP, Rauh JL, Bonventre PF, Buncher CR, Beiting A. 1982. The epidemiology of genital colonization with Staphylococcus aureus. Ann Intern Med 96:940–944. doi: 10.7326/0003-4819-96-6-940. [DOI] [PubMed] [Google Scholar]
- 181.Parsonnet J, Hansmann MA, Seymour JL, Delaney ML, Dubois AM, Modern PA, Jones MB, Wild JE, Onderdonk AB. 2010. Persistence survey of toxic shock syndrome toxin-1 producing Staphylococcus aureus and serum antibodies to this superantigen in five groups of menstruating women. BMC Infect Dis 10:249. doi: 10.1186/1471-2334-10-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Parsonnet J, Hansmann MA, Delaney ML, Modern PA, Dubois AM, Wieland-Alter W, Wissemann KW, Wild JE, Jones MB, Seymour JL, Onderdonk AB. 2005. Prevalence of toxic shock syndrome toxin 1-producing Staphylococcus aureus and the presence of antibodies to this superantigen in menstruating women. J Clin Microbiol 43:4628–4634. doi: 10.1128/JCM.43.9.4628-4634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hardy L, Jespers V, De Baetselier I, Buyze J, Mwambarangwe L, Musengamana V, van de Wijgert J, Crucitti T. 2017. Association of vaginal dysbiosis and biofilm with contraceptive vaginal ring biomass in African women. PLoS One 12:e0178324. doi: 10.1371/journal.pone.0178324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hardy L, Jespers V, Van den Bulck M, Buyze J, Mwambarangwe L, Musengamana V, Vaneechoutte M, Crucitti T. 2017. The presence of the putative Gardnerella vaginalis sialidase A gene in vaginal specimens is associated with bacterial vaginosis biofilm. PLoS One 12:e0172522. doi: 10.1371/journal.pone.0172522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dastgheyb SS, Otto M. 2015. Staphylococcal adaptation to diverse physiologic niches: an overview of transcriptomic and phenotypic changes in different biological environments. Future Microbiol 10:1981–1995. doi: 10.2217/fmb.15.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Veeh RH, Shirtliff ME, Petik JR, Flood JA, Davis CC, Seymour JL, Hansmann MA, Kerr KM, Pasmore ME, Costerton JW. 2003. Detection of Staphylococcus aureus biofilm on tampons and menses components. J Infect Dis 188:519–530. doi: 10.1086/377001. [DOI] [PubMed] [Google Scholar]
- 187.Mausner EV, Yitta S, Slywotzky CM, Bennett GL. 2011. Commonly encountered foreign bodies and devices in the female pelvis: MDCT appearances. AJR Am J Roentgenol 196:W461–W470. doi: 10.2214/AJR.10.5119. [DOI] [PubMed] [Google Scholar]
- 188.Kovar WR, Giblin HJ, Roddy JM. 1959. Vaginal tampons and their relationship to the cervix uteri as determined by roentgenography. Obstet Gynecol 13:269–272. [PubMed] [Google Scholar]
- 189.Schlievert PM, Blomster DA, Kelly JA. 1984. Toxic shock syndrome Staphylococcus aureus: effect of tampons on toxic shock syndrome toxin 1 production. Obstet Gynecol 64:666–671. [PubMed] [Google Scholar]
- 190.Reiser RF, Hinzman SJ, Bergdoll MS. 1987. Production of toxic shock syndrome toxin 1 by Staphylococcus aureus restricted to endogenous air in tampons. J Clin Microbiol 25:1450–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schlievert PM. 2020. Effect of non-absorbent intravaginal menstrual/contraceptive products on Staphylococcus aureus production of the superantigen TSST-1. Eur J Clin Microbiol Infect Dis 39:31–38. doi: 10.1007/s10096-019-03685-x. [DOI] [PubMed] [Google Scholar]
- 192.Schlievert PM. 1995. Comparison of cotton and cotton/rayon tampons for effect on production of toxic shock syndrome toxin. J Infect Dis 172:1112–1114. doi: 10.1093/infdis/172.4.1112. [DOI] [PubMed] [Google Scholar]
- 193.Parsonnet J, Modern PA, Giacobbe KD. 1996. Effect of tampon composition on production of toxic shock syndrome toxin-1 by Staphylococcus aureus in vitro. J Infect Dis 173:98–103. doi: 10.1093/infdis/173.1.98. [DOI] [PubMed] [Google Scholar]
- 194.Tierno PM Jr, Hanna BA. 1985. In vitro amplification of toxic shock syndrome toxin-1 by intravaginal devices. Contraception 31:185–194. doi: 10.1016/0010-7824(85)90033-2. [DOI] [PubMed] [Google Scholar]
- 195.Tierno PM Jr, Hanna BA. 1998. Viscose rayon versus cotton tampons. J Infect Dis 177:824–826. doi: 10.1086/517804. [DOI] [PubMed] [Google Scholar]
- 196.Hill DR, Davis CC, Osborn TW III. 2010. Intravaginal and in vitro temperature changes with tampons of differing composition and absorbency. J Biomed Mater Res B Appl Biomater 92:535–541. doi: 10.1002/jbm.b.31550. [DOI] [PubMed] [Google Scholar]
- 197.van der Bijl P, de Blois AM, van Eyk AD, Thompson IO. 2000. Permeability of vaginal mucosa to water at normal and elevated temperatures. SADJ 55:206–210. [PubMed] [Google Scholar]
- 198.Hickey RJ, Abdo Z, Zhou X, Nemeth K, Hansmann M, Osborn TW III, Wang F, Forney LJ. 2013. Effects of tampons and menses on the composition and diversity of vaginal microbial communities over time. BJOG 120:695–704. doi: 10.1111/1471-0528.12151. [DOI] [PubMed] [Google Scholar]
- 199.Merriman JA, Mueller EA, Cahill MP, Beck LA, Paller AS, Hanifin JM, Ong PY, Schneider L, Babineau DC, David G, Lockhart A, Artis K, Leung DY, Schlievert PM. 2016. Temporal and racial differences associated with atopic dermatitis Staphylococcus aureus and encoded virulence factors. mSphere 1:e00295-16. doi: 10.1128/mSphere.00295-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Magrid MO, Gregor J. 1942. The intravaginal tampon in menstrual hygiene; a clinical study. Med Rec 155:316–320. [Google Scholar]
- 201.Onderdonk AB, Zamarchi GR, Walsh JA, Mellor RD, Munoz A, Kass EH. 1986. Methods for quantitative and qualitative evaluation of vaginal microflora during menstruation. Appl Environ Microbiol 51:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nowak-Sadzikowska J, Bulanda M, Heczko PB. 1994. Effect of vaginal tampons on bacterial flora of the lower genitalia. Pol Tyg Lek 49:207–209. [PubMed] [Google Scholar]
- 203.Chow AW, Bartlett KH, Percival-Smith R, Morrison BJ. 1984. Vaginal colonization with Staphylococcus aureus, positive for toxic-shock marker protein, and Escherichia coli in healthy women. J Infect Dis 150:80–84. doi: 10.1093/infdis/150.1.80. [DOI] [PubMed] [Google Scholar]
- 204.Larsen B. 1993. Vaginal flora in health and disease. Clin Obstet Gynecol 36:107–121. doi: 10.1097/00003081-199303000-00016. [DOI] [PubMed] [Google Scholar]
- 205.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. 2007. The human microbiome project. Nature 449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Springer Nature. 2019. After the Integrative Human Microbiome Project, what’s next for the microbiome community? Nature 569:599. doi: 10.1038/d41586-019-01674-w. [DOI] [PubMed] [Google Scholar]
- 207.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.MacPhee RA, Miller WL, Gloor GB, McCormick JK, Hammond JA, Burton JP, Reid G. 2013. Influence of the vaginal microbiota on toxic shock syndrome toxin 1 production by Staphylococcus aureus. Appl Environ Microbiol 79:1835–1842. doi: 10.1128/AEM.02908-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pierson JD, Hansmann MA, Davis CC, Forney LJ. 2018. The effect of vaginal microbial communities on colonization by Staphylococcus aureus with the gene for toxic shock syndrome toxin 1 (TSST-1): a case-control study. Pathog Dis 76:fty015. doi: 10.1093/femspd/fty015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jacquemond I, Muggeo A, Lamblin G, Tristan A, Gillet Y, Bolze PA, Bes M, Gustave CA, Rasigade JP, Golfier F, Ferry T, Dubost A, Abrouk D, Barreto S, Prigent-Combaret C, Thioulouse J, Lina G, Muller D. 2018. Complex ecological interactions of Staphylococcus aureus in tampons during menstruation. Sci Rep 8:9942. doi: 10.1038/s41598-018-28116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Noyes N, Cho KC, Ravel J, Forney LJ, Abdo Z. 2018. Associations between sexual habits, menstrual hygiene practices, demographics and the vaginal microbiome as revealed by Bayesian network analysis. PLoS One 13:e0191625. doi: 10.1371/journal.pone.0191625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Quan L, Morita R, Kawakami S. 2010. Toxic shock syndrome toxin-1 (TSST-1) antibody levels in Japanese children. Burns 36:716–721. doi: 10.1016/j.burns.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 213.Kansal RG, Aziz RK, Kotb M. 2005. Modulation of expression of superantigens by human transferrin and lactoferrin: a novel mechanism in host-Streptococcus interactions. J Infect Dis 191:2121–2129. doi: 10.1086/430386. [DOI] [PubMed] [Google Scholar]
- 214.Norrby-Teglund A, Kaul R, Low DE, McGeer A, Andersson J, Andersson U, Kotb M. 1996. Evidence for the presence of streptococcal-superantigen-neutralizing antibodies in normal polyspecific immunoglobulin G. Infect Immun 64:5395–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Stolz SJ, Davis JP, Vergeront JM, Crass BA, Chesney PJ, Wand PJ, Bergdoll MS. 1985. Development of serum antibody to toxic shock toxin among individuals with toxic shock syndrome in Wisconsin. J Infect Dis 151:883–889. doi: 10.1093/infdis/151.5.883. [DOI] [PubMed] [Google Scholar]
- 216.Vergeront JM, Stolz SJ, Crass BA, Nelson DB, Davis JP, Bergdoll MS. 1983. Prevalence of serum antibody to staphylococcal enterotoxin F among Wisconsin residents: implications for toxic-shock syndrome. J Infect Dis 148:692–698. doi: 10.1093/infdis/148.4.692. [DOI] [PubMed] [Google Scholar]
- 217.Parsonnet J, Goering RV, Hansmann MA, Jones MB, Ohtagaki K, Davis CC, Totsuka K. 2008. Prevalence of toxic shock syndrome toxin 1 (TSST-1)-producing strains of Staphylococcus aureus and antibody to TSST-1 among healthy Japanese women. J Clin Microbiol 46:2731–2738. doi: 10.1128/JCM.00228-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Latham RH, Haslam BT, Dewitt C, Skolnick M, Smith CB. 1983. Histocompatibility leukocyte antigens in patients with toxic-shock syndrome. J Infect Dis 147:783. doi: 10.1093/infdis/147.4.783. [DOI] [PubMed] [Google Scholar]
- 219.Krogman A, Tilahun A, David CS, Chowdhary VR, Alexander MP, Rajagopalan G. 2017. HLA-DR polymorphisms influence in vivo responses to staphylococcal toxic shock syndrome toxin-1 in a transgenic mouse model. HLA 89:20–28. doi: 10.1111/tan.12930. [DOI] [PubMed] [Google Scholar]
- 220.Rasigade JP, Thomas D, Perpoint T, Peyramond D, Chidiac C, Etienne J, Vandenesch F, Lina G, Ferry T. 2011. T-cell response to superantigen restimulation during menstrual toxic shock syndrome. FEMS Immunol Med Microbiol 62:368–371. doi: 10.1111/j.1574-695X.2011.00808.x. [DOI] [PubMed] [Google Scholar]
- 221.Kimber I, Nookala S, Davis CC, Gerberick GF, Tucker H, Foertsch LM, Dearman RJ, Parsonnet J, Goering RV, Modern P, Donnellen M, Morel J, Kotb M. 2013. Toxic shock syndrome: characterization of human immune responses to TSST-1 and evidence for sensitivity thresholds. Toxicol Sci 134:49–63. doi: 10.1093/toxsci/kft099. [DOI] [PubMed] [Google Scholar]
- 222.Vu BG, Stach CS, Kulhankova K, Salgado-Pabón W, Klingelhutz AJ, Schlievert PM. 2015. Chronic superantigen exposure induces systemic inflammation, elevated bloodstream endotoxin, and abnormal glucose tolerance in rabbits: possible role in diabetes. mBio 6:e02554-14. doi: 10.1128/mBio.02554-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Given WD. 1986. Toxic shock syndrome associated with diaphragm use. W V Med J 82:171–173. [PubMed] [Google Scholar]
- 224.Baehler EA, Dillon WP, Cumbo TJ, Lee RV. 1982. Prolonged use of a diaphragm and toxic shock syndrome. Fertil Steril 38:248–250. doi: 10.1016/s0015-0282(16)46467-8. [DOI] [PubMed] [Google Scholar]
- 225.Mitchell MA, Bisch S, Arntfield S, Hosseini-Moghaddam SM. 2015. A confirmed case of toxic shock syndrome associated with the use of a menstrual cup. Can J Infect Dis Med Microbiol 26:218–220. doi: 10.1155/2015/560959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Klug CD, Keay CR, Ginde AA. 2009. Fatal toxic shock syndrome from an intrauterine device. Ann Emerg Med 54:701–703. doi: 10.1016/j.annemergmed.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 227.Nonfoux L, Chiaruzzi M, Badiou C, Baude J, Tristan A, Thioulouse J, Muller D, Prigent-Combaret C, Lina G. 2018. Impact of currently marketed tampons and menstrual cups on Staphylococcus aureus growth and toxic shock syndrome toxin 1 production in vitro. Appl Environ Microbiol 84:e00351-18. doi: 10.1128/AEM.00351-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Leidy LE. 1994. Possible role of the pessary in the etiology of toxic shock syndrome. Med Anthropol Q 8:198–208. doi: 10.1525/maq.1994.8.2.02a00040. [DOI] [Google Scholar]
- 229.Dart RC, Levitt MA. 1985. Toxic shock syndrome associated with the use of the vaginal contraceptive sponge. JAMA 253:1877. doi: 10.1001/jama.253.13.1877b. [DOI] [PubMed] [Google Scholar]
- 230.Faich G, Pearson K, Fleming D, Sobel S, Anello C. 1986. Toxic shock syndrome and the vaginal contraceptive sponge. JAMA 255:216–218. [PubMed] [Google Scholar]
- 231.Smith CB, Noble V, Bensch R, Ahlin PA, Jacobson JA, Latham RH. 1982. Bacterial flora of the vagina during the menstrual cycle: findings in users of tampons, napkins, and sea sponges. Ann Intern Med 96:948–951. doi: 10.7326/0003-4819-96-6-948. [DOI] [PubMed] [Google Scholar]
- 232.Tierno PM, Hanna BA. 1983. Enzymic hydrolysis of tampon carboxymethylcellulose and toxic shock syndrome. Lancet i:1379–1380. doi: 10.1016/s0140-6736(83)92155-4. [DOI] [PubMed] [Google Scholar]
- 233.Schlievert PM. 1996. Effect of Merocel vaginal sponge on growth of Staphylococcus aureus and production of toxic shock syndrome-associated toxins. J Am Coll Surg 183:19–24. [PubMed] [Google Scholar]
- 234.Sciali AR. 2001. Tampons, dioxins, and endometriosis. Reprod Toxicol 15:231–238. doi: 10.1016/s0890-6238(01)00134-4. [DOI] [PubMed] [Google Scholar]
- 235.DeVito MJ, Schecter A. 2002. Exposure assessment to dioxins from the use of tampons and diapers. Environ Health Perspect 110:23–28. doi: 10.1289/ehp.0211023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Branch F, Woodruff TJ, Mitro SD, Zota AR. 2015. Vaginal douching and racial/ethnic disparities in phthalates exposures among reproductive-aged women: National Health and Nutrition Examination Survey 2001-2004. Environ Health 14:57. doi: 10.1186/s12940-015-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Daum RS, Ito T, Hiramatsu K, Hussain F, Mongkolrattanothai K, Jamklang M, Boyle-Vavra S. 2002. A novel methicillin-resistance cassette in community-acquired methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J Infect Dis 186:1344–1347. doi: 10.1086/344326. [DOI] [PubMed] [Google Scholar]
- 238.Herold BC, Immergluck LC, Maranan MC, Lauderdale DS, Gaskin RE, Boyle-Vavra S, Leitch CD, Daum RS. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 239.Diep BA, Chambers HF, Graber CJ, Szumowski JD, Miller LG, Han LL, Chen JH, Lin F, Lin J, Phan TH, Carleton HA, McDougal LK, Tenover FC, Cohen DE, Mayer KH, Sensabaugh GF, Perdreau-Remington F. 2008. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med 148:249–257. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- 240.Tattevin P, Diep BA, Jula M, Perdreau-Remington F. 2009. Methicillin-resistant Staphylococcus aureus USA300 clone in long-term care facility. Emerg Infect Dis 15:953–955. doi: 10.3201/eid1506.080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yarwood JM, McCormick JK, Paustian ML, Kapur V, Schlievert PM. 2002. Repression of the Staphylococcus aureus accessory gene regulator in serum and in vivo. J Bacteriol 184:1095–1101. doi: 10.1128/jb.184.4.1095-1101.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Novick RP. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 243.Salgado-Pabón W, Schlievert PM. 2014. Models matter: the search for an effective Staphylococcus aureus vaccine. Nat Rev Microbiol 12:585–591. doi: 10.1038/nrmicro3308. [DOI] [PubMed] [Google Scholar]
- 244.Roetzer A, Jilma B, Eibl MM. 2017. Vaccine against toxic shock syndrome in a first-in-man clinical trial. Expert Rev Vaccines 16:81–83. doi: 10.1080/14760584.2017.1268921. [DOI] [PubMed] [Google Scholar]
- 245.Giantonio BJ, Alpaugh RK, Schultz J, McAleer C, Newton DW, Shannon B, Guedez Y, Kotb M, Vitek L, Persson R, Gunnarsson PO, Kalland T, Dohlsten M, Persson B, Weiner LM. 1997. Superantigen-based immunotherapy: a phase I trial of PNU-214565, a monoclonal antibody-staphylococcal enterotoxin A recombinant fusion protein, in advanced pancreatic and colorectal cancer. J Clin Oncol 15:1994–2007. doi: 10.1200/JCO.1997.15.5.1994. [DOI] [PubMed] [Google Scholar]
- 246.Cone LA, Woodard DR, Byrd RG, Schulz K, Kopp SM, Schlievert PM. 1992. A recalcitrant, erythematous, desquamating disorder associated with toxin-producing staphylococci in patients with AIDS. J Infect Dis 165:638–643. doi: 10.1093/infdis/165.4.638. [DOI] [PubMed] [Google Scholar]
- 247.Kravitz GR, Dries DJ, Peterson ML, Schlievert PM. 2005. Purpura fulminans due to Staphylococcus aureus. Clin Infect Dis 40:941–947. doi: 10.1086/428573. [DOI] [PubMed] [Google Scholar]
- 248.Assimacopoulos AP, Strandberg KL, Rotschafer JH, Schlievert PM. 2009. Extreme pyrexia and rapid death due to Staphylococcus aureus infection: analysis of 2 cases. Clin Infect Dis 48:612–614. doi: 10.1086/597009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Manders SM, Heymann WR, Atillasoy E, Kleeman J, Schlievert PM. 1996. Recurrent toxin-mediated perineal erythema. Arch Dermatol 132:57–60. [PubMed] [Google Scholar]
- 250.Strom MA, Hsu DY, Silverberg JI. 2017. Prevalence, comorbidities and mortality of toxic shock syndrome in children and adults in the USA. Microbiol Immunol 61:463–473. doi: 10.1111/1348-0421.12539. [DOI] [PubMed] [Google Scholar]
- 251.Bright R, Cope J. 2004. Tampon-related toxic shock syndrome (TSS) continues to peak among adolescent girls; a nationwide hospital study. Int Soc Pharmacoecon Outcomes Res 7:352. doi: 10.1016/S1098-3015(10)62474-6. [DOI] [Google Scholar]
- 252.Reingold A, Manos M, Hurley L. 1998. TSS in a Californian subgroup: an assessment of temporal trends in diagnosed hospitalized cases, 1981-1996. Int Congr Symp Ser 229:27–29. [Google Scholar]
- 253.Petitti DB, Reingold AL. 1991. Recent trends in the incidence of toxic shock syndrome in northern California. Am J Public Health 81:1209–1211. doi: 10.2105/ajph.81.9.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Petitti DB, Reingold A, Chin J. 1986. The incidence of toxic shock syndrome in Northern California. 1972 through 1983. JAMA 255:368–372. [PubMed] [Google Scholar]
- 255.Todd JK, Kurtz B, Combs P, Todd A, Anderson J. 1998. Epidemiology of TSS in Colorado 1970-1996. Int Congr Symp Ser 229:24–26. [Google Scholar]
- 256.Wiesenthal AM, Ressman M, Caston SA, Todd JK. 1986. Toxic shock syndrome in hospitalized patients with Staphylococcus aureus infection. Infection 14:86. doi: 10.1007/bf01644450. [DOI] [PubMed] [Google Scholar]
- 257.Linnemann CC Jr, Knarr D. 1986. Increasing incidence of toxic shock syndrome in the 1970s. Am J Public Health 76:566–567. doi: 10.2105/ajph.76.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Helms CM, Lengeling RW, Pinsky RL, Myers MG, Koontz FP, Klassen LW, Wintermeyer LA. 1981. Toxic shock syndrome: a retrospective study of 25 cases from Iowa. Am J Med Sci 282:50–60. doi: 10.1097/00000441-198109000-00001. [DOI] [PubMed] [Google Scholar]
- 259.Jacobson JA, Nichols CR, Kasworm EM. 1985. Toxic shock syndrome in Utah—1976 to 1983. West J Med 143:337–341. [PMC free article] [PubMed] [Google Scholar]
- 260.Lange JL, Steinbuch M. 2005. Trends in the incidence of toxic shock syndrome. Presented at the 43rd Annual Meeting of the Infectious Diseases Society of America, San Francisco, CA. [Google Scholar]
- 261.Centers for Disease Control and Prevention. 1980. Follow-up on toxic-shock syndrome—United States. MMWR Morb Mortal Wkly Rep 29:441–445. [Google Scholar]
- 262.National Research Council. 1982. Toxic shock syndrome—assessment of current information and future research needs. National Academy Press, Washington, DC. [Google Scholar]
- 263.Anonymous. 1942. Sanitary pads and tampons. Consum Rep 1942:157–160. [Google Scholar]
- 264.Anonymous. 1949. Sanitary pads and tampons—technical report. Consum Rep 1949:352–355. [Google Scholar]
- 265.US Office of Management and Budget. 1987. Regulatory program of the United States Government. Menstrual tampons; proposed user labeling April 1, 1987–March 31, 1988. U.S. Office of Management and Budget, Washington, DC. [Google Scholar]
- 266.US Food and Drug Administration. 2019. Part 801—Labeling. Subpart H—Special requirements for specific devices. Sec. 801.430—User labeling for menstrual tampons. Code of Federal Regulations, title 21, vol 8. Revised as of 1 April 2019. US Food and Drug Administration, Department of Health and Human Services, Washington, DC: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=801.430. [Google Scholar]
- 267.US Food and Drug Administration. 2019. Part 807—Establishment registration and device listing for manufacturers and initial importers of devices. Subpart E—Premarket notification procedures. 21 CFR 807. Revised as of 1 April 2019. US Food and Drug Administration, Department of Health and Human Services, Washington, DC: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=807&showFR=1&s. [Google Scholar]
- 268.The Human Microbiome Consortium. 2012. Structure, function, and diversity of the healthy microbiome. Nature 486:207–214. 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.US Food and Drug Administration. 1995. Compliance Policy Guidelines (CPG): CPG Sec. 345.300 Menstrual sponges. Center for Devices and Radiological Health, US Food and Drug Administration, Washington, DC: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-345300-menstrual-sponges. [Google Scholar]
- 270.US Food and Drug Administration. 2019. Title 21, chapter I, subchapter H—Medical devices; 21 CFR 812 Investigational device exemptions. Updated 19 September 2019. US Food and Drug Administration Department of Health and Human Services, Washington, DC: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=812. [Google Scholar]