Abstract
Acetaminophen (APAP) overdose is the leading cause of acute liver failure in the United States and APAP-induced hepatotoxicity is initiated by formation of a reactive metabolite which depletes hepatic glutathione and forms protein adducts. Studies over the years have established the critical role of c-Jun N terminal kinase (JNK) and its mitochondrial translocation, as well as mitochondrial oxidant stress and subsequent induction of the mitochondrial permeability transition in APAP pathophysiology. However, it is now evident that mitochondrial responses to APAP overdose are more nuanced than appreciated earlier, with multiple levels of control, for example, to dose of APAP. In addition, mitochondrial dynamics, as well as the organelle’s importance in recovery and regeneration after APAP-induced liver injury is also being recognized, which are exciting new areas with significant therapeutic potential. Thus, this review examines the temporal course of hepatocyte mitochondrial responses to an APAP overdose with an emphasis on mechanistic response to various trigger checkpoints such as NAPQI-mitochondrial protein adduct formation and activated JNK translocation. Mitochondrial dynamics, the organelle’s role in recovery after APAP and emerging areas of research which promise to provide further insight into modulation of APAP pathophysiology by these fascinating organelles will also be discussed.
Keywords: Liver, acetaminophen, mitochondria, JNK, ROS, mitochondrial dynamics
Introduction
Acetaminophen (APAP; N-acetyl-p-aminophenol) is a widely used analgesic worldwide and is the most common drug ingredient in the United states according to the Consumer Healthcare Products Association, present in more than 500 different medicines, both prescription and over the counter. While safe at therapeutic doses, an overdose of APAP can result in significant hepatotoxicity and is the most common cause of acute liver failure in the United States (Lee, 2004). While therapeutic doses of APAP are mainly glucuronidated and sulfated followed by excretion through the kidney, a minor percentage is metabolized by the cytochrome P450 system to form a reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) (Ramachandran and Jaeschke, 2018). Since the low amounts of NAPQI generated with therapeutic doses APAP are efficiently scavenged by hepatic glutathione stores, no liver injury is seen with these doses. During an APAP overdose, however, generation of NAPQI is significantly elevated, overwhelming hepatic glutathione stores and resulting in depletion of liver glutathione, which sets the stage for a cascade of events culminating initially in necrosis of hepatocytes closest to the central vein with areas of necrosis subsequently radiating outward. The mitochondria are a critical early target for NAPQI adduct formation, with the early mitochondrial oxidant stress activating the Mitogen Activated Protein (MAP) kinase JNK in the cytosol (Ramachandran et al., 2018). Translocation of activated JNK from the cytosol to the mitochondria (Hanawa et al., 2008) then amplifies the mitochondrial oxidant stress and induces mitochondrial dysfunction with subsequent hepatocyte necrosis (Ramachandran and Jaeschke, 2019b). While NAPQI induced mitochondrial adduct formation and subsequent mitochondrial oxidant stress has been well studied over the last decades (Knight et al., 2002; Knight et al., 2001; Myers et al., 1995; Pumford et al., 1990), the discovery of the amplifying role of JNK was a more recent event (Gunawan et al., 2006; Hanawa et al., 2008). Thus, it is pertinent to examine the early mitochondrial events in the context of JNK activation, which typically occurs by 6 hours in human hepatocytes, with mitochondrial translocation by 15 h (Xie et al, 2014). In mice however, events are accelerated, with JNK activation within 60 minutes after a 300mg/kg dose of APAP, andmitochondrial translocation evident by 2h. This would help in better understanding the signaling cascade induced by NAPQI mediated mitochondrial adducts (Figure 1).
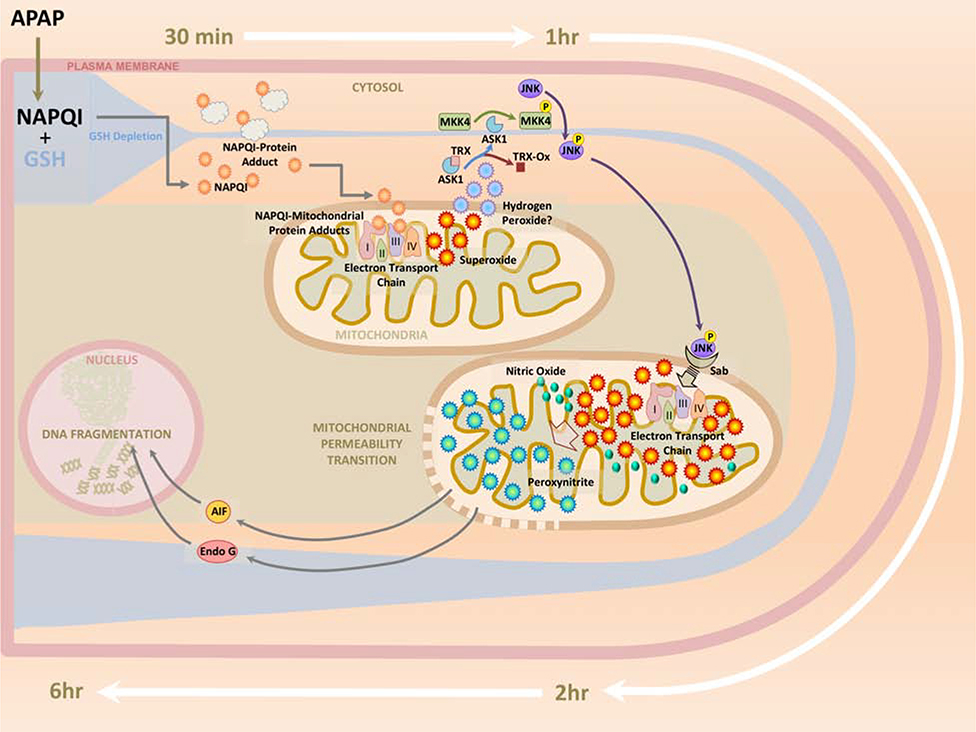
Figure 1: A temporal representation of signaling events targeting mitochondria after a 300mg/kg acetaminophen overdose in the mouse.
Reaction of NAPQI generated after an APAP overdose with cellular GSH results in its rapid depletion within 30 minutes, when NAPQI-protein adducts in the liver are detectable. NAPQI reaction with mitochondrial proteins cause rapid subsequent elevation of mitochondrial protein adducts, accompanied by mild oxidant stress through interference with the electron transport chain. This activates the mitogen activated protein (MAP) kinase c-jun N-terminal kinase (JNK) in the cytosol within 1hr, followed by its translocation to the mitochondria within 2h. The subsequent amplification of mitochondrial oxidant stress along with generation of peroxynitrite by reaction of superoxide with nitric oxide results in induction of the mitochondrial permeability transition and release of endonuclease G and Apoptosis inducing factor (AIF) into the cytosol. By 6h after APAP, these proteins translocate to the nucleus, inducing DNA fragmentation and subsequent cell necrosis.
1. Early Mitochondrial changes after APAP overdose
Due to the reactivity of NAPQI, one of the very early events after its formation and depletion of hepatic glutathione stores is the formation of NAPQI adducts on cellular proteins. Studies focused on mitochondrial function demonstrated that NAPQI binding to mitochondrial proteins correlates with APAP toxicity (Jaeschke et al., 2003). In addition, mitochondrial protein adducts also seem to be responsible for the APAP-induced mitochondrial dysfunction (Hu et al., 2016b). The importance of mitochondrial protein adduct formation to APAP induced hepatotoxicity is illustrated by experiments which used exposure to the species-specific non-toxic regioisomer of APAP, N-acetyl-m-aminophenol (AMAP), which did not form mitochondrial adducts in mice, hamsters and cell lines (Matthews et al., 1997; Myers et al., 1995; Tirmenstein and Nelson, 1989). However, AMAP-induced mitochondrial adduct formation was demonstrated in human hepatocytes, where the regioisomer was indeed toxic (Xie et al., 2015). While glutathione depletion occurs rapidly (within 30 minutes) after a 300mg/kg dose in mice (McGill et al., 2013; McGill et al., 2012), studies examining mitochondrial adducts and functional parameters are typically carried out much later making it difficult to differentiate changes inducing JNK activation and translocation from those induced by JNK translocation. Early studies using radioactive APAP demonstrated highest levels of covalent binding in the cytosolic and ER fractions by 2 hours after a dose of 375mg/kg APAP in mice, but levels in mitochondria were also evident, being approximately 65% of that in the cytosol (Jollow et al., 1973). Subsequent proteomic studies at the 2h time point after a dose of 350mg/kg APAP in mice revealed a number of mitochondrial protein-adducts, including on mitochondrial aldehyde dehydrogenase, the α subunit of ATP synthetase, and glutathione peroxidase (Qiu et al., 1998). Modification of ATP synthase could influence protein activity and decreases in ATP synthase enzyme activity have been shown after APAP overdose (Parmar et al., 1995). However, these measurements were carried out at the 24h time point in the rat model (Parmar et al., 1995), which demonstrates significantly lower amounts of mitochondrial protein adducts and is not a good model in the human context (McGill et al., 2012). Very rapid drops in ATP content and oxygen consumption in the range of minutes have been demonstrated in hepatocytes directly exposed to 400μM NAPQI (Andersson et al., 1990), though the relevance of this time course to in-vivo exposure to APAP is questionable since it is unlikely that hepatocytes would be exposed to such concentrations of NAPQI generated extracellularly in vivo. However, drops in hepatic ATP content have been demonstrated by 90 minutes after a 500mg/kg dose of APAP in the mouse (Jaeschke, 1990), which probably indicates an accelerated time course due to the higher dose. Adduct formation on glutathione peroxidase within 2h (Qiu et al., 1998) is likely to influence mitochondrial anti-oxidant capacity, but this seems to be a bystander effect, since mice deficient in glutathione peroxidase showed no exacerbation of APAP-induced liver injury (Knight et al., 2002). While a more recent proteomic analysis identified additional protein modified after APAP exposure including the voltage gated ion channel VDAC2 as well as antioxidant enzymes such as peroxiredoxin 6 and redox sensitive chaperone PARK7 (Bruderer et al., 2015), a major caveat is that it was carried out on 3D Human Liver Microtissues exposed to concentration of APAP up to 10mM over a 3 day period, which does not match the in vivo acute injury seen with typical APAP overdose, although glutathione depletion, adduct formation and mitochondrial dysfunction is delayed in human hepatocytes when compared to mice (Xie et al., 2014).
Experiments in isolated mitochondria indicated that exposure to APAP inhibited NADH- and succinate-linked respiration reversibly, while spectral changes of cytochromes suggest that inhibition was at the Complex III region of the respiratory chain (Ramsay et al., 1989). However, experiments in isolated mouse hepatocytes exposed to 5mM APAP for 60 minutes also showed decrease in mitochondrial complex I and II mediated respiration without significant effect on complex III (Burcham and Harman, 1991). However, these experiments need cautious interpretation since they were carried out in hepatocytes in suspension cultures which artificially increase susceptibility of cells to APAP induced cell death when compared to adherent cells (Jaeschke et al., 2010). Interestingly, while Complex II of the respiratory chain was found to be susceptible to direct exposure to NAPQI when experiments were carried out in vitro (Lee et al., 2015), only half the mice treated with APAP in vivo showed inhibition of complex II activity 4 hours after a 450mg/kg dose (Lee et al., 2015). However, bypassing complex II in the respiratory chain using methylene blue resulted in significant protection against APAP-induced liver necrosis in vivo (Lee et al., 2015) indicating the modulation of electron transport chain complexes could influence APAP-induced injury. Our recent preliminary data on mitochondrial changes very early after an APAP overdose indicates that while total adducts in the liver are detectable by 15 minutes after an overdose of 300mg/kg in mice, significant elevations in mitochondrial protein adducts were evident between 30–60 min (Nguyen et al., 2019), immediately after glutathione depletion. This occurred prior to JNK activation in the cytosol at 60 minutes, without significant change in electron transport chain parameters, indicating that mitochondrial respiratory function is maintained even after initial adduct formation, which is a requirement for JNK activation in the cytosol (Figure 1). Subsequent experiments indicate that antimycin-induced directional superoxide release from Complex III, preferentially to the intermembrane space of the mitochondria also activates cytosolic JNK along a similar time course as APAP treatment (Ramachandran et al, unpublished data). This raises the possibility that initial NAPQI-induced mitochondrial adduct formation induces release of superoxide from complex III of the electron transport chain towards the cytosolic compartment, which then results in JNK activation without altering mitochondrial bioenergetics. Interestingly, a proteomic analysis of APAP-induced changes in mitochondrial protein expression identified complex III as the most upregulated within the ETC within 2h after a 300mg/kg dose in mice (Stamper et al., 2011).
Activation of JNK in the cytosol is not a direct effect of cytosolic oxidant stress, but involves several intermediates including thioredoxin, apoptosis signal-regulating kinase 1 (ASK1), Mixed-lineage kinase 3 (MLK3) and Mitogen activated protein kinase kinase 4 (MKK4) (Ramachandran and Jaeschke, 2019b). NAPQI mediated mitochondrial adduct formation also results in activation of glycogen synthase kinase-3β (GSK3β) in the cytosol, followed by its translocation to the mitochondria with a time course slightly earlier than that of JNK after a 300mg/kg dose of APAP in mice (Shinohara et al., 2010). Silencing GSK3β significantly reduced JNK activation and protected mice against APAP hepatotoxicity (Shinohara et al., 2010), suggesting that GSK3β functions upstream of JNK, probably at a level parallel to ASK1 (Shinohara et al., 2010). In addition to these kinases, Bax also translocates to the mitochondria along a similar time course as JNK (Bajt et al., 2008; El-Hassan et al., 2003). Bax translocation to mitochondria after an APAP overdose is probably induced by JNK activation, since phosphorylation of the 14–3-3 proteins (which normally tether Bax in the cytosol) by JNK promotes Bax translocation to mitochondria (Tsuruta et al., 2004).
2. The mitochondria after JNK translocation
Mitochondrial alterations subsequent to JNK, Bax and GSK3β translocation are one of the most intensively studied steps of the APAP-induced hepatocyte necrosis cascade. While binding partners and downstream signaling for JNK after APAP has been a focus of the Kaplowitz group, the lack of APAP-specific information for Bax and GSK3β requires extrapolation from other contexts. The mitochondrial outer membrane scaffold protein Sab has been identified as a binding partner for activated JNK (Win et al., 2011) and data from cardiomyocytes indicates that the N-terminal domain of GSK3β may function as a mitochondrial targeting sequence to facilitate binding to VDAC2 on the mitochondrial outer membrane (Tanno et al., 2014), while Bax likely directly inserts into the outer membrane (Westphal et al., 2011). Signal transduction after JNK-Sab interaction on the mitochondrial outer membrane involves release of SHP1 (PTPN6) from Sab in the inside of the mitochondrial outer membrane, which leads to its activation and transfer to the inner membrane mediated by the platform protein DOK4 (Win et al., 2016). SHP1 then dephosphorylates P-Y419Src (active) to its inactive form, resulting in inhibition of the electron transport chain and formation of reactive oxygen species (Win et al., 2016) (Figure 2).The NADH ubiquinone flavoprotein 2 at Tyr 193 of complex I and succinate dehydrogenase A at Tyr 215 of complex II are targets of Src phosphorylation and inhibition of Src activity decreased activity of the NADH dehydrogenase-ubiquinone oxidoreductase system in complex I (Lim et al., 2016). While exogenous expression of GSK3β in the mitochondria itself was shown to decrease complex I activity and ATP production as well as increase reactive oxygen species production (King et al., 2008), this was in neuroblastoma cells and so it is not clear if the same effects will be seen in hepatocytes. While evidence for Bax interaction with subunits of mitochondrial Complex I have been presented (Kim et al., 2014), the deficiency of Bax does not influence APAP-induced mitochondrial ROS generation (Bajt et al., 2008), and while these mice showed early protection against APAP hepatotoxicity by 6 h, liver necrosis was similar to wild type mice at later 24h time points (Bajt et al., 2008). This suggests that alternate pathways for completion of necrotic cell death exist if Bax translocation is prevented in a milieu of oxidative stress. The above-mentioned proteins are not the only molecules migrating to mitochondria after APAP. Transfer of iron from the lysosomes to mitochondria has been demonstrated between 2 and 4 hours in hepatocytes exposed to APAP in culture (Kon et al., 2010). Lysosomal instability was also evident by 3h after APAP overdose in mice in vivo (Woolbright et al., 2012) and further studies in isolated primary hepatocytes reveal that iron translocation into mitochondrial after APAP occur through the calcium uniporter (Hu et al., 2016a).
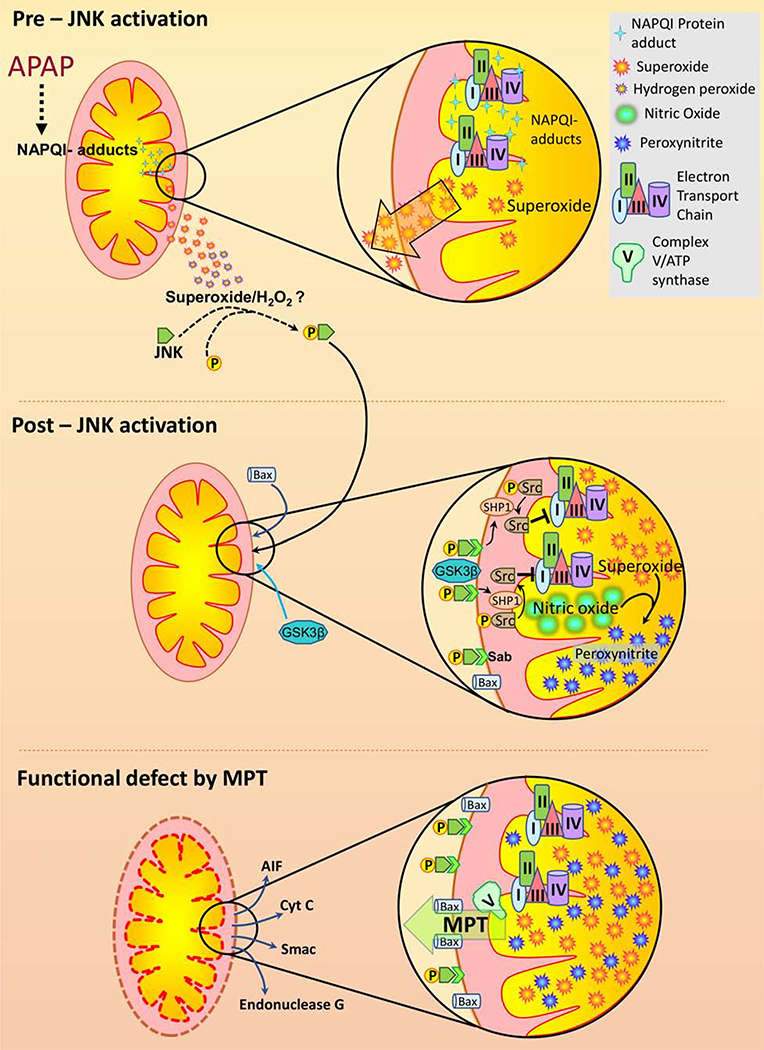
Figure 2. Mitochondrial response to an APAP overdose:
NAPQI protein adduct formation on mitochondrial proteins is a relatively early event after and APAP overdose. While complete mechanistic details are still to be worked out, adduct formation causes elevated superoxide production, especially towards the cytosol, probably from complex III of the respiratory chain. This early superoxide release seems to occur without appreciable mitochondrial dysfunction, with bioenergetic parameters being maintained. The cytosolic release of mitochondrial derived free radicals ultimately activates the mitogen activated protein (MAP) kinase c-jun N-terminal kinase (JNK) through several intermediates which have been reviewed elsewhere (Ramachandran and Jaeschke, 2019b). Subsequent translocation of JNK as well as other proteins such as Bax and GSK3β activate a signaling cascade which is likely distinct from the early mechanism of superoxide elevation. This post-JNK translocation phase is induced by release of SHP1 from its binding partner Sab on the outer membrane following JNK-Sab binding. This in turn dephosphorylates Src, which then results in inhibition of mitochondrial electron transport and enhanced superoxide formation. Superoxide reaction with nitric oxide results in peroxynitrite formation, which exacerbates mitochondrial dysfunction. This amplification of mitochondrial oxidant stress after JNK translocation ultimately results in activation of the mitochondrial permeability transition pore (MPTP) opening facilitated by Bax on the mitochondrial outer membrane. Release of proteins such as endonuclease G and AIF from mitochondria through the MPTP ultimately causes nuclear DNA fragmentation and hepatocyte necrosis after their translocation to the nucleus.
Thus, the overall consequence of activation and translocation of JNK and its partners to the mitochondria is an increased generation of superoxide from the organelle (Yan et al., 2010), and the critical role played by JNK in ROS formation is evident in the protection against APAP hepatotoxicity by blockade of JNK activation and translocation (Akakpo et al., 2019; Huo et al., 2017; Saito et al., 2010a). The importance of mitochondrial superoxide is evident in the exacerbation of APAP hepatoxicity seen in mice with partial deficiency of mitochondrial localized manganese superoxide dismutase (MnSOD) (Fujimoto et al., 2009; Ramachandran et al., 2011b) and further confirmed by robust protection afforded by mitochondrially targeted superoxide dismutase mimetics such as Mito-TEMPO (Du et al., 2017a; Du et al., 2019). Recent studies also highlight the importance of modulation of mitochondrial bioenergetics in influencing mitochondrial events post JNK translocation as seen in mice deficient in pyruvate dehydrogenase kinase 4, which showed a rapid alteration (by 2h) of mitochondrial uncoupling protein 2 (UCP2) levels after APAP and were protected against liver injury despite JNK translocation to mitochondria (Duan et al., 2020).
Mechanistically, however, it is the reaction product of mitochondrial generated superoxide with nitric oxide to form peroxynitrite (Cover et al., 2005; Knight et al., 2001; Saito et al., 2010b), which further amplifies mitochondrial dysfunction. Formation of peroxynitrite in centrilobular hepatocytes was identified by immunostaining for nitro-tyrosine modified proteins after an APAP overdose in mice (Hinson et al., 1998). Evidence that significant peroxynitrite formation occurs subsequent to JNK activation and translocation arises from studies which revealed nitro-tyrosine staining in sinusoidal endothelial cell staining within 1 hour after a 300mg/kg dose of APAP in mice, with hepatocyte staining only by 4h post APAP (Knight et al., 2001). The source of mitochondrial nitric oxide for peroxynitrite formation after APAP has been unclear, with various nitric oxide synthase (NOS) inhibitors having either no effect or exacerbating APAP induced liver injury (Hinson et al., 2002) and iNOS deficient mice showing injury similar to wild type animals (Saito et al., 2010a). Though a definite molecular source is yet to be conclusively determined, currently, the most plausible candidate seems to be neuronal NOS (nNOS), a putative mitochondrial NOS (Finocchietto et al., 2009). Pharmacological inhibition of nNOS was shown to protect hepatocytes against APAP-induced necrosis in vitro (Banerjee et al., 2015) and nNOS deficient mice were also protected against APAP-induced liver injury (Agarwal et al., 2012). Consequences of peroxynitrite formation are widespread, ranging from post translational modification and inhibition of MnSOD (Agarwal et al., 2011), which would further compromise mitochondrial superoxide scavenging, to mitochondrial DNA modification and loss (Cover et al., 2005). The critical role played by peroxynitrite-induced protein modification in APAP-hepatotoxicity is evident from the fact that scavenging peroxynitrite by resveratrol (Du et al., 2015) or mitochondrial glutathione (Knight et al., 2002; Saito et al., 2010b) protects against liver injury.
3. Mitochondrial permeability transition- effect of dose
It is now clear that amplification of mitochondrial oxidative and nitrosative stress by JNK translocation facilitates induction of the mitochondrial permeability transition and loss of membrane potential (Kon et al., 2004; Masubuchi et al., 2005; Ramachandran et al., 2011a). While these events were considered to be terminal for mitochondrial function, with subsequent loss of functional capacity, it is now being recognized that these changes are much more nuanced than earlier appreciated with the dose of APAP modulating the extent of damage. The mitochondrial permeability transition pore (MPTP) on the inner mitochondrial membrane has been functionally recognized to allow movement of molecules of less than 1.5kDa across the membrane (Bernardi et al., 1994) and its regulation by cyclophilin D from within the mitochondrial matrix is also well characterized (Baines et al., 2005; Karch and Molkentin, 2014). The molecular identity of the MPTP has been intensively investigated over the last decades and while studies are ongoing, an emerging candidate is the c-subunit of ATP synthase (Mnatsakanyan et al., 2017) though roles for other proteins such as the adenine nucleotide transporter are still being clarified. Recent studies using reconstituted purified monomeric ATP synthase from porcine heart mitochondria into vesicles with mitochondrial inner membrane composition indicate that the ATP synthase monomer is sufficient for MPTP activity (Mnatsakanyan et al., 2019). However, there is possibly some difference between tissues, since liver mitochondrial MPTP seems to be mediated by the adenine nucleotide transporter (ANT) and an additional cyclosporin inhibitable component (Karch et al., 2019) which is yet to be identified conclusively. However, these identified components could also be functioning as part of a large multi protein complex, since ANT has been shown to interact and form a complex with F1FO ATP synthase (Chen et al., 2004; Mnatsakanyan et al., 2017). In spite of the investigations on the inner mitochondrial membrane pore, contributors to the permeabilization of the outer membrane are mainly Bax and the protein Bak (Karch et al., 2013; Karch and Molkentin, 2015). However, Bax regulated outer membrane permeabilization, while acting in synergy with inner membrane permeabilization, also seems to be separately regulated in certain contexts. For example, activation of the inner membrane permeability transition and loss of mitochondrial membrane potential can occur in absence of Bax and Bak (Karch et al., 2013), and Bax has been shown to form two distinct types of channels which can fine-tune outer membrane permeabilization (Lin et al., 2011).
The protection against APAP induced liver injury afforded by pharmacological inhibition of cyclophilin D in vitro (Kon et al., 2004) implicated the mitochondrial permeability transition (MPT) in mitochondrial dysfunction after APAP. However, this protection was transient, evident at 6h but disappearing by 16h after APAP (Kon et al., 2004) suggesting that sustained upstream signaling can bypass the blockade mediated by cyclophilin D inhibition. The relevance of dose was evident in in vivo studies, where mice lacking cyclophilin D were protected against liver injury when exposed to APAP at 200mg/kg (Ramachandran et al., 2011a), but were not protected at the higher 600mg/kg dose (LoGuidice and Boelsterli, 2011). Interestingly, an in vivo study comparing a low 150mg/kg dose of APAP with 300mg/kg in mice demonstrated transient JNK activation and reversible induction of the MPTP at the lower dose with irreversible MPTP at the higher dose (Hu et al., 2016b). This indicates that sustained JNK activation is required to maintain necrotic cell signaling after APAP overdose and induction of the MPTP is not the terminal event for mitochondrial dysfunction. Such transient opening of the MPTP are now recognized as having possible physiological functions in various systems (Boyman et al., 2019; Mnatsakanyan et al., 2017). With regards to JNK localization on mitochondria, rigorous time course examinations are lacking, but our preliminary data indicates that activated phospho-JNK on mitochondria gradually disappears by 24h after a 300mg/kg dose of APAP in mice, indicating an additional level of control by modulating duration of P-JNK localization on mitochondria. MAP kinases are typically inactivated by dephosphorylation by mitogen-activated protein kinase phosphatases (Keyse, 2000) and mitogen-activated protein kinase phosphatase 1 (MKP-1) is responsible for dephosphorylation of JNK (Wancket et al., 2012). Interestingly, MKP-1 deficient mice showed an increased susceptibility with prolonged JNK activation (Wancket et al., 2012), suggesting that MKP-1 restricts APAP-induced necrosis and hence its influence on duration of JNK activation provides fine control of the dose response to APAP-induced mitochondrial oxidant stress. Ultimately, in the face of persistent JNK activation and sustained mitochondrial oxidant stress, induction of the mitochondrial permeability transition results in release of inter membrane proteins such as apoptosis inducing factor (AIF) and endonuclease G, which translocate to the nucleus to induce DNA fragmentation and hepatocyte necrosis. These aspects have been extensively reviewed recently (Jaeschke et al., 2019b; Ramachandran and Jaeschke, 2019a) and hence preclude discussion in this mitochondria-focused review. Nevertheless, irreversible opening of the MPTP, accompanied by mitochondrial matrix swelling and release of intermembrane proteins causing DNA fragmentation, represent the point of no return for APAP-induced cell death.
4. Mitochondrial dynamics, biogenesis and its role in liver recovery after APAP-induced injury
While mitochondrial morphology was not a focus of early studies examining its role in cellular energy homeostasis, evidence from recent decades have established that mitochondria are dynamic organelles undergoing significant changes in morphology which are interlinked with its function. Thus, mitochondria undergo fission and fusion during maintenance of cellular homeostasis, which can be perturbed in pathophysiological conditions. APAP overdose was shown to induce elevations in the mitochondrial fission protein Drp1 and its translocation to mitochondria after APAP overdose resulting in mitochondrial fission (Ramachandran et al., 2013). Drp1 translocation and mitochondrial fission are downstream of JNK activation and mitochondrial translocation, since mice lacking Sab, the JNK binding partner on the mitochondrial outer membrane, did not show Drp1 translocation (Dara et al., 2015). Drp1 mediated mitochondrial fission could influence later events such as the MPTP, since Drp1 can interact with and reciprocally regulate Bax (Karbowski et al., 2002; Wang et al., 2015; Wu et al., 2011). Considering the earlier discussed translocation of lysosomal iron to mitochondria after APAP, it is also interesting to note that iron has been suggested to modulate mitochondrial dynamics (Upadhyay and Agarwal, 2019), albeit studied in neuronal systems. An excess of mitochondrial iron seems to facilitate mitochondrial fission (Upadhyay and Agarwal, 2019), which is what is evident after APAP. Delving deeper into mitochondrial structural features, cristae are mitochondrial inner membrane invaginations which increase inner membrane surface area and host components of the ETC. Recent evidence now highlights structural alterations in cristae which influence function of the electron transport chain (ETC) allowing adaptation to cellular perturbations. It has been shown that the arrangement of ETC complexes vary between normal cellular conditions and situations of stress, which facilitate formation of ETC super-complexes to modulate reactive oxygen production and ATP generation (Baker et al., 2019). In this context, it is interesting to note that APAP was also suggested to block assembly of mitochondrial respiratory super complexes by modulation of the mitochondrial negative regulator MCJ, which ultimately decreased ATP production and enhanced ROS generation (Barbier-Torres et al., 2017). Thus, the remodeling of cristae and mitochondria can regulate mitochondrial efficiency and influence cellular bioenergetic status (Baker et al., 2019), which would have significant consequences on subsequent cellular viability.
While the roles of mitochondria in APAP-induced liver injury have been extensively investigated as detailed above, APAP overdose also triggers adaptive responses which attempt to maintain homeostasis in the face of mitochondrial dysfunction by removal of damaged mitochondria (Figure 3). Such autophagic responses are important, since they facilitate removal of APAP-protein adducts (Ni et al., 2016) and limit APAP induced injury (Ni et al., 2012). A specialized version of autophagy targets mitochondria and removes damaged mitochondrial by mitophagy (Jaeschke et al., 2019a; Ni et al., 2012; Ni et al., 2013) through a PINK1-Parkin signaling pathway (Wang et al., 2019) and inhibition of this process results in exacerbation of APAP-induced liver injury (Baulies et al., 2015). While the dose of APAP would dictate the effectiveness of limiting liver injury by the adaptive mechanisms, it is now becoming evident that patient prognosis after an APAP overdose is not only dependent on the extent of injury, but also on the liver’s inherent capacity to regenerate (Schmidt and Dalhoff, 2005). APAP hepatotoxicity-induced liver regeneration involves a complex interplay of several signaling mediators, including growth factors, cytokines, angiogenic factors, and other mitogenic pathways (Bhushan and Apte, 2019). Mitochondrial biogenesis has been recognized to play an important role in recovery after an APAP overdose (Jaeschke et al., 2019a; Ramachandran and Jaeschke, 2019b; Ramachandran et al., 2018), especially in surviving cells bordering the areas of centrilobular necrosis (Du et al., 2017b). This spatially defined enhancement of mitochondrial biogenesis plays a critical role in liver recovery after APAP, with pharmacological activation of mitochondrial biogenesis facilitating liver recovery and regeneration (Du et al., 2017b). Centrilobular necrosis is a characteristic of APAP-induced liver injury and illustrates the importance of spatial heterogeneity of hepatocytes to drug-induced liver injury. The enhanced susceptibility of centrilobular hepatocytes to APAP hepatotoxicity has been attributed to several reasons, including increased expression of cyp2E1, the prominent cytochrome P450 enzyme metabolizing APAP, as well as lower levels of glutathione stores (Sezgin et al., 2018). However, another putative reason for this spatial divergence in susceptibility, which has been relatively neglected, is the heterogeneity of mitochondria in hepatocytes from the central and portal regions. Zonal mitochondrial heterogeneity within the liver has been well recognized from an anatomical perspective for several decades (Ferri et al., 2005; Gebhardt and Matz-Soja, 2014; Guzman et al., 1995) but the role this plays in influencing pathophysiology such as drug-induced liver injury is not well understood and would be interesting to study.
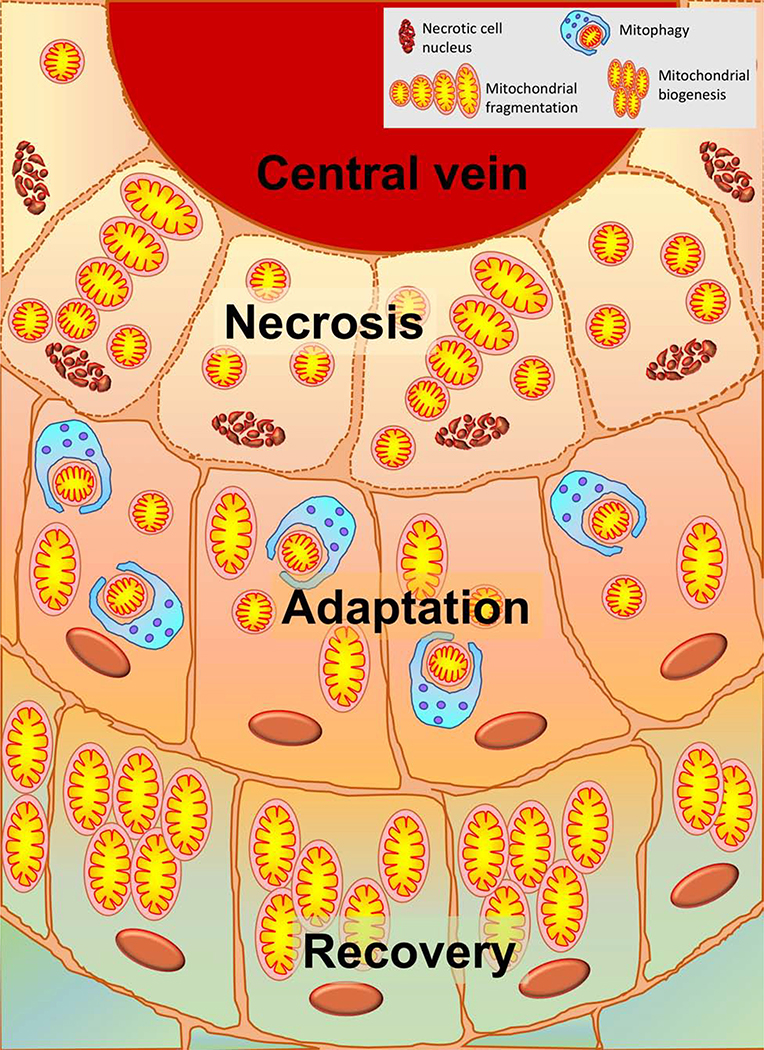
Figure 3: Spatial distinctions of mitochondrial response to APAP overdose:
APAP overdose is characterized by centrilobular necrosis initiated in hepatocytes around the central vein. These cells will have significant mitochondrial dysfunction and morphological changes such as mitochondrial fission. Cells immediately adjacent to those undergoing necrosis will be attempting adaptation by removing damaged mitochondria undergoing fission by mitophagy and may recover if adaptation is successful. Surviving cells at the outer border, will induce mitochondrial biogenesis in order to facilitate recovery and regeneration of hepatocytes lost by necrosis.
Conclusions
In summary, while extensive investigation over the years have highlighted the critical role of mitochondrial dysfunction and oxidant stress in APAP-induced hepatotoxicity, the somewhat naive concept of the organelle as a silent gatekeeper allowing downstream events to occur once JNK is activated and translocates to it is clearly rather simplistic. If anything, the mitochondria function as a highly regulated and selective gatekeepers, with nuanced responses dictated by multiple stimuli, JNK translocation being one among them. Recent evidence also indicates that mechanisms of mitochondrial oxidant stress immediately after NAPQI-adduct formation, which initially result in JNK activation are mechanistically distinct from those inducing free radical formation from the ETC subsequent to JNK translocation. Other emerging areas of research including the role of mitochondrial dynamics as well as zonal heterogeneity in dictating regenerative responses after APAP are poised to provide exciting information which could facilitate development of late acting therapeutics to prevent development of liver failure after an APAP overdose.
Acknowledgements
Work in the authors’ laboratory was supported by a grant from McNeil Consumer Health Care, Inc., the National Institutes of Health grant R01 DK102142, and the National Institute of General Medical Sciences (P20 GM103549 and P30 GM118247) from the National Institutes of Health.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal R, Hennings L, Rafferty TM, Letzig LG, McCullough S, James LP, MacMillan-Crow LA, Hinson JA, 2012. Acetaminophen-induced hepatotoxicity and protein nitration in neuronal nitric-oxide synthase knockout mice. J Pharmacol Exp Ther 340, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R, MacMillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, Hinson JA, 2011. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther 337, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akakpo JY, Ramachandran A, Duan L, Schaich MA, Jaeschke MW, Freudenthal BD, Ding WX, Rumack BH, Jaeschke H. Delayed Treatment With 4-Methylpyrazole Protects Against Acetaminophen Hepatotoxicity in Mice by Inhibition of c-Jun n-Terminal Kinase. Toxicol Sci. 2019. July 1;170(1):57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson BS, Rundgren M, Nelson SD, Harder S, 1990. N-acetyl-p-benzoquinone imine-induced changes in the energy metabolism in hepatocytes. Chem Biol Interact 75, 201–211. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD, 2005. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H, 2008. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther 324, 8–14. [DOI] [PubMed] [Google Scholar]
- Baker N, Patel J, Khacho M, 2019. Linking mitochondrial dynamics, cristae remodeling and supercomplex formation: How mitochondrial structure can regulate bioenergetics. Mitochondrion 49, 259–268. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Melnyk SB, Krager KJ, Aykin-Burns N, Letzig LG, James LP, Hinson JA, 2015. The neuronal nitric oxide synthase inhibitor NANT blocks acetaminophen toxicity and protein nitration in freshly isolated hepatocytes. Free Radic Biol Med 89, 750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier-Torres L, Iruzubieta P, Fernandez-Ramos D, Delgado TC, Taibo D, Guitierrez-de-Juan V, Varela-Rey M, Azkargorta M, Navasa N, Fernandez-Tussy P, Zubiete-Franco I, Simon J, Lopitz-Otsoa F, Lachiondo-Ortega S, Crespo J, Masson S, McCain MV, Villa E, Reeves H, Elortza F, Lucena MI, Hernandez-Alvarez MI, Zorzano A, Andrade RJ, Lu SC, Mato JM, Anguita J, Rincon M, Martinez-Chantar ML, 2017. The mitochondrial negative regulator MCJ is a therapeutic target for acetaminophen-induced liver injury. Nat Commun 8, 2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulies A, Ribas V, Nunez S, Torres S, Alarcon-Vila C, Martinez L, Suda J, Ybanez MD, Kaplowitz N, Garcia-Ruiz C, Fernandez-Checa JC, 2015. Lysosomal Cholesterol Accumulation Sensitizes To Acetaminophen Hepatotoxicity by Impairing Mitophagy. Sci Rep 5, 18017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P, Broekemeier KM, Pfeiffer DR, 1994. Recent progress on regulation of the mitochondrial permeability transition pore; a cyclosporin-sensitive pore in the inner mitochondrial membrane. J Bioenerg Biomembr 26, 509–517. [DOI] [PubMed] [Google Scholar]
- Bhushan B, Apte U, 2019. Liver Regeneration after Acetaminophen Hepatotoxicity: Mechanisms and Therapeutic Opportunities. Am J Pathol 189, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman L, Coleman AK, Zhao G, Wescott AP, Joca HC, Greiser BM, Karbowski M, Ward CW, Lederer WJ, 2019. Dynamics of the mitochondrial permeability transition pore: Transient and permanent opening events. Arch Biochem Biophys 666, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruderer R, Bernhardt OM, Gandhi T, Miladinovic SM, Cheng LY, Messner S, Ehrenberger T, Zanotelli V, Butscheid Y, Escher C, Vitek O, Rinner O, Reiter L, 2015. Extending the limits of quantitative proteome profiling with data-independent acquisition and application to acetaminophen-treated three-dimensional liver microtissues. Mol Cell Proteomics 14, 1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcham PC, Harman AW, 1991. Acetaminophen toxicity results in site-specific mitochondrial damage in isolated mouse hepatocytes. J Biol Chem 266, 5049–5054. [PubMed] [Google Scholar]
- Chen C, Ko Y, Delannoy M, Ludtke SJ, Chiu W, Pedersen PL, 2004. Mitochondrial ATP synthasome: three-dimensional structure by electron microscopy of the ATP synthase in complex formation with carriers for Pi and ADP/ATP. J Biol Chem 279, 31761–31768. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H, 2005. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther 315, 879–887. [DOI] [PubMed] [Google Scholar]
- Dara L, Johnson H, Suda J, Win S, Gaarde W, Han D, Kaplowitz N, 2015. Receptor interacting protein kinase 1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology 62, 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Farhood A, Jaeschke H, 2017a. Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch Toxicol 91, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, McGill MR, Xie Y, Bajt ML, Jaeschke H, 2015. Resveratrol prevents protein nitration and release of endonucleases from mitochondria during acetaminophen hepatotoxicity. Food Chem Toxicol 81, 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Ramachandran A, McGill MR, Mansouri A, Asselah T, Farhood A, Woolbright BL, Ding WX, Jaeschke H, 2017b. Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity. Food Chem Toxicol 108, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Ramachandran A, Weemhoff JL, Woolbright BL, Jaeschke AH, Chao X, Ding WX, Jaeschke H, 2019. Mito-tempo protects against acute liver injury but induces limited secondary apoptosis during the late phase of acetaminophen hepatotoxicity. Arch Toxicol 93, 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Ramachandran A, Akakpo JY, Woolbright BL, Zhang Y, Jaeschke H, 2020. Mice deficient in pyruvate dehydrogenase kinase 4 are protected against acetaminophen-induced hepatotoxicity. Toxicol Appl Pharmacol 387, 114849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, Lee PC, Hinton RH, Price SC, Kass GE, 2003. Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: roles of cytochrome c, Bax, Bid, and caspases. Toxicol Appl Pharmacol 191, 118–129. [DOI] [PubMed] [Google Scholar]
- Ferri D, Moro L, Mastrodonato M, Capuano F, Marra E, Liquori GE, Greco M, 2005. Ultrastructural zonal heterogeneity of hepatocytes and mitochondria within the hepatic acinus during liver regeneration after partial hepatectomy. Biol Cell 97, 277–288. [DOI] [PubMed] [Google Scholar]
- Finocchietto PV, Franco MC, Holod S, Gonzalez AS, Converso DP, Antico Arciuch VG, Serra MP, Poderoso JJ, Carreras MC, 2009. Mitochondrial nitric oxide synthase: a masterpiece of metabolic adaptation, cell growth, transformation, and death. Exp Biol Med (Maywood) 234, 1020–1028. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Kumagai K, Ito K, Arakawa S, Ando Y, Oda S, Yamoto T, Manabe S, 2009. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol Pathol 37, 193–200. [DOI] [PubMed] [Google Scholar]
- Gebhardt R, Matz-Soja M, 2014. Liver zonation: Novel aspects of its regulation and its impact on homeostasis. World J Gastroenterol 20, 8491–8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N, 2006. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology 131, 165–178. [DOI] [PubMed] [Google Scholar]
- Guzman M, Bijleveld C, Geelen MJ, 1995. Flexibility of zonation of fatty acid oxidation in rat liver. Biochem J 311 ( Pt 3), 853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N, 2008. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem 283, 13565–13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA, Bucci TJ, Irwin LK, Michael SL, Mayeux PR, 2002. Effect of inhibitors of nitric oxide synthase on acetaminophen-induced hepatotoxicity in mice. Nitric Oxide 6, 160–167. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Pike SL, Pumford NR, Mayeux PR, 1998. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol 11, 604–607. [DOI] [PubMed] [Google Scholar]
- Hu J, Kholmukhamedov A, Lindsey CC, Beeson CC, Jaeschke H, Lemasters JJ, 2016a. Translocation of iron from lysosomes to mitochondria during acetaminophen-induced hepatocellular injury: Protection by starch-desferal and minocycline. Free Radic Biol Med 97, 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Ramshesh VK, McGill MR, Jaeschke H, Lemasters JJ, 2016b. Low Dose Acetaminophen Induces Reversible Mitochondrial Dysfunction Associated with Transient c-Jun N-Terminal Kinase Activation in Mouse Liver. Toxicol Sci 150, 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y, Win S, Than TA, Yin S, Ye M, Hu H, Kaplowitz N, 2017. Antcin H Protects Against Acute Liver Injury Through Disruption of the Interaction of c-Jun-N-Terminal Kinase with Mitochondria. Antioxid Redox Signal 26, 207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, 1990. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther 255, 935–941. [PubMed] [Google Scholar]
- Jaeschke H, Duan L, Nguyen N, Ramachandran A, 2019a. Mitochondrial damage and biogenesis in acetaminophen-induced liver injury. Liver Research 3, 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Knight TR, Bajt ML, 2003. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett 144, 279–288. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Ramachandran A, Chao X, Ding WX, 2019b. Emerging and established modes of cell death during acetaminophen-induced liver injury. Arch Toxicol 93, 3491–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Yan HM, Ramachandran A, 2010. Reactive nitrogen species in acetaminophen-induced mitochondrial damage and toxicity in mouse hepatocytes: a cautionary note on the impact of cell culture conditions. Chem Res Toxicol 23, 1853–1854; author reply 1855–1858. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB, 1973. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther 187, 195–202. [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ, 2002. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol 159, 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch J, Bround MJ, Khalil H, Sargent MA, Latchman N, Terada N, Peixoto PM, Molkentin JD, 2019. Inhibition of mitochondrial permeability transition by deletion of the ANT family and CypD. Sci Adv 5, eaaw4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch J, Kwong JQ, Burr AR, Sargent MA, Elrod JW, Peixoto PM, Martinez-Caballero S, Osinska H, Cheng EH, Robbins J, Kinnally KW, Molkentin JD, 2013. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. Elife 2, e00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch J, Molkentin JD, 2014. Identifying the components of the elusive mitochondrial permeability transition pore. Proc Natl Acad Sci U S A 111, 10396–10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch J, Molkentin JD, 2015. Regulated necrotic cell death: the passive aggressive side of Bax and Bak. Circ Res 116, 1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse SM, 2000. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol 12, 186–192. [DOI] [PubMed] [Google Scholar]
- Kim EM, Park JK, Hwang SG, Kim WJ, Liu ZG, Kang SW, Um HD, 2014. Nuclear and cytoplasmic p53 suppress cell invasion by inhibiting respiratory complex-I activity via Bcl-2 family proteins. Oncotarget 5, 8452–8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TD, Clodfelder-Miller B, Barksdale KA, Bijur GN, 2008. Unregulated mitochondrial GSK3beta activity results in NADH: ubiquinone oxidoreductase deficiency. Neurotox Res 14, 367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H, 2002. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther 303, 468–475. [DOI] [PubMed] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H, 2001. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci 62, 212–220. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ, 2004. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40, 1170–1179. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Uchiyama A, Jaeschke H, Lemasters JJ, 2010. Lysosomal iron mobilization and induction of the mitochondrial permeability transition in acetaminophen- induced toxicity to mouse hepatocytes. Toxicol Sci 117, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Imaizumi N, Chamberland SR, Alder NN, Boelsterli UA, 2015. Targeting mitochondria with methylene blue protects mice against acetaminophen-induced liver injury. Hepatology 61, 326–336. [DOI] [PubMed] [Google Scholar]
- Lee WM, 2004. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology 40, 6–9. [DOI] [PubMed] [Google Scholar]
- Lim S, Smith KR, Lim ST, Tian R, Lu J, Tan M, 2016. Regulation of mitochondrial functions by protein phosphorylation and dephosphorylation. Cell Biosci 6, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SH, Perera MN, Nguyen T, Datskovskiy D, Miles M, Colombini M, 2011. Bax forms two types of channels, one of which is voltage-gated. Biophys J 101, 2163–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGuidice A, Boelsterli UA, 2011. Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin D-regulated permeability transition. Hepatology 54, 969–978. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Suda C, Horie T, 2005. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol 42, 110–116. [DOI] [PubMed] [Google Scholar]
- Matthews AM, Hinson JA, Roberts DW, Pumford NR, 1997. Comparison of covalent binding of acetaminophen and the regioisomer 3’-hydroxyacetanilide to mouse liver protein. Toxicol Lett 90, 77–82. [DOI] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H., 2013. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol 269, 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H, 2012. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol 264, 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnatsakanyan N, Beutner G, Porter GA, Alavian KN, Jonas EA, 2017. Physiological roles of the mitochondrial permeability transition pore. J Bioenerg Biomembr 49, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnatsakanyan N, Llaguno MC, Yang Y, Yan Y, Weber J, Sigworth FJ, Jonas EA, 2019. A mitochondrial megachannel resides in monomeric F1FO ATP synthase. Nat Commun 10, 5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers TG, Dietz EC, Anderson NL, Khairallah EA, Cohen SD, Nelson SD, 1995. A comparative study of mouse liver proteins arylated by reactive metabolites of acetaminophen and its nonhepatotoxic regioisomer, 3’-hydroxyacetanilide. Chem Res Toxicol 8, 403–413. [DOI] [PubMed] [Google Scholar]
- Nguyen N, Du K, Ramachandran A, Jaeschke H, 2019. Mitochondrial Protein Adduct and Superoxide Generation are Prerequisites for Early Activation of C-Jun N-Terminal Kinase within the Cytosol after an Acetaminophen Overdose in Mice. Hepatoloy 70, 1222A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX, 2012. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology 55, 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, McGill MR, Chao X, Du K, Williams JA, Xie Y, Jaeschke H, Ding WX, 2016. Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J Hepatol 65, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Williams JA, Jaeschke H, Ding WX, 2013. Zonated induction of autophagy and mitochondrial spheroids limits acetaminophen-induced necrosis in the liver. Redox Biol 1, 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar DV, Ahmed G, Khandkar MA, Katyare SS, 1995. Mitochondrial ATPase: a target for paracetamol-induced hepatotoxicity. Eur J Pharmacol 293, 225–229. [DOI] [PubMed] [Google Scholar]
- Pumford NR, Hinson JA, Benson RW, Roberts DW, 1990. Immunoblot analysis of protein containing 3-(cystein-S-yl)acetaminophen adducts in serum and subcellular liver fractions from acetaminophen-treated mice. Toxicol Appl Pharmacol 104, 521–532. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Benet LZ, Burlingame AL, 1998. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem 273, 17940–17953. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Jaeschke H, 2018. Acetaminophen Toxicity: Novel Insights Into Mechanisms and Future Perspectives. Gene Expr 18, 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Jaeschke H, 2019a. Acetaminophen Hepatotoxicity. Semin Liver Dis 39, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Jaeschke H, 2019b. Acetaminophen hepatotoxicity: A mitochondrial perspective. Adv Pharmacol 85, 195–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H, 2011a. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res 45, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H, 2011b. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 251, 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H, 2013. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology 58, 2099–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Duan L, Akakpo JY, Jaeschke H, 2018. Mitochondrial dysfunction as a mechanism of drug-induced hepatotoxicity: current understanding and future perspectives. J Clin Transl Res 4, 75–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay RR, Rashed MS, Nelson SD, 1989. In vitro effects of acetaminophen metabolites and analogs on the respiration of mouse liver mitochondria. Arch Biochem Biophys 273, 449–457. [DOI] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H, 2010. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 246, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H., 2010b. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology 51, 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LE, Dalhoff K, 2005. Alpha-fetoprotein is a predictor of outcome in acetaminophen-induced liver injury. Hepatology 41, 26–31. [DOI] [PubMed] [Google Scholar]
- Sezgin S, Hassan R, Zuhlke S, Kuepfer L, Hengstler JG, Spiteller M, Ghallab A, 2018. Spatio-temporal visualization of the distribution of acetaminophen as well as its metabolites and adducts in mouse livers by MALDI MSI. Arch Toxicol 92, 2963–2977. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Ybanez MD, Win S, Than TA, Jain S, Gaarde WA, Han D, Kaplowitz N, 2010. Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem 285, 8244–8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper BD, Mohar I, Kavanagh TJ, Nelson SD, 2011. Proteomic analysis of acetaminophen-induced changes in mitochondrial protein expression using spectral counting. Chem Res Toxicol 24, 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno M, Kuno A, Ishikawa S, Miki T, Kouzu H, Yano T, Murase H, Tobisawa T, Ogasawara M, Horio Y, Miura T, 2014. Translocation of glycogen synthase kinase-3beta (GSK-3beta), a trigger of permeability transition, is kinase activity-dependent and mediated by interaction with voltage-dependent anion channel 2 (VDAC2). J Biol Chem 289, 29285–29296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD, 1989. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3’-hydroxyacetanilide, in mouse liver. J Biol Chem 264, 9814–9819. [PubMed] [Google Scholar]
- Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, Yoshioka K, Masuyama N, Gotoh Y, 2004. JNK promotes Bax translocation to mitochondria through phosphorylation of 14–3-3 proteins. EMBO J 23, 1889–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay M, Agarwal S, 2019. Ironing the mitochondria: Relevance to its dynamics. Mitochondrion 50, 82–87. [DOI] [PubMed] [Google Scholar]
- Wancket LM, Meng X, Rogers LK, Liu Y, 2012. Mitogen-activated protein kinase phosphatase (Mkp)-1 protects mice against acetaminophen-induced hepatic injury. Toxicol Pathol 40, 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ni HM, Chao X, Ma X, Rodriguez YA, Chavan H, Wang S, Krishnamurthy P, Dobrowsky R, Xu DX, Jaeschke H, Ding WX, 2019. Double deletion of PINK1 and Parkin impairs hepatic mitophagy and exacerbates acetaminophen-induced liver injury in mice. Redox Biol 22, 101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wang P, Liu B, Zhao J, Pang Q, Agrawal SG, Jia L, Liu FT, 2015. Dynamin-related protein Drp1 is required for Bax translocation to mitochondria in response to irradiation-induced apoptosis. Oncotarget 6, 22598–22612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal D, Dewson G, Czabotar PE, Kluck RM, 2011. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta 1813, 521–531. [DOI] [PubMed] [Google Scholar]
- Win S, Than TA, Han D, Petrovic LM, Kaplowitz N, 2011. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem 286, 35071–35078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S, Than TA, Min RW, Aghajan M, Kaplowitz N, 2016. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology 63, 1987–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Ramachandran A, McGill MR, Yan HM, Bajt ML, Sharpe MR, Lemasters JJ, Jaeschke H, 2012. Lysosomal instability and cathepsin B release during acetaminophen hepatotoxicity. Basic Clin Pharmacol Toxicol 111, 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Zhou F, Zhang Z, Xing D, 2011. Bax is essential for Drp1-mediated mitochondrial fission but not for mitochondrial outer membrane permeabilization caused by photodynamic therapy. J Cell Physiol 226, 530–541. [DOI] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H., 2014. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol Appl Pharmacol 279, 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Du K, Dorko K, Kumer SC, Schmitt TM, Ding WX, Jaeschke H, 2015. Mitochondrial protein adducts formation and mitochondrial dysfunction during N-acetyl-m-aminophenol (AMAP)-induced hepatotoxicity in primary human hepatocytes. Toxicol Appl Pharmacol 289, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HM, Ramachandran A, Bajt ML, Lemasters JJ, Jaeschke H, 2010. The oxygen tension modulates acetaminophen-induced mitochondrial oxidant stress and cell injury in cultured hepatocytes. Toxicol Sci 117, 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]