Abstract
In major depressive disorder (MDD) and remitted MDD (rMDD) alterations in cortisol and inflammation are associated with cognitive difficulties, but these relationships have not been investigated in HIV. We used secondary data from a placebo-controlled, cross-over study of cognitive performance following a probe of the hypothalamic-pituitary-adrenal (HPA) axis (low dose hydrocortisone; LDH 10mg) in 65 people with HIV (PWH; 36 women). Using placebo data, we examined sex-specific associations between two biomarkers – basal afternoon salivary cortisol and salivary inflammatory cytokines - cognition, and rMDD. Salivary cortisol and inflammatory biomarkers were sampled across the 5-hour study. The panel of inflammatory markers included interleukin (IL)-6, IL-8, IL-1β, tumor necrosis factor-(TNF)-α, CRP, interferon gamma-induced protein (IP-10), monocyte chemotactic protein (MCP)-1, monokine induced by interferon (MIG), matrix metalloproteinase MMP-9, and MMP-1. Learning, memory, attention/concentration, and executive function were assessed 30 minutes and 4 hours after the placebo intervention; visuospatial ability was also assessed 30 minutes after the placebo intervention. For women but not men with HIV, basal cortisol concentrations were higher in rMDD versus noMDD groups, and related to poorer learning and memory. For men and women with HIV, basal inflammatory cytokines were higher in rMDD versus noMDD groups, but were negatively related to cognition independent of rMDD status. Cortisol and cytokines relate to cognition in PWH, but the associations depended on sex, rMDD status, and their interaction.
Keywords: HIV, Cognition, Cortisol, Inflammation, Depression
1. Introduction
Depression rates are higher in people with HIV (PWH) versus the general U.S. population (Do et al., 2014). The prevalence of a lifetime diagnosis of Major Depressive Disorder (MDD) was 32.4% in a large-sample of women with HIV (WWH) versus 22.9% in the general population of women (Cook et al., 2018). In some (Turner et al., 2003), but not all reports (Rubin et al., 2019), WWH have higher depression rates and more severe depressive symptoms versus men with HIV (MWH)(Aljassem et al., 2016; Semple et al., 1996). Depressive symptoms are associated with cognitive dysfunction in HIV-uninfected individuals and in PWH (Maki et al., 2015; Rubin et al., 2017a; Rubin et al., 2019). A meta-analysis revealed moderate executive function, attention, and memory deficits in active MDD and small-to-moderate executive function and attention deficits in remitted MDD (rMDD), suggesting that certain deficits occur independently of episodes of low mood in MDD (Rock et al., 2014; Snyder, 2013). This line of work has not been extended to HIV, nor have the mechanisms underlying the rMDD-cognition association been studied in HIV.
The hypothalamic-pituitary-adrenal (HPA) axis mediates the longer-acting portion of the stress response and its actions are largely mediated by the release of glucocorticoids (GCs), primarily cortisol in humans. The relationship between the HPA axis and MDD has been reported and alterations in the HPA axis during MDD may be an under-recognized determinant of cognition in PWH, and a novel target for therapeutic interventions. Given the strong inflammatory presence in the CNS of PWH (Dahl et al., 2014; Spudich, 2016), such an association could also provide important insight for other somatic diseases and disorders that include a chronic inflammatory component (e.g., cardiovascular disease, diabetes, obesity). The HPA axis regulates itself through an inhibitory feedback loop which receives regulatory input from the prefrontal and limbic (e.g., hippocampal) regions. The negative effects of GCs on cognition are mediated by the binding of cortisol to GC receptors in the prefrontal cortex (PFC) and hippocampal formation (HI)(McEwen et al., 2015). HPA axis alterations including excessive release of cortisol and changes in GC receptor availability and function, can influence cognitive abilities subserved by these brain regions (Valdez et al., 2016). To this end, WWH exhibit GC function alterations, similar to women with MDD (Bekhbat et al., 2018). Individuals with active MDD and rMDD also show elevated cortisol concentrations including modest cortisol awakening response elevations (Knorr et al., 2010) and persistent cortisol elevations (Vreeburg et al., 2009) longitudinally and impairment on cognitive abilities mediated by the PFC and HI (Rock et al., 2014). Studies demonstrate sex differences in basal cortisol concentrations in HIV-uninfected individuals with MDD although the patterns are not always consistent (Hinkelmann et al., 2012; Matsuzaka et al., 2013; Young and Korszun, 2010). These inconsistencies could be the product of age, sex steroid hormones concentrations, or interactions among these and other factors.
HPA axis alterations can also influence cognition through immunomodulatory effects, including regulation of cytokine production. In homeostasis, the HPA axis activates innate immunity and promotes development of protective immune responses such as the production of anti-inflammatory cytokines and the inhibition of proinflammatory cytokines (e.g., TNF-α, IL-1, IL-6)(Silverman and Sternberg, 2012). Conversely, HPA axis perturbations can impair cognition through cytokine-driven neuroinflammation. Communication between the HPA axis and immune system is bidirectional. GCs promote proinflammatory cytokines and proinflammatory cytokines stimulate GC release. For example, high cortisol concentrations have anti-inflammatory actions and proinflammatory cytokines (e.g., IL-1α) can reduce GC receptor translocation and function (Pariante et al., 1999). Like PWH, individuals with MDD show increased proinflammatory cytokine concentrations (IL-1, IL-6, TNF-α), increased acute-phase proteins, and increased chemokines and adhesion molecule expression (Miller et al., 2009; Norcini Pala et al., 2016). A causal role for inflammation in MDD is demonstrated in studies where treatment with interferon-alpha induces depressive symptoms, an effect associated with elevated adrenocorticotropic hormone and cortisol concentrations (Capuron et al., 2003). Albeit counterintuitive, increased inflammation and cortisol hypersecretion (marker of GC receptor resistance) are seen in the same depressed individuals (Pariante, 2017). Thus, comorbid HIV and rMDD may exacerbate cognitive difficulties in PWH through HPA axis and immune function alterations, particularly through heightened proinflammatory cytokine concentrations.
HPA axis investigations in PWH have been conducted on predominantly or exclusively male samples. HPA axis alterations include elevated basal cortisol concentrations (over 2-hours and 2-years intervals), attenuated cortisol responsivity to behavioral and corticotropin-releasing hormone challenges, and alterations in the diurnal rhythm of cortisol secretion (Chittiprol et al., 2009; Zapanti et al., 2008). Given marked sex differences in HPA axis activity including women exhibiting smaller and more variable cortisol responses to psychosocial stressors, (Kajantie and Phillips, 2006; Kudielka and Kirschbaum, 2005), it is important to examine the role of the HPA axis in HIV+ men and women separately.
Alterations in cortisol and inflammation correlate to cognitive difficulties in MDD and may contribute to the heterogeneous patterns of HIV-associated cognitive impairment where there is a high prevalence of lifetime MDD. We examined associations between cortisol, inflammation, cognition, and rMDD by using secondary data from a pharmacological challenge study using low-dose hydrocortisone (LDH 10mg) in PWH (Rubin et al., 2018b; Rubin et al., 2017b). Briefly, WWH (50% with rMDD) and MWH (33% with rMDD) participated in a cross-over study and were administered either a single dose of LDH or placebo and then completed cognitive tests 30 minutes and again 4 hours later. Cortisol was sampled throughout the 5-hour study (12pm-5pm). Approximately one month later, they were crossed over to the other intervention. Primary analyses focused on the cognitive effects of LDH among men and women separate. Briefly, LDH improved learning and memory 4 hours following treatment which was larger than any benefit due to practice in women but not men. Using the same data, we now examine sex-specific associative relationships between cortisol, inflammation, cognition, and rMDD by drawing on data from the placebo day. Given prior data showing that LDH has stronger effects on cognition in WWH versus MWH, we hypothesized that the extent to which cortisol and inflammation would be associated with cognition would be stronger in WWH with rMDD.
2. Methods
2.1. Participants
Eighty-one PWH (36 women) participated in a randomized, double-blind, placebo-controlled, cross-over pharmacologic challenge study focusing on cognitive changes following an HPA axis probe (low-dose hydrocortisone; LDH 10mg) versus placebo (Rubin et al., 2018b; Rubin et al., 2017b). Participants were aged 18-45 with English as their first language and used the same antiretrovirals for ≥ three months. The majority of participants were free from current antidepressant medication use (97%). Participants did not have a: lifetime history of any psychotic disorder, neurological condition impacting cognition (e.g., loss of consciousness >1 hour), body mass index > 40, history of substance use disorder in the past 6 months excluding alcohol/nicotine, an inability to abstain from illicit substances 24 hours prior to testing via urine toxicology screen.
2.2. Study Procedures
Qualifying participants via phone screen were evaluated at an initial visit (Session 1) where participants provided informed consent and completed a Diagnostic and Statistical Manual of Mental Disorders (SCID) IV interview (used to determine rMDD status), toxicology screen, vitals assessment, and questionnaires including the Center for Epidemiologic Studies Depression Scale (CES-D), PTSD Checklist-Civilian Version (PCL-C), Perceived Stress Scale-10 (PSS-10), Pittsburgh Sleep Quality Index (PSQI), and Childhood Trauma Questionnaire (CTQ). In a cross-over design, qualifying participants (based on Session 1 data) participated in the pharmacologic challenge study (Sessions 2 and 3; one month apart; women tested in the early follicular phase of the menstrual cycle only) whereby they completed cognitive tests at two time points; one 30 minutes after and again 4 hours later. Cortisol was sampled throughout the study (12pm-6pm) to measure basal afternoon cortisol and to account for diurnal cortisol variations (Petrovsky and Harrison, 1998; Stalder et al., 2016).
2.3. Cognitive Measures
Learning and memory: Hopkins Verbal learning Test (HVLT-R; learning = Trial 1 learning and total learning across Trials 1–3; memory=delayed recall). Attention and concentration: Letter-Number Sequencing (LNS) attention condition from the Wechsler Adult Intelligence Scale IV (total correct), Trail Making Test (TMT) Part A (time to completion), and computerized Stroop congruent trials (accuracy). Executive function: LNS working memory condition (total correct), TMT Part B (time to completion)(Reitan, 1978), and Stroop incongruent trials (accuracy). Visuospatial ability: Line Orientation Task (LOT) from the Repeatable Battery for the Assessment of Neuropsychological Status (total correct). At Sessions 2 and 3, all tests were administered at the 30 minute and 4 hour time points except Stroop and Line Orientation (30 minute time point only).
2.4. Saliva Collection, Cortisol, and Cytokines
During Sessions 2 and 3, salivary samples were obtained at 10 time points (35 and 20 minutes before pill administration and 30, 60, 90, 180, 210, 240, 270, and 300 minutes after pill administration). See (Rubin et al., 2018b; Rubin et al., 2017b) for details regarding saliva collection procedures. Samples were assayed for cortisol with an ELISA kit by Salimetrics. Salivary cytokines were assessed at three time points (20 minutes before pill administration and 30 and 240 minutes after pill administration). Nine cytokines were assessed in the original panel of markers including IL-6, IL-8, IL-1β, tumor necrosis factor-(TNF)-α, CRP, interferon gamma-induced protein (IP-10), monocyte chemotactic protein (MCP)-1, monokine induced by interferon (MIG), matrix metalloproteinase MMP-9, and MMP-1. Saliva was assayed following manufacturer’s procedure using a MILLIPLEX MAP human high sensitivity T cell panel immunology multiplex assay (Millipore, Billerica, MA) to detect IL-6, IL-8, IL-1β, and TNF-α (standard curves 0.18–7500pg/mL; average interplate CV 9.9%); MILLIPLEX MAP standard sensitivity cytokine to detect IP-10 and MCP-1 (standard curve 3.2–10,000 pg/mL; average interplate CV 9%); and R&D systems custom 5-plex to detect CRP, MIG, and MMP-9 (144–96,000 pg/mL; average interplate CV 12.7%). All samples (standards, experimental) were tested in duplicate. Milliplex results were acquired on a Labscan 200 analyzer (Luminex, Austin, Tx) using Bio-Plex manager software 6.1 (Bio-Rad, Hercules, CA). To calculate the concentration from the fluorescence intensity of the bead measurements, a 5-point logistic regression curve was used. Samples below the level of detection were assigned one-half the lowest detectable value for that analyte. All cytokine values were log transformed.
2.5. Statistical analysis
To determine whether rMDD and noMDD differed in basal afternoon cortisol concentrations (placebo day) in PWH, we conducted mixed-effects regression models (MRM) where rMDD status, time (treated categorically), and the two-way interaction were the predictors. To examine associations between cortisol, inflammatory markers, and cognition, we conducted Pearson correlations. For inflammatory markers, we computed the median cytokine value across the three placebo day values (Rubin et al., 2018b). Analyses were stratified by sex given our previous findings with this data (Rubin et al., 2018b; Rubin et al., 2017b). Analyses were conducted in SAS (v.9.4 for Windows; SAS); significance was set at P<0.05. Effect sizes for trends are also provided given the sample size. Cohen’s d effect sizes (small effect = 0.2; medium effect = 0.5; large effect = 0.8) were calculated using pooled standard deviations and estimated means adjusted for age.
3. Results
3.1. Sex-specific associations of rMDD status with basal afternoon cortisol and inflammatory biomarkers in HIV.
Among WWH, cortisol concentrations were higher in rMDD versus noMDD (P=0.03; Fig 1A, left panel), and this group difference did not vary over time (P=0.21). Based on these findings, we computed cortisol area under the curve (Pruessner et al., 2003) and not surprising cortisol AUC was higher among rMDD (M=56.8, SD=37.7) versus noMDD (median=34.9 IQR[interquartile range]=27.7)(P=0.02). Several inflammatory markers including IL-8, CRP, IL-6, and TNF-α, were also higher in the comorbid group of women (P’s<0.05; Fig 1A, right panel).
Figure 1.
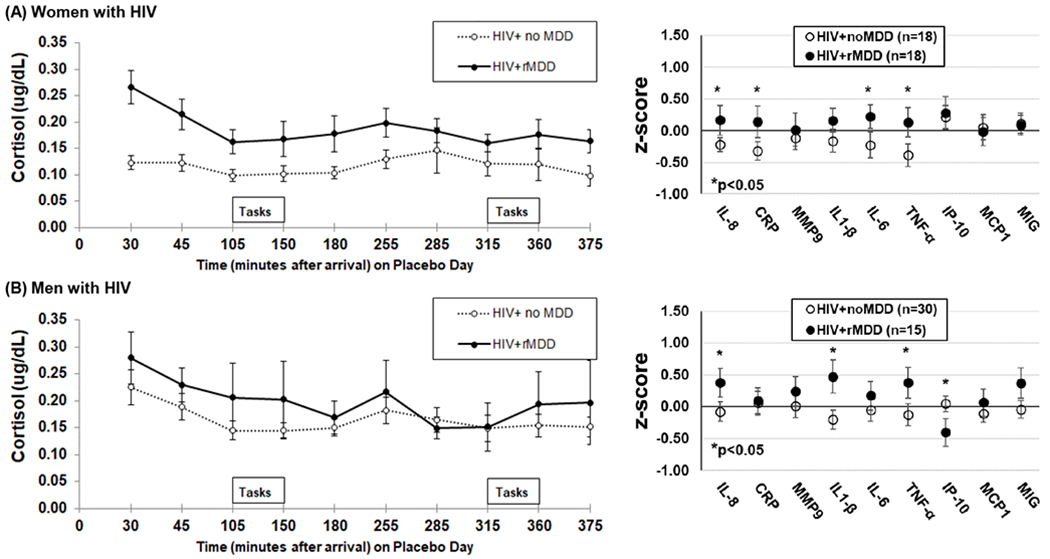
(A) Women with HIV and rMDD (n=18) have higher concentrations of endogenous salivary cortisol across the afternoon (p=0.03) and show elevated salivary inflammatory markers (median of 3 values between 12pm and 5pm) versus women with HIV and no MDD (n=18); (B) men with HIV and rMDD (n=15) and men with HIV and no MDD (n=30) have a similar profile of endogenous salivary cortisol across the afternoon (p=0.58). *P<0.05
Among MWH, cortisol concentrations did not differ by rMDD status (P=0.58; Fig 1B) and did not change across time as a function of rMDD status (P=0.82). Cortisol AUC was the same for rMDD (Median=50.6, IQR=26.9) and noMDD (Median=49.4, IQR=26.4) (P=0.85). For inflammatory markers, IL-8, TNF-α, IL-1 β and IP-10 were higher in the rMDD versus noMDD (P’s<0.05).
Given that prior and current trauma are also associated with reduced HPA axis output despite elevated concentrations of inflammation, we reran these analyses first controlling for childhood trauma using the CTQ total score and then PTSD symptom burden using the PCLC total score. The pattern of rMDD status findings among WWH and MWH did not change after controlling for either of these factors. Additionally, the pattern of findings persisted after controlling for viral load and CD4 count.
3.2. Sex-specific associative relationships of cognition with basal afternoon cortisol and inflammatory markers in HIV: Effect of rMDD
Cortisol concentrations did not change over time, so we examined cortisol AUC in relation to cognition at the 30-minute and 4-hour test session. Among WWH, higher cortisol concentrations were associated with lower performance at the 30-minute and 4-hour test sessions on HVLT total learning, with somewhat higher associations at the 4-hour session (r=−0.36, P=0.03; r=−0.46, P=0.005, respectively) and delayed recall (r=−0.37, P=0.03; r=−0.55, P<0.001). These associations were being driven by rMDD (Fig 2) although a formal test of differences between correlation coefficients between rMDD and noMDD using Fisher’s r-to-z transformation missed statistical significance for HVLT total learning (Z=−1.92, P=0.05) and delayed recall (Z=−1.77, P=0.07) at the 30-minute test session and were not significant at the 4-hour session (P’s>0.33). Higher concentrations of several inflammatory markers were also associated with lower performance, especially learning and memory (Fig 3A). For example, higher median CRP, IL-6, and IP-10 concentrations were associated with lower HVLT delay recall (P’s<0.05). In contrast to the cortisol findings, associations between inflammatory markers and cognition were not overwhelmingly driven by MDD status. The exceptions were that higher IP-10 and MCP-1 associating with lower HVLT total learning (r=−0.52, P=0.02; r=−0.57, P=0.01, respectively) and delayed recall (r=−0.59, P=0.009; r=−0.50, P=0.04, respectively) to a greater degree among those with rMDD than those without a history of MDD (P’s>0.09).
Figure 2.
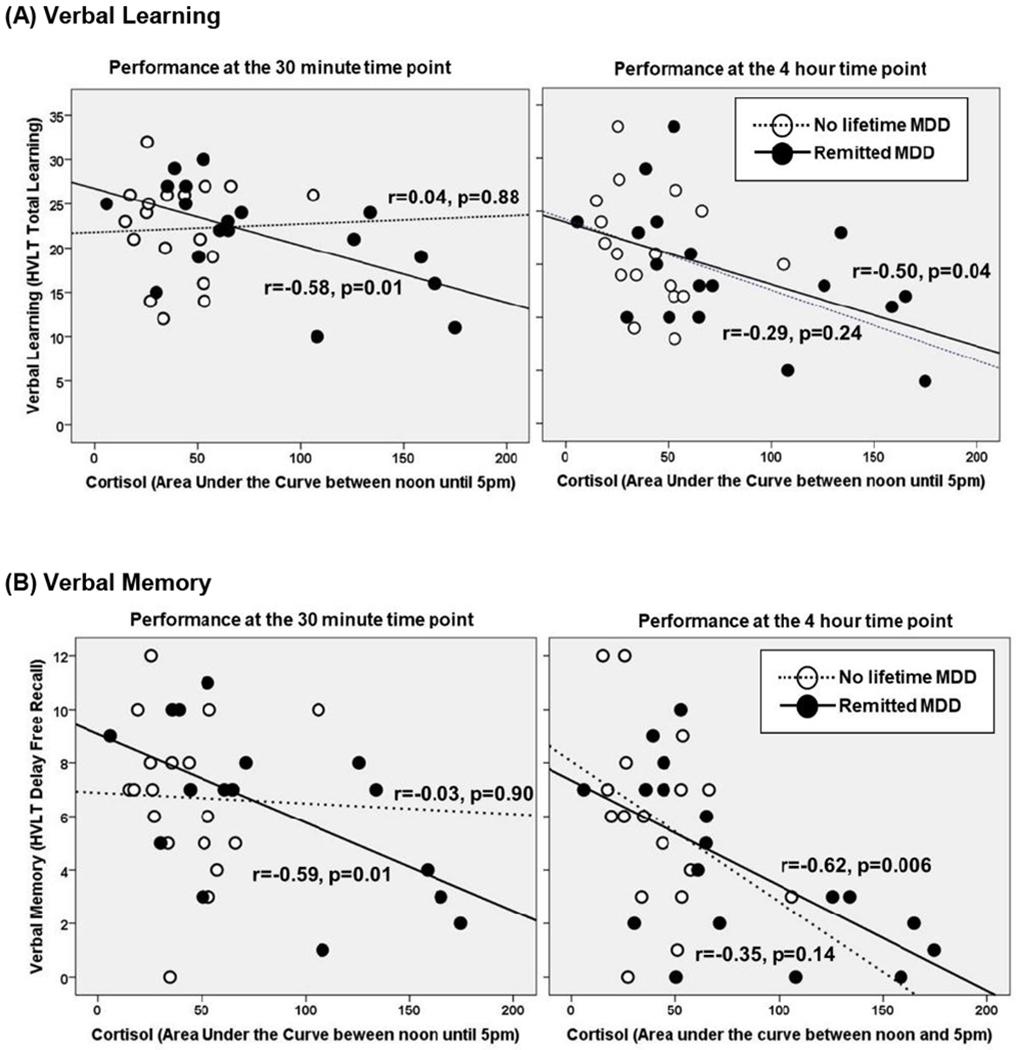
There is a strong association between basal salivary cortisol concentrations and (A) verbal learning and (B) memory among women with HIV and rMDD.
Figure 3.
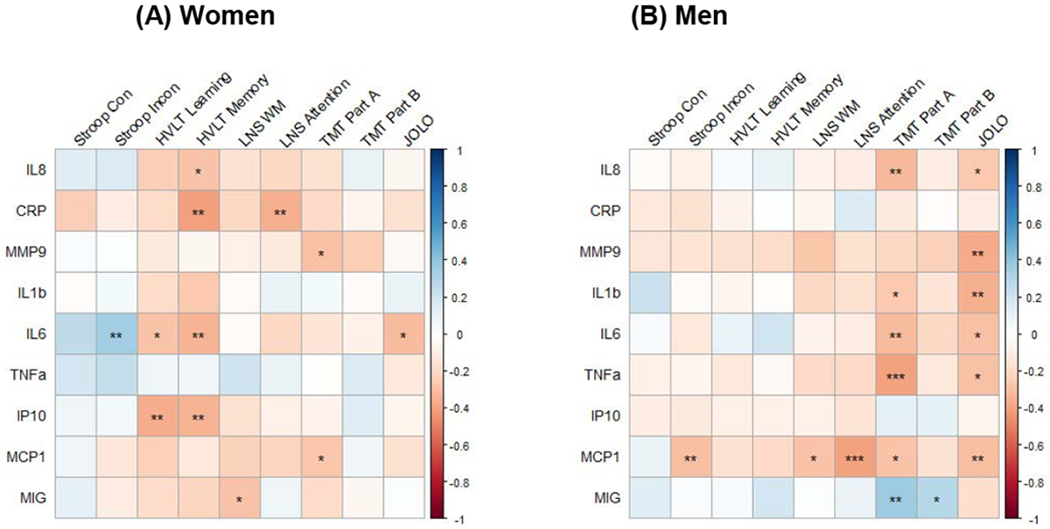
Associations between salivary inflammatory markers and cognition in (A) women with HIV and (B) men with HIV
Note. *P<0.10; ** P<0.05; *** P<0.01; Con=Congruent; Incon=incongruent; HVLT=Hopkins Verbal Learning Test; LNS=Letter Number Sequencing; TMT=Trail Making Test; JOLO=judgement of lines orientation
Among MWH, inflammatory markers but not cortisol were associated with cognition, particularly TMT Part A and LOT (both associated with 6 of 9 markers). Among the inflammatory markers, MCP1 was associated with 5 of 9 cognitive outcomes. Most associations were inverse ones (Fig 3B). For example, higher concentrations of IL-8, IL-6, IL-1β, TNF-α, and MCP-1 and lower MIG concentrations were associated with lower TMT Part B performance. Additionally, IL-8, MMP-9, IL-1β, and MCP-1 were associated with lower LOT performance (P’s<0.05). There were no associations on the HVLT, and inflammatory marker-cognition associations were not driven by MDD status.
Although there were no significant differences between rMDD and noMDD among WWH or MWH, small-to-medium effect sizes were found in favor of those without MDD on LNS working memory (Cohen’s d=−0.58), incongruent Stroop trials (d=−0.25), and LOT (d=−0.24) among WWH; and LOT (d=−0.56), TMT Part A and B (d=−0.41, d=−0.35), HVLT total learning (d=−0.35), Stroop incongruent trials (d=−0.30) and delay recall (d=−0.26) among MWH. When combining men and women those without MDD performed better than rMDD on LOT (P=0.02; d=−0.52). Small effects were found in favor of those without MDD on LNS working memory (Cohen’s d=−0.24) and TMT Part A and B (d=−0.22, d=−0.34).
4. Discussion
We examined whether cognition in HIV varies in relation to cortisol and inflammatory markers, and whether history of depression influences those relationships. Our findings elucidate the potential endocrine and immune mechanisms linking history of depression with cognitive function, and show that these mechanisms differ by depression history and biological sex in a population living with a chronic inflammatory condition.
Our analyses inform our understanding of whether associations of basal afternoon cortisol, inflammatory markers, and cognition differed by rMDD status in PWH. Consistent with other studies in HIV-uninfected women with rMDD (Halbreich et al., 1984; Matsuzaka et al., 2013; Pariante and Miller, 2001; Young and Korszun, 2010), basal cortisol concentrations were reliably elevated across 10 time points in rMDD versus noMDD WWH. The higher basal cortisol concentrations in rMDD were clinically significant, as they were associated with lower cognitive performance, particularly learning and memory. These relationships were reliable over time at the 30-minute and 4-hour test sessions. In contrast to these significant findings in women, we found no basal afternoon cortisol elevations in rMDD which is consistent with some (Hinkelmann et al., 2012) but not all studies (Halbreich et al., 1984; Matsuzaka et al., 2013; Young and Korszun, 2010); and no associations of rMDD with cortisol concentrations or associations of cortisol concentrations and cognition. These findings suggest that some of the heterogeneity in cognition in HIV is associated with alterations in HPA axis function that occur with mental health disorders such as rMDD, particularly in women. This is consistent with a previous study in WWH that demonstrated similar effects of HIV and depression on GC biology (Bekhbat et al., 2018).
Basal inflammatory marker concentrations differed by rMDD status for both sexes with elevated concentrations of select markers in rMDD. For both MWH and WWH, IL-8 and TNF-α concentrations differed by MDD status with higher concentrations among rMDD. However, IL-1β and IP-10 differed by MDD status only in women, and CRP and IL-6 differed by MDD status only in men. For each marker, rMDD demonstrated higher concentrations than no MDD. A causal relationship between inflammatory cytokines and depression is shown in studies where administration of proinflammatory cytokines, like interferon (IFN)-γ, IFN-α, and TNF-α, induced MDD-like behavior in patients (Chiu et al., 2017; Schlaak et al., 2012) and sickness-like behavior in animals (Ma et al., 2016; Stepanichev et al., 2014). Furthermore, many of the proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α are increased in the serum of patients with MDD (Liu et al., 2012; Rizavi et al., 2016). Meta-analyses also show there is an association between increased proinflammatory cytokines and MDD (Dowlati et al., 2010; Hiles et al., 2012). Those specific markers were not differentially correlated with cognition in rMDD. Instead, for most inflammatory markers, higher concentrations (i.e., IL-6, IL-9, CRP, and IP10) were associated with poorer cognition in WWH generally - independent of rMDD status except in a few cases - and were strongest and most reliably observed across different markers for memory. Overall, these findings are consistent with previous studies showing that peripheral systemic inflammatory markers including IL-6, IL-1β, IP-10, and CRP are associated with cognition among PWH (Cohen et al., 2011; Correia et al., 2013; Rubin et al., 2018a). For example, IL-6 and CRP are associated with global cognitive impairment and CRP is also related to executive function, attention/working memory, and psychomotor speed performance (Rubin et al., 2018a). IL-1β and IP-10 are also associated with psychomotor speed (Cohen et al., 2011) and IP-10 with learning (Correia et al., 2013). Inflammatory cytokines have many different properties beyond their role in inflammatory response to infection, including effects on synaptic transmission, neuronal excitability, and astrocyte function (and blood brain barrier permeability)(Vezzani and Viviani, 2015). Additionally, inflammatory cytokines have downstream effects on neural networks that can result in disrupted or altered cognition (Köhler et al., 2017; Marsland et al., 2017). Our findings add to that literature by showing that the association between inflammatory biomarkers and cognition is independent of rMDD status for both MWH and WWH, even though many cytokines are elevated in PWH with a history of depression. We do not know if these findings generalize to active MDD or older PWH with rMDD.
Study strengths include 10 time points of saliva for measuring cortisol, and three time points to assess inflammatory markers. Limitations include the small number of rMDD and noMDD and the number of exploratory correlations without adjustment for the family-wise error rate as these analyses were done for heuristic purposes. Small sample sizes also limited our ability to conduct sensitivity analyses in PWH who were virally suppressed which is important for future work as HIV replication is a major driver of neuroendocrine dysregulation and inflammation. Additionally, we opted to collect salivary inflammatory markers rather than plasma or basal markers of neuroinflamation using cerebral spinal fluid (CSF). Our goal was the collection of assessments of both cortisol and inflammation by noninvasive means. Future studies are warranted to determine whether the same patterns of results would emerge with plasma or CSF markers of inflammation as the vast majority of studies use peripheral measurements of plasma or serum cytokines in HIV and MDD. While saliva has good utility detecting inflammatory change to acute stressors (Slavish et al., 2015), the utility of saliva as a reliable index of basal systemic inflammation is debated due to some of the independent sources of flux in salivary cytokines such as oral hygiene (Engeland, 2019). However, this may be of rather limited concern as thirty-five individuals in our sample had an oral exam at the time of study entry as part of a substudy. The majority (71%) did not have any oral lesions and there were no differences between men and women in our study with respect to the number of lesions. We also included current smokers and cannabis users which are substances that can alter HPA axis responsivity/sensitivity (van Leeuwen et al., 2011). Controlling for these factors does not change the pattern of results and these factors did not differ by rMDD status. Lastly, larger-scale studies are needed to verify the correlational patterns, to examine the possibility of a causal relationship between cortisol and cognition in those women, and to test the moderating effects of sex as a biological variable while controlling for necessary differences between the sexes. In sum, our findings provide preliminary evidence for the role of HPA axis and inflammation in comorbid HIV and rMDD which occurs in approximately 34% of WWH (Cook et al., 2018). More generally, results suggest that interventions to improve cognition in PWH should take into account mental health history and biological sex.
Table 1.
Demographic and clinical characteristics for remitted depression (rMDD) and no history of depression at enrollment visit, Session 1 by biological sex.
| WWH (n=36) |
MWH (n=45) |
|||||
|---|---|---|---|---|---|---|
| No rMDD (n=18) | rMDD (n=18) | p-value | No rMDD (n=30) | rMDD (n=15) | p-value | |
| Socio-demographic factors | ||||||
| Age | 35.7 (8.2) | 37.5 (6.6) | 0.48 | 30.9 (8.8) | 35.3 (8.1) | 0.11 |
| Education | 0.44 | 0.43 | ||||
| <High school | 7 (39) | 7 (39) | 5 (17) | 5 (33) | ||
| High school graduate | 5 (28) | 8 (44) | 9 (30) | 3 (20) | ||
| >High school | 6 (33) | 3 (17) | 16 (53) | 7 (47) | ||
| Black, not Hispanic | 17 (94) | 16 (89) | 0.40 | 29 (97) | 13 (87) | 0.21 |
| Unemployed | 8 (44) | 12 (67) | 0.19 | 23 (77) | 9 (60) | 0.25 |
| Risky health behaviors | ||||||
| Currently smoking | 6 (22) | 10 (55) | 0.19 | 21 (70) | 11 (73) | 0.82 |
| Number of Alcohol drinks/week | 0.47 (0.68) | 0.47 (0.79) | 1.00 | 1.3 (1.4) | 1.4 (2.3) | 0.85 |
| Current marijuana use | 5 (28) | 3 (17) | 0.43 | 18 (60) | 9 (60) | 1.00 |
| Current cocaine use | 0 (0) | 2 (11) | 0.15 | 0 (0) | 1 (7) | 0.16 |
| Ever dependent/abuse alcohol | 0 (0) | 2 (11) | 0.15 | 3 (10) | 1 (7) | 0.71 |
| Ever dependent/abuse substances | 4 (22) | 9 (50) | 0.09 | 7 (23) | 4 (27) | 0.81 |
| Psychological profile | ||||||
| CES-D | 13.8 (9.1) | 17.9 (8.8) | 0.17 | 12.4 (7.4) | 10.3 (6.5) | 0.35 |
| PCL-C | 32.8 (14.5) | 38.2 (14.5) | 0.28 | 26.7 (8.0) | 24.3 (7.4) | 0.35 |
| PSS-10 | 21.7 (5.9) | 22.8 (5.8) | 0.55 | 19.9 (5.5) | 18.4 (5.2) | 0.31 |
| CTQ | ||||||
| Emotional abuse | 10.2 (6.1) | 12.9 (5.7) | 0.17 | 9.2 (4.7) | 10.1 (5.6) | 0.55 |
| Physical abuse | 8.9 (4.9) | 12.4 (7.1) | 0.10 | 8.7 (4.1) | 10.2 (6.2) | 0.33 |
| Sexual abuse | 6.4 (3.9) | 11.9 (7.9) | 0.01 | 8.5 (5.1) | 9.8 (7.6) | 0.50 |
| Emotional neglect | 11.2 (4.5) | 13.9 (4.4) | 0.08 | 11.1 (4.9) | 11.7 (5.7) | 0.74 |
| Physical neglect | 8.4 (3.9) | 10.8 (4.6) | 0.10 | 8.7 (3.5) | 8.5 (4.5) | 0.83 |
| Current antidepressant use | 2 (1.5) | 1 (1.5) | 0.55 | 0 (0) | 0 (0) | - |
| PSQI total score | 7.2 (3.2) | 8.5 (3.6) | 0.22 | 5.9 (2.6) | 6.7 (2.9) | 0.38 |
| Clinical characteristics | ||||||
| Body mass index | 27.5 (5.2) | 29.5 (5.3) | 0.27 | 23.5 (4.6) | 22.9 (4.0) | 0.68 |
| Years living with HIV | 12.4 (6.6) | 12.0 (5.6) | 0.86 | 7.7 (5.9) | 9.0 (7.2) | 0.51 |
| Efavirenz use | 8 (38) | 5 (20) | 0.17 | 14 (40) | 6 (38) | 0.87 |
| Dolutegravir use | 2 (9) | 3 (12) | 0.79 | 1 (3) | 1 (6) | 0.56 |
| Raltegravir use | 4 (19) | 1 (4) | 0.10 | 2 (6) | 3 (19) | 0.15 |
| Elvitegravir use | 1 (5) | 0 (0) | 0.27 | 20 (7) | 0 (0) | 0.05 |
| Medication (MASRI) missing ≥1 dose in the last month± | 9 (50) | 7 (39) | 0.51 | 15 (50) | 5 (33) | 0.29 |
| CD4 Count (cells/μl), median(IQR) | 486 (286) | 487 (520) | 0.93 | 493 (364) | 567 (314) | 0.79 |
| Viral Load (HIV RNA (cp/ml)) | 0.77 | 0.67 | ||||
| Undetectable | 7 (39) | 8 (44) | 11 (39) | 7 (50) | ||
| Lowest detectable limit (20) | 4 (22) | 5 (27) | 8 (29) | 2 (14) | ||
| < 10,000 | 4 (22) | 4 (22) | 7 (25) | 3 (21) | ||
| ≥10,000 | 3 (17) | 1 (6) | 2 (7) | 2 (14) | ||
Note. WAmong HIV+ women, No rMDD differed from rMDD at p<0.05; IQR=lnterquartile Range. Current use=use in the last month. CES-D= Center for Epidemiologic Studies Depression Scale; PCL-C=PTSD Checklist-Civilian Version; PSS-10=Perceived Stress Scale-10; PSQI=Pittsburgh Sleep Quality Index; CTQ=Childhood Trauma Questionnaire; MASRI= Medication Adherence±visual analogue scale for proportion of doses taken in the last month; WWH=women with HIV; MWH=men with HIV
HIGHLIGHTS.
Cognition in HIV varies in relation to basal and exogenous cortisol.
History of depression and sex influences those relationships.
Both hormonal and inflammatory mechanisms are implicated.
Acknowledgments
Funding
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Numbers K01MH098798 (Rubin), R21MH099978 (Rubin), R01MH113512 (Rubin), U54 AG062334, and R01MH110364. The project described was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000050. This project was also supported in part by a University of Illinois at Chicago Campus Review Board (CRB) Grant (Rubin) and a Chicago Developmental Center for AIDS Research pilot grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank Bruni Hirsch, Alana Aziz-Bradley, Jacob Ellis, Sheila D’Sa, Shannon Dowty, Lauren Drogos, Lacey Wisslead, Aleksa Anderson, and Preet Dhillon for their assistance with this study and Raha Dastgheyb for help with creating Figure 4. We would also like to thank Kathleen Weber and the CORE Center at John H. Stroger Jr Hospital of Cook County for help in recruiting participants to the present study. We would also like to thank all of our participants, for without you this work would not be possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethnical standards of the institution and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest/ Financial disclosures
All coauthors have nothing to disclose.
References
- Aljassem K, Raboud JM, Hart TA, Benoit A, Su D, Margolese SL, Rourke SB, Rueda S, Burchell A, Cairney J, Shuper P, Loutfy MR, Team OCSR, 2016. Gender Differences in Severity and Correlates of Depression Symptoms in People Living with HIV in Ontario, Canada. Journal of the International Association of Providers of AIDS Care 15, 23–35. [DOI] [PubMed] [Google Scholar]
- Bekhbat M, Mehta CC, Kelly SD, Vester A, Ofotokun I, Felger J, Wingood G, Anastos K, Gustafson DR, Kassaye S, Milam J, Aouizerat B, Weber K, Golub ET, Moore MF, Diclemente R, Fischl M, Kempf MC, Maki P, Neigh GN, 2018. HIV and symptoms of depression are independently associated with impaired glucocorticoid signaling. Psychoneuroendocrinology 96, 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH, 2003. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry 160, 1342–1345. [DOI] [PubMed] [Google Scholar]
- Chittiprol S, Kumar AM, Shetty KT, Kumar HR, Satishchandra P, Rao RS, Ravi V, Desai A, Subbakrishna DK, Philip M, Satish KS, Kumar M, 2009. HIV-1 clade C infection and progressive disruption in the relationship between cortisol, DHEAS and CD4 cell numbers: a two-year follow-up study. Clin Chim Acta 409, 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WC, Su YP, Su KP, Chen PC, 2017. Recurrence of depressive disorders after interferon-induced depression. Transl Psychiatry 7, e1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, de la Monte S, Gongvatana A, Ombao H, Gonzalez B, Devlin KN, Navia B, Tashima KT, 2011. Plasma cytokine concentrations associated with HIV/hepatitis C coinfection are related to attention, executive and psychomotor functioning. J Neuroimmunol 233, 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JA, Burke-Miller JK, Steigman PJ, Schwartz RM, Hessol NA, Milam J, Merenstein DJ, Anastos K, Golub ET, Cohen MH, 2018. Prevalence, Comorbidity, and Correlates of Psychiatric and Substance Use Disorders and Associations with HIV Risk Behaviors in a Multisite Cohort of Women Living with HIV. AIDS Behav 22, 3141–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia S, Cohen R, Gongvatana A, Ross S, Olchowski J, Devlin K, Tashima K, Navia B, Delamonte S, 2013. Relationship of plasma cytokines and clinical biomarkers to memory performance in HIV. Journal of Neuroimmunology 265, 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW, 2014. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS 28, 2251–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do AN, Rosenberg ES, Sullivan PS, Beer L, Strine TW, Schulden JD, Fagan JL, Freedman MS, Skarbinski J, 2014. Excess burden of depression among HIV-infected persons receiving medical care in the united states: data from the medical monitoring project and the behavioral risk factor surveillance system. PloS one 9, e92842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL, 2010. A meta-analysis of cytokines in major depression. Biol Psychiatry 67, 446–457. [DOI] [PubMed] [Google Scholar]
- Engeland CG, Bosch JA, Rohleder N, 2019. Salivary biomarkers in psychoneuroimmunology. Current Opinion in Behavioral Sciences 28, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Asnis GM, Zumoff B, Nathan RS, Shindledecker R, 1984. Effect of age and sex on cortisol secretion in depressives and normals. Psychiatry Res 13, 221–229. [DOI] [PubMed] [Google Scholar]
- Hiles SA, Baker AL, de Malmanche T, Attia J, 2012. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: exploring the causes of heterogeneity. Brain Behav Immun 26, 1180–1188. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Botzenhardt J, Muhtz C, Agorastos A, Wiedemann K, Kellner M, Otte C, 2012. Sex differences of salivary cortisol secretion in patients with major depression. Stress 15, 105–109. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI, 2006. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31, 151–178. [DOI] [PubMed] [Google Scholar]
- Knorr U, Vinberg M, Kessing LV, Wetterslev J, 2010. Salivary cortisol in depressed patients versus control persons: a systematic review and meta-analysis. Psychoneuroendocrinology 35, 1275–1286. [DOI] [PubMed] [Google Scholar]
- Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctôt KL, Carvalho AF, 2017. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatrica Scandinavica 135, 373–387. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C, 2005. Sex differences in HPA axis responses to stress: a review. Biol Psychol 69, 113–132. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ho RC, Mak A, 2012. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord 139, 230–239. [DOI] [PubMed] [Google Scholar]
- Ma K, Zhang H, Baloch Z, 2016. Pathogenetic and Therapeutic Applications of Tumor Necrosis Factor-alpha (TNF-alpha) in Major Depressive Disorder: A Systematic Review. Int J Mol Sci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, Weber KM, Manly J, Richardson J, Alden C, Anastos K, 2015. Cognitive function in women with HIV: findings from the Women’s Interagency HIV Study. Neurology 84, 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Kuan DCH, Sheu LK, Krajina K, Kraynak TE, Manuck SB, Gianaros PJ, 2017. Systemic inflammation and resting state connectivity of the default mode network. Brain, Behavior, and Immunity 62, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaka H, Maeshima H, Kida S, Kurita H, Shimano T, Nakano Y, Baba H, Suzuki T, Arai H, 2013. Gender differences in serum testosterone and cortisol in patients with major depressive disorder compared with controls. Int J Psychiatry Med 46, 203–221. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C, 2015. Mechanisms of stress in the brain. Nat Neurosci 18, 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL, 2009. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcini Pala A, Steca P, Bagrodia R, Helpman L, Colangeli V, Viale P, Wainberg ML, 2016. Subtypes of depressive symptoms and inflammatory biomarkers: An exploratory study on a sample of HIV-positive patients. Brain Behav Immun 56, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, 2017. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. Eur Neuropsychopharmacol 27, 554–559. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH, 2001. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry 49, 391–404. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Pearce BD, Pisell TL, Sanchez CI, Po C, Su C, Miller AH, 1999. The proinflammatory cytokine, interleukin-1alpha, reduces glucocorticoid receptor translocation and function. Endocrinology 140, 4359–4366. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, Harrison LC, 1998. The chronobiology of human cytokine production. International reviews of immunology 16, 635–649. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH, 2003. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28, 916–931. [DOI] [PubMed] [Google Scholar]
- Reitan R, 1978. Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Neuropsychology Laboratories, Inc., Tuscon, AZ. [Google Scholar]
- Rizavi HS, Ren X, Zhang H, Bhaumik R, Pandey GN, 2016. Abnormal gene expression of proinflammatory cytokines and their membrane-bound receptors in the lymphocytes of depressed patients. Psychiatry Res 240, 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, Blackwell AD, 2014. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med 44, 2029–2040. [DOI] [PubMed] [Google Scholar]
- Rubin LH, Benning L, Keating SM, Norris PJ, Burke-Miller J, Savarese A, Kumanan KN, Awadalla S, Springer G, Anastos K, Young M, Milam J, Valcour VG, Weber KM, Maki PM, 2018a. Variability in C-reactive protein is associated with cognitive impairment in women living with and without HIV: a longitudinal study. J Neurovirol 24, 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Cook JA, Springer G, Weber KM, Cohen MH, Martin EM, Valcour VG, Benning L, Alden C, Milam J, Anastos K, Young MA, Gustafson DR, Sundermann EE, Maki PM, 2017a. Perceived and post-traumatic stress are associated with decreased learning, memory, and fluency in HIV-infected women. AIDS 31, 2393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Phan KL, Keating SM, Maki PM, 2018b. A single low-dose of hydrocortisone enhances cognitive functioning in HIV-infected women. AIDS 32, 1983–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Phan KL, Keating SM, Weber KM, Maki PM, 2017b. Brief Report: Low-Dose Hydrocortisone Has Acute Enhancing Effects on Verbal Learning in HIV-Infected Men. J Acquir Immune Defic Syndr 75, e65–e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Springer G, Martin EM, Seaberg EC, Sacktor NC, Levine A, Valcour VG, Young MA, Becker JT, Maki PM, Neuropsychology Working Groups of the Women’s InterAgency, H.I.V.S., the Multicenter, A.C.S., 2019. Elevated depressive symptoms are a stronger predictor of executive dysfunction in HIV-infected women than men. J Acquir Immune Defic Syndr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaak JF, Trippler M, Hoyo-Becerra C, Erim Y, Kis B, Wang B, Scherbaum N, Gerken G, 2012. Selective hyper-responsiveness of the interferon system in major depressive disorders and depression induced by interferon therapy. PLoS One 7, e38668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Straits-Troster K, Atkinson JH, McCutchan JA, Grant I, 1996. Social and psychological characteristics of HIV-infected women and gay men. HIV Neurobehavioral Research Center (HNRC) Group. Women Health 24, 17–41. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Sternberg EM, 2012. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci 1261, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavish DC, Graham-Engeland JE, Smyth JM, Engeland CG, 2015. Salivary markers of inflammation in response to acute stress. Brain Behav Immun 44, 253–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, 2013. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull 139, 81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich SS, 2016. Immune activation in the central nervous system throughout the course of HIV infection. Current opinion in HIV and AIDS 11, 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wust S, Dockray S, Smyth N, Evans P, Hellhammer DH, Miller R, Wetherell MA, Lupien SJ, Clow A, 2016. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 63, 414–432. [DOI] [PubMed] [Google Scholar]
- Stepanichev M, Dygalo NN, Grigoryan G, Shishkina GT, Gulyaeva N, 2014. Rodent models of depression: neurotrophic and neuroinflammatory biomarkers. BioMed research international 2014, 932757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BJ, Laine C, Cosler L, Hauck WW, 2003. Relationship of gender, depression, and health care delivery with antiretroviral adherence in HIV-infected drug users. J Gen Intern Med 18, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez AN, Rubin LH, Neigh GN, 2016. Untangling the Gordian knot of HIV, stress, and cognitive impairment. Neurobiol Stress 4, 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen AP, Creemers HE, Greaves-Lord K, Verhulst FC, Ormel J, Huizink AC, 2011. Hypothalamic-pituitary-adrenal axis reactivity to social stress and adolescent cannabis use: the TRAILS study. Addiction 106, 1484–1492. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Viviani B, 2015. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96, 70–82. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW, 2009. Major depressive disorder and hypothalamic-pituitaryadrenal axis activity: results from a large cohort study. Arch Gen Psychiatry 66, 617–626. [DOI] [PubMed] [Google Scholar]
- Young E, Korszun A, 2010. Sex, trauma, stress hormones and depression. Mol Psychiatry 15, 23–28. [DOI] [PubMed] [Google Scholar]
- Zapanti E, Terzidis K, Chrousos G, 2008. Dysfunction of the hypothalamic-pituitary-adrenal axis in HIV infection and disease. Hormones (Athens) 7, 205–216. [DOI] [PubMed] [Google Scholar]


