Abstract
Adherence to chronic disease medication regimens depends in part on successful self-regulation. However, the overall benefit of interventions targeting self-regulatory mechanisms is not well-understood. Accordingly, we conducted a meta-review of meta-analyses assessing the effect of interventions targeting self-regulation on medication adherence. For this meta-review, meta-analyses appearing between January 2006 and March 2019 were eligible if they included experimental trials that assessed the effect of an intervention targeting self-regulation on adherence to chronic disease medication. A systematic literature search of multiple databases for published and unpublished literature identified 16,001 abstracts. Twelve meta-analyses met eligibility criteria and had variable quality according to AMSTAR 2 item completion (M = 50%; range: 31-66%). Overall, meta-reviews showed small to medium effect sizes for interventions that targeted self-monitoring, provided personalized feedback on adherence, or involved complete self-management. Other interventions, such as goal setting, barrier identification and problem solving, and stress management showed little evidence of improving adherence. Only a limited number of self-regulation intervention components were able to be evaluated. Additional research is needed to advance the understanding of the efficacy of adherence interventions focused on self-regulation by expanding the scope of self-regulation elements targeted (e.g., emotion regulation).
Keywords: meta-review, chronic disease, medication adherence, self-regulation, behavioural intervention
Chronic disease self-management involves a continuum of behaviours, including linkage to care and retention in care as well as initiation and maintenance of medication adherence, recommended lifestyle modifications, and other prevention efforts (Newman, Steed, & Mulligan, 2004; Stirratt et al., 2018). Medication adherence is a cornerstone of effective self-management of many chronic diseases, including asthma, cardiovascular diseases, and HIV/AIDS. It reflects the alignment of patient medication-taking behaviour with a regimen agreed upon by the patient and provider (Vrijens et al., 2012). From a clinical and public health standpoint, medication adherence is an important self-management behaviour with significant consequences for physical health and quality of life (Mannheimer et al., 2005; Mojtabai & Olfson, 2003; Sokol, McGuigan, Verbrugge, & Epstein, 2005). Research on individuals with chronic conditions consistently indicate the problem of inadequate adherence, with estimates of adherence ranging from 40% to 60% across diverse populations, conditions, and measurement approaches (Stirratt et al., 2018).
Development of effective and sustainable behaviour change interventions to support lifelong adherence to chronic disease medication is therefore a public health priority. Many approaches to improving adherence focus on reducing the complexity of managing adherence behaviour through simplified regimens, removing barriers to medication access, providing prompts to reduce the likelihood of forgetting doses, problem-solving to mitigate medication side effects, or through adherence support provided by health care providers (Fenerty, West, Davis, Kaplan, & Feldman, 2012; Ingersoll & Cohen, 2008; Neiman et al., 2017; Thompson et al., 2012).
For independent-living adults, effective self-management for chronic conditions depends, at least in part, on daily and long-term adherence behaviour. Adherence self-management, therefore, requires self-regulation to achieve desired goals related to the health condition (Bosworth, Blalock, Hoyle, Czajkowski, & Voils, 2018; Clark, Gong, & Kaciroti, 2014; Weidner, Sieverding, & Chesney, 2016). Self-regulation is believed to be a core principle of successful change for a broad range of behaviours (Eisenberg et al., 2018; Miller et al., 2018; Rothman, Baldwin, Hertel, & Fuglestad, 2011; Williams et al., 2018), including medication adherence (Jackson, Eliasson, Barber, & Weinman, 2014; Leventhal, Diefenbach, & Leventhal, 1992; Williams, Rodin, Ryan, Grolnick, & Deci, 1998). Although definitions of self-regulation differ in the literature, the concept typically refers to effortful modulation of cognition, affect, or self-related processing in the service of achieving a behavioural goal (Hofmann, Schmeichel, & Baddeley, 2012; Moore et al., 2016). Interventions that seek to build self-regulation have employed a number of tactics, including goal setting, action planning, identification of barriers and problem solving to overcome barriers, self-monitoring, attentional control, the ability to inhibit habitual responses, delay of gratification, and emotional regulation (Maes & Karoly, 2005; Teixeira et al., 2015).
The most prominent theoretical frameworks regarding health behaviours all include self-regulatory components. For example, social cognitive theory (SCT) posits that an important determinant of health behaviour is the health goals that individuals create, along with the plans and strategies devised for achieving these goals (Bandura, 2004). Based on this, interventions that include goal setting and planning, both elements of self-regulation, should improve adherence. Similarly, temporal self-regulation theory (TST) considers a somewhat larger array of self-regulatory components to account for health behaviours (e.g., goal planning, inhibition of prepotent responses) with a focus on the differing cost-benefit profiles of heath behaviours for the short vs. the long term (Hall & Fong, 2007). Critically, many of these leading models (particularly SCT) do not include various potentially relevant aspects of self-regulation. For instance, emotion regulation is a key part of self-regulation and may be centrally implicated in health behaviour (DeStano, Gross & Kubzansky, 2013). Furthermore, the core aspects of these models are often not actually related to self-regulatory mechanisms (e.g. expectancy that hypertensive medication will lower one’s blood pressure, belief that one is susceptible to suffering a future stroke or heart attack). Given the known importance of the far-reaching construct of self-regulation, the large body of accumulated research should not only be evaluated with respect to testing particular aspects of well-established theories of health behaviour, but also with respect to the broad array of all potentially relevant self-regulatory components that may drive health behaviours.
A growing number of clinical trials have sought to improve medication adherence. Reviews of progress to date suggest that there is substantial room for improvement in how to design and deliver adherence interventions that are impactful, sustained, and cost-effective (Conn & Ruppar, 2017; Nieuwlaat et al., 2014; Wiecek et al., 2019). In particular, little is known about whether interventions that include components designed to impact self-regulation are successful in doing so, and whether these changes result in improved adherence.
To advance the science of medication adherence interventions, we conducted a synthesis of evidence from meta-analyses exploring interventions that target self-regulation to promote medication adherence for chronic diseases. This synthesis was drawn directly from and extends the findings of a ‘parent’ systematic meta-review, focused on self-regulation interventions for health behaviours such as physical activity and smoking that are broadly linked to chronic disease prevention or treatment (Hennessy, Johnson, Acabchuk, McCloskey, & Stewart-James, 2019). Ideally, according to the mechanistic approach of the Science of Behavior Change (SOBC) initiative (Sumner, Beauchaine, & Nielsen, 2018), intervention research should regularly measure potential self-regulatory mechanisms as psychological targets by which medication adherence may be improved. The SOBC establishes the importance of identifying mechanisms of change and applies an experimental medicine approach to behavioural research to guide intervention development (Nielsen et al., 2018). The SOBC approach stresses the importance of not only positing theoretical mechanisms of change (e.g., self-monitoring, self-efficacy, cognitive flexibility, emotion regulation ability) for particular behavioural interventions but also of measuring changes in those hypothesized mechanisms. Unfortunately, however, the vast majority of interventions with medication adherence as an outcome have not measured any hypothesized mechanisms (Edmondson et al., 2018). Therefore, the present meta-review addresses self-regulatory components of interventions using instead the taxonomy of behaviour change techniques (BCTs), a theory-driven system that supports researchers in identifying and testing potential active ingredients in interventions that may drive change in behaviours through their impact on theory-derived intrapersonal and interpersonal processes (Michie & Johnston, 2012). The most recent taxonomy of BCTs includes 93 intervention components that are hierarchically organized into 16 groups that emerged through hierarchical cluster analytic techniques. Clusters were identified in the domain of self-regulation, such as “feedback and monitoring” (e.g., self-monitoring of behaviour), “goals and planning” (e.g., goal setting), and “regulation” (e.g., down-regulating negative emotion) (Michie et al., 2013). By focusing on medication adherence outcomes, we aim to disentangle the effectiveness of self-regulatory intervention components for this important health behaviour as well as identify the impact of other factors specific to medication adherence on intervention success (e.g., intervention delivery mode, use of reminder systems). Thus, this analysis seeks to further the understanding of mechanistic pathways that can improve intervention effectiveness in medication adherence.
Method
Overview
A broad, overarching meta-review was conducted to explore self-regulatory BCTs in interventions spanning health promoting behaviours linked to chronic disease incidence (PROSPERO No. CRD42017074018). We abstracted meta-analyses from the parent meta-review for inclusion in the current review, in addition to conducting an updated search for articles published between January 2017 and March 2019. A separate protocol for this medication adherence review was registered prior to the extraction of data unique to the specific aims of this review and synthesis of results (PROSPERO CRD42019127979). Prior to each stage of the review process (for both the parent meta-review and the current review), researchers experienced in systematic review processes trained undergraduate and graduate students on the protocol, screening instruments, data extraction, and assessment of the quality process. Literature screening was facilitated by EPPI-Reviewer4 (Thomas, Brunton, & Graziosi, 2018) and data extraction, including AMSTAR 2 quality assessment, was conducted using the survey function in RedCAP (Harris et al., 2009) and in Excel. Analysis was conducted using Stata 15.1 (StataCorp, 2017). Data from this analysis are available through the Open Science Framework (https://osf.io/9usxt/?view_only=f104bf0d8a6b4dd595c9ccb4dd3327d1).
Inclusion Criteria
The parent meta-review sought to identify specific self-regulation mechanisms that link interventions with health behaviour change and/or resulting health outcomes across a wide range of health behaviours and chronic conditions. Thus, eligible meta-analyses 1) evaluated trials of any type of health behaviour intervention and 2) quantitatively assessed the effect of self-regulation on behaviour change (e.g., reported on the relationship between a change in self-regulation and a health behaviour or, as a close proxy, examined whether a particular self-regulatory intervention component served as a moderator of effect size). To determine which intervention features were close proxies of self-regulation mechanisms, senior members of the parent meta-review team reviewed and discussed the most current behaviour change taxonomy for intervention techniques relevant to self-regulation, as well as previous versions of the taxonomy (Michie, Abraham, Whittington, McAteer & Gupta, 2009; Michie et al., 2011; Michie, Hyder, Walia & West, 2011). These discussions resulted in consensus that interventions including any of the following 11 BCTs were most relevant to self-regulatory processes and eligible for the review: (1) goal setting, (2) prompt review of goals, (3) prompt self-monitoring, (4) emotional control training, (5) prompt self-talk, (6) stress management, (7) action planning, (8) barrier identification/problem-solving, (9) relapse prevention/coping planning, (10) time management, and (11) provision of feedback. BCTs from other behaviour change taxonomies and closely related intervention methods, such as “inhibitory control training,” also received consideration. Any additional candidate self-regulatory intervention components found during coding were discussed by the team and included in the review if consensus was reached. Systematic reviews without meta-analysis were ineligible. To be relevant to current practice, meta-analyses published before 2006 were ineligible unless they were linked to an updated review published after that date. Any type of publication status or language of publication was eligible.
Meta-analyses eligible for the parent meta-review were categorized into different health behaviours. Reviews that were considered eligible for the present medication adherence meta-review were those that focused on improving adherence to chronic disease medications. To avoid overlap with a recent meta-review on diabetes management (Captieux et al., 2018), we excluded reviews focused specifically on diabetes medication adherence.
Search and Screening Process
For the parent meta-review, two reference librarians were consulted to refine the search strategy, and two team members searched seven electronic databases (hosts) from database inception until August 2017: PubMed, Education Resources Information Center (ERIC), EMBASE (Scopus), PsycINFO, CINAHL, Cochrane Database of Abstracts of Reviews of Effects (DARE), and the Cochrane Database of Systematic Reviews. The Appendix shows the search strategy for PubMed for the original search; this strategy was modified, as needed, to suit other databases.
Screening for the parent meta-review commenced after a training period: 10% of identified papers were screened independently and in duplicate at the title/abstract level. Training for full-text screening commenced with eight team members independently screening the same 15 reviews, and the remaining full texts were divided among the team of eight reviewers for screening. The first 25 reviews in each set were screened independently and in duplicate (to assess screener agreement). Junior team members were paired with another team member for double screening, and senior team members screened the remaining full texts independently.
After completion of the parent-review, included meta-analyses were identified that focused specifically on chronic disease medication adherence. In order to confirm eligibility of adherence-focused meta-analyses abstracted from the parent meta-review, three senior team members thematically categorized the reviews through discussion. In addition to utilizing eligible records from the search process for the parent meta-review, an information retrieval specialist conducted an updated database search in March 2019 for the present review that was specific to the focus of medication adherence outcomes. We limited the electronic database searches to PubMed, Cochrane Database of Systematic Reviews, and PsycINFO. A PubMed Similar Articles search was also performed using the 9 initially included meta-analyses (May 7, 2019). The cited references and reference lists of these meta-analyses were retrieved from Web of Science and Scopus (May 7 and May 8, respectively). Endnote was used to de-duplicate records. The bibliographies of eligible reviews and meta-reviews were assessed for additional eligible publications. For the updated search in this meta-review, screening of titles/abstracts was conducted independently and in duplicate and screening of full texts was conducted by a single screener, with excluded articles reviewed by a second screener (accelerated screening process; Hennessy, Johnson & Keenan, 2019).
Data Extraction
Reviews were first coded for the items pre-specified in the parent meta-review (e.g., review population characteristics, effect sizes for eligible relationships, information about meta-analytic methods). Eligible self-regulation mechanisms related to the domains of regulation of cognition, affect, or self-related processing were included. More specifically, meta-analyses that explored 11 particular intervention features that could be considered relevant proxies of self-regulation were included. These features included goal setting, prompt review of goals, prompt self-monitoring, emotional control training, prompt self-talk, stress management, action planning, barrier identification/problem solving, relapse prevention/coping planning, and time management (Michie et al., 2013).
For this meta-review, additional items were collected as variables coded at the aggregate (review level) as percentages of studies in the review: (1) control group type; (2) intervention dose (duration), (3) mode of intervention delivery; (4) whether the self-regulation intervention also included other components (yes/no) such as reminder systems, case management or referral, adherence incentives, or an additional support system (e.g., directly observed therapy, individual medication managers, treatment assistants, home visits, peer support); (5) the chronic condition studied; (6) adherence measurement approach (e.g., self-report, electronic); and (6) time from start to end of intervention. We had planned to collect time to adherence follow-up, but aside from information on intervention and study length, this level of detail for individual studies (or in aggregate across reviews) was typically not available.
For the original process of data collection (items coded for the parent review that are reported in this review), the first third (n = 22) of 66 meta-analyses were coded independently and in duplicate by three independent reviewers using a standardized coding form. For the remaining studies, one coder independently extracted data that were checked for accuracy by one senior team member. Discrepancies were resolved through discussion and consensus. For the additional items of interest in this review, all meta-analyses were coded independently and in duplicate by a team of two independent reviewers using a standardized coding form, and discrepancies were resolved through discussion and consensus.
Assessment of Meta-Analysis Quality
Two independent reviewers used the AMSTAR 2 instrument (Shea et al., 2017) to assess the quality of the included meta-analyses; a third were rated independently and in duplicate, followed by discrepancy resolution. For the remaining studies, one coder independently assessed risk of bias, and a senior team member checked the ratings for accuracy. Discrepancies were discussed and resolved by consensus.
AMSTAR-2 items with No/Yes options were given scores of 0/2, respectively, while items with “No”/“Partial Yes”/“Yes” options were given scores of 0/1/2, respectively. The AMSTAR-2 score for each meta-analysis represents the proportion of items satisfying the total number of relevant quality dimensions such that a score of 100% signifies that all quality dimensions were satisfied. A total calculation for each review was estimated using applicable AMSTAR-2 items only (e.g., in cases where reviews only included RCTs, the questions related to proper synthesis and quality rating of non-randomized studies of interventions were irrelevant). The present meta-review reports the results for all 12 meta-analyses, with qualifiers based on the AMSTAR-2 proportion scoring.
Narrative synthesis
We conducted a narrative synthesis describing interventions that included self-regulation mechanisms in the meta-analyses and the quantitative results linking these interventions to adherence outcomes. Given the diversity of analyses across the included reviews, we present effect sizes and other statistical findings in the metrics originally presented in the reviews. The corrected covered area (CCA) was estimated to determine the relative coverage or overlap of primary studies in the included meta-analyses and was used as a diagnostic tool to interpret outcomes (Pieper, Antoine, Mathes, Neugebauer, & Eikermann, 2014).
Results
Eight reviews were found eligible from the parent meta-review and included in this review. Our updated search identified 3,803 unique studies. Only four of these studies was eligible for inclusion (see Figure 1). Thus, 12 eligible meta-analyses were included, representing 704,004 participants (range: 1,806 - 568,811).
Figure 1.
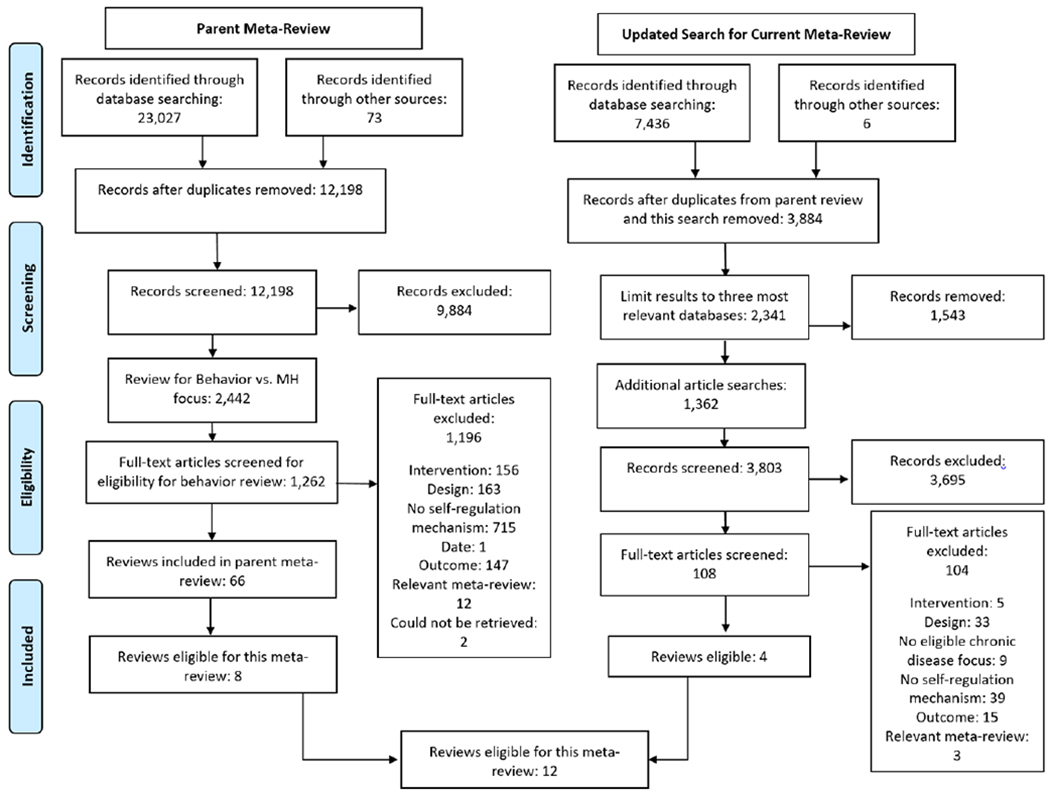
Literature screening and selection process
Five of the reviews included multiple chronic diseases. The remaining seven restricted eligible patient populations to a single chronic disease: hypertension (l = 3), coronary artery disease (l = 1), asthma (l = 1), HIV/AIDS (l = 1), and unspecified illness/diagnosis (l = 1). The majority of reviews focused on adults (58%), with one focused on older adults (60 or more years old), and four reviews did not specify age restrictions. Apart from one review that restricted eligible participant populations to African Americans and another that restricted eligible populations to cis-gender women, reviews were broad in their demographic inclusion criteria. Seven reviews reported intervention duration ranges: mean (SD) of minimum and maximum intervention duration, respectively, were 1.6 week (1.7) and 65 weeks (27.1). Seven reviews reported study duration ranges. The mean (SD) of minimum and maximum study duration, respectively, were 4.0 weeks (2.3) and 71.3 weeks (22.7). Of the studies included in the eight reviews that reported the type of control/comparison groups, the majority used inactive/assessment-only control groups (on average 81% across reviews); while a few used informational control and active control groups (7% and 11%, respectively). Eleven of the 12 reviews reported on the different types of medication adherence measures that primary studies used, including electronic, pharmacy, pill count, self-report, and “biological measures.” Across the meta-analyses, the included studies most often used self-report to measure medication adherence (M = 48% of studies in a meta-analysis, SD = 20%) and fewer utilized electronic (M = 30%, SD = 32%), pill count (M = 19%, SD = 12%), and pharmacy refill (M = 19%, SD = 7%). Only a single review reported including studies that measured adherence using biological measures (M = 6%, SD = 3%).
Across reviews, six intervention approaches to improving self-regulation were assessed, including: self-monitoring behaviour and/or outcomes (l= 6), personalized feedback (l = 5), goal setting (l = 4), barrier identification (l =6), stress management (l = 4), and self-management interventions (l = 1). Some of the reviews also examined non-self-regulatory intervention components such as the use of reminder systems (l = 8), social support (l = 8), adherence incentives (l = 5), home visits (l = 4), case management/referrals (l = 1), and directly observed therapy (l = 1). Reviews reported a wide variety of intervention delivery modes; most often, in-person (individual or group-delivered) and telephone-based approaches were reported in reviews. Reviews also noted some instances of mailed, mHealth, online, and text message-based delivery systems. See Tables 1 and 2 for characteristics of included reviews.
Table 1.
Characteristics of reviews included in meta-review of self-regulation mechanisms on medication adherence
| Citation | Funding reported | Review aim | Eligible study designs | Age, gender, race of eligible populations | Clinical status | Included studies (k) | Participants (n) | Self-regulation component examineda | Proportion of AMSTAR 2 items satisfied |
|---|---|---|---|---|---|---|---|---|---|
| (Chase et al., 2016) | Yes | Describe and quantify the overall effectiveness of MA intervention research among CAD patients, and to explore potential moderators of intervention effectiveness. | Not reported | 18+ years | Patients with CAD diagnosis | 24 | 18,839 | 1, 2, 4 | 0.33 |
| (Conn & Ruppar, 2017) | Yes | Assess 1) What are the average effects of interventions on adherence? 2) Do intervention effects vary depending on study design or sample characteristics? 3) Do intervention effects vary depending on intervention characteristics? | Not reported | Adults | Predominantly physical health problems in cardiac populations. Excluded: contraceptive/sexual function medications, samples with major psychiatric or substance abuse problems and incarcerated/institutionalized persons | 739 | 568,811 | 1, 2, 3, 4, 5 | 0.31 |
| (Conn et al., 2009) | No | Investigate the effectiveness of interventions to improve MA in older adults: What are the overall effects of MA interventions to improve adherence behaviour? What are the overall effects of MA interventions on participants’ knowledge about their medications, management of medications, disease symptoms, health outcomes, systolic and diastolic blood pressure, health care services utilisation, and quality of life? Do sample demographics, intervention components, or adherence measurement methodologies moderate the effect of interventions on MA behaviour? | RCT | Mean age of sample at least 60 years | Sample with physical health condition requiring at least one prescription medication. | 38 | 11,827 | 2 | 0.41 |
| (Conn et al., 2015) | Yes | Examine: (1) What is the overall average effect of interventions designed to increase adherence among subjects with HTN? (2) Do effects of interventions vary depending on sample and study characteristics? (3) Do the effects vary depending on intervention features? (4) What risks of bias are present in studies, and what influence do they have on effect sizes? | Not reported | Adults | Hypertension | 101 | 34,272 | 2, 4, 5 | 0.44 |
| (Demonceau et al., 2013) | Yes | Identify RCTs containing empirical data on the efficacy of interventions to enhance adherence to prescribed medications, as assessed by electronic medication-event monitoring methods. | RCT | Not restricted | Not restricted | 79 | 5,237 | 5 | 0.66 |
| (Denford et al., 2014) | Yes | Update previous systematic reviews of interventions targeting asthma self-care in adults with asthma and explore the association between specific BCTs and change in asthma morbidity, unscheduled health care use, and adherence to preventative asthma medication. | RCT | 18+ years | Asthma diagnosis | 38 | 7,883 | 1, 4, 5 | 0.63 |
| Fletcher et al., 2015 | No | Synthesize the literature to determine the effect of self-monitoring of BP on medication adherence, medication persistence, and lifestyle factors in people with hypertension. | RCT, QED | Not restricted | Participants with hypertension that were receiving care in ambulatory/outpatient settings | 28 | 7,021 | 2 | 0.53 |
| (Kassavou & Sutton, 2018) | Yes | Conduct a comprehensive effect-size analysis of IVR and SMS interventions to promote adherence to cardio-metabolic medication, to identify components of these interventions that are associated with their effectiveness, and to discuss possible mechanisms of action. | RCT | Adults aged 18 years and over | Participants prescribed oral medication to treat or prevent cardio-metabolic conditions. Adults whose main prescription was insulin to manage T1D were excluded. | 17 | 38,671 | 4 | 0.56 |
| (Pellowski et al., 2019) | Yes | Identify and categorise behavioural interventions to improve ART adherence for cis-gender women living with HIV, determine efficacy of RCTs testing ART adherence interventions for this population, and identify key moderators of the efficacy of these interventions. | RCT; Longitudinal | Cis-gender women | Living with HIV | 14 | 1,806 | 1, 3, 4 | 0.59 |
| (Ruppar et al., 2017) | Yes | Conduct a comprehensive search and systematic review of intervention studies testing interventions to improve adherence to BP medications among Black adults with hypertension. | RCT | 18+ years, identify as Black | Hypertension | 37 | 5,228 | 2 | 0.59 |
| (Sakakibara et al., 2017) | Yes | Describe self-management interventions used to improve risk factor control in stroke patients and quantitatively assess their effects on overall risk factor control from lifestyle behaviour (i.e., physical activity, diet and nutrition, stress management, smoking, alcohol, and medication adherence) and medical risk factors (i.e., blood pressure, cholesterol, blood glucose) and on individual risk factors. | RCT | 18+ years | History of stroke or ischemic attack | 14 | 2,316 | 6 | 0.31 |
| (Seewoodharry, Maconachie, Gillies, Gottlob, and McLean, 2017) | Yes | Explore whether feedback, guided by subjective or objective adherence measures, improves adherence. | RCT | Not restricted | Not restricted | 24 | 2,093 | 5 | 0.63 |
Note. BCT = Behaviour change technique. BP = Blood pressure. CAD = Coronary artery disease. HTN = Hypertension. MA = Medication adherence. NR = Not reported. QED = Quasi-experimental design. RCT = Randomized controlled trial.
1 = Goal setting; 2 = Prompt self-monitoring; 3 = Stress management; 4 = Barrier identification/Problem solving; 5 = Feedback on performance; 6 = Self-management interventions.
Table 2.
Study and intervention characteristics of primary studies included in reviews
| Number of Reviews (l) | M (SD) | |
|---|---|---|
| Delivery modes reported | 11 | |
| In-person, individual | 10 | |
| In-person, group | 8 | |
| App-based | 0 | |
| Mhealth | 1 | |
| Online, including email | 7 | |
| Telephone | 11 | |
| Text messages | 5 | |
| Study and Intervention duration reported | 7 | |
| Intervention length min. (weeks) | 1.55 (1.68) | |
| Intervention length max. (weeks) | 64.90 (27.05) | |
| Study length min. (weeks) | 4.03 (2.26) | |
| Study length max. (weeks) | 71.34 (22.72) | |
| Control/Comparison type reporteda | 8 | |
| Inactive/asssessment only (% of primary studies) | 0.81 (0.16) | |
| Information-only (% of primary studies) | 0.07 (0.10) | |
| Active (% of primary studies) | 0.11 (0.14) | |
| Intervention components reported | 9 | |
| Reminder systems | 8 | |
| Case management/referral | 1 | |
| Adherence incentives | 5 | |
| Additional support systems reported | 9 | |
| Directly observed therapy | 1 | |
| Home visits | 4 | |
| Individual medication managers | 0 | |
| Social support | 8 | |
| Treatment assistants | 0 | |
| Adherence measures reporteda | 11 | |
| Electronic (% of primary studies) | 0.30 (0.32) | |
| Pharmacy (% of primary studies) | 0.19 (0.07) | |
| Pill count (% of primary studies) | 0.19 (0.12) | |
| Self-report (% of primary studies) | 0.48 (0.20) | |
| “Other” type (% of primary studies) | 0.06 (0.03) | |
Note. Inactive/assessment-only control groups included standard/usual care.
l = number of reviews. M = mean. SD = Standard deviation
At the review level, % of primary studies that reported these variables.
Quality assessment
Overall, reviews achieved 50% item completion on the AMSTAR 2 (range: 31-66%). The majority of reviews clearly reported inclusion criteria (78%). Only one review had preregistered a protocol (Fletcher, Hartmann-Boyce, Hinton, & McManus, 2015), and one review reported funding sources for included studies (Ruppar, Dunbar-Jacob, Mehr, Lewis, & Conn, 2017). Search strategies varied in their comprehensiveness, with only a single review reporting an AMSTAR 2 defined completely comprehensive search (searched at least two databases, provided key word and/or search strategy, conducted search within 24 months of completion of the review, consulted content experts in the field, justified publication restrictions, searched for grey literature when relevant, and searched the bibliographies of included studies and trial/study registries). Five reviews reported adequate search methods (searched at least two databases, provided key word and/or search strategy, and justified publication restrictions). All reviews searched at least two databases and provided their key word and search strategy, and all but two reviews reported double and independent coding of data. Three reviews reported selecting studies in duplicate. Only one review provided a complete list of excluded reviews with exclusion reasons, but nine (82%) included a detailed PRISMA flow diagram of the study selection process.
The majority of meta-analyses (66%) included studies in good (33%) or excellent (33%) detail. Assessment of study quality, using AMSTAR 2 criteria, varied across reviews but was of higher quality in the reviews that only addressed RCTs (42% received a “Partial Yes” rating, and 42% received a “Yes” rating). Of the four reviews that included non-randomized studies of interventions, none used an appropriate tool to assess study quality. A similar picture emerged for the analysis methods. For reviews that included RCTs, the majority (83%) used appropriate meta-analytic methods, while none of the four reviews that also included non-randomized intervention studies used appropriate methods to combine those outcomes. The majority of reviews adequately examined heterogeneity (92%) and potential for publication bias (75%). Reviews were generally less rigorous in quantitatively assessing effects of risk of bias on their outcomes of interest (58% did so) and in incorporating risk of bias results into a discussion of their results (33% incorporated these findings). The majority of reviews (92%) reported potential conflicts of interest. See Figure 2 for an overall summary and the supplemental files for ratings of each item for each review.
Figure 2.
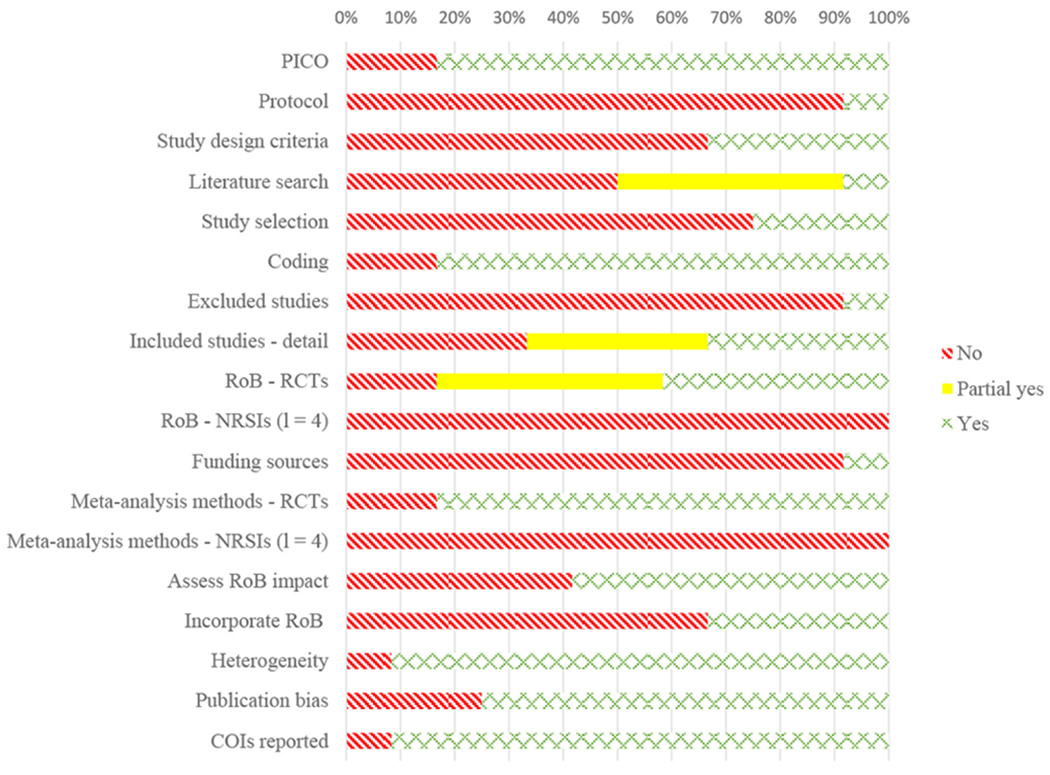
Summary of review quality assessment (AMSTAR 2) across included reviews (l = 12).
Note: PICO = Patient, Intervention, Comparison, Outcome (inclusion criteria categories); RoB = Risk of Bias; RCT = Randomized Controlled Trial; NRSI = Non-Randomized Studies of Interventions
Overlap
Across the 12 reviews, the CCA was low (2%); however, four reviews had over 75% overlap of primary studies with at least one other review (Chase, Bogener, Ruppar, & Conn, 2016; Conn et al., 2009; Conn, Ruppar, Chase, Enriquez, & Cooper, 2015; Ruppar et al., 2017). The reviews with higher overlap were authored by nearly identical author teams, and the CCA for these studies was 6%. The largest review in this set (Conn & Ruppar, 2017) included 739 studies and focused broadly on medication adherence across diverse groups of interventions and populations. The overlap is likely high among these reviews because some of the primary studies from the largest and broader review was used to further address questions about distinct sub-populations of participants, including in the context of coronary artery disease (Chase et al., 2016), among hypertensive black adults (Ruppar et al., 2017), older adults (Conn et al., 2009), and among hypertensive patients (Conn et al., 2015). Thus, we have included each review in our synthesis, by comparing and contrasting findings from the largest review to findings from particular smaller reviews that covered the same/similar population and outcome.
Intervention Mechanisms and Outcomes
The majority of the included meta-analyses indicated that inclusion of a self-regulation intervention component did not produce statistically significant improvements in adherence (see Figure 3 for overall results and supplemental files for effect size data for each study). In comparison, a meta-review on diabetes self-management programs described short-term increases in glycemic control, with a small but statistically significant improvement in related health behaviours, which including medication adherence (Captieux et al., 2018). However, there was some evidence of efficacy for a few self-regulatory intervention approaches, certain types of reminder systems with older populations, and some modes of intervention delivery. As described in the following paragraphs, only self-monitoring in two reviews, personalized feedback in one review, and self-management interventions in one review were found to produce significantly improved medication adherence among intervention participants. The remaining interventions targeting self-regulation mechanisms examined were not related to medication adherence outcomes in any of the other reviews, including in those reviews with high overlap.
Figure 3.
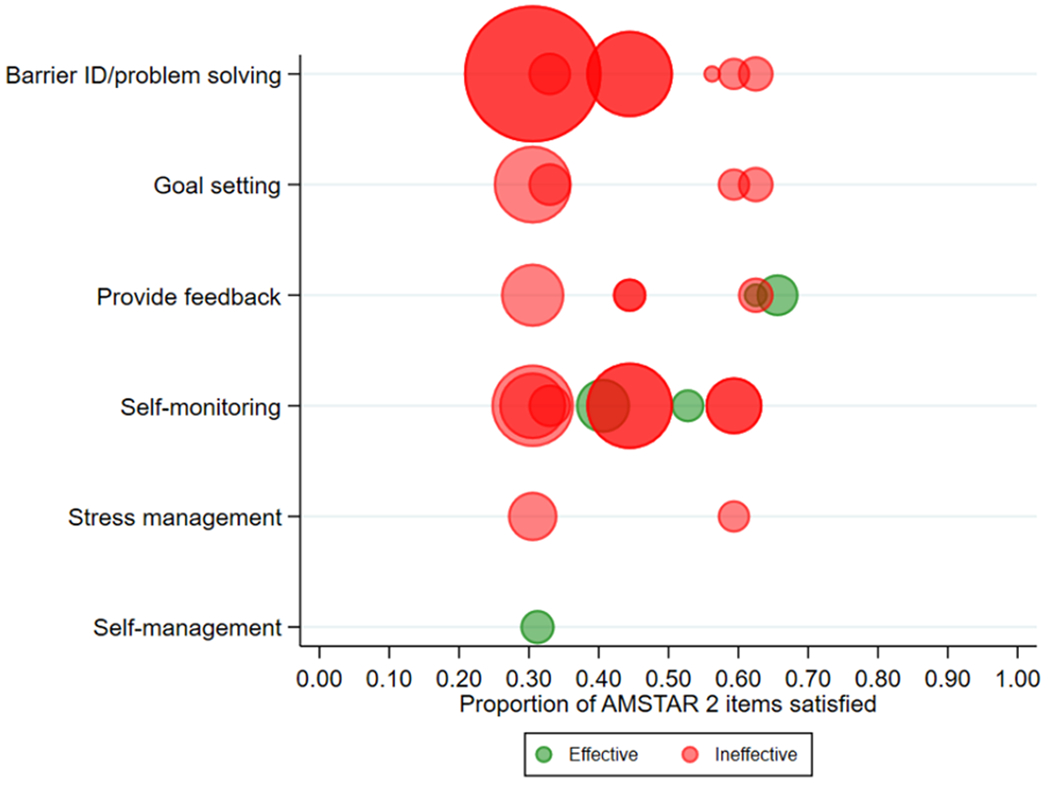
Quality and supportiveness of data from meta-analyses for effective (green) or ineffective (red) interventions targeting individual self-regulation mechanisms for improving medication adherence. Bubbles for each meta-analysis are sized proportional to the numbers of studies each meta-analysis included.
Self-monitoring.
There was mixed evidence for self-monitoring as a mechanism of behaviour change related to medication adherence. In their review of 28 studies, Fletcher et al. (2015) found that self-monitoring of blood pressure significantly improved medication adherence outcomes among individuals with hypertension (k = 13; d = 0.21 [0.08, 0.34]). Similarly, in their review of 38 studies, Conn et al. (2009) found that interventions that directed elderly patients to self-monitor symptoms related to medications (including symptom improvement and medication side effects) significantly improved their participants’ medication adherence compared to interventions that did not have this feature (d = 1.18 vs. d = 0.30). In contrast, reviews of patients with hypertension (k = 101; Conn et al., 2015), patients with CAD (k = 28; Chase et al., 2016), adults with physical health and cardiac problems (k = 739; Conn & Ruppar, 2017), and Black adults with hypertension (k = 37) did not find evidence for significant improvement of medication adherence outcomes through the use of self-monitoring. However, the quality of both reviews that indicated self-monitoring was effective and those that did not find evidence of effectiveness were similarly mixed. Thus, given the inconsistent findings and the quality of meta-reviews, the evidence regarding the efficacy of patient self-monitoring for improving medication adherence remains inconclusive.
Personalized feedback.
The evidence was also mixed for personalized feedback as an effective ingredient of adherence interventions. Nevertheless, the accumulated evidence suggests that this intervention component likely promotes health behaviour change related to medication use. In their review of 79 studies, Demonceau et al. (2013) found that interventions using an electronic medication adherence monitoring system that also gave participants feedback on adherence behaviour significantly improved participant medication adherence outcomes relative to no-feedback interventions (k = 22; 19.8% improvement [10.7%, 28.9%] vs. 10.3% improvement [7.5%, 13.1%]). This sole component was more important than other intervention components examined as moderators. In contrast, the four other reviews which examined the provision of feedback did not find evidence for a significant increase in medication adherence. Notably, these other reviews differed from the review by Demonceau et a. (2013) in important ways. The Denford et al. (2014) review synthesized a much smaller number of primary studies (k = 38) and focused on adults with asthma. The Seewoodharry et al. review (2017) only included six studies in their meta-analysis and combined diverse populations (patients with asthma, HIV/AIDS, or osteoporosis). Finally, although the Conn et al. (2015) and Conn and Ruppar (2017) reviews examined specific populations of adults with cardiac health issues and were very large reviews (k = 101 and k = 739, respectively), they satisfied far fewer of the AMSTAR 2 criteria than did the Demonceau et al. (2013) review (44% and 31% versus 66%, respectively). In sum, the evidence suggests that electronic medication adherence monitoring that gives personalized feedback to patients may be an effective intervention tool, while other forms of feedback, or for particular health conditions (i.e., asthma), may not produce improved medication adherence.
Self-management interventions.
The current evidence suggests that self-management interventions do increase medication adherence, though with a generally small effect size. Sakakibara et al. (2017) reviewed 14 RCTs among patients who had previously had a stroke. They found that self-management interventions significantly increased medication adherence outcomes compared to control groups (k = 5; d = 0.31 [0.07, 0.56]).
Reminder systems.
The inclusion of patient reminders as feature of interventions was associated with improved adherence only for a subset of reminder types. Of the eight reviews that reported that primary studies utilized some sort of reminder system for participants, three also quantitatively tested whether use of a reminder system produced significant changes in overall intervention effects on medication adherence (Conn et al., 2009; Conn et al., 2015; Pellowski et al., 2018). In one review that examined reminders for older adults (Conn et al., 2009), the authors found that interventions which included “a stimulus to take medication, such as an electronic device that makes a sound each time medications should be administered” (p. 457), were significantly more effective (k = 3; d = 1.06, p < .01) than interventions without this feature (k = 38; d = 0.30). Another review (Conn et al., 2015) of adults with hypertension examined medication administration calendars and concluded that these calendars did not produce significantly higher outcomes (k = 10; d = 0.23, SE = 0.09) than interventions without this feature (k = 98; d = 0.30, SE = 0.04). A third review (Pellowski et al., 2018) examined whether the inclusion of Wisepill, an electronic medication dispenser, produced better outcomes among cis-gender women living with HIV but found that the inclusion of Wisepill did not significantly improve participant outcomes (d = −1.05, p = 0.29) among the two studies that used this tool. Yet, it is unclear whether the studies used the Wisepill simply as an adherence measure or if they also utilized its reminder capabilities for participants. In sum, although it seems that electronic reminders could be effective for forgetting to take medication among older adults, the type of stimulus may matter (e.g., in-the-moment auditory cues vs. administrative calendars). Yet, very few reviews of self-regulation mechanisms in medication adherence also quantitatively examined reminder systems.
Mode of intervention delivery.
Findings for delivery mode of interventions had mixed results. Three aspects of delivery mode were considered: interventions administered to groups vs. individuals, interventions delivered face-to-face vs. some other method (e.g., mail, by computer), and interventions delivered by different type of healthcare provider (e.g., nurses, pharmacists, physicians). Although seven reviews attempted to quantitatively examine whether delivery mode was a factor in the success of interventions, two did not examine this aspect (Demonceau et al., 2013; Fletcher et al., 2015), and one stated there was not enough evidence to do so (Denford, Taylor, Campbell, & Greaves, 2014).
First, in terms of groups versus individual administration, findings were mixed. One review (Pellowski et al., 2018) indicated that the seven interventions with a group component had significantly larger effect sizes as compared to individual administration (β = 0.47, p = 0.049). In contrast, in the large review by Conn and Ruppar (2017), there were no significant differences among adults between interventions delivered to individuals versus groups.
Second, there was also mixed evidence across medical conditions and patient ages suggesting that face-to-face mode of delivery may improve efficacy. For the largest review of this set of overlapping reviews (Conn & Ruppar, 2017), interventions delivered face-to-face were more effective than interventions delivered in other ways (i.e., computer, telephone, mail, text messages, written materials. The five reviews with high overlap examined differences in delivery mode for their particular populations of interest and found that among adult patients with coronary artery disease, mail-delivered interventions were less effective than interventions without mail delivery, but that there were no differences between telephone, written materials, or face-to-face interventions (Chase et al., 2016), and that among African American adults, interventions delivered using an interactive discussion had reduced effects when compared to interventions delivered in part by computer (Ruppar et al., 2017). However, specifically among older adult participants, there was no difference between direct face-to-face delivery and indirect (e.g., technologically mediated, mail-based) delivery (Conn et al., 2009). Similarly, among adults with hypertension, interventions delivered face-to-face were not significantly more effective than interventions delivered in other formats, such as by mail or telephone (Conn et al., 2015). Finally, one review (Kassavou & Sutton, 2018) found that interactive voice response (IVR) or short message service (SMS) interventions nearly doubled the odds (OR = 1.89) of adult patient adherence to cardio-metabolic medications when compared to usual care.
Third, the current evidence is insufficient to make firm conclusions about the impact of type of health care professional as interventionist, but there is some indication that nurse- and pharmacist-administered interventions may be associated with improved efficacy. Five reviews examined the impact of the personnel delivering the intervention, while another stated there was not enough evidence to do so (Denford et al., 2013). Personnel occupation had no significant effect on outcomes among general participants (Demonceau et al., 2013) and in older adults (Conn et al., 2009). For adult patients with coronary artery disease (Chase et al., 2016), interventions with nurses produced higher effects than those without, but there was no difference for pharmacist or physician inclusion/exclusion. Although one review (Ruppar et al., 2017) found that there were no significant differences by provider for African American adults, the broader review of any type of medication adherence interventions (Conn & Ruppar, 2017) found that when pharmacists delivered the intervention, there were significantly higher results among participants.
Discussion
Self-regulation is believed to be a core principle of successful behaviour change (Eisenberg et al., 2018; Miller et al., 2018; Rothman et al., 2011; Williams et al., 2018). Increasing evidence suggests that interventions designed to improve elements of self-regulation can promote a range of chronic disease-related outcomes, including improved health care utilization and health related quality of life (Allegrante, Wells, & Peterson, 2019). Relationships between self-regulation focused interventions and medication adherence have been less clear. In this analysis, improved adherence outcomes were associated with interventions that included self-monitoring and personalized feedback. Personalized feedback, in particular, showed the most promising effects for improving adherence. Yet, there was little overall evidence that interventions targeting other aspects of self-regulation were associated with improved medication adherence.
From a theoretical perspective, these findings suggest that a subset of self-regulatory mechanisms of change are implicated in successful medication adherence, consistent with the inclusion of self-regulation as an important component of multiple leading theories regarding health behaviour change (e.g., SCT, TST, HBM; Bandura, 2001; Hall & Fong, 2007; Rosenstock et al., 1988). These findings should be considered in light of a large meta-analysis examining the relationship of theory application to improved medication adherence in chronic disease-focused interventions (Conn, Enriquez, Ruppar, & Chan, 2016). In that analysis, effect sizes varied considerably depending on the theory or framework used, with the highest for studies utilizing the health belief model (Rosenstock, Strecher, & Becker, 1988) and among the lowest for studies that utilized a self-regulation framework. In the 11 comparisons in which a self-regulation framework was cited as the basis for the intervention, the overall effect size was not statistically significant (Conn et al., 2016). In contrast to that previous theory-based analysis of interventions, the present meta-review of particular techniques to improve behaviour revealed that two aspects within the self-regulatory domain showed evidence of efficacy. These two aspects—personalized feedback and self-monitoring—deserve further attention in future mechanism-focused intervention research to better understand why, for which specific behaviours, and for whom they improve medication adherence.
It is important to note, however, that the meta-analyses included in this meta-review were only able to examine a limited number of constructs related to self-regulation. For example, it has been posited that emotion regulation (e.g., successfully reducing negative emotions in the context of stress) may be important for managing goal-directed behaviour. However, the included meta-analyses did not provide sufficient information to assess adherence interventions designed to promote emotion regulation. Based on the evidence available from the meta-analyses reviewed, we are unable to draw conclusions about the relationships between inclusion of the full array of variables falling under the umbrella of self-regulation, and to assess these elements as they relate to adherence outcomes. Intervention and synthesis research is needed that addresses a wider array of all possible self-regulatory mechanisms.
It is not possible to determine with the existing data whether null to small effect sizes were due to self-regulatory processes being potentially unimportant causal determinants of chronic disease adherence, or whether the interventions designed to influence those processes were insufficiently strong to produce meaningful changes in self-regulatory processes. Future intervention research in this area would benefit greatly from following the recommendations of the previously mentioned SOBC initiative by measuring changes in specific self-regulatory constructs targeted by BCTs. These self-regulatory changes should then be tested as potential mediators to further specify and test the putative causal pathways linking self-regulation interventions to behavioural outcomes. This systematic approach, championed by the SOBC initiative, can identify and isolate causal pathways in behaviour change interventions.
Across the reviewed meta-analyses, there were mixed findings regarding the impacts of reminder systems and mode of intervention administration on adherence behaviour. Yet, there appears to be some accumulation of evidence—albeit mixed—supporting stronger outcomes in interventions delivered in a face-to-face format. Because the meta-analyses reviewed included many multi-component interventions, it is not clear whether the mode of administration interacted specifically with self-regulation intervention components to influence adherence outcomes. In addition to more formal assessment of mediation models to assess mechanisms of change, we also suggest future research that identifies potential intervention moderators, such as mode of delivery. Differences in study efficacy as a function of administration mode must also be assessed, considering potential feasibility, cost, and cost-effectiveness. Small differences in efficacy between in-person versus e- and m-Health-based intervention administration may be outweighed by the capacity, for instance, for greater reach to patient populations that might most benefit from adherence support.
Medication adherence behaviour also depends on the social environment in which it occurs (Kardas, Lewek, & Matyjaszczyk, 2013). For example, one of the meta-analyses focused on hypertension noted socioeconomic status as a moderator of intervention effectiveness but also noted a lack of studies that included relevant variables such as educational attainment (Conn et al., 2015). This meta-review was unable to determine factors at the social and structural level, such as lack of insurance coverage for medications and transportation barriers to pick up medicine, that might interact with self-regulation interventions to impact behaviour. Additionally, this review was not able to differentiate effects on intentional versus unintentional non-adherence. Intentional non-adherence, driven by cognitive beliefs such as skipping medications to avoid side effects or not taking HIV medications in front of others due to stigma concerns, would be approached differently in terms of intervention strategies than those seek to reduce unintentional forgetting of doses, which would likely benefit more from efforts to improve self-regulation. A single individual can have both intentional and unintentional non-adherence (Gadkari & McHorney, 2012), and the degree to which one versus the other influences behaviour changes over time, making a tailored approach based on non-adherence intentionality challenging. Because of this, we were not able to analyze for intentionality of non-adherence, but suggest this as another potentially important avenue for future research.
Findings should be interpreted in light of several potential limitations. First, the majority of meta-analyses included in the review limited study inclusion to randomized controlled trials. For this reason, rigorous studies utilizing quasi-experimental designs may be underrepresented. Second, the inclusion criteria for this analysis limited meta-analyses to those that included a behavioural adherence outcome, which would exclude meta-analyses that focused solely on clinical outcomes but did not include an adherence outcome measurement. Given the close relationship between adherence and clinical outcomes for many chronic diseases, this restriction may also have influenced findings. Third, although a systematic and comprehensive search was conducted, there may be some meta-analyses that were missed. Fourth, while meta-reviews are able to provide an overarching perspective on a topic, this is often at the expense of more granular analyses that occur at the individual study level. Thus, while this review provided an overview of several variables that may influence the association between self-regulation interventions and adherence outcomes such as intervention dose, chronic condition studied, type of adherence measurement, and type of control group, we are not able to detail specific interaction effects of these variables.
Medication adherence is an important public health issue, and there have been significant advances made in our understanding of how best to support adherence to chronic disease medication. Additional research is needed, however, regarding how best to intervene to support self-regulation in ways that are feasible, acceptable, and impactful across a range of populations. Future research synthesis should address whether these changes, alone and in combination with other approaches, produces meaningful and sustained changes in medication adherence.
Supplementary Material
Acknowledgements:
This work was supported by the National Institutes of Health (NIH) Science of Behavior Change Common Fund Program through an award administered by the National Institute on Aging (U24AG052175), the National Institute of Nursing Research (R21NR018348), and the National Heart Lung and Blood Institute (R21HL145970). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Rebecca L. Acabchuk and Blair T. Johnson as well as our literature screeners and coders: Emily Betterton, Meiko Howell, Sahar Iqbal, Kiana McDavid, Lindsay Roethke, and Jania Stewart-James.
Footnotes
Disclosure statement:
The authors have no conflicts of interest to disclose.
References
- Allegrante JP, Wells MT, & Peterson JC (2019). Interventions to support behavioral self-management of chronic diseases. Annual Review of Public Health, 40, 127–146. doi: 10.1146/annurev-publhealth-040218-044008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A (2001). Social Cognitive Theory: An agentic perspective. Annual Review of Psychology, 52, 1–26. [DOI] [PubMed] [Google Scholar]
- Bandura A (2004). Health promotion by social cognitive means. Health Education & Behavior, 31(2), 143–164. [DOI] [PubMed] [Google Scholar]
- Bosworth HB, Blalock DV, Hoyle RH, Czajkowski SM, & Voils CI (2018). The role of psychological science in efforts to improve cardiovascular medication adherence. American Psychologist, 73(8), 968–980. doi: 10.1037/amp0000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Captieux M, Pearce G, Parke HL, Epiphaniou E, Wild S, Taylor SJC, & Pinnock H (2018). Supported self-management for people with type 2 diabetes: A meta-review of quantitative systematic reviews. BMJ Open, 8(12), e024262. doi: 10.1136/bmjopen-2018-024262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase JD, Bogener JL, Ruppar TM, & Conn VS (2016). The effectiveness of medication adherence interventions among patients with coronary artery disease: A meta-analysis. The Journal of Cardiovascular Nursing, 31(4), 357–366. doi: 10.1097/JCN.0000000000000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NM, Gong M, & Kaciroti N (2014). A model of self-regulation for control of chronic disease. Health Education & Behavior, 41(5), 499–508. doi: 10.1177/1090198114547701 [DOI] [PubMed] [Google Scholar]
- Conn VS, Enriquez M, Ruppar TM, & Chan KC (2016). Meta-analyses of theory use in medication adherence intervention research. American Journal of Health Behavior, 40(2), 155–171. doi: 10.5993/AJHB.40.2.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS, Hafdahl AR, Cooper PS, Ruppar TM, Mehr DR, & Russell CL (2009). Interventions to improve medication adherence among older adults: Meta-analysis of adherence outcomes among randomized controlled trials. The Gerontologist, 49(4), 447–462. doi: 10.1093/geront/gnp037 [DOI] [PubMed] [Google Scholar]
- Conn VS, & Ruppar TM (2017). Medication adherence outcomes of 771 intervention trials: Systematic review and meta-analysis. Preventive Medicine, 99, 269–276. doi: 10.1016/j.ypmed.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS, Ruppar TM, Chase JA, Enriquez M, & Cooper PS (2015). Interventions to improve medication adherence in hypertensive patients: Systematic review and meta-analysis. Current Hypertension Reports, 17(12), 94. doi: 10.1007/s11906-015-0606-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonceau J, Ruppar T, Kristanto P, Hughes DA, Fargher E, Kardas P, … Vrijens B (2013). Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: A systematic literature review and meta-analysis. Drugs, 73(6), 545–562. doi: 10.1007/s40265-013-0041-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denford S, Taylor RS, Campbell JL, & Greaves CJ (2014). Effective behavior change techniques in asthma self-care interventions: Systematic review and meta-regression. Health Psychology, 33(7), 577–587. doi: 10.1037/a0033080 [DOI] [PubMed] [Google Scholar]
- DeSteno D, Gross JJ, & Kubzansky L (2013). Affective science and health: the importance of emotion and emotion regulation. Health Psychology, 32(5), 474–486. doi: 10.1037/a0030259 [DOI] [PubMed] [Google Scholar]
- Edmondson D, Falzon L, Sundquist KJ, Julian J, Meli L, Sumner JA, & Kronish IM (2018). A systematic review of the inclusion of mechanisms of action in NIH-funded intervention trials to improve medication adherence. Behavior Research & Therapy, 101, 12–19. doi: 10.1016/j.brat.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg IW, Bissett PG, Canning JR, Dallery J, Enkavi AZ, Whitfield-Gabrieli S, … Poldrack RA (2018). Applying novel technologies and methods to inform the ontology of self-regulation. Behavior Research & Therapy, 101, 46–57. doi: 10.1016/j.brat.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenerty S, West C, Davis S, Kaplan S, & Feldman S (2012). The effect of reminder systems on patients’ adherence to treatment. Patient Preference & Adherence, 6, 127. doi: 10.2147/PPA.S26314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher BR, Hartmann-Boyce J, Hinton L, & McManus RJ (2015). The effect of self-monitoring of blood pressure on medication adherence and lifestyle factors: A systematic review and meta-analysis. American Journal of Hypertension, 28(10), 1209–1221. doi: 10.1093/ajh/hpv008 [DOI] [PubMed] [Google Scholar]
- Gadkari AS, & McHorney CA (2012). Unintentional non-adherence to chronic prescription medications: how unintentional is it really? BMC Health Services Research, 12, 98–98. doi: 10.1186/1472-6963-12-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PA, & Fong GT (2007). Temporal self-regulation theory: A model for individual health behavior. Health Psychology Review, 1(1), 6–52. doi: 10.1080/17437190701492437 [DOI] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (redcap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy EA, Johnson BT, & Keenan C (2019). Best Practice Guidelines and Essential Methodological Steps to Conduct Rigorous and Systematic Meta-Reviews. Applied Psychology: Health and Well Being, 11(3), 353–381. doi: 10.1111/aphw.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W, Schmeichel BJ, & Baddeley AD (2012). Executive functions and self-regulation. Trends in Cognitive Sciences, 16(3), 174–180. doi: 10.1016/j.tics.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Ingersoll KS, & Cohen J (2008). The impact of medication regimen factors on adherence to chronic treatment: A review of literature. Journal of Behavioral Medicine, 31(3), 213–224. doi: 10.1007/s10865-007-9147-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C, Eliasson L, Barber N, & Weinman J (2014). Applying com-b to medication adherence: A suggested framework for research and interventions. European Health Psychologist, 16(1), 7–17. [Google Scholar]
- Kardas P, Lewek P, & Matyjaszczyk M (2013). Determinants of patient adherence: A review of systematic reviews. Frontiers in Pharmacology, 4, 91. doi: 10.3389/fphar.2013.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal H, Diefenbach M, & Leventhal EA (1992). Illness cognition: Using common sense to understand treatment adherence and affect cognition interactions. Cognitive Therapy and Research, 16(2), 143–163. doi: 10.1007/BF01173486 [DOI] [Google Scholar]
- Maes S, & Karoly P (2005). Self-regulation assessment and intervention in physical health and illness: A review. Applied Psychology, 54(2), 267–299. doi: 10.1111/j.1464-0597.2005.00210.x [DOI] [Google Scholar]
- Mannheimer SB, Matts J, Telzak E, Chesney M, Child C, Wu AW, … Terry Beirn Community Programs for Clinical Research on AIDS. (2005). Quality of life in HIV-infected individuals receiving antiretroviral therapy is related to adherence. AIDS Care, 17(1), 10–22. doi: 10.1080/09540120412331305098 [DOI] [PubMed] [Google Scholar]
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, & French DP (2011). A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychology & Health, 26(11), 1479–1498. doi: 10.1080/08870446.2010.540664 [DOI] [PubMed] [Google Scholar]
- Michie S, & Johnston M (2012). Theories and techniques of behaviour change: Developing a cumulative science of behaviour change. Health Psychology Review, 6(1), 1–6. doi: 10.1080/17437199.2012.654964 [DOI] [Google Scholar]
- Michie S, Abraham C, Whittington C, McAteer J, & Gupta S (2009). Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychology, 28(6), 690–701. doi: 10.1037/a0016136 [DOI] [PubMed] [Google Scholar]
- Michie S, Hyder N, Walia A, & West R (2011). Development of a taxonomy of behaviour change techniques used in individual behavioural support for smoking cessation. Addictive Behaviors, 36(4), 315–319. doi: 10.1016/j.addbeh.2010.11.016 [DOI] [PubMed] [Google Scholar]
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, … Wood CE (2013). The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine, 46(1), 81–95. doi: 10.1007/s12160-013-9486-6 [DOI] [PubMed] [Google Scholar]
- Miller AL, Gearhardt AN, Fredericks EM, Katz B, Shapiro LF, Holden K, … Lumeng JC (2018). Targeting self-regulation to promote health behaviors in children. Behavior Research & Therapy, 101, 71–81. doi: 10.1016/j.brat.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojtabai R, & Olfson M (2003). Medication costs, adherence, and health outcomes among medicare beneficiaries. Health Affairs, 22(4), 220–229. doi: 10.1377/hlthaff.22.4.220 [DOI] [PubMed] [Google Scholar]
- Moore SM, Schiffman R, Waldrop-Valverde D, Redeker NS, McCloskey DJ, Kim MT, … Grady P (2016). Recommendations of common data elements to advance the science of self-management of chronic conditions. Journal of Nursing Scholarship, 48(5), 437–447. doi: 10.1111/jnu.12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman AB, Ruppar T, Ho M, Garber L, Weidle PJ, Hong Y, … Thorpe PG (2017). Cdc grand rounds: Improving medication adherence for chronic disease management - innovations and opportunities. MMWR Morbidity & Mortality Weekly Report, 66(45), 1248–1251. doi: 10.15585/mmwr.mm6645a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S, Steed L, & Mulligan K (2004). Self-management interventions for chronic illness. The Lancet, 364(9444), 1523–1537. doi: 10.1016/S0140-6736(04)17277-2 [DOI] [PubMed] [Google Scholar]
- Nielsen L, Riddle M, King JW, Aklin WM, Chen W, Clark D, … Weber W (2018). The NIH science of behavior change program: Transforming the science through a focus on mechanisms of change. Behaviour Research & Therapy, 101, 3–11. doi: 10.1016/j.brat.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, … Jack S (2014). Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews(11). doi: 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellowski JA, Price DM, Harrison AD, Tuthill EL, Myer L, Operario D, & Lurie MN (2018). A systematic review and meta-analysis of antiretroviral therapy (art) adherence interventions for women living with HIV. AIDS & Behavior, 23(8), 1998–2013. doi: 10.1007/s10461-018-2341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper D, Antoine S-L, Mathes T, Neugebauer EA, & Eikermann M (2014). Systematic review finds overlapping reviews were not mentioned in every other overview. Journal of Clinical Epidemiology, 67(4), 368–375. doi: 10.1016/j.jclinepi.2013.11.007 [DOI] [PubMed] [Google Scholar]
- Rosenstock IM, Strecher VJ, & Becker MH (1988). Social learning theory and the Health Belief Model. Health Education Quarterly, 15(2), 175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- Rothman AJ, Baldwin AS, Hertel AW, & Fuglestad PT (2011). Self-regulation and behavior change: Disentangling behavioral initiation and behavioral maintenance In Vohs KD & Baumeister RF (Eds.), Handbook of Self-Regulation: Research, Theory, and Applications (Vol. 2, pp. 106–122). New York: Guilford Publications. [Google Scholar]
- Ruppar TM, Dunbar-Jacob JM, Mehr DR, Lewis L, & Conn VS (2017). Medication adherence interventions among hypertensive black adults: A systematic review and meta-analysis. Journal of Hypertension, 35(6), 1145–1154. doi: 10.1097/HJH.0000000000001260 [DOI] [PubMed] [Google Scholar]
- Sakakibara BM, Kim AJ, & Eng JJ (2017). A Systematic Review and Meta-Analysis on Self-Management for Improving Risk Factor Control in Stroke Patients. International Journal of Behavioral Medicine, 24(1), 42–53. doi: 10.1007/s12529-016-9582-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, … Kristjansson E (2017). Amstar 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. British Medical Journal, 358, j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol MC, McGuigan KA, Verbrugge RR, & Epstein RS (2005). Impact of medication adherence on hospitalization risk and healthcare cost. Medical Care, 43(6), 521–530. doi: 10.1097/01.mlr.0000163641.86870.af [DOI] [PubMed] [Google Scholar]
- StataCorp L (2017). Stata statistical software. College Station TX. [Google Scholar]
- Stirratt MJ, Curtis JR, Danila MI, Hansen R, Miller MJ, & Gakumo CA (2018). Advancing the science and practice of medication adherence. Journal of General Intern Medicine, 33(2), 216–222. doi: 10.1007/s11606-017-4198-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Beauchaine T, & Nielsen L (2018). A mechanism-focused approach to the science of behavior change: An introduction to the special issue. Behaviour Research and Therapy, 101, 1–2. doi: 10.1016/j.brat.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira PJ, Carraca EV, Marques MM, Rutter H, Oppert JM, De Bourdeaudhuij I, … Brug J (2015). Successful behavior change in obesity interventions in adults: A systematic review of self-regulation mediators. BMC Medicine, 13, 84. doi: 10.1186/s12916-015-0323-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Brunton J, & Graziosi S (2018). Eppi-reviewer 4: Software for research synthesis. Eppi-centre software. London: Social Science Research Unit, UCL Institute of Education; 2010. [Google Scholar]
- Thompson MA, Mugavero MJ, Amico KR, Cargil VA, Chang LW, Gross R, … Nachega JB (2012). Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: Evidence-based recommendations from an international association of physicians in AIDS care panel. Annals of Internal Medicine, 156(11), 817–833. doi: 10.7326/0003-4819-156-11-201206050-00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, … Team, A. B. C. P. (2012). A new taxonomy for describing and defining adherence to medications. British Journal of Clinical Pharmacology, 73(5), 691–705. doi: 10.1111/j.1365-2125.2012.04167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner G, Sieverding M, & Chesney MA (2016). The role of self-regulation in health and illness. Psychology Health & Medicine, 21(2), 135–137. doi: 10.1080/13548506.2015.1115528 [DOI] [PubMed] [Google Scholar]
- Wiecek E, Tonin FS, Torres-Robles A, Benrimoj SI, Fernandez-Llimos F, & Garcia-Cardenas V (2019). Temporal effectiveness of interventions to improve medication adherence: A network meta-analysis. PLoS One, 14(3), e0213432. doi: 10.1371/journal.pone.0213432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC, Rodin GC, Ryan RM, Grolnick WS, & Deci EL (1998). Autonomous regulation and long-term medication adherence in adult outpatients. Health Psychology, 17(3), 269–276. doi: 10.1037//0278-6133.17.3.269 [DOI] [PubMed] [Google Scholar]
- Williams L, Pines A, Goldstein-Piekarski AN, Rosas LG, Kullar M, Sacchet MD, … Ma J (2018). The engage study: Integrating neuroimaging, virtual reality and smartphone sensing to understand self-regulation for managing depression and obesity in a precision medicine model. Behavior Research & Therapy, 101, 58–70. doi: 10.1016/j.brat.2017.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


