Abstract
Pectin extracted via hydrodynamic cavitation in water only from waste lemon peel and further isolated via freeze drying displays significant antibacterial activity against Staphylococcus aureus, a Gram positive pathogen which easily contaminates food. The antibacterial effect of the new IntegroPectin is largely superior to that of commercial citrus pectin, opening the way to advanced applications of a new bioproduct now obtainable in large amounts and at low cost from citrus juice industry's waste.
Keywords: pectin, antibacterial, citrus flavonoids, hydrodynamic cavitation, IntegroPectin
Bacteria vs. lemons! Pectin extracted via hydrodynamic cavitation in water only from waste lemon peel and further isolated via freeze drying displays significant antibacterial activity against Staphylococcus aureus, a Gram positive pathogen which easily contaminates food.
The ability to control bacterial growth and colonization is of extreme practical importance in several fields, including prevention of food contamination in the food chain,1 in healthcare2 and even in the protection of cultural heritage to reduce biodeterioration processes.3 In the last years, the emergence of multi‐drug resistant strains occurred in several animals and environments, including wild and uncontaminated ones.4 One of the most frequent antibiotic resistant strain causing food poisoning is the Gram positive Staphylococcus aureus.1, 2
Intense researches are devoted in several countries to find new, harmless and cost‐effective compounds and materials alternative to antibiotics to prevent the spread of bacteria, especially in foodstuff,5, 6 food contact surfaces,7 and in food processing environments, including hydrophobic coatings preventing or minimizing condensation which creates a number of hazards in food manufacturing facilities.8
Amid natural antibacterials, the surprising (in vitro) antibacterial properties of pectin were first reported by Russian scholars in the late 1990s.9 The team found that amid four food fibers (wheat bran, soya isolate, soybean flour, and pectin), pectin is the only food fiber showing bactericidal activity on the most widely distributed pathogenic and opportunistic microorganisms, inactivating therapeutic bacteriophages at high concentration (>2 per cent), and decreasing the antimicrobial activity of antibiotic penicillins.9
These findings remained almost ignored until the second decade of the 2000s, when a number of studies started to report a number of new pectin‐based substances with high bactericidal activity.10, 11, 12
Developed in the context of the emerging bioeconomy of pectin,13 these studies focused in particular on the antimicrobial effect of Modified Citrus Pectin (MCP). The latter is a form of pectin obtained from the controlled enzymatic degradation of citrus pectin with low molecular weight (<15 kDa) and a degree of esterification <5 % which, contrary to unmodified pectin, easily undergoes intestinal absorption.14
In 2015, scholars in Lebanon reported the antibacterial activity of citrus pectin against S. aureus,11 a virulent pathogen which frequently contaminates food, including milk, cheese, meat, and dairy preparations.15 Pectin showed the highest antibacterial activity at pH 6 with minimum inhibitory concentration (MIC) values ranging from 0.39 mg mL−1 to 3.125 mg mL−1.11
We have lately reported the discovery of IntegroPectin, namely citrus pectin isolated from orange16 or lemon17 peel via controlled hydrodynamic cavitation carried out on waste orange peel or waste lemon peel in water only.
Colored in yellow, and bearing a delicate fragrance pointing to the presence of lemon peel terpenes, IntegroPectin isolated via lyophilization of the water phase extract upon hydrodynamic cavitation in 120 L water of 34 kg waste peel of organically grown Siracusa lemons obtained by an in‐line extractor at a juice factory, has exceptionally high antioxidant and non‐cytotoxic activity.17
We now report that lemon IntegroPectin shows also significant antimicrobial activity against S. aureus, considerably higher than commercial citrus pectin obtained via hydrolysis with mineral acids of dried lemon peel, followed by prolonged separation and purification of the inevitably degraded pectic polymer.18
The antimicrobial activities of lemon IntegroPectin obtained using the same process described in previous studies16, 17 and commercial citrus pectin (galacturonic acid ≥74.0 %, dried basis) purchased from Sigma‐Aldrich (Merck Life Science, Milan, Italy) were tested against Staphylococcus aureus ATCC 25923 strain.
The antibacterial activity was preliminarily assessed through the disc diffusion antibiotic sensitivity assay.19 Briefly, a concentrated bacterial suspension (107 cells) was spread onto the Luria Bertani [hereinafter named as LB and composed of (g L−1): sodium chloride, 10; yeast extract, 5; tryptone, 10] agar plates (containing 20 % agar, hereafter named LB‐agar). IntegroPectin and commercial pectin were dissolved in sterile distilled water, with 20 μL solutions spotted directly on the bacterial lawn. Plates were incubated at 37 °C overnight (ca. 16 hours). Antibacterial activity was evaluated as growth inhibition halos around the spots. The growth of the strain was inhibited in the presence of both pectins (data not shown).
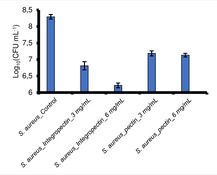
Moreover, to evaluate the actual number of viable Colony Forming Units (CFU) per milliliter of culture, the broth microdilution method was carried out. S. aureus cells were challenged in the presence of 3 and 6 mg mL−1 of either IntegroPectin or commercial pectin in liquid rich LB medium for 24 h at 37 °C under mechanical shacking (180 rpm). A non‐challenged bacterial suspension was incubated in parallel and used as control. After 24 h, all cultures were serially diluted and aliquots (20 μL) of opportune dilutions were spotted onto mannitol salt agar plates, recovered at 37 °C under static mode.20 The number of viable CFU mL−1 is reported (Figure 1) in logarithmic (Log10) scale with standard deviation (n=3).
Figure 1.
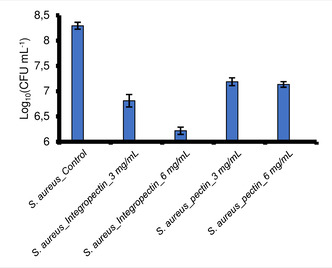
Viable cells of Staphylococcus aureus ATCC 25923 in the presence of lemon IntegroPectin and of commercial citrus pectin.
Evaluation of viable bacterial count shows that both pectin and IntegroPectin inhibited S. aureus growth, with a decrease in the number of viable cells in the range 1 to 2 log units, respectively, when the concentration was 3 mg mL−1. In closer detail, the Log10 (CFU) went from 8.3 for the control to 6.8 and 7.2 for IntegroPectin and commercial citrus pectin, respectively (Figure 1 and 2).
Figure 2.
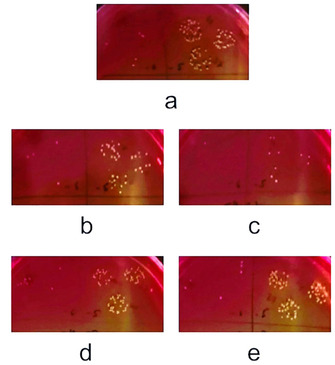
MSA plates depicting CFU of S. aureus unchallenged culture (a), and after challenge with either 3 or 6 mg mL−1 of lemon IntegroPectin (b,c), and of commercial citrus pectin (d,e).
A higher difference in antibacterial activity of commercial pectin and lemon IntegroPectin was noted when cultures were challenged with 6 mg mL−1. Indeed, in presence 6 mg mL−1 of commercial pectin the Log10 (CFU) remained almost unvaried (from 7.2 to 7.1); differently, doubling the concentration of lemon IntegroPectin, the viable bacterial count decreased from 6.8 to 6.2, therefore highlighting the greater antimicrobial potential of lemon IntegroPectin as compared to commercial pectin.
A detailed analysis of the yellow and perfumed IntegroPectin is in course and will be reported shortly.
We ascribe the aforementioned difference between IntegroPectin and commercial citrus pectin to the combined antibacterial action of lemon oil (present in IntegroPectin in the form of a highly stable nanoemulsion in the aqueous phase after the hydrocavitation process),16 and of the water‐soluble lemon flavonoids found in abundant amount at the surface of lyophilized IntegroPectin where they are responsible also for the powerful antioxidant activity of lemon IntegroPectin.17
Lemon oil is known to exert antibacterial activity against food‐borne bacteria, though being the least effective in reducing S. aureus strain when compared to mandarine (the best), grapefruit and orange oil.21 Reported in the early 1970s,22 the antibacterial action of citrus bioflavonoids, hydroxylated phenolic molecules synthesized by plants in response to microbial infection,23 today is the subject of intense anti‐infective applied research.24 Oral antimicrobial formulations whose active ingredients are soluble citrus bioflavonoids, for instance, are successfully commercialized.25 Lemon flavonoids including the flavanones hesperidin (hesperetin 7‐O‐beta‐rutinoside) and eriocitrin (eriodictyol 7‐O‐beta‐rutinoside), and the flavone diosmin (diosmetin 7‐O‐rutinoside) exert numerous and different biological functions, including antioxidative, anti‐inflammatory, antiviral, antiproliferative, antimutagenic, antiallergic, and anticarcinogenic activities.26 Hesperidin and eriocitrin are chiefly contained in the albedo and in the flavedo and to a lesser extent in the pulp (levels in the albedo are 2‐fold higher than in the flavedo, and 4–6‐fold higher than the levels detected in pulp), whereas poorly soluble diosmin is almost entirely contained in the flavedo and in the albedo,26 suggesting that waste lemon peel is an ideal source of these important biomolecules.
In conclusion, we have discovered that lemon pectin obtained via hydrodynamic cavitation of citrus industry's waste lemon peel (IntegroPectin) displays significant antibacterial activity against Staphylococcus aureus, a Gram positive pathogen which easily contaminates food.
The antibacterial effect of the new IntegroPectin is largely superior to that of commercial citrus pectin, opening the way to advanced applications of a new bioproduct now obtainable in large amounts and at low cost from citrus juice industry's waste.
In the context of the emerging bioeconomy of lemon,27 these results, added to the lack of cytotoxicity of this new biomaterial, open the route to numerous new applications of this new biomaterial, including (but not limited to) antibacterial pectin‐based coatings for food packaging and new antibacterial aerogel biomaterials.28
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This article is dedicated to the memory of Professor Vassili Nesterenko (1934–2008) for all he did to promote the use of pectin to reduce contamination levels in people living in regions affected by nuclear fallout. Thanks to Campisi Italia (Siracusa, Italy) for the gift of waste lemon peel from which the IntegroPecting used in this study was obtained.
A. Presentato, A. Scurria, L. Albanese, C. Lino, M. Sciortino, M. Pagliaro, F. Zabini, F. Meneguzzo, R. Alduina, D. Nuzzo, R. Ciriminna, ChemistryOpen 2020, 9, 628.
Contributor Information
Prof. Rosa Alduina, Email: valeria.alduina@unipa.it.
Dr. Domenico Nuzzo, Email: domenico.nuzzo@irib.cnr.it.
Dr. Rosaria Ciriminna, Email: rosaria.ciriminna@cnr.it.
References
- 1. Nerín C., Aznar M., Carrizo D., Trends Food Sci. Technol. 2016, 48, 63. [Google Scholar]
- 2. Vitale M., Galluzzo P., Buffa P. G., Carlino E., Spezia O., Alduina R., Antibiotics 2019, 8, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barresi G., Cammarata M., Palla F., Biotechnology and Conservation of Cultural Heritage, F. Palla, G. Barresi (Eds.), Springer, Cham, 2014; pp. 49–65. [Google Scholar]
- 4. Alduina R., Gambino D., Presentato A., Gentile A., Sucato A., Savoca D., Filippello S., Visconti G., Caracappa G., Vicari D., Arculeo M., Antibiotics 2020, 9, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ciriminna R., Meneguzzo F., Delisi R., Pagliaro M., ChemistrySelect 2017, 2, 1360. [Google Scholar]
- 6. Tiwari B. K., Valdramidis V. P., O’ Donnell C. P., Muthukumarappan K., Bourke P., Cullen P. J., J. Agric. Food Chem. 2009, 57, 5987. [DOI] [PubMed] [Google Scholar]
- 7. Torres Dominguez E., Nguyen P. H., Hunt H. K., Mustapha A., Compr. Rev. Food Sci. Food Saf. 2019, 18, 1825. [DOI] [PubMed] [Google Scholar]
- 8. Bartholomew G. W., Food Safety Magazine 2015, 21 (4), 46. [Google Scholar]
- 9. Men'shikov D. D., Lazareva E. B., Popova T. S., Shramko L. U., Tokaev I. S., Zalogueva G. V., Gaponova I. N., Antibiot. Khimioter. 1997, 42, 10. [PubMed] [Google Scholar]
- 10. Kukovinets O. S., Mudarisova R. K., Plekhanova D. F., Tarasova A. V., Abdullin M. I., Russ. J. Appl. Chem. 2014, 87, 1524. [Google Scholar]
- 11. Abdel-Massih R. M., Hawach V., Daoud Z., J. Nutr. Food Sci. 2015, 5, 4. [Google Scholar]
- 12. Dahdouh E., El-Khatib S., Baydoun E., Abdel-Massih R. M., Med. Chem. 2017, 13, 682. [DOI] [PubMed] [Google Scholar]
- 13. Ciriminna R., Chavarría-Hernández N., Rodríguez Hernández A., Pagliaro M., Biofuel. Biopr. Bioref. 2015, 9, 368. [Google Scholar]
- 14. Courts F. L., PharmaNutrition 2013, 1, 22. [Google Scholar]
- 15. Vitale M., Gaglio S., Galluzzo P., Cascone G., Piraino C., Di Marco Lo Presti V., Alduina R., Foodborne Pathog. Dis. 2018, 15, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meneguzzo F., Brunetti C., Fidalgo A., Ciriminna R., Delisi R., Albanese L., Zabini F., Gori A., dos Santos Nascimento L. B., De Carlo A., Ferrini F., Ilharco L. M., Pagliaro M., Processes 2019, 7, 581. [Google Scholar]
- 17. Nuzzo D., Cristaldi L., Sciortino M., Albanese L., Scurria A., Zabini F., Lino C., Pagliaro M., Meneguzzo F., Di Carlo M., Ciriminna R., ChemistrySelect 2020, 5, 5066. [Google Scholar]
- 18. Ciriminna R., Fidalgo A., Delisi R., Ilharco L. M., Pagliaro M., Agro-Food-Ind. Hi-Tech 2016, 27 (5), 17. [Google Scholar]
- 19. Dresler C., Saladino M. L., Demirbag C., Caponetti E., Chillura Martino D. F., Alduina R., Int. Biodeterior. Biodegrad. 2017, 125, 150. [Google Scholar]
- 20. Presentato A., Piacenza E., Darbandi A., Anikovskiy M., Cappelletti M., Zannoni D., Turner R. J., Sci. Rep. 2018, 8, 3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dabbah R., Edwards V. M., Moats W. A., Appl. Microbiol. 1970, 19, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramaswamy A. S., Jayaraman S., Sirsi M., Rao K. H., Indian J. Exp. Biol. 1972, 10, 72. [PubMed] [Google Scholar]
- 23. Kumar S., Pandey A. K., Sci. World J. 2013, 2013, 162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cushnie T. P., Lamb A. J., Int. J. Antimicrob. Agents 2005, 26, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hooper S. J., Lewis M. A. O., Wilson M. J., Williams D. W., Br. Dent. J. 2011, 210, E22. [DOI] [PubMed] [Google Scholar]
- 26. Del Río J. A., Fuster M. D., Gómez P., Porras I., García-Lidón A., Ortuño A., Food Chem. 2004, 84, 457. [Google Scholar]
- 27. Ciriminna R., Fidalgo A., Scurria A., Sciortino M., Lino C., Meneguzzo F., Ilharco L. M., Pagliaro M., Adv. Sustainable Syst. 2020, 2000006. [Google Scholar]
- 28. Groult S., Budtova T., Eur. Polym. J. 2018, 108, 250. [DOI] [PubMed] [Google Scholar]


